Abstract
We performed pre-embedding electron microscopic study for visualizing the antigen and genome of severe fever with thrombocytopenia syndrome (SFTS) virus in the cytoplasm of macrophages of the human splenic red pulp, both requesting preheating treatment of sections. To pursue this, coated glass slides with unique characteristics are needed. Namely, during staining they must prevent detaching off sections, but after staining the sections must be transferred to epoxy resin. Aminopropyltriexoxysilane-coated glass slides, widely used for immunostaining, were resistant to transfer to epoxy resin. In contrast, coated glass slides designated as Thinlayer Advanced Cytology Assay System (TACAS) were suitable for this purpose. The technique is also applicable to the coated glass slide-requiring cytology practice, in which immunocytochemical evaluation is needed after cell transfer to another glass slide.
Keywords: electron microscopy, immunohistochemistry, in situ hybridization, severe fever with thrombocytopenia syndrome virus, TACAS slides
I. Introduction
In pursuing the diagnostic and research activity, we occasionally need to transfer histo/cytological preparations from one to another. For preventing detaching off sections, coated glass slides are widely utilized, but they themselves make a barrier for transferring sections. The purpose of the present study is to solve this inconsistency.
Severe fever with thrombocytopenia syndrome (SFTS) caused by SFTS virus belonging to the genus Phlebovirus in the Bunyavirus family was first reported in China [11, 12], and lethal cases of SFTS have been accumulated also in Japan [2, 8]. In addition to B-lymphocytes, macrophages are known to be the target cell of SFTS virus [3, 5]. We happened to experience an autopsy case of SFTS, and positive signals were obtained with both immunostaining for SFTS viral antigen and in situ hybridization (ISH) for SFTS viral RNA in the cytoplasm of macrophages of the splenic red pulp in formalin-fixed, paraffin-embedded sections. Both techniques request pre-heating procedures for obtaining positive signals, and the use of coated glass slides is thus essential. Reportedly, SFTS virus is round in shape and measures 80–100 nm in size [7]. In order to confirm the specificity of the techniques, we planned to visualize the signals at the electron microscopic level. Pre-embedding electron microscopy needs section transfer to epoxy resin [1]. We then reached the technical point of conquer described above.
II. Materials and Methods
Case of analysis
We analyzed the SFTS virus-infected spleen, obtained at autopsy (September, 2013) in Tokushima Prefectural Naruto Hospital, Naruto, Tokushima, Japan. The 86-year-old female complaining of high fever and paraplegia died of SFTS in four days, and the diagnosis was confirmed by virus isolation in the blood. Platelet count was 8×104/μL and white blood count was 1,600/μL. Tick bite was seen on the back. The spleen weighing 60 g was routinely fixed in 10% formalin and embedded in paraffin wax.
Coated glass slides
Paraffin sections at 3 μm thickness were mounted on eight kinds of commercially available coated glass slides, including those designated as Silane S (Muto Pure Chemicals, Tokyo, Japan), Silane (Muto), New Silane II (Muto), New Silane III (Muto), Amino Propyltriethoxy Silane (APS, Matsunami Glass, Kishiwada, Japan), Poly-L-Lysine (PLL, Matsunami), Matsunami Adhesive Slide (MAS, Matsunami), and Thinlayer Advanced Cytology Assay System (TACAS, Medical & Biological Laboratories, Nagoya, Japan). The main component in Silane S, Silane, New Silane II, New Silane III and APS comprises aminopropyltriethoxysilane. The main component of PLL is poly-L-lysine. The components of MAS and TACAS, secured by the patent, are not open to the public. MAS has enriched density of amino residues on the surface. In the positively charged TACAS slides, the coated areas are encircled (13 mm in diameter), and the non-coated surface was covered with a black sheet [4]. For applying to the TACAS slides, the splenic sections were previously trimmed to be contained within the TACAS circle. Non-coated glass slides (Muto) were also utilized for comparison. A total of five samples were evaluated under each condition.
