Abstract
Evolutionary developmental biology (evo-devo) has provided invaluable contributions to our understanding of the mechanistic relationship between genotypic and phenotypic change. Similarly, evolutionary ecology has greatly advanced our understanding of the relationship between the phenotype and the environment. To fully understand the evolution of organismal diversity, a thorough integration of these two fields is required. This integration remains highly challenging because model systems offering a rich ecological and evolutionary background, together with the availability of developmental genetic tools and genomic resources, are scarce. In this review, we introduce the semi-aquatic bugs (Gerromorpha, Heteroptera) as original models well suited to study why and how organisms diversify. The Gerromorpha invaded water surfaces over 200 mya and diversified into a range of remarkable new forms within this new ecological habitat. We summarize the biology and evolutionary history of this group of insects and highlight a set of characters associated with the habitat change and the diversification that followed. We further discuss the morphological, behavioral, molecular and genomic tools available that together make semi-aquatic bugs a prime model for integration across disciplines. We present case studies showing how the implementation and combination of these approaches can advance our understanding of how the interaction between genotypes, phenotypes and the environment drives the evolution of distinct morphologies. Finally, we explain how the same set of experimental designs can be applied in other systems to address similar biological questions.
Keywords: semi-aquatic bugs, evo-devo, evolutionary ecology, multidisciplinarity, functional genomics
Introduction
Understanding how and why organisms diversify is a major goal in evolutionary biology [1–4]. To understand organismal diversification, we need to dissect how the genotype encodes the phenotype (morphology, physiology and behavior) during development, how changes in the genotype are associated with changes in the phenotype and how the environment shapes the phenotype throughout evolution. Therefore, a strong integration of evolutionary developmental biology (Evo-devo) with evolutionary ecology is required if we want to gain a comprehensive and thorough understanding of the origin of organismal diversity [5–7].
Evo-devo has contributed greatly to our understanding of the developmental genetic mechanisms underlying phenotypic evolution. Among the major achievements of evo-devo was the discovery of Hox genes and the striking extent of their conservation [8]. This, together with the discovery that gene composition in distant taxa is largely similar, led to the now established paradigm that the same genetic toolkit is reused throughout evolution [2]. This was paradoxical with the observation that there are ‘endless forms most beautiful’ in nature, but it is now established that new developmental programs can emerge largely through co-option of preexisting regulatory gene networks via changes in how they are regulated and deployed (‘old genes play new tricks’) [9]. These lines of research provided great insights into the construction of a genotype–phenotype map, leading to the emergence of a set of useful concepts. Among these are the optimal pleiotropy and modularity concepts. Evo-devo studies established that because of optimal pleiotropy, the gain or loss of a trait will likely proceed by the co-option of a local regulator that responds to global cues and controls a battery of effector genes, rather than a major upstream regulator or multiple downstream effectors [10–13]. However, this does not exclude the possibility that evolution may co-opt pleiotropic regulators in some contexts [14, 15]. Also thanks to evo-devo studies, we now know that there is a strong modularity in the genotype–phenotype relationship such that organisms are made of sub-components that are interdependent yet can evolve as independent modules [16–18]. Although evo-devo has made tremendous contributions to our understanding of the genotype–phenotype map, we do not wish to state that everything is explained. For example, recently, there has been accumulating evidence that new lineage-specific genes can play a role in the development and evolution of morphological structures [19–21], meaning that both the co-option of old genes together with the emergence of new genes and regulatory modules might underlie the evolution of phenotypic variability.
One of the major aspects of evolutionary theory that evo-devo failed to integrate so far is the link between developmental genetic mechanisms and the evolutionary history of the phenotype [10, 11, 22, 23]. A key challenge for the next years to come is how to connect these developmental changes, ultimately, to organismal fitness and ecology. Two major difficulties have been hindering such integration efforts. First is the paucity of ecological model organisms where we can successfully apply state-of-the-art tools of molecular and gene function analyses. Model systems that can offer a rich ecological and evolutionary foundation combined with the tractability to developmental genetic studies are desperately needed. The increase in model species will be helpful not only to elucidate the mechanisms that are specific to particular taxa but also to pinpoint which of these principles can be generalizable for a better understanding of the evolutionary process as a whole [5]. Richer and more accurate lessons can be learned from comparative approaches of phenotypic evolution across a spectrum of species. The second challenge of marrying natural systems with developmental genetics has been, until recently, the difficulty of generating genomic resources. The emergence and democratization of next-generation sequencing technologies provides an invaluable opportunity for large-scale unbiased molecular genetic studies in nonstandard systems. It is now possible to have transcriptome and genome sequences of natural systems where the ecology is well understood [24, 25].
Toward an integrative approach
In this review, we show examples of some successful case studies with varied model systems that integrated across the scales. We introduce the semi-aquatic bugs as a set of natural model organisms that offer both a rich ecological and evolutionary context, along with the amenability to a spectrum of standard tools for functional studies [14, 26–28]. We also expose a set of experimental designs that can help integrate developmental and evolutionary genetics with ecology. We show how such approaches can be generalized to other systems to help understand how the environment, phenotype and genotype interact to generate the remarkable diversity of organismal forms.
The bony armor of three-Spine sticklebacks
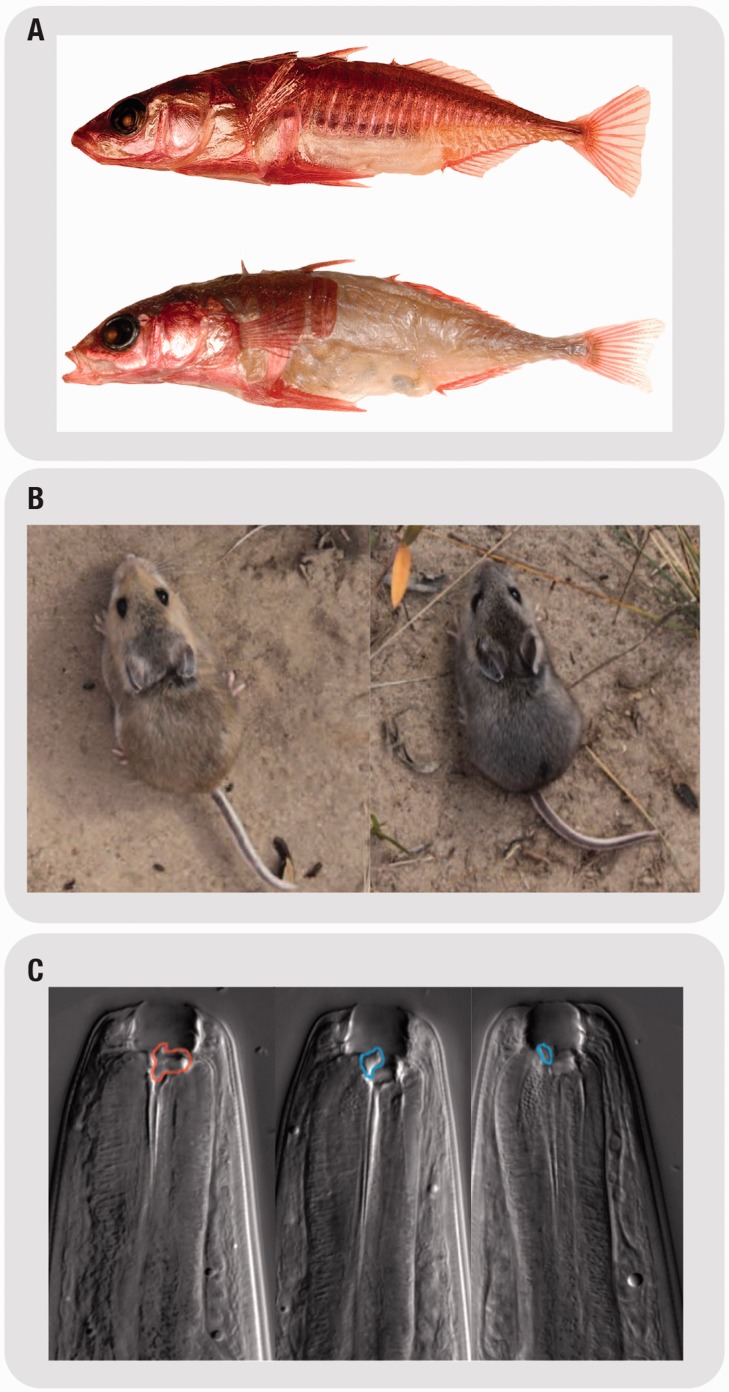
