Abstract
It has been recently reported that the centrosome of neurons does not have microtubule nucleating activity. Microtubule nucleation requires γ-tubulin as well as its recruiting proteins, GCP-WD/NEDD1 and CDK5RAP2 that anchor γ-tubulin to the centrosome. Change in the localization of these proteins during in vivo development of brain, however, has not been well examined. In this study we investigate the localization of γ-tubulin, GCP-WD and CDK5RAP2 in developing cerebral and cerebellar cortex with immunofluorescence. We found that γ-tubulin and its recruiting proteins were localized at centrosomes of immature neurons, while they were lost at centrosomes in mature neurons. This indicated that the loss of microtubule nucleating activity at the centrosome of neurons is due to the loss of γ-tubulin-recruiting proteins from the centrosome. RT-PCR analysis revealed that these proteins are still expressed after birth, suggesting that they have a role in microtubule generation in cell body and dendrites of mature neurons. Microtubule regrowth experiments on cultured mature neurons showed that microtubules are nucleated not at the centrosome but within dendrites. These data indicated the translocation of microtubule-organizing activity from the centrosome to dendrites during maturation of neurons, which would explain the mixed polarity of microtubules in dendrites.
Keywords: GCP-WD/NEDD1, CDK5RAP2, γ-tubulin, microtubule, centrosome
I. Introduction
Dendrites are subcellular compartments of neurons that receive information from other neurons. Growth of dendrites and maintenance of their morphology are critical for brain development and health, because dendritic arborization is directly linked to the neuronal function, and progressive regression of dendritic tree undergoes with increasing age [17]. To understand the mechanisms for dendritic growth and maintenance, it is important to know how microtubules within dendrites are generated and regulated.
Microtubules in dendrites have unique characteristics in several aspects. Firstly, they are not associated with centrosomes [24], whereas those of general cells are attached to the centrosome with their minus-ends. Second, microtubules within dendrites are oriented randomly [1, 19], whereas those in general cells are radially arranged so that the plus-ends are orienting periphery. It is not well known how such unique microtubule arrays are generated in dendrites.
It was believed that microtubules in dendrites are generated at the centrosome, but cleaved into short filaments and transported into dendrites [2]. This idea could explain the unique arrays of microtubules in dendrites described above. Recently, however, this belief has been challenged by several studies reporting that neuronal centrosomes do not have ability to generate microtubules. We previously reported that γ-tubulin as well as microtubule-anchoring centrosomal protein, ninein, were not localized at the centrosomes of cultured rodent neurons, but were found being scattered as fine granules throughout the cell body and dendrites [13]. Stiess et al. [21] reported that the centrosome of primary cultured hippocampal neurons lost its function as a microtubule organizing center and that axons still extended after laser ablation of the centrosome. In Drosophila neurons, centrioles were reported not surrounded by γ-tubulin, and ablation of centrioles did not impair the microtubule polarity in dendrites [12]. These studies showed that centrosomes do not contribute to the dendritic microtubule organization.
In the present study, to confirm in vivo the loss of γ-tubulin from the centrosome of neurons, and to address why the centrosome loses γ-tubulin, we investigated the expression of γ-tubulin and its recruiting proteins, GCP-WD/NEDD1 and CDK5RAP2 during the development of mouse brain. GCP-WD and CDK5RAP2 are well known γ-tubulin-recruiting proteins that are localized at the centrosome in general interphase cells and bind to γ-tubulin ring complex (γTuRC) [5, 9, 18]. GCP-WD and CDK5RAP2, together with many kinds of kinases, make γTuRC change conformation so that the complex acts as a scaffold for α/β-tubulin dimers to initiate polymerization [5, 15].
We found all three proteins were localized at centrosomes in undifferentiated stem cells and in immature neural progenitors, but were not detected at the centrosome of mature neurons. This suggests that the loss of γ-tubulin is due to the loss of γ-tubulin recruiting proteins. RT-PCR analysis, however, showed continuing expression of these molecules in adult cerebral cortex. Given that GCP-WD and CDK5RAP2 are γTuRC activating protein as well [5, 18], we hypothesize that mature neurons still have microtubule nucleating activity at non-centrosomal sites. After depolymerization of microtubules of cultured neurons, we found newly generated short microtubules appearing within the cell body and dendrites. Our findings suggest trans-localization of microtubule nucleating proteins from centrosome to dendrites, which results in acentrosomal nucleation of microtubules in dendrites.
II. Materials and Methods
Animals
Rabbits (Japanese White) for immunization and mice (ICR) for histological analysis and cell culture were obtained from Japan SLC, Inc. (Shizuoka, Japan). All animal experiments were conducted according to the Principles of Laboratory Animal Care (NIH publication No. 85-23) and to the guidelines of the Animal Experiment Committee of Sophia University.
Antibodies
Antibodies used are as listed below: mouse monoclonal antibody to α-tubulin (clone B-5-1-2, Sigma, St. Louis, MO) [1:500], mouse monoclonal antibody to neuron specific isoform of β-tubulin (clone TUJ-1, Covance, Berkeley, CA) [1:200], mouse monoclonal antibody to γ-tubulin (clone GTU-88, Sigma) [1:500] and rabbit polyclonal antibody to pericentrin (Covance) [1:500]. Antibody to GCP-WD [1:500] were generated in rabbit by immunizing with recombinant histidine-tagged C-terminal peptide (550–660) that were expressed in Escherichia coli using PET21c vector system (Novagen, Madison, WI).
Cell culture and microtubule regrowth experiment
Cells were obtained from E13.5 mice cerebral cortex and cultured as described in elsewhere [22]. Briefly, trypsin treated cortices were dissociated and plated onto plastic dishes. Cells were cultured for 2 weeks in NeuroBasal medium supplemented with B27 (Invitrogen, Carlsbad, CA). To destroy microtubules, culture medium was replaced by ice-cold medium containing 10 mg/l nocodazole. They were kept on ice for 45 min. After washing with cold medium without nocodazol five times, they were incubated in normal medium at 37°C for 3 or 10 min. Then cells were treated with 0.2% Triton, 0.1 mM Taxol in PHEM solution (60 mM PIPES, 10 mM EGTA, 25 mM HEPES, 2 mM MgCl2, pH 6.9) for 1 min and fixed with 4% paraformaldehyde (PFA) in sodium-phosphate buffer (pH 7.4) for 30 min. Cells were double stained with antibody to α-tubulin and antibody to pericentrin as described below.
