Abstract
Purpose
To determine the 12-year risk of developing an ipsilateral breast event (IBE) for women with ductal carcinoma in situ (DCIS) of the breast treated with surgical excision (lumpectomy) without radiation.
Patients and Methods
A prospective clinical trial was performed for women with DCIS who were selected for low-risk clinical and pathologic characteristics. Patients were enrolled onto one of two study cohorts (not randomly assigned): cohort 1: low- or intermediate-grade DCIS, tumor size 2.5 cm or smaller (n = 561); or cohort 2: high-grade DCIS, tumor size 1 cm or smaller (n = 104). Protocol specifications included excision of the DCIS tumor with a minimum negative margin width of at least 3 mm. Tamoxifen (not randomly assigned) was given to 30% of the patients. An IBE was defined as local recurrence of DCIS or invasive carcinoma in the treated breast. Median follow-up time was 12.3 years.
Results
There were 99 IBEs, of which 51 (52%) were invasive. The IBE and invasive IBE rates increased over time in both cohorts. The 12-year rates of developing an IBE were 14.4% for cohort 1 and 24.6% for cohort 2 (P = .003). The 12-year rates of developing an invasive IBE were 7.5% and 13.4%, respectively (P = .08). On multivariable analysis, study cohort and tumor size were both significantly associated with developing an IBE (P = .009 and P = .03, respectively).
Conclusion
For patients with DCIS selected for favorable clinical and pathologic characteristics and treated with excision without radiation, the risks of developing an IBE and an invasive IBE increased through 12 years of follow-up, without plateau. These data help inform the treatment decision-making process for patients and their physicians.
INTRODUCTION
The optimal clinical management for women with newly diagnosed ductal carcinoma in situ (DCIS; intraductal carcinoma) of the breast is controversial, with variable patterns of practice.1–7 Although most women with DCIS of the breast present with an asymptomatic finding on routine screening mammography, population-based studies demonstrate a wide range of treatments.4–7 Local treatment options for DCIS include surgical excision (lumpectomy) with or without radiation treatment, unilateral mastectomy, and even bilateral mastectomies. Systemic therapy options include adjuvant tamoxifen for hormone receptor–positive DCIS tumors. In randomized clinical trials, adding radiation treatment or tamoxifen after lumpectomy has been shown to reduce recurrence rates, with low rates of distant metastases and high rates of overall survival (OS), regardless of initial treatment.8–19
The Eastern Cooperative Oncology Group–American College of Radiology Imaging Network (ECOG-ACRIN; formerly the Eastern Cooperative Oncology Group) Cancer Research Group E5194 study prospectively enrolled onto a nonrandomized clinical trial those patients for whom surgical excision alone (without radiation) was thought to be a reasonable treatment option on the basis of low-risk clinical and pathologic characteristics. A previous analysis from the ECOG-ACRIN E5194 study reported 5- and 7-year outcomes.20 The present report provides updated results from the ECOG-ACRIN E5194 study, including 10- and 12-year outcomes.
PATIENTS AND METHODS
Study Design
The ECOG-ACRIN E5194 study was a prospective, nonrandomized clinical trial. Patients were enrolled onto protocol through ECOG-ACRIN and the North Central Cancer Treatment Group (now part of the Alliance for Clinical Trials in Oncology) from April 1997 to October 2002. The study protocol was approved by the respective institutional review boards of the participating centers at which the patients were enrolled. All patients gave written informed consent.
Detailed information on the trial design and conduct has previously been reported.20,21 A brief summary is as follows. The protocol study included two cohorts of patients (not randomly assigned) with low-risk clinical and pathologic characteristics: cohort 1: low- or intermediate-grade DCIS, tumor size 2.5 cm or smaller (cohort 1; n = 561 patients); or cohort 2: high-grade DCIS, tumor size 1 cm or smaller (cohort 2; n = 104 patients). Cohort assignment and study enrollment were based on clinical evaluation and pathology assessment from the treating institution.
All patients underwent surgical excision (lumpectomy) of the primary DCIS tumor. Protocol specifications included surgical excision with a minimum negative margin width of at least 3 mm or no tumor on re-excision. The surgical specimen was sequentially sectioned and completely embedded at the treating institution to determine the pathologic characteristics of the DCIS tumor. Hormone receptor status was not collected at the time of study entry. A negative postoperative mammogram was required for any patient presenting with suspicious calcifications on preoperative mammography. Radiation treatment was not allowed. In May 2000, the study was amended to allow treatment with adjuvant tamoxifen in an optional, nonrandomized fashion. In the event of disease recurrence or progression, treatment was given at the discretion of the treating physicians.
A total of 665 patients were evaluated for the present analysis (n = 561 patients in cohort 1; and n = 104 patients in cohort 2). After the first report of the E5194 study, subsequent central pathology review determined that five patients had invasive carcinoma, and these patients were excluded from the present analysis. Evaluation of the study results either with or without inclusion of these five patients did not differ substantially (data not shown).
Pathology Evaluation
Pathology findings have been scored in three ways. First, pathology findings were recorded from the institutional pathology assessment on entry into the parent study protocol. Second, in the parent study, central pathology review (also referred to as the first central pathology review) was performed using previously specified criteria.22 Tumor size was evaluated on central pathology review of 601 patients (n = 501 for cohort 1; and n = 100 for cohort 2). Thereafter, the College of American Pathologists (CAP) published a new set of guidelines for reporting DCIS tumor specimens, now widely used in clinical practice.23 Using the CAP guidelines, a second central pathology review was performed concurrently by two expert breast pathologists (S.S.B. and F.L.B.) to characterize the DCIS tumors of 500 patients (75% of the overall group) for whom pathology specimens were available (Appendix, online only).24
Statistical Methods
The primary end point of the study was an ipsilateral breast event (IBE). An IBE was defined as the first local recurrence of DCIS or invasive carcinoma in the treated (ipsilateral) breast. In addition to the primary end point of any IBE, separate analyses were also performed for the subsets of invasive IBE (with or without associated DCIS) and DCIS-only IBE. The time to an IBE was defined as the time from the last (definitive) surgery until the first evidence of an IBE.
The Kaplan-Meier method was used to estimate time to event distributions. SEs were estimated using the Greenwood formula. CIs were constructed using the normal approximation on the probability scale, and are given at the 95% level. Cox proportional hazard methods were used to estimate hazard ratios (HRs) and tests for significance for event times. All P values are two-sided. Tamoxifen use was considered as a time-dependent covariate. Follow-up cutoff was at the time of last reported disease evaluation, ipsilateral mastectomy, initiation of chemotherapy, or death. The median follow-up time was 12.3 years, with 346 patients and 217 patients observed for at least 10 and 12 years, respectively.
RESULTS
Patient, Tumor, and Treatment Characteristics
Table 1 details patient, tumor, and treatment characteristics. The patients enrolled onto study showed generally more favorable clinical and pathologic characteristics than required by the study protocol. The median tumor size was 6 mm for cohort 1 and 7 mm for cohort 2. The minimum negative margin width was 5 mm or greater for 64% and 69% of the patients in each cohort, respectively, and 10 mm or greater for 21% and 24% of the patients, respectively. The median patient age was 60 years and 58 years, respectively. Tamoxifen was given in a nonrandomized fashion to 30% of the patients (31% for cohort 1% and 24% for cohort 2).