Pre-embedding immunoelectron microscopy
Pre-embedding immunoelectron microscopy [1] for visualizing SFTS viral antigen was performed as follows. Endogenous peroxidase activity was quenched with 0.3% hydrogen peroxide in methanol for 30 min at room temperature. Hydrated heat-assisted epitope retrieval was applied using a pressure pan cooker in 10 mM citrate buffer, pH 6.0, for 10 min. Anti-SFTS virus mouse monoclonal antibody (clone: 1C3, diluted at 1:2,000, raised in the Department of Virology, Institute of Tropical Medicine, Nagasaki University, Nagasaki) was incubated overnight at room temperature. As the second layer reagent, Simple Stain MAX-PO (Nichirei Bioscience, Tokyo, Japan) was applied for 1 hr at room temperature. The reaction products were visualized in 50 mM Tris-HCl buffer, pH 7.6, containing 20 mg/dl diaminobenzidine tetrahydrochloride (DAB) and 0.006% hydrogen peroxide. The sections were sequentially treated with 1% osmium tetroxide in 10 mM phosphate-buffered saline (PBS), pH 7.2, for 1 hr at room temperature, dehydrated in a graded series of ethanol, and embedded in epoxy resin (EPON812; Oken Shoji, Tokyo, Japan) with the inverted gelatin capsule method. The opposite surface of the fully dehydrated stained sections was briefly heated by a burner during the tissue transfer procedure. Ultrathin sections were cut with an ultramicrotome (Ultracut N; Reichert-Nissei, Tokyo, Japan) at 100 nm thickness, put on the copper grid, and observed on a transmission electron microscope (H-7650; Hitachi, Tokyo, Japan).
In situ hybridization (ISH) at the electron microscopic level
SFTS viral RNA was detected with ISH, the AT tailing method, for high-sensitivity signal detection [6]. Paraffin sections of the infected spleen were heated using pressure pan cooker with 10 mM citrate buffer, pH 6.0, for 10 min, followed by digestion with 0.1 μg/mL proteinase K for 15 min at 37°C. The sections were hybridized overnight at 50°C with 0.01 pmol/mL AT-tailed oligonucleotide antisense cocktail probes for the L, M and S segments of viral RNA: 5'-CACTACTAGTGTGACCACTCTTGAGTCTGG CCACTCAGAC(ATx10)-3' for the L segment, 5'-CACC ACCACCTGCATAACAGAGGGTAGTGAAGTGAAGCCA(ATx10)-3' for the M segment and 5'-GTGCTTATCTGA ATAGGCCTTGAACCAGGCGTGGAACTCC(ATx10)-3' for the S segment. After hybridization, the sections were rinsed in 1× saline sodium citrate (SSC) and 0.1× SSC for 10 min at 55°C, respectively. Gene Frame (Thermo Fisher Scientific, Yokohama, Japan) was attached to each slide for exposure to the AT tailing mixture consisting nucleotide, biotin-16-dUTP and Gene Taq DNA polymerase for 10 min at 60°C. The GenPoint system (Dako, Carpinteria, CA, USA) was employed for signal amplification. The reaction products were visualized in the DAB solution. Pre-embedding electron microscopy was carried out, as previously described.
Ethical issue
The study on SFTS study was approved by the Ethical Committee of the Clinical and Epidemiological Study, Fujita Health University, Toyoake (approval number #15-109).
III. Results and Discussion
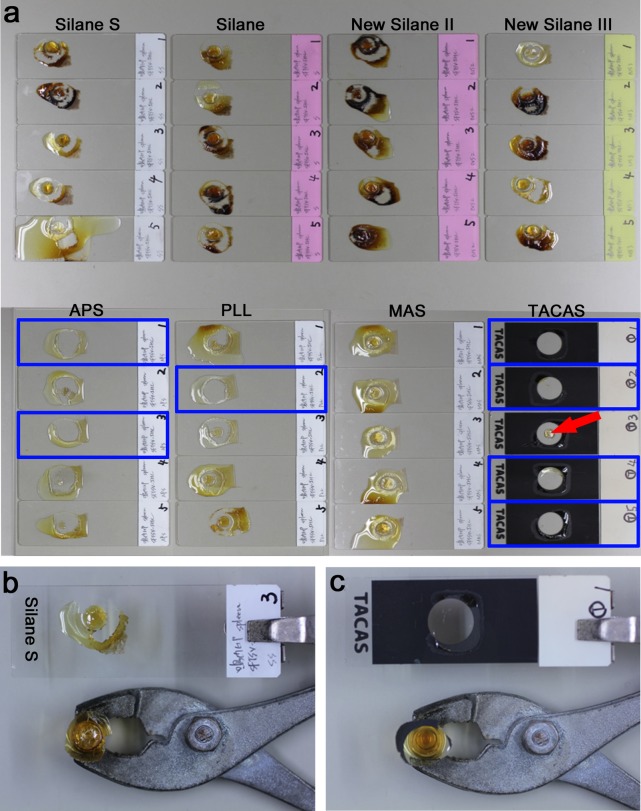
All the eight kinds of coated glass slides prevented section detachment during the staining procedure using preheating, while sections were detached off non-coated slides. Section transfer after staining was succeeded in four of five sections mounted on TACAS slides, two of five sections mounted on APS slides, and one of five sections mounted on PLL slides: no tissue part remained on the glass slides. Sections mounted on Silane S, Silane, New Silane II, New Silane III and MAS slides were scarcely transferred to the epoxy resin. Actually, only the peripheral part of the sections were transferred, and the central main part remained on the glass slides (Fig. 1a). MAS slides were especially resistant to transfer. The surface of the EPON blocks unsuccessful for section transfer was irregular and burnt (Fig. 1b), while smooth surfaced EPON blocks were seen when the transfer was successful (Fig. 1c). Table 1 summarizes the percentage of tissue areas (mean±standard deviation) left over the eight kinds of the glass slides. Apparently, TACAS slides gave the best result.