Immunostainings
Mice were deeply anesthetized with ether and perfused with phosphate buffered saline (PBS), and subsequently with 4% PFA in 0.1 M sodium phosphate buffer (pH 7.4). Brains were removed and further fixed for 4 hr at 4°C. After immersed in 20% sucrose/PBS, they were embedded in OCT compound and cryo-cut at 12 μm. Sections were treated with 0.1% Triton-X100/PBS for 30 min and subsequently with 10% fetal bovine serum, 0.1% Triton-X100 in PBS for 1 hr. They were then incubated with first antibody for 1 hr, washed in 0.1% Triton-X100/PBS three times and then with secondary antibody for 1 hr. Washed sections were observed with laser confocal microscope (LSM700, Carl Zeiss, Oberkochen, Germany) or with fluorescent microscope (AxioVert200M, Carl Zeiss). Secondary antibodies used were Alexa Fluor 568 goat anti-rabbit IgG (H+L) (Invitrogen) [1:200] and Alexa Fluor 488 goat ant-mouse IgG (H+L) (Invitrogen) [1:200]. All antibodies were diluted in 10% fetal bovine serum, 0.1% Triton-X100/PBS.
Western blotting
Forebrains of E13.5 mice were homogenized in 5 volume of Laemmli’s sodium dodecyl sulfate (SDS) sample buffer and boiled. 10 μg of protein was subjected to SDS-polyacrylamide gel electrophoresis and proteins in the gel were transferred to an Immobilon-FL membrane (EMD Millipore, Billerica, MA) by a semi-dry electroblotter. The membranes were blocked with Odyssey Blocking Buffer (LI-COR, Lincoln, NE) and incubated with an anti-GCP-WD antibody generated in this study (1:200 dilution in the same blocking buffer). The membranes were washed in 0.1% Tween-20/PBS and then incubated with Alexa Fluor 680 anti-rabbit IgG (Invitrogen) diluted in the blocking buffer. The blots were washed and visualized using Odyssey Infrated Imaging System (LI-COR).
RT-PCR
Total RNA was extracted from cerebral cortex of embryonic day 13.5 (E13.5), E17.5, postnatal 1 week (P1W) and P10W mice with acid phenol-guanidinium thiocyanate-chloroform extraction method described in [16]. 1 μg of RNAs were glyoxylated in the presence of ethidium bromide and electrophoresed to verify that the same amount and quality of RNA was obtained from each sample. RT-PCR was performed with PrimeScript One Step RT-PCR Kit (Takara Bio, Shiga, Japan). Thermal-cycle reactions were carried out 25 cycles, which we chose so that none of the products reached a plateau. The following oligonucleotide primers were used: telencephalin, 5'-GGCCAAAAATGTGGCCGTCACG-3' (sense), 5'-CGCT CACGACGACTGTTTTGAC-3' (antisense); TUBG1, 5'-ACGCCGATGTCTGAGGAG-3' (sense); TUBG2, 5'-TGGC AGGAGTTCCTCTCAGT-3' (sense); TUBG1 and 2, 5'-CCATGCTCCGACAGGTAGAT-3' (antisense); GCP-WD, 5'-CAGCGTTTCGGATAATGGAG-3' (sense), 5'-GGTT TTCACGGGAGATTTCA-3' (antisense); CDK5RAP2, 5'-ACATCCCCAGAATGAACTGC-3' (sense), 5'-TGACGT CAGAGAGCTGTGC-3' (antisense).
For calculation of relative expression level, intensity of each band was measured using Image J software (http://rsb.info.nih.gov/ij/), and was normalized with the intensity of the band of total RNA.
III. Results
Localization of γ-tubulin, GCP-WD and CDK5RAP2 in : developing cerebral cortex
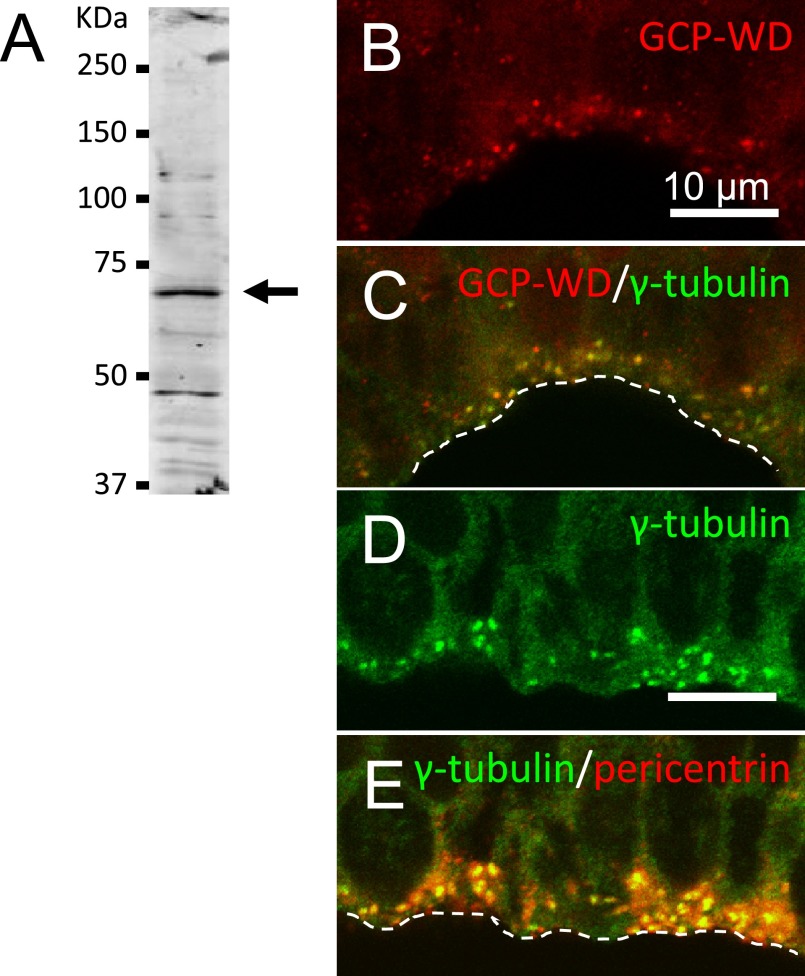
The antibody to GCP-WD that we generated in a rabbit immunized with recombinant C-terminal peptide reacted with a protein of approximately 71 KDa in E14.5 mouse forebrain (Fig. 1A). This size matches the calculated molecular mass of mouse GCP-WD (71.2 KDa).
Fig. 1. .