Table 1.
Patient, Tumor, and Treatment Characteristics
| Characteristic | Cohort 1,a No. (%) (n = 561) | Cohort 2,a No. (%) (n = 104) |
|---|---|---|
| Age, years | ||
| ≤ 39 | 13 (2) | 4 (4) |
| 40-49 | 93 (17) | 21 (20) |
| 50-59 | 166 (30) | 30 (29) |
| 60-69 | 146 (26) | 28 (27) |
| ≥ 70 | 143 (25) | 21 (20) |
| Race/ethnicityb | ||
| White | 519 (93) | 95 (95) |
| Hispanic | 8 (1) | 1 (1) |
| Black | 16 (3) | 4 (4) |
| Other | 14 (3) | 0 (0) |
| Menopausal statusc | ||
| Premenopausal | 134 (24) | 29 (28) |
| Postmenopausal | 427 (76) | 75 (72) |
| Tumor size, mmd | ||
| ≤ 5 | 226 (40) | 28 (27) |
| 6-10 | 231 (41) | 61 (59) |
| > 10 | 104 (19) | 15 (14) |
| Minimum negative margin width, mm | ||
| < 1e | 9 (2) | 2 (2) |
| 1-2.9e | 10 (2) | 2 (2) |
| 3-4.9 | 184 (33) | 28 (27) |
| 5-9.9 | 239 (43) | 47 (45) |
| ≥ 10f | 119 (21) | 25 (24) |
| Method of detectionb | ||
| Microcalcifications | 399 (71) | 88 (85) |
| Density or mass | 93 (17) | 4 (4) |
| Both | 40 (7) | 7 (7) |
| Incidental finding | 19 (3) | 5 (5) |
| Other | 8 (1) | 0 (0) |
| Bloody nipple dischargeb | ||
| Yes | 12 (2) | 1 (1) |
| No | 541 (98) | 102 (99) |
| Tamoxifen use | ||
| Yes | 174 (31) | 25 (24) |
| No | 387 (69) | 79 (76) |
| Hormone replacement therapy before study entryb | ||
| Yes | 239 (43) | 47 (45) |
| No | 315 (57) | 57 (55) |
| Treating institution gradeg | ||
| Low | 281 (50) | 0 (0) |
| Intermediate | 280 (50) | 0 (0) |
| High | 0 (0) | 104 (100) |
| CAP gradeh | ||
| Low | 61 (15) | 2 (2) |
| Intermediate | 249 (59) | 19 (24) |
| High | 110 (26) | 59 (74) |
Abbreviation: CAP, College of American Pathologists.
Cohort 1 defined as low- or intermediate-grade ductal carcinoma in situ (DCIS), tumor size 2.5 cm or smaller. Cohort 2 defined as high-grade DCIS, tumor size 1.0 cm or smaller. Cohort assignment was based on clinical evaluation and pathology assessment from the treating institution at the time of enrollment onto the study.
Unknown cases and cases that were not evaluated were excluded.
Patients younger than age 50 years were assumed to be premenopausal when menopausal status was not recorded.
Tumor size was based on central pathology review, when this information was available.
Cases with negative margin width less than 3 mm were determined on central pathology review.
Cases with no tumor on re-excision are included.
Grade as determined on pathology assessment from the treating institution at the time of enrollment onto study.
Grade as determined on central pathology review using current CAP guidelines. Cases that were not evaluated were excluded.
IBEs and Other Outcomes
Overall, there were 99 IBEs (n = 74 in cohort 1; n = 25 in cohort 2), of which 51 (52%) were an invasive IBE (n = 39 in cohort 1; n = 12 in cohort 2). The 12-year rates of developing an IBE were 14.4% for cohort 1 and 24.6% for cohort 2 (P = .003; Fig 1 and Table 2). The 12-year rates for developing an invasive IBE were 7.5% and 13.4%, respectively (P = .08; Fig 1 and Table 3). The risks of developing an IBE and an invasive IBE increased over time through 12 years of follow-up, without plateau. For cohort 1, the rate of developing an IBE was approximately 1.2% per year through year 12, and for an invasive IBE, approximately 0.6% per year through year 12. No differences were seen between the two cohorts for the 12-year rates of OS (84.0% v 82.8%; P = .96) or contralateral breast cancer events (6.7% v 12.0%; P = .16; Appendix Table A1, online only).
Fig 1.

Ipsilateral breast events (IBEs) for cohort 1 and cohort 2. Cohort 1 was defined as low- or intermediate-grade ductal carcinoma in situ (DCIS), tumor size 2.5 cm or smaller. Cohort 2 was defined as high-grade DCIS, tumor size 1.0 cm or smaller. Cohort assignment was based on clinical evaluation and pathology assessment from the treating institution at the time of enrollment. The numbers at risk are given beneath the x-axis. (A) Any IBE. (B) Subset of invasive IBE. (C) Subset of DCIS-only IBE.
Table 2.
IBE Rates for Clinically Relevant Subsets of Patients
| Variable | No. of Patients | 5 Years |
10 Years |
12 Years |
P | |||
|---|---|---|---|---|---|---|---|---|
| % | 95% CI | % | 95% CI | % | 95% CI | |||
| Cohort 1 | 561 | 6.0 | 4.0 to 8.1 | 12.5 | 9.5 to 15.4 | 14.4 | 11.2 to 17.6 | .003 |
| Cohort 2 | 104 | 15.0 | 8.0 to 22.0 | 24.6 | 15.7 to 33.4 | 24.6 | 15.7 to 33.4 | |
| Age, years | ||||||||
| ≤ 49 | 131 | 11.7 | 6.2 to 17.3 | 16.8 | 10.3 to 23.4 | 18.9 | 11.9 to 26.0 | .51 |
| 50-69 | 370 | 6.0 | 3.5 to 8.5 | 13.5 | 9.8 to 17.2 | 15.1 | 11.2 to 19.0 | |
| ≥ 70 | 164 | 7.1 | 3.1 to 11.2 | 14.1 | 8.1 to 20.1 | 15.7 | 9.0 to 22.3 | |
| Minimum negative margin width, mm | ||||||||
| < 5 | 235 | 7.6 | 4.1 to 11.1 | 14.9 | 10.0 to 19.7 | 17.0 | 11.7 to 22.2 | .85 |
| 5-9 | 286 | 7.1 | 4.0 to 10.1 | 13.5 | 9.2 to 17.8 | 15.2 | 10.6 to 19.8 | |
| ≥ 10 | 144 | 8.0 | 3.5 to 12.6 | 15.3 | 9.1 to 21.5 | 16.3 | 9.9 to 22.8 | |
| Tumor size, mm* | ||||||||
| ≤ 5 | 254 | 6.1 | 3.1 to 9.2 | 10.7 | 6.7 to 14.7 | 11.3 | 7.2 to 15.4 | .01 |
| 6-10 | 292 | 6.6 | 3.7 to 9.6 | 15.5 | 11.0 to 20.0 | 17.8 | 12.9 to 22.7 | |
| > 10 | 119 | 12.5 | 6.4 to 18.6 | 20.0 | 12.3 to 27.8 | 22.9 | 14.5 to 31.3 | |
| CAP grade† | ||||||||
| Low | 63 | 5.1 | 0 to 10.8 | 12.5 | 3.8 to 21.3 | 12.5 | 3.8 to 21.3 | .16 |
| Intermediate | 268 | 6.3 | 3.3 to 9.4 | 13.2 | 8.9 to 17.6 | 15.1 | 10.4 to 19.9 | |
| High | 169 | 11.1 | 6.2 to 15.9 | 20.6 | 14.2 to 27.0 | 20.6 | 14.2 to 27.0 | |
Abbreviations: CAP, College of American Pathologists; IBE, ipsilateral breast event.