Marine three-spine sticklebacks have repeatedly invaded new freshwater environments, which resulted in the reduction of the extensive bony armor found in the marine ancestor (Figure 1A). The loss of armor in fresh water populations is mapped down to the Ectodysplasin (EDA) signaling pathway [32]. This was done through a combination of quantitative trait loci mapping, sequencing and transgenic experiments. However, this is only part of the story, and understanding the adaptive significance of armor morphology is important to understanding phenotypic diversification. Further experimental tests were performed to connect the phenotype to the underlying environmental pressures. Barret and colleagues conducted a common garden transplantation experiment where they introduced marine sticklebacks, with heavy armor, into freshwater ponds. Strikingly after one generation, the fresh water morphology, with reduced armor plate, dominated the pond. This was the result of a rapid spread of the EDA locus containing the allele responsible for armor reduction [29]. Reduced armor is thought to be connected to increased growth rate and lower risk of predation, although this conclusion awaits more solid experimental support (Figure 1A). Interestingly, there was also an unexpected strong deleterious effect of the low-plated allele during early stages of development. This low-plated allele only increased in frequency after the lateral plates have been formed. This is a powerful example of antagonistic pleiotropy where one allele has differential effects in different fitness relevant traits, reinforcing the conclusion that fitness effects must be tested rather than assumed. By combining developmental genetics tools with tests of fitness in the field, this case study represents an example of a firm demonstration of how changes in developmental genetic pathways can be associated with the emergence of adaptive phenotypes in nature.
Figure 1.
Model systems where the integration between ecology and evo-devo was successful. (A) Sticklebacks: On top is the full-plated marine morph, bellow is the low-plated morph. Adapted from [29]. (B) Deer mice: on the left is the lighter color morph, on the right it is the darker prairie morph; both morphs are standing on a sandy background, the lighter morph blends in with the background while the dark phenotype is more conspicuous. Adapted from [30]. (C) Pristionchus: On the left is the toothless morph (red circle) whereas on the middle and right images predatory morph showing teeth-like structures (blue circles), either with right or left symmetry. Adapted from [31].
Developmental genetic and adaptive basis of color pattern in deer mice
The Sand Hills of Nebraska are characterized by clear-colored sand in contrast to the darker surrounding prairies (Figure 1B). Mice found in the sand hills have a lighter coat, and mice found in prairies have darker coat. The match between coat color and the substrate the mice live on is thought to be a cryptic adaptation to predation by hovering hawks [33]. The acquisition of light-colored coat, as an adaptation to the sandy hills, is largely driven by a cis-regulatory mutation in the Agouti locus [34, 35]. This genetic change led to an increase in the level and area of expression of Agouti across the skin of the mouse, preventing melanocyte maturation and resulting in the development of a lighter coat. This group of researchers went further to test the fitness of the different colored morphs (using plasticine models in the field), and showed that the light coat color of deer mice, which recently colonized the light-colored soil, provides a strong selective advantage against visually hunting predators [30]. This type of approach, which integrates developmental and quantitative genetics with field experiments and test of fitness, has been quite successful in pinpointing the genetic changes underlying the emergence of a derived trait and at the same time, connect this trait to the specific selective pressure that shape it in nature.
Evolution of adaptive mouth morphology in the worm Pristionchus
The nematode Pristionchus pacificus shows two alternative mouth morphologies, one of them associated with bacteriovorous feeding and the other showing novel teeth-like structures associated with predatory behavior, sometimes on conspecifics [36] (Figure 1C). This example of polyphenism, where distinct morphs are produced through the same genotype, is associated with nutritional resources, whereby scarcity of bacteria induces the development of the predatory morph [31]. Further comparative analyses across different populations and phylogenetically distinct species led to the identification of the eud-1 gene that acts as a developmental switch for the formation of the predatory morphs. EUD-1 action is dose dependent and is necessary and sufficient to control the dimorphism of feeding forms [37]. These two morphs coexist in P. pacificus but can become genetically fixed through genetic accommodation in different species (high expression versus low expression of eud-1). EUD-1 emerged following a lineage-specific duplication characteristic to Pristionchus, and executes a developmental switch for morphological plasticity in the adult stage, showing that regulatory pathways can evolve by terminal addition of new genes [37]. This is another example of how developmental mechanisms can generate adaptive phenotypes that can be favored by natural selection.
These examples demonstrate that it is possible to address the question of the origin of organismal diversity both from developmental and ecological perspectives. The conclusions reached through such approaches are more comprehensive, as they inform us about how developmental genetic pathways can generate the phenotype, and how selection can act on such phenotypes in nature.
Semi-aquatic bugs as natural models for integrating developmental genetics with ecology
Semi-aquatic bugs (Heteroptera, Gerromorpha) are a well-characterized model organism in behavior [28], biophysics (hydrodynamics of water surface locomotion [39–41]), evolutionary ecology [42], and are an emerging model for developmental genetics [14, 26–28]. This multitude of topics makes them an ideal model system for integrative research toward understanding why and how organisms diversify, offering the opportunity for integrating across disciplines, from genome organization through genes, molecular and developmental mechanisms to phenotypic and ecological effects. The phenotypes available in nature are not always adaptive; some of the traits might be neutral and emerge via drift, and some others might be present due to developmental constraints and pleiotropy, so that selection on one trait causes the evolution of another. Further studying these types of traits is of paramount importance if we want to have a general theory for the evolution of development [9, 43]. In this review, though, we will focus on the examples available so far for the model focusing on traits that are adaptive.
Biology and evolutionary history of the semi-aquatic bugs
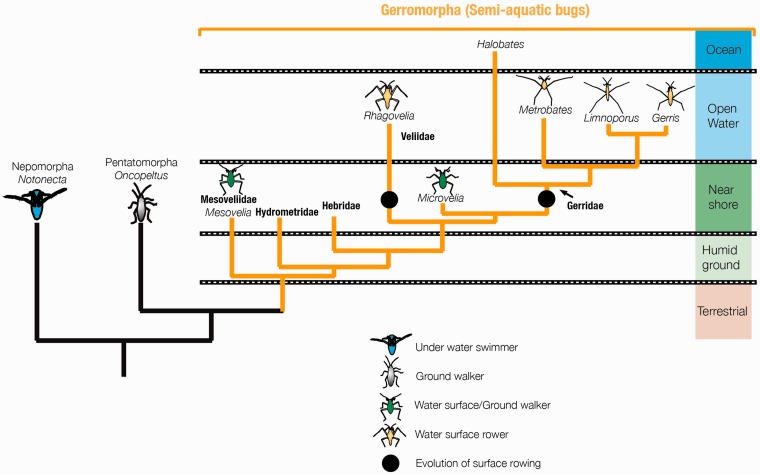
The semi-aquatic bugs have long fascinated scientists with their seemingly effortless ability to move on water surfaces. They have conquered water surfaces worldwide including the open oceans, and represent by far the group of insects the most dominating in these habitats (Figure 2) [44]. It is thought that this habitat diversification occurred in a stepwise manner, where the ancestors of the group occupied solid wet substrate, followed by the invasion of the shore–water intersection, and finally there was the diversification into the open-water habitats. Basally branching lineages occupy transitional zones and can walk both on water and land, while derived lineages have specialized in open-water zones and propel their body by means of surface rowing (Gerris, Limnoporus, Rhagovelia and Metrobates) [40, 41] (Figure 2).