A: Westernblot analysis of the anti-GCP-WD antibody used in this study. Forebrains of E14.5 mice were homogenized in Laemmli’s SDS sample buffer and 10 μg of protein was subjected to Western blot. The antibody strongly reacted with protein of approximately 71 KDa (arrow) that matches the calculated molecular mass of mouse GCP-WD. B–E: Confocal laser microscopy for the localization of γ-tubulin and its recruiting proteins in the ventricular zone of cerebral cortex of E13.5 mouse. γ-Tubulin, pericentrin and GCP-WD were co-localized at the centrosomes situated at the ventricular surface (dashed lines) of neuroepithelial cells.
Pyramidal neurons of cerebral cortex are born in the ventricular zone by cell division of neuronal stem cells, of which the centrosomes are located close to the ventricular surface. When we observed ventricular zone of E13.5 mice, intense staining with γ-tubulin, GCP-WD, and CDK5RAP2 were detected at the centrosomes marked by pericentrin (Fig. 1B–E, CDK5RAP2 not shown). All of pericentrin-positive centrosomes were positive for γ-tubulin, GCP-WD, and CDK5RAP2. These observation were consistent with the previous reports for GCP-WD [10] and for CDK5RAP2 [3].
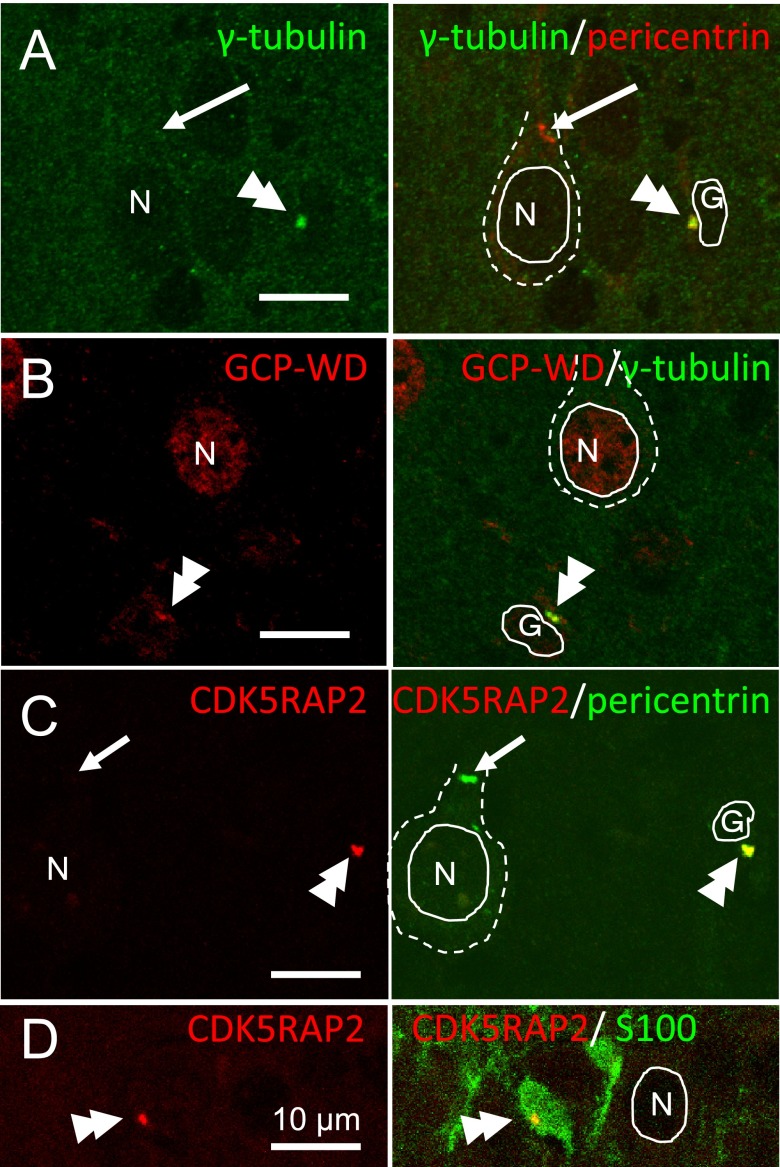
In cerebral cortex of postnatal 10 days mice, most of the cells in cortical plate are matured neurons. When we examined pyramidal neurons in the cortical plate, centrosomes were negative to γ-tubulin, GCP-WD, and CDK5RAP2 (arrows in Fig. 2A and 2C). Centrosomes of cells of less than 5% in cortical plate were positive to these proteins (double arrowheads in Fig. 2A–D). For example, we found 7 CDK5RAP2 positive cells (3.8%) out of 184 cells in cortical plate of P10 cerebral cortex, all of the 7 cells were positive to antibody to glial marker, S100 (Fig. 2D).
Fig. 2. .
Confocal laser microscopy for the localization of γ-tubulin and its recruiting proteins in the cortical plate of cerebral cortex of P10 mouse. A, B and C: γ-Tubulin, pericentrin, CDK5RAP2 and GCP-WD were co-localized at the centrosome of glial cells (double arrowheads), but γ-tubulin, GCP-WD and CDK5RAP2 were not detected at the pericentrin-positive centrosomes (arrows) of pyramidal neurons. D: Double staining with CDK5RAP2 and glial marker, S100 revealed that CDK5RAP2 positive centrosomes were included in S100 positive cells. Cell nuclei are outlined with solid lines and cell bodies are outlined with dashed lines. Nuclei of neurons are labeled with N, and those of glia are with G.
Localization of γ-tubulin, GCP-WD and CDK5RAP2 in : developing cerebellar cortex
We observed cerebellar cortex of postnatal 10 days mice, which contains both undifferentiated and differentiated neurons. External granular layer (EGL) of cortex is occupied by proliferating and migrating granular cells that are not yet fully differentiated. Purkinje cell layer (PCL) is occupied by matured Purkinje neurons, which extend long and arborizing dendrites into molecular layer (ML). Internal granular layer (IGL) is occupied by differentiated granule cells, of which dendrites are short.
