Abstract
Purpose
Survivors of childhood cancer carry a substantial burden of long-term morbidity; personal risk awareness is critical to ensure survivors' engagement in early detection/management of complications. The impact of education provided in survivorship clinics on survivors' understanding of their personal health risks is unclear.
Methods
Patients diagnosed with cancer at age 21 years or younger and at 2 or more years off therapy completed questionnaires about awareness of personal risk for therapy-related complications at T0 (first survivorship clinic visit) and at T1 to T5 (subsequent visits). After questionnaire completion at each clinic visit, survivors received education tailored to personal risk.
Results
A total of 369 survivors completed 1,248 visits (median, three visits; range, one to six visits). The median age at cancer diagnosis was 11 years (range, 0 to 21 years); the median age at T0 was 24 years (range, 5 to 57 years); 38% were white; 45% had leukemia; and 34% received hematopoietic cell transplantation. The cohort was at risk for a median of six (range, one to nine) complications. Awareness increased from 38.6% at T0 to 66.3% at T3. Generalized estimating equations (that adjusted for diagnosis, hematopoietic cell transplantation, race/ethnicity, and patient/parent education) showed significant gains in awareness from T0 to T1 (P < .001), T1 to T2 (P = .03), and T2 to T3 (P < .001) but no significant gain thereafter through T5 (P = .7). Predictors of low awareness included education less than a college degree (odds ratio [OR], 1.9; P = .02), longer time from diagnosis (OR, 1.03/year; P = .04), diagnosis of leukemia (OR, 2.1; P = .004), nonwhite race (OR, 2.8; P < .001), and risk for six or fewer complications (OR, 2.1; P = .002).
Conclusion
Risk-based education in a survivorship clinic significantly increases awareness of personal health risk through three sessions, with saturation thereafter. Vulnerable populations with minimal gain in awareness identified in this study could inform targeted interventions.
INTRODUCTION
Contemporary risk-based therapy for childhood cancer has resulted in 5-year survival rates that now exceed 80%1; increasing attention, therefore, is being focused on the health and well-being of the growing population of long-term survivors.2 Treatment-related chronic health conditions are well described in this population and include vital organ compromise,3–9 endocrine disturbances,10–12 neurocognitive impairment,13–15 issues related to fertility and reproduction,16–18 and subsequent malignant neoplasms19–23; the cumulative incidence of severe or life-threatening chronic health conditions approaches 40% by 30 years from diagnosis.24
Because many chronic health conditions do not become clinically apparent for years after therapeutic exposure, ongoing risk-based care for survivors of childhood cancer is imperative.25–27 Specialized long-term follow-up (LTFU) programs have been established at many pediatric oncology centers to provide survivors with risk-directed care aimed at decreasing morbidity and mortality through targeted health promotion, early identification of therapy-related complications, and timely interventions to ameliorate those complications.25,28,29 These programs rely on LTFU guidelines,30–32 including the Children's Oncology Group (COG) Long-Term Follow-Up Guidelines,33,34 to direct provision of risk-based survivorship care. The COG guidelines are accompanied by lay educational materials (Health Links)35 to inform survivors of exposure-specific health risks and promote health-protective behaviors.
Previous studies to evaluate adult survivors of childhood cancer have identified significant knowledge deficits,36–38 and only 35% of survivors have acknowledged that past therapies could cause serious health problems.36 Survivors who lack awareness about their health risks may fail to seek adequate follow-up care. Therefore, a major focus of specialized LTFU programs has been to improve awareness of risk for chronic health conditions in survivors. Physicians and nurses in these specialized LTFU programs typically provide education to survivors about their treatment history and health risks.39–42 However, the impact of specialized education provided in these programs on survivors' awareness of their personal health risks is unclear. The current study addressed this gap in knowledge by assessing the trajectory of change in survivors' awareness of personal risk for developing therapy-related complications after receipt of targeted education at up to five survivorship clinic visits; predictors of those who lacked risk awareness were also examined.
METHODS
Study Participants
Participants were enrolled in the survivorship clinic at a single institution between December 2005 and September 2013. Eligibility for clinic participation included a diagnosis of cancer at age 21 or younger, in remission, and off cancer therapy for at least 2 years. Institutional review board approval for the study was obtained; all participants and/or their parents provided informed consent/assent.
Health Knowledge Questionnaire
Patients were eligible if they completed a health knowledge questionnaire at their initial survivorship clinic visit and had not previously attended any specialized LTFU program. Thus, questionnaire completion occurred before provision of any tailored education by the clinician at the baseline visit (T0) and at up to five subsequent LTFU visits (T1 through T5; Data Supplement). To accurately evaluate change in awareness of personal therapy-related health risks over time, and to ensure that the individual who completed the questionnaire was present for all teaching, questionnaires completed by individuals other than the baseline respondent were excluded from the analysis at subsequent time points. The questionnaire, available in both English and Spanish, consisted of 19 items that assessed the survivors' knowledge of their cancer therapy and awareness of therapy-related health risks. For this analysis, we focused on the following item: “Have you ever been told that you might experience any of the following problems as a result of the treatment that you received for this illness?” Potential therapy-related complications were listed with the response choices yes, no, or don't know. At-risk patients who answered yes were categorized as aware; those who answered no or don't know were categorized as lacking awareness. For survivors younger than 13 years or those with cognitive impairment, the questionnaire was completed by the survivor's parent/caregiver; survivors between 13 and 15 years completed the questionnaire with assistance from their parent/caregiver; survivors 16 years or older completed the questionnaire independently.
