ABSTRACT
In Escherichia coli or Salmonella enterica, the stress-associated mammalian hormones epinephrine (E) and norepinephrine (NE) trigger a signaling cascade by interacting with the QseC sensor protein. Here we show that Vibrio cholerae, the causative agent of cholera, exhibits a specific response to E and NE. These catecholates (0.1 mM) enhanced the growth and swimming motility of V. cholerae strain O395 on soft agar in a medium containing calf serum, which simulated the environment within the host. During growth, the hormones were converted to degradation products, including adrenochrome formed by autooxidation with O2 or superoxide. In E. coli, the QseC sensor kinase, which detects the autoinducer AI-3, also senses E or NE. The genome of V. cholerae O395 comprises an open reading frame coding for a putative protein with 29% identity to E. coli QseC. Quantitative reverse transcriptase PCR (qRT-PCR) experiments revealed increased transcript levels of the qseC-like gene and of pomB, a gene encoding a structural component of the flagellar motor complex, under the influence of E or NE. Phentolamine blocks the response of E. coli QseC to E or NE. A V. cholerae mutant devoid of the qseC-like gene retained the phentolamine-sensitive motility in the presence of E, whereas NE-stimulated motility was no longer inhibited by phentolamine. Our study demonstrates that V. cholerae senses the stress hormones E and NE. A sensor related to the histidine kinase QseC from E. coli is identified and is proposed to participate in the sensing of NE.
IMPORTANCE Vibrio cholerae is a Gram-negative bacterium that may cause cholera, a severe illness with high mortality due to acute dehydration caused by diarrhea and vomiting. Pathogenic V. cholerae strains possess virulence factors like the cholera toxin (CTX) and the toxin-coregulated pilus (TCP) produced in response to signals provided by the host. In pathogenic enterobacteria, the stress-associated hormones epinephrine (E) and norepinephrine (NE) of the human host act as signal molecules for the production of virulence factors and promote bacterial growth by the sequestration of iron from the host. Here we show that V. cholerae, like some enterobacteria, benefits from these stress hormones and possesses a sensor to recognize them.
INTRODUCTION
Stress enhances the susceptibility of a mammalian host to infection by bacteria. The stress-associated mammalian hormones epinephrine (E) and norepinephrine (NE), for example, support the growth (1–3) and motility (4, 5) of the enterobacteria Escherichia coli, Salmonella enterica serovar Typhimurium, and Klebsiella pneumoniae (6), of Pseudomonas aeruginosa (7, 8), and of some Vibrio species (9) in a serum-based bacteriostatic medium. As E and NE share structural similarities to the bacterial catechol siderophores—a benzene ring with two adjacent hydroxyl groups—it was suggested that these stress hormones may be involved in the binding and uptake of iron by bacteria, thus promoting growth or motility (1–5). Recently, the mechanism by which catecholamine hormones bind to the ferric ion in complex with lactoferrin or transferrin was elucidated. The catecholamines are able to reduce Fe(III) to Fe(II), which exhibits a lower affinity for transferrin or lactoferrin than Fe(III) does (10). In E. coli, NE works as a transcription factor, enhancing the expression of the siderophore enterobactin and, as a direct consequence, enhancing iron uptake (11). Structural similarities between bacterial siderophores and the catecholamine hormones, together with the availability of E and NE in the host gut, strongly suggest that pathogenic bacteria are able to use these compounds as pseudosiderophores for the uptake of iron. The ability to sense E and NE is an example of interkingdom communication since bacterial gene expression patterns are influenced by host hormones. E and NE are sensed via the two-component system QseBC, with QseC as the receptor for these catecholates and QseB as its cognate response regulator, which initiates transcription of different virulence-associated genes (4, 12).
Some Vibrio cholerae strains cause the severe illness cholera in humans, and thus it was of interest to investigate whether this pathogen also responds to the stress hormones E and NE. Using V. cholerae strain RIMD2203102, Nakano et al. (9) did not observe improved growth in the presence of E or NE. Yet, the genome of V. cholerae O1 classical strain O395 revealed the presence of an open reading frame with significant homology to qseC from E. coli. Here we show that a derivative of the classical V. cholerae O395 strain, V. cholerae O395-N1 (13), specifically responds to catecholate hormones. A mutant variant of this strain lacking the qseC-like gene was constructed, and it exhibited diminished motility on serum-containing soft agar. The significance of our findings with respect to the stress-dependent responses of V. cholerae in the host is discussed.
MATERIALS AND METHODS
Chemicals, bacterial strains, and oligonucleotides.
Unless stated otherwise, chemicals were purchased from Sigma-Aldrich Chemie GmbH, Germany. Oligonucleotides were custom synthesized by Eurofins MWG Operon, Germany. Yeast extract, tryptone, and Bacto agar were purchased from Becton, Dickinson, USA. The strains and plasmids used in this study are listed in Table 1.
TABLE 1.
Strains and plasmids
| Strain or plasmid | Characteristicsa | Reference or source |
|---|---|---|
| Strains | ||
| V. cholerae O395-N1 | ΔctxA Strr | 13 |
| V. cholerae O395-N1 ΔpomAB | ΔctxA Strr ΔpomAB | 26 |
| V. cholerae O395-N1 ΔqseC | ΔctxA Strr ΔqseC | This work |
| E. coli TOP10 | Invitrogen | |
| E. coli β3914 | 20 | |
| Plasmids | ||
| pJET1.2 | CloneJET, Fermentas | |
| pJET-ΔqseC | This work | |
| pSW7848 | 19 | |
| pSW7848-qseC | This work |
Strr, streptomycin resistance.
Sequence alignments.
Amino acid sequences of QseC homologs were obtained from the NCBI BLAST database by using the algorithm blastp (protein-protein BLAST) with the query sequence QseC from the E. coli K-12 substrain W3110 (GenBank accession no. YP_491218.1). Multiple sequence alignments of 40 sequences for QseC were performed using the ClustalW algorithm (14) implemented in the BioEdit Sequence Alignment Editor (version 7.0.5.3) (15), with default settings. Identical and similar residues were identified with BioEdit using an identity and similarity threshold of 83% and a Blosum62 (16) scoring matrix. Sequence analyses, drawings of plasmid charts, and designs of oligonucleotides were performed using Geneious Pro 5.4.6.
Cultivation media.
LB medium (1% [wt/vol] tryptone, 0.5% [wt/vol] yeast extract, and 1% [wt/vol] NaCl) was used for the cultivation of V. cholerae. For growth on solid medium, 1.5% (wt/vol) Bacto agar was added before the autoclaving. To monitor the formation of adrenochrome from epinephrine, V. cholerae was grown in a modified M9 medium containing 7 g liter−1 Na2HPO4, 3 g liter−1 KH2PO4, 0.5 g liter−1 NaCl, 1 g liter−1 NH4Cl, 0.1 mM CaCl2, 1 mM MgSO4, 20 mg liter−1 each of methionine, threonine, histidine, and leucine, and 1 mg liter−1 thiamine, and 0.2% (wt/vol) glucose as a carbon source. To 20 ml of medium, 50 μg ml−1 streptomycin and 0.1 mM epinephrine were added at the start of the experiment. Serum-based SAPI medium was used for growth experiments and motility assays in the presence of catecholamine hormones (17). SAPI medium is a salt-based medium containing 0.05% (wt/vol) NH4NO3, 0.025% (wt/vol) KH2PO4, and 0.025% (wt/vol) KCl dissolved in 680 ml deionized H2O. The pH was adjusted to 7.2 to 7.5 with 5 M HCl. After the autoclaving, 0.1% (vol/vol) sterile 1 M MgSO4, 0.277% (vol/vol) sterile 1 M glucose, and 1% (vol/vol) sterile 1 M HEPES with a pH of 7.5 were added. Prior to the experiments, 30% (vol/vol) untreated fetal calf serum (FCS) (Seromed) or adult calf serum (ACS) (Sigma) was added. For motility experiments, 0.25% (wt/vol) Bacto agar was added prior to the autoclaving. To inactivate the bacteriostatic compounds, serum was heated to 60°C and incubated at this temperature for 30 min (heat-treated serum). This treatment inactivates heat-sensitive viruses and mycoplasms and reduces the amount of heat-sensitive components in the serum, e.g., vitamins, growth factors, and the mammalian complement system. Growth was also monitored in RPMI 1640 (FG 1385; Biochrom), which is used as a cultivation medium for eukaryotic cells (18). It contains NaCl, KCl, amino acids, vitamins, glucose as a carbon source, and 20 mM HEPES as a buffer substance. Phenol red was added as a pH indicator. Heat-treated fetal calf serum (30% [vol/vol]) was added prior to inoculation with V. cholerae.