Figure 2.
The common ancestor of semi-aquatic bugs invaded the water surface and diversified into a range of new forms within this previously unexploited ecological niche. Within the Gerromorpha, there are several groups with differing water-walking phenotypes. The Mesoveliidae (Mesovelia) and most of the Vellidae (Microvelia) walk both on land and water, whereas some Veliidae (Rhagovelia) and all the Gerridae specialize in water surface rowing and are unable to walk on land.
The transition to water surface habitats required the ability of these insects to support their body weight on water and to overcome the constraints imposed by the hydrodynamics of the fluid substrate. The evolution of water-repellent bristles and the diversification in the leg morphologies (length, shape and allometry) are two important events that were critical to the water surface invasion and are associated with the stepwise habitat transitions [39, 41, 44].
The bristles of water-walking bugs, owing to their specific size, arrangement and distribution, act as nonwetting structures capable of exploiting water surface tension by trapping air between the leg and water surface [39, 41, 45]. The evolution of water repellency and the modifications in bristles morphology throughout the evolution of semi-aquatic bugs shows how tinkering with preexisting traits can have a spectacular impact on the ecology and adaptation of natural populations and can fuel species diversification.
The diversification into different water surface niches is associated with modifications in the morphology of locomotory appendages, which represent some of the most diversified traits in the group. Derived lineages, which have specialized exclusively in water-surface locomotion through rowing (all Gerridae and some Veliidae), have long legs compared with their basally branching relatives, but they also evolved longer mid-legs relative to rear-legs. This derived leg plan enabled the evolution of rowing as a novel mode of locomotion on the fluid. The mid-legs function as propelling oars and the rear-legs function as steering rudders. These modifications in the morphology and the function of the legs enabled these animals to generate efficient propulsion on the water–air interface, and have been key to their diversification [40, 41, 44].
These morphological traits are easily quantifiable. Measurements with appropriate statistical tools can become a foundation for further studies, such as association mapping, comparative developmental analyses, genetic screens of functional tests and fitness assays. Characterizing the specific genetic changes that underlie the emergence and diversification of these traits together with the description of the selective pressures acting on them is key to understanding why and how semi-aquatic bugs diversified.
Genomic resources
One of the most exciting technological advances that made research on natural systems much more accessible compared with a decade ago is the emergence of next-generation sequencing [24]. Genomes are now more affordable and international efforts for the sequencing of thousands of genomes are underway. The first genome of a water strider, Gerris buenoi, has been sequenced and assembled thanks to the i5K community effort, and the manual annotation of this genome is underway [46, 47]. We also can afford generating and annotating whole transcriptomes in many species, allowing access to genetic sequence resources and freeing researchers from the daunting task of cloning individual genes. These genomic resources are of great importance because we can readily explore coding and noncoding sequences to generate candidate genes. Transcriptome-scale techniques are rapidly developing (e.g. RNAseq) that allow for groups of genes, genetic pathways or variation in gene expression to be connected to tissue- and species-specific phenotypes [48]. As for genome-scale techniques, association mapping approaches that correlate genomic variation with phenotype variation allow for the discovery of regions under selection and candidate genes underlying the emergence and diversification of these traits, paving the way for further studies of their function [49].
Connecting the genotype to the phenotype
Integrating evo-devo with ecology requires model systems that allow for tests of gene expression and function from the one hand, and for tests of the function of the trait on the other hand. These tests will connect the phenotype to its developmental and genetic basis, identify changes in developmental genetic processes underlying evolutionary changes in the phenotype and finally link the phenotype to its ecological and fitness value.
A first step is to identify candidate genes that are potentially required for shaping the phenotype during development. This can be done on a focal species to establish the genotype–phenotype relationship before expanding the analysis across species. Next-generation sequencing allows us to conduct comparative transcriptomics analyses (between tissues and/or developmental stages) or genome association mapping (between the trait of interest and genomic variability) to identify candidate genes and genetic pathways underlying the phenotype of interest.
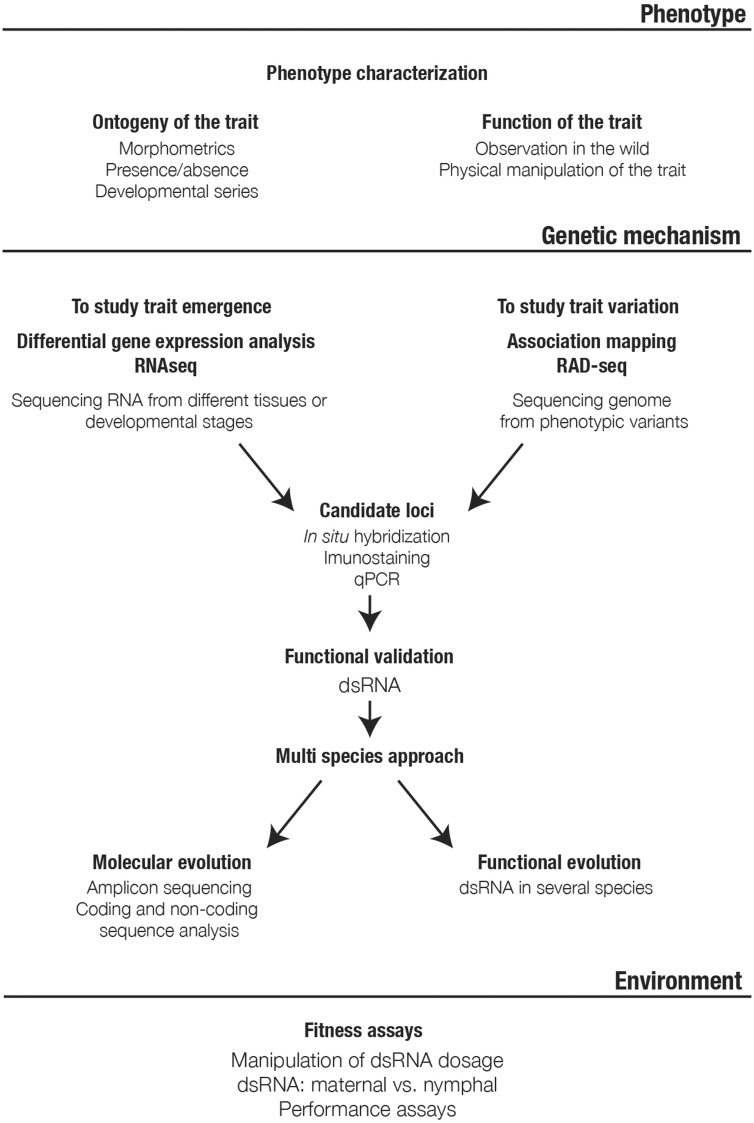
These methods result in a list, often a long one, of genes that are potentially important for the development of the structure of interest. Screens of gene expression, either in situ hybridization or through quantitative methods such as PCR, can help narrow this list down to those genes with experimentally validated levels and patterns of expression that match the phenotype (Figure 3). Finally, tests of genes function, using techniques of gene knockdown through RNA interference, allow an accurate description of the role of genes in shaping the phenotype during development [14, 26–28]. When the trait is shaped early during embryogenesis, analyses of the role of the candidate genes underlying their development is conducted using parental RNAi (Figure 4). This timing-directed application of the technique is achieved through injection of adult females with a solution of double-stranded RNA artificially synthesized based on the sequence of the gene of interest (see [14, 26–28] for methods), which is transferred to the developing oocytes in the ovary. The associated modifications to the phenotype are scored in the embryonic progeny of the injected females [14, 26–28]. This technique is greatly optimized in semi-aquatic bugs such that we can perform a ‘one-female-one-gene’ rapid screen of dozens of genes with a good efficiency (Figure 4). When the trait of interest is shaped later during ontogeny, namely during nymphal development, nymphal RNAi is a more appropriate approach [14]. This late application of the technique allows the normal development of other processes and singles out the timing relevant to the trait of interest.