Tailored Health Education
After completion of the questionnaire at each LTFU clinic visit, a nurse practitioner or physician provided education tailored to the survivor's personal risk for therapy-related late effects per the COG-LTFU Guidelines. Tailored education included a review of the patient's cancer treatment summary (Data Supplement) and followed a structured format guided by personalized teaching materials that contained simplified health promotion messages derived from and corresponding to the COG Health Links.35 Teaching materials used for each survivorship clinic visit were customized to individual survivors on the basis of age, sex, and therapeutic exposures. Study clinicians were involved in initial development and implementation of the materials or were trained in their standardized application upon joining the study team. Tailored materials were provided to clinicians by the research assistant, which prompted consistent use. Several strategies previously shown to enhance comprehension of patient education materials43–45 were incorporated into the design; these included organization of recommendations by body system, use of simplified fonts and bulleted lists, personalization, use of visual cues (icons) to introduce each topic, use of the second person (you) throughout, and preparation/presentation of materials in the survivor's preferred language (Data Supplement).
Main Outcome Measure: Awareness of Health Risks
Therapeutic exposures were abstracted from medical records. Exposure-related health risks were determined according to an algorithm that was based on the COG-LTFU Guidelines.33 For example, survivors who received anthracycline chemotherapy were categorized as at risk for both cardiac dysfunction and subsequent malignant neoplasms, because the COG-LTFU Guidelines specify an association between anthracyclines and each of these complications (Data Supplement).34 Each survivor's responses regarding their awareness of health risks were compared with their actual exposure-related health risks for nine therapy-related complications that are highly prevalent and/or associated with potentially serious health consequences (ie, cardiac dysfunction, pulmonary compromise, neurocognitive impairment, low bone mineral density, sensory impairment, renal impairment, thyroid problems, fertility problems, and subsequent malignant neoplasms).24,46,47 Awareness was defined as the proportion of personal risk of all complications correctly identified by the survivor at each time point.
Statistical Analyses
Descriptive statistics were used to summarize demographic and clinical characteristics of study participants, knowledge of their therapeutic exposures, and awareness of personal risk for therapy-related complications (ie, proportion correct). Generalized estimating equations were used to examine overall awareness of personal health risks (ie, proportion of personal risk correctly identified for all at-risk complications). Survivors were classified into three groups (lowest, middle, and highest tertile of awareness) according to their overall level of awareness of risk for therapy-related complications at T0. Logistic regression was used to determine clinical and sociodemographic predictors (ie, diagnosis, sex, race/ethnicity, language, age at diagnosis and at study entry, time from diagnosis, educational level, number of at-risk complications) of low awareness (ie, being in the lowest tertile of awareness). Data were analyzed with SAS (SAS Institute, Cary, NC), version 9.3.
RESULTS
Participant Characteristics
Three hundred sixty-nine patients met eligibility criteria for the study (Table 1) and completed a total of 1,248 clinic visits; the median age at diagnosis was 11 years (range, 0 to 21 years); the median age at study entry was 24 years (range, 5 to 57 years). Overall, 53% were male, 38% were non-Hispanic white, and 45% had a diagnosis of leukemia. The median time between two consecutive visits was 1 year (Data Supplement); all patients who completed the questionnaire at each time point had completed all prior time points (ie, there were no missing education visits). Participants at T1, T2, T3, and T4 were older at diagnosis and study entry than nonparticipants at these time points; time from diagnosis was longer for participants than nonparticipants at T4 only. There was no substantial difference in participant characteristics and no statistically significant difference in change in awareness between patients who did and did not complete T5 (Data Supplement).