Construction of a chromosomal deletion mutant in V. cholerae.
The upstream and downstream regions of the qseC-related gene (GenBank accession no. ABQ19142.1; denoted “sensor protein QseC, provisional”) were amplified from the genomic DNA of V. cholerae O395-N1 using primer pairs qseC up fwd BamHI and qseC up rev (upstream fragment) and qseC do fwd and qseC do rev (downstream fragment). In a second step, the up and down fragments were joined using primers qseC up fwd BamHI and qseC do rev (see Table S1 in the supplemental material). For the two reactions, Phusion high-fidelity DNA polymerase was used according to the manufacturer's protocol. After purification, the 244-bp fragment was ligated into pJET1.2 using the CloneJET kit by Fermentas and transformed into chemically competent E. coli TOP10. The plasmid pJET-ΔqseC was isolated from E. coli TOP10 and was digested using the enzymes BamHI and XbaI according to the manufacturer's protocol. Then, it was ligated into the linearized suicide plasmid pSW7848 (19) and transformed into E. coli β3914 (20). V. cholerae and E. coli β3914(pSW7848-qseC) were costreaked from LB plates with the appropriate antibiotics and diaminopimelic acid onto LB plates containing no antibiotics but containing 200 μM diaminopimelic acid. The agar plates were incubated for 6 h at 37°C. Cells were harvested with 2 ml LB medium, and serial dilutions (10−1 to 10−3) were prepared in LB medium. From each dilution, 100 μl was spread on an LB agar plate containing streptomycin (50 μg/ml), chloramphenicol (25 μg/ml), and glucose (1% [wt/vol]). Plates were incubated overnight at 37°C, and single colonies were streaked on LB plates containing 50 μg/ml streptomycin and 10 mM arabinose. These plates were incubated at room temperature until colonies appeared; these were tested by PCR (OneTaq polymerase; NEB) using primers qseC up fwd and qseC do rev (see Table S1). The expected length of the PCR product from a V. cholerae mutant strain lacking the qseC-like gene is 240 bp. We identified two clones carrying the deletion (see Fig. S1 in the supplemental material).
Monitoring the growth of V. cholerae.
Bacterial growth experiments were performed with 5 ml liquid serum-SAPI medium or RPMI 1640 medium in sterile test tubes (160 by 16 mm; Assistent) under aerobic conditions. The optical densities of the bacterial cultures at a wavelength of 600 nm (OD600) were determined. One milliliter of a V. cholerae overnight culture was centrifuged at a relative centrifugal force (RCF) of 10,416 for 1 min. The bacterial pellet was resuspended in an equal amount of SAPI medium. This culture was used to inoculate 5 ml of serum-SAPI medium to an OD600 of 0.01. Growth was monitored by measuring the OD600s at specific times.
Motility on soft agar plates.
To prepare the soft agar plates, warm SAPI soft agar medium (14 ml) was mixed with 6 ml adult calf serum and 20 μg μl−1 streptomycin in sterile 50-ml reaction tubes. A hormone (0.1 mM epinephrine or norepinephrine) or 0.35 mM phentolamine was added as indicated in the legend to Fig. 9. The media were briefly vortexed and poured into sterile petri dishes. These plates were kept closed overnight at room temperature and dried with partially opened lids under a sterile bench for about 1 h prior to handling. Five milliliters of LB medium supplemented with streptomycin was inoculated with a single colony of V. cholerae, and bacteria were grown overnight at 37°C. Fresh LB medium was inoculated with the overnight cultures to an OD600 of 0.01, and aerobic growth was continued until an OD600 of 0.5 was reached. Cells were briefly centrifuged and resuspended in an equal amount of SAPI medium. Aliquots (1.25 μl) were spotted on the soft agar plates, with six spots for each condition. Care was taken to obtain spots with similar diameters at the start of the experiment. The soft agar plates were incubated at 37°C.
FIG 9.
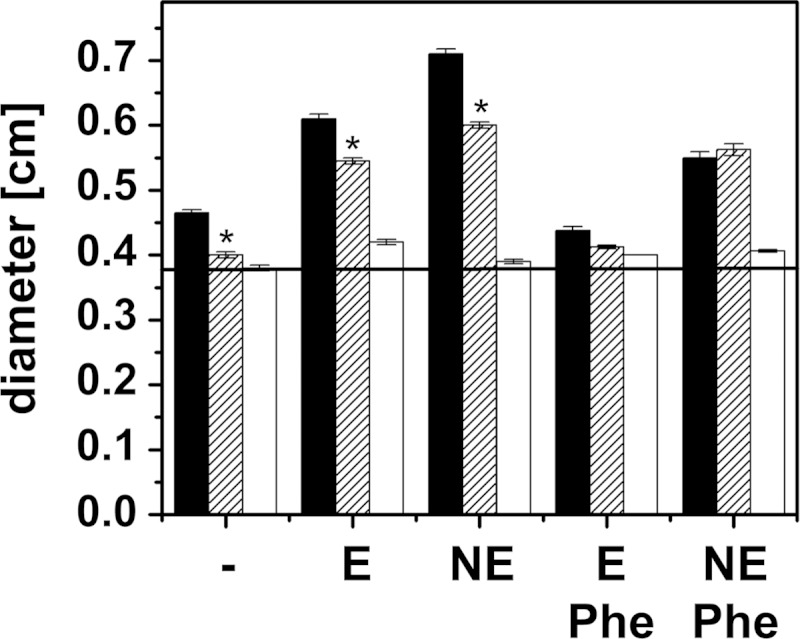
Motility and catecholate response of the V. cholerae mutant lacking the qseC-like gene. Motility in serum-containing soft agar was studied with the V. cholerae reference strain, the mutant lacking the qseC-like gene, and V. cholerae ΔpomAB devoid of the flagellar stator proteins PomA and PomB. Diameters of colonies were determined after 24 h. V. cholerae reference strain, black bars; V. cholerae ΔqseC, hatched bars; V. cholerae ΔpomAB, white bars. V. cholerae ΔpomAB is immotile due to the lack of the flagellar stator components PomAB, and it served as an indicator for colony growth in the absence of motility. No addition, −; 0.1 mM epinephrine, E; 0.1 mM norepinephrine, NE; 0.35 mM phentolamine, Phe. The averages and standard errors from eight experiments are presented. P values were calculated using Student's t test. *, P < 0.06.
Statistical analysis.
Statistical analysis (Student's t test) and graphical presentation of data were performed using Origin 8.0.
Isolation of total RNA from V. cholerae.
Five milliliters of fresh LB medium supplemented with 50 μg ml−1 streptomycin was inoculated with a single colony of V. cholerae. After the cells were grown aerobically overnight at 37°C, 1 ml of the bacterial culture was centrifuged and the bacterial pellet was resuspended in an equal volume of SAPI medium. Five milliliters of serum-SAPI medium (30% [vol/vol] adult calf serum) supplemented with 0.1 mM E, NE, or FeSO4 was inoculated with the overnight culture to an OD600 of 0.01. Addition of FeSO4 was required in the control reaction mixture to obtain comparable amounts of mRNA from the different V. cholerae cultures. Cells were grown aerobically (180 rpm) at 37°C until they reached an OD600 of 0.5 to 0.7. Enzymatic lysis and proteinase K digestion were performed according to the manufacturer's protocol using the RNeasy minikit (Qiagen). The RNA yields from V. cholerae grown with 0.1 mM FeSO4, 0.1 mM E, or 0.1 mM NE were 0.39 μg μl−1, 0.18 μg μl−1, and 0.77 μg μl−1, respectively. The quality of the isolated RNA was assessed by gel electrophoresis in 0.75% LE GP agarose (Biozym) with 1× Tris-acetate-EDTA (TAE) buffer, 7.4% (vol/vol) formaldehyde, and 1-fold-concentrated GelRed from a 10,000-fold-concentrated stock solution (Biotium). The RiboRuler high-range RNA ladder (Thermo Scientific) was used as the molecular standard. For the synthesis of cDNA, the SuperScript III first-strand synthesis system for reverse transcriptase PCR (RT-PCR) (Invitrogen) was used according to the manufacturer's protocol, using 8 μl of each RNA preparation. The random hexamer primers (50 ng/μl) supplied by the manufacturer were used in the synthesis reaction.
qRT-PCR.