Figure 3.
Experimental design workflow that allows us to connect genotype, phenotype and environment.
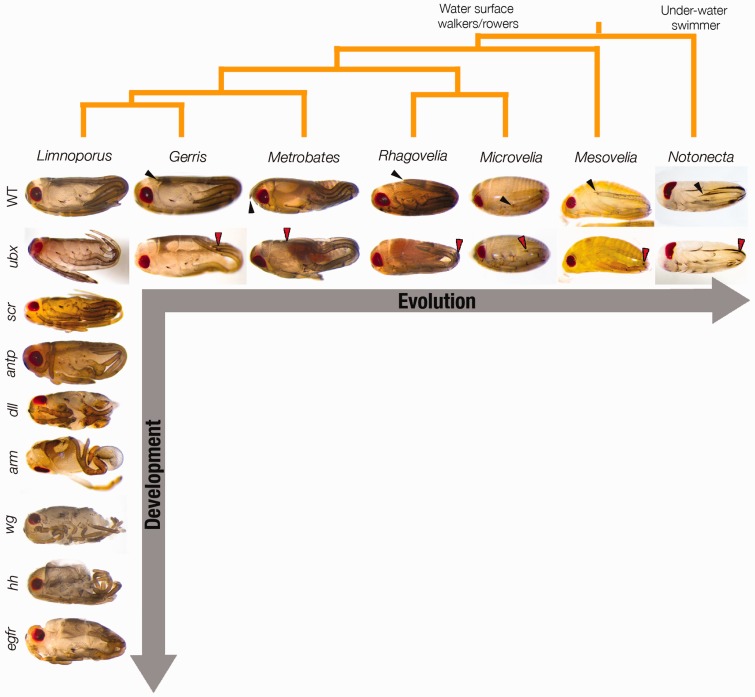
Figure 4.
RNAi screen of multiple developmental genes (Hox genes, signaling molecules and transcription factors) allows uncovering gene function during development within the same species. RNAi analyses across multiple semi-aquatic bugs and outgroups (Notonecta) helps link changes in the function of the same gene to changes in the associated phenotypes across these species. Even when the phenotype is spectacular (e. g. arm (armadillo), dpp (decapentaplegic)), embryos reach the end of development, allowing a detailed description of gene function. (A colour version of this figure is available online at: http://bfg.oxfordjournals.org)
Connecting changes in the genotype to changes in the phenotype
A broad sampling and comparative analysis across species is important to describing general evolutionary mechanisms [5]. In the Gerromorpha, we have been able to establish developmental genetics tools across a number of species and outgroups (Figure 4) [14, 26–28]. This allows us to go beyond a single species analysis and reconstruct differences in gene expression and function across all these species, and associate these genetic differences with evolutionary modifications of the phenotype. The study of the genetic and developmental basis of the traits that facilitated water surface invasion in the different Gerromorpha species (Figures 3 and 4) will show what are the genetic changes that underlie the evolution of these traits throughout the diversification of the group. Studies of gene expression and function coupled with studies of protein (coding) and regulatory (noncoding) sequence of the genes in the different species will allow for correlations between genetic changes with important evolutionary events for the diversification and adaptation of the group. These types of studies will provide historical insights into the process of evolution by identifying when, and in which lineage (species), gene function changed and its impact on the phenotype [50]. By using this approach, we will be able to find the genes and/or pathways underlying the development of the trait and then be able to gain insight into the phenotype-coincident and phenotype-causative changes. The semi-aquatic bugs are all the more interesting for this type of approach, as we can manipulate genes across many species and at various developmental stages (Figure 4).
Tests of traits function
To integrate evo-devo with ecology, one of the most important criteria for the choice of a model system is its ability to provide a strong link to the selective forces that shape the phenotype in nature [6]. The semi-aquatic bugs are an example of such a model, exhibiting a large array of phenotypes that are tightly associated with life on the water surface [44]. A key entry to understanding the fitness value of such phenotypes is through testing their importance to the biology of the organism as a whole. Close observations in the field coupled with behavioral tests in laboratory setting can represent simple, yet powerful methods to uncovering the role of specific traits (Figure 3).
An example of a striking adaptation in water striders is the departure from the common ancestral relative leg length where the hind-legs are the longest, to a derived morphology where the mid-legs are now the longest [44]. This morphology is associated with the evolution of rowing as a novel mode of locomotion on the fluid but also enables some striking interactions either between the sexes or competition between males, or in relation to predation [39, 41, 51]. Observations in the wild revealed that sexual selection favors males with longer legs in the large water strider Aquarius enlongatus [51]. The males are territorial and guard egg-laying sites while fighting and chasing other males away. Larger males with longer legs often dominate and call fertile females to the egg-laying site by producing ripples on the water surface [51, 52]. Another prominent function of reversed leg length is that it confers the ability to jump high in the air in response to predation attempts coming from underneath the water surface [41, 44].
The basic understanding of the functions of the traits and how it impacts the interaction of organisms with their environment allows the identification of gene functions that affect not only the phenotype but also which genes matter the most in a given ecological interaction. This understanding in turn offers a deeper understanding of the role and nature of the selective forces shaping the phenotype. Detailed morphological and behavioral characterization can help determine whether the trait evolved neutrally through drift, conferring no advantage to the organism, or whether its evolution is correlated with selection on another trait (developmental constraint), or if indeed the trait confers fitness benefits to the organism.
More elaborate ways of studying trait function can then follow. Trait manipulation together with behavioral tests after the manipulation can provide insight into the importance of the trait for the biology of the animal and can inform about the fitness value acquired through the evolution of the trait. Manipulation can be achieved by simple physical interference with its operation, provided that it does not affect other essential parts of the body. For example, Rowe and colleagues tied the antennae of males of the water strider Rheumatobates rileyi to test their role in male–females interaction before copulation. They found that when they tie the antennae together, males can no longer grasp the females during pre-mating struggles and fail to mate [53]. This is an example of simple, yet informative approach to manipulate trait function. However, although this method informs the function of the trait, it does not say much about the genetic mechanisms that shape this trait.