Table 1.
Participant Characteristics at Baseline
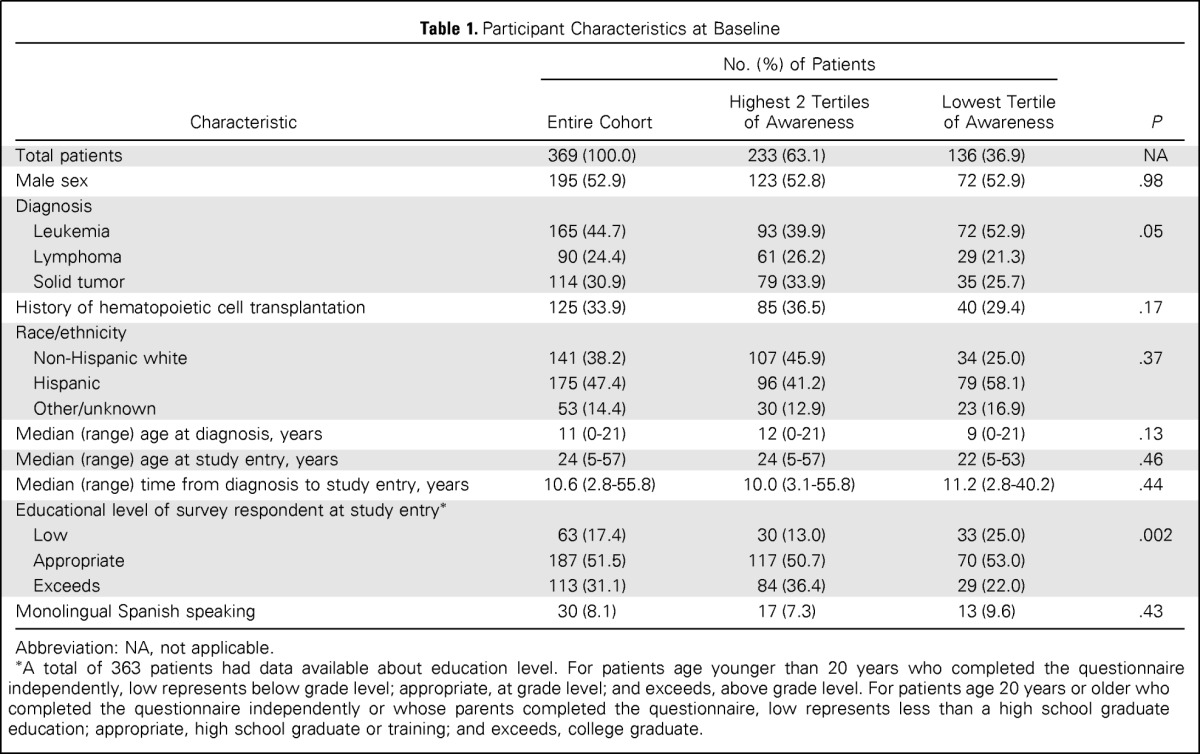
| Characteristic | No. (%) of Patients |
P | ||
|---|---|---|---|---|
| Entire Cohort | Highest 2 Tertiles of Awareness | Lowest Tertile of Awareness | ||
| Total patients | 369 (100.0) | 233 (63.1) | 136 (36.9) | NA |
| Male sex | 195 (52.9) | 123 (52.8) | 72 (52.9) | .98 |
| Diagnosis | ||||
| Leukemia | 165 (44.7) | 93 (39.9) | 72 (52.9) | .05 |
| Lymphoma | 90 (24.4) | 61 (26.2) | 29 (21.3) | |
| Solid tumor | 114 (30.9) | 79 (33.9) | 35 (25.7) | |
| History of hematopoietic cell transplantation | 125 (33.9) | 85 (36.5) | 40 (29.4) | .17 |
| Race/ethnicity | ||||
| Non-Hispanic white | 141 (38.2) | 107 (45.9) | 34 (25.0) | .37 |
| Hispanic | 175 (47.4) | 96 (41.2) | 79 (58.1) | |
| Other/unknown | 53 (14.4) | 30 (12.9) | 23 (16.9) | |
| Median (range) age at diagnosis, years | 11 (0-21) | 12 (0-21) | 9 (0-21) | .13 |
| Median (range) age at study entry, years | 24 (5-57) | 24 (5-57) | 22 (5-53) | .46 |
| Median (range) time from diagnosis to study entry, years | 10.6 (2.8-55.8) | 10.0 (3.1-55.8) | 11.2 (2.8-40.2) | .44 |
| Educational level of survey respondent at study entry* | ||||
| Low | 63 (17.4) | 30 (13.0) | 33 (25.0) | .002 |
| Appropriate | 187 (51.5) | 117 (50.7) | 70 (53.0) | |
| Exceeds | 113 (31.1) | 84 (36.4) | 29 (22.0) | |
| Monolingual Spanish speaking | 30 (8.1) | 17 (7.3) | 13 (9.6) | .43 |
Abbreviation: NA, not applicable.
A total of 363 patients had data available about education level. For patients age younger than 20 years who completed the questionnaire independently, low represents below grade level; appropriate, at grade level; and exceeds, above grade level. For patients age 20 years or older who completed the questionnaire independently or whose parents completed the questionnaire, low represents less than a high school graduate education; appropriate, high school graduate or training; and exceeds, college graduate.
Two hundred eighty-three survivors (76.7%) completed the questionnaire independently; 17 (4.6%) completed the questionnaire with parent/caregiver assistance; and the questionnaire was completed by parent/caregiver alone for 69 of the survivors (18.7%). Because the large majority of survivors completed the questionnaire independently, the term survivors is used to generically refer to responses from all questionnaire respondents in this report.
Therapeutic Exposures
Of the 369 patients in the cohort, 352 (95.4%) received chemotherapy, 209 (56.6%) received radiation, 336 (91.1%) underwent surgery (including central line insertion), and 125 (33.9%) underwent hematopoietic cell transplantation (HCT) as part of their cancer treatment. At baseline, survivors were able to correctly identify general categories of their therapeutic exposures with a high degree of accuracy (ie, any chemotherapy, 98.3%; any radiation, 98.6%; any surgery, 83.8%; HCT, 98.4%). Survivors' abilities to identify these broad categories of therapeutic exposures did not change significantly at subsequent LTFU clinic visits.
Health Risks
The cohort was at risk for a median of six (range, one to nine) of the nine late complications evaluated in this study. (The proportion of patients at risk for each complication by clinic visit is presented in the Data Supplement.) All patients were at risk for at least one complication; 10% were at-risk for 3 or fewer; 66%, for four to eight; and 24%, for all nine complications (Fig 1; Data Supplement).
Fig 1.
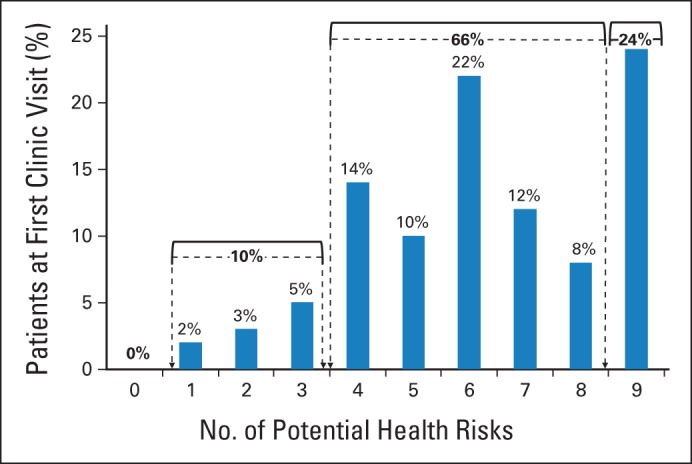
Potential health risks per patient.
Awareness of Personal Health Risks
Seventy percent of the patients were seen primarily by a nurse practitioner, and 30%, by a physician. Survivors' awareness of their risk for therapy-related complications improved after three clinic visits (Fig 2). At baseline, awareness ranged from a low of 25% for renal dysfunction to a high of 63% for fertility problems; after three clinic visits, awareness remained lowest (35%) for renal dysfunction and highest (82%) for fertility problems. The largest net gain in awareness was for neurocognitive impairment (44% awareness at baseline; 81%, after three visits), whereas the smallest net gain in awareness was for renal dysfunction (10%).
Fig 2.