Tumor size was based on central pathology review, when this information was available. Results are similar for tumor size on the basis of institutional pathology assessment at enrollment onto the study (P = .03; data not shown).
Restricted to the subset of 500 cases for which a central pathology review was performed using current CAP guidelines.
Table 3.
Invasive IBE Rates for Clinically Relevant Subsets of Patients
| Variable | No. of Patients | 5 Years |
10 Years |
12 Years |
P | |||
|---|---|---|---|---|---|---|---|---|
| % | 95% CI | % | 95% CI | % | 95% CI | |||
| Cohort 1 | 561 | 2.7 | 1.3 to 4.1 | 6.4 | 4.2 to 8.6 | 7.5 | 5.1 to 10.0 | .08 |
| Cohort 2 | 104 | 5.3 | 0.8 to 9.7 | 13.4 | 5.9 to 20.9 | 13.4 | 5.9 to 20.9 | |
| Age, years | ||||||||
| ≤ 49 | 131 | 4.8 | 1.1 to 8.6 | 8.5 | 3.4 to 13.5 | 9.6 | 4.1 to 15.0 | .43 |
| 50-69 | 370 | 3.4 | 1.5 to 5.4 | 7.6 | 4.7 to 10.5 | 8.8 | 5.7 to 12.0 | |
| ≥ 70 | 164 | 0.7 | 0 to 2.1 | 6.2 | 1.7 to 10.8 | 6.2 | 1.7 to 10.8 | |
| Minimum negative margin width, mm | ||||||||
| < 5 | 235 | 2.8 | 0.6 to 4.9 | 8.1 | 4.3 to 11.9 | 8.1 | 4.3 to 11.9 | .64 |
| 5-9 | 286 | 3.4 | 1.2 to 5.5 | 7.8 | 4.4 to 11.3 | 9.6 | 5.7 to 13.4 | |
| ≥ 10 | 144 | 3.1 | 0.1 to 6.0 | 5.6 | 1.6 to 9.6 | 6.7 | 2.2 to 11.2 | |
| Tumor size, mm* | ||||||||
| ≤ 5 | 254 | 2.5 | 0.5 to 4.5 | 6.3 | 3.1 to 9.5 | 6.3 | 3.1 to 9.5 | .40 |
| 6-10 | 292 | 2.9 | 0.9 to 4.9 | 8.5 | 5.0 to 12.0 | 10.3 | 6.3 to 14.3 | |
| > 10 | 119 | 4.8 | 0.7 to 8.8 | 7.2 | 2.0 to 12.4 | 8.7 | 2.8 to 14.6 | |
| CAP grade† | ||||||||
| Low | 63 | 1.8 | 0 to 5.3 | 5.5 | 0 to 11.6 | 5.5 | 0 to 11.6 | .19 |
| Intermediate | 268 | 2.0 | 0.3 to 3.8 | 5.4 | 2.4 to 8.4 | 6.7 | 3.2 to 10.1 | |
| High | 169 | 4.5 | 1.2 to 7.7 | 11.7 | 6.4 to 17.0 | 11.7 | 6.4 to 17.0 | |
Abbreviations: CAP, College of American Pathologists; IBE, ipsilateral breast event.
Tumor size was based on central pathology review, when this information was available. Results are similar for tumor size on the basis of institutional pathology assessment at enrollment onto the study (P = .40; data not shown).
Restricted to the subset of 500 cases for which a central pathology review was performed using current CAP guidelines.
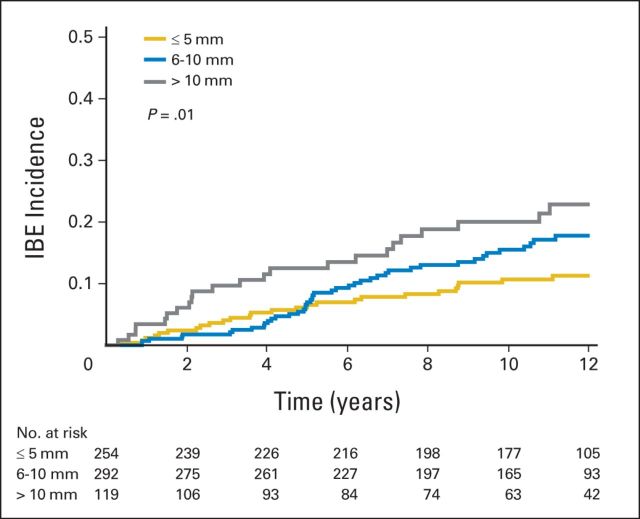
The rates of developing an IBE and an invasive IBE as a function of clinically relevant subsets of patients are shown in Tables 2 and 3, respectively. Tumor size was statistically significantly associated with developing an IBE (P = .01; Fig 2 and Table 2). The results of similar analyses restricted to the patients enrolled in cohort 1 are given in Appendix Tables A2 and A3 (online only).
Fig 2.
Ipsilateral breast events (IBEs) according to tumor size. The numbers at risk are given beneath the x-axis.
We found that study cohort and tumor size were both statistically significantly associated with developing an IBE using multivariable Cox proportional hazards model. The hazard ratio (HR) for cohort 2 was 1.84 compared with cohort 1 (P = .009). Using a tumor size of 5 mm or less as the reference group, the HRs were 1.42 (95% CI, 0.88 to 2.29) for tumor size 6 to 10 mm and 2.11 (95% CI, 1.23 to 3.62) for tumor size greater than 10 mm (P = .03). Variables not statistically significant were age, menopausal status, minimum negative margin width, method of detection, bloody nipple discharge, tamoxifen use, and prior hormone therapy (all P > .25). The HR for tamoxifen use (compared with no tamoxifen use) was 0.66 (95% CI, 0.40 to 1.06; P = .09) with tamoxifen evaluated as a single variable in a proportional hazards model.
By using a multivariable Cox proportional hazards model for developing an invasive IBE, we found that only the study cohort was borderline significant (P = .08). No other factor was statistically significant (all P > .26).
Central Pathology Evaluation
For the subset of 500 patients for which central pathology review had been performed using current CAP guidelines for DCIS, the 12-year rates of developing an IBE were 12.5% for low-grade DCIS, 15.1% for intermediate-grade DCIS, and 20.6% for high-grade DCIS (P = .16; Table 2 and Appendix Fig A1, online only). The 12-year rates of developing an invasive IBE were 5.5%, 6.7%, and 11.7%, respectively (P = .19; Table 3 and Appendix Fig A1). Comedo necrosis and the University of Southern California/Van Nuys Prognostic Index (USC/VNPI) were not significantly associated with developing an IBE or invasive IBE (all P > .13).25 Appendix Tables A4 and A5 (online only) show analyses of IBE rates that compare current CAP grading with older grading classifications. Fewer DCIS tumors were classified as low grade using CAP guidelines compared with older grading classifications.