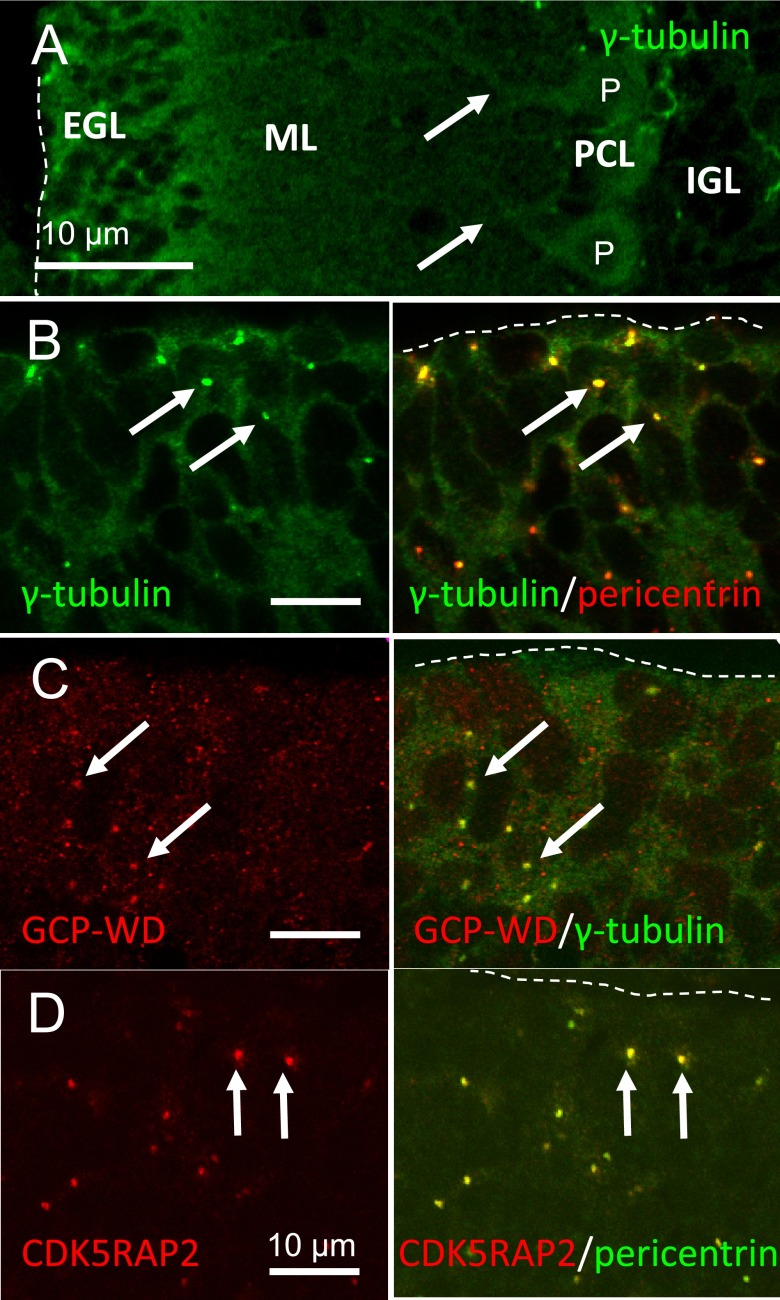
When a sagittal section of P10 cerebellar cortex was stained with an antibody to γ-tubulin (Fig. 3A), intense immunoreactivity was found at EGL and PCL. In Purkinje neurons, cytoplasm of cell body and proximal region of dendrites were intensely stained (arrows in Fig. 3A). Staining of ML was moderate, while staining of IGL was weak. Presence of γ-tubulin at the dendrites of Purkinje neurons suggests its role in dendritic growth.
Fig. 3. .
Confocal laser microscopy for the localization of γ-tubulin and its recruiting proteins in P10 cerebellar cortex. A: A sagittal section was stained with an antibody to γ-tubulin. Low-magnified observation showed the intense expression of γ-tubulin at EGL and PCL. Cytoplasm of cell body and dendrites (arrows) of Purkinje neurons was stained. B, C and D: γ-Tubulin and its recruiting proteins in the external granular layer of P10 cerebellar cortex. They were all co-localized at the centrosome of granular neurons (arrows). Pial surfaces are outlined with dashed lines. EGL, external granular layer; ML, molecular layer; PCL, Purkinje cell layer; IGL, internal granular layer; P, Purkinje neuron.
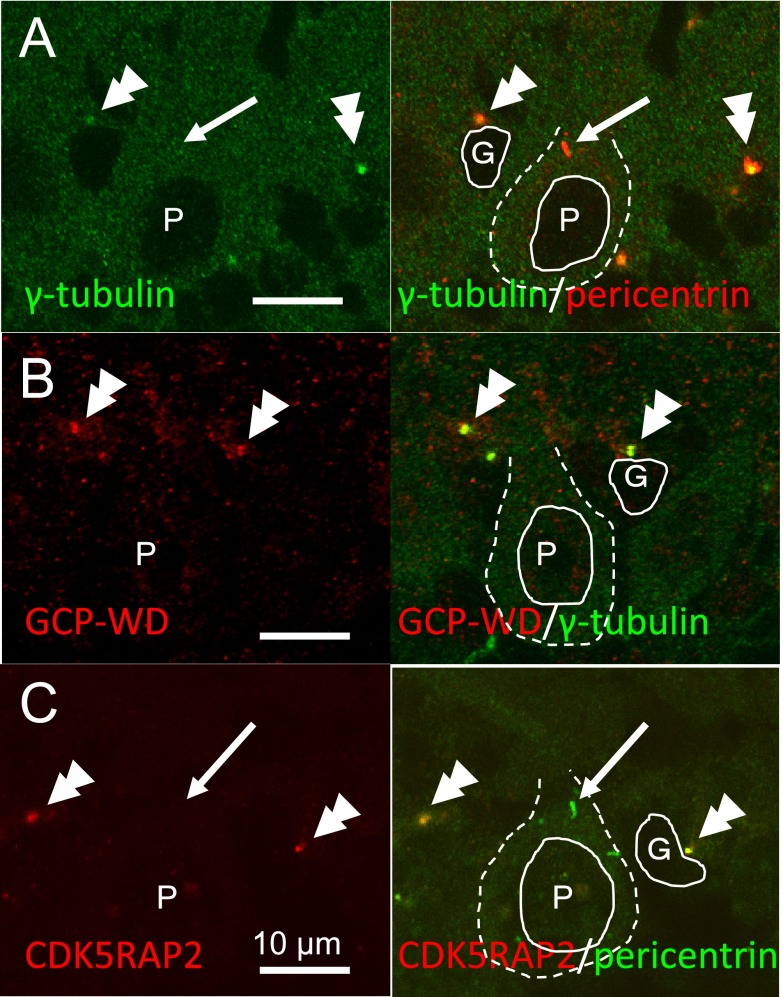
All centrosomes of cells in EGL were stained with γ-tubulin, GCP-WD, and CDK5RAP2 (Fig. 3). Purkinje neurons (label P in Fig. 4A, B and C), as identified by possessing large round nucleus with large cytoplasm and thick dendrites, however, were not stained for these proteins at the pericentrin-positive centrosomes (long arrows in Fig. 4). Cells adjacent to the Purkinje neurons (double arrowheads in Fig. 4), probably Golgi glial cells, showed intense staining for γ-tubulin, GCP-WD, and CDK5RAP2 at the centrosomes.