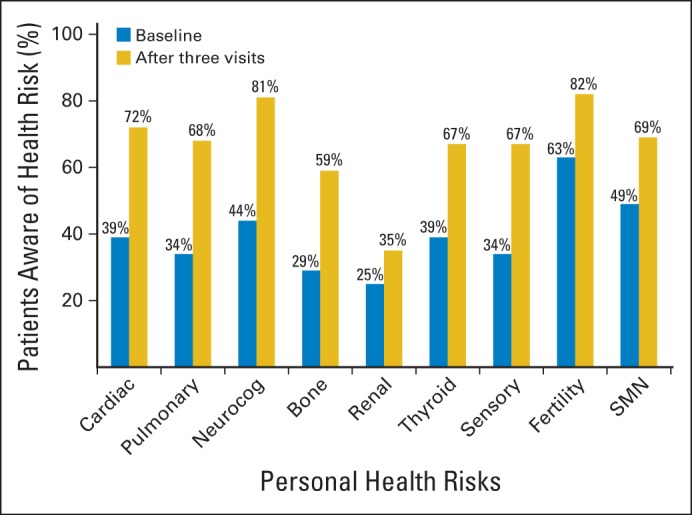
Awareness of personal health risks by complication type at study entry and after three education sessions. SMN, subsequent malignant neoplasm.
By using generalized estimating equations with adjustment for primary cancer diagnosis, history of HCT, race/ethnicity, and patient/parental educational level, we found that overall awareness of personal health risks (ie, proportion of personal risk correctly identified for all at-risk complications) increased from 38.6% at T0 to 66.4% at T3 (Fig 3). There was a significant gain in awareness from T0 to T1 (P < .001), T1 to T2 (P = .03), and T2 to T3 (P < .001), but no significant gain occurred thereafter through T5 (P = .7). When the analysis was limited to patients who completed all study time points (n = 88), the trajectory of increasing awareness was similar (Data Supplement). Although 42% of survivors at baseline had no or minimal (< 25%) awareness of their personal health risks, and although only 14% of survivors at baseline were aware of all or nearly all (> 75%) of their personal health risks, only 13% of survivors had no or minimal awareness after three educational sessions, and 40% of survivors were aware of all or nearly all of their personal health risks after three educational sessions (Data Supplement).
Fig 3.
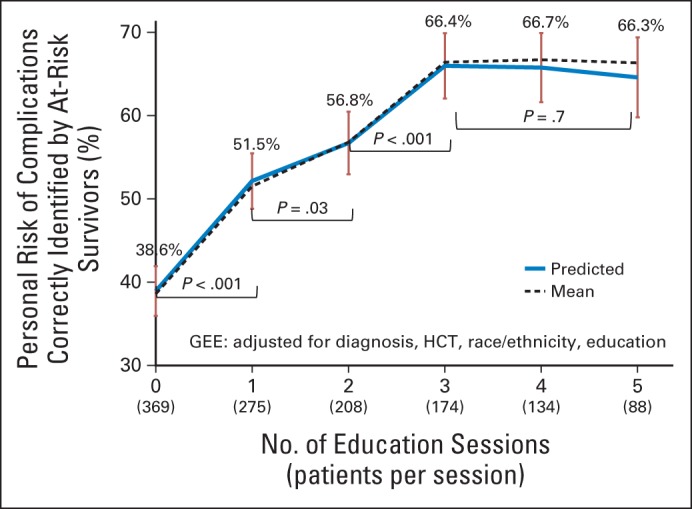
Trajectory of awareness of personal risk for late complications by number of education sessions. GEE, generalized estimating equations; HCT, hematopoietic cell transplantation.
Trajectory of Awareness of Personal Health Risks
Survivors in the lowest tertile of awareness at T0 demonstrated a steeper gain in awareness over time compared with survivors in the higher tertiles of awareness (P < .001; Fig 4). Change in awareness did not significantly differ by clinician type (physician v nurse practitioner; P = .66; Data Supplement).
Fig 4.
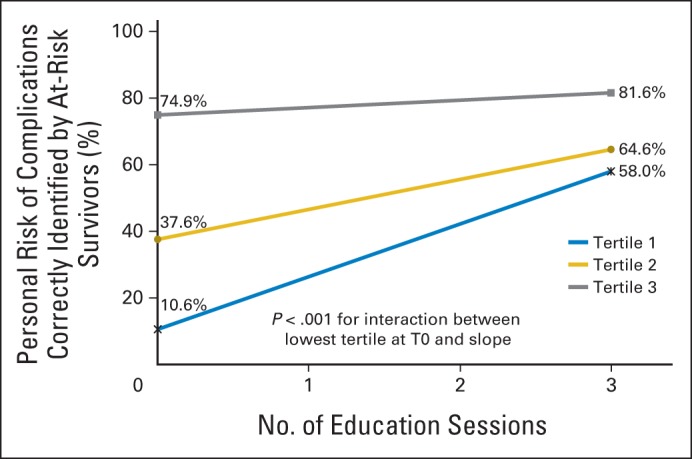
Awareness of personal risk for late complications by tertile.
Predictors for Lowest Tertile of Awareness of Personal Health Risks
In a multivariable logistic regression model adjusted for respondent type (patient v parent/caregiver) and history of HCT (yes/no), factors significantly associated with being in the lowest tertile of awareness at baseline included lower educational level (ie, less than high school graduate if age 20 years or older, or below grade level if age < 20 years; odds ratio [OR], 1.9; 95% CI, 1.2 to 3.4; P = .02), longer time from diagnosis (OR, 1.03/year; 95% CI, 1.0 to 1.06; P = .04), diagnosis of leukemia (OR, 2.1; 95% CI, 1.2 to 3.1; P = .004; referent group: solid tumors and lymphoma), nonwhite race (OR, 2.8; 95% CI, 1.6 to 4.5; P < .001); and at risk for six or fewer complications (OR, 2.1; 95% CI, 1.3 to 3.4, P = .002).