A SensiFAST SYBR and fluorescein kit (Bioline) was used to determine the expression level of specific target genes in a quantitative reverse transcriptase PCR (qRT-PCR). Each reaction mix with a volume of 20 μl was prepared with 500 nM each primer (final concentration) (see Table S1 in the supplemental material) and 2.5 ng cDNA. qRT-PCRs were performed in a CFX96 cycler (Bio-Rad) using a two-step protocol with the following thermal cycling parameters: 95°C for 2 min (polymerase activation), with 40 cycles at 95°C for 10 s (denaturation) and 60°C for 20 s (primer annealing and extension). Following the last amplification cycle, a melting curve was generated by heating from 65°C to 95°C in increments of 0.5°C/s. Calculations of primer efficiencies were performed with CFX Manager software (Bio-Rad). The efficiencies of primers with regard to binding to rssA, pomB, and qseC were tested as triplets with different cDNA concentrations (0.0025 ng, 0.025 ng, 0.25 ng, and 2.5 ng). All primer pairs had an R2 of 1, with efficiencies of 97.8% (rrsA), 95.5% (pomB), and 97.3% (qseC) (see Fig. S2 in the supplemental material). Each gene and condition was measured in triplicate. The threshold cycle (CT) values of pomB and qseC were normalized using rssA, a housekeeping gene coding for the 16S rRNA. Expression ratios were calculated using the efficiency (E)-corrected Pfaffl equation below (21), with Etarget as the amplification efficiency of pomB or qseC, Ereference as the amplification efficiency of rssA, CP as the crossing point (the point at which the fluorescence rises above the background fluorescence), ΔCPtarget as the CP deviation of the control minus the sample of the target gene transcript, and ΔCPreference as the CP deviation of the control minus the sample of the reference gene transcript. The values of rssA, pomB, and qseC, obtained for V. cholerae cultivated in serum-SAPI with 0.1 mM FeSO4, were used as controls. The Pfaffl equation is as follows: .
Analysis of catecholates in serum-based medium by electrochemical detection.
V. cholerae was grown aerobically in 30 ml serum-SAPI (ACS) under shaking (180 rpm) at 37°C in the presence of 50 μg ml−1 streptomycin and either 0.1 mM E or NE. The OD600 at the beginning of the experiment was adjusted to 0.01 with a liquid overnight culture of V. cholerae. For this purpose, the OD600 of the overnight culture was determined, and 1 ml of the culture was centrifuged for 1 min at an RCF of 8.609. The supernatant was discarded, and the bacterial pellet was resuspended in SAPI medium. As controls, serum-SAPI (ACS) medium with either 0.1 mM E or NE but without the addition of V. cholerae was included. At the points in time indicated in Fig. 6, aliquots of the cultivation medium were taken and centrifuged. The supernatant was filtered through a 0.2-μm filter device and was shock frozen in liquid nitrogen. Samples were then stored at −80°C. The detection of E and NE in serum-SAPI medium was performed by high-performance liquid chromatography (HPLC) with electrochemical detection. The sample preparation with alumina extraction was adapted from the method first described by Anton and Sayre (22). In brief, 1 ml of serum-SAPI (ACS) and 1,000 pg of an internal standard (dihydroxybenzylamine; Thermo Fisher Scientific) were added to extraction tubes containing 20 mg Al2O3 previously activated with 600 μl 2 M Tris-EDTA buffer (pH 8.7). Samples were thoroughly mixed in an overhead shaker for 10 min and afterwards were washed 3 times with 1 ml of 16.5 mM Tris-EDTA buffer (pH 8.1), followed by centrifugation for 1 min (1,000 × g, 4°C). The catecholates were eluted by addition of 120 μl 0.2 M HClO4, short mixing, and centrifuging for 1 min (1,000 × g, 4°C). Thirty microliters of the eluate was injected into the HPLC system (Thermo Fisher Scientific Dionex UltiMate 3000), which consisted of an autosampler (WPS-3000TBSL), a pump (ISO-3100BM), a degasser (SRD-3200), and an electrochemical detector (Coulochem III) with a conditioning cell (model 50210A) and an analytical cell (model 5011A). The potentials of the cells were set at 300 mV, 50 mV, and −250 mV. The separation was performed with a reversed-phase column (Recipe). Cat-A-Phase II was used as the mobile phase, with a flow rate of 1 ml min−1.
FIG 6.
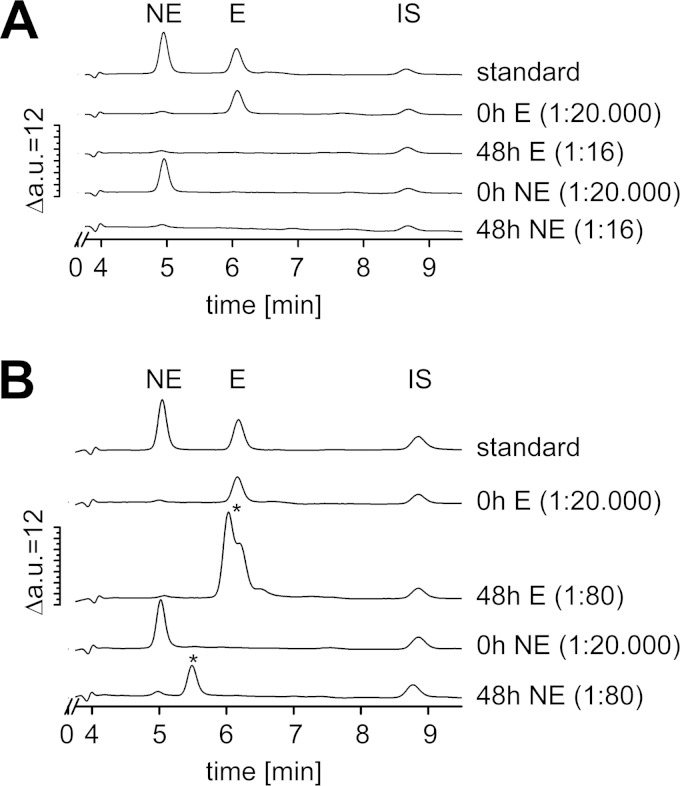
Detection of epinephrine and norepinephrine in serum-SAPI in the absence (A) or presence (B) of V. cholerae. Catecholates were analyzed by HPLC combined with electrochemical detection. (A) Supernatants from serum-SAPI without bacteria after 0 h or 48 h; (B) supernatants from V. cholerae grown in serum-SAPI after 0 h and 48 h of growth. At the start of the experiment (t = 0 h), 0.1 mM norepinephrine (NE) (1.7 × 104 ng ml−1) or 0.1 mM epinephrine (E) (1.8 × 104 ng ml−1) was added. Standards were as follows: 1,000 pg ml−1 norepinephrine, 1,000 pg ml−1 epinephrine, and 1,000 pg ml−1 internal standard (IS) in serum-SAPI. In the chromatograms, norepinephrine and epinephrine eluted at 4.9 min and 6.0 min, respectively. Note the different dilutions of the measured supernatants. Asterisks indicate the peaks assigned to unknown compounds. a.u., arbitrary units.
Analysis of catecholates in minimal medium by UV-Vis detection.
V. cholerae was grown aerobically under shaking conditions (180 rpm) at 30°C in modified M9 medium with or without 0.1 mM E. The starting OD600 was adjusted to 0.05 using an inoculum of V. cholerae cells from an overnight culture in LB medium, which had been centrifuged (7,000 rpm), washed with M9, collected by centrifugation, and resuspended in M9 medium. To monitor the fate of E in the absence of V. cholerae and to exclude the formation of adrenochrome from a source other than E, a control with E but without inoculum and a control with inoculum but without E were handled in parallel manners. At the time points indicated in Fig. 7, aliquots were taken, OD600 values were determined, and cells were removed by centrifugation (7,000 rpm). The supernatant was passed through a polytetrafluoroethylene (PTFE) filter (pore size, 0.2 μm; Amchro), and 50 μl was immediately loaded onto an HPLC system (Bischoff) consisting of a pump (type 3350), an analytical cell, and a UV-visible (UV-Vis) multiple-wavelength detector (DAD-4L). UV-Vis spectra for HPLC peak analysis were obtained using a diode array DAD-3000 detector (Bischoff). If necessary, dilutions were prepared in eluent. The eluent was continuously degassed (Gastorr TG-14), and the column was heated to 30°C (S4010 oven; Sykam). E was detected at 279 nm and adrenochrome was detected at 480 nm (23) following isocratic separation on the reversed-phase ProntoSIL-120-3-C18 AQ column (Bischoff) with the catecholamine phase A eluent. To prepare 1.04 liters of eluent, 11.22 g sodium acetate (trihydrate), 6.73 g citric acid (water free), and 0.048 g disodium acetate were dissolved in 0.935 liter of deionized H2O and filtered (pore size, 2 to 3 μm; Rotilab 114 A). If required, the pH (30°C) was adjusted to 4.35, and 0.105 liter of methanol was added. The final pH was 4.4. The flow rate was varied, with 0.3 ml min−1 from 0 to 1 min, a gradient (0.3 ml min−1 to 0.7 ml min−1) from 1 to 3 min, and 0.7 ml min−1 from 3 to 7 min. Concentrations of adrenochrome and E were determined with the help of a linear regression curve using standards in eluent ranging from 1 μM to 500 μM adrenochrome or 10 μM to 10 mM E.