Fig. 4. .
Confocal laser microscopy for the localization of γ-tubulin and its recruiting proteins in the Purkinje cell layer of P10 cerebellar cortex. γ-Tubulin, CDK5RAP2, GCP-WD and pericentrin were co-localized at the centrosome of glial cells (double arrowheads), but γ-tubulin, GCP-WD and CDK5RAP2 were not detected at the pericentrin-positive centrosomes (arrows) of Purkinje neurons. Cell nuclei are outlined with solid lines and cell bodies are outlined with dashed lines. Nuclei of Purkinje neurons are labeled with P, and those of glia are with G.
mRNA expression of γ-tubulin, GCP-WD and CDK5RAP2
Loss of immunostaining for γ-tubulin, GCP-WD, and CDK5RAP2 at the centrosome of differentiated neurons does not necessary mean that these proteins are not expressed at all. We examined mRNA expression of γ-tubulin, GCP-WD, and CDK5RAP2 in postnatal cerebral cortex, in which most of the cells were negative for these protein with immunostaining.
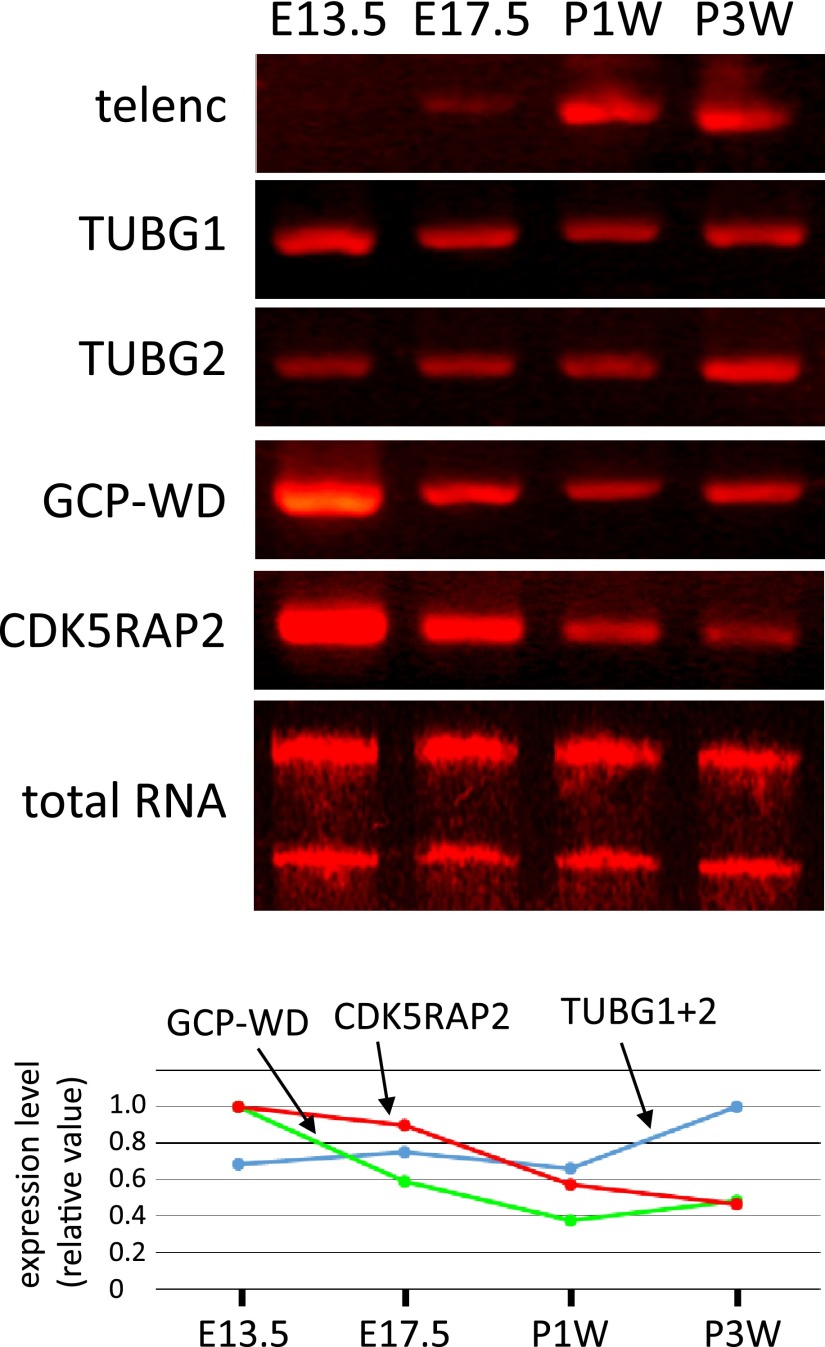
RT-PCR analysis of telencephalin demonstrated the differentiation of neurons at around perinatal period (Fig. 5). The ubiquitously expressed conventional isoform of γ-tubulin, TUBG1, was expressed in nearly equal amount during the development of cerebral cortex. Expression of brain-specific isoform, TUBG2 [25], was also observed throughout the development, with slight tendency to increase with development. Although expression of both GCP-WD and CDK5RAP2 decreased after E13.5, they kept being expressed in substantial amount up to 3 weeks after birth. Semi-quantitative evaluation revealed that the expression level of GCP-WD decreased earlier than CDK5RAP2.
Fig. 5. .
RT-PCR analysis for the expression of two isoforms of γ-tubulin, GCP-WD and CDK5RAP2 during the development of cerebral cortex. RNA was extracted from cerebral cortex of indicated age and subjected to one-step RT-PCR reactions. Telencephalin is a molecular marker for the development of dendrites. Ethidium bromide staining of total RNAs showed the quantity and quality of RNAs examined. Relative expression level was calculated from the intensity of each band and was normalized with the intensity of the band of total RNA.
Regrowth of microtubules from the cytoplasm of matured neurons
Sustained expression of γ-tubulin, GCP-WD, and CDK5RAP2 in matured brain suggests the possibility that these proteins are localized at the other sites than the centrosome in neurons and remain functional.