DISCUSSION
In this cohort of survivors of childhood cancer who attended a specialized LTFU clinic, in which each survivor received clinician-delivered education tailored to their specific risk of therapy-related complications, we found that survivors' awareness of personal health risks significantly increased after each of the first three consecutive clinic visits and plateaued thereafter. To our knowledge, this is the first study to evaluate childhood cancer survivors' changes in awareness about their personal health risks over time.
Consistent with previous studies,36,48,49 survivors in our cohort were able to identify their therapeutic exposures within broad categories (ie, chemotherapy, radiation, surgery, transplantation) with a high degree of accuracy at study entry; we found no significant change in survivors' awareness about their exposures after receipt of tailored education over multiple time points.
Also consistent with previous studies, we showed that a considerable proportion of survivors of childhood cancer underestimate their risk for therapy-related complications.48–51 At baseline, our cohort correctly identified only 39% of the complications for which they were at risk, and only 14% of the survivors were aware of all or nearly all (> 75%) of their personal health risks. Awareness of risk for therapy-related complications improved significantly after three education sessions, such that the cohort could correctly identify 66% of the complications for which they were at risk, and 40% of the survivors were aware of all or nearly all of their personal health risks. However, despite repeatedly receiving tailored education in a specialized survivorship program, fully 60% of survivors failed to achieve awareness of greater than 75% of the complications for which they were at risk.
Self-awareness of health risks is important, because survivors of childhood cancer need to serve as advocates for their own health across settings within the health care system. As time from diagnosis increases, survivors become less likely to receive cancer-related care.52,53 When survivors enter young adulthood, the vast majority of their medical care is delivered by primary care providers, who are often unfamiliar with therapy-related risks.53 Thus, survivors who lack awareness of their personal health risks may fail to seek and receive the appropriate risk-directed follow-up care necessary for prevention and early detection of late-onset therapy-related complications.25,26,29,54–58
We found that survivors' awareness of their specific therapy-related risks was lowest at baseline for renal dysfunction (25%) and low bone mineral density (29%) and highest for fertility problems (63%). The largest net gain in awareness after three clinic visits was for neurocognitive impairment (37% net gain); awareness also increased similarly for pulmonary dysfunction (34% net gain), cardiac dysfunction and sensory impairment (33% net gain for each), and low bone mineral density (30% net gain). It is possible that screening tests for these complications (eg, neurocognitive testing, pulmonary function tests, echocardiograms, visual acuity testing, audiograms, and dual energy x-ray absorptiometry scans) may have served to raise awareness and improve recall of risk for these complications beyond the awareness gained through targeted education, whereas some complications for which the net gain in awareness was lower (ie, renal dysfunction and thyroid dysfunction) had associated screening tests (ie, laboratory testing) that may have been less memorable to the survivors. The net gain in awareness for fertility problems was also low (19%). However, because baseline awareness about fertility problems was already high, the lower gain in awareness may represent a ceiling effect. It is also possible that clinicians placed more emphasis on some complications than others and that certain complications may seem more relevant to life concerns of the predominantly young adult survivor population evaluated in this study (eg, fertility and its implications for intimate relationships and family planning,16,17,59 heart/lung function and its association with the ability to participate in recreational activities that require physical exertion,5,6,60 neurocognitive function and its relationship to academic and occupational success13–15,59).
We found that certain subpopulations were at particular risk of being in the lowest tertile of awareness at study entry. These included survivors who were farther from diagnosis, were of nonwhite race, had lower educational levels, were at risk for six or fewer complications, and had a diagnosis of leukemia. It is possible that survivors from these groups received less information about their therapy-related risks in previous encounters with the health care system, possibly because the health care providers had a lower level of concern about the actual risks (eg, survivors at risk for fewer complications or those with a history of lower-risk leukemia).54,57,61,62 It is also possible that the survivors simply did not understand or recall the information that was provided.36,48–51 Encouragingly, we found that survivors in the lowest tertile of awareness at baseline derived the most benefit from the educational sessions, as evidenced by their steeper gain in awareness over time.
Strengths of the study include the prospective design, the size and heterogeneity of the cohort, the availability of detailed medical records that allowed identification of risk profiles for individual survivors, the measurement of change in survivors' awareness of therapy-related health risks over time, and the strategy that provided for a full year between provision of education and each subsequent measurement, which allowed for the first time evaluation of long-term retention of risk awareness in survivors. Enduring acquisition of risk awareness by the survivors is substantiated by the fact that, although there was no additional increase in awareness after three clinic visits, awareness remained stable and did not decline over time.
The findings in this study need to be considered in the context of its limitations. The education was clinician delivered, and, although standardized materials were prepared for each patient, it is possible that there was variability among clinicians in the way that the educational content was presented to patients. To account for this, we looked at differences in awareness over time on the basis of the patient's primary clinician type (nurse practitioner v physician) and found no statistically significant differences. Finally, this study represents a single-institution experience in a specialized LTFU program for survivors of childhood cancer. The results are possibly not generalizable to survivors of childhood cancer observed at other specialized programs; however, use of the COG-LTFU Guidelines did standardize the education provided in the visits.
These limitations notwithstanding, we found that awareness of personal risk for long-term complications improved after each of three clinician-delivered tailored educational sessions and stabilized thereafter, with no additional improvement or decline. Although overall awareness of personal health risks improved from 39% to 66% over time, fully 60% of the survivors remained unaware of most (> 75%) of their personal therapy-related health risks despite repeated provision of tailored, personalized health education in a specialized LTFU program. Future studies, therefore, are needed to determine ways to improve uptake of childhood cancer survivors' awareness of their therapy-related health risks, particularly for the vulnerable subpopulations most likely to lack awareness.
Supplementary Material
Footnotes
See accompanying editorial on page 3849
Supported in part through the generosity of the Lincy, Bandai, Hearst, Graham Family, Rite Aid, Altschul, and Newman's Own Foundations, and by the Sam Bottleman Estate.