Fig. 1. .
Evaluation of coated glass slides optimal for immunoelectron microscopy. (a) Results of transfer of immunostained paraffin sections mounted on the coated glass slides to epoxy resin (EPON). Five sections were evaluated under each condition. The vertical row represents Silane S, Silane, New Silane II and New Silane III slides in the upper panels, and APS, PLL, MAS and TACAS slides in the lower panels. Sections, particularly located in the center of the circle rimmed by the gelatin capsule, often remain on the glass slides, and often show brown color (burnt by heat). No sections remain on TACAS slides, except for one (arrow). Successfully transferred cases are framed in blue. (b) A section mounted on a Silane S slide after trial of tissue transfer. Tissue section mostly remains on the glass slide, and the surface of EPON block appears to be irregular and rough. (c) A section mounted on a TACAS slide after trial of tissue transfer. No tissue section remains on the glass slide, and the EPON block is smooth-surfaced.
Table 1 .
The percentage of tissue areas (mean±standard deviation) left over the eight kinds of the glass slides and the number of slides showing successful transfer
| Silane S | Silane | New Silane II | New Silane III | APS | PLL | MAS | TACAS | |
|---|---|---|---|---|---|---|---|---|
| Areas failed to be transferred (%) Mean±standard deviation |
60.4±9.9 | 70.8±5.0 | 66.4±9.9 | 62.0±4.8 | 21.2±13.8 | 51.6±20.6 | 97.2±2.2 | 8.0±12.8 |
| The number of slides showing successful transfer |
0/5 | 0/5 | 0/5 | 0/5 | 2/5 | 1/5 | 0/5 | 4/5 |
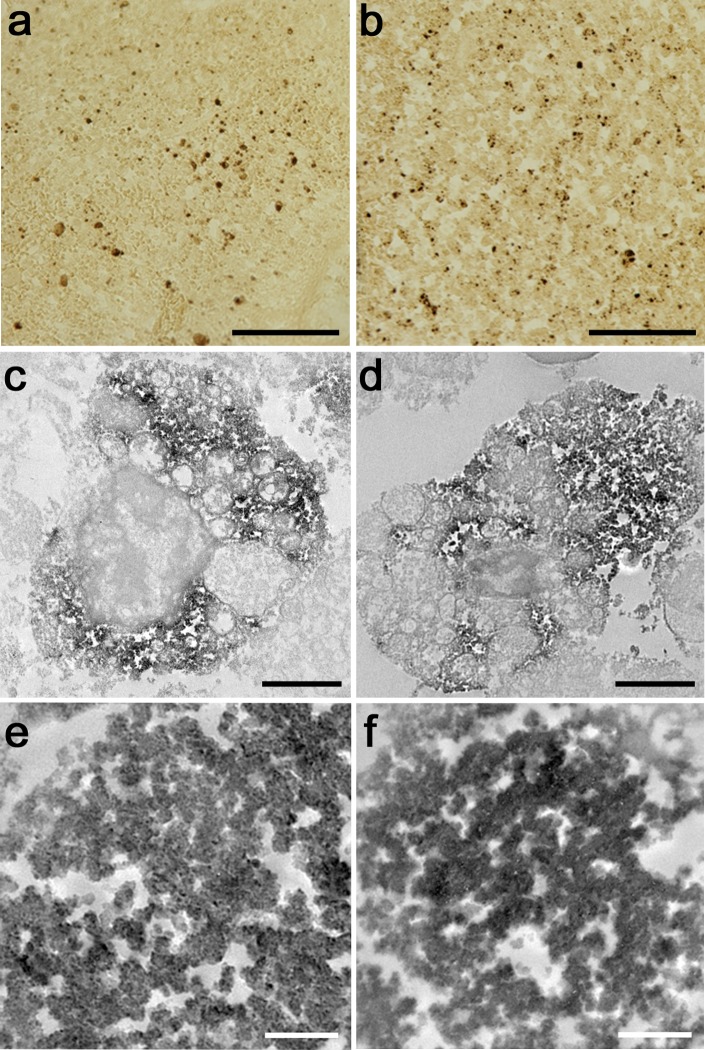
SFTS virus was visualized at the ultrastructural level by the two histochemical techniques, immunostaining and ISH. In the cytoplasm of macrophages in the splenic red pulp, dot-like positivity of SFTS viral signals were visualized at light microscopic level (Fig. 2a and b). At the ultrastructural level, clusters of round-shaped viral particles, around 100 nm in diameter, were visualized (Fig. 2c–f). The size of the SFTS virus was compatible with the previously reported electron microscopic study [7]. In the present study, the SFTS viral particles were demonstrated in the cytoplasm of splenic macrophages, which are reported to be a target of this lethal virus [3, 5].
