Abstract
Purpose
Children receiving CNS-directed therapy for cancer are at risk for cognitive problems, with few available empirically supported interventions. Cognitive problems indicate neurodevelopmental disruption that may be modifiable with intervention. This study evaluated short-term efficacy of a computerized cognitive training program and neural correlates of cognitive change.
Patient and Methods
A total of 68 survivors of childhood acute lymphoblastic leukemia (ALL) or brain tumor (BT) with identified cognitive deficits were randomly assigned to computerized cognitive intervention (male, n = 18; female, n = 16; ALL, n = 23; BT, n = 11; mean age ± standard deviation, 12.21 ± 2.47 years) or waitlist (male, n = 18; female, n = 16; ALL, n = 24; BT, n = 10; median age ± standard deviation, 11.82 ± 2.42 years). Intervention participants were asked to complete 25 training sessions at home with weekly, telephone-based coaching. Cognitive assessments and functional magnetic resonance imaging scans (intervention group) were completed pre- and postintervention, with immediate change in spatial span backward as the primary outcome.
Results
Survivors completing the intervention (n = 30; 88%) demonstrated greater improvement than controls on measures of working memory (mean ± SEM; eg, Wechsler Intelligence Scale for Children [fourth edition; WISC-IV] spatial span backward, 3.13 ± 0.58 v 0.75 ± 0.43; P = .002; effect size [ES], 0.84), attention (eg, WISC-IV spatial span forward, 3.30 ± 0.71 v 1.25 ± 0.39; P = .01; ES, 0.65), and processing speed (eg, Conners' Continuous Performance Test hit reaction time, −2.10 ± 1.47 v 2.54 ± 1.25; P = .02; ES, .61) and showed greater reductions in reported executive dysfunction (eg, Conners' Parent Rating Scale III, −6.73 ± 1.51 v 0.41 ± 1.53; P = .002; ES, 0.84). Functional magnetic resonance imaging revealed significant pre- to post-training reduction in activation of left lateral prefrontal and bilateral medial frontal areas.
Conclusion
Study findings show computerized cognitive training is feasible and efficacious for childhood cancer survivors, with evidence for training-related neuroplasticity.
INTRODUCTION
Children treated for brain tumors (BTs) or acute lymphoblastic leukemia (ALL) experience declines in intellectual functioning1–4 associated with reduced academic, social, and vocational attainment.5–8 Impairments in attention, working memory (WM; temporary storage and manipulation of information), and processing speed contribute to intellectual declines.9–13 Treatment-related brain changes, including reduced white matter volumes in frontal regions, are associated with attention and WM performance among childhood cancer survivors.14–18 As survival rates rise,19,20 efforts to improve neurocognitive outcomes become imperative. However, there are few empirically supported interventions that ameliorate cognitive impairments arising secondary to childhood cancer. Some evidence supports the efficacy of stimulant medications21,22 or therapist-delivered cognitive remediation23–25; however, stimulant use is limited by medical contraindication and parental preference,26–29 and therapist-delivered interventions are associated with low participation rates as a result of high time investment and logistic challenges.23–25
Computerized cognitive training programs target specific cognitive processes using repetitive exercises of graded difficulty. Advantages of computerized training include remote administration affording greater geographic reach, reduced time burden with scheduling flexibility, engaging interfaces for children, easy progress monitoring, and few medical contraindications.30–35 Cogmed (http://www.cogmed.com) is a computerized WM intervention, with demonstrated efficacy for developmental and acquired attention disorders30–33; improvements have been achieved on measures of attention, WM, and executive functions, with benefits persisting months after training. Functional magnetic resonance imaging (fMRI) with healthy adults completing Cogmed suggests training-based neuroplasticity, with increased activity in regions well established for supporting WM.36,37 Hardy et al34 demonstrated feasibility and acceptability of Cogmed among cancer survivors in a pilot study not powered to evaluate efficacy. We recently replicated feasibility and acceptability in a larger geographically dispersed and socioeconomically varied cancer survivor group.35
In our study, we used a randomized, single-blind (psychological examiner), waitlist-controlled design to investigate the efficacy and neural correlates of change associated with computerized cognitive training in children who received CNS-directed therapy for a BT or ALL. We hypothesized Cogmed participants would demonstrate greater short-term improvement on performance- and rater-based measures of WM relative to waitlisted cancer survivors and would demonstrate increased activity in prefrontal and parietal cortices, supporting WM after training.
PATIENTS AND METHODS
Participants
Eligible participants were childhood BT or ALL survivors who received cranial irradiation and/or intrathecal chemotherapy and had completed treatment at least 1 year before, without disease recurrence. Participants had to be English speakers and between ages 8 and 16 years, with intelligence quotient (IQ) ≥ 70. Children were excluded for history of premorbid CNS injury or disease (eg, traumatic brain injury, epilepsy), preexisting attention deficit hyperactivity disorder (ADHD), psychotropic medications within 2 weeks of enrollment, motor or sensory deficit precluding valid testing or completion of the intervention, or psychological condition precluding or taking precedence over cognitive intervention. This study was conducted at St Jude Children's Research Hospital between December 2010 and December 2013, as approved by the institutional review board, and written informed consent was obtained before participation.
Procedures
To avoid potential biases, patients were recruited consecutively in order of upcoming appointments until 60 evaluable participants reached the post-intervention time point. At the first visit, patients completed screening or pre-intervention cognitive assessment to determine eligibility. WM problems were defined by digit span, letter-number sequencing, or spatial span performance (Wechsler Intelligence Scale for Children [fourth edition; WISC-IV]38) > one standard deviation below the normative mean or the individual's IQ (Wechsler Abbreviated Scale of Intelligence [WASI]39). Participants were also required to be appropriate for neuroimaging without sedation (eg, no orthodontic appliances or known claustrophobia). Qualifying participants were randomly assigned to computerized training (Cogmed) or a waitlist. Group random assignment was performed at a 1:1 ratio and stratified by diagnosis (ALL v BT), age (8 to 11 v 12 to 16 years), and sex. Block random assignment was performed by computer.40
Participants randomly assigned to intervention completed neuroimaging during the same visit as cognitive assessment. Computers and/or Internet access were provided as needed. The Cogmed intervention group was asked to complete 25 training sessions at home over 5 to 9 weeks. Training sessions consisted of visual–spatial and verbal WM exercises presented as games, with each session lasting approximately 30 to 45 minutes. Exercise difficulty was adjusted based on performance. Training progress was monitored over the Internet. Weekly coaching telephone calls were used to provide feedback and help maintain motivation. Participants demonstrating slower-than-desired progress (ie, score gain < 20 after 20 sessions) were offered five additional sessions. Eight of 16 participants offered additional sessions agreed, with a range of 26 to 30 completed sessions.
Approximately 10 weeks after baseline assessment, all study participants completed postintervention/waitlist cognitive assessments and neuroimaging examinations (intervention group). Six months later, all participants had a final cognitive assessment, and control participants were offered the intervention. Incentives were offered to encourage continued participation. Both groups were provided equal incentives to minimize motivational differences. Participants received $10 gift cards after completing nine, 17, and 25 sessions (or 2, 4, and 6 weeks for controls), as well as after completing preintervention or pre-waitlist, postintervention or post-waitlist, and 6-month follow-up appointments.
Cognitive Measures
Participants were assessed with the same battery of cognitive measures at study outset and 10 weeks and 6 months postintervention or post-waitlist. All measures had age-specific norms from representative standardization samples and demonstrated reliability and validity. Psychological examiners who performed testing were blind to participants' group status.
An abbreviated IQ was derived from the WASI39 vocabulary and matrix reasoning subtests. This abbreviated IQ has normative mean of 100, standard deviation of 15, and is highly correlated with a full IQ.41,42
WISC-IV integrated spatial span, digit span, and letter-number sequencing were the performance-based WM measures.38 Change in spatial span backward from pre- to immediately postintervention was the primary outcome, because it is a nontrained WM task used to assess Cogmed training effects in children with ADHD.31 Other performance-based and parent measures were secondary outcomes. For spatial span, the examiner taps sequences of blocks, and the participant repeats block taps in the same order to measure attention (spatial span forward) or in reverse order to measure WM (spatial span backward). Digit span includes digit span forward (participant repeats digits verbatim) and digit span backward (participant repeats digits in reverse order). For letter-number sequencing, the examiner presents sequences of numbers and letters, after which the participant repeats the numbers in ascending order followed by the letters in alphabetic order. These tasks each provide an age-standardized score, with a mean of 10 and standard deviation of 3.
The Conners' Continuous Performance Test II (CPT-II) is a computerized measure of sustained attention.43 Letters are presented on a computer screen, and children press the space bar as quickly and accurately as possible for any letter except the letter X. The CPT program computes an omission score, as an index of inattention, and reaction time. Scores are age-standardized T-scores, with a mean of 50 and standard deviation of 10.
Reading fluency and math fluency subtests of the Woodcock Johnson III (WJ-III)44 were administered. Reading fluency requires the participant to read simple sentences and decide if they are true. Math fluency requires the participant to solve simple mathematic calculations. Both subtests measure the number of items correctly completed in 3 minutes. Scores are age standardized, with a mean of 100 and standard deviation of 15.
The Conners' Parent Rating Scale 3 (CPRS-3)45 is a parent-reported measure consisting of 110 items rated on a scale from 0 (not true at all) to 3 (very much true). Primary scales of interest were inattention and executive functioning. Scaled scores are age and sex standardized, with a mean of 50 and standard deviation of 10.
The Behavior Rating Inventory of Executive Function (BRIEF)46 is a parent questionnaire consisting of 86 items rated as occurring never, sometimes, or often. Primary scales of interest were WM and metacognitive index. All scaled scores are age and sex standardized, with a mean of 50 and standard deviation of 10.
Neuroimaging
fMRI examination was designed to explore neural correlates of WM performance and response to Cogmed intervention. Neuroimaging was completed during baseline and postintervention visits for participants in the intervention group. Before scanning, participants watched a presentation about scanning procedures, practiced fMRI tasks, and tried the manual response mechanism. All scans were completed on 3 Tesla Magnets (Trio and Skyra models; Siemens Medical Systems, Malvern, PA). Conventional MRIs were used to identify morphologic abnormalities, facilitate spatial normalization of brain images, and visualize fMRI results.
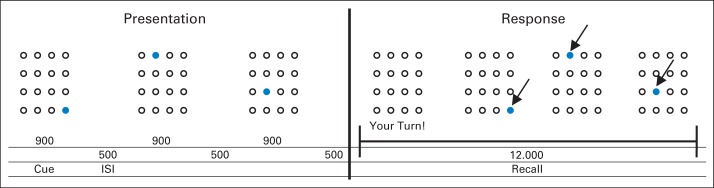
During fMRI, participants completed a grid-based spatial WM task used in prior investigations of Cogmed with healthy adults.36 The block-design task consisted of WM trials during which the participant was required to remember, and subsequently reproduce, the location and order of a series of cues presented transiently in a grid. WM trials included three (low load) or five cues (high load). The WM trials were separated by control trials, during which the participant selected five stationary cues presented in the grid. Stimuli were presented at the back of the magnet with an LCD projector and viewed via a mirror on the head coil (Appendix and Appendix Fig A1, online only, provide neuroimaging acquisition details).
Statistical Analyses
A sample size of 30 was targeted for each group to afford 80% power to detect a medium size effect (0.65) between groups on WM measures using a one-sided significance level of .05. Demographic and clinical variables were compared between groups. To identify change in cognitive abilities associated with training, repeated-measures analyses of variance were conducted. Effect sizes were computed comparing pre- with postintervention change scores between groups using Cohen's d.47 All reported P values are two sided.
Neuroimaging analysis targeted three areas: patterns of activation before training to elucidate WM impairment in cancer survivors, changes in activation after intervention to identify potential mechanisms of training-related WM changes, and evidence for neural phenotypes at baseline that may predict response to Cogmed intervention. Functional images were analyzed with statistical parametric mapping48 via a two-level analysis. In the first-level analysis, data were analyzed according to a fixed-effect generalized linear model, with task-induced activity represented by a boxcar function convolved with canonic hemodynamic response function. Contrasts selecting for activation of interest were set in a model, and contrast images from each participant were used as a variable in a second-level random-effects analysis (Appendix and Appendix Fig A2, online only, provides fMRI data analysis details).