Presented in part at the European Symposium on Late Complications After Childhood Cancer, Edinburgh, United Kingdom, September 15, 2014.
Authors' disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Disclosures provided by the authors are available with this article at www.jco.org.
AUTHOR CONTRIBUTIONS
Conception and design: Wendy Landier, Smita Bhatia
Financial support: Smita Bhatia
Administrative support: Wendy Landier, Smita Bhatia
Provision of study materials or patients: Wendy Landier, Karla Wilson, Saro Armenian, Julie A. Wolfson, Smita Bhatia
Collection and assembly of data: Wendy Landier, Golnaz Namdar, Liton Francisco, Karla Wilson, Claudia Herrera, Saro Armenian, Julie A. Wolfson, Smita Bhatia
Data analysis and interpretation: Wendy Landier, Yanjun Chen, Can-Lan Sun, F. Lennie Wong, Smita Bhatia
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Impact of Tailored Education on Awareness of Personal Risk for Therapy-Related Complications Among Childhood Cancer Survivors
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Wendy Landier
Research Funding: Merck, Sharpe, & Dohme (Inst)
Yanjun Chen
No relationship to disclose
Golnaz Namdar
No relationship to disclose
Liton Francisco
No relationship to disclose
Karla Wilson
No relationship to disclose
Claudia Herrera
No relationship to disclose
Saro Armenian
No relationship to disclose
Julie A. Wolfson
No relationship to disclose
Can-Lan Sun
No relationship to disclose
F. Lennie Wong
No relationship to disclose
Smita Bhatia
No relationship to disclose
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.Mariotto AB, Rowland JH, Yabroff KR, et al. Long-term survivors of childhood cancers in the United States. Cancer Epidemiol Biomarkers Prev. 2009;18:1033–1040. doi: 10.1158/1055-9965.EPI-08-0988. [DOI] [PubMed] [Google Scholar]
- 3.Mertens AC, Yasui Y, Liu Y, et al. Pulmonary complications in survivors of childhood and adolescent cancer: A report from the Childhood Cancer Survivor Study. Cancer. 2002;95:2431–2441. doi: 10.1002/cncr.10978. [DOI] [PubMed] [Google Scholar]
- 4.Motosue MS, Zhu L, Srivastava K, et al. Pulmonary function after whole lung irradiation in pediatric patients with solid malignancies. Cancer. 2012;118:1450–1456. doi: 10.1002/cncr.26371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mulder RL, Thonissen NM, van der Pal HJ, et al. Pulmonary function impairment measured by pulmonary function tests in long-term survivors of childhood cancer. Thorax. 2011;66:1065–1071. doi: 10.1136/thoraxjnl-2011-200618. [DOI] [PubMed] [Google Scholar]
- 6.Mulrooney DA, Yeazel MW, Kawashima T, et al. Cardiac outcomes in a cohort of adult survivors of childhood and adolescent cancer: Retrospective analysis of the Childhood Cancer Survivor Study cohort. BMJ. 2009;339:b4606. doi: 10.1136/bmj.b4606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lipshultz SE, Adams MJ, Colan SD, et al. Long-term cardiovascular toxicity in children, adolescents, and young adults who receive cancer therapy: Pathophysiology, course, monitoring, management, prevention, and research directions—A scientific statement from the American Heart Association. Circulation. 2013;128:1927–1995. doi: 10.1161/CIR.0b013e3182a88099. [DOI] [PubMed] [Google Scholar]
- 8.Shankar SM, Marina N, Hudson MM, et al. Monitoring for cardiovascular disease in survivors of childhood cancer: Report from the Cardiovascular Disease Task Force of the Children's Oncology Group. Pediatrics. 2008;121:e387–e396. doi: 10.1542/peds.2007-0575. [DOI] [PubMed] [Google Scholar]
- 9.Jones DP, Spunt SL, Green D, et al. Renal late effects in patients treated for cancer in childhood: A report from the Children's Oncology Group. Pediatr Blood Cancer. 2008;51:724–731. doi: 10.1002/pbc.21695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sklar CA. Growth and neuroendocrine dysfunction following therapy for childhood cancer. Pediatr Clin North Am. 1997;44:489–503. doi: 10.1016/s0031-3955(05)70487-9. [DOI] [PubMed] [Google Scholar]
- 11.Nandagopal R, Laverdiere C, Mulrooney D, et al. Endocrine late effects of childhood cancer therapy: A report from the Children's Oncology Group. Horm Res. 2008;69:65–74. doi: 10.1159/000111809. [DOI] [PubMed] [Google Scholar]
- 12.Chemaitilly W, Sklar CA. Endocrine complications in long-term survivors of childhood cancers. Endocr Relat Cancer. 2010;17:R141–R159. doi: 10.1677/ERC-10-0002. [DOI] [PubMed] [Google Scholar]
- 13.Ellenberg L, Liu Q, Gioia G, et al. Neurocognitive status in long-term survivors of childhood CNS malignancies: A report from the Childhood Cancer Survivor Study. Neuropsychology. 2009;23:705–717. doi: 10.1037/a0016674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krull KR, Annett RD, Pan Z, et al. Neurocognitive functioning and health-related behaviors in adult survivors of childhood cancer: A report from the Childhood Cancer Survivor Study. Eur J Cancer. 2011;47:1380–1388. doi: 10.1016/j.ejca.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krull KR, Brinkman TM, Li C, et al. Neurocognitive outcomes decades after treatment for childhood acute lymphoblastic leukemia: A report from the St Jude Lifetime Cohort Study. J Clin Oncol. 2013;31:4407–4415. doi: 10.1200/JCO.2012.48.2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kenney LB, Cohen LE, Shnorhavorian M, et al. Male reproductive health after childhood, adolescent, and young adult cancers: A report from the Children's Oncology Group. J Clin Oncol. 2012;30:3408–3416. doi: 10.1200/JCO.2011.38.6938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Metzger ML, Meacham LR, Patterson B, et al. Female reproductive health after childhood, adolescent, and young adult cancers: Guidelines for the assessment and management of female reproductive complications. J Clin Oncol. 2013;31:1239–1247. doi: 10.1200/JCO.2012.43.5511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wallace WH. Oncofertility and preservation of reproductive capacity in children and young adults. Cancer. 2011;117(suppl 10):2301–2310. doi: 10.1002/cncr.26045. [DOI] [PubMed] [Google Scholar]