FIG 7.
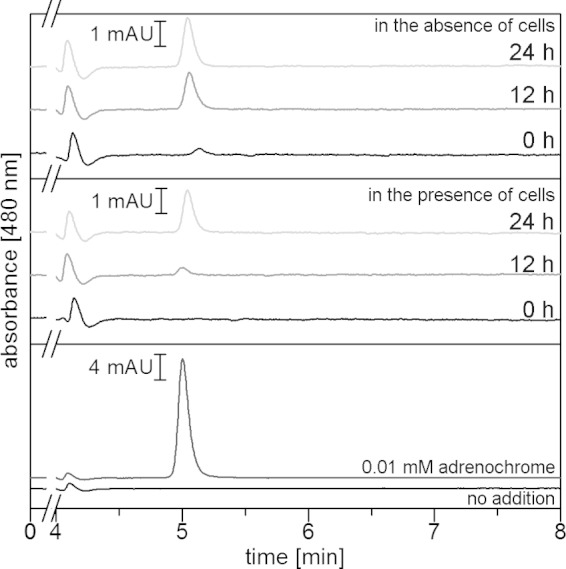
Adrenochrome formation in minimal medium in the absence or presence of V. cholerae. Adrenochrome was separated by HPLC and quantified from the area of the peak eluting at 5.0 min. In the top panel (medium with 0.1 mM E in the absence of cells) and middle panel (medium with 0.1 mM E in the presence of cells), aliquots were taken at 0 h, 12 h, or 24 h and subjected to HPLC. In the bottom panel, the cell-free supernatant of V. cholerae cells was grown to the stationary phase in minimal medium without added E. Cell-free supernatant, black trace; cell-free supernatant with 0.01 mM adrenochrome added immediately before analysis, dark-gray trace. mAU, arbitrary units (103).
RESULTS
Epinephrine and norepinephrine stimulate the growth of V. cholerae.
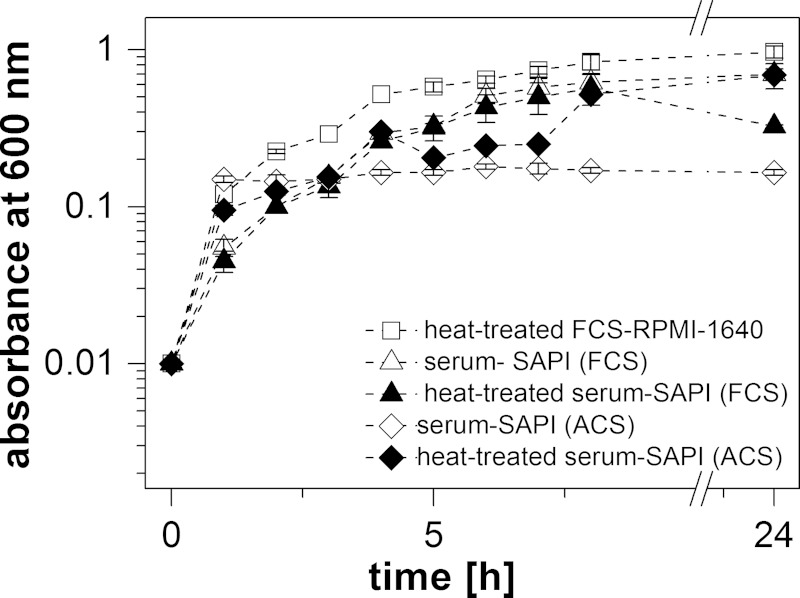
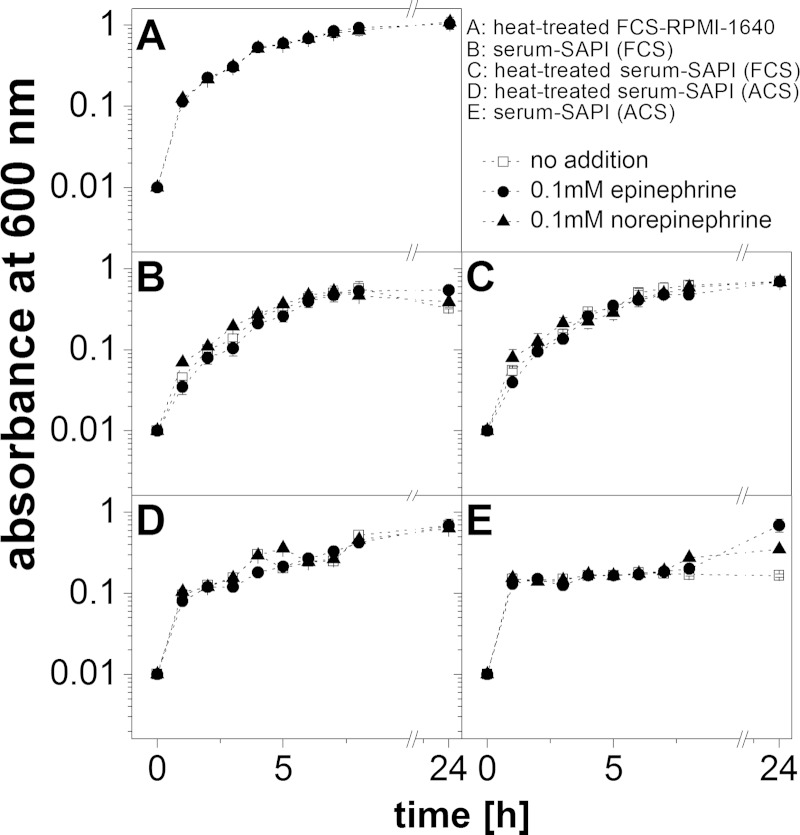
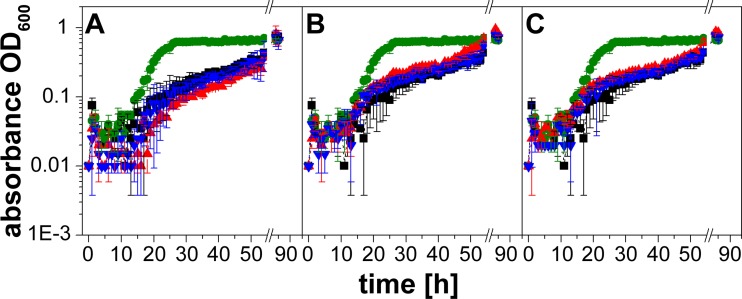
To simulate the environment in a eukaryotic host, V. cholerae was treated with the stress hormones epinephrine (E) and norepinephrine (NE) in a serum-based medium that contained bacteriostatic compounds like the complement system (24). The upper intestinal tract is a microaerobic environment with O2 tensions decreasing from the highest physiological concentration (160 torr or 21% O2 partial pressure) to 58 torr in the stomach and 32 torr in the midduodenum (determined in mice [25]). We therefore chose aerobic conditions and first tested the growth of V. cholerae in different serum-based media without added E or NE. As expected, the highest growth yields were observed with a medium (FCS-RPMI 1640) containing heat-treated fetal calf serum (FCS) (Fig. 1). The lowest growth yields were observed in serum-SAPI with adult calf serum (ACS) that had not been heated. We next asked if E or NE (each 0.1 mM) stimulated growth in a native or a heat-treated serum-based medium. Stimulation was observed in the presence of native ACS (Fig. 2E), where the final absorbances at 600 nm were 0.69 (E), 0.35 (NE), and 0.17 (no hormone added). Lower hormone concentrations (0.1 to 10 μM) did not significantly enhance the growth of V. cholerae in this medium (Fig. 3). Native calf serum contains iron-chelating proteins, like transferrin, which greatly diminish the concentration of free-iron cations, thereby preventing the growth of bacteria. Studies performed with E. coli indicated that E and NE liberate iron from specific iron-binding proteins in the serum-based medium (10), thereby stimulating bacterial growth. Iron clearly accelerated the growth of V. cholerae in the serum-SAPI (ACS) medium (Fig. 3). The levels of growth of V. cholerae with 0.1 mM E or NE, without added hormone, or with 0.26 mM FeSO4 were compared. The first results obtained with serum-SAPI (ACS) (Fig. 2E) revealed that the effect of E or NE (each 0.1 mM) was observed after a prolonged incubation. When the experiments were repeated, growth was monitored continuously for 55 h, and the final cell densities were determined after 85 h. At this time, all cultures grown in serum-SAPI (ACS) reached similar cell densities, but there were profound differences with respect to the growth rate and the onset of growth (Fig. 4). In the presence of FeSO4, cell growth commenced after 10 h and was shortly followed by V. cholerae cells grown in NE. In the medium with no additions or with E added, cells exhibited pronounced lag phases and showed similar growth behaviors up to 40 h. At this time, cells with E showed a slight increase in growth rate compared to that of the control with no hormones added (Fig. 4). These findings prompted us to test whether the catecholate hormones E and NE affected the motility of V. cholerae and the expression of the qseC-like gene. We also investigated the fate of these compounds in the serum and studied the phenotype of a mutant devoid of the qseC-like gene. Since the response to the hormones was most obvious in the medium with native adult calf serum (serum-SAPI), this medium was chosen for further studies and will be referred to as serum-SAPI.