RESULTS
Participants
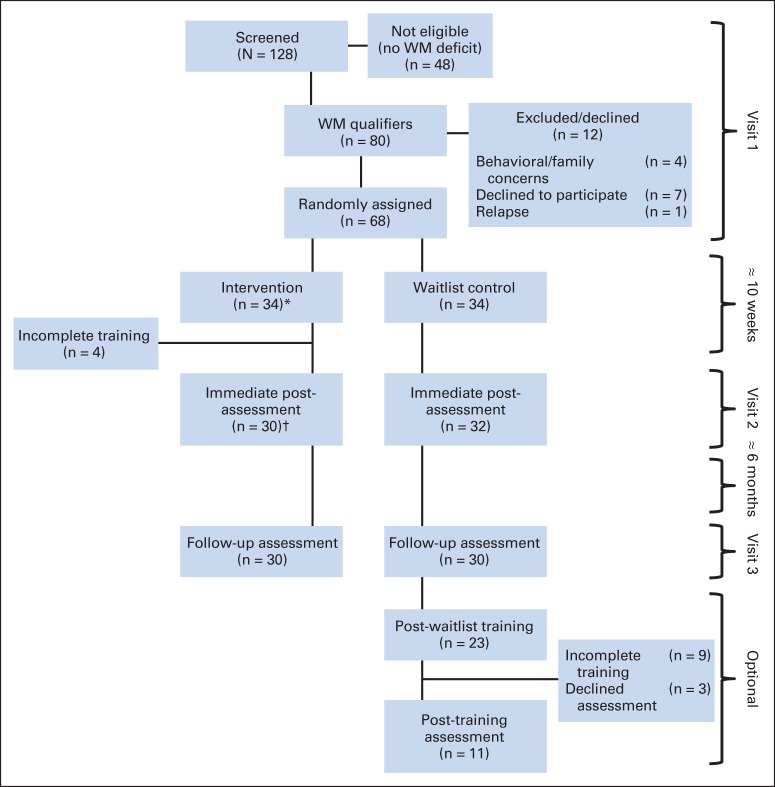
Feasibility and acceptability of this trial have been reported elsewhere35 and are briefly summarized here. Of 128 patients screened, 80 qualified based on WM problems. Among qualifiers, five were excluded, seven declined participation, and 68 were randomly assigned (34 in each group). Of those randomly assigned to the intervention, 30 (88%) completed at least 20 of 25 sessions (a priori criterion for compliance),31,34 and all returned for postintervention assessments. There were no significant differences between patients who completed the intervention and patients who dropped out early based on demographic, clinical, or cognitive performance to suggest limits to generalizability of findings. fMRI examinations were completed by 91% and 93% of participants at preintervention and postintervention, respectively. Of those randomly assigned to the control group, 32 returned for post-waitlist assessments (Fig 1).
Fig 1.
CONSORT diagram. WM, working memory. (*) Completed visit one functional magnetic resonance imaging (fMRI; n = 31); one participant supplied partial preintervention fMRI data because of fatigue. (†) Completed visit two fMRI (n = 28).
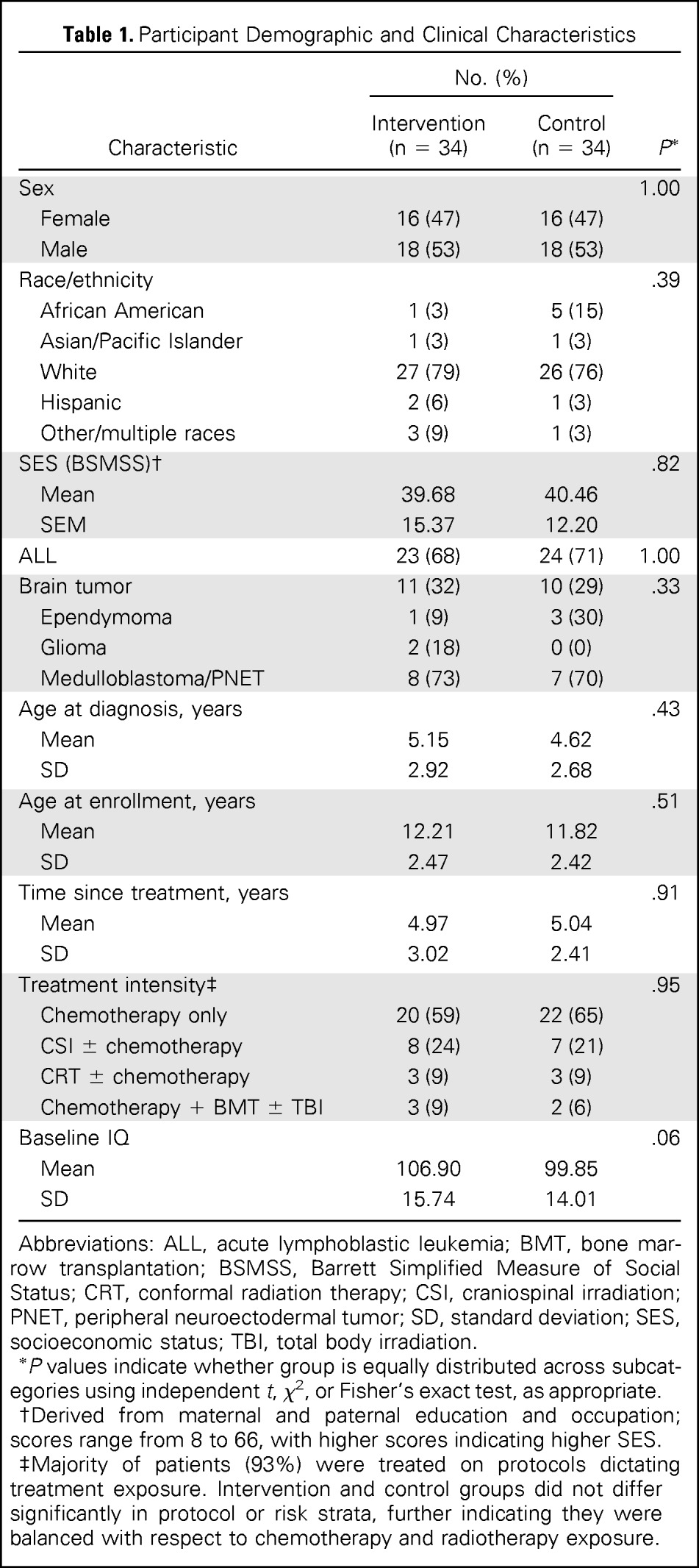
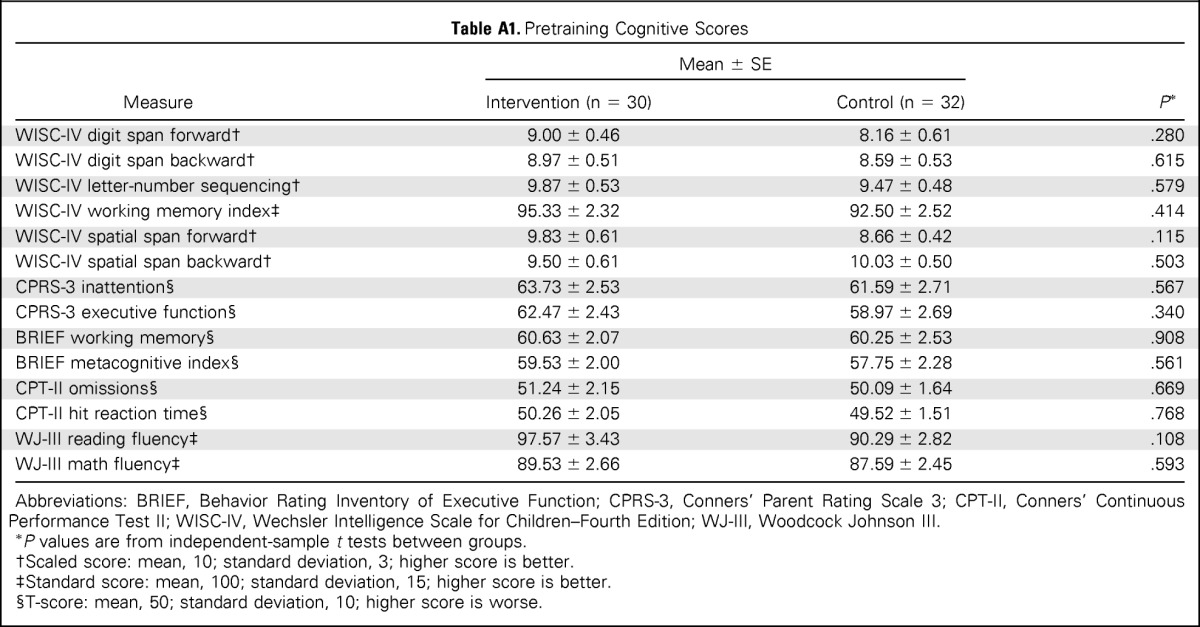
Study participants were balanced by sex (male, 53%) and largely white (78%; Table 1). Approximately two thirds (69%) of the sample were treated for ALL, often with chemotherapy only (87%). A majority of participants with BTs were treated with cranial irradiation (73%). Participants were on average age 12 years and 5 years from completion of treatment. Intervention and control groups were balanced with respect to sex, age, and diagnosis; there were no group differences in socioeconomic status, age at diagnosis, time since treatment, or treatment intensity. A trend for a higher baseline IQ among the intervention group (106.9 v 99.9; P = .06) was the only baseline difference in cognitive performance (Appendix Table A1, online only).
Table 1.
Participant Demographic and Clinical Characteristics
| Characteristic | No. (%) |
P* | |
|---|---|---|---|
| Intervention (n = 34) | Control (n = 34) | ||
| Sex | 1.00 | ||
| Female | 16 (47) | 16 (47) | |
| Male | 18 (53) | 18 (53) | |
| Race/ethnicity | .39 | ||
| African American | 1 (3) | 5 (15) | |
| Asian/Pacific Islander | 1 (3) | 1 (3) | |
| White | 27 (79) | 26 (76) | |
| Hispanic | 2 (6) | 1 (3) | |
| Other/multiple races | 3 (9) | 1 (3) | |
| SES (BSMSS)† | .82 | ||
| Mean | 39.68 | 40.46 | |
| SEM | 15.37 | 12.20 | |
| ALL | 23 (68) | 24 (71) | 1.00 |
| Brain tumor | 11 (32) | 10 (29) | .33 |
| Ependymoma | 1 (9) | 3 (30) | |
| Glioma | 2 (18) | 0 (0) | |
| Medulloblastoma/PNET | 8 (73) | 7 (70) | |
| Age at diagnosis, years | .43 | ||
| Mean | 5.15 | 4.62 | |
| SD | 2.92 | 2.68 | |
| Age at enrollment, years | .51 | ||
| Mean | 12.21 | 11.82 | |
| SD | 2.47 | 2.42 | |
| Time since treatment, years | .91 | ||
| Mean | 4.97 | 5.04 | |
| SD | 3.02 | 2.41 | |
| Treatment intensity‡ | .95 | ||
| Chemotherapy only | 20 (59) | 22 (65) | |
| CSI ± chemotherapy | 8 (24) | 7 (21) | |
| CRT ± chemotherapy | 3 (9) | 3 (9) | |
| Chemotherapy + BMT ± TBI | 3 (9) | 2 (6) | |
| Baseline IQ | .06 | ||
| Mean | 106.90 | 99.85 | |
| SD | 15.74 | 14.01 | |
Abbreviations: ALL, acute lymphoblastic leukemia; BMT, bone marrow transplantation; BSMSS, Barrett Simplified Measure of Social Status; CRT, conformal radiation therapy; CSI, craniospinal irradiation; PNET, peripheral neuroectodermal tumor; SD, standard deviation; SES, socioeconomic status; TBI, total body irradiation.
P values indicate whether group is equally distributed across subcategories using independent t, χ2, or Fisher's exact test, as appropriate.
Derived from maternal and paternal education and occupation; scores range from 8 to 66, with higher scores indicating higher SES.
Majority of patients (93%) were treated on protocols dictating treatment exposure. Intervention and control groups did not differ significantly in protocol or risk strata, further indicating they were balanced with respect to chemotherapy and radiotherapy exposure.
