Abstract
In recent years, our knowledge of the conserved master-switch gene doublesex (dsx) and its function in regulating the development of dimorphic traits in insects has deepened considerably. Here, a comprehensive overview is given on the properties of the male- and female-specific dsx transcripts yielding DSXF and DSXM proteins in Drosophila melanogaster, and the many downstream targets that they regulate. As insects have cell-autonomous sex determination, it was assumed that dsx would be expressed in every somatic cell, but recent research showed that dsx is expressed only when a cell is required to show its sexual identity through function or morphology. This spatiotemporal regulation of dsx expression has not only been established in D. melanogaster but in all insect species studied. Gradually, it has been appreciated that dsx could no longer be viewed as the master-switch gene orchestrating sexual development and behaviour in each cell, but instead should be viewed as the interpreter for the sexual identity of the cell, expressing this identity only on request, making dsx the central nexus of insect sex determination.
Keywords: doublesex, sex determination, insects, dimorphism, transcription factor
Introduction
In many animals, males and females have distinct gender-related appearances such as size differences, ornamentation and colour. Some species have such extreme sexual dimorphisms, that it is sometimes hard to identify them as belonging to the same species based on phenotype alone. These extreme phenotypic differences make sexual dimorphism one of the most intriguing aspects of animal morphology, physiology and behaviour. This diversity is reflected in the underlying molecular mechanisms by an array of systems, from sex-specific gonadal hormones sealing sexual fate in mammals and other vertebrates, to cell-autonomous auto-regulatory splicing loops that maintain the sexual state in insects (reviewed in [1, 2]). It had not been realized that the basis of sex determination harbours a common theme, until a large family of similar transcription factors was discovered. In Drosophila melanogaster and Caenorhabditis elegans, the homologous genes doublesex (dsx) and male abnormal-3 (mab-3) were found, and more doublesex/mab-3-related genes (Dmrt genes) were subsequently identified in many other metazoa.
In insects, the sex determination cascade regulates the sex-specific expression and splicing of genes required for sex-specific development and behaviour. The primary signals are extremely variable in the insect order [3] but all relay their signal through a number of genes to regulate the sex-specific splicing of dsx resulting in male and female proteins [4–29]. These dsx splicing factors are conserved in many species (reviewed in [30, 31]) but in Lepidoptera and possibly Coleoptera different mechanisms operate (reviewed in [29, 32]). As all insects have cell-autonomous sex determination, the sex determining cascade operates on a cell-to-cell basis and features a memory function [15].
For many years, research into insect sex determination has focused only on the presence and position of dsx in the sex determining cascade, but its function in sexual differentiation was studied primarily in D. melanogaster. However, recently, several papers have been published that focus on the function of dsx in the differentiation of many extreme sexual traits in non-model insects species. In this review, we synthesize the research on D. melanogaster dsx and combine it with the description of the current status of dsx research in non-model organisms. We describe some major properties of the dsx gene and the male- and female-specific proteins, DSXM and DSXF, which are translated from their sex specifically spliced transcripts. We outline the functional domains of these proteins and how these domains aid the mechanism by which dsx maintains its function as an integral part of insect sex determination pathways. In addition, the role of doublesex in development, which results in such widely divergent sex-specific and species-specific morphologies, will be discussed. Ultimately, we identify a common pattern in all insect dsx research that changes our view of the role of dsx in determining sex.
Characteristics of doublesex
Discovery of doublesex and mab-3 led to identification of a family of DM-genes
In 1965, a recessive mutation was described in Drosophila that causes genetical males and females to develop as intersexes. Appropriately, this mutation, and consequently the whole gene, was termed doublesex (dsx) [33]. Molecular analysis revealed that the bifunctional nature of this gene and its role in somatic sexual differentiation is achieved by sex-specific alternative splicing of the dsx transcript resulting in sex-specific proteins [24, 34]. In the same period, and also based on the occurrence of a mutant phenotype, the male abnormal-3 (mab-3) gene was identified in C. elegans as required for male-specific functions [35]. Both genes share a DNA-binding motif that has a common evolutionary origin in sexual development, evidenced by the fact that the Drosophila male DSX protein is able to direct male-specific neuroblast differentiation in C. elegans [36, 37]. This DNA-binding motif was named dsx/mab-3-domain (DM-domain). Subsequently, a large array of proteins containing a DM-domain was identified in mammals and other vertebrates, resulting in the description of a large family of homologous DM-genes. These DM-genes were found to be expressed in gonad-precursor cells in both mice and chicken and may control testis development in particular [38]. All vertebrate and invertebrate genomes contain multiple DM-domain proteins some of which are not directly involved in sex determination (reviewed by [39]) but also function in other developmental processes (reviewed by [40, 41]. Still, this family of dsx/mab-3-related genes (Dmrt genes) appears to be involved in sex-specific differentiation in all cases studied, thus evidencing a common theme at the basis of the mechanism of sex determination.
Dsx has a DNA-binding domain and two oligomerization domains
The dsx gene and all its orthologs found so far contain two oligomerization domains: the DM domain, a sex-independent domain shared with all Dmrt genes (see above), and the sex-specific OD2 domain that is restricted to dsx and its orthologs (Figure 1) [42, 43]. The DM domain consists of a DNA binding domain (DBD) and an oligomerization domain (OD1). It features both a novel zinc module containing intertwined CCHC and HCCC zinc-binding sites that bind to the DNA minor groove, and a nascent alpha-helix structure [36, 44]. The OD2 domain consists of a common non-sex-specific N-terminus and a sex-specific C-terminus resulting from sex-specific splicing of the dsx transcripts. In Drosophila, both DSXM and DSXF proteins form asymmetric homodimers at low concentration and tetramers and higher oligomers at higher concentrations, but on binding to DNA, only DSX dimers are formed [42, 43, 45]. OD1 and DBD are together required for specific binding to DNA as a dimer [43] and both full-length DSX proteins have similar DNA binding properties [46, 47], as the OD2 domain plays no role in DNA binding. This indicates that both DSX homodimers recognize and bind to the exact same DNA sequence and thus can compete with each other for DNA binding [42, 43]. Apart from OD1, the entire OD2 domain is independently involved in the dimerization of the full-length protein by coiled-coil interactions [43]. The observed 3-fold stronger dimerization of DSXM over DSXF is likely caused by the male-specific C-terminus in OD2 [48]. Apparently, both common regions of the DSXF and DSXM oligomerization domains form dimers or tetramers by holding both ends of the protein together. The ease with which the two common domains dimerize is reflected in the formation of heterodimers when both DSXF and DSXM are present, which then inhibits their respective activity, at least in vitro [42, 43]. It is unclear if this could result in an effective dsx-null mutant in vivo. Still, in the normal DSXF or DSXM homodimers, OD1 forms the dimeric DNA binding unit, and OD2 regulates the sex-specific functionality of the protein by sex-specific interaction with the transcriptional machinery, or maybe by forming sex-specific regulatory structures by increasing DNA binding cooperativity when DSX binds multiple regulatory sites of its target genes [43].
Figure 1:
Overview of the male and female D. melanogaster DSX protein showing the functional protein domains. At the N-terminus, the doublesex/mab3-domain (DM-domain) consisting of the DBD and the first oligodimerization domain (OD1). Towards the C-terminus, the second oligomerization domain (OD2): with first, the OD2 common region that is present in both male and female isoforms; and second the male-specific OD2 domain; and the female-specific OD2 domain. Not drawn to scale. (A colour version of this figure is available online at: http://bfg.oxfordjournals.org)
DSXF requires intersex and sometimes hermaphrodite in Drosophila
DSXF requires two co-factors for its female-specific function, which are encoded by the genes intersex (ix) and hermaphrodite (her) [49–51]. It was suggested by Siegal and Baker ([52] and references therein) that DSXF requires IX because DSXF lacks a transactivation domain. IX has a proline-, glycine-, glutamate- and serine-rich region, which resembles some known transcriptional activation domains [50]. DSXM, on the other hand, contains a longer OD2 domain with a proline- and serine-rich tail, suggesting that DSXM alone has the same functionality as the combination of DSXF and IX [52]. Still, ix seems to be transcribed in males as well [51], but its function in males is unknown.