FIG 1.
Growth of V. cholerae in different serum-based media. Cells were grown aerobically for 24 h at 37°C. The OD600 was measured every hour for 8 h after inoculation. Averages and standard deviations of results from two experiments are shown.
FIG 2.
Effect of catecholamines on growth of V. cholerae in different serum-based media. Cells were grown aerobically for 24 h at 37°C. The OD600 was measured every hour for 8 h after inoculation. Averages and standard deviations of results from two experiments are shown.
FIG 3.
Growth of V. cholerae in adult calf serum is not affected by 0.1 μM to 10 μM epinephrine or norepinephrine. Serum-SAPI (ACS) medium was supplemented with 0.1 μM (A), 1 μM (B), or 10 μM (C) epinephrine or norepinephrine. Cells were grown aerobically for 85 h at 37°C. The OD600 was measured every hour for the first 54 h. Control without addition, black; 0.26 mM FeSO4, green; norepinephrine, blue; epinephrine, red. Experiments were performed as triplets, and the outliers were omitted. Averages and standard errors were calculated from two experiments.
FIG 4.
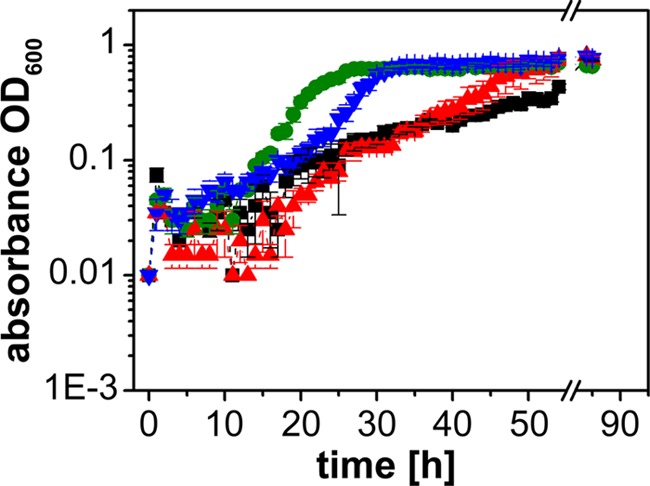
Growth of V. cholerae in adult calf serum is enhanced in the presence of 0.1 mM epinephrine or norepinephrine. Cells were grown aerobically in serum-SAPI (ACS) for 85 h at 37°C. The OD600 was measured every hour for the first 54 h. Control without addition, black; 0.26 mM FeSO4, green; 0.1 mM norepinephrine, blue; 0.1 mM epinephrine, red. Experiments were performed in triplicate, and outliers were omitted. Averages and standard errors were calculated from two experiments.
Increased motility of V. cholerae in the presence of epinephrine or norepinephrine.
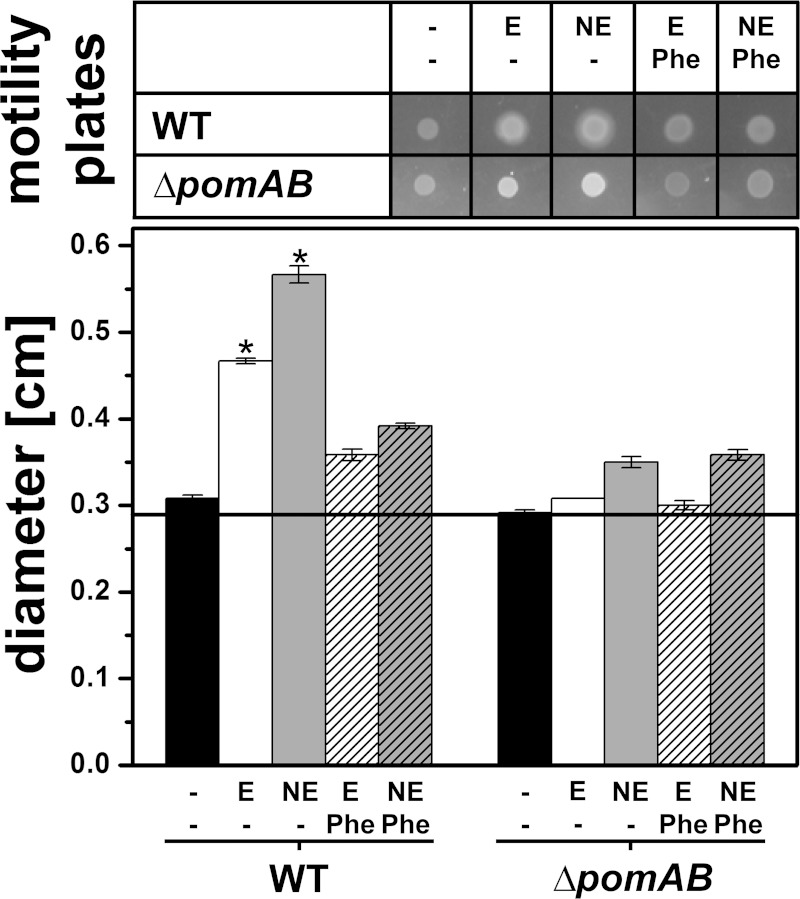
The effect of the catecholates E and NE (each 0.1 mM) on the motility of V. cholerae was studied using serum-SAPI soft agar (Fig. 5). We included the pomAB deletion strain lacking a functional flagellum (26) as the control since the stimulation of growth by the catecholates might increase the colony size even in the absence of cellular motility. After 24 h of incubation, the increased spreading of the cells was detected in E-treated and NE-treated wild-type cells compared to spreading in the wild-type cells without catecholates. With the pomAB deletion strain, colony size increased only slightly with E or NE, which was probably caused by growth stimulation in the presence of the catecholates. Importantly, the addition of phentolamine, a reversible, nonselective α-adrenergic antagonist (27), reduced motility rings of the E-treated and NE-treated wild-type cells to diameters observed with the pomAB deletion strain (Fig. 5).
FIG 5.
Epinephrine or norepinephrine increases the motility of V. cholerae in soft agar with serum-SAPI (ACS). Diameters of growth zones of the V. cholerae reference strain and its variant lacking the pomA and pomB genes were determined after 24 h. The top panel shows typical motility rings under the indicated conditions. In the lower panel, the averages and standard errors from six experiments are presented. P values were calculated using Student's t test (*, P ≤ 0.012). Black bars, control without addition; E, 0.1 mM epinephrine; NE, 0.1 mM norepinephrine; Phe, 0.35 mM phentolamine. V. cholerae ΔpomAB lacks the flagellar stator components PomAB and serves as an indicator for colony size in the absence of swimming motility. WT, wild type.
Detection of epinephrine and norepinephrine in bacterial supernatants using HPLC.