DISCUSSION
This study has demonstrated that for patients with favorable DCIS who were selected on the basis of clinical and pathologic characteristics and treated with surgical excision without radiation, the risks of developing an IBE and an invasive IBE increased over time through 12 years of follow-up (Fig 1 and Tables 2 and 3). The 12-year rates of developing an IBE were 14.4% for cohort 1 and 24.6% for cohort 2 (P = .003; Fig 1 and Table 2), and the 12-year rates of developing an invasive IBE were 7.5% and 13.4%, respectively (P = .08; Fig 1 and Table 3). No clearly defined plateau was observed for either cohort of patients. Individual patients and their physicians will need to decide if these 12-year risks are acceptable, and whether or not to forego adding adjuvant treatment after surgical excision.
The ECOG-ACRIN E5194 study was a nonrandomized, prospective clinical trial for women with DCIS who were selected for low-risk clinical and pathologic features as identified at the time of enrollment. The majority of enrolled patients were postmenopausal and had small (tumor size ≤ 1 cm), mammographically detected DCIS excised with negative margins of resection (Table 1). These characteristics are similar to those of patients with DCIS in population-based studies.4,5 Therefore, the findings in this study are relevant to contemporary practice.
The optimal treatment for women with newly diagnosed DCIS continues to be debated. National guidelines in the United States allow for a wide range of local treatment options, including surgical excision (lumpectomy) with or without radiation, unilateral mastectomy, and even bilateral mastectomies,26,27 but no consensus approach has developed. For patients considered low risk, national guidelines include the option for surgical excision alone (without radiation) as acceptable local treatment, although what constitutes low risk is not well defined.
Five randomized clinical trials have consistently demonstrated that adding radiation treatment after surgical excision for patients with DCIS reduces the risk of local recurrence in the ipsilateral breast by approximately half.8–16 The Early Breast Cancer Trialists' Collaborative Trial Group (EBCTCG) meta-analysis combined data from four of the randomized trials of radiation treatment after surgical excision.17 Two randomized clinical trials have demonstrated that adding tamoxifen reduces the risk of all breast cancer events (ipsilateral plus contralateral) for hormone receptor–positive DCIS tumors.9,10,14,18,19 Although the risk of recurrence is decreased by adding radiation and tamoxifen after surgical excision, no improvement in the rates of distant metastases and OS have been shown in any randomized clinical trial.
The risk of local recurrence after surgical excision without radiation has been reported in a number of randomized and nonrandomized, prospective clinical trials.8–16,28 The increasing risk of local recurrence over time as seen in the current analysis of ECOG-ACRIN E5194 is consistent with other prospective studies, both randomized and nonrandomized. For patients treated with surgical excision without radiation in randomized clinical trials, the 10-year rate of local recurrence has been reported as approximately 24% to 30%.9,11,14,15 In the randomized RTOG 9804 study, adding radiation treatment after lumpectomy reduced the 7-year rate of local recurrence from 6.7% to 0.9% (P < .001).16 In a nonrandomized, prospective study, Wong et al28 reported that the 10-year rate of local recurrence was 15.6% after surgical excision without radiation. The various prospective clinical trials differ with respect to a number of protocol-defined parameters (eg, tumor grade, definition of negative surgical margins, use of tamoxifen), which may, at least in part, account for the reported differences seen in local recurrence rates between studies.
Even though the risk of recurrence is reduced by adding radiation and tamoxifen after surgical excision, ongoing efforts continue in an attempt to identify patients with favorable disease at presentation who have a sufficiently low risk of local recurrence after surgical excision that omitting adjuvant therapy is reasonable. The majority of women with DCIS present with an asymptomatic finding of microcalcifications on routine screening mammography. As most women with newly diagnosed DCIS are eligible for breast conservation surgery, an important aspect of clinical decision making is often whether or not to add radiation treatment, which includes an assessment of the risks of developing a local recurrence and an invasive local recurrence after surgical excision alone without radiation. The lack of survival benefit from adding adjuvant treatments and the small, but real, potential risk of adverse effects are reasons commonly given to omit adjuvant treatments (radiation and tamoxifen) after surgical excision. Therefore, the risk-benefit ratio may favor omitting adjuvant treatment if a cohort of patients could be prospectively identified at presentation with a sufficiently low risk of recurrence after surgical excision alone.
Pathologic grade commonly influences clinical management decisions for the treatment of DCIS. Since the design of this study, guidelines for determining the grade of DCIS have changed, resulting in fewer DCIS tumors classified as low grade (Table 1). In this study, CAP grading was not associated with the risks of developing a local recurrence and an invasive local recurrence, and thus, is not clinically useful in identifying a low-risk cohort of patients (Tables 2 and 3; Appendix Fig A1).
Early efforts to identify a cohort of patients with low-risk disease focused on using clinical and pathologic characteristics. However, prospective clinical trials, randomized and nonrandomized, have not reproducibly and reliably identified patients with low-risk DCIS, especially with long-term follow-up of at least 10 years. More recent efforts have focused on using molecular markers to determine the risk of recurrence after surgical excision.21,29–33 The value of molecular markers compared with traditional clinical and pathologic features of DCIS remains an area of great interest.
This study has several notable strengths. First, the present clinical trial prospectively identified and enrolled patients with DCIS who were selected for treatment using surgical excision without radiation on the basis of low-risk clinical and pathologic features at presentation. Second, patients enrolled onto this study have characteristics similar to those of patients in population-based studies, and therefore, this study is relevant to contemporary practice. Third, this study reports long-term follow-up of greater than 10 years, which is especially important after treatment for potentially lower-risk DCIS because of the known risk of late and ongoing local recurrence (Fig 1; Tables 2 and 3). Finally, there was a sufficient number of patients to perform analyses for some clinically meaningful patient subgroups (Tables 2 and 3).
This study has a number of potential limitations. First, the trial design was a nonrandomized cohort study. Thus, the impact of adding radiation or tamoxifen after surgical excision could not be assessed. Second, tamoxifen was administered to approximately 30% of patients in a nonrandomized fashion. Despite this limitation, tamoxifen use was associated with a decreased risk of local recurrence (HR, 0.66; P = .09), which is consistent with results from randomized clinical trials. Third, the number of patients in cohort 2 was relatively small (n = 104), limiting the statistical power in this group of patients. Finally, the numbers of patients in some clinically relevant subsets were small (Tables 2 and 3).
In summary, this study has demonstrated that the risks of developing an IBE and an invasive IBE increased over time through 12 years of follow-up, without plateau, for patients with DCIS of the breast who were selected for favorable clinical and pathologic characteristics and treated with surgical excision without radiation. Individual patients and their physicians will need to decide if these 12-year risks are acceptable, and to judge whether or not to add adjuvant treatment after surgical excision. Not all patients and their physicians will agree on what is considered too high a risk of developing an IBE or an invasive IBE to recommend observation after surgical excision, or what risk is considered too low to justify adding radiation treatment. However, this study provides 12-year data to begin those discussions, and to help inform the treatment decision-making process.