The requirement for IX in female development may be conserved in insect sex determination as ix seems conserved through the metazoans. In transgenic D. melanogaster, expression of an ix ortholog of Megaselia scalaris or Bombyx mori completely or partially rescues the sexual development of mutant ix females [52]. However, apart from a study in B. mori [53], no follow-up research has been done on the presence and function of ix homologs in insects, and the requirement of IX for DSXF function outside Drosophila is unknown.
Her encodes a zinc-finger protein and is expressed independently of the sex determination cascade [54]. It is involved upstream in the D. melanogaster cascade, in parallel to or downstream of, dsx [49]. Homologs of her have not been reported outside Drosophila, indicating that at least some of the interactants of D. melanogaster DSXF are derived. No association of HER and DSXM has ever been reported.
Dsx expression is regulated by HOX genes in D. melanogaster
The research on the sex combs, a sex- and species-specific morphological trait in Drosophila, has put the role of dsx as master-regulator into a new perspective. The sex comb is a recently evolved male trait found only in a small subset of Drosophila species [55]. Sex comb development requires the expression of the HOX gene Sex combs reduced (Scr) and dsx in a tightly restricted, sex-specific pattern at a critical time in development. In species without sex combs, Scr is expressed at equal levels in males and females throughout development [55, 56]. In D. melanogaster, in the absence of DSXM or DSXF, Scr is expressed at an intermediate level, which is sufficient to activate partial sex comb development [57]. Relative to this level, Scr is actively upregulated by DSXM, both Scr and DSXM being then required for sex comb development. In contrast, DSXF is involved in repressing Scr in a female-specific pattern [57, 58]. On the other hand, a knockdown of Scr results in a strong reduction of dsx expression, indicating that SCR in turn is required for dsx expression. Hence, Scr and dsx form a positive feedback loop [57]. It was recently shown that early expression of Scr does not require DSX [58], making it not entirely clear how the feedback loop of Scr and dsx may precisely function, but the study by Tanaka et al. is the first that has identified a regulator of dsx expression after realization of sex determination [57].
The occurrence of interactions between DSX and a HOX gene is supported by studies on the dynamics of dsx expression in the posterior pupal abdomen [59, 60]. Dsx is expressed at low levels through the developing abdomen and is highly enriched in the posterior abdomen of both sexes compared with the anterior abdomen. In male pupae, dsx levels are even higher when compared with female pupae, and this coincides with the highest levels of Abd-B in this region. Disruption of Abd-B expression results in lower dsx expression, while ectopic expression of Abd-B leads to higher dsx expression, indicating that, as observed with Scr, the HOX-gene Abd-B regulates dsx expression. However, no indication was found here of dsx-mediated regulation of Abd-B expression [59, 60]. These studies gave the first hints that dsx expression may not be continuous and ubiquitous, but only observed when and where required. Yet, more research is required to identify additional (HOX-) genes that regulate dsx in a spatiotemporal manner and more importantly, to determine the possible evolutionary conservation in the regulation of dsx in different insect taxa.
Doublesex function in sexual differentiation
Regulation of dimorphic trait development by DSX in Drosophila
The mechanisms by which DSXF and DSXM can regulate sexual dimorphism have been studied primarily in D. melanogaster and appear diverse. In some cases, DSXF and DSXM are antagonistic, but in other cases, both have a context-dependent instructive function (reviewed by [77]). In this section, we will highlight some of the research on the target genes of DSX and their mode of regulation in D. melanogaster. A summary of the DSX target genes and the activating or repressing mode of regulation is given in Table 1.
Table 1:
Overview of the different regulatory actions of dsx in sexual differentiation
| Species name | Morphology | Genes | Sex | DSX isoform | Activation / Repression | Reference |
|---|---|---|---|---|---|---|
| Drosophila melanogaster | Yolk protein | Yolk proteins (Yp) | Female | DSXF + IX | + | [46, 50, 51, 54, 61–63] |
| Male | DSXM | − | ||||
| Drosophila melanogaster | Pigmentation | bric-a-brac (bab) | Female | DSXF + IX | + | [64, 65] |
| Male | DSXM | − | ||||
| Drosophila melanogaster | Sensory organs | no. of Gustation Sensory Organs | Female | DSXF + IX | − | [66] |
| Male | DSXM | + | ||||
| Drosophila melanogaster | Genitalia | branchless (bnl) | Female | DSXF + IX | − | [67] |
| Drosophila melanogaster | Genitalia | wingless (wg) + decapentaplegic (dpp) | Female | DSXF + IX | + | [68] |
| Male | DSXM | + | ||||
| Drosophila melanogaster | Pheromones | desaturase-F (dsat-F) | Female | DSXF + IX | + | [69] |
| Drosophila melanogaster | Abdomen | wingless (wg) | Male | DSXM | − | [70] |
| Drosophila melanogaster | Abdomen | extramacrochetae (emc) | Female | DSXF + IX | − | [60] |
| Male | DSXM | + | ||||
| Drosophila melanogaster | Sex combs | Sex combs reduced | Female | DSXF + IX | (−) | [55–58] |
| Male | DSXM | + | ||||
| Bombyx mori | Egg yolk precursor | Vitellogenin (vg) | Female | DSXF (+ IX) | + | [81, 82] |
| Male | DSXM | − | ||||
| Bombyx mori | Fat body | Hexamerin | Female | DSXF (+ IX) | + | [72] |
| Bombyx mori | Pheromones | Pheromone binding protein (PBP) | Female | DSXF (+ IX) | − | [71, 72] |
| Male | DSXM | + | ||||
| Tribolium castaneum | Egg yolk precursor | Vitellogenin (vg) | Female | DSXF | + | [12] |
| Male | DSXM | − | ||||
| Bombyx mori | Abdomen | Abd-B + spitz (spi) | Male | DSXM | + | [73] |
| Nasonia vitripennis | Wing size | n.a. | Male | DSXM | n.a. | [74] |
| Onthophagus taurus | Horns | n.a. | Female | DSXF | − | [10] |
| Male | DSXM | + | ||||
| Onthophagus sagittarius | Horns | n.a. | Female | DSXF | + | [10] |
| Male | DSXM | − | ||||
| Trypoxylus dichotomus | Thorasic horns | n.a. | Male | DSXM | + | [11] |
| Trypoxylus dichotomus | Head horns | n.a. | Female | DSXF | − | [11] |
| Male | DSXM | + | ||||
| Cyclommatus metallifer | Mandible growth | Juvenile hormone sensitivity | Female | DSXF | − | [75] |
| Male | DSXM | + | ||||
| Papilio polytes | Mimicry | n.a. | Female | DSXF | n.a. | [76] |
| Bactrocera dorsalis | Yolk protein | Yolk protein 1 (Yp1) | Female | DSXF | + | [21] |
The interaction of DSX and IX has been shown in D. melanogaster [50]. A ‘+’ signifies a promoting action of the associated DSX isoform on the target gene(s), while a ‘−’ indicates a repressing action of the associated DSX isoform on the target gene(s). When the direct downstream target genes are unknown, this is indicated with n.a. (not available), similar for cases where the mode of DSX regulation is unknown.
Yolk protein genes
The first identification of transcriptional regulation by DSX proteins came from studies on the yolk protein genes (Yp), showing the binding of DSXF and DSXM [36, 46, 61] to the fat body enhancer (FBE) in between two Yp [78, 79] thereby regulating their expression. Together with IX and HER, DSXF promotes expression of both genes while DSXM alone represses gene expression [46, 50, 51, 61, 62, 63]. A 13-bp palindromic sequence ([G/A]NNAC[A/T]A[T/A]GTNN[C/T]) was identified in the FBE consisting of two motifs around a central A/T [42]. Based on these data, Luo et al. defined a palindromic consensus motif (GCAACAATGTTGC) in the genomes of different Drosophila species and some other Dipterans [80]. A number of D. melanogaster genes that were known DSX targets were found to be associated with this sequence, and putative novel DSX targets were identified. By examining more distantly related species, Luo et al. also showed that a 13-bp sequence with variation around the core palindromic sequence ACA[A/T]TGT is present in B. mori and the mosquitos Aedes aegypti, Anopheles gambiae and Culex pipiens. However, no such motifs were found in Tribolium castaneum, Apis mellifera and Nasonia vitripennis and other insect species, indicating that DSX-binding sites are evolving within the insect order [80] and that further studies into the binding sites of DSX proteins are required.