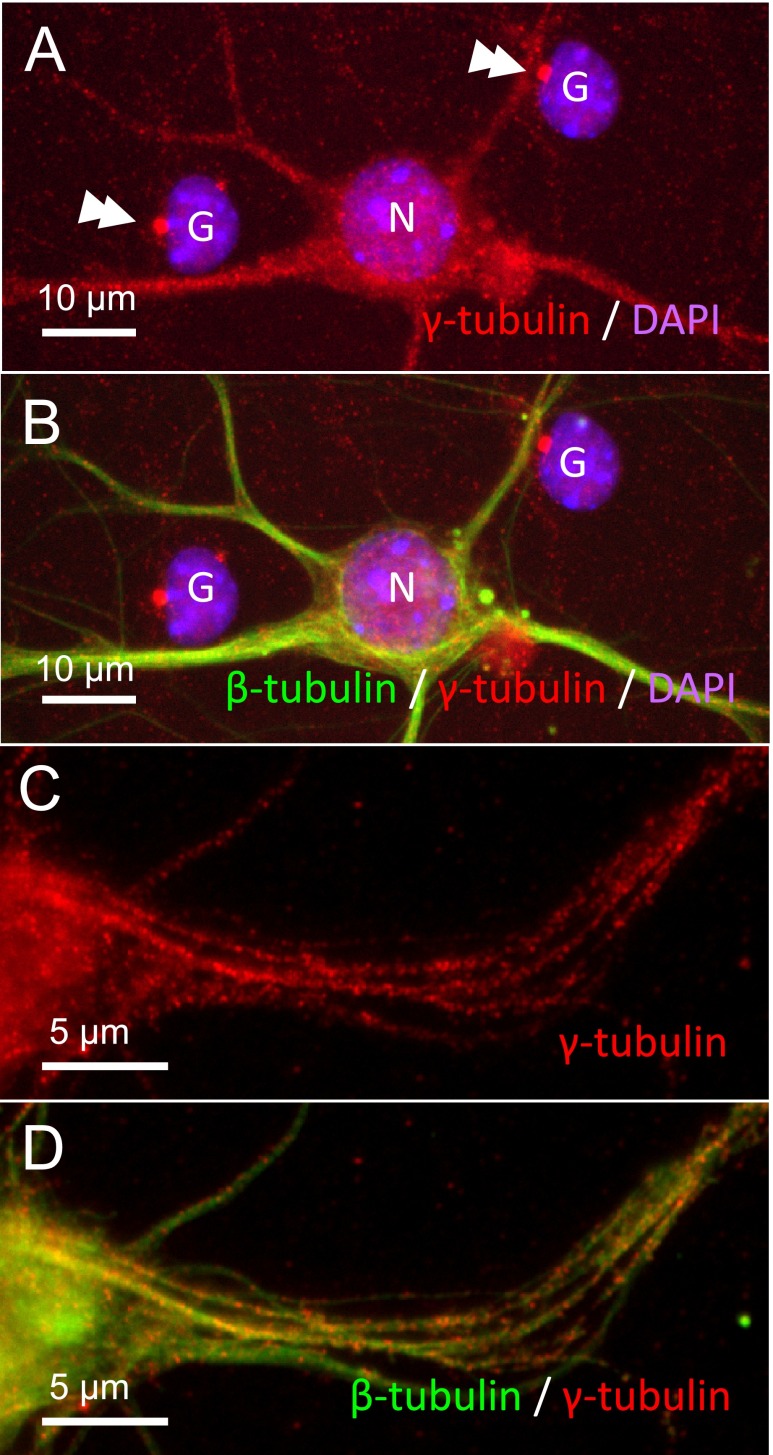
Functional γTuRC, in many cases, are recruited onto intracellular structures such as centrosome, Golgi apparatus or lateral side of pre-existing microtubules [9]. Cytosolic γTuRC is thought to have no microtubule nucleating activity. We examined whether acentrosomal γ-tubulin is associated with any structures in neurons. We cultured cerebral cortical neurons for 12 days in vitro (12DIV). Cytosolic γTuRCs were washed out with detergent before fixation. In glial cells contaminated in this cultures, γ-tubulin was detected at the centrosomes but not at the cytoplasm (Fig. 6A and B). In neurons, in contrast, diffuse staining was observed in the cell body and dendrites. Close observation of dendrites that were double-stained for γ-tubulin and neuron-specific isoform of β-tubulin revealed that γ-tubulin was associated with microtubules (Fig. 6C and D).
Fig. 6. .
γ-Tubulin distribution in primary cultured cortical neurons and astroglia. 12DIV cells were treated with detergent before fixation to washout cytosolic γTuRCs. A and B: Fluorescent microscopic observation of immunostaining for γ-tubulin revealed the localization of γ-tubulin at the centrosomes of glial cells (double arrowheads). The cytoplasm of glial cells was not stained, whereas cell body and dendrites of neurons were diffusely stained. Nuclei of the neuron is labeled with N, and those of glia with G. Antibody to neuron-specific isoform of β-tubulin stained the cell body and dendrites of a neuron. B and C: Close observation of dendrites with confocal laser microscope revealed that γ-tubulin were associated with microtubules.