Fig. 2. .
Visualization of SFTS virus in the splenic red pulp at the light microscopic (a and b) and ultrastructural (c–f) levels. a, c, e: immunostaining using a monoclonal antibody against SFTS viral antigen at the light microscopic (a) and ultrastructural (c and e) levels. b, d, f: in situ hybridization for SFTS virus RNA at the light microscopic (b) and ultrastructural (d and f) levels. Granular cytoplasmic reactivity is observed in the cytoplasm of macrophages in the splenic red pulp (a–d). Both ultrastructural techniques demonstrate round-shaped viral particles, around 100 nm in diameter and densely labeled with DAB product-related osmium black. Bars=100 μm (a and b), 2 μm (c and d) and 500 nm (e and f).
Of note is the fact that viral particles were identified at the ultrastructural level using formalin-fixed, paraffin-embedded sections. Microorganisms possess rigid particulated structures, which are relatively tolerant to the harsh process for light microscopic observation. One of the authors have reported ultrastructural visualization of pathogenic particles using routinely prepared paraffin sections [9, 10].
We showed that the TACAS slides, primarily used for the cytological evaluation [4], were quite useful in tissue transfer after histochemical stains employing preheating procedures. The magical features of the TACAS slides, preventing section detachment during staining and accelerating tissue transfer after staining, can also easily be applied to immnocytochemical evaluation after cell transfer of cytological specimens mounted on the TACAS slides. It is usually difficult for us to utilize the cell transfer technique when the cytology samples, particularly liquid-type (urine or effusion) specimens, were smeared on aminopropyltrimethoxysilane-coated glass slides.
IV. Acknowledgments
The authors are grateful to Mr. Gen Niimi and Mr. Tomihiko Ide, Division of Electron Microscopy, Institute of Joint Research, Fujita Health University, Toyoake, Aichi, Japan, for their skillful technical assistance. Ms. Yukika Hasegawa, Ms. Sayaka Takeuchi, Ms. Mika Maeshima, and Ms. Chikayo Yashiro, Department of Pathology, Fujita Health University School of Medicine, Toyoake, are cordially acknowledged for their positive cooperation in our research activity.
V. Conflict of Interest
The corresponding author (Y. T.) is a technical consultant of Medical & Biological Laboratories, Nagoya, Japan, and the TACAS slides were provided by the same company. However, regarding the present study, there is no conflict of interest between the authors and the company.