Abdominal pigmentation genes
In D. melanogaster, males show conspicuous abdominal pigmentation, which is absent in females. Just as with Yp expression regulation, DSXF and DSXM act antagonistically in controlling this sex-specific abdomen pigmentation [64, 65]. The bric-a-brac (bab) gene is a repressor of abdominal pigmentation, whereas the HOX gene Abdominal-B (Abd-b) is an activator of abdominal pigmentation and, in addition, represses bab expression. DSXF promotes bab expression by binding to a dimorphic cis-regulatory-element (CRE), thereby overruling the repressing action of Abd-B on bab. This results in repression of posterior pigmentation in females. DSXM binds to the same CRE to directly repress bab expression and consequently, promotes male-specific pigmentation [65]. This mechanism of sexually dimorphic pigmentation has only arisen in the D. melanogaster species group and evolved through multiple fine-scale changes within the dimorphic CRE [65].
Pheromone genes
In Drosophilids with dimorphic pheromone production, female-specific pheromones are produced via the activity of the desaturase DESAT-F under control of DSXF in combination with other cis-regulatory factors [69]. DSXM does not repress dsatF expression, indicating that DSX does not always act antagonistically. DSXF binds a CRE upstream of dsatF to control dimorphic expression, but it is unclear whether DSXM binds to the same CRE but without effect. Within the Drosophilids, frequent evolutionary changes in this CRE site partly explain the gain and loss of direct DSXF regulation of dsatF, which is correlated with transitions from dimorphic to monomorphic expression of dsatF [69].
Gustatory sense organs genes
The effect of dsx on shaping dimorphic tissues can be dependent on spatial determinants as was discovered in examining the sex-specific development of gustatory sense organs (GSOs) in the foreleg of D. melanogaster [66]. Males have more GSOs than females on Segments 1–4 of the tarsus (T1-T4), and this is controlled by dsx. In T1 and T3, DSXM promotes the number of GSOs in males, whereas DSXF seems to have no effect in females. In T2, DSXM stimulates the number of GSOs in males, whereas DSXF represses the formation of GSOs in females, and in T4, DSXF represses the development of two GSOs but DSXM has no effect [66]. Thus, DSXM and DSXF have apparently different modes of action within close tissue proximity. To fully understand this developmental process, more research is required to determine the specific target genes and the regulatory mechanism exerted by DSXF and DSXM on these GSO target genes.
Genital and abdominal development genes
The sex-specific differentiation of the genital imaginal disc is actively regulated by both DSXF and DSXM in two separate steps [68]. The growth of the genital primordia is regulated on a non-cell-autonomous base by the activity of wingless (wg) and decapentaplegic (dpp) in the anterior/posterior border [68]. The expression of wg and dpp is under sex-specific control of DSXF or DSXM in conjunction with Abdominal-A (ABD-A) and ABD-B, resulting in growth of either the female or the male genital primordium. Then, the differentiation of the genital primordia is also controlled by DSX, but on a cell-autonomous basis, by regulating the actions of wg and dpp in a sex-specific way [68, 81, 82].
Another cell-autonomous signalling system used by DSX to further regulate the development of a major portion of the internal adult male genitalia is by regulating the sex-specific expression of branchless (bnl) [67]. In male cells, bnl is expressed to recruit additional mesodermal cells to the male genital disc, which then develop male-specific structures. In female cells, DSXF actively represses bnl expression, most likely by directly binding to the upstream region of bnl that contains multiple putative DSX-binding sites [67].
Further shaping of the male-specific posterior abdomen is also controlled by DSXM in conjunction with ABD-B. The reduction of adult male segment A7 is achieved by DSXM through repression of wg and promotion of extramacrochetae (emc) [60, 70]. Female development of the posterior abdomen is controlled by DSXF together with ABD-B by repressing emc [60].
Concluding remark
All these studies indicate that DSXF and DSXM indeed bind to the same DSX-binding site or dimorphic CRE sites of their targets genes, but their mode of actions likely depends on the sex-specific OD2 that probably interacts with HOX-genes, other transcription factors or regulatory proteins. Small changes in binding sites can then have a huge effect on the sex-specific regulation of that particular gene, making evolutionary changes in dimorphic traits relatively straightforward.
Regulation of dimorphic trait development by DSX in other insects
The occurrence of orthologs of dsx and the conservation of its function in insects outside of Drosophilids has been known for years. However, many of the studied cases show the presence of multiple protein isoforms of dsx, with some isoforms being non-sex-specific and (partially) missing the OD2 domain [4, 10, 12, 13, 76, 83]. This contrasts with the case of Drosophila, which features only one male- and one female-specific isoform [24]. In the past couple of years, the search for the dsx target genes in other insect species has been boosted by the availability of many complete genome sequences [29], and in some cases, the function of (some of) the dsx isoforms has been identified. In this section, the spatiotemporal regulation of dsx and the downstream targets of DSX are discussed in non-model insect species, see also Table 1.
Silk moths—female-specific genes
In B. mori, one female and one male isoform were published in 2001 [6, 7]. Recently, however, more male and female isoforms have been discovered but the functional difference between all these proteins is still unclear [84, 85]. Ectopic expression of DSXF1 in males has no effect on morphology, which may suggest the requirement for a co-factor such as IX [53, 71]. Transgenic females expressing dsxM1 do show intersex phenotypes [71], as DSXM1 has no requirement for IX. The presence of a DSXF1 isoform in males activates the expression of the female-specific genes vitellogenin (vg) and hexameric storage protein 1 (sp1), and represses the expression of the male-specific gene pheromone-binding protein (pbp) [72]. As expected, ectopic expression of DSXM1 in females showed the reverse pattern for vg and pbp expression [71]. Apparently, DSXF1 activates vg expression while DSXM1 represses vg expression by binding to the palindromic core sequence (ACATTGT) in the promoter region of vg [71, 72]. The activation of vg and sp1 expression by DSXF was also shown in the wild silk moths Antheraea assama and Antheraea mylitta [83].
Bombyx mori—abdominal morphology genes
In B. mori, only females have a chitin plate, which is formed by degeneration of the eighth abdominal segment (A8) and is essential for copulation. Expression of dsxM1 in transgenic females results in the formation of an abnormal chitin plate, indicating that the normal formation of a male-specific A8 is under developmental control of DSXM1 [73]. Moreover, these transgenic females show an increase of Abd-B expression in their posterior abdomen, resembling that of wild-type males. This suggests that DSXM1 induces Abd-B expression [73], but it is unknown whether Abd-B is required for dsx expression in B. mori as it is in D. melanogaster. In addition to Abd-B regulation, DSXM1 also upregulates the expression of the epidermal growth factor receptor ligand Spitz (Spi) to activate EGFR signalling, which is required for cell proliferation of A8 segment cells in males [73]. The observation that ectopic dsxM1 expression leads to an intermediate phenotype of the A8 might be due to the presence of both DSXF and DSXM in the cells of the transgenic individuals, which may lead to the formation of heterodimers. As described in the section ‘Dsx has a DNA-binding domain and two oligomerization domains’, the formation of DSX heterodimers could hinder their function and ectopic expression of DSXF protein in a male D. melanogaster background shows that DSXF and DSXM compete with each other for target genes [51]. The observed effect of ectopic expression of DSXM or DSXF on the regulation of downstream targets in this section and the section ‘Silk moths—female-specific genes’ may, therefore, not be entirely biologically correct.