Intervention
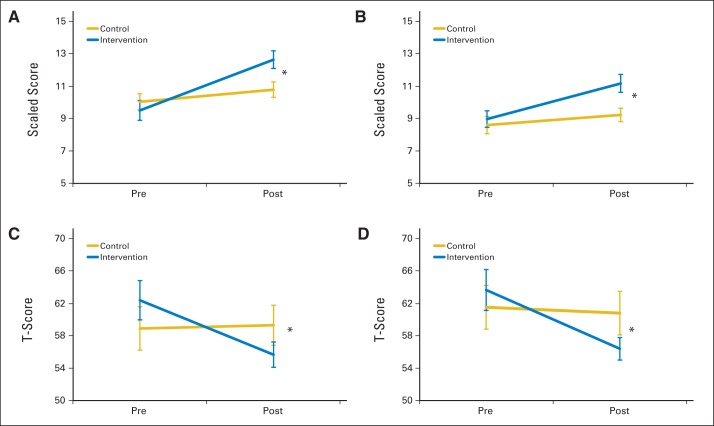
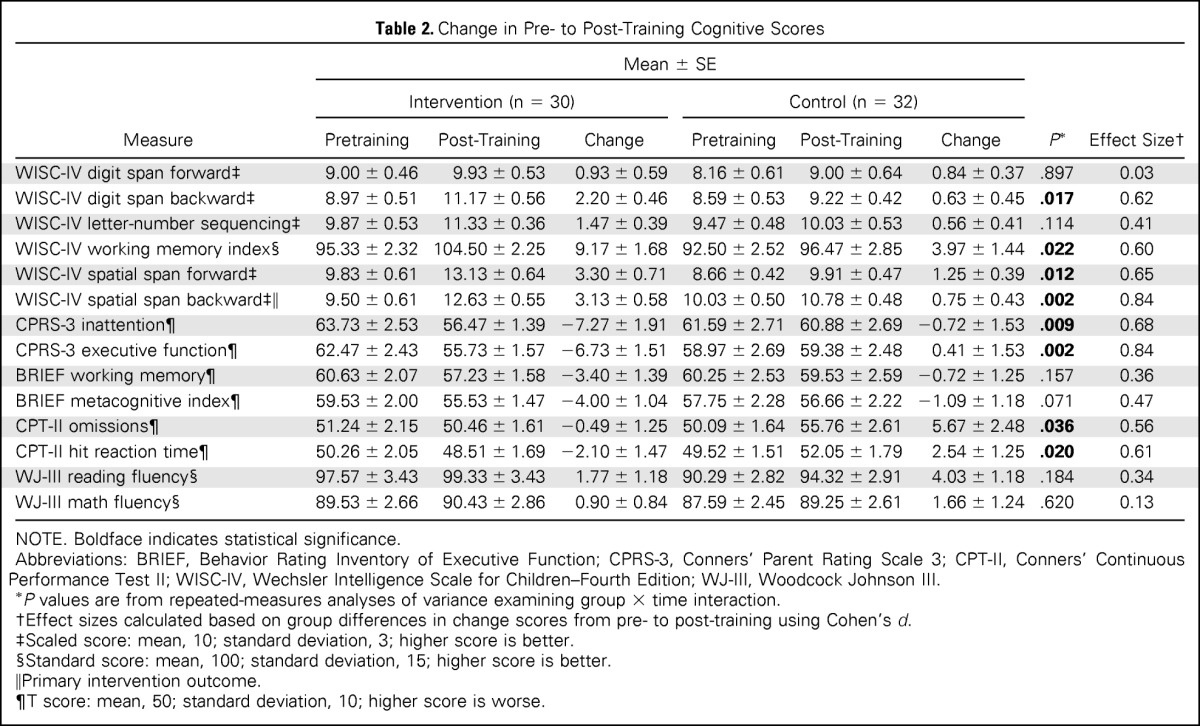
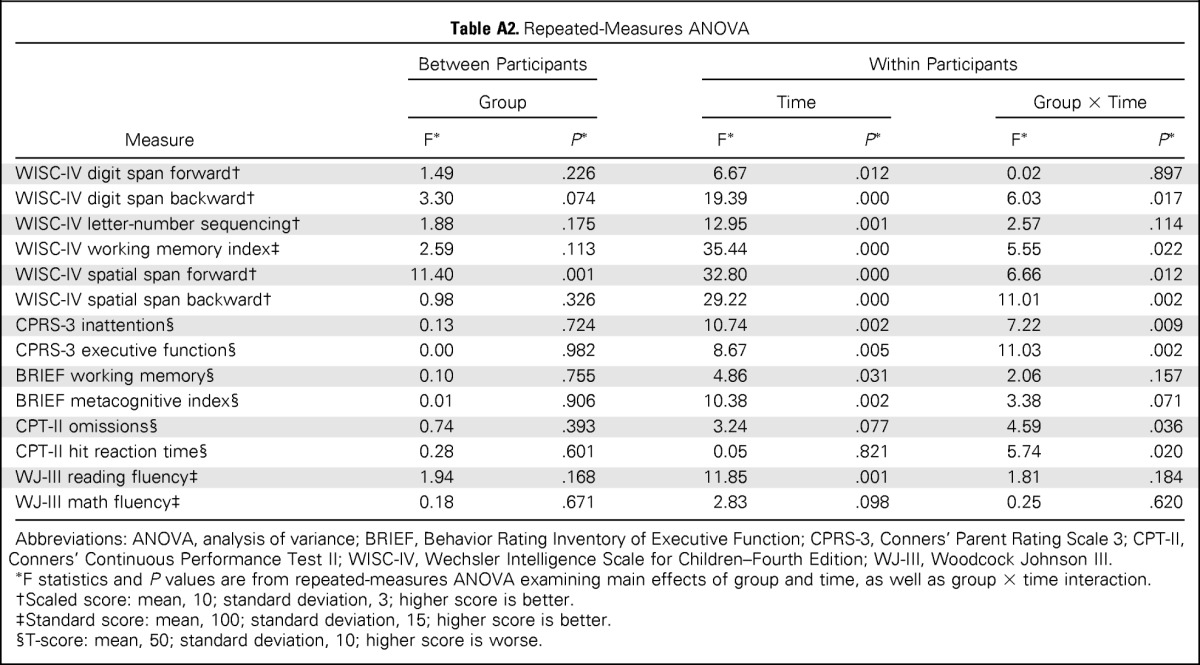
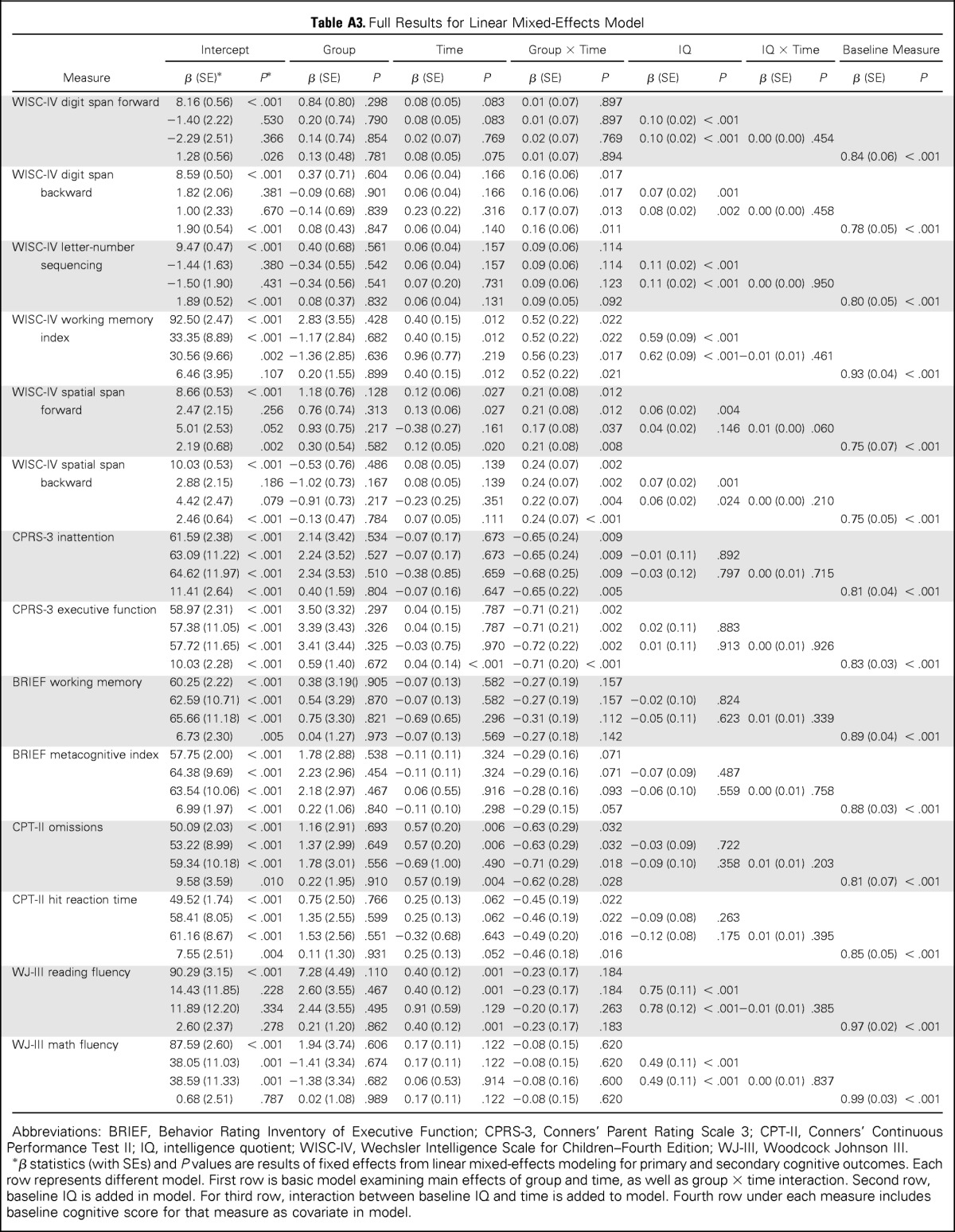
For the primary outcome measure—spatial span backward—the intervention group demonstrated greater short-term improvement than the control group, as indicated by a significant group × time interaction (P = .002; Table 2; Fig 2). The intervention group also demonstrated greater short-term improvement than the control group on secondary measures of attention (WISC-IV spatial span forward, P = .012; CPT-II omissions, P = .036), WM (WISC-IV digit span backward, P = .017; WISC-IV working memory index, P = .022), and processing speed (CPT-II reaction time, P = .020; Table 2; Fig 2). Parents of intervention participants reported greater reduction in inattention and executive dysfunction than parents of control group participants (CPRS-3 inattention, P = .009; CPRS-3 executive function, P = .002; Fig 2; Appendix Table A2, online only, provides full model). There was no difference in change in academic fluency between groups (WJ-III reading and math fluency). To account for IQ at baseline, linear mixed-effects models including group, time, group × time, baseline IQ, and baseline IQ × time were created, with all significant group × time interactions remaining significant (Appendix Table A3, online only).
Table 2.