VI. References
- 1.D’Alessandro D., Mattii L., Moscato S., Bernardini N., Segnani C., Dolfi A. and Bianchi F. (2004) Immunohistochemical demonstration of the small GTPase RhoA on epoxy-resin embedded sections. Micron 35; 287–296. [DOI] [PubMed] [Google Scholar]
- 2.Hiraki T., Yoshimitsu M., Suzuki T., Goto Y., Higashi M., Yokoyama S. and Yonezawa S. (2014) Two autopsy cases of severe fever with thrombocytopenia syndrome (SFTS) in Japan: A pathognomonic histological feature and unique complication of SFTS. Pathol. Int. 64; 569–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jin C., Liang M., Ning J., Gu W., Jiang H., Wu W., Zhang F., Li C., Zhang Q., Zhu H., Chen T., Han Y., Zhang W., Zhang S., Wang Q., Sun L., Liu Q., Li J., Wang T., Wei Q., Wang S., Deng Y., Qin C. and Li D. (2012) Pathogenesis of emerging severe fever with thrombocytopenia syndrome virus in C57/BL6 mouse model. Proc. Natl. Acad. Sci. U S A 109; 10053–10058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kuramoto H., Iwami Y., Sugimoto N., Kato C., Sugahara T. and Iida M. (2012) Application of a new liquid-based procedure (TACAS) for the screening of cervical cancer: a preliminary study. Acta Cytol. 56; 74–79. [DOI] [PubMed] [Google Scholar]
- 5.Liu S., Chai C., Wang C., Amer S., Lv H., He H., Sun J. and Lin J. (2014) Systematic review of severe fever with thrombocytopenia syndrome: virology, epidemiology, and clinical characteristics. Rev. Med. Virol. 24; 90–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nakajima N., Ionescu P., Sato Y., Hashimoto M., Kuroita T., Takahashi H., Yoshikura H. and Sata T. (2003) In situ hybridization AT-tailing with catalyzed signal amplification for sensitive and specific in situ detection of human immunodeficiency virus-1 mRNA in formalin-fixed and paraffin-embedded tissues. Am. J. Pathol. 162; 381–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Park S-W., Han M-G., Yun S-M., Park C., Lee W-J. and Ryou J (2014) Severe fever with thrombocytopenia syndrome virus, South Korea, 2013. Emerg. Infect. Dis. 20; 1880–1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Takahashi T., Maeda K., Suzuki T., Ishido A., Shigeoka T., Tominaga T., Kamei T., Honda M., Ninomiya D., Sakai T., Senba T., Kaneyuki S., Sakaguchi S., Satoh A., Hosokawa T., Kawabe Y., Kurihara S., Izumikawa K., Kohno S., Azuma T., Suemori K., Yasukawa M., Mizutani T., Omatsu T., Katayama Y., Miyahara M., Ijuin M., Doi K., Okuda M., Umeki K., Saito T., Fukushima K., Nakajima K., Yoshikawa T., Tani H., Fukushi S., Fukuma A., Ogata M., Shimojima M., Nakajima N., Nagata N., Katano H., Fukumoto H., Sato Y., Hasegawa H., Yamagishi T., Oishi K., Kurane I., Morikawa S. and Saijo M. (2014) The first identification and retrospective study of Severe Fever with Thrombocytopenia Syndrome in Japan. J. Infect. Dis. 209; 816–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tsutsumi Y., Kawai K., Hori S. and Osamura R. Y. (1991) Ultrastructural visualization of human papillomavirus DNA in verrucous and precancerous squamous lesions. Acta Pathol. Jpn. 41; 757–762. [DOI] [PubMed] [Google Scholar]
- 10.Tsutsumi Y. (1994) Application of the immunoperoxidase method for histopathological diagnosis of infectious diseases. Acta Histochem. Cytochem. 27; 547–560. [Google Scholar]
- 11.Xu B., Liu L., Huang X., Ma H., Zhang Y., Du Y., Wang P., Tang X., Wang H., Kang K., Zhang S., Zhao G., Wu W., Yang Y., Chen H., Mu F. and Chen W. (2011) Metagenomic analysis of fever, thrombocytopenia and leukopenia syndrome (FTLS) in Henan Province. China: discovery of a new bunyavirus. PLoS. Pathog. 7; e1002369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yu X. J., Liang M. F., Zhang S. Y., Liu Y., Li J. D., Sun Y. L., Zhang L., Zhang Q. F., Popov V. L., Li C., Qu J., Li Q., Zhang Y. P., Hai R., Wu W., Wang Q., Zhan F. X., Wang X. J., Kan B., Wang S. W., Wan K. L., Jing H. Q., Lu J. X., Yin W. W., Zhou H., Guan X. H., Liu J. F., Bi Z. Q., Liu G. H., Ren J., Wang H., Zhao Z., Song J. D., He J. R., Wan T., Zhang J. S., Fu X. P., Sun L. N., Dong X. P., Feng Z. J., Yang W. Z., Hong T., Zhang Y., Walker D. H., Wang Y. and Li D. X. (2011) Fever with thrombocytopenia associated with a novel bunyavirus in China. N. Engl. J. Med. 364; 1523–1532. [DOI] [PMC free article] [PubMed] [Google Scholar]