Tribolium castaneum—vitellogenin gene
A more direct approach was used to identify dsx target genes in T. castaneum [12]. Knocking down different dsx isoforms revealed a number of target genes, including vg, which is also a dsx target in D. melanogaster and B. mori with a similar regulation. DSXF increases vg expression, whereas DSXM represses vg expression [12]. In addition, the presence of a 13-bp consensus sequence, with the palindromic core ACA[A/T]TGT, was identified in eight more target genes, suggesting that these genes might be direct dsx targets as well [12]. The presence of the 13-bp consensus binding site is noteworthy, as this motif was not identified in T. castaneum by Luo et al. [80].
Horned beetles—exaggerated horn development
The regulation of dsx in exaggerated beetle horn development has been studied in two beetle genera, dung beetles (Onthophagus) and rhinoceros beetles (Trypoxylus) [10, 11]. The location and size of horn development differs between species and sexes in both genera, and also depends on the nutritional status of the male. In Onthophagus taurus, males in good nutritional condition have large horns, whereas males in bad condition have small horns. Knockdown of dsx resulted in a significant reduction in horn size in both types of males, but was more dramatic in large males. This suggests that dsx function depends on nutritional condition. Female dung beetles have a posterior ridge on the head that is proportional to body size. After larval dsx knockdown, this ridge develops into small horns, particularly in large females [10]. Thus, in O. taurus, DSXM promotes horn development while DSXF inhibits horn development, and both are influenced by nutritional status. However, in the closely related Onthophagus sagittarius, females have a single thoracic horn and a posterior head horn that both lack in males. Dsx knockdown in females results in a reduction of thoracic horn size, development of male-specific anterior head horns and transformation of the large single female posterior head horn to a smaller branched horn. In males, dsx knockdown results in development of a small thoracic horn, and development of a large, branched posterior horn, but it has no effect on the anterior head horn [10]. The function of DSXM and DSXF are, therefore, reversed in O. sagittarius compared with O. taurus in development of the thoracic horn growth. In addition, the DSXM function is reversed for posterior head horn, but DSXF promotes transformation of the posterior head horn. This suggests that DSX is not simply promoting horn growth in Onthophagus but features complex regulation. Besides, DSX can quickly reverse its function or gain novel functions as seen from these two closely related species [10]. A similar complex regulation by DSX on horn development was seen in Trypoxylus dichotomus [11]. Here, females have no horns at all, and males have a thoracic horn and a head horn. Dsx knockdown reduces the size of the head horn in males, while the thoracic horn disappears completely. In females, dsx knockdown results in the development of a small head horn, suggesting that DSX regulates horn formation in different ways for the two horn morphologies [11].
Stag beetle—exaggerated mandible growth
The developmental interaction of dsx regulation and nutrition was studied in greater detail in the stag beetle (Cyclommatus metallifer) [75]. The mandibles are an exaggerated male trait and, as in Onthophagus, mandible size is correlated with the body size of the male. Knockdown of dsx resulted in an intersex phenotype in males and females, the mandible size being dramatically reduced in males but slightly increased in females. The sensitivity of the mandibular tissue to Juvenile Hormone (JH) was previously shown in males, but ectopic expression of JH in female mandibular tissue did not lead to an increase in mandible size, suggesting a sex-specific tissue response to JH during development [86]. Supplementing a JH analog (JHA) to dsx knockout females induced mandible growth, which implicates that DSXF sensitizes mandibular tissue to JH at a level comparable with male tissue sensitivity [75]. Dsx knockdown in males leads to a slight decreased sensitivity of supplemented JHA. The timing of increased dsxF and dsxM expressions during the prepupal stages when mandible growth takes place in males, but is inhibited in females, is precise. This suggests that DSXF inhibits mandible growth by repressing the sensitivity to JH, whereas DSXM promotes mandible growth by enhancing JH sensitivity [75].
Nasonia vitripennis—wing size
In the parasitic wasp, Nasonia, wing size is sex and species specific. Nasonia vitripennis males have small wings and cannot fly, whereas Nasonia giraulti males have large wings and do fly. The region responsible for this difference, ws1, was mapped using positional cloning to the 5′ UTR of dsx [74]. The ws1 region of N. giraulti, containing only the dsx 5′ UTR and no coding regions, was then backcrossed into a N. vitripennis background, resulting in a wing size increase that accounted for 44% of the inter-species difference [74]. An increase of DSXM expression was found in the developing wings of individuals with the vitripennis ws1 (ws1v) locus relative to individuals with the giraulti ws1 (ws1g) locus in the same background. This difference was not found in male legs or whole pre-pupae [74], suggesting that cis-regulation of dsx expression possibly by HOX genes could also have an effect on dimorphic and species-specific traits in other insect species.
Papilio polytes—mimicry
Recently, new research suggested another role for dsx in development, more precisely in sex-limited mimicry in the butterfly Papilio polytes [76]. The males of this species all have the same non-mimetic wing pattern, whereas the females have a wing pattern that either resembles the male-like non-mimetic pattern or mimics one of the different patterns in the toxic genus Pachliopta. Multiple genetic approaches pointed to dsx as the central gene in the female wing polymorphism and three female-specific isoforms were found, two expressed in the wings and one in the body. No splicing differences were found between the mimetic forms, and it seems that it is the variation in dsx expression levels of the two isoforms in the mimetic versus non-mimetic wings that controls the differences in wing pattern variation in females [76]. Strikingly, dsx expression in the mimetic wings has a strong spatial correlation to the adult wing patterns, showing that spatiotemporal dsx expression probably regulates the different mimetic forms, most likely by involving other regulatory elements [76, 87]. In addition to dsx expression differences, a number of coding changes were found between the mimetic and non-mimetic alleles located predominantly in the first exon, but not in the DM-domain. These coding changes possibly lead to different protein structures, as the protein structure predictions shows that the non-mimetic DSX isoforms fold like other insects DSX proteins, whereas the mimetic DSX protein isoform structures are atypical. The allelic differences between the two forms are maintained by the reduced recombination caused by an inversion polymorphism of dsx [76]. Apparently, dsx has evolved a mechanism to regulate different phenotypes within one sex in addition to its normal function.
Concluding remarks
The comparison of Drosophila DSX targets and dsx regulation with that of other insects shows a partial overlap in target genes. For example, yolk proteins and vg are conserved female-specific genes that show the same mode of antagonistic regulation by DSX in different insect species. These conserved genes often even contain identical DSX binding sites. For other, more species-specific dimorphic traits, the DSX binding sites are unknown but, again, the regulatory role of DSX is often antagonistic. This indicates that in insects other than Drosophila also, DSXM and DSXF bind to the same binding site but probably assemble different additional factors for their sex-specific function. Evolutionary changes, therefore, are not restricted to the DNA binding sites of the target genes, but can also take place on the sex-specific OD2 part and the additional factors, which might thus explain the diversity found in dimorphic traits.
Spatio-temporal regulation of DSX
The general idea that emerged from earlier studies, primarily on D. melanogaster, was that dsx regulates sex-specific morphologies by either repressing or activating the expression of its target genes, while, as a sex determination master-switch, it was cell autonomous and expected to be expressed ubiquitously. However, more recent studies have shown that dsx isoforms are not expressed constitutively, but are under complex spatiotemporal regulation, for example, by the somatic gonad identification [88], in the central nervous system [89, 90], during neuron development [91], coordinating dimorphic axon guidance [92], regulating female receptivity [93], controlling female post-mating behaviour [94] and specifying male courtship behaviour (reviewed by [95]).
Specifically, Robinett et al. established a D. melanogaster strain with a GAL4 insertion into the dsx gene, allowing them to visualize all cells that express dsx during development [96]. This demonstrated that dsx is not expressed in all cells but rather forms a mosaic of expression patterns in developing and adult individuals, regardless of their chromosomal sex composition (XX or XY). The sex determination cascade appears to specify the sex of each cell, which is maintained as a molecular memory system (reviewed in [30]), but the regulation of dsx expression sets the developmental route [96]. Shortly hereafter, two studies in Drosophila showed that HOX genes are responsible for strict regulation of dsx expression (see the section ‘Dsx expression is regulated by HOX genes in D. melanogaster’).