- 19.Bhatia S, Robison LL, Oberlin O, et al. Breast cancer and other second neoplasms after childhood Hodgkin's disease. N Engl J Med. 1996;334:745–751. doi: 10.1056/NEJM199603213341201. [DOI] [PubMed] [Google Scholar]
- 20.Bhatia S, Louie AD, Bhatia R, et al. Solid cancers after bone marrow transplantation. J Clin Oncol. 2001;19:464–471. doi: 10.1200/JCO.2001.19.2.464. [DOI] [PubMed] [Google Scholar]
- 21.Bhatia S, Yasui Y, Robison LL, et al. High risk of subsequent neoplasms continues with extended follow-up of childhood Hodgkin's disease: Report from the Late Effects Study Group. J Clin Oncol. 2003;21:4386–4394. doi: 10.1200/JCO.2003.11.059. [DOI] [PubMed] [Google Scholar]
- 22.Socie G, Baker KS, Bhatia S. Subsequent malignant neoplasms after hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2012;18(suppl 1):S139–S150. doi: 10.1016/j.bbmt.2011.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bowers DC, Nathan PC, Constine L, et al. Subsequent neoplasms of the CNS among survivors of childhood cancer: A systematic review. Lancet Oncology. 2013;14:E321–E328. doi: 10.1016/S1470-2045(13)70107-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oeffinger KC, Mertens AC, Sklar CA, et al. Chronic health conditions in adult survivors of childhood cancer. N Engl J Med. 2006;355:1572–1582. doi: 10.1056/NEJMsa060185. [DOI] [PubMed] [Google Scholar]
- 25.American Academy of Pediatrics Section on Hematology/Oncology Children's Oncology Group. Long-term follow-up care for pediatric cancer survivors. Pediatrics. 2009;123:906–915. doi: 10.1542/peds.2008-3688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hewitt M, Weiner SL, Simone JV, editors. Childhood Cancer Survivorship: Improving Care and Quality of Life. Washington, DC: National Academies Press; 2003. [PubMed] [Google Scholar]
- 27.Oeffinger KC. Longitudinal risk-based health care for adult survivors of childhood cancer. Curr Probl Cancer. 2003;27:143–167. doi: 10.1016/s0147-0272(03)00031-x. [DOI] [PubMed] [Google Scholar]
- 28.Eshelman-Kent D, Kinahan KE, Hobbie W, et al. Cancer survivorship practices, services, and delivery: A report from the Children's Oncology Group (COG) nursing discipline, adolescent/young adult, and late effects committees. J Cancer Surviv. 2011;5:345–357. doi: 10.1007/s11764-011-0192-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Landier W, Wallace WH, Hudson MM. Long-term follow-up of pediatric cancer survivors: Education, surveillance, and screening. Pediatr Blood Cancer. 2006;46:149–158. doi: 10.1002/pbc.20612. [DOI] [PubMed] [Google Scholar]
- 30.Dutch Childhood Oncology Group Richtlijn follow-up na kinderkanker meer dan 5 jaar na diagnose [in Dutch] Den Haag/Amsterdam, the Netherlands, SKION. 2010. http://www.skion.nl/
- 31.United Kingdom Children's Cancer Study Group Late Effects Group. Therapy-based long-term follow-up practice statement, 2011. http://www.cclg.org.uk/
- 32.Scottish Intercollegiate Guidelines Network. Long-term follow-up of survivors of childhood cancer: A national clinical guideline, 2004. http://www.sign.ac.uk/
- 33.Landier W, Bhatia S, Eshelman DA, et al. Development of risk-based guidelines for pediatric cancer survivors: The Children's Oncology Group Long-Term Follow-Up Guidelines from the Children's Oncology Group Late Effects Committee and Nursing Discipline. J Clin Oncol. 2004;22:4979–4990. doi: 10.1200/JCO.2004.11.032. [DOI] [PubMed] [Google Scholar]
- 34.Children's Oncology Group. Monrovia, CA: Children's Oncology Group; 2013. Long-term follow-up guidelines for survivors of childhood, adolescent, and young adult cancers (version 4.0) http://www.survivorshipguidelines.org/ [Google Scholar]
- 35.Eshelman D, Landier W, Sweeney T, et al. Facilitating care for childhood cancer survivors: Integrating children's oncology group long-term follow-up guidelines and health links in clinical practice. J Pediatr Oncol Nurs. 2004;21:271–280. doi: 10.1177/1043454204268875. [DOI] [PubMed] [Google Scholar]
- 36.Kadan-Lottick NS, Robison LL, Gurney JG, et al. Childhood cancer survivors' knowledge about their past diagnosis and treatment: Childhood Cancer Survivor Study. JAMA. 2002;287:1832–1839. doi: 10.1001/jama.287.14.1832. [DOI] [PubMed] [Google Scholar]
- 37.Blacklay A, Eiser C, Ellis A. Development and evaluation of an information booklet for adult survivors of cancer in childhood: The United Kingdom Children's Cancer Study Group Late Effects Group. Arch Dis Child. 1998;78:340–344. doi: 10.1136/adc.78.4.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Byrne J, Lewis S, Halamek L, et al. Childhood cancer survivors' knowledge of their diagnosis and treatment. Ann Intern Med. 1989;110:400–403. doi: 10.7326/0003-4819-110-5-400. [DOI] [PubMed] [Google Scholar]