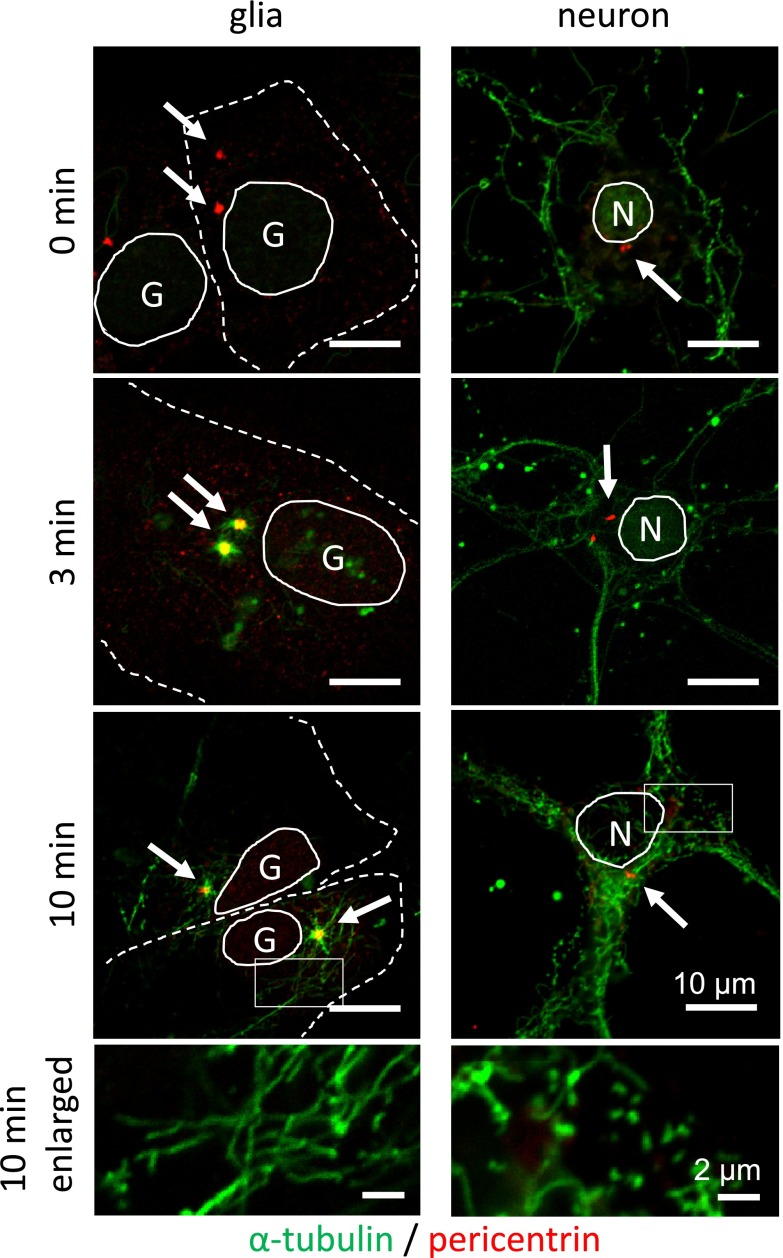
To test whether microtubules are nucleated at the other sites than the centrosome in neurons, we performed microtubule regrowth experiment on 12DIV neurons (Fig. 7). After treatment with ice-cold nocodazol, we confirmed that almost all microtubules in cell body and dendrites are destroyed except for those in axons running around the neuron (Fig. 7, 0 min). Then nocodazol was washed out and the cells were incubated in normal medium at 37°C. At 3 min after wash, short microtubules were observed at the centrosome of glial cells, but were not observed around the centrosome of neurons. At 10 min, microtubules in glial cells grew longer to form radial array emanating from the centrosome. In neurons, short microtubules were observed at non-centosomal sites in cell body and dendrites (Fig. 7, 10 min and 10 min enlarged). This observation supports the idea that microtubule nucleation takes place at acentrosomal sites of neurons, although the acentrosomal nucleation in neurons was slower than the centrosomal nucleation in glial cells.
Fig. 7. .
Microtubule regrowth experiments. 12DIV neurons were treated with nocodazol to destroy microtubules (0 min), and were washed and incubated for indicated time. Microtubules were stained with antibody to α-tubulin and the centrosomes are visualized with antibody to pericentrin (arrows). Cells were observed with confocal laser microscope. Cell nuclei are outlined with solid lines and glial cell periphery is outlined with dashed lines. Nuclei of glial cells are labeled with G, and those of neurons with N. In glial cells, short microtubules appeared at the centrosome at 3 min and they grew longer at 10 min. In neurons short microtubules appeared at 10 min within cell body and dendrites. The bottom panels are enlarged images of the rectangles of the above panels.
IV. Discussion
Centrosomes are the main microtubule organizing center (MTOC) in general cells. γ-Tubulin is an indispensable component of MTOCs that contributes to microtubule nucleation [8]. It forms γTuRC with other components and is embedded in the pericentriolar materials, of which pericentrin is one of the major component. Beside the centrosome, about 80% of γTuRCs are found in cytosol [11], but they are thought to have no microtubule nucleating activity [15, 23]. Activation of γTuRCs requires molecular interactions with kinases and recruiting proteins [9]. GCP-WD and CDK5RAP2 are well known recruiting proteins [5, 18]. Depletion of these proteins resulted in the loss of γTuRC at the centrosome and microtubule nucleating activity of the centrosome.
It was believed that microtubules in neurons are also generated at the centrosome and then cleaved and transported into dendrites [2, 19]. This was a good explanation of random polarity of dendritic microtubules, because cleaved short microtubules in cell body could be transported by motor proteins with either polarity. Recent studies, however, showed that γTuRC is not concentrated at the centrosome of neurons [13] and that the centrosome of neurons does not possess microtubule nucleating activity [21].
The first question addressed here is why γTuRC is lost at the centrosome of neurons. We confirmed in vivo the loss of γ-tubulin from the centrosome during neuronal maturation. Centrosomes of mature neurons in cortical plate of cerebral cortex and of mature Purkinje neurons were not stained for γ-tubulin. γ-Tubulin recruiting proteins, GCP-WD and CDK5RAP2, were also lost from the centrosomes of these neurons, indicating that the loss of γ-tubulin from the centrosome is due to the loss of recruiting proteins from it. As the mRNA for these proteins were found to be expressed in matured brains, these proteins are supposed to translocate from the centrosome to the cytoplasm. The reason why these proteins loose the binding affinity to the centrosome in mature neurons is unknown.
Loss of γ-tubulin and microtubule nucleating activity of the centrosome led us question where and how neuronal microtubules are generated. One possibility is that microtubules increase their number by cycles of severing of pre-existing microtubules and polymerization at their ends. Katanin and spastin are thought to be involved in this process [20]. This process, however, could not explain the random polarity of dendritic microtubules, because it is unlikely that severed microtubules rotate their polarity within dendrites. Alternatively, microtubules can be possibly generated by acentrosomal nucleation. If microtubules are generated de novo within dendrites, they would be in random polarity. Acentrosomal nucleation of microtubules are well known in some differentiated non-neuronal cells. Microtubule nucleation is found at Golgi apparatus of retinal pigment epithelial cells [6] and from the nuclear membrane of muscle cells [4]. Recent study in Drosophila demonstrated the cytoplasmic and Golgi-associated nucleation of microtubules in neurons [12, 14]. Acentrosomal microtubule nucleation was indeed observed in cell body of cultured hippocampal neurons using EB3-GFP tracking [21].
The present microtubule regrowth experiments demonstrate the nucleation of microtubules in dendrites. Short microtubules were generated within the cell body and in the distal region of dendrites. These microtubules, however, were only found after 10 min in neurons, while they appeared at the centrosome in glial cells within 3 min. This suggests more intense activation of γTuRC at the centrosome of glial cells than in the cell body and dendrites of neurons. As γ-tubulin was found associated with pre-existing microtubules in dendrites, it is possible that some kind of protein complexes like augmin complex [7], which is an anchor structure for γTuRC onto spindle microtubules might be involved in the anchoring of γTuRC onto dendritic microtubules. We do not know whether such an anchoring complex involves GCP-WD and/or CDK5RAP2, but we suppose anchoring on dendritic microtubules activates γTuRC to nucleate microtubules. These microtubules could have random orientation, which would explain random polarity of microtubules in dendrites.