Evolution of doublesex
One of the questions now arising is how to reconcile the maintenance of the function of dsx as the nexus of sex determination with its ever-evolving ability to regulate a variety of sexual morphologies. When compiling the current data, three levels become apparent on which selection for sexual dimorphic traits can act. First, selection can result in changes in the coding region of dsx itself. On the one hand, dsx is expected to be under strong purifying selection, as deleterious mutations would have a devastating effect on reproduction. On the other hand, DSX is also involved in male courtship behaviour and genital development, both of which are often under sexual (positive) selection. Previous studies found only evidence for purifying selection, mainly in the common regions [19, 97], but, recently, multiple other studies also evidenced the role of positive selection in the evolution of dsx [76, 98, 99]. These modifications can have an effect on the secondary and tertiary structure of the DSX protein [76] and may lead to changes in the dimerization process and the interaction of DSX with other transcription factors [99]. Particularly, the male-specific exon accumulates the majority of the non-synonymous mutations over longer evolutionary time frames [98], which is in accordance with the fact that female genital morphology is more conserved than male genital morphology. As the common region is primarily under purifying selection, the main functionality of dsx can be maintained [19, 98, 99].
Second, in addition to selective forces acting on dsx directly, the cis-regulatory elements (CRE) of DSX target genes are also under selection [65, 69, 100]. Moreover, the idea that some DSX regions involved in dimerization and DNA binding are under positive selection [99], suggests that the evolution of the DSX binding domains and the DSX binding sites may even go hand-in-hand. In some studied cases, for instance the Drosophila pheromone genes, proto-sequences for the sexually dimorphic CRE sites are already present in monomorphic ancestor species and a relatively small number of mutations are required to make the transition to a DSX-sensitive CRE [65, 99–102].
Third, the observation that spatiotemporal expression of dsx is one of the main regulators for dimorphic characters indicates that selection on CRE in the dsx promoter region may be of huge importance for the evolution of new sex-specific traits [74]. However, this area of research is less advanced, as only the HOX-genes Abd-B and Scr are now known to be implicated in dsx expression regulation [57, 58, 60, 70], and their mechanism of interaction is largely unknown.
DSX is not a master-switch but a central nexus
Doublesex has thus far been regarded as the final master-switch gene in the sex determination cascade of all studied insects. However, in the past couple of years, it has become clear that dsx is not the final master-switch in the sex determination cascades but rather the central switch at the interface of sex determination and sexual differentiation. The sex determination cascades start with a primary sex-determining signal, which is highly variable in insect species (e.g. csd, maternal imprinting or M-factor). This signal results in sex-specific splicing of downstream genes (Sexlethal, tra) (Figure 2) [30]. When the female state is induced, this state is memorized in the cell by a positive feedback-splicing loop [15]. Only in the female state, transformer (tra) splicing results in a functional TRA protein, which then splices dsx into a female-specific transcript. In males, no functional TRA protein is produced and dsx is spliced by default into a male-specific transcript [2]. In other systems, dsx splicing is instructed by P-element somatic inhibitor+mRNA-binding protein (PSI+IMP) [32]. Sexual differentiation starts when the sex-specific information provided by DSXF or DSXM to its target genes is used to differentiate tissues during development, leading to sex-specific traits.
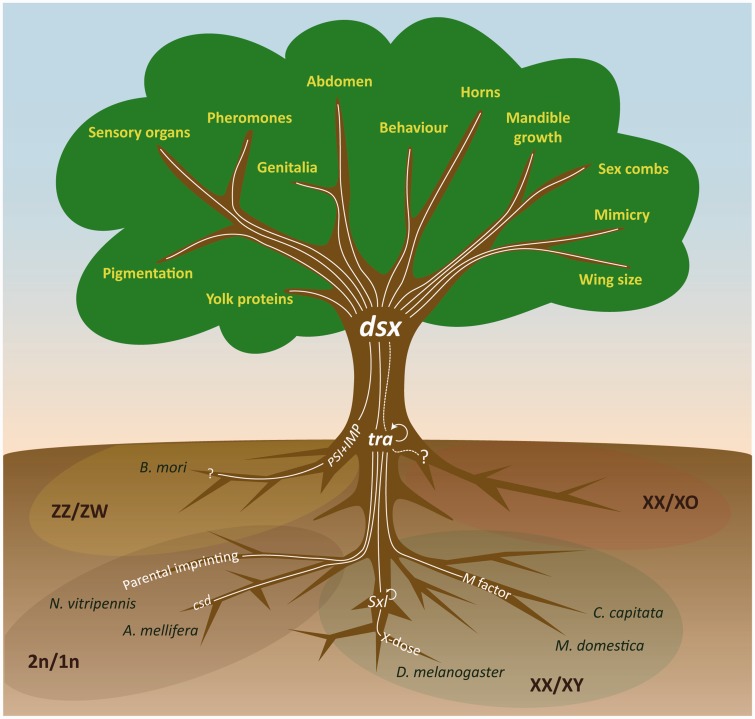
Figure 2:
Doublesex (dsx) is at the interface of sex determination and sexual differentiation in insects. The different mechanisms of sex determination in insects are like the root of a tree and their effects are not noticeable until dsx is required for sex-specific instructions in the cell. The regulation by dsx on dimorphic traits development is diverse and extends to all aspects of sexual differentiation like the branches on a tree. ZZ/ZW; XX/XY; XX/XO and 2n/1n (haplodiploidy) represent the different chromosomal sex determining systems. Many sex determining systems converge on tra (sometimes termed feminizer) resulting in splicing of tra into a male- or female-specific transcript (reviewed in [29]). Only in females this transcript results in a functional TRA protein, which then splices dsx into a female-specific transcript. In males, no functional TRA protein is produced and dsx is spliced by default into a male-specific transcript. Cellular memory of the sex is maintained in most species by auto regulation of tra, but in Drosophila this is taken over by Sxl (reviewed in [30]). The dsx transcripts yield DSXM and DSXF proteins that regulate the downsteam targets in a sex-specific manner. In B. mori, tra appears not present and dsx is spliced by default in the female-specific form. In B. mori males, a P-element somatic inhibitor (PSI) and IGF-II mRNA-binding protein (IMP) act together to splice dsx into the male-specific transcript (reviewed in [32]). In XX/XO individuals, little is known about the sex determination mechanisms. (A colour version of this figure is available online at: http://bfg.oxfordjournals.org)
Extending the metaphor of a tree as proposed by Clough and Oliver [103] to include all studied insect species, the difference between sex determination and sexual differentiation can be envisioned by comparing the variety of primary signals with the root of a tree (Figure 2). These signals and the resulting cascades operate underground and their actions are not (yet) visible. When dsx is expressed, it receives sex-specific input from multiple signal transferring genes, including tra and PSI+IMP [30, 32], ultimately resulting in either DSXM or DSXF. The transition from the roots (sex determining cascades) to the branches (sexual differentiation) is in the trunk of the tree, which is represented by the two DSX proteins (Figure 2). All the sex-determining actions in the roots take place during early development, and the effects of disrupting the sex-specific input to dsx in adults can be minor [104].
As has been summarized in this review, the regulation by DSXM or DSXF on dimorphic trait development is diverse and extends to all aspects of, often visible, sexual differentiation like the branches on the tree (Figure 2). As insects have cell-autonomous sex determination, it was expected that the entire tree was present in all cells, so that depending on the outcome of the sex determination cascade, either DSXM or DSXF protein would be found in all somatic cells to regulate their target genes. However, growing evidence suggests that regulation of dsx itself may be at the basis of sex- and species-specific morphological differences. Only when a cell needs sex-specific information, dsx is ordered to present this information. This also suggests that the role of the positive feedback-splicing loop may be of even more importance than previously thought [2]. After all, it is this cell-autonomous auto regulation that maintains the memory (male or female) of the cell should it require this information during development [15]. Therefore, as also noted by Clough and Oliver for Drosophila [103], in all studied insects dsx is not part of the sex determining cascade in the roots but rather represents the trunk of the tree connecting roots and branches. It gets the input from the omnipresent feeding tree root (the sex determination cascades), but, only at a specific time and place, dsx relays this information through the tree trunk to the required target genes, resulting in visible dimorphic traits in the tree branches. Taken together, dsx is not the sex determining master-switch gene but only a nexus for sexual differentiation.