Change in Pre- to Post-Training Cognitive Scores
| Measure | Mean ± SE |
P* | Effect Size† | |||||
|---|---|---|---|---|---|---|---|---|
| Intervention (n = 30) |
Control (n = 32) |
|||||||
| Pretraining | Post-Training | Change | Pretraining | Post-Training | Change | |||
| WISC-IV digit span forward‡ | 9.00 ± 0.46 | 9.93 ± 0.53 | 0.93 ± 0.59 | 8.16 ± 0.61 | 9.00 ± 0.64 | 0.84 ± 0.37 | .897 | 0.03 |
| WISC-IV digit span backward‡ | 8.97 ± 0.51 | 11.17 ± 0.56 | 2.20 ± 0.46 | 8.59 ± 0.53 | 9.22 ± 0.42 | 0.63 ± 0.45 | .017 | 0.62 |
| WISC-IV letter-number sequencing‡ | 9.87 ± 0.53 | 11.33 ± 0.36 | 1.47 ± 0.39 | 9.47 ± 0.48 | 10.03 ± 0.53 | 0.56 ± 0.41 | .114 | 0.41 |
| WISC-IV working memory index§ | 95.33 ± 2.32 | 104.50 ± 2.25 | 9.17 ± 1.68 | 92.50 ± 2.52 | 96.47 ± 2.85 | 3.97 ± 1.44 | .022 | 0.60 |
| WISC-IV spatial span forward‡ | 9.83 ± 0.61 | 13.13 ± 0.64 | 3.30 ± 0.71 | 8.66 ± 0.42 | 9.91 ± 0.47 | 1.25 ± 0.39 | .012 | 0.65 |
| WISC-IV spatial span backward‡‖ | 9.50 ± 0.61 | 12.63 ± 0.55 | 3.13 ± 0.58 | 10.03 ± 0.50 | 10.78 ± 0.48 | 0.75 ± 0.43 | .002 | 0.84 |
| CPRS-3 inattention¶ | 63.73 ± 2.53 | 56.47 ± 1.39 | −7.27 ± 1.91 | 61.59 ± 2.71 | 60.88 ± 2.69 | −0.72 ± 1.53 | .009 | 0.68 |
| CPRS-3 executive function¶ | 62.47 ± 2.43 | 55.73 ± 1.57 | −6.73 ± 1.51 | 58.97 ± 2.69 | 59.38 ± 2.48 | 0.41 ± 1.53 | .002 | 0.84 |
| BRIEF working memory¶ | 60.63 ± 2.07 | 57.23 ± 1.58 | −3.40 ± 1.39 | 60.25 ± 2.53 | 59.53 ± 2.59 | −0.72 ± 1.25 | .157 | 0.36 |
| BRIEF metacognitive index¶ | 59.53 ± 2.00 | 55.53 ± 1.47 | −4.00 ± 1.04 | 57.75 ± 2.28 | 56.66 ± 2.22 | −1.09 ± 1.18 | .071 | 0.47 |
| CPT-II omissions¶ | 51.24 ± 2.15 | 50.46 ± 1.61 | −0.49 ± 1.25 | 50.09 ± 1.64 | 55.76 ± 2.61 | 5.67 ± 2.48 | .036 | 0.56 |
| CPT-II hit reaction time¶ | 50.26 ± 2.05 | 48.51 ± 1.69 | −2.10 ± 1.47 | 49.52 ± 1.51 | 52.05 ± 1.79 | 2.54 ± 1.25 | .020 | 0.61 |
| WJ-III reading fluency§ | 97.57 ± 3.43 | 99.33 ± 3.43 | 1.77 ± 1.18 | 90.29 ± 2.82 | 94.32 ± 2.91 | 4.03 ± 1.18 | .184 | 0.34 |
| WJ-III math fluency§ | 89.53 ± 2.66 | 90.43 ± 2.86 | 0.90 ± 0.84 | 87.59 ± 2.45 | 89.25 ± 2.61 | 1.66 ± 1.24 | .620 | 0.13 |
NOTE. Boldface indicates statistical significance.
Abbreviations: BRIEF, Behavior Rating Inventory of Executive Function; CPRS-3, Conners' Parent Rating Scale 3; CPT-II, Conners' Continuous Performance Test II; WISC-IV, Wechsler Intelligence Scale for Children–Fourth Edition; WJ-III, Woodcock Johnson III.
P values are from repeated-measures analyses of variance examining group × time interaction.
Effect sizes calculated based on group differences in change scores from pre- to post-training using Cohen's d.
Scaled score: mean, 10; standard deviation, 3; higher score is better.
Standard score: mean, 100; standard deviation, 15; higher score is better.
Primary intervention outcome.
T score: mean, 50; standard deviation, 10; higher score is worse.
Fig 2.
Pre- to post-training cognitive scores. (A) Wechsler Intelligence Scale for Children (fourth edition; WISC-IV) spatial span backward; (B) WISC-IV digit span backward; (C) Conners' Parent Rating Scale III (CPRS-3) executive function; (D) CPRS-3 inattention. (*) P < .05 group × time interaction on repeated-measures analysis of variance.
Neuroimaging
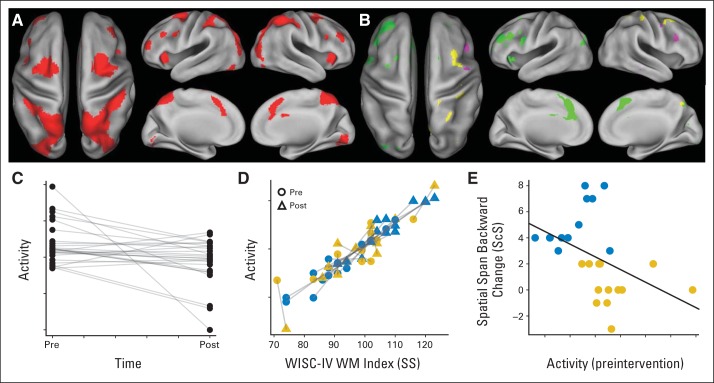
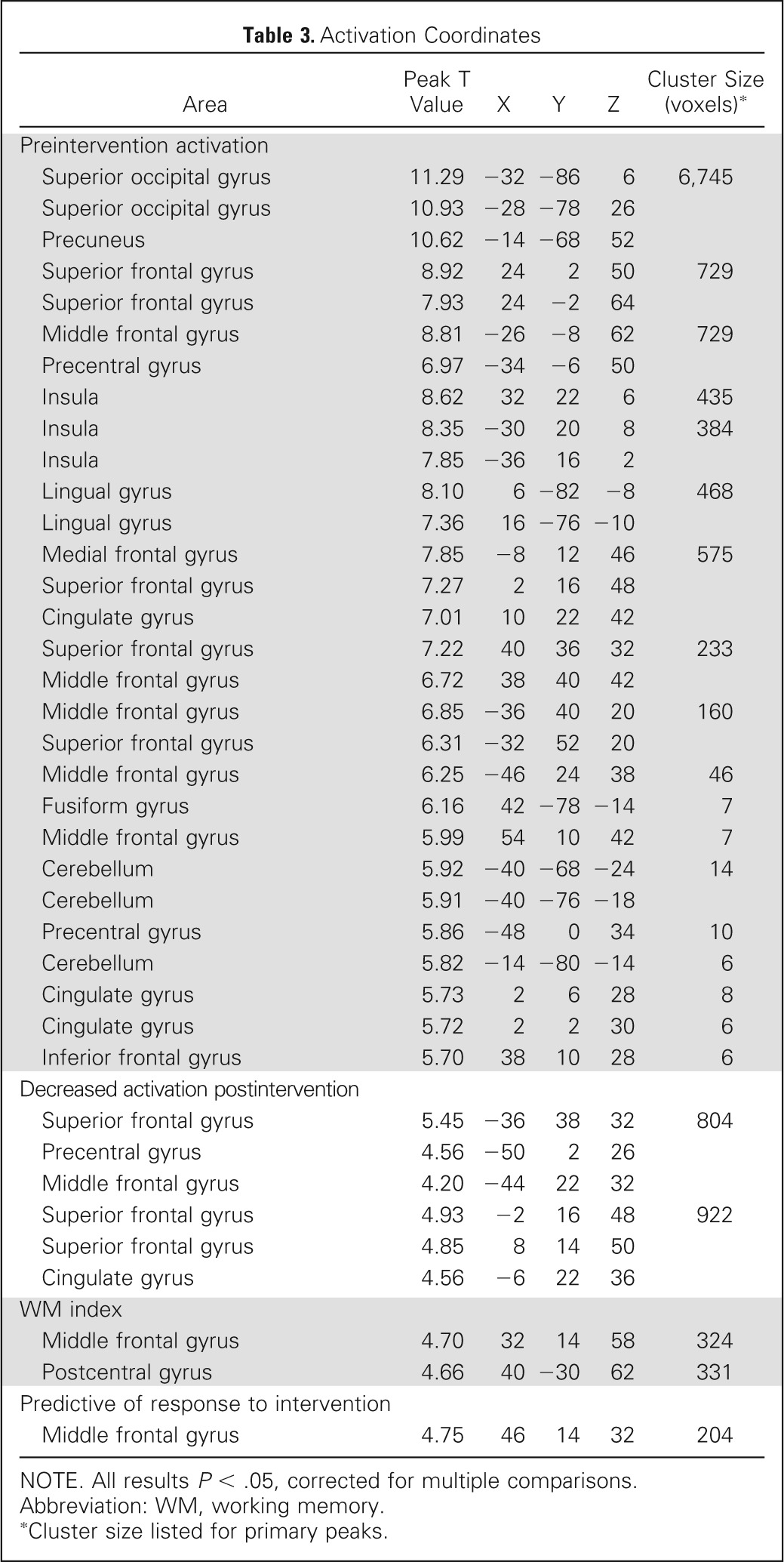
The pattern of preintervention activation during the WM task was consistent with the neuroimaging literature, revealing a bilateral frontal-parietal network (Table 3; Fig 3).49 Activation was robust in dorsal visual stream, including occipital and parietal lobes, ventral and dorsolateral prefrontal cortex, middle and superior frontal gyri, and anterior cingulate cortex (shown in red in Fig 3A). A majority of participants completing fMRI were right-handed (90%), and findings were unchanged with handedness as a covariate in the fMRI model. Activation decreased after training (shown in green in Figs 3B and 3C), with extensive changes in left lateral prefrontal, left cingulate, and bilateral medial frontal areas. Task activity in frontal and parietal regions previously shown to support spatial WM49 was significantly associated with performance on the WISC-IV WM index (digit span and letter-number sequencing) measured outside of the MRI (Fig 3D). Change in WM scores after intervention was not significantly associated with change in activation in any brain areas, but lower preintervention activation in a right dorsolateral prefrontal subregion was predictive of positive intervention response based on spatial span backward performance (Fig 3E).
Table 3.
Activation Coordinates
| Area | Peak T Value | X | Y | Z | Cluster Size (voxels)* |
|---|---|---|---|---|---|
| Preintervention activation | |||||
| Superior occipital gyrus | 11.29 | −32 | −86 | 6 | 6,745 |
| Superior occipital gyrus | 10.93 | −28 | −78 | 26 | |
| Precuneus | 10.62 | −14 | −68 | 52 | |
| Superior frontal gyrus | 8.92 | 24 | 2 | 50 | 729 |
| Superior frontal gyrus | 7.93 | 24 | −2 | 64 | |
| Middle frontal gyrus | 8.81 | −26 | −8 | 62 | 729 |
| Precentral gyrus | 6.97 | −34 | −6 | 50 | |
| Insula | 8.62 | 32 | 22 | 6 | 435 |
| Insula | 8.35 | −30 | 20 | 8 | 384 |
| Insula | 7.85 | −36 | 16 | 2 | |
| Lingual gyrus | 8.10 | 6 | −82 | −8 | 468 |
| Lingual gyrus | 7.36 | 16 | −76 | −10 | |
| Medial frontal gyrus | 7.85 | −8 | 12 | 46 | 575 |
| Superior frontal gyrus | 7.27 | 2 | 16 | 48 | |
| Cingulate gyrus | 7.01 | 10 | 22 | 42 | |
| Superior frontal gyrus | 7.22 | 40 | 36 | 32 | 233 |
| Middle frontal gyrus | 6.72 | 38 | 40 | 42 | |
| Middle frontal gyrus | 6.85 | −36 | 40 | 20 | 160 |
| Superior frontal gyrus | 6.31 | −32 | 52 | 20 | |
| Middle frontal gyrus | 6.25 | −46 | 24 | 38 | 46 |
| Fusiform gyrus | 6.16 | 42 | −78 | −14 | 7 |
| Middle frontal gyrus | 5.99 | 54 | 10 | 42 | 7 |
| Cerebellum | 5.92 | −40 | −68 | −24 | 14 |
| Cerebellum | 5.91 | −40 | −76 | −18 | |
| Precentral gyrus | 5.86 | −48 | 0 | 34 | 10 |
| Cerebellum | 5.82 | −14 | −80 | −14 | 6 |
| Cingulate gyrus | 5.73 | 2 | 6 | 28 | 8 |
| Cingulate gyrus | 5.72 | 2 | 2 | 30 | 6 |
| Inferior frontal gyrus | 5.70 | 38 | 10 | 28 | 6 |
| Decreased activation postintervention | |||||
| Superior frontal gyrus | 5.45 | −36 | 38 | 32 | 804 |
| Precentral gyrus | 4.56 | −50 | 2 | 26 | |
| Middle frontal gyrus | 4.20 | −44 | 22 | 32 | |
| Superior frontal gyrus | 4.93 | −2 | 16 | 48 | 922 |
| Superior frontal gyrus | 4.85 | 8 | 14 | 50 | |
| Cingulate gyrus | 4.56 | −6 | 22 | 36 | |
| WM index | |||||
| Middle frontal gyrus | 4.70 | 32 | 14 | 58 | 324 |
| Postcentral gyrus | 4.66 | 40 | −30 | 62 | 331 |
| Predictive of response to intervention | |||||
| Middle frontal gyrus | 4.75 | 46 | 14 | 32 | 204 |
NOTE. All results P < .05, corrected for multiple comparisons.
Abbreviation: WM, working memory.
Cluster size listed for primary peaks.
Fig 3.