A more informative way about trait function and the role of developmental genetic mechanisms in shaping the trait is through genetic manipulation [14]. This is only possible if the system allows for manipulation of gene function, either through simple or sophisticated tools (Figures 3 and 4). One of the most prominent obstacles to this method, which we believe discourages the evo-devo community from applying it widely, is the pleiotropic nature of many developmental genes [54, 55]. Although techniques such as RNAi are specific to the target gene, this specificity fails in discriminating between distinct tissues and developmental stages and may lead to the disruption of other potential functions of the same genes that are not associated with the trait under study. However, there are many ways that can allow overcoming pleiotropy and manipulating traits, through RNAi, for example, without major consequences on other essential functions of the organism. This tool, although sometimes difficult, is applicable in many contexts and can yield important insights into trait evolution [14]. One aspect of the biology of the semi-aquatic bugs which is helpful in overcoming this problem, is the nature of their development, which proceeds directly through molts without metamorphosis. The most essential structures are established at the end of embryogenesis, and the hatched first-instar nymphs resemble adults to a great extent. Interestingly, a large number of traits are shaped during nymphal development, and therefore allow for trait manipulation, sometimes through targeting highly pleiotropic genes, without disrupting other essential functions [14]. This is done using nymphal RNAi, which only affects postembryonic processes. Adults emerging from such manipulations are viable, show trait reduction and can be subjected to tests of performance guided by the preestablished basic knowledge of trait function (see example below).
Two examples of integrative case studies in the semi-aquatic bugs
In the previous section of this review, we highlight a number of resources and tools that are key to merging evolutionary developmental biology with evolutionary ecology using semi-aquatic bugs as model system. Here, we present two case studies showing how the implementation and combination of these approaches can advance our understanding of how the interaction between genotypes, phenotypes and the environment drives the evolution of distinct morphologies.
Diversification of the leg plan during the diversification of the Gerromorpha
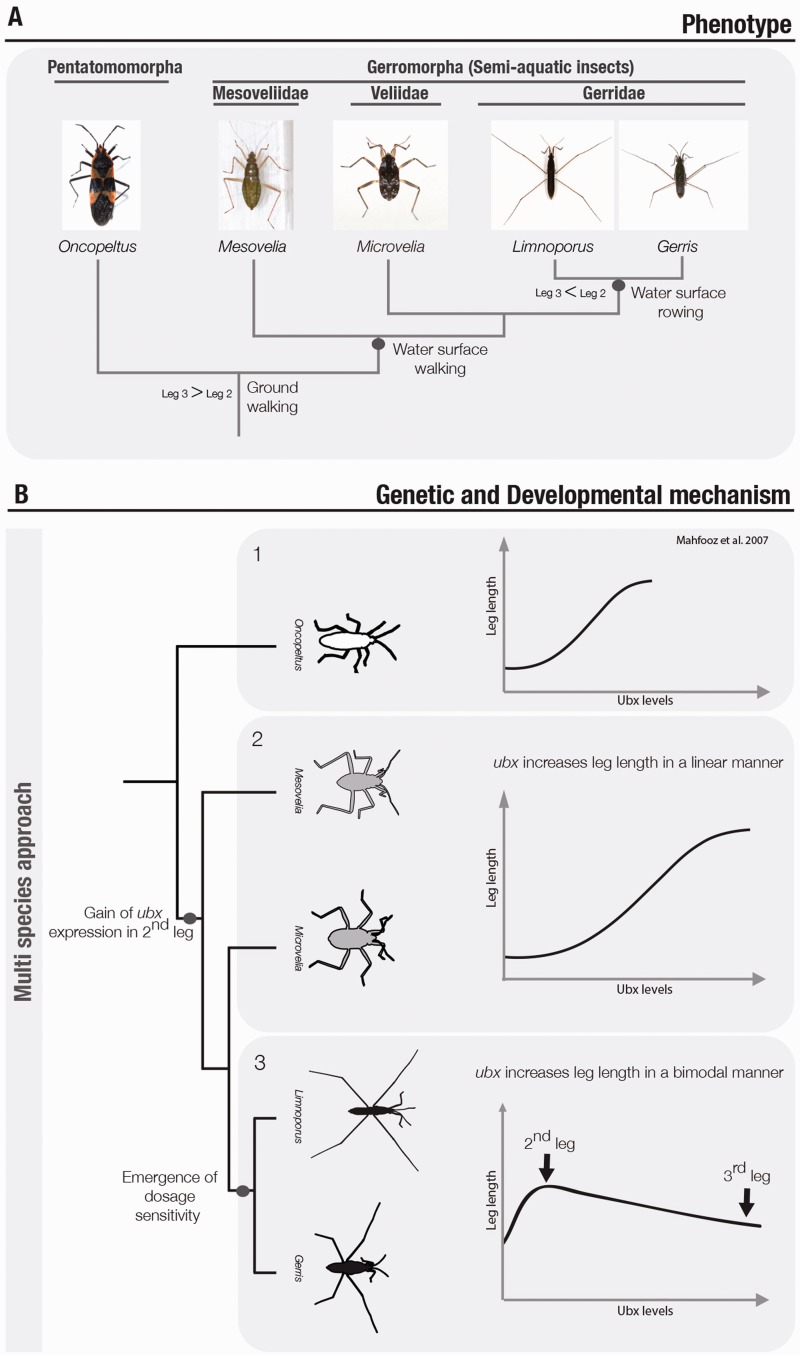
Invasion of water surfaces required new modes of locomotion (walking and rowing on the water surface), which are associated with the diversification of leg morphologies (Figure 5A). The environmental transition is associated with gradual morphological changes and the transition from walking to rowing. The most basally branching group the Mesoveliidae are water walkers (near shore and land–water interface). Veliidae, a paraphyletic group, includes both water walkers and rowers (on the open water). Water walkers maintain the ancestral appendage plan where the mid-leg is shorter than the hind-leg (L2<L3) whereas water rowers show a derived leg plan where the mid-leg is longer then the hind-leg (L2>L3). This derived leg plan is thought to have evolved multiple independent times in the Veliidae lineage and once in the ancestor of Gerridae.
Figure 5.
Diversification of the leg plan during Gerromorpha diversification. (A) Schematic phylogeny of the species used in Khila et al. 2014. Mesovellidae and most Vellidae are water walkers while all Gerridae are water rowers. Water walkers show an increase in leg length relative to body size when compared with the terrestrial outgroups. Water rowers show an increase in relative leg length but also a reversal in leg-length where the mid-leg is longer than the hind-leg. (B) Two evolutionary steps in Ubx function and regulation were mapped to the nearshore–water interface and to the open-water surface transitions. (1) In terrestrial species Ubx is expressed only in the hind-leg and its function is to lengthen it. (2) The first change is the Ubx gain of expression in the mid-leg of the ancestor of water walkers, which led to the increase in length of both legs. (3) Then, tissue sensitiveness to Ubx levels emerged in the ancestor of the Gerridae, where high or low levels of Ubx result in shorter leg (like the case of hind-leg), whereas moderate levels result in a longer leg (case of mid-leg). The X-axis represents Ubx intraspecific levels of expression.
In terrestrial insects, the activity of the Hox gene Ultrabithorax (Ubx) is restricted to the third thoracic segment and functions to lengthen the rear legs that this segment bears. Ubx expression and function was analyzed in six representative species of semi-aquatic bugs. While Ubx expression and function in rear legs is conserved between terrestrial and aquatic bugs, a novel domain of expression in the mid-legs evolved in the common ancestor of all semi-aquatic bugs (Figure 5B). This gain of expression has different phenotypic effects in water walkers and water rowers. In water walkers, Ubx lengthens both rear legs and mid-legs, whereas in water rowers, Ubx shortens the rear-legs and lengthens the mid-legs [26, 28]. This opposing response of leg tissue to Ubx is mediated through bimodal response to Ubx protein levels in water rowers, whereby high levels of Ubx slow down leg growth and low levels promote leg growth [27] (Figure 5B). Importantly, this bimodal response did not evolve in the water walkers despite the preexisting differences in Ubx levels between mid- and hind-legs. This is a demonstration that tinkering with the levels of protein expression and the emergence of tissue sensitivity to these levels can drive adaptive phenotypic change [27] (Figure 5B). This multi-species approach has been instrumental in mapping changes in Ubx expression and function across the entire phylogeny of the group, and associated these changes with the ecological transition to the nearshore–water interface and to the open-water surface.