V. Acknowledgments
We thank Mr. Koyo Ide (Sophia University, Tokyo, Japan) for his technical assistance in Western blot analysis. This study was funded by the Ministry of Science, Education, Sports and Culture of Japan (grant no. 20500315).
VI. References
- 1.Baas P. W., Deitch J. S., Black M. M. and Banker G. A. (1988) Polarity orientation of microtubules in hippocampal neurons: uniformity in the axon and nonuniformity in the dendrite. Proc. Natl. Acad. Sci. U S A 85; 8335–8339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baas P. W. and Joshit H. C. (1992) γ-tubulin distribution in the neuron: implications for the origins of neuritic microtubules. J. Cell Biol. 119; 171–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buchman J. J., Tseng H. C., Zhou Y., Frank C. L., Xie Z. and Tsai L. H. (2010) Cdk5rap2 interacts with pericentrin to maintain the neural progenitor pool in the developing neocortex. Neuron 66; 386–402. [DOI] [PubMed] [Google Scholar]
- 4.Bugnard E., Zaal K. J. M. and Ralston E. (2005) Reorganization of microtubule nucleation during muscle differentiation. Cell Motil. Cytoskelton 60; 1–13. [DOI] [PubMed] [Google Scholar]
- 5.Choi Y.-K., Liu P., Sze S. K., Dai C. and Qi R. Z. (2010) CDK5RAP2 stimulates microtubule nucleation by the γ-tubulin ring complex. J. Cell Biol. 191; 1089–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Efimov A., Kharitonov A., Efimova N., Loncarek J., Miller P. M., Andreyeva N., Gleeson P., Galjart N., Maia A. R., McLeod I. X., Yates J. R. 3rd., Maiato H., Khodjakov A., Akhmanove A. and Kaverina I. (2007) Asymmetric CLASP-dependent nucleation of noncentrosomal microtubules at the trans-Golgi network. Dev. Cell 12; 917–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goshima G., Mayer M., Zhang N., Sturman N. and Vale R. D. (2008) Augmin: a protein complex required for centrosome-independent microtubule generation within the spindle. J. Cell Biol. 181; 421–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kollman J. M., Merdes A., Mourey L. and Agard D. A. (2011) Microtubule nucleation by γ-tubulin complexes. Nature Rev. Mol. Cell Biol. 12; 709–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin T.-C., Neuner A. and Schiebel E. (2014) Targeting of γ-tubulin complexes to microtubule organizing centers: conservation and divergence. Trend. Cell Biol. 25; 296–307. [DOI] [PubMed] [Google Scholar]
- 10.Manning J. A., Paul A., Colussi P. A., Koblar S. A. and Kumar S. (2008) Nedd1 expression as a marker of dynamic centrosomal localization during mouse embryonic development. Histochem. Cell Biol. 129; 751–764. [DOI] [PubMed] [Google Scholar]
- 11.Moudjou M., Bordes N., Paintrand M. and Bornens M. (1996) γ-Tubulin in mammalian cells: the centrosomal and the cytosolic forms. J. Cell Sci. 109; 875–887. [DOI] [PubMed] [Google Scholar]
- 12.Nguyen M. M., Stone M. C. and Rolls M. M. (2011) Microtubules are organized independently of the centrosome in Drosophila neurons. Neural Dev. 6; 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ohama Y. and Hayashi K. (2009) Relocalization of a microtubule-anchoring protein, ninein, from the centrosome to dendrites during differentiation of mouse neurons. Histochem. Cell Biol. 132; 515–524. [DOI] [PubMed] [Google Scholar]
- 14.Ori-McKenney K. M., Jan L. Y. and Jan Y.-N. (2012) Golgi outposts shape dendrite morphology by functioning as sites of acentrosomal microtubule nucleation in neurons. Neuron 76; 921–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Remy M-H., Merdes A. and Gregory-Pauron L. (2013) Assembly of gamma-tubulin ring complexes: implications for cell biology and disease. Progr. Mol. Biol. Transl. Sci. 117; 511–530. [DOI] [PubMed] [Google Scholar]
- 16.Sambrook, J. and Russell, D. W. (2001) Molecular Cloning. A Latoratory Manual 3rd ed. Cold Spring Harbor Laboratory Press, Cold Sprin Harbor, NY. [Google Scholar]
- 17.Scheibel M. E., Lindsay R. D., Tomiyasu U. and Scheibel A. B. (1975) Progressive dendritic changes in aging human cortex. Exp. Neurol. 47; 392–403. [DOI] [PubMed] [Google Scholar]
- 18.Sdelci S., Schutz M., Pinyol R., Bertran T. R., Regue L., Caelles C., Vemos I. and Roig J. (2012) Nek9 phosphorylation of NEDD1/GCP-WD contributes to Plk1 control of γ-tubulin recruitment to the mitotic centrosome. Curr. Biol. 22; 1516–1523. [DOI] [PubMed] [Google Scholar]
- 19.Sharp D. J., Yu W. and Baas P. W. (1995) Transport of dendritic microtubules establishes their nonuniform polarity orientation. J. Cell Biol. 130; 93–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sharp D. J. and Ross J. L. (2012) Microtubule-severing enzymes at the cutting edge. J. Cell Sci. 125; 2561–2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stiess M., Maghelli N., Kapitein L. C., Gomis-Ruth S., Wilsch-Brauninger M., Hoogenraad C. C., Tolic-Norrelykke I. M. and Bradke F. (2010) Axon extension occurs independently of centrosomal microtubule nucleation. Science 327; 704–707. [DOI] [PubMed] [Google Scholar]
- 22.Takahashi D., Yu W., Baas P. W., Kawai-Hirai R. and Hayashi K. (2007) Rearrangement of microtubule polarity orientation during conversion of dendrites to axons in cultured pyramidal neurons. Cell Motil. Cytoskel. 64; 347–359. [DOI] [PubMed] [Google Scholar]
- 23.Wiese C. and Zheng Y. (2006) Microtubule nucleation: γ-tubulin and beyond. J. Cell Sci. 119; 4143–4153. [DOI] [PubMed] [Google Scholar]
- 24.Yu W., Centonze V. E., Ahmad F. J. and Baas P. W. (1993) Microtubule nucleation and release from the neuronal centrosome. J. Cell Biol. 122; 349–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yuba-Kubo A., Kubo A., Hata M. and Tsukita S. (2005) Gene knockout analysis of two γ-tubulin isoforms in mice. Dev. Biol. 282; 361–373. [DOI] [PubMed] [Google Scholar]