To elucidate the fate of E and NE in the supernatants of a serum-containing medium, we determined the amount of these catecholate hormones using HPLC combined with electrochemical detection. E and NE recovered both from the serum-SAPI medium without bacteria and from the culture supernatants eluted at the expected retention times at 6.0 min and 4.9 min, respectively, and at the expected amount (around 2 × 104 ng ml−1) at the start of the experiments (Fig. 6A and B). Analyses were performed immediately after addition of the catecholates (0 h) and after 48 h. The catecholamines (dilution, 1:16) were no longer detectable in serum-SAPI, which was incubated for 48 h in the absence of bacteria. However, in the supernatant from the bacterial cultures treated with E (dilution, 1:80), small amounts of E (around 1% of the initial amount at 0 h) were still detectable at the expected retention time (6.0 min) after 48 h. In addition, a second peak appeared shortly before the E peak (retention time, 5.8 min) (Fig. 6B). Similarly, in the supernatant from the bacterial cultures treated with NE (dilution, 1:80), an unknown compound appeared. The retention time was 5.4 min, which was approximately between the retention times of NE and E (Fig. 6B). NE could not be detected in these 1:80-diluted supernatants after 48 h.
Conversion of epinephrine to adrenochrome during cultivation of V. cholerae in minimal medium.
HPLC analysis of catecholates from a highly complex organic matrix like serum-SAPI required an extraction step that may result in (partial) loss of catecholate-derived conversion products. Moreover, the identification of conversion products in serum-containing medium is hampered by many other low-molecular-weight compounds. To further characterize the conversion product(s) of catecholates, we followed the fate of 0.1 mM E in minimal medium in the presence or absence of V. cholerae. Using this medium rather than serum-SAPI allowed direct injection of cell-free supernatants into an HPLC system connected to a UV-Vis detector operating at multiple wavelengths. In Fig. 7, chromatograms recorded at 480 nm demonstrate the formation of adrenochrome in minimal medium with 0.1 mM E in the absence or presence of V. cholerae cells. The peak assigned to adrenochrome based on its retention time exhibited a UV-Vis spectrum that was comparable to the spectrum of the adrenochrome standard. Most likely, adrenochrome was formed during the oxidation of E with superoxide, O2−. In fact, the reaction of E with superoxide under stoichiometric formation of adrenochrome is the principle behind a spectrophotometric assay for quantification of superoxide (28). In the medium with 0.1 mM E, which was freshly inoculated with V. cholerae cells at 0 h, the adrenochrome concentration was below the detection limit. After 12 h, when the cells had reached the late exponential phase (OD600, 0.4), the medium contained 0.15 μM adrenochrome, and its concentration increased to 0.76 μM after 24 h when the stationary phase was reached (OD600, 0.5) (Fig. 7, middle). During this time, we did not detect a significant decrease in the concentration of E, as was determined in parallel in aliquots that were diluted in eluent prior to HPLC analysis. In the control performed in the absence of cells, larger amounts of adrenochrome were detected. Immediately after addition of E, the medium already contained 0.15 μM adrenochrome, increasing to 0.80 μM and 0.98 μM adrenochrome after 12 and 24 h, respectively (Fig. 7, top). Again, there was no significant change in E concentration, but it should be noted that the maximum amount of adrenochrome detected represented only 1% of the initial amount of E added to the medium, a value that lay within the standard deviation of quantification of E. Adrenochrome formation from E in minimal medium was slowed down in the presence of growing V. cholerae cells compared to that in the cell-free control. We considered that the adrenochrome detected in culture supernatants may represent a metabolite excreted by V. cholerae but not a conversion product from E added at the beginning of the growth experiment. To exclude this, the supernatant from a culture in the late stationary phase grown in the absence of E was analyzed by HPLC (Fig. 7, bottom). No adrenochrome was detected. When adrenochrome (10 μM) was added to this supernatant, the expected peak eluting at 5 min was observed. The peak area corresponded to a concentration of 9.4 μM adrenochrome based on a calibration with standards in eluent (Fig. 7, bottom).
QseC-related proteins in V. cholerae strains.
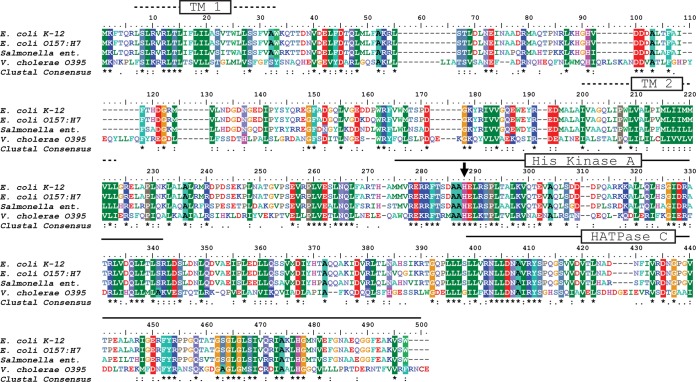
Some bacteria are able to sense catecholamine hormones by the two-component system QseBC described for E. coli (29) and S. enterica serovar Typhimurium (4). Using QseC from E. coli (GenBank accession no. YP_491218) as a query in a protein-protein BLAST, we found that QseC-related proteins are present in a variety of important human, animal, and plant pathogens. The QseC proteins from E. coli and S. enterica serovar Typhimurium exhibit 80% identity and 89% similarity (Fig. 8). With E. coli QseC as the query, a QseC-related protein was found in V. cholerae (29% identity, 47% similarity) that was highly conserved in different strains (V. cholerae O1 classical O395, O1 El Tor B33, and O1 bv. El Tor). The genome of V. cholerae B33 harbors the cholera toxin (CTX) prophage typical for the El Tor biovar type but with ctxAB genes coding for the cholera toxin of the classical biovar type (30). The sequences deposited under GenBank accession no. EEO17765.1 (V. cholerae B33) and AJZ98549.1 (V. cholerae El Tor) represent QseC-like proteins starting with a Met residue equivalent to Met 25 in the QseC-like protein in V. cholerae strain O395 (GenBank accession no. ABQ19142.1). We considered this very unlikely, as this would correspond to a truncation of the first transmembrane helix (TM1) of the histidine kinase (Fig. 8) (31). Twenty-four codons upstream of the transcription start site of the gene encoding QseC-like protein from V. cholerae B33 or El Tor as assigned by GenBank, a Leu codon (UUG) that most likely represents an alternative start codon was found (32). This is followed by codons for hydrophobic amino acid residues that are conserved in the QseC-like protein from V. cholerae strain O395. Compared with typical QseC proteins (Fig. 8), the conserved His (H241) in the His kinase A domain, which undergoes autophosphorylation catalyzed by the histone ATPase (HATPase) c domain in response to a signal (33), was identified in the QseC-like proteins from all examined V. cholerae strains.
FIG 8.
Alignment of QseC sequences from enterobacteria with a putative sensor histidine kinase in V. cholerae. Using E. coli K-12 QseC as the input, sequence comparisons were performed using an identity and similarity threshold of 83% and the BLOSUM62 scoring matrix. E. coli K-12 QseC (GenBank accession no. YP_491218.1), E. coli O157:H7 QseC (GenBank accession no. AAG58160.1) (98% identity, 99% similarity), and Salmonella enterica QseC (GenBank accession no. WP_023185439.1) (80% identity, 89% similarity) are compared. The putative sensor histidine kinase in V. cholerae O1 classical strain O395 (GenBank accession no. ABQ19142.1) (29% identity, 47% similarity) was also included. The predicted transmembrane helices TM1 and TM2, the conserved His residue accepting a phosphoryl group (arrow) within the His kinase A domain, and the C-terminal histone ATPase c domain (HATPase C) are indicated.
Properties of the V. cholerae mutant lacking the qseC-like gene.
A chromosomal knockout of the qseC-like gene was created without introduction of a resistance marker, and the resulting mutant strain was studied with respect to growth and motility in serum-based medium. Its growth behavior was comparable to that of the V. cholerae wild-type strain with respect to rates and stimulation in the presence of E and NE. This was in marked contrast to the motility in serum-containing soft agar, which was severely impaired in the mutant strain even in the absence of catecholates. After 24 h, the motility ring of the mutant was only slightly larger than the colony diameter of the immotile V. cholerae ΔpomAB strain (Fig. 9).
However, addition of E or NE stimulated the motility of V. cholerae lacking the qseC-like gene, although to a smaller extent than observed with the V. cholerae reference strain. We next asked if phentolamine, the α-adrenergic antagonist, had an effect on the E- or NE-stimulated motility of the mutant devoid of the qseC-like gene. With the V. cholerae wild type, phentolamine inhibited E- and NE-stimulated motility, which is in accord with the data shown in Fig. 5. The situation was different with the mutant lacking the qseC-like gene. In the presence of E, phentolamine diminished the motility rings from 0.55 cm to 0.42 cm, indicating some competition between E and phentolamine and suggesting the presence of an additional sensor protein in V. cholerae lacking the qseC-like gene. In marked contrast, the stimulation of motility by NE of the V. cholerae mutant strain was not prevented by phentolamine; the diameters were 0.60 cm with NE compared to 0.58 cm with NE and phentolamine (Fig. 9). This indicated that the QseC-like protein participated in the NE-mediated stimulation of motility in V. cholerae in serum-containing medium.