Key points.
DSXM or DSXF regulates sex-specific morphologies by either repressing or activating the expression of its target genes.
This mode of activation or repression by DSX differs between target genes, sexes and species.
Changes of cis-regulatory-elements in the promoter regions of target genes can lead quickly to novel dimorphic binding sites for DSX resulting in fast evolution of dimorphic phenotypes.
Dsx was thought to be ubiquitously expressed and cell autonomous, however dsx expression is regulated spatiotemporally, often by HOX-genes, and provides sexual information to the cell only when required.
Funding
This work is part of the research program Innovational Research Incentives Scheme, financed by the Netherlands Organization for Scientific Research (NWO) Grant No. ALW 863.13.014 to ECV. We thank two anonymous reviewers for their constructive comments and suggestions.
Biographies
Eveline C. Verhulst is a postdoctoral fellow at the Laboratory of Genetics of Wageningen University. Her research interests are the evolution of sex determining mechanisms and sexual dimorphism in particularly haplodiploids.
Louis van de Zande is an associate professor at the Evolutionary Genetics Group of the Groningen Institute for Evolutionary Life Sciences of the University of Groningen, the Netherlands. His research focuses on the functional molecular aspects of biological complexity such as life history evolution (including ageing, photoperiodic diapause induction and sex determination).
References
- 1.Wilhelm D, Palmer S, Koopman P. Sex determination and gonadal development in mammals. Physiol Rev 2007;87:1–28. [DOI] [PubMed] [Google Scholar]
- 2.Verhulst EC, Van de Zande L, Beukeboom LW. Insect sex determination: it all evolves around transformer. Curr Opin Genet Dev 2010;20:376–83. [DOI] [PubMed] [Google Scholar]
- 3.Sánchez L. Sex-determining mechanisms in insects. Int J Dev Biol 2008;52:837–56. [DOI] [PubMed] [Google Scholar]
- 4.Cho S, Huang ZY, Zhang J. Sex-specific splicing of the honeybee doublesex gene reveals 300 million years of evolution at the bottom of the insect sex-determination pathway. Genetics 2007;177:1733–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oliveira DCSG, Werren JH, Verhulst EC, et al. Identification and characterization of the doublesex gene of Nasonia . Insect Mol Biol 2009;18:315–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ohbayashi F, Suzuki MG, Mita K, et al. A homologue of the Drosophila doublesex gene is transcribed into sex-specific mRNA isoforms in the silkworm, Bombyx mori. Comp Biochem Phys B 2001;128:145–58. [DOI] [PubMed] [Google Scholar]
- 7.Suzuki MG, Ohbayashi F, Mita K, et al. The mechanism of sex-specific splicing at the doublesex gene is different between Drosophila melanogaster and Bombyx mori . Insect Biochem Mol Biol 2001;31:1201–11. [DOI] [PubMed] [Google Scholar]
- 8.Shukla JN, Nagaraju J. Doublesex: a conserved downstream gene controlled by diverse upstream regulators. J Genet 2010;89:341–56. [DOI] [PubMed] [Google Scholar]
- 9.Sugimoto TN, Fujii T, Kayukawa T, et al. Expression of a doublesex homologue is altered in sexual mosaics of Ostrinia scapulalis moths infected with Wolbachia. Insect Biochem Mol Biol 2010;40:847–54. [DOI] [PubMed] [Google Scholar]
- 10.Kijimoto T, Moczek AP, Andrews J. Diversification of doublesex function underlies morph-, sex-, and species-specific development of beetle horns. Proc Natl Acad Sci USA 2012;109:20526–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ito Y, Harigai A, Nakata M, et al. The role of doublesex in the evolution of exaggerated horns in the Japanese rhinoceros beetle. EMBO Rep 2013;14:561–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shukla JN, Palli SR. Doublesex target genes in the red flour beetle, Tribolium castaneum. Sci Rep 2012;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Salvemini M, Mauro U, Lombardo F, et al. Genomic organization and splicing evolution of the doublesex gene, a Drosophila regulator of sexual differentiation, in the dengue and yellow fever mosquito Aedes aegypti. BMC Evol Biol 2011;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dübendorfer A, Hediger M, Burghardt G, et al. Musca domestica, a window on the evolution of sex-determining mechanisms in insects. Int J Dev Biol 2002;46:75–9. [PubMed] [Google Scholar]
- 15.Pane A, Salvemini M, Delli Bovi P, et al. The transformer gene in Ceratitis capitata provides a genetic basis for selecting and remembering the sexual fate. Development 2002;129:3715–25. [DOI] [PubMed] [Google Scholar]
- 16.Saccone G, Salvemini M, Polito LC. The transformer gene of Ceratitis capitata: a paradigm for a conserved epigenetic master regulator of sex determination in insects. Genetica 2011;139:99–111. [DOI] [PubMed] [Google Scholar]
- 17.Scali C, Catteruccia F, Li Q, et al. Identification of sex-specific transcripts of the Anopheles gambiae doublesex gene. J Exp Biol 2005;208:3701–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ruiz MF, Stefani RN, Mascarenhas RO, et al. The gene doublesex of the fruit fly Anastrepha obliqua (Diptera, Tephritidae). Genetics 2005;171:849–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ruiz MF, Eirín-López JM, Stefani RN, et al. The gene doublesex of Anastrepha fruit flies (Diptera, Tephritidae) and its evolution in insects. Dev Genes Evol 2007;217:725–31. [DOI] [PubMed] [Google Scholar]
- 20.Permpoon R, Aketarawong N, Thanaphum S. Isolation and characterization of doublesex homologues in the Bactrocera species: B. dorsalis (Hendel) and B. correcta (Bezzi) and their putative promoter regulatory regions. Genetica 2011;139:113–27. [DOI] [PubMed] [Google Scholar]
- 21.Chen SL, Dai SM, Lu KH, et al. Female-specific doublesex dsRNA interrupts yolk protein gene expression and reproductive ability in oriental fruit fly, Bactrocera dorsalis (Hendel). Insect Biochem Mol Biol 2008;38:155–65. [DOI] [PubMed] [Google Scholar]
- 22.Lagos D, Ruiz MF, Sánchez L, et al. Isolation and characterization of the Bactrocera oleae genes orthologous to the sex determining Sex-lethal and doublesex genes of Drosophila melanogaster. Gene 2005;348:111–21. [DOI] [PubMed] [Google Scholar]
- 23.Shearman DCA, Frommer M. The Bactrocera tryoni homologue of the Drosophila melanogaster sex-determination gene doublesex. Insect Mol Biol 1998;7:355–66. [DOI] [PubMed] [Google Scholar]
- 24.Burtis KC, Baker BS. Drosophila doublesex gene controls somatic sexual differentiation by producing alternatively spliced mRNAs encoding related sex-specific polypeptides. Cell 1989;56:997–1010. [DOI] [PubMed] [Google Scholar]
- 25.Concha C, Li F, Scott MJ. Conservation and sex-specific splicing of the doublesex gene in the economically important pest species Lucilia cuprina. J Genet 2010;89:279–85. [DOI] [PubMed] [Google Scholar]
- 26.Sievert V, Kuhn S, Traut W. Expression of the sex determining cascade genes Sex-lethal and doublesex in the phorid fly Megaselia scalaris . Genome 1997;40:211–4. [DOI] [PubMed] [Google Scholar]
- 27.Kuhn S, Sievert V, Traut W. The sex-determining gene doublesex in the fly Megaselia scalaris: conserved structure and sex-specific splicing. Genome 2000;43:1011–20. [DOI] [PubMed] [Google Scholar]
- 28.Hediger M, Burghardt G, Siegenthaler C, et al. Sex determination in Drosophila melanogaster and Musca domestica converges at the level of the terminal regulator doublesex . Dev Genes Evol 2004;214:29–42. [DOI] [PubMed] [Google Scholar]
- 29.Geuverink E, Beukeboom LW. Phylogenetic distribution and evolutionary dynamics of the sex determination genes doublesex and transformer in insects. Sex Dev 2014;8:38–49. [DOI] [PubMed] [Google Scholar]