Sexual antagonism and the evolution of sex-specific traits
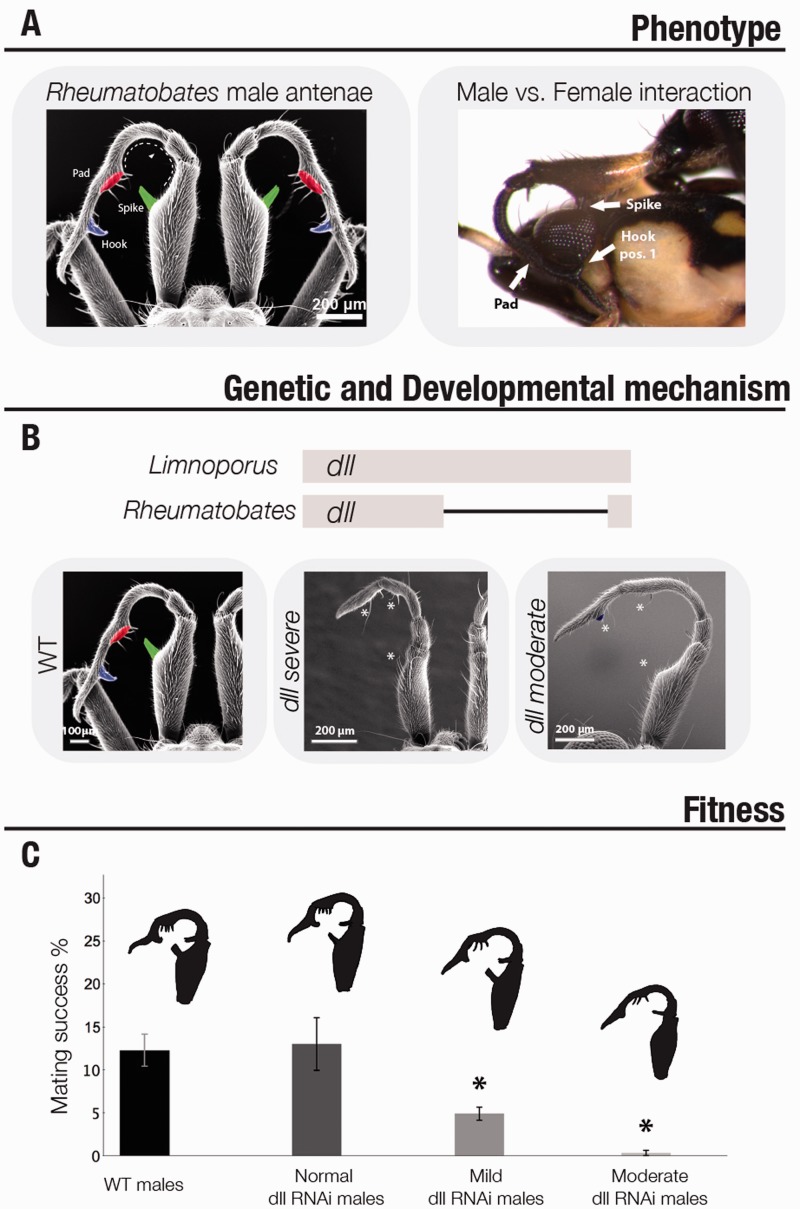
Mating systems in many water striders are characterized by conflict between the sexes, whereby males gain in fitness with increased mating frequency whereas females pay fitness costs [38]. This divergence in interest between the sexes results in the evolution of adaptations and counter adaptations that increase sexual dimorphisms. In Rheumatobates rileyi, females resist to costly mating through vigorous struggles aiming at rejecting male mating attempts. Males on the other hand evolved morphological structures that increase their mating success, such as modified antennae acting as grasping structures [53] (Figure 6A). Modifications of male antennae into grasping structures appear during the 4th and 5th nymphal instars. RNAseq allowed the identification of the homeodomain-containing protein Distal-less (Dll) as a potential gene underlying the modifications observed in the male antennae [14] (Figure 6B). RNAi knock-down resulted in the removal or reduction of a set of specific traits that distinguish male antennae from those of females, whereas the same treatment had no effect on female antennae (Figure 6B). A comparative analysis in other water striders where male antennae are not modified revealed that Dll was co-opted in Rheumatobates during late nymphal development to modify antenna into grasping structures. In this example, understanding the basic role of male antennae in grasping females for mating, and uncovering a gene responsible for shaping this phenotype provided the opportunity for genetic manipulation. Although Dll is known for its essential role in specifying appendages early in the embryo, we were able to overcome this pleiotropic role by directing RNAi injection at later developmental stages. RNAi phenotypes, which ranged from normal to severe, resulted in viable adult males with reduced modifications of the antennae (Figure 6B). Mating tests using the different classes of males (normal, mild and severe) revealed that reduction of grasping structures resulted in a corresponding reduction in mating success (Figure 6C). This demonstrates selection on male antennae to become modified and the role of Dll in shaping these modifications [14]. These results are a true example of integration between fields, linking an evolutionary change in morphology, the fitness advantage of this change and its underlying genetic basis.
Figure 6.
Evolution of Rheumatobates antennal appendages. (A) The male modified antennae are shown on the left. The novel structures are shown in green (spike), red (pad) and purple (hook). These modifications act as female grasping structures as depicted on the right image. (B) Rheumatobates species show a deletion in the distal-less gene. Nymphal RNAi knock-down of this gene leads to the loss of these modifications in males. RNAi phenotypes, which ranged from normal to severe, resulted in viable adult males with reduced modifications of the antennae. (C) Performance tests using the different classes of RNAi males (normal, mild and severe) revealed that reduction of grasping structures resulted in a corresponding reduction in mating success.
Expanding this integrative approach to other systems
With the available tools and approaches summarized above, we have been able to address some important biological questions using the semi-aquatic bugs as models. However, we believe that similar approaches can be applied in other organisms, both in traditional developmental genetics systems (e.g. flies, mice) and nonstandard model systems (e.g. guppies, beetles). With the development of next-generation sequencing tools together with the realization that more emphasis on the environment is needed in evo-devo studies, it will be possible to move classical developmental models toward ecology and vice versa.
Classical laboratory model organisms are often chosen because they allow the development of powerful molecular tools. The main limit is that the ecological and the evolutionary history of a specific trait are often unknown or ignored. Specially in these systems where a plethora of functional and transgenic tools are available to genetically engineer individuals, morphological and behavioral studies are necessary to uncover the functional and ecological basis of a trait. Drosophila trichomes and denticles, resulting from the extension of epidermal cells, are classical examples that contributed greatly to our understanding of the developmental mechanisms of phenotypic variation [56, 57]. These patterns are highly different between species, and the genetic mechanisms underlying this interspecific variation are changes in the cis-regulation of the shavenbaby (svb) gene [56, 57]. Unfortunately, until today nobody assessed the function of these trichomes; therefore, we do not know if these cis-regulatory changes had an impact on the evolution of the group. In this particular example, the community can build on the detailed knowledge accumulated throughout the years about the developmental mechanisms underlying trichome formation and diversification. Field observations could provide important insight into differences in trichome patterns and species specificities in terms of biology, reproduction, food and habitat preference and egg-laying behavior. With a wide range of genetic tools available in flies, trait manipulation should be accessible in a more specific and controlled manner. This should allow a good test of why closely related flies exhibit clear differences in the patterns of these structures. Doing more detailed morphological and behavioral analysis will also help to decipher if this trait evolved neutrally, if its change is correlated with selection on another trait, or if it confers fitness benefits to the larvae. This set of approaches should be helpful in understanding how the building of these structures matters for organismal biology, and therefore bring a more complete insight into species diversification.