Functional neuroimaging. (A) Preintervention activation during Olesen working memory (WM) task (contrast: WM trials > control trials in random-effects group analysis; t test P < .05 with family wise error [FWE] correction). (B) Neural correlates of WM ability and training. Green denotes areas of decreased activation after WM intervention (contrast: WM trials > control trials in random-effects group analysis; paired t test P < .05 with FWE correction). Yellow denotes areas where activity was positively associated with Wechsler Intelligence Scale for Children–Fourth Edition (WISC-IV) WM index (regression: WM trials > control trials v WM index in random-effects analysis; P < .05 with FWE correction). Purple denotes area where low preintervention activity (contrast: WM trials > control trials) predicted good response to intervention (median split on spatial span backward change; P < .05 with cluster correction). (C) Activity (fixed-effects parameter estimate for each participant) of green left middle frontal gyrus cluster in (B) showing significant groupwise decrease in activation after intervention. (D) Activity of yellow right postcentral gyrus cluster in (B) versus WM index scores. Blue symbols identify patients who responded to intervention (median split on WM index change). SS, standard score (mean = 100, standard deviation = 15). Gold symbols identify nonresponders. (E) Change in spatial span backward score versus preintervention activity for purple right frontal cluster in (B). ScS, scaled score (mean = 10, standard deviation = 3). Dots are colored as in (D).
DISCUSSION
Study findings show computerized cognitive training is feasible and efficacious for childhood cancer survivors experiencing cognitive late effects. High acceptability and training compliance have been reported35 and are consistent with other computerized cognitive intervention studies,34,50 suggesting better participation than therapist-delivered cognitive interventions.23–25 Training improved short-term measures of attention, WM, and processing speed. Caregivers also reported a significant reduction in inattention and executive dysfunction. These findings are particularly relevant to childhood cancer survivors for whom IQ declines have been attributed to interruption of normal development of attention, WM, and processing speed.12,51 A reduction in fMRI prefrontal and parietal activation from pre- to postintervention demonstrates training-induced neuroplasticity, perhaps indicative of increased neural efficiency for systems known to support WM.
Study results indicate computerized cognitive training is an efficacious, portable, and less time intensive alternative to existing interventions, offering a significant advancement in the management of cognitive late effects. The Internet-based training platform allows for greater geographic reach and flexibility in scheduling, contributing to intervention disseminability. Study findings may alter management of cognitive late effects, whereby a greater number of childhood cancer survivors can now access an efficacious intervention within their home. Effect sizes for attention and WM measures were similar to those of stimulant medications for treatment of ADHD52–54 and resulted in normalized performance.
Current neuroimaging findings may provide insight into cognitive rehabilitation more broadly, including clues regarding mechanisms that underlie training-based behavioral change.55 For example, the observed post-training reduction in prefrontal activation, particularly in the left hemisphere during completion of a spatial WM task, may suggest reduced reliance on compensatory strategies, such as verbal rehearsal. Training-related activation changes in individuals with positive intervention response may allow for real-time biofeedback to facilitate greater intervention-associated gains. In addition, baseline neuroimaging findings that predict intervention response may help guide individualized intervention selection.
Of note, fMRI findings were not consistent with the a priori hypothesis of increased activation in prefrontal and parietal brain regions. Current study findings are more consistent with the larger cognitive rehabilitation literature reporting decreased activation after training on higher cognitive tasks, including WM.56–60 The primary mechanism proposed to underlie activation decrease is increased neural efficiency, potentially related to a change in cognitive processes, because childhood cancer survivors rely less on compensatory strategies and more on a well-established functional network.61 This mechanism is supported by the fact that activation was relatively unchanged by training in the right prefrontal and parietal areas, where activation was significantly correlated with WM performance. Divergent findings could reflect differences in the study populations. In the study by Olesen et al,36 fMRI participants were healthy adults without cognitive deficits, whereas current participants were childhood cancer survivors with known WM impairment. It is possible our participants had developed compensatory strategies for long-standing cognitive late effects such that a reduction in fMRI activation reflected change to a more efficient, normalized, neural activation pattern. This interpretation is also consistent with the novel finding that lower preintervention activation in the right dorsolateral prefrontal cortex was predictive of a positive response to intervention.
Primate and clinical research has established the active ingredients for successful cognitive training are intensity and adaptivity.62,63 This principle is well supported by randomized controlled trials demonstrating superiority of Cogmed over similar, but nonadaptive, computerized training or commercially available videogames.30,31,34,64–68 Accordingly, we did not use an active control group in our study because it would not add to design novelty, was not scientifically warranted, and would have reduced the likelihood controls would complete Cogmed training offered off-study.34 Although we would not anticipate nonspecific intervention benefits (eg, increased social support) to improve performance-based cognitive outcomes or fMRI findings, without an active control group, the possibility cannot be eliminated.
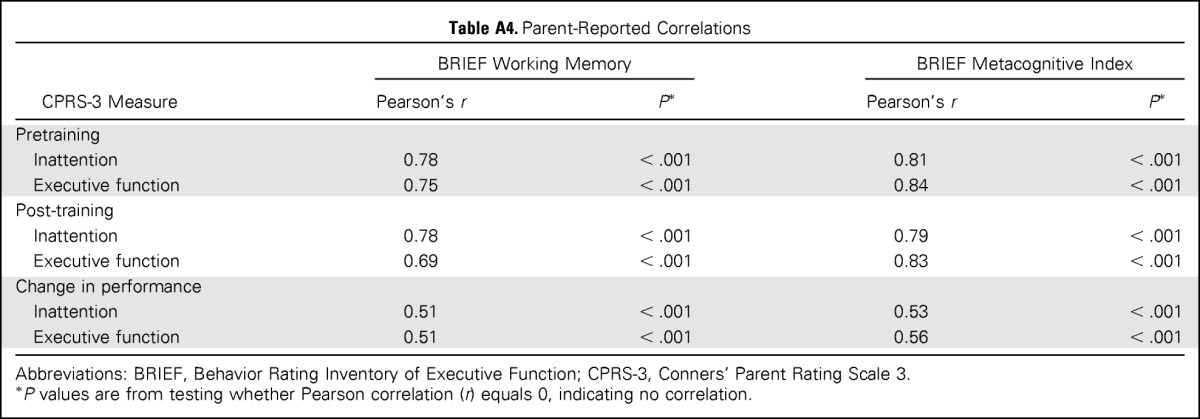
Some methodologic issues limit study conclusions and offer direction for future study. There was mixed evidence for generalizability of cognitive benefits, with improvement in processing speed but not academic fluency. Although Cogmed has been associated with improvements in reading comprehension69 and mathematic ability30 in nononcology samples, it will be important to assess the functional impact of cognitive change, including academic gains, among childhood cancer survivors. Parent-reported measures, although subject to rater biases,21 showed similar group findings and trends; however, findings for only one of two measures (CPRS-3 but not BRIEF) reached statistical significance. Post-hoc correlation analyses indicate measures were tapping similar constructs, with one more sensitive to change than the other (Appendix Table A4, online only), highlighting measurement nuances. Although the neuroimaging findings are compelling with respect to identifying mechanisms of change, fMRI examination of the control group would have allowed for better teasing apart of intervention and developmental effects. Future studies should investigate maintenance of cognitive gains, combining empirically validated interventions to assess potential therapeutic synergism and efficacy of intervention before the emergence of cognitive problems.
Supplementary Material
Acknowledgment
We thank the patients and their families who volunteered their time to participate in this study and Melissa Jones, MS, and Charlene Phillips, MSN, for their work in functional magnetic resonance imaging data acquisition.
Appendix
Neuroimaging Details
Regarding functional magnetic resonance imaging (fMRI) scan acquisition, neuroimaging was completed during baseline and postintervention visits for participants in the intervention group. Before scanning, participants watched a presentation about scanning procedures and practiced fMRI tasks, including the MRI-compatible response device. All scans were completed on 3 Tesla Magnets (Trio and Skyra models; Siemens Medical Systems). Conventional MRIs were used to identify morphologic abnormalities, facilitate spatial normalization of brain images, and visualize fMRI results. Whole-brain functional images were acquired with T2*-weighted echo planar imaging pulse sequences (repetition time, 2 seconds; echo time, 30 milliseconds; field of view, 192 mm; matrix, 64 × 64; bandwidth, 2,055 Hz/pixel; 32 slices; slice thickness, 3.5 mm). fMRI images were acquired in planes parallel to the anterior and posterior commissure lines. Stimuli were presented at the back of the magnet with an LCD projector and viewed via a mirror on the head coil.
Olesen Working Memory Task
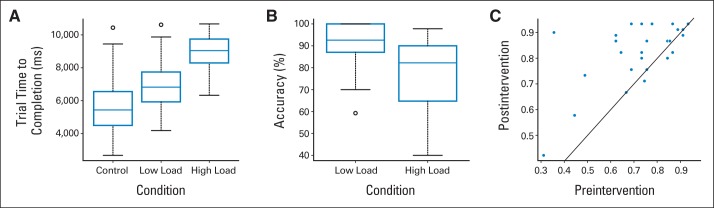
During fMRI, participants completed a block-design spatial working memory (WM) task used by Olesen et al36 in prior investigations of Cogmed with healthy adults (Appendix Fig A1). This task has been shown to activate brain areas typically associated with WM (middle and inferior frontal gyri, superior and inferior parietal cortices, and cingulate gyrus) and was sensitive to training-induced changes in healthy adults.37 Participants were presented with a 4 × 4 grid of circles. A set number of cues (solid blue circles) were presented sequentially in randomized locations. The participant's job was to repeat the pattern of cues. All task parameters were taken from Olesen et al (cue duration, 900 milliseconds; interstimulus interval, 500 milliseconds; response block, 12 seconds; intertrial duration, 5 seconds),33 except for the number of cues that were adjusted for a young population (low load, three cues instead of five; high load, five cues instead of seven). The control condition consisted of five solid green cues on the top two rows that appeared in sequential order. These cues remained illuminated until deselected by the participants. The fMRI experiment consisted of three sessions of 12 trials each (three low load, three high load, and six control trials in randomized order). Participants responded using a modified videogame controller. Performance on the task was scored using three calculated measures: trial time to completion, accuracy (percentage of correct responses regardless of order), and more strict order (percentage of correct responses in correct order).
fMRI Analysis
fMRI imaging data were preprocessed (motion corrected, slice time corrected, normalized, and smoothed [6-mm Gaussian kernel]) using SPM8 software (http://www.fil.ion.ucl.ac.uk/spm/software/spm8/).48 Standard statistical parametric mapping two-level general linear model analyses were performed. The first-level analysis consisted of fixed-effect general linear model analysis for each participant. Low-load, high-load, and control-condition trials were modeled as boxcar functions convolved with canonic hemodynamic response function. The contrast of interest was WM (low load plus high load) trials greater than control trials. Participant contrast images were then used in second-level random-effects analyses. The primary analysis consisted of a paired t test with performance covariates of interest (in magnet accuracy, clinical spatial span backward score and Wechsler Intelligence Scale for Children [fourth edition; WISC-IV] WM index) using pre- and postintervention imaging. Secondary analysis consisted of a one-sample t test on preintervention imaging with covariates of interest (change in spatial span backward score) to examine the predictive ability of preintervention patterns of brain activity. In addition, difference images (post- minus preintervention contrast images) were created for each participant and entered into a one-sample t test with covariates of interest (change in spatial span backward and change in WISC-IV WM index) to evaluate if change in bold signal predicted cognitive outcomes. Significance level for all tests was set at P < .05, with family-wise error correction for multiple comparisons at voxel level. A cluster-size threshold of five voxels was applied to all statistical parametric mapping after family-wise error correction.
Olesen WM Task Performance
Reaction time increased parametrically with trial type (Appendix Fig A2A). Accuracy decreased parametrically between the low- and high-load conditions (Appendix Fig A2B). There was no difference in reaction time pre- to postintervention. Accuracy and strict order (Appendix Fig A2C) were improved after the intervention (Wilcoxon signed-rank P < .01) for the high-load condition. Note that performance for most patients was at or above the line of identity (solid line in Appendix Fig A2C), indicating improved high-load performance after intervention.
Supplemental fMRI Results
There was no significant relationship between the pre- to postintervention change in activation and the pre- to postintervention change in clinical measures of WM performance. Post hoc testing of handedness (parent-reported left-handedness, n = 3) showed no effect of handedness on imaging results.
Table A1.