- 30.Bopp D, Saccone G, Beye M. Sex determination in insects: variations on a common theme. Sex Dev 2014;8:20–8. [DOI] [PubMed] [Google Scholar]
- 31.Scott MJ, Pimsler ML, Tarone AM. Sex determination mechanisms in the Calliphoridae (blow flies). Sex Dev 2014;8:29–37. [DOI] [PubMed] [Google Scholar]
- 32.Nagaraju J, Gopinath G, Sharma V, et al. Lepidopteran sex determination: a cascade of surprises. Sex Dev 2014;8:104–12. [DOI] [PubMed] [Google Scholar]
- 33.Hildreth PE. Doublesex, a recessive gene that tranforms both males and females of Drosophila into intersexes. Genetics 1965;51:659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baker BS, Wolfner MF. A molecular analysis of doublesex, a bifunctional gene that controls both male and female sexual differentiation in Drosophila melanogaster . Genes Dev 1988;2:477–89. [DOI] [PubMed] [Google Scholar]
- 35.Shen MM, Hodgkin J. mab-3, a gene required for sex-specific yolk protein expression and a male-specific lineage in C. elegans. Cell 1988;54:1019–31. [DOI] [PubMed] [Google Scholar]
- 36.Erdman SE, Burtis KC. The Drosophila doublesex proteins share a novel zinc finger related DNA binding domain. EMBO J 1993;12:527–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Raymond CS, Shamu CE, Shen MM, et al. Evidence for evolutionary conservation of sex-determining genes. Nature 1998;391:691–5. [DOI] [PubMed] [Google Scholar]
- 38.Raymond CS, Kettlewell JR, Hirsch B, et al. Expression of Dmrt1 in the genital ridge of mouse and chicken embryos suggests a role in vertebrate sexual development. Dev Biol 1999;215:208–20. [DOI] [PubMed] [Google Scholar]
- 39.Hong C-S, Park B-Y, Saint-Jeannet J-P. The function of Dmrt genes in vertebrate development: It is not just about sex. Dev Biol 2007;310:1–9. [DOI] [PubMed] [Google Scholar]
- 40.Kopp A. Dmrt genes in the development and evolution of sexual dimorphism. Trends Genet 2012;28:175–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bellefroid EJ, Leclère L, Saulnier A, et al. Expanding roles for the evolutionarily conserved Dmrt sex transcriptional regulators during embryogenesis. Cell Mol Life Sci 2013;70:3829–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Erdman SE, Chen HJ, Burtis KC. Functional and genetic characterization of the oligomerization and DNA binding properties of the Drosophila doublesex proteins. Genetics 1996;144:1639–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.An W, Cho S, Ishii H, et al. Sex-specific and non-sex-specific oligomerization domains in both of the doublesex transcription factors from Drosophila melanogaster . Mol Cell Biol 1996;16:3106–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhu L, Wilken J, Phillips NB, et al. Sexual dimorphism in diverse metazoans is regulated by a novel class of intertwined zinc fingers. Genes Dev 2000;14:1750–64. [PMC free article] [PubMed] [Google Scholar]
- 45.Cho S, Wensink PC. Purification and physical properties of the male and female doublesex proteins of Drosophila. Proc Natl Acad Sci USA 1996;93:2043–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Burtis KC, Coschigano KT, Baker BS, et al. The doublesex proteins of Drosophila melanogaster bind directly to a sex-specific yolk protein gene enhancer. EMBO J 1991;10:2577–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cho S, Wensink PC. DNA binding by the male and female doublesex proteins of Drosophila melanogaster. J Biol Chem 1997;272:3185–9. [DOI] [PubMed] [Google Scholar]
- 48.Cho S, Wensink PC. Linkage between oligomerization and DNA binding in Drosophila doublesex proteins. Biochemistry 1998;37:11301–8. [DOI] [PubMed] [Google Scholar]
- 49.Pultz MA, Baker BS. The dual role of hermaphrodite in the Drosophila sex determination regulatory hierarchy. Development 1995;121:99–111. [DOI] [PubMed] [Google Scholar]
- 50.Garrett-Engele CM, Siegal ML, Manoli DS, et al. intersex, a gene required for female sexual development in Drosophila, is expressed in both sexes and functions together with doublesex to regulate terminal differentiation. Development 2002;129:4661–75. [DOI] [PubMed] [Google Scholar]
- 51.Waterbury JA, Jackson LL, Schedl P. Analysis of the doublesex female protein in Drosophila melanogaster: role on sexual differentiation and behavior and dependence on intersex . Genetics 1999;152:1653–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Siegal ML, Baker BS. Functional conservation and divergence of intersex, a gene required for female differentiation in Drosophila melanogaster. Dev Genes Evol 2005;215:1–12. [DOI] [PubMed] [Google Scholar]
- 53.Arunkumar KP, Nagaraju J. Drosophila intersex orthologue in the silkworm, Bombyx mori and related species. Genetica 2011;139:141–7. [DOI] [PubMed] [Google Scholar]
- 54.Li H, Baker BS. Her, a gene required for sexual differentiation in Drosophila, encodes a zinc finger protein with characteristics of ZFY-like proteins and is expressed independently of the sex determination hierarchy. Development 1998;125:225–35. [DOI] [PubMed] [Google Scholar]
- 55.Barmina O, Kopp A. Sex-specific expression of a HOX gene associated with rapid morphological evolution. Dev Biol 2007;311:277–86. [DOI] [PubMed] [Google Scholar]
- 56.Barmina O, Gonzalo M, McIntyre LM, et al. Sex- and segment-specific modulation of gene expression profiles in Drosophila . Dev Biol 2005;288:528–44. [DOI] [PubMed] [Google Scholar]
- 57.Tanaka K, Barmina O, Sanders LE, et al. Evolution of sex-specific traits through changes in HOX-dependent doublesex expression. PLoS Biol 2011;9:e1001131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Devi TR, Shyamala B. Male- and female-specific variants of doublesex gene products have different roles to play towards regulation of Sex combs reduced expression and sex comb morphogenesis in Drosophila. J Biosci (Bangalore) 2013;38:455–60. [DOI] [PubMed] [Google Scholar]
- 59.Wang W, Yoder JH. Hox-mediated regulation of doublesex sculpts sex-specific abdomen morphology in Drosophila . Dev Dyn 2012;241:1076–90. [DOI] [PubMed] [Google Scholar]
- 60.Foronda D, Martín P, Sánchez-Herrero E. Drosophila Hox and sex-determination genes control segment elimination through EGFR and extramacrochetae activity. PLoS Genet 2012;8:e1002874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Coschigano KT, Wensink PC. Sex-specific transcriptional regulation by the male and female doublesex proteins of Drosophila . Genes Dev 1993;7:42–54. [DOI] [PubMed] [Google Scholar]
- 62.An W, Wensink PC. Three protein binding sites form an enhancer that regulates sex- and fat body-specific transcription of Drosophila yolk protein genes. EMBO J 1995;14:1221–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li H, Baker BS. hermaphrodite and doublesex function both dependently and independently to control various aspects of sexual differentiation in Drosophila . Development 1998;125:2641-51. [DOI] [PubMed] [Google Scholar]
- 64.Kopp A, Duncan I, Carroll SB. Genetic control and evolution of sexually dimorphic characters in Drosophila . Nature 2000;408:553–9. [DOI] [PubMed] [Google Scholar]
- 65.Williams TM, Selegue JE, Werner T, et al. The regulation and evolution of a genetic switch controlling sexually dimorphic traits in Drosophila . Cell 2008;134:610–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mellert DJ, Robinett CC, Baker BS. doublesex functions early and late in gustatory sense organ development. PLoS One 2012;7:e51489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ahmad SM, Baker BS. Sex-specific deployment of FGF signaling in Drosophila recruits mesodermal cells into the male genital imaginal disc. Cell 2002;109:651–61. [DOI] [PubMed] [Google Scholar]