In natural systems, a primary difficulty to exporting more sophisticated tools in their integrity, such as transgenesis and genome editing, to a wide range of organisms resides in the endless differences in the specificities of each model system. However, some lineages, although difficult as laboratory models, are indispensible to address specific evolutionary questions that are not applicable to standard models. A prominent example is the evolution of eusociality that characterizes all ants [58]. The sophisticated tools established in powerful models are difficult to establish in most ants because of the nature of their biology and difficulty to obtain fertile progeny in the laboratory. Yet, a strong research community has been putting a large effort for several decades to understand ant social evolution from a number of angles, including theory [59], development [60, 61], evolution [62] and ecology [58]. Other lineages, such as beetles and a number of Hemiterans and Hymenopterans, are more tractable for more general tools like RNAi. Knocking down the expression of a gene using RNAi reveals the role of this gene in shaping the phenotype during organismal ontogeny. In addition, the recently and fast expanding genome editing technology based on CRISPR/Cas9 is applicable to virtually all species, and is therefore a promising tool to fully address these questions [63]. Therefore, natural models can be highly informative about the evolutionary process, provided that some basic, but important, tools are available.
We believe that the integrative approaches outlined in this review and, in particular, the ability to manipulate traits can, and should, be applied to a broader number of model organisms to better understand their importance for organismal biology. With this approach, we can correlate which genetic changes lead to phenotypic changes and ultimately which phenotypic changes impact the ability of an organism to interact with its environment. By addressing these same questions across different timescales (across morphs, sexes, populations, species and genera), we can begin to address the long-standing question in evolutionary developmental biology, that is, whether the mechanisms of developmental change are the same at the micro- and macro-evolutionary scale [11].
Key points.
Combining evo-devo and ecological approaches can bring a more comprehensive understanding of organismal diversity
To allow integration, models should provide a clear ecological context together with tractability to developmental genetic tools
The semi-aquatic bugs are good models for integrative studies
The approach used in semi-aquatic bugs can be generalized to other models.
Acknowledgements
The authors would like to thank members of the Khila lab and two anonymous reviewers for helpful comments on the manuscript.
Biographies
Emilia Santos is a developmental and evolutionary geneticist who is interested in the study of adaptation and morphological innovations.
Chloe Berger is a developmental and evolutionary geneticist who is interested in the genomics of adaptation.
Peter Refki is a developmental and evolutionary geneticist who is interested in the study of adaptation and species diversification.
Abderrahman Khila is developmental and evolutionary geneticist who is interested in the study of adaptation and species diversification.
Funding
This work was supported by an ERC-CoG grant to A.K., by a Swiss National Foundation Early Post-Doc Mobility fellowship to M.E.S., and a PhD fellowship from Universite Lyon1 to P.R.
References
- 1.Darwin C. On the Origin of Species by Means of Natural Selection, or, The Preservation of Favoured Races in the Struggle for Life. London: J. Murray, 1859. [PMC free article] [PubMed] [Google Scholar]
- 2.Carroll SB, Grenier JK, Weatherbee SD. From DNA to Diversity: Molecular Genetics and the Evolution of Animal Design. Oxford: Blackwell Science, 2001. [Google Scholar]
- 3.Grant PR, Grant BR. How and Why Species Multiply: The Radiation of Darwin’s Finches. Princeton: Princeton University Press, 2008. [Google Scholar]
- 4.Schluter D. The Ecology of Adaptive Radiation. Oxford: Oxford University Press, 2000. [Google Scholar]
- 5.Haag ES, Lenski RE. L’enfant terrible at 30: the maturation of evolutionary developmental biology. Development 2011;138:2633–7. [DOI] [PubMed] [Google Scholar]
- 6.Mallarino R, Abzhanov A. Paths less traveled: evo-devo approaches to investigating animal morphological evolution. Annu Rev Cell Dev Biol 2012;28:743–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sommer R. The future of evo–devo: model systems and evolutionary theory. Nat Rev Genet 2009;10:416–22. [DOI] [PubMed] [Google Scholar]
- 8.Ferrier DEK, Holland PWH. Ancient origin of the Hox gene cluster. Nat Rev Genet 2001;2:33–8. [DOI] [PubMed] [Google Scholar]
- 9.Carroll SB. Evo-devo and an expanding evolutionary synthesis: a genetic theory of morphological evolution. Cell 2008;134:25–36. [DOI] [PubMed] [Google Scholar]
- 10.Stern DL. Evolution, Development, & the Predictable Genome. Greenwood Village: Roberts and Company, 2011. [Google Scholar]
- 11.Nunes MDS, Arif S, Schlötterer C, et al. A perspective on micro-evo-devo: progress and potential. Genetics 2013;195:625–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lovell JT, Juenger TE, Michaels SD, et al. Pleiotropy of FRIGIDA enhances the potential for multivariate adaptation. Proc R Soc B Biol Sci 2013;280:20131043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kopp A. Metamodels and phylogenetic replication: a systematic approach to the evolution of developmental pathways. Evolution 2009;63:2771–89. [DOI] [PubMed] [Google Scholar]
- 14.Khila A, Abouheif E, Rowe L. Function, developmental genetics, and fitness consequences of a sexually antagonistic trait. Science 2012;336:585–9. [DOI] [PubMed] [Google Scholar]
- 15.Moczek AP, Rose DJ. Differential recruitment of limb patterning genes during development and diversification of beetle horns. Proc Natl Acad Sci USA 2009;106:8992–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beldade P, Brakefield PM. The genetics and evo-devo of butterfly wing patterns. Nat Rev Genet 2002;3:442–52. [DOI] [PubMed] [Google Scholar]
- 17.Parsons KJ, Márquez E, Albertson RC. Constraint and opportunity: the genetic basis and evolution of modularity in the cichlid mandible. Am Nat 2012;179:64–78. [DOI] [PubMed] [Google Scholar]
- 18.Marshall CR, Orr H A, Patel NH. Morphological innovation and developmental genetics. Proc Natl Acad Sci USA 1999;96:9995–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martin A, Reed RD. Wingless and aristaless2 define a developmental ground plan for moth and butterfly wing pattern evolution? Mol Biol Evol 2010;27:2864–78. [DOI] [PubMed] [Google Scholar]
- 20.Khalturin K, Hemmrich G, Fraune S, et al. More than just orphans: are taxonomically-restricted genes important in evolution. Trends Genet 2009;25:404–13. [DOI] [PubMed] [Google Scholar]
- 21.Milde S, Hemmrich G, Anton-Erxleben F, et al. Characterization of taxonomically restricted genes in a phylum-restricted cell type. Genome Biol 2009;10:R8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Raff R. Evo-devo: the evolution of a new discipline. Nat Rev Genet 2000;307:302–7. [DOI] [PubMed] [Google Scholar]