Appendix
Pathology Evaluation
For the present analysis, there were data available from the central pathology review using the current College of American Pathologists ductal carcinoma in situ guidelines.23,24 The central pathology review was performed concurrently by two expert breast pathologists (S.S.B. and F.L.B.) for 500 patients (75% of the overall group) for whom pathology specimens were available. Ductal carcinoma in situ pattern, grade, and comedo necrosis were scored. Of the 500 evaluated patients, 343 had undergone central pathology review as part of a prior study.21,24 Subsequently, central pathology review was performed for 157 new patients, as well as blinded re-evaluation of 145 previously evaluated patients. For the cases evaluated twice, grade for this study was scored as the highest grade on either of the evaluations, and comedo necrosis was scored as present if present on either evaluation.
Patients with (n = 500) and without (n = 165) central pathology review were similar for distribution of cohort, age, menopausal status, minimum negative margin width, and method of detection. Tumor size in patients without central pathology review was slightly smaller than that in patients with central pathology review (tumor size ≤ 5 mm for 46% v 36%, respectively).
Table A1.
Contralateral Breast Cancer Event Rates
| Variable | No. | 5 Years |
10 Years |
12 Years |
P | |||
|---|---|---|---|---|---|---|---|---|
| % | 95% CI | % | 95% CI | % | 95% CI | |||
| Contralateral breast cancer | ||||||||
| Overall group | 665 | 3.7 | 2.2 to 5.1 | 6.1 | 4.1 to 8.0 | 7.5 | 5.3 to 9.7 | |
| Cohort 1 | 561 | 3.6 | 2.0 to 5.2 | 5.3 | 3.4 to 7.3 | 6.7 | 4.4 to 9.0 | .16 |
| Cohort 2 | 104 | 4.0 | 0.2 to 7.8 | 10.4 | 3.9 to 16.9 | 12.0 | 4.9 to 19.1 | |
| Invasive contralateral breast cancer | ||||||||
| Overall group | 665 | 2.6 | 1.3 to 3.8 | 4.6 | 2.9 to 6.3 | 5.8 | 3.8 to 7.7 | |
| Cohort 1 | 561 | 2.3 | 1.0 to 3.6 | 3.8 | 2.1 to 5.5 | 4.9 | 2.9 to 6.9 | .06 |
| Cohort 2 | 104 | 4.0 | 0.2 to 7.8 | 9.0 | 3.0 to 15.0 | 10.6 | 3.9 to 17.3 | |
| DCIS-only contralateral breast cancer | ||||||||
| Overall group | 665 | 1.1 | 0.3 to 2.0 | 1.5 | 0.5 to 2.5 | 1.8 | 0.7 to 2.9 | |
| Cohort 1 | 561 | 1.3 | 0.4 to 2.3 | 1.6 | 0.5 to 2.6 | 1.8 | 0.6 to 3.0 | .66 |
| Cohort 2 | 104 | 0 | 0 to 0 | 1.5 | 0 to 4.5 | 1.5 | 0.0 to 4.5 | |
Abbreviation: DCIS, ductal carcinoma in situ.
Table A2.
IBE Rates for Clinically Relevant Subsets of Patients
| Variable | No. | 5 Years |
10 Years |
12 Years |
P | |||
|---|---|---|---|---|---|---|---|---|
| % | 95% CI | % | 95% CI | % | 95% CI | |||
| Age, years | ||||||||
| ≤ 49 | 106 | 6.8 | 1.9 to 11.7 | 9.9 | 4.1 to 15.7 | 12.5 | 5.8 to 19.2 | .66 |
| 50-69 | 312 | 5.1 | 2.6 to 7.6 | 12.1 | 8.3 to 16.0 | 13.9 | 9.8 to 18.1 | |
| ≥ 70 | 143 | 7.5 | 3.0 to 12.0 | 15.4 | 8.7 to 22.1 | 17.2 | 9.8 to 24.5 | |
| Minimum negative margin width, mm | ||||||||
| < 5 | 203 | 7.3 | 3.6 to 11.0 | 13.2 | 8.2 to 18.1 | 15.5 | 10.1 to 21.0 | .87 |
| 5-9 | 239 | 5.4 | 2.4 to 8.3 | 12.1 | 7.6 to 16.5 | 14.0 | 9.1 to 18.9 | |
| ≥ 10 | 119 | 5.3 | 1.2 to 9.4 | 12.1 | 5.9 to 18.3 | 13.4 | 6.8 to 19.9 | |
| Tumor size, mm* | ||||||||
| ≤ 5 | 226 | 5.0 | 2.1 to 7.9 | 8.0 | 4.3 to 11.6 | 8.6 | 4.8 to 12.5 | < .001 |
| 6-10 | 231 | 4.3 | 1.5 to 7.0 | 14.1 | 9.1 to 19.1 | 16.9 | 11.4 to 22.4 | |
| > 10 | 104 | 12.3 | 5.8 to 18.8 | 19.7 | 11.5 to 28.0 | 23.3 | 14.0 to 32.5 | |
| CAP grade† | ||||||||
| Low | 61 | 3.5 | 0 to 8.2 | 11.0 | 2.7 to 19.3 | 11.0 | 2.7 to 19.3 | .61 |
| Intermediate | 249 | 6.4 | 3.3 to 9.6 | 12.9 | 8.4 to 17.4 | 14.9 | 9.9 to 19.8 | |
| High | 110 | 6.6 | 1.9 to 11.4 | 15.9 | 8.7 to 23.1 | 15.9 | 8.7 to 23.1 | |
NOTE. Analysis restricted to patients enrolled in cohort 1 (n = 561).
Abbreviations: CAP, College of American Pathologists; IBE, ipsilateral breast event.
Tumor size was based on central pathology review, when this information was available.
Restricted to the subset of cases for which a central pathology review was performed using current CAP guidelines.
Table A3.
Invasive IBE Rates for Clinically Relevant Subsets of Patients
| Variable | No. | 5 Years |
10 Years |
12 Years |
P | |||
|---|---|---|---|---|---|---|---|---|
| % | 95% CI | % | 95% CI | % | 95% CI | |||
| Age, years | ||||||||
| ≤ 49 | 106 | 2.9 | 0 to 6.2 | 5.1 | 0.7 to 9.5 | 6.4 | 1.4 to 11.4 | .57 |
| 50-69 | 312 | 3.4 | 1.3 to 5.4 | 6.8 | 3.8 to 9.7 | 8.1 | 4.8 to 11.4 | |
| ≥ 70 | 143 | 0.8 | 0 to 2.4 | 7.1 | 2.0 to 12.3 | 7.1 | 2.0 to 12.3 | |
| Minimum negative margin width, mm | ||||||||
| < 5 | 203 | 2.2 | 0.1 to 4.3 | 6.3 | 2.7 to 10.0 | 6.3 | 2.7 to 10.0 | .46 |
| 5-9 | 239 | 3.1 | 0.8 to 5.4 | 7.4 | 3.7 to 11.0 | 9.4 | 5.2 to 13.6 | |
| ≥ 10 | 119 | 2.7 | 0 to 5.8 | 4.7 | 0.7 to 8.8 | 6.0 | 1.3 to 10.7 | |
| Tumor size, mm* | ||||||||
| ≤ 5 | 226 | 2.3 | 0.3 to 4.3 | 4.8 | 1.9 to 7.8 | 4.8 | 1.9 to 7.8 | .17 |
| 6-10 | 231 | 1.9 | 0.1 to 3.7 | 7.9 | 4.0 to 11.7 | 10.0 | 5.5 to 14.5 | |
| > 10 | 104 | 5.4 | 0.8 to 10.0 | 6.8 | 1.5 to 12.0 | 8.5 | 2.3 to 14.8 | |
| CAP grade† | ||||||||
| Low | 61 | 1.8 | 0 to 5.3 | 5.5 | 0 to 11.6 | 5.5 | 0 to 11.6 | .69 |
| Intermediate | 249 | 2.2 | 0.3 to 4.1 | 5.3 | 2.2 to 8.3 | 6.6 | 3.1 to 10.2 | |
| High | 110 | 2.9 | 0 to 6.2 | 9.3 | 3.5 to 15.2 | 9.3 | 3.5 to 15.2 | |
NOTE. Analysis restricted to patients enrolled in cohort 1 (n = 561).