- 39.Hudson MM, Hester A, Sweeney T, et al. A model of care for childhood cancer survivors that facilitates research. J Pediatr Oncol Nurs. 2004;21:170–174. doi: 10.1177/1043454204264388. [DOI] [PubMed] [Google Scholar]
- 40.Nathan PC, Hayes-Lattin B, Sisler JJ, et al. Critical issues in transition and survivorship for adolescents and young adults with cancers. Cancer. 2011;117(suppl 10):2335–2341. doi: 10.1002/cncr.26042. [DOI] [PubMed] [Google Scholar]
- 41.Hobbie WL, Hollen PJ. Pediatric nurse practitioners specializing with survivors of childhood cancer. J Pediatr Health Care. 1993;7:24–30. doi: 10.1016/0891-5245(93)90023-b. [DOI] [PubMed] [Google Scholar]
- 42.Carlson CA, Hobbie WL, Brogna M, et al. A multidisciplinary model of care for childhood cancer survivors with complex medical needs. J Pediatr Oncol Nurs. 2008;25:7–13. doi: 10.1177/1043454207311741. [DOI] [PubMed] [Google Scholar]
- 43.Aldridge MD. Writing and designing readable patient education materials. Nephrol Nurs J. 2004;31:373–377. [PubMed] [Google Scholar]
- 44.Centers for Disease Control and Prevention. Scientific and Technical Information: Simply Put (ed 2) Atlanta, GA: Centers for Disease Control and Prevention; 1999. [Google Scholar]
- 45.Doak CC, Doak LG, Root JH. Teaching Patients With Low Literacy Skills (ed 2) Philadelphia, PA: JB Lippincott; 1996. [Google Scholar]
- 46.Landier W, Armenian SH, Lee J, et al. Yield of screening for long-term complications using the Children's Oncology Group Long-Term Follow-Up Guidelines. J Clin Oncol. 2012;30:4401–4408. doi: 10.1200/JCO.2012.43.4951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hudson MM, Ness KK, Gurney JG, et al. Clinical ascertainment of health outcomes among adults treated for childhood cancer. JAMA. 2013;309:2371–2381. doi: 10.1001/jama.2013.6296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bashore L. Childhood and adolescent cancer survivors' knowledge of their disease and effects of treatment. J Pediatr Oncol Nurs. 2004;21:98–102. doi: 10.1177/1043454203262754. [DOI] [PubMed] [Google Scholar]
- 49.Ford JS, Chou JF, Sklar CA. Attendance at a survivorship clinic: Impact on knowledge and psychosocial adjustment. J Cancer Surviv. 2013;7:535–543. doi: 10.1007/s11764-013-0291-9. [DOI] [PubMed] [Google Scholar]
- 50.Hudson MM, Tyc VL, Srivastava DK, et al. Multi-component behavioral intervention to promote health protective behaviors in childhood cancer survivors: The protect study. Med Pediatr Oncol. 2002;39:2–1. doi: 10.1002/mpo.10071. discussion 2. [DOI] [PubMed] [Google Scholar]
- 51.Cherven B, Mertens A, Meacham LR, et al. Knowledge and risk perception of late effects among childhood cancer survivors and parents before and after visiting a childhood cancer survivor clinic. J Pediatr Oncol Nurs. 2014;31:339–349. doi: 10.1177/1043454214532022. [DOI] [PubMed] [Google Scholar]
- 52.Oeffinger KC, Mertens AC, Hudson MM, et al. Health care of young adult survivors of childhood cancer: A report from the Childhood Cancer Survivor Study. Ann Fam Med. 2004;2:61–70. doi: 10.1370/afm.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nathan PC, Greenberg ML, Ness KK, et al. Medical care in long-term survivors of childhood cancer: A report from the Childhood Cancer Survivor Study. J Clin Oncol. 2008;26:4401–4409. doi: 10.1200/JCO.2008.16.9607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Suh E, Daugherty CK, Wroblewski K, et al. General internists' preferences and knowledge about the care of adult survivors of childhood cancer: A cross-sectional survey. Ann Intern Med. 2014;160:11–17. doi: 10.7326/M13-1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Oeffinger KC. Childhood cancer survivors and primary care physicians. J Fam Pract. 2000;49:689–690. [PubMed] [Google Scholar]
- 56.Bhatia S, Landier W. Evaluating survivors of pediatric cancer. Cancer J. 2005;11:340–354. doi: 10.1097/00130404-200507000-00010. [DOI] [PubMed] [Google Scholar]
- 57.Oeffinger KC, Hudson MM, Landier W. Survivorship: Childhood cancer survivors. Prim Care. 2009;36:743–780. doi: 10.1016/j.pop.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 58.Hewitt M, Greenfield S, Stovall E, editors. From Cancer Patient to Cancer Survivor: Lost in Transition. Washington, DC: National Academies Press; 2006. [Google Scholar]
- 59.Gurney JG, Krull KR, Kadan-Lottick N, et al. Social outcomes in the Childhood Cancer Survivor Study cohort. J Clin Oncol. 2009;27:2390–2395. doi: 10.1200/JCO.2008.21.1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ness KK, Mertens AC, Hudson MM, et al. Limitations on physical performance and daily activities among long-term survivors of childhood cancer. Ann Intern Med. 2005;143:639–647. doi: 10.7326/0003-4819-143-9-200511010-00007. [DOI] [PubMed] [Google Scholar]
- 61.Meacham LR, Edwards PJ, Cherven BO, et al. Primary care providers as partners in long-term follow-up of pediatric cancer survivors. J Cancer Surviv. 2012;6:270–277. doi: 10.1007/s11764-012-0224-z. [DOI] [PubMed] [Google Scholar]
- 62.McCabe MS, Partridge AH, Grunfeld E, et al. Risk-based health care, the cancer survivor, the oncologist, and the primary care physician. Semin Oncol. 2013;40:804–812. doi: 10.1053/j.seminoncol.2013.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