Pretraining Cognitive Scores
| Measure | Mean ± SE |
P* | |
|---|---|---|---|
| Intervention (n = 30) | Control (n = 32) | ||
| WISC-IV digit span forward† | 9.00 ± 0.46 | 8.16 ± 0.61 | .280 |
| WISC-IV digit span backward† | 8.97 ± 0.51 | 8.59 ± 0.53 | .615 |
| WISC-IV letter-number sequencing† | 9.87 ± 0.53 | 9.47 ± 0.48 | .579 |
| WISC-IV working memory index‡ | 95.33 ± 2.32 | 92.50 ± 2.52 | .414 |
| WISC-IV spatial span forward† | 9.83 ± 0.61 | 8.66 ± 0.42 | .115 |
| WISC-IV spatial span backward† | 9.50 ± 0.61 | 10.03 ± 0.50 | .503 |
| CPRS-3 inattention§ | 63.73 ± 2.53 | 61.59 ± 2.71 | .567 |
| CPRS-3 executive function§ | 62.47 ± 2.43 | 58.97 ± 2.69 | .340 |
| BRIEF working memory§ | 60.63 ± 2.07 | 60.25 ± 2.53 | .908 |
| BRIEF metacognitive index§ | 59.53 ± 2.00 | 57.75 ± 2.28 | .561 |
| CPT-II omissions§ | 51.24 ± 2.15 | 50.09 ± 1.64 | .669 |
| CPT-II hit reaction time§ | 50.26 ± 2.05 | 49.52 ± 1.51 | .768 |
| WJ-III reading fluency‡ | 97.57 ± 3.43 | 90.29 ± 2.82 | .108 |
| WJ-III math fluency‡ | 89.53 ± 2.66 | 87.59 ± 2.45 | .593 |
Abbreviations: BRIEF, Behavior Rating Inventory of Executive Function; CPRS-3, Conners' Parent Rating Scale 3; CPT-II, Conners' Continuous Performance Test II; WISC-IV, Wechsler Intelligence Scale for Children–Fourth Edition; WJ-III, Woodcock Johnson III.
P values are from independent-sample t tests between groups.
Scaled score: mean, 10; standard deviation, 3; higher score is better.
Standard score: mean, 100; standard deviation, 15; higher score is better.
T-score: mean, 50; standard deviation, 10; higher score is worse.
Table A2.
Repeated-Measures ANOVA
| Measure | Between Participants |
Within Participants |
||||
|---|---|---|---|---|---|---|
| Group |
Time |
Group × Time |
||||
| F* | P* | F* | P* | F* | P* | |
| WISC-IV digit span forward† | 1.49 | .226 | 6.67 | .012 | 0.02 | .897 |
| WISC-IV digit span backward† | 3.30 | .074 | 19.39 | .000 | 6.03 | .017 |
| WISC-IV letter-number sequencing† | 1.88 | .175 | 12.95 | .001 | 2.57 | .114 |
| WISC-IV working memory index‡ | 2.59 | .113 | 35.44 | .000 | 5.55 | .022 |
| WISC-IV spatial span forward† | 11.40 | .001 | 32.80 | .000 | 6.66 | .012 |
| WISC-IV spatial span backward† | 0.98 | .326 | 29.22 | .000 | 11.01 | .002 |
| CPRS-3 inattention§ | 0.13 | .724 | 10.74 | .002 | 7.22 | .009 |
| CPRS-3 executive function§ | 0.00 | .982 | 8.67 | .005 | 11.03 | .002 |
| BRIEF working memory§ | 0.10 | .755 | 4.86 | .031 | 2.06 | .157 |
| BRIEF metacognitive index§ | 0.01 | .906 | 10.38 | .002 | 3.38 | .071 |
| CPT-II omissions§ | 0.74 | .393 | 3.24 | .077 | 4.59 | .036 |
| CPT-II hit reaction time§ | 0.28 | .601 | 0.05 | .821 | 5.74 | .020 |
| WJ-III reading fluency‡ | 1.94 | .168 | 11.85 | .001 | 1.81 | .184 |
| WJ-III math fluency‡ | 0.18 | .671 | 2.83 | .098 | 0.25 | .620 |
Abbreviations: ANOVA, analysis of variance; BRIEF, Behavior Rating Inventory of Executive Function; CPRS-3, Conners' Parent Rating Scale 3; CPT-II, Conners' Continuous Performance Test II; WISC-IV, Wechsler Intelligence Scale for Children–Fourth Edition; WJ-III, Woodcock Johnson III.
F statistics and P values are from repeated-measures ANOVA examining main effects of group and time, as well as group × time interaction.
Scaled score: mean, 10; standard deviation, 3; higher score is better.
Standard score: mean, 100; standard deviation, 15; higher score is better.
T-score: mean, 50; standard deviation, 10; higher score is worse.
Table A3.
Full Results for Linear Mixed-Effects Model
| Measure | Intercept |
Group |
Time |
Group × Time |
IQ |
IQ × Time |
Baseline Measure |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β (SE)* | P* | β (SE) | P | β (SE) | P | β (SE) | P | β (SE) | P | β (SE) | P | β (SE) | P | |
| WISC-IV digit span forward | 8.16 (0.56) | < .001 | 0.84 (0.80) | .298 | 0.08 (0.05) | .083 | 0.01 (0.07) | .897 | ||||||
| −1.40 (2.22) | .530 | 0.20 (0.74) | .790 | 0.08 (0.05) | .083 | 0.01 (0.07) | .897 | 0.10 (0.02) | < .001 | |||||
| −2.29 (2.51) | .366 | 0.14 (0.74) | .854 | 0.02 (0.07) | .769 | 0.02 (0.07) | .769 | 0.10 (0.02) | < .001 | 0.00 (0.00) | .454 | |||
| 1.28 (0.56) | .026 | 0.13 (0.48) | .781 | 0.08 (0.05) | .075 | 0.01 (0.07) | .894 | 0.84 (0.06) | < .001 | |||||
| WISC-IV digit span backward | 8.59 (0.50) | < .001 | 0.37 (0.71) | .604 | 0.06 (0.04) | .166 | 0.16 (0.06) | .017 | ||||||
| 1.82 (2.06) | .381 | −0.09 (0.68) | .901 | 0.06 (0.04) | .166 | 0.16 (0.06) | .017 | 0.07 (0.02) | .001 | |||||
| 1.00 (2.33) | .670 | −0.14 (0.69) | .839 | 0.23 (0.22) | .316 | 0.17 (0.07) | .013 | 0.08 (0.02) | .002 | 0.00 (0.00) | .458 | |||
| 1.90 (0.54) | < .001 | 0.08 (0.43) | .847 | 0.06 (0.04) | .140 | 0.16 (0.06) | .011 | 0.78 (0.05) | < .001 | |||||
| WISC-IV letter-number sequencing | 9.47 (0.47) | < .001 | 0.40 (0.68) | .561 | 0.06 (0.04) | .157 | 0.09 (0.06) | .114 | ||||||
| −1.44 (1.63) | .380 | −0.34 (0.55) | .542 | 0.06 (0.04) | .157 | 0.09 (0.06) | .114 | 0.11 (0.02) | < .001 | |||||
| −1.50 (1.90) | .431 | −0.34 (0.56) | .541 | 0.07 (0.20) | .731 | 0.09 (0.06) | .123 | 0.11 (0.02) | < .001 | 0.00 (0.00) | .950 | |||
| 1.89 (0.52) | < .001 | 0.08 (0.37) | .832 | 0.06 (0.04) | .131 | 0.09 (0.05) | .092 | 0.80 (0.05) | < .001 | |||||
| WISC-IV working memory index | 92.50 (2.47) | < .001 | 2.83 (3.55) | .428 | 0.40 (0.15) | .012 | 0.52 (0.22) | .022 | ||||||
| 33.35 (8.89) | < .001 | −1.17 (2.84) | .682 | 0.40 (0.15) | .012 | 0.52 (0.22) | .022 | 0.59 (0.09) | < .001 | |||||
| 30.56 (9.66) | .002 | −1.36 (2.85) | .636 | 0.96 (0.77) | .219 | 0.56 (0.23) | .017 | 0.62 (0.09) | < .001 | −0.01 (0.01) | .461 | |||
| 6.46 (3.95) | .107 | 0.20 (1.55) | .899 | 0.40 (0.15) | .012 | 0.52 (0.22) | .021 | 0.93 (0.04) | < .001 | |||||
| WISC-IV spatial span forward | 8.66 (0.53) | < .001 | 1.18 (0.76) | .128 | 0.12 (0.06) | .027 | 0.21 (0.08) | .012 | ||||||
| 2.47 (2.15) | .256 | 0.76 (0.74) | .313 | 0.13 (0.06) | .027 | 0.21 (0.08) | .012 | 0.06 (0.02) | .004 | |||||
| 5.01 (2.53) | .052 | 0.93 (0.75) | .217 | −0.38 (0.27) | .161 | 0.17 (0.08) | .037 | 0.04 (0.02) | .146 | 0.01 (0.00) | .060 | |||
| 2.19 (0.68) | .002 | 0.30 (0.54) | .582 | 0.12 (0.05) | .020 | 0.21 (0.08) | .008 | 0.75 (0.07) | < .001 | |||||
| WISC-IV spatial span backward | 10.03 (0.53) | < .001 | −0.53 (0.76) | .486 | 0.08 (0.05) | .139 | 0.24 (0.07) | .002 | ||||||
| 2.88 (2.15) | .186 | −1.02 (0.73) | .167 | 0.08 (0.05) | .139 | 0.24 (0.07) | .002 | 0.07 (0.02) | .001 | |||||
| 4.42 (2.47) | .079 | −0.91 (0.73) | .217 | −0.23 (0.25) | .351 | 0.22 (0.07) | .004 | 0.06 (0.02) | .024 | 0.00 (0.00) | .210 | |||
| 2.46 (0.64) | < .001 | −0.13 (0.47) | .784 | 0.07 (0.05) | .111 | 0.24 (0.07) | < .001 | 0.75 (0.05) | < .001 | |||||
| CPRS-3 inattention | 61.59 (2.38) | < .001 | 2.14 (3.42) | .534 | −0.07 (0.17) | .673 | −0.65 (0.24) | .009 | ||||||
| 63.09 (11.22) | < .001 | 2.24 (3.52) | .527 | −0.07 (0.17) | .673 | −0.65 (0.24) | .009 | −0.01 (0.11) | .892 | |||||
| 64.62 (11.97) | < .001 | 2.34 (3.53) | .510 | −0.38 (0.85) | .659 | −0.68 (0.25) | .009 | −0.03 (0.12) | .797 | 0.00 (0.01) | .715 | |||
| 11.41 (2.64) | < .001 | 0.40 (1.59) | .804 | −0.07 (0.16) | .647 | −0.65 (0.22) | .005 | 0.81 (0.04) | < .001 | |||||
| CPRS-3 executive function | 58.97 (2.31) | < .001 | 3.50 (3.32) | .297 | 0.04 (0.15) | .787 | −0.71 (0.21) | .002 | ||||||
| 57.38 (11.05) | < .001 | 3.39 (3.43) | .326 | 0.04 (0.15) | .787 | −0.71 (0.21) | .002 | 0.02 (0.11) | .883 | |||||
| 57.72 (11.65) | < .001 | 3.41 (3.44) | .325 | −0.03 (0.75) | .970 | −0.72 (0.22) | .002 | 0.01 (0.11) | .913 | 0.00 (0.01) | .926 | |||
| 10.03 (2.28) | < .001 | 0.59 (1.40) | .672 | 0.04 (0.14) | < .001 | −0.71 (0.20) | < .001 | 0.83 (0.03) | < .001 | |||||
| BRIEF working memory | 60.25 (2.22) | < .001 | 0.38 (3.19() | .905 | −0.07 (0.13) | .582 | −0.27 (0.19) | .157 | ||||||
| 62.59 (10.71) | < .001 | 0.54 (3.29) | .870 | −0.07 (0.13) | .582 | −0.27 (0.19) | .157 | −0.02 (0.10) | .824 | |||||
| 65.66 (11.18) | < .001 | 0.75 (3.30) | .821 | −0.69 (0.65) | .296 | −0.31 (0.19) | .112 | −0.05 (0.11) | .623 | 0.01 (0.01) | .339 | |||
| 6.73 (2.30) | .005 | 0.04 (1.27) | .973 | −0.07 (0.13) | .569 | −0.27 (0.18) | .142 | 0.89 (0.04) | < .001 | |||||
| BRIEF metacognitive index | 57.75 (2.00) | < .001 | 1.78 (2.88) | .538 | −0.11 (0.11) | .324 | −0.29 (0.16) | .071 | ||||||
| 64.38 (9.69) | < .001 | 2.23 (2.96) | .454 | −0.11 (0.11) | .324 | −0.29 (0.16) | .071 | −0.07 (0.09) | .487 | |||||
| 63.54 (10.06) | < .001 | 2.18 (2.97) | .467 | 0.06 (0.55) | .916 | −0.28 (0.16) | .093 | −0.06 (0.10) | .559 | 0.00 (0.01) | .758 | |||
| 6.99 (1.97) | < .001 | 0.22 (1.06) | .840 | −0.11 (0.10) | .298 | −0.29 (0.15) | .057 | 0.88 (0.03) | < .001 | |||||
| CPT-II omissions | 50.09 (2.03) | < .001 | 1.16 (2.91) | .693 | 0.57 (0.20) | .006 | −0.63 (0.29) | .032 | ||||||
| 53.22 (8.99) | < .001 | 1.37 (2.99) | .649 | 0.57 (0.20) | .006 | −0.63 (0.29) | .032 | −0.03 (0.09) | .722 | |||||
| 59.34 (10.18) | < .001 | 1.78 (3.01) | .556 | −0.69 (1.00) | .490 | −0.71 (0.29) | .018 | −0.09 (0.10) | .358 | 0.01 (0.01) | .203 | |||
| 9.58 (3.59) | .010 | 0.22 (1.95) | .910 | 0.57 (0.19) | .004 | −0.62 (0.28) | .028 | 0.81 (0.07) | < .001 | |||||
| CPT-II hit reaction time | 49.52 (1.74) | < .001 | 0.75 (2.50) | .766 | 0.25 (0.13) | .062 | −0.45 (0.19) | .022 | ||||||
| 58.41 (8.05) | < .001 | 1.35 (2.55) | .599 | 0.25 (0.13) | .062 | −0.46 (0.19) | .022 | −0.09 (0.08) | .263 | |||||
| 61.16 (8.67) | < .001 | 1.53 (2.56) | .551 | −0.32 (0.68) | .643 | −0.49 (0.20) | .016 | −0.12 (0.08) | .175 | 0.01 (0.01) | .395 | |||
| 7.55 (2.51) | .004 | 0.11 (1.30) | .931 | 0.25 (0.13) | .052 | −0.46 (0.18) | .016 | 0.85 (0.05) | < .001 | |||||
| WJ-III reading fluency | 90.29 (3.15) | < .001 | 7.28 (4.49) | .110 | 0.40 (0.12) | .001 | −0.23 (0.17) | .184 | ||||||
| 14.43 (11.85) | .228 | 2.60 (3.55) | .467 | 0.40 (0.12) | .001 | −0.23 (0.17) | .184 | 0.75 (0.11) | < .001 | |||||
| 11.89 (12.20) | .334 | 2.44 (3.55) | .495 | 0.91 (0.59) | .129 | −0.20 (0.17) | .263 | 0.78 (0.12) | < .001 | −0.01 (0.01) | .385 | |||
| 2.60 (2.37) | .278 | 0.21 (1.20) | .862 | 0.40 (0.12) | .001 | −0.23 (0.17) | .183 | 0.97 (0.02) | < .001 | |||||