Abbreviations: CAP, College of American Pathologists; IBE, ipsilateral breast event.
Tumor size was based on central pathology review, when this information was available.
Restricted to the subset of cases for which a central pathology review was performed using current CAP guidelines.
Table A4.
IBE Rates and Subset of Invasive IBE Rates Comparing Current CAP Grading to Older Grading Classifications
| Variable | No. | 5 Years |
10 Years |
12 Years |
P | |||
|---|---|---|---|---|---|---|---|---|
| % | 95% CI | % | 95% CI | % | 95% CI | |||
| IBE rates | ||||||||
| Grade* | ||||||||
| Low | 63 | 5.1 | 0 to 10.8 | 12.5 | 3.8 to 21.3 | 12.5 | 3.8 to 21.3 | .16 |
| Intermediate | 268 | 6.3 | 3.3 to 9.4 | 13.2 | 8.9 to 17.6 | 15.1 | 10.4 to 19.9 | |
| High | 169 | 11.1 | 6.2 to 15.9 | 20.6 | 14.2 to 27.0 | 20.6 | 14.2 to 27.0 | |
| Grade† | ||||||||
| Low | 287 | 5.1 | 2.5 to 7.7 | 10.5 | 6.7 to 14.2 | 12.1 | 8.0 to 16.2 | .02 |
| Intermediate | 239 | 7.6 | 4.1 to 11.1 | 17.7 | 12.5 to 22.9 | 19.1 | 13.6 to 24.5 | |
| High | 64 | 17.9 | 8.3 to 27.5 | 23.9 | 12.9 to 35.0 | 23.9 | 12.9 to 35.0 | |
| Grade‡ | ||||||||
| Low | 281 | 5.6 | 2.8 to 8.3 | 12.3 | 8.2 to 16.3 | 14.0 | 9.5 to 18.4 | .01 |
| Intermediate | 280 | 6.5 | 3.5 to 9.5 | 12.7 | 8.5 to 16.9 | 14.9 | 10.2 to 19.5 | |
| High | 104 | 15.0 | 8.0 to 22.0 | 24.6 | 15.7 to 33.4 | 24.6 | 15.7 to 33.4 | |
| Subset of invasive IBE rates | ||||||||
| Grade* | ||||||||
| Low | 63 | 1.8 | 0 to 5.3 | 5.5 | 0 to 11.6 | 5.5 | 0 to 11.6 | .19 |
| Intermediate | 268 | 2.0 | 0.3 to 3.8 | 5.4 | 2.4 to 8.4 | 6.7 | 3.2 to 10.1 | |
| High | 169 | 4.5 | 1.2 to 7.7 | 11.7 | 6.4 to 17.0 | 11.7 | 6.4 to 17.0 | |
| Grade† | ||||||||
| Low | 287 | 1.8 | 0.2 to 3.4 | 5.3 | 2.5 to 8.1 | 5.8 | 2.8 to 8.8 | .02 |
| Intermediate | 239 | 3.2 | 0.9 to 5.6 | 8.9 | 5.0 to 12.9 | 9.7 | 5.5 to 13.9 | |
| High | 64 | 10.2 | 2.5 to 18.0 | 16.7 | 6.6 to 26.8 | 16.7 | 6.6 to 26.8 | |
| Grade‡ | ||||||||
| Low | 281 | 1.5 | 0 to 3.0 | 5.4 | 2.5 to 8.3 | 5.9 | 2.9 to 9.0 | .11 |
| Intermediate | 280 | 3.9 | 1.5 to 6.2 | 7.4 | 4.1 to 10.8 | 9.1 | 5.4 to 12.9 | |
| High | 104 | 5.3 | 0.8 to 9.7 | 13.4 | 5.9 to 20.9 | 13.4 | 5.9 to 20.9 | |
NOTE. Cases with unknown information were excluded from analysis.
Abbreviations: CAP, College of American Pathologists; IBE, ipsilateral breast event.
Second central pathology review using current CAP guidelines.
First central pathology review.22
Institutional pathology assessment at the time of study entry.
Table A5.
IBE Rates and Subset of Invasive IBE Rates Comparing Current CAP Grading to Older Grading Classifications
| Variable | No. | 5 Years |
10 Years |
12 Years |
P | |||
|---|---|---|---|---|---|---|---|---|
| % | 95% CI | % | 95% CI | % | 95% CI | |||
| IBE rates | ||||||||
| Grade* | ||||||||
| Low | 61 | 3.5 | 0 to 8.2 | 11.0 | 2.7 to 19.3 | 11.0 | 2.7 to 19.3 | .61 |
| Intermediate | 249 | 6.4 | 3.3 to 9.6 | 12.9 | 8.4 to 17.4 | 14.9 | 9.9 to 19.8 | |
| High | 110 | 6.6 | 1.9 to 11.4 | 15.9 | 8.7 to 23.1 | 15.9 | 8.7 to 23.1 | |
| Grade† | ||||||||
| Low | 286 | 5.1 | 2.5 to 7.7 | 10.5 | 6.7 to 14.3 | 12.2 | 8.0 to 16.3 | .25 |
| Intermediate | 191 | 7.9 | 3.9 to 11.9 | 17.3 | 11.5 to 23.1 | 19.0 | 12.9 to 25.0 | |
| High | 14 | 7.1 | 0 to 20.6 | 15.6 | 0 to 35.6 | 15.6 | 0 to 35.6 | |
| Grade‡ | ||||||||
| Low | 281 | 5.6 | 2.8 to 8.3 | 12.3 | 8.2 to 16.3 | 14.0 | 9.5 to 18.4 | .72 |
| Intermediate | 280 | 6.5 | 3.5 to 9.5 | 12.7 | 8.5 to 16.9 | 14.9 | 10.2 to 19.5 | |
| Subset of invasive IBE rates | ||||||||
| Grade* | ||||||||
| Low | 61 | 1.8 | 0 to 5.3 | 5.5 | 0 to 11.6 | 5.5 | 0 to 11.6 | .69 |
| Intermediate | 249 | 2.2 | 0.3 to 4.1 | 5.3 | 2.2 to 8.3 | 6.6 | 3.1 to 10.2 | |
| High | 110 | 2.9 | 0 to 6.2 | 9.3 | 3.5 to 15.2 | 9.3 | 3.5 to 15.2 | |
| Grade† | ||||||||
| Low | 286 | 1.8 | 0.2 to 3.4 | 5.3 | 2.5 to 8.1 | 5.8 | 2.8 to 8.8 | .21 |
| Intermediate | 191 | 4.0 | 1.1 to 7.0 | 9.1 | 4.7 to 13.5 | 10.0 | 5.3 to 14.7 | |
| High | 14 | 7.1 | 0 to 20.6 | 15.6 | 0 to 35.6 | 15.6 | 0 to 35.6 | |
| Grade‡ | ||||||||
| Low | 281 | 1.5 | 0 to 3.0 | 5.4 | 2.5 to 8.3 | 5.9 | 2.9 to 9.0 | .22 |
| Intermediate | 280 | 3.9 | 0.8 to 6.2 | 7.4 | 4.1 to 10.8 | 9.1 | 5.4 to 12.9 | |
NOTE. Analysis restricted to patients enrolled in cohort 1 (n = 561). Cases with unknown information were excluded from analysis.