Expression of qseC and pomB is enhanced in the presence of catecholamines.
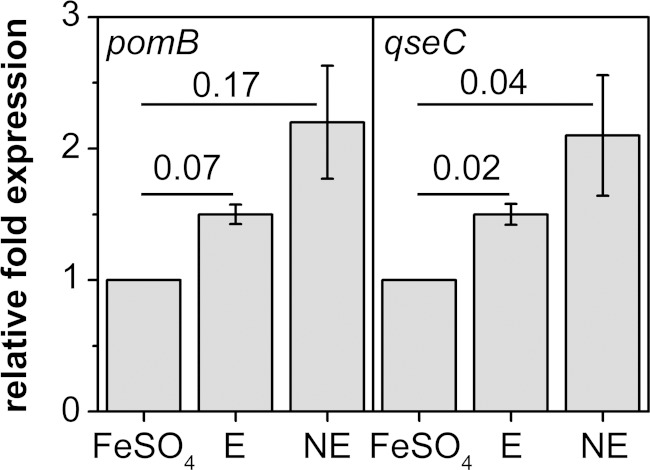
To demonstrate the expression of the qseC-like gene in V. cholerae and to study if expression is modulated by E and NE, we performed qRT-PCR on qseC. In addition, we followed pomB expression in response to E or NE since expression of flagellar genes is known to be stimulated upon catecholate treatment (4). Expression levels of the qseC-related gene and of pomB were compared with the level of rssA, a constitutively expressed household gene. Total RNA was extracted from cells grown in serum-SAPI with E or NE added, but despite many attempts, we never obtained RNA in sufficient quantity and quality from cells grown in serum-SAPI only. We therefore used cells grown in the absence of hormones but in the presence of 0.1 mM FeSO4 as a negative control for qRT-PCR experiments to study the effect of E and NE on qseC and pomB expression. All qRT-PCR products exhibited the expected lengths, and expression of the three target genes was confirmed in LB medium and in serum-SAPI with hormones or FeSO4 added (see Fig. S2 in the supplemental material). The expression of qseC was approximately doubled in cells treated with NE and was slightly increased in cells treated with E (Fig. 10). For pomB gene expression, the results were similar. Analysis of the swarming behavior of the qseC deletion mutant indicated that this strain had lost a sensor protein for NE that interacts with phentolamine. It is noteworthy that qseC expression of the reference strain was highest in the presence of NE, which is in support of this notion.
FIG 10.
Expression of pomB and qseC under the influence of epinephrine or norepinephrine. The relative fold expressions of pomB and qseC were calculated using the Pfaffl equation with the housekeeping gene rssA as a reference. As a control for the expressions of pomB and qseC without added hormones, cells grown with 0.1 mM FeSO4 were used. Expression levels of pomB and qseC from epinephrine- and norepinephrine-treated cells are given relative to those in the control. Averages and standard deviations were calculated from technical triplicates. The numbers are P values obtained by Student's t test.
DISCUSSION
Stress increases the risk of infection by pathogenic microorganisms. The human pathogen enterohemorrhagic E. coli (EHEC) (12, 29) and the human or animal pathogen S. enterica serovar Typhimurium (4, 5, 34–36) possess the membrane-embedded QseC receptor, which senses the catecholamine hormones epinephrine (E) and norepinephrine (NE) or bacterial autoinducer 3 (AI-3). The binding of one of these compounds leads to an expression of virulence genes in bacteria (29). Nakano et al. (9) investigated the effect of E or NE on the growth of different Vibrio species under iron-limiting conditions in serum-containing media. With V. cholerae strain O395-N1, we observed that growth was impaired in serum-SAPI containing adult calf serum but was not completely prevented and may be enhanced by adding a 0.1 mM concentration of either E or NE. This indicated a specific response of V. cholerae to catecholamine hormones (or their iron complexes [10]), which was further supported by our finding that the motility of V. cholerae was enhanced in the presence of E or NE. The addition of phentolamine, a reversible, nonselective α-adrenergic antagonist (27), reduced the motility of E-treated or NE-treated V. cholerae, indicating the presence of an adrenergic receptor that responds to NE or E, similar to the QseC receptor described in E. coli (27).
Searching the genomes of different V. cholerae strains for homologs of the QseC protein in E. coli revealed the presence of QseC-like proteins in V. cholerae strains O395, B33, and O1 bv. El Tor. V. cholerae O395 also possesses a putative response regulator protein (GenBank accession no. YP_001214980) with 44% sequence identity and 67% sequence similarity to QseB from E. coli (GenBank accession no. EDU66021.1). V. cholerae O395, devoid of the qseC-like gene, exhibited reduced motility on serum-SAPI compared to the reference strain, but motility was still enhanced by E or NE, suggesting the presence of additional systems for catecholate sensing, like QseE, BasS, or CpxA (37). Expression levels of the pomB gene coding for a structural component of the flagellar stator complex (38) and the qseC-like gene were increased when V. cholerae was exposed to E or NE. This indicates an NE- or E-triggered signal transduction chain in V. cholerae, which might be related to the QseC-dependent signaling cascades described for S. enterica (4) and E. coli (29, 39). In V. cholerae, E or NE might bind to a membrane-embedded receptor encoded by the qseC-related gene, resulting in its autophosphorylation. The QseC-like receptor is proposed to transfer its phosphate to its cognate response regulator, similar to QseB, which triggers the expression of flagellar genes, including pomB. This may be accomplished via upregulation of FlrA, the master regulator of flagellar gene expression in V. cholerae (40). We were interested in the fate of NE or E during the growth of V. cholerae in serum-SAPI and adapted a protocol for extraction and separation of NE and E by HPLC in a serum-containing medium. The two compounds were clearly separated, with NE eluting at 4.9 min and E eluting at 6.0 min. The recoveries for the two hormones from the serum-based medium with or without bacterial inoculum at the start of the experiments (0 h) were satisfactory (approximately 2 × 104 ng ml−1 each), and they corresponded to the 0.1 mM hormone added to each. In supernatants from V. cholerae that had reached the stationary phase (a dilution of 1:80 after 48 h), NE could no longer be detected and the amount of E was drastically diminished. A completely unexpected finding was that in serum-SAPI medium incubated at 37°C with shaking and without bacteria, neither NE nor E could be detected after 48 h (even in 1:16 dilutions). This indicated degradation of the hormones in serum-SAPI in the absence of cells, which was delayed in serum-SAPI with growing bacteria. In the presence of V. cholerae cells, E was stabilized and was still detectable after 48 h. One can speculate that a binding protein in the native serum with a high affinity for NE or E, similar to lipocalin (41), may diminish the free-hormone concentration. In growing cultures, this binding protein, as a growth substrate, would be degraded by bacteria under release of its bound ligand, giving rise to the seemingly increased NE and E concentrations in serum-based medium with V. cholerae cells. This is considered unlikely, as the applied extraction method prior to HPLC analyses involved precipitation of proteins with perchloric acid, which releases NE or E from a binding protein in the serum. We favor another explanation for the observed stabilization of catecholates by V. cholerae growing in serum-SAPI. Distinct conversion products of NE (eluting at 5.4 min) and of E (eluting at 5.8 min) were detected in the V. cholerae cultures grown for 48 h. Most likely, these compounds are formed by oxidation of NE or E by O2 or, in a faster reaction, by an extracellular superoxide, O2−, formed by V. cholerae during respiration (42). In the case of E, a possible candidate is leucochrome, which further reacts to adrenochrome (28). Adrenochrome formation was confirmed in supernatants from V. cholerae cells in minimal M9 medium. The improved stability of E in M9 medium compared to that in serum-SAPI was due to the shorter incubation time in M9 (24 h in M9 versus 48 h in serum-SAPI) and the lower temperature (30°C in M9 versus 37°C in serum-SAPI) (43).