| WJ-III math fluency | 87.59 (2.60) | < .001 | 1.94 (3.74) | .606 | 0.17 (0.11) | .122 | −0.08 (0.15) | .620 | ||||||
| 38.05 (11.03) | .001 | −1.41 (3.34) | .674 | 0.17 (0.11) | .122 | −0.08 (0.15) | .620 | 0.49 (0.11) | < .001 | |||||
| 38.59 (11.33) | .001 | −1.38 (3.34) | .682 | 0.06 (0.53) | .914 | −0.08 (0.16) | .600 | 0.49 (0.11) | < .001 | 0.00 (0.01) | .837 | |||
| 0.68 (2.51) | .787 | 0.02 (1.08) | .989 | 0.17 (0.11) | .122 | −0.08 (0.15) | .620 | 0.99 (0.03) | < .001 | |||||
Abbreviations: BRIEF, Behavior Rating Inventory of Executive Function; CPRS-3, Conners' Parent Rating Scale 3; CPT-II, Conners' Continuous Performance Test II; IQ, intelligence quotient; WISC-IV, Wechsler Intelligence Scale for Children–Fourth Edition; WJ-III, Woodcock Johnson III.
β statistics (with SEs) and P values are results of fixed effects from linear mixed-effects modeling for primary and secondary cognitive outcomes. Each row represents different model. First row is basic model examining main effects of group and time, as well as group × time interaction. Second row, baseline IQ is added in model. For third row, interaction between baseline IQ and time is added to model. Fourth row under each measure includes baseline cognitive score for that measure as covariate in model.
Table A4.
Parent-Reported Correlations
| CPRS-3 Measure | BRIEF Working Memory |
BRIEF Metacognitive Index |
||
|---|---|---|---|---|
| Pearson's r | P* | Pearson's r | P* | |
| Pretraining | ||||
| Inattention | 0.78 | < .001 | 0.81 | < .001 |
| Executive function | 0.75 | < .001 | 0.84 | < .001 |
| Post-training | ||||
| Inattention | 0.78 | < .001 | 0.79 | < .001 |
| Executive function | 0.69 | < .001 | 0.83 | < .001 |
| Change in performance | ||||
| Inattention | 0.51 | < .001 | 0.53 | < .001 |
| Executive function | 0.51 | < .001 | 0.56 | < .001 |
Abbreviations: BRIEF, Behavior Rating Inventory of Executive Function; CPRS-3, Conners' Parent Rating Scale 3.
P values are from testing whether Pearson correlation (r) equals 0, indicating no correlation.
Fig A1.
Block-design spatial working memory task designed by Olesen et al.36 ISI, interstimulus interval.
Fig A2.
Working memory task (designed by Olesen et al36) performance. (A) Trial time versus trial type; (B) accuracy versus load; (C) high-load performance (order).
Footnotes
Supported in part by the National Cancer Institute (Core Grant No. P30 CA21765 to St Jude Cancer Center Support), American Cancer Society (Grant No. RSGPB-11-009-01-CPPB to H.M.C.), and American Lebanese Syrian Associated Charities. Cogmed software was provided by Pearson Education (New York, NY) for research purposes.
Presented in part at the 49th Annual Meeting of the American Society of Clinical Oncology, Chicago, IL, May 31-June 4, 2013; the Annual Meeting of the Society for Neuro-Oncology, San Francisco, CA, November 21-24, 2013; and the Annual Meeting of the International Neuropsychological Society, Seattle, WA, February 12-15, 2014.
Pearson Education did not play a role in the design or conduct of the study; analysis or interpretation of the data; or preparation, review, or approval of the manuscript.
Authors' disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
Clinical trial information: NCT01217996.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Disclosures provided by the authors are available with this article at www.jco.org.
AUTHOR CONTRIBUTIONS
Conception and design: Heather M. Conklin, Robert J. Ogg, Jason M. Ashford, Kristina K. Hardy, Thomas E. Merchant, Sima Jeha, Hui Zhang
Financial support: Heather M. Conklin
Administrative support: Heather M. Conklin, Robert J. Ogg, Jason M. Ashford
Provision of study materials or patients: Heather M. Conklin, Robert J. Ogg, Thomas E. Merchant, Sima Jeha
Collection and assembly of data: Heather M. Conklin, Jason M. Ashford, Matthew A. Scoggins, Kellie N. Clark, Karen Martin-Elbahesh, Thomas E. Merchant
Data analysis and interpretation: Heather M. Conklin, Robert J. Ogg, Jason M. Ashford, Matthew A. Scoggins, Ping Zou, Lu Huang, Hui Zhang
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Computerized Cognitive Training for Amelioration of Cognitive Late Effects Among Childhood Cancer Survivors: A Randomized Controlled Trial
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Heather M. Conklin
No relationship to disclose
Robert J. Ogg
No relationship to disclose
Jason M. Ashford
No relationship to disclose
Matthew A. Scoggins
No relationship to disclose
Ping Zou
No relationship to disclose
Kellie N. Clark
No relationship to disclose
Karen Martin-Elbahesh
No relationship to disclose
Kristina K. Hardy
No relationship to disclose
Thomas E. Merchant
No relationship to disclose
Sima Jeha
No relationship to disclose
Lu Huang
No relationship to disclose
Hui Zhang
No relationship to disclose
REFERENCES
- 1.Moleski M. Neuropsychological, neuroanatomical, and neurophysiological consequences of CNS chemotherapy for acute lymphoblastic leukemia. Arch Clin Neuropsychol. 2000;15:603–630. [PubMed] [Google Scholar]
- 2.Moore BD., 3rd Neurocognitive outcomes in survivors of childhood cancer. J Pediatr Psychol. 2005;30:51–63. doi: 10.1093/jpepsy/jsi016. [DOI] [PubMed] [Google Scholar]
- 3.Mulhern RK, Butler RW. Neurocognitive sequelae of childhood cancers and their treatment. Pediatr Rehabil. 2004;7:1–14. doi: 10.1080/13638490310001655528. [DOI] [PubMed] [Google Scholar]
- 4.Ris MD, Noll RB. Long-term neurobehavioral outcome in pediatric brain-tumor patients: Review and methodological critique. J Clin Exp Neuropsychol. 1994;16:21–42. doi: 10.1080/01688639408402615. [DOI] [PubMed] [Google Scholar]
- 5.Crom DB, Lensing SY, Rai SN, et al. Marriage, employment, and health insurance in adult survivors of childhood cancer. J Cancer Surviv. 2007;1:237–245. doi: 10.1007/s11764-007-0026-x. [DOI] [PubMed] [Google Scholar]
- 6.Haupt R, Fears TR, Robinson LL, et al. Educational attainment in long-term survivors of acute lymphoblastic leukemia. JAMA. 1994;272:1427–1432. [PubMed] [Google Scholar]
- 7.Mitby PA, Robison LL, Whitton JA, et al. Utilization of special education services and educational attainment among long-term survivors of childhood cancer: A report from the Childhood Cancer Survivor Study. Cancer. 2003;97:1115–1126. doi: 10.1002/cncr.11117. [DOI] [PubMed] [Google Scholar]
- 8.Mostow EN, Byrne J, Connelly RR, et al. Quality of life in long-term survivors of CNS tumors of childhood and adolescence. J Clin Oncol. 1991;9:592–599. doi: 10.1200/JCO.1991.9.4.592. [DOI] [PubMed] [Google Scholar]
- 9.Conklin HM, Ashford JM, Howarth RA, et al. Working memory performance among childhood brain tumor survivors. J Int Neuropsychol Soc. 2012;18:996–1005. doi: 10.1017/S1355617712000793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mabbott DJ, Penkman L, Witol A, et al. Core neurocognitive functions in children treated for posterior fossa tumors. Neuropsychology. 2008;22:159–168. doi: 10.1037/0894-4105.22.2.159. [DOI] [PubMed] [Google Scholar]
- 11.Reeves CB, Palmer SL, Reddick WE, et al. Attention and memory function among pediatric patients with medulloblastoma. J Pediatr Psychol. 2006;31:272–280. doi: 10.1093/jpepsy/jsj019. [DOI] [PubMed] [Google Scholar]
- 12.Schatz J, Kramer JH, Ablin A, et al. Processing speed, working memory, and IQ: A developmental model of cognitive deficits following cranial radiation therapy. Neuropsychology. 2000;14:189–200. doi: 10.1037//0894-4105.14.2.189. [DOI] [PubMed] [Google Scholar]
- 13.Waber DP, Pomeroy SL, Chiverton AM, et al. Everyday cognitive function after craniopharyngioma in childhood. Pediatr Neurol. 2006;34:13–19. doi: 10.1016/j.pediatrneurol.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 14.Carey ME, Haut MW, Reminger SL, et al. Reduced frontal white matter volume in long-term childhood leukemia survivors: A voxel-based morphometry study. AJNR Am J Neuroradiol. 2008;29:792–797. doi: 10.3174/ajnr.A0904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mulhern RK, White HA, Glass JO, et al. Attentional functioning and white matter integrity among survivors of malignant brain tumors of childhood. J Int Neuropsychol Soc. 2004;10:180–189. doi: 10.1017/S135561770410204X. [DOI] [PubMed] [Google Scholar]
- 16.Qiu D, Kwong DL, Chan GC, et al. Diffusion tensor magnetic resonance imaging finding of discrepant fractional anisotropy between the frontal and parietal lobes after whole-brain irradiation in childhood medulloblastoma survivors: Reflection of regional white matter radiosensitivity? Int J Radiat Oncol Biol Phys. 2007;69:846–851. doi: 10.1016/j.ijrobp.2007.04.041. [DOI] [PubMed] [Google Scholar]
- 17.Reddick WE, Taghipour DJ, Glass JO, et al. Prognostic factors that increase the risk for reduced white matter volumes and deficits in attention and learning for survivors of childhood cancers. Pediatr Blood Cancer. 2014;61:1074–1079. doi: 10.1002/pbc.24947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Law N, Bouffet E, Laughlin S, et al. Cerebello-thalamo-cerebral connections in pediatric brain tumor patients: Impact on working memory. Neurimage. 2011;56:2238–2248. doi: 10.1016/j.neuroimage.2011.03.065. [DOI] [PubMed] [Google Scholar]
- 19.Jemal A, Siegel R, Ward E, et al. Cancer statistics. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 20.Pui CH, Campana D, Pei D, et al. Treating childhood acute lymphoblastic leukemia without cranial irradiation. N Engl J Med. 2009;360:2730–2741. doi: 10.1056/NEJMoa0900386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Conklin HM, Reddick WE, Ashford J, et al. Long-term efficacy of methylphenidate in enhancing attention regulation, social skills, and academic abilities of childhood cancer survivors. J Clin Oncol. 2010;28:4465–4472. doi: 10.1200/JCO.2010.28.4026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mulhern RK, Khan RB, Kaplan S, et al. Short-term efficacy of methylphenidate: A randomized, double-blind, placebo-controlled trial among survivors of childhood cancer. J Clin Oncol. 2004;22:4795–4803. doi: 10.1200/JCO.2004.04.128. [DOI] [PubMed] [Google Scholar]