Abbreviations: CAP, College of American Pathologists; IBE, ipsilateral breast event.
Second central pathology review using current CAP guidelines.
First central pathology review.22
Institutional pathology assessment at the time of study entry.
Fig A1.

Ipsilateral breast events (IBEs) according to grade as scored using current College of American Pathology guidelines. Analyses exclude cases not evaluated. The numbers at risk are given beneath the x-axis. (A) Any IBE. (B) Subset of invasive IBE.
Footnotes
Supported in part by Public Health Service Grants No. CA180820, CA180794, CA189859, CA180864, CA180795, CA180844, CA180802, CA25224 from the National Cancer Institute, National Institutes of Health, Department of Health and Human Services, and by the Breast Cancer Research Foundation.
Presented in part at the San Antonio Breast Cancer Symposium, San Antonio, TX, December 9-13, 2014.
The contents of this manuscript are solely the responsibility of the authors, and do not necessarily represent the official views of the National Cancer Institute.
Authors' disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
Clinical trial information: NCT00002934.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Disclosures provided by the authors are available with this article at www.jco.org.
AUTHOR CONTRIBUTIONS
Conception and design: Lawrence J. Solin, Robert Gray, Lorie L. Hughes, William C. Wood, James N. Ingle
Administrative support: Lawrence J. Solin, Joseph A. Sparano
Provision of study materials or patients: Lawrence J. Solin, Robert Gray, Lorie L. Hughes, William C. Wood, James N. Ingle, Edith A. Perez, Joseph A. Sparano
Collection and assembly of data: Lawrence J. Solin, Robert Gray, Lorie L. Hughes, Mary Ann Lowen, Sunil S. Badve, Frederick L. Baehner, James N. Ingle, Edith A. Perez
Data analysis and interpretation: Lawrence J. Solin, Robert Gray, Lorie L. Hughes, Frederick L. Baehner, James N. Ingle, Edith A. Perez, Abram Recht, Joseph A. Sparano, Nancy E. Davidson
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Surgical Excision Without Radiation for Ductal Carcinoma in Situ of the Breast: 12-Year Results From the ECOG-ACRIN E5194 Study
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Lawrence J. Solin
No relationship to disclose
Robert Gray
Research Funding: Abbott Molecular, Agios, Amgen, AstraZeneca, Bristol-Myers Squibb, Boehringer Ingelheim, Celgene, Genentech, Genomic Health, Genzyme, GlaxoSmithKline, ImClone Systems, Janssen Pharmaceuticals, Kanisa Pharmaceuticals, Millennium Pharmaceuticals, Nodality, Onyx Pharmaceuticals, OSI Pharmaceuticals, Pfizer, Sanofi, Sequent, Syndax Pharmaceuticals
Lorie L. Hughes
No relationship to disclose
William C. Wood
Consulting or Advisory Role: Genomic Health
Travel, Accommodations, Expenses: Teva Neuroscience
Mary Ann Lowen
No relationship to disclose
Sunil S. Badve
No relationship to disclose
Frederick L. Baehner
Employment: Genomic Health
Stock or Other Ownership: Genomic Health
James N. Ingle
No relationship to disclose
Edith A. Perez
No relationship to disclose
Abram Recht
Consulting or Advisory Role: CareCore National, US Oncology
Research Funding: Genomic Health (Inst)
Joseph A. Sparano
No relationship to disclose
Nancy E. Davidson
No relationship to disclose
REFERENCES
- 1.Allegra CJ, Aberle DR, Ganschow P, et al. National Institutes of Health State-of-the-Science Conference statement: Diagnosis and management of ductal carcinoma in situ September 22-24, 2009. J Natl Cancer Inst. 2010;102:161–169. doi: 10.1093/jnci/djp485. [DOI] [PubMed] [Google Scholar]
- 2.Burstein HJ, Polyak K, Wong JS, et al. Ductal carcinoma in situ of the breast. N Engl J Med. 2004;350:1430–1441. doi: 10.1056/NEJMra031301. [DOI] [PubMed] [Google Scholar]
- 3.Schwartz GF, Solin LJ, Olivotto IA, et al. Consensus conference on the treatment of in situ ductal carcinoma of the breast, April 22-25, 1999. Cancer. 2000;88:946–954. [PubMed] [Google Scholar]
- 4.Zujewski JA, Harlan LC, Morrell DM, et al. Ductal carcinoma in situ: Trends in treatment over time in the US. Breast Cancer Res Treat. 2011;127:251–257. doi: 10.1007/s10549-010-1198-z. [DOI] [PubMed] [Google Scholar]
- 5.Dodwell D, Clements K, Lawrence G, et al. Radiotherapy following breast-conserving surgery for screen-detected ductal carcinoma in situ: Indications and utilisation in the UK—Interim findings from the Sloane Project. Br J Cancer. 2007;97:725–729. doi: 10.1038/sj.bjc.6603945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baxter NN, Virnig BA, Durham SB, et al. Trends in the treatment of ductal carcinoma in situ of the breast. J Natl Cancer Inst. 2004;96:443–448. doi: 10.1093/jnci/djh069. [DOI] [PubMed] [Google Scholar]
- 7.Smith GL, Smith BD, Haffty BG. Rationalization and regionalization of treatment for ductal carcinoma in situ of the breast. Int J Radiat Oncol Biol Phys. 2006;65:1397–1403. doi: 10.1016/j.ijrobp.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 8.Fisher B, Dignam J, Wolmark N, et al. Lumpectomy and radiation therapy for the treatment of intraductal breast cancer: Findings from National Surgical Adjuvant Breast and Bowel Project B-17. J Clin Oncol. 1998;16:441–452. doi: 10.1200/JCO.1998.16.2.441. [DOI] [PubMed] [Google Scholar]
- 9.Fisher B, Land S, Mamounas E, et al. Prevention of invasive breast cancer in women with ductal carcinoma in situ: An update of the National Surgical Adjuvant Breast and Bowel Project experience. Semin Oncol. 2001;28:400–418. doi: 10.1016/s0093-7754(01)90133-2. [DOI] [PubMed] [Google Scholar]
- 10.Wapnir IL, Dignam JJ, Fisher B, et al. Long-term outcomes of invasive ipsilateral breast tumor recurrences after lumpectomy in NSABP B-17 and B-24 randomized clinical trials for DCIS. J Natl Cancer Inst. 2011;103:478–488. doi: 10.1093/jnci/djr027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Donker M, Litière S, Werutsky G, et al. Breast-conserving treatment with or without radiotherapy in ductal carcinoma in situ: 15-year recurrence rates and outcome after a recurrence, from the EORTC 10853 randomized phase III trial. J Clin Oncol. 2013;31:4054–4059. doi: 10.1200/JCO.2013.49.5077. [DOI] [PubMed] [Google Scholar]