- 68.Keisman EL, Christiansen AE, Baker BS. The sex determination gene doublesex regulates the A/P organizer to direct sex-specific patterns of growth in the Drosophila genital imaginal disc. Dev Cell 2001;1:215–25. [DOI] [PubMed] [Google Scholar]
- 69.Shirangi TR, Dufour HD, Williams TM, et al. Rapid evolution of sex pheromone-producing enzyme expression in Drosophila . PLoS Biol 2009;7:e1000168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang W, Kidd BJ, Carroll SB, et al. Sexually dimorphic regulation of the Wingless morphogen controls sex-specific segment number in Drosophila. Proc Natl Acad Sci USA 2011;108:11139–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Suzuki MG, Funaguma S, Kanda T, et al. Role of the male BmDSX protein in the sexual differentiation of Bombyx mori . Evol Dev 2005;7:58–68. [DOI] [PubMed] [Google Scholar]
- 72.Suzuki MG, Funaguma S, Kanda T, et al. Analysis of the biological functions of a doublesex homologue in Bombyx mori. Dev Genes Evol 2003;213:345–54. [DOI] [PubMed] [Google Scholar]
- 73.Duan J, Xu H, Ma S, et al. Ectopic expression of the male BmDSX affects formation of the chitin plate in female Bombyx mori. Mol Reprod Dev 2014;81:240–7. [DOI] [PubMed] [Google Scholar]
- 74.Loehlin DW, Oliveira DCSG, Edwards R, et al. Non-coding changes cause sex-specific wing size differences between closely related species of Nasonia. PLoS Genet 2010;6:e1000821-e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gotoh H, Miyakawa H, Ishikawa A, et al. Developmental link between sex and nutrition; doublesex regulates sex-specific mandible growth via Juvenile Hormone signaling in stag beetles. PLoS Genet 2014;10:e1004098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kunte K, Zhang W, Tenger-Trolander A, et al. doublesex is a mimicry supergene. Nature 2014;507:229–32. [DOI] [PubMed] [Google Scholar]
- 77.Christiansen AE, Keisman EL, Ahmad SM, et al. Sex comes in from the cold: the integration of sex and pattern. Trends Genet 2002;18:510–6. [DOI] [PubMed] [Google Scholar]
- 78.Shepherd B, Garabedian MJ, Hung M-C, et al. Developmental control of Drosophila yolk protein 1 gene by cis-acting DNA elements. Cold Spring Harbor Symp Quant Biol 1985;50:521–6. [DOI] [PubMed] [Google Scholar]
- 79.Garabedian MJ, Shepherd BM, Wensink PC. A tissue-specific transcription enhancer from the Drosophila yolk protein 1 gene. Cell 1986;45:859–67. [DOI] [PubMed] [Google Scholar]
- 80.Luo SD, Shi GW, Baker BS. Direct targets of the D. melanogaster DSXF protein and the evolution of sexual development. Development 2011;138:2761–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Keisman EL, Baker BS. The Drosophila sex determination hierarchy modulates wingless and decapentaplegic signaling to deploy dachshund sex-specifically in the genital imaginal disc. Development 2001;128:1643–56. [DOI] [PubMed] [Google Scholar]
- 82.Sánchez L, Gorfinkiel N, Guerrero I. Sex determination genes control the development of the Drosophila genital disc, modulating the response to Hedgehog, Wingless and Decapentaplegic signals. Development 2001;128:1033–43. [DOI] [PubMed] [Google Scholar]
- 83.Shukla JN, Nagaraju J. Two female-specific DSX proteins are encoded by the sex-specific transcripts of dsx, and are required for female sexual differentiation in two wild silkmoth species, Antheraea assama and Antheraea mylitta (Lepidoptera, Saturniidae). Insect Biochem Mol Biol 2010;40:672–82. [DOI] [PubMed] [Google Scholar]
- 84.Shukla JN, Jadhav S, Nagaraju J. Novel female-specific splice form of dsx in the silkworm, Bombyx mori. Genetica 2011;139:23–31. [DOI] [PubMed] [Google Scholar]
- 85.Duan J, Xu H, Guo H, et al. New insights into the genomic organization and splicing of the doublesex gene, a terminal regulator of sexual differentiation in the silkworm Bombyx mori . PLoS One 2013;8:e79703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gotoh H, Cornette R, Koshikawa S, et al. Juvenile hormone regulates extreme mandible growth in male Stag beetles. PLoS One 2011;6:e21139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Loehlin DW, Carroll SB. Evolutionary biology: sex, lies and butterflies. Nature 2014;507:172–3. [DOI] [PubMed] [Google Scholar]
- 88.Hempel LU, Oliver B. Sex-specific doublesexM expression in subsets of Drosophila somatic gonad cells. BMC Dev Biol 2007;7:113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lee G, Hall JC, Park JH. Doublesex gene expression in the central nervous system of Drosophila melanogaster. J Neurogenet 2002;16:229–48. [DOI] [PubMed] [Google Scholar]
- 90.Sanders LE, Arbeitman MN. Doublesex establishes sexual dimorphism in the Drosophila central nervous system in an isoform-dependent manner by directing cell number. Dev Biol 2008;320:378–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rideout EJ, Dornan AJ, Neville MC, et al. Control of sexual differentiation and behavior by the doublesex gene in Drosophila melanogaster . Nat Neurosci 2010;13:458–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mellert DJ, Knapp J-M, Manoli DS, et al. Midline crossing by gustatory receptor neuron axons is regulated by fruitless, doublesex and the Roundabout receptors. Development 2010;137:323–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhou C, Pan Y, Robinett Carmen C, et al. Central brain neurons expressing doublesex regulate female receptivity in Drosophila . Neuron 2014;83:149–63. [DOI] [PubMed] [Google Scholar]
- 94.Rezával C, Nojima T, Neville Megan C, et al. Sexually dimorphic octopaminergic neurons modulate female postmating behaviors in Drosophila . Curr Biol 2014;24:725–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Dauwalder B. The roles of fruitless and doublesex in the control of male courtship. Int Rev Neurobiol 2011, 87–105. [DOI] [PubMed] [Google Scholar]
- 96.Robinett CC, Vaughan AG, Knapp J-M, et al. Sex and the single cell. II. There is a time and place for sex. PLoS Biol 2010;8:e1000365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Permpoon R, Thanaphum S. Isolation and characterization of oligomerization domain I and II coding regions of doublesex genes in agricultural fruit flies (Diptera: Tephritidae). Eur J Entomol 2010;107. [Google Scholar]
- 98.Hughes AL. Runaway evolution of the male-specific exon of the doublesex gene in Diptera. Gene 2011;472:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sobrinho IS, de Brito RA. Positive and purifying selection influence the evolution of doublesex in the Anastrepha fraterculus species group. PLoS One 2012;7:e33446-e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Rogers WA, Salomone JR, Tacy DJ, et al. Recurrent modification of a conserved cis-regulatory element underlies fruit fly pigmentation diversity. PLoS Genet 2013;9:e1003740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gompel N, Carroll SB. Genetic mechanisms and constraints governing the evolution of correlated traits in drosophilid flies. Nature 2003;424:931–5. [DOI] [PubMed] [Google Scholar]
- 102.Jeong S, Rokas A, Carroll SB. Regulation of body pigmentation by the Abdominal-B Hox protein and its gain and loss in Drosophila evolution. Cell 2006;125:1387–99. [DOI] [PubMed] [Google Scholar]
- 103.Clough E, Oliver B. Genomics of sex determination in Drosophila. Brief Funct Genomics 2012; 11:387–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Verhulst EC, Beukeboom LW, van de Zande L. Maternal control of haplodiploid sex determination in the wasp Nasonia. Science 2010; 328:620–3. [DOI] [PubMed] [Google Scholar]