- 23.Butler RW, Copeland DR, Fairclough DL, et al. A multicenter, randomized clinical trial of cognitive remediation program for childhood survivors of pediatric malignancy. J Consult Clin Psychol. 2008;76:367–378. doi: 10.1037/0022-006X.76.3.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Patel SK, Katz ER, Richardson R, et al. Cognitive and problem solving training in children with cancer: A pilot project. J Pediatr Hematol Oncol. 2009;31:670–677. doi: 10.1097/MPH.0b013e3181b25a1d. [DOI] [PubMed] [Google Scholar]
- 25.Moore IM, Hockenberry MJ, Anhault C, et al. Mathematics intervention for prevention of neurocognitive deficits in childhood leukemia. Pediatr Blood Cancer. 2012;59:278–284. doi: 10.1002/pbc.23354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Conklin HM, Khan RB, Reddick WE, et al. Acute neurocognitive response to methylphenidate among survivors of childhood cancer: A randomized, double-blind, cross-over trial. J Pediatr Psychol. 2007;32:1127–1139. doi: 10.1093/jpepsy/jsm045. [DOI] [PubMed] [Google Scholar]
- 27.Conklin HM, Lawford J, Jasper BW, et al. Side effects of methylphenidate in survivors of childhood cancer: A randomized double-blind, placebo-controlled trial. Pediatrics. 2009;124:226–233. doi: 10.1542/peds.2008-1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Conklin HM, Helton S, Ashford J, et al. Predicting methylphenidate response in long-term survivors of childhood cancer: A randomized, double-blinded, placebo-controlled, cross-over trial. J Pediatr Psychol. 2010;35:144–155. doi: 10.1093/jpepsy/jsp044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jasper BW, Conklin HM, Lawford J, et al. Growth effects of methylphenidate among childhood cancer survivors: A 12-month case-matched open-label study. Pediatr Blood Cancer. 2009;52:39–43. doi: 10.1002/pbc.21770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Holmes J, Gathercole SE, Dunning DL. Adaptive training leads to sustained enhancement of poor working memory in children. Dev Sci. 2009;12:F9–F15. doi: 10.1111/j.1467-7687.2009.00848.x. [DOI] [PubMed] [Google Scholar]
- 31.Klingberg T, Fernell E, Olesen P, et al. Computerized training of working memory in children with ADHD: A randomized, controlled trial. J Am Acad Child Adolesc Psychiatry. 2005;44:177–186. doi: 10.1097/00004583-200502000-00010. [DOI] [PubMed] [Google Scholar]
- 32.Westerberg H, Jacobaeus H, Hirvikoski T, et al. Computerized working memory training after stroke: A pilot study. Brain Inj. 2007;21:21–29. doi: 10.1080/02699050601148726. [DOI] [PubMed] [Google Scholar]
- 33.Grunewaldt KH, Løhaugen GC, Austeng D. Working memory training improves cognitive function in VLBW preschoolers. Pediatrics. 2013;131:747–754. doi: 10.1542/peds.2012-1965. [DOI] [PubMed] [Google Scholar]
- 34.Hardy KK, Willard VW, Allen TM. Working memory training in survivors of pediatric cancer: A randomized pilot study. Psychooncology. 2013;22:1856–1865. doi: 10.1002/pon.3222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cox LE, Ashford JM, Clark KN, et al. Feasibility and acceptability of a remotely-administered computerized intervention to address cognitive late effects among childhood cancer survivors. Neuro Oncol Pract. 2015;2:70–77. doi: 10.1093/nop/npu036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Olesen PJ, Westerberg H, Klingberg T. Increased prefrontal and parietal activity after training of working memory. Nat Neurosci. 2004;7:75–79. doi: 10.1038/nn1165. [DOI] [PubMed] [Google Scholar]
- 37.Westerberg H, Klingberg T. Changes in cortical activity after training of working memory: A single-subject analysis. Physiol Behav. 2007;92:186–192. doi: 10.1016/j.physbeh.2007.05.041. [DOI] [PubMed] [Google Scholar]
- 38.Wechsler D. Wechsler Intelligence Scale for Children (ed 4, integrated) San Antonio, TX: Pearson Corporation; 2004. [Google Scholar]
- 39.Wechsler D. Wechsler Abbreviated Scale of Intelligence. San Antonio, TX: Harcourt Assessment; 1999. [Google Scholar]
- 40.Zelen M. The randomization and stratification of patients to clinical trials. J Chronic Dis. 1974;27:365–375. doi: 10.1016/0021-9681(74)90015-0. [DOI] [PubMed] [Google Scholar]
- 41.Wechsler D. Wechsler Intelligence Scale for Children (ed 3) San Antonio, TX: Psychological Corporation; 1991. [Google Scholar]
- 42.Wechsler D. Wechsler Adult Intelligence Scale (ed 3) San Antonio, TX: Harcourt Assessment; 1999. [Google Scholar]
- 43.Conners CK. Conners' Continuous Performance Test II. San Antonio, TX: Pearson Corporation; 2004. [Google Scholar]
- 44.Woodcock RW, McGrew KS, Mather N. Woodcock-Johnson III Tests of Achievement. Itasca, IL: Riverside Publishing; 2001. [Google Scholar]
- 45.Conners CK. Conners' Rating Scales (ed 3) Toronto, Ontario, Canada: Multi-Health Systems; 2008. [Google Scholar]
- 46.Gioia GA, Isquith PK, Guy SC, et al. Behavior Rating Inventory of Executive Function. Odessa, FL: Psychological Assessment Resources; 2000. [Google Scholar]
- 47.Cohen J. Statistical Power Analyses for the Behavioral Sciences (ed 2) Hillsdale, NJ: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- 48.Friston KJ, Holmes AP, Worsley KJ, et al. Statistical parametric maps in functional imaging: A general linear approach. Hum Brain Mapp. 1995;2:189–210. [Google Scholar]
- 49.Nee DE, Brown JW, Askren MK, et al. A meta-analysis of executive components of working memory. Cereb Cortex. 2013;23:264–282. doi: 10.1093/cercor/bhs007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kesler SR, Lacayo NJ, Jo B. A pilot study of an online cognitive rehabilitation program for executive function skills in children with cancer-related brain injury. Brain Inj. 2011;25:101–112. doi: 10.3109/02699052.2010.536194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Palmer SL, Armstrong C, Onar-Thomas A, et al. Processing speed, attention, and working memory after treatment for medulloblastoma: An international, prospective, and longitudinal study. J Clin Oncol. 2013;31:3494–3500. doi: 10.1200/JCO.2012.47.4775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Barnett R, Maruff P, Vance A, et al. Abnormal executive function in attention deficit hyperactivity disorder: The effect of stimulant medication and age on spatial working memory. Psychol Med. 2001;31:1107–1115. doi: 10.1017/s0033291701004172. [DOI] [PubMed] [Google Scholar]
- 53.Bedard AC, Martinussen R, Ickowicz A, et al. Methylphenidate improves visual-spatial memory in children with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2004;43:260–268. doi: 10.1097/00004583-200403000-00006. [DOI] [PubMed] [Google Scholar]
- 54.Kempton S, Vance A, Maruff P, et al. Executive function and attention deficit hyperactivity disorder: Stimulant medication and better executive function performance in children. Psychol Med. 1999;29:527–538. doi: 10.1017/s0033291799008338. [DOI] [PubMed] [Google Scholar]
- 55.Patel R, Spreng RN, Turner GR. Functional brain changes following cognitive and motor skills training: A quantitative meta-analysis. Neurorehabil Neural Repair. 2013;27:187–199. doi: 10.1177/1545968312461718. [DOI] [PubMed] [Google Scholar]
- 56.Kelly C, Foxe JJ, Garavan H. Patterns of normal human brain plasticity after practice and their implications for neurorehabilitation. Arch Phys Med Rehabil. 2006;87(suppl):S20–S29. doi: 10.1016/j.apmr.2006.08.333. [DOI] [PubMed] [Google Scholar]
- 57.Garavan H, Kelley D, Rosen A, et al. Practice-related functional activation changes in a working memory task. Microsc Res Tech. 2000;51:54–63. doi: 10.1002/1097-0029(20001001)51:1<54::AID-JEMT6>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 58.Jansma JM, Ramsey NF, Slagter HA, et al. Functional anatomical correlates of controlled and automatic processing. J Cogn Neurosci. 2001;13:730–743. doi: 10.1162/08989290152541403. [DOI] [PubMed] [Google Scholar]
- 59.Sayala S, Sala JB, Courtney SM. Increased neural efficiency with repeated performance of a working memory task is information-type dependent. Cereb Cortex. 2006;16:609–617. doi: 10.1093/cercor/bhj007. [DOI] [PubMed] [Google Scholar]
- 60.Hempel A, Giesel FL, Caraballo NM, et al. Plasticity of cortical activation related to working memory during training. Am J Psychiatry. 2004;161:745–747. doi: 10.1176/appi.ajp.161.4.745. [DOI] [PubMed] [Google Scholar]
- 61.Poldrack RA. Is “efficiency” a useful concept in cognitive neuroscience? Dev Cog Neurosci. 2015;11:12–17. doi: 10.1016/j.dcn.2014.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Diamond A, Lee K. Interventions shown to aid executive function development in children 4 to 12 years old. Science. 2011;333:959–963. doi: 10.1126/science.1204529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Klingberg T. The Overflowing Brain: Information Overload and the Limits of Working Memory. New York, NY: Oxford University Press; 2008. [Google Scholar]
- 64.Klingberg T, Forssberg H, Westerberg H. Training of working memory in children with ADHD. J Clin Exper Neuropsych. 2002;24:781–791. doi: 10.1076/jcen.24.6.781.8395. [DOI] [PubMed] [Google Scholar]
- 65.Dunning DL, Holmes J, Gathercole SE. Does working memory training lead to generalized improvements in children with low working memory? A randomized controlled trial. Dev Sci. 2013;16:915–925. doi: 10.1111/desc.12068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Green CT, Long DL, Green D, et al. Will working memory training generalize to improve off-task behavior in children with attention-deficit/hyperactivity disorder? Neurotherapeutics. 2012;9:639–648. doi: 10.1007/s13311-012-0124-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chacko A, Feirsen N, Bedard A, et al. Cogmed working memory training for youth with ADHD: A closer examination of efficacy utilizing evidence-based criteria. J Clin Child Adol Psychol. 2013;42:769–783. doi: 10.1080/15374416.2013.787622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Thorell LB, Lindqvist S, Bergman Nutley S, et al. Training and transfer effects of executive functions in preschool children. Dev Sci. 2009;12:106–133. doi: 10.1111/j.1467-7687.2008.00745.x. [DOI] [PubMed] [Google Scholar]
- 69.Dahlin KI. Effects of working memory training on reading in children with special needs. Read Writ. 2011;24:479–491. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.