- 23.Gilbert SF. The morphogenesis of evolutionary developmental biology. Int J Dev Biol 2003;47:467–77. [PubMed] [Google Scholar]
- 24.Hawkins RD, Hon GC, Ren B. Next-generation genomics: an integrative approach. Nat Rev Genet 2010;11:476–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ungerer MC, Johnson LC, Herman MA. Ecological genomics: understanding gene and genome function in the natural environment. Heredity (Edinb) 2008;100:178–83. [DOI] [PubMed] [Google Scholar]
- 26.Khila A, Abouheif E, Rowe L. Evolution of a novel appendage ground plan in water striders is driven by changes in the Hox gene Ultrabithorax. PLoS Genet 2009;5:e1000583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Refki PN, Armisén D, Crumière AJJ, et al. Emergence of tissue sensitivity to Hox protein levels underlies the evolution of an adaptive morphological trait. Dev Biol 2014;392:441–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khila A, Abouheif E, Rowe L. Comparative functional analyses of ultrabithorax reveal multiple steps and paths to diversification of legs in the adaptive of semi-aquatic insects. Evolution 2014;68:2159–70. [DOI] [PubMed] [Google Scholar]
- 29.Barrett RDH, Rogers SM, Schluter D. Natural selection on a major armor gene in threespine stickleback. Science 2008;322:2006–8. [DOI] [PubMed] [Google Scholar]
- 30.Linnen CR, Poh YP, Peterson BK, et al. Adaptive evolution of multiple traits through multiple mutations at a single gene. Science 2013;339:1312–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bento G, Ogawa A, Sommer RJ. Co-option of the hormone-signalling module dafachronic acid-DAF-12 in nematode evolution. Nature 2010;466:494–7. [DOI] [PubMed] [Google Scholar]
- 32.Colosimo PF, Hosemann KE, Balabhadra S, et al. Widespread parallel evolution in sticklebacks by repeated fixation of ectodysplasin alleles. Science 2005;307:1928–33. [DOI] [PubMed] [Google Scholar]
- 33.Vignieri SN, Larson JG, Hoekstra HE. The selective advantage of crypsis in mice. Evolution 2010;64:2153–8. [DOI] [PubMed] [Google Scholar]
- 34.Manceau M, Domingues VS, Mallarino R, et al. The developmental role of Agouti in color pattern evolution. Science 2011;331:1062–5. [DOI] [PubMed] [Google Scholar]
- 35.Linnen C, Kingsley E, Jensen J, et al. On the origin and spread of an adaptive allele in deer mice. Science 2009;325:1095–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Serobyan V, Ragsdale EJ, Sommer RJ. Adaptive value of a predatory mouth-form in a dimorphic nematode. Proc R Soc B Biol Sci 2014;281:20141334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ragsdale EJ, Müller MR, Rödelsperger C, et al. A developmental switch coupled to the evolution of plasticity acts through a sulfatase. Cell 2014;155:922–33. [DOI] [PubMed] [Google Scholar]
- 38.Arnqvist G, Rowe L. Sexual Conflict. Princeton: Princeton University Press, 2005. [Google Scholar]
- 39.Hu DL, Bush JWM. The hydrodynamics of water-walking arthropods. J Fluid Mech 2010;644:5–33. [Google Scholar]
- 40.Hu DL, Chan B, Bush JWM. The hydrodynamics of water strider locomotion. Nature 2003;424:663–6. [DOI] [PubMed] [Google Scholar]
- 41.Andersen NM. A comparative study of locomotion on the water surface in semiaquatic bugs (Insecta, Hemiptera, Gerromorpha). Vidensk. Meddr dansk naturh. Foren 1976:37–396. [Google Scholar]
- 42.Preziosi RF, Daphne FJ. Sexual size dimorphism and selection in the wild in the waterstrider aquarius remigis: lifetime fecundity selection on female total length and its components. Evolution 1997;51:467–74. [DOI] [PubMed] [Google Scholar]
- 43.Laland K, Uller T, Feldman M, et al. Does evolutionary theory need a rethink? Nature 2014;514:161–4. [DOI] [PubMed] [Google Scholar]
- 44.Andersen NM. The Semiaquatic Bugs (Hemiptera: Gerromorpha) . Klampenborg: Scandinavian Science Press LTD, 1982. [Google Scholar]
- 45.Gao X, Jiang L. Water-repellent legs of water striders. Nature 2004;432:36. [DOI] [PubMed] [Google Scholar]
- 46.i5K Consortium. The i5K Initiative: advancing arthropod genomics for knowledge, human health, agriculture, and the environment. J Hered 2013;104:595–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Robinson GE, Hackett KJ, Purcell-Miramontes M, et al. Creating a Buzz about insect genomes. Science 2011;331:1386. [DOI] [PubMed] [Google Scholar]
- 48.Wang Z, Gerstein M, Snyder M. RNA-Seq: a revolutionary tool for transcriptomics. Nat Rev Genet 2009;10:57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Davey JW, Davey JL, Blaxter ML, et al. RADSeq: next-generation population genetics. Brief Funct Genomics 2010;9:416–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dean AM, Thornton JW. Mechanistic approaches to the study of evolution: the functional synthesis. Nat Rev Genet 2007;8:675–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hayashi K. The Evolution of Mating System and Sexual Dimorphism in the Water Strider Aquarius Elongatus. Osaka: Kinki University, 1995. [Google Scholar]
- 52.Spence J, Wilcox RS. The mating system of two hybridizing species of water striders (Gerridae). Behav Ecol Sociobiol 1986;19:87–95. [Google Scholar]
- 53.Rowe L, Westlake K, Currie D. Functional significance of elaborate secondary sexual traits and their evolution in the water strider genus Rheumatobates. Can Entomol 2006;577:568–77. [Google Scholar]
- 54.Tomoyasu Y, Denell RE. Larval RNAi in Tribolium (Coleoptera) for analyzing adult development. Dev Genes Evol 2004;214:575–8. [DOI] [PubMed] [Google Scholar]
- 55.Sacher R, Stergiou L, Pelkmans L. Lessons from genetics: interpreting complex phenotypes in RNAi screens. Curr Opin Cell Biol 2008;20:483–9. [DOI] [PubMed] [Google Scholar]
- 56.Frankel N, Erezyilmaz DF, McGregor AP, et al. Morphological evolution caused by many subtle-effect substitutions in regulatory DNA. Nature 2011;474:598–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sucena E, Delon I, Jones I, et al. Regulatory evolution of shavenbaby/ovo underlies multiple cases of morphological parallelism. Nature 2003;424:935–8. [DOI] [PubMed] [Google Scholar]
- 58.Hölldobler BO, Wilson E. The Ants. Cambridge MA: Belknap Press, 1990. [Google Scholar]
- 59.Nowak MA, Tarnita CE, Wilson EO. The evolution of eusociality. Nature 2010;466:1057–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Khila A, Abouheif E. Reproductive constraint is a developmental mechanism that maintains social harmony in advanced ant societies. Proc Natl Acad Sci USA 2008;105:17884–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rajakumar R, San Mauro D, Dijkstra MB, et al. Ancestral developmental potential facilitates parallel evolution in ants. Science 2012;335:79–82. [DOI] [PubMed] [Google Scholar]
- 62.Hamilton WD. The genetical evolution of social behaviour. II. J Theor Biol 1964;7:17–52. [DOI] [PubMed] [Google Scholar]
- 63.Gilles AF, Averof M. Functional genetics for all: engineered nucleases, CRISPR and the gene editing revolution. Evodevo 2014;5:43. [DOI] [PMC free article] [PubMed] [Google Scholar]