- 12.Bijker N, Meijnen P, Peterse JL, et al. Breast-conserving treatment with or without radiotherapy in ductal carcinoma-in-situ: Ten-year results of European Organisation for Research and Treatment of Cancer randomized phase III trial 10853—A study by the EORTC Breast Cancer Cooperative Group and EORTC Radiotherapy Group. J Clin Oncol. 2006;24:3381–3387. doi: 10.1200/JCO.2006.06.1366. [DOI] [PubMed] [Google Scholar]
- 13.Bijker N, Peterse JL, Duchateau L, et al. Risk factors for recurrence and metastasis after breast-conserving therapy for ductal carcinoma-in-situ: Analysis of European Organization for Research and Treatment of Cancer Trial 10853. J Clin Oncol. 2001;19:2263–2271. doi: 10.1200/JCO.2001.19.8.2263. [DOI] [PubMed] [Google Scholar]
- 14.Cuzick J, Sestak I, Pinder SE, et al. Effect of tamoxifen and radiotherapy in women with locally excised ductal carcinoma in situ: Long-term results from the UK/ANZ DCIS trial. Lancet Oncol. 2011;12:21–29. doi: 10.1016/S1470-2045(10)70266-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Warnberg F, Garmo H, Emdin S, et al. Effect of radiotherapy after breast-conserving surgery for ductal carcinoma in situ: 20 years follow-up in the randomized SweDCIS trial. J Clin Oncol. 2014;32:3613–3618. doi: 10.1200/JCO.2014.56.2595. [DOI] [PubMed] [Google Scholar]
- 16.McCormick B, Winter K, Hudis C, et al. RTOG 9804: A prospective randomized trial for good-risk ductal carcinoma in situ comparing radiotherapy with observation. J Clin Oncol. 2015;33:709–715. doi: 10.1200/JCO.2014.57.9029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Correa C, McGale P, Taylor C, et al. Overview of the randomized trials of radiotherapy in ductal carcinoma in situ of the breast. J Natl Cancer Inst Monogr. 2010;41:162–177. doi: 10.1093/jncimonographs/lgq039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fisher B, Dignam J, Wolmark N, et al. Tamoxifen in treatment of intraductal breast cancer: National Surgical Adjuvant Breast and Bowel Project B-24 randomised controlled trial. Lancet. 1999;353:1993–2000. doi: 10.1016/S0140-6736(99)05036-9. [DOI] [PubMed] [Google Scholar]
- 19.Allred DC, Anderson SJ, Paik S, et al. Adjuvant tamoxifen reduces subsequent breast cancer in women with estrogen receptor-positive ductal carcinoma in situ: A study based on NSABP protocol B-24. J Clin Oncol. 2012;30:1268–1273. doi: 10.1200/JCO.2010.34.0141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hughes LL, Wang M, Page DL, et al. Local excision alone without irradiation for ductal carcinoma in situ of the breast: A trial of the Eastern Cooperative Oncology Group. J Clin Oncol. 2009;27:5319–5324. doi: 10.1200/JCO.2009.21.8560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Solin LJ, Gray R, Baehner FL, et al. A multigene expression assay to predict local recurrence risk for ductal carcinoma in situ of the breast. J Natl Cancer Inst. 2013;105:701–710. doi: 10.1093/jnci/djt067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Page DL, Anderson TJ, Rogers LW. Carcinoma in situ (CIS) In: Page DL, Anderson TJ, editors. Diagnostic Histopathology of the Breast (ed 1) Edinburgh, UK: Churchill Livingstone; 1987. pp. 157–192. [Google Scholar]
- 23.Lester SC, Bose S, Chen YY, et al. Protocol for the examination of specimens from patients with ductal carcinoma in situ of the breast. Arch Pathol Lab Med. 2009;133:15–25. doi: 10.5858/133.1.15. [DOI] [PubMed] [Google Scholar]
- 24.Badve SS, Gray RJ, Baehner FL, et al. Correlation between the DCIS score and traditional clinicopathologic features in the prospectively designed E5194 clinical validation study. J Clin Oncol. 2012;30:50s. (abstr 1005) [Google Scholar]
- 25.Silverstein MJ, Lagios MD. Choosing treatment for patients with ductal carcinoma in situ: Fine tuning the University of Southern California/Van Nuys Prognostic Index. J Natl Cancer Inst Monogr. 2010;41:193–196. doi: 10.1093/jncimonographs/lgq040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.NCCN Clinical Practice Guidelines in Oncology. Breast Cancer. http://www.nccn.org/professionals/physician_gls/pdf/breast.pdf.
- 27.Moran MS, Bai HX, Harris EE, et al. ACR appropriateness criteria ductal carcinoma in situ. Breast J. 2012;18:8–15. doi: 10.1111/j.1524-4741.2011.01197.x. [DOI] [PubMed] [Google Scholar]
- 28.Wong JS, Chen YH, Gadd MA, et al. Eight-year update of a prospective study of wide excision alone for small low- or intermediate-grade ductal carcinoma in situ (DCIS) Breast Cancer Res Treat. 2014;143:343–350. doi: 10.1007/s10549-013-2813-6. [DOI] [PubMed] [Google Scholar]
- 29.Rakovitch E, Nofech-Mozes S, Hanna W, et al. A population-based validation study of the DCIS Score predicting recurrence risk in individuals treated by breast-conserving surgery alone. Breast Cancer Res Treat. 2015;152:389–398. doi: 10.1007/s10549-015-3464-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kerlikowske K, Molinaro AM, Gauthier ML, et al. Biomarker expression and risk of subsequent tumors after initial ductal carcinoma in situ diagnosis. J Natl Cancer Inst. 2010;102:627–637. doi: 10.1093/jnci/djq101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rakovitch E, Nofech-Mozes S, Hanna W, et al. HER2/neu and Ki-67 expression predict non-invasive recurrence following breast-conserving therapy for ductal carcinoma in situ. Br J Cancer. 2012;106:1160–1165. doi: 10.1038/bjc.2012.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Williams KE, Barnes NL, Cramer A, et al. Molecular phenotypes of DCIS predict overall and invasive recurrence. Ann Oncol. 2015;26:1019–1025. doi: 10.1093/annonc/mdv062. [DOI] [PubMed] [Google Scholar]
- 33.Sakr RA, Andrade VP, Chandarlapaty S, et al. Molecular predictors for type of recurrence following conservative treatment for DCIS. Cancer Res. 2012:72. (abstr PD04-05) [Google Scholar]