We propose that compared to bacteria in the cell-free medium, growing bacteria provide a more reducing environment that slows down the oxidative degradation of NE or E. First, O2 tension is lowered due to the metabolic activity of the bacteria; second, reactive oxygen species (ROS) like superoxide, which accelerate the oxidative degradation of NE or E (28) and which are formed during the initial oxidation step (44), are eliminated by ROS-protective enzymes like the periplasmic superoxide dismutase of V. cholerae (42). To the best of our knowledge, qseC expression levels in bacteria exposed to NE or E during growth have not yet been correlated with the actual concentrations of these signaling compounds in the medium. Our results indicate that NE or E concentrations decrease during growth in serum-SAPI and that the two hormones give rise to conversion products that might also have signaling capacity. In summary, we demonstrated that V. cholerae benefits from catecholate hormones when growing in a serum-based medium, which simulates the environment within the host. E or NE released by a stressed host might give V. cholerae an advantage in the colonization of the small intestine by improving iron sequestration and by increasing motility. Future studies should address whether E or NE has an effect on virulence factor and toxin production in V. cholerae.
Supplementary Material
ACKNOWLEDGMENTS
We thank Didier Mazel for providing E. coli strains π3813 and β3914 and the suicide plasmid pSW7848. We thank Felix Haukap from the Institute of Animal Husbandry and Animal Breeding, University of Hohenheim, for experimental assistance.
We thank the Life Science Center, University of Hohenheim, for financial support. This work was also supported by Deutsche Forschungsgemeinschaft grant FR 1321/5-1 (to J.S.).
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JB.00345-15.
REFERENCES
- 1.Freestone PP, Williams PH, Haigh RD, Maggs AF, Neal CP, Lyte M. 2002. Growth stimulation of intestinal commensal Escherichia coli by catecholamines: a possible contributory factor in trauma-induced sepsis. Shock 18:465–470. doi: 10.1097/00024382-200211000-00014. [DOI] [PubMed] [Google Scholar]
- 2.Freestone PP, Haigh RD, Lyte M. 2007. Specificity of catecholamine-induced growth in Escherichia coli O157:H7, Salmonella enterica and Yersinia enterocolitica. FEMS Microbiol Lett 269:221–228. doi: 10.1111/j.1574-6968.2006.00619.x. [DOI] [PubMed] [Google Scholar]
- 3.Freestone PP, Lyte M, Neal CP, Maggs AF, Haigh RD, Williams PH. 2000. The mammalian neuroendocrine hormone norepinephrine supplies iron for bacterial growth in the presence of transferrin or lactoferrin. J Bacteriol 182:6091–6098. doi: 10.1128/JB.182.21.6091-6098.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bearson BL, Bearson SM. 2008. The role of the QseC quorum-sensing sensor kinase in colonization and norepinephrine-enhanced motility of Salmonella enterica serovar Typhimurium. Microb Pathog 44:271–278. doi: 10.1016/j.micpath.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 5.Bearson BL, Bearson SM, Lee IS, Brunelle BW. 2010. The Salmonella enterica serovar Typhimurium QseB response regulator negatively regulates bacterial motility and swine colonization in the absence of the QseC sensor kinase. Microb Pathog 48:214–219. doi: 10.1016/j.micpath.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 6.Belay T, Sonnenfeld G. 2002. Differential effects of catecholamines on in vitro growth of pathogenic bacteria. Life Sci 71:447–456. doi: 10.1016/S0024-3205(02)01683-1. [DOI] [PubMed] [Google Scholar]
- 7.Hegde M, Wood TK, Jayaraman A. 2009. The neuroendocrine hormone norepinephrine increases Pseudomonas aeruginosa PA14 virulence through the las quorum-sensing pathway. Appl Microbiol Biotechnol 84:763–776. doi: 10.1007/s00253-009-2045-1. [DOI] [PubMed] [Google Scholar]
- 8.Li W, Lyte M, Freestone PP, Ajmal A, Colmer-Hamood JA, Hamood AN. 2009. Norepinephrine represses the expression of toxA and the siderophore genes in Pseudomonas aeruginosa. FEMS Microbiol Lett 299:100–109. doi: 10.1111/j.1574-6968.2009.01739.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nakano M, Takahashi A, Sakai Y, Kawano M, Harada N, Mawatari K, Nakaya Y. 2007. Catecholamine-induced stimulation of growth in Vibrio species. Lett Appl Microbiol 44:649–653. doi: 10.1111/j.1472-765X.2007.02136.x. [DOI] [PubMed] [Google Scholar]
- 10.Sandrini SM, Shergill R, Woodward J, Muralikuttan R, Haigh RD, Lyte M, Freestone PP. 2010. Elucidation of the mechanism by which catecholamine stress hormones liberate iron from the innate immune defense proteins transferrin and lactoferrin. J Bacteriol 192:587–594. doi: 10.1128/JB.01028-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burton CL, Chhabra SR, Swift S, Baldwin TJ, Withers H, Hill SJ, Williams P. 2002. The growth response of Escherichia coli to neurotransmitters and related catecholamine drugs requires a functional enterobactin biosynthesis and uptake system. Infect Immun 70:5913–5923. doi: 10.1128/IAI.70.11.5913-5923.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sperandio V, Torres AG, Kaper JB. 2002. Quorum sensing Escherichia coli regulators B and C (QseBC): a novel two-component regulatory system involved in the regulation of flagella and motility by quorum sensing in E. coli. Mol Microbiol 43:809–821. doi: 10.1046/j.1365-2958.2002.02803.x. [DOI] [PubMed] [Google Scholar]
- 13.Mekalanos JJ, Swartz DJ, Pearson GD, Harford N, Groyne F, de Wilde M. 1983. Cholera toxin genes: nucleotide sequence, deletion analysis and vaccine development. Nature 306:551–557. doi: 10.1038/306551a0. [DOI] [PubMed] [Google Scholar]
- 14.Thompson JD, Higgins DG, Gibson TJ. 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hall TA. 1999. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp Ser 41:95–98. [Google Scholar]
- 16.Henikoff S, Henikoff JG. 1992. Amino acid substitution matrices from protein blocks. Proc Natl Acad Sci U S A 89:10915–10919. doi: 10.1073/pnas.89.22.10915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Freestone PP, Lyte M. 2008. Microbial endocrinology: experimental design issues in the study of interkingdom signalling in infectious disease. Adv Appl Microbiol 64:75–105. [DOI] [PubMed] [Google Scholar]
- 18.Lindl T. 2002. Zell- und Gewebekultur, 5th ed Spektrum Akademischer Verlag, Heidelberg, Germany. [Google Scholar]
- 19.Demarre G, Guérout AM, Matsumoto-Mashimo C, Rowe-Magnus DA, Marlière P, Mazel D. 2005. A new family of mobilizable suicide plasmids based on broad host range R388 plasmid (IncW) and RP4 plasmid (IncPalpha) conjugative machineries and their cognate Escherichia coli host strains. Res Microbiol 156:245–255. doi: 10.1016/j.resmic.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 20.Le Roux F, Binesse J, Saulnier D, Mazel D. 2007. Construction of a Vibrio splendidus mutant lacking the metalloprotease gene vsm by use of a novel counterselectable suicide vector. Appl Environ Microbiol 73:777–784. doi: 10.1128/AEM.02147-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pfaffl MW. 2001. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Anton AH, Sayer DF. 1962. A study of the factors affecting the aluminum oxide-trihydroxyindole procedure for the analysis of catecholamines. J Pharmacol Exp Ther 138:360–375. [PubMed] [Google Scholar]
- 23.Remião F, Milhazes N, Borges F, Carvalho F, Bastos ML, Lemos-Amado F, Domingues P, Ferrer-Correia A. 2003. Synthesis and analysis of aminochromes by HPLC-photodiode array. Adrenochrome evaluation in rat blood. Biomed Chromatogr 17:6–13. [DOI] [PubMed] [Google Scholar]
- 24.Lyte M. 2004. Microbial endocrinology and infectious disease in the 21st century Trends Microbiol 12:14–20. doi: 10.1016/j.tim.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 25.He G, Shankar RA, Chzhan M, Samouilov A, Kuppusamy P, Zweier JL. 1999. Noninvasive measurement of anatomic structure and intraluminal oxygenation in the gastrointestinal tract of living mice with spatial and spectral EPR imaging. Proc Natl Acad Sci U S A 96:4586–4591. doi: 10.1073/pnas.96.8.4586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gosink K, Häse CC. 2000. Requirements for conversion of the Na+-driven flagellar motor of Vibrio cholerae to the H+-driven motor of Escherichia coli. J Bacteriol 182:4234–4240. doi: 10.1128/JB.182.15.4234-4240.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Clarke MB, Hughes DT, Zhu C, Boedeker EC, Sperandio V. 2006. The QseC sensor kinase: a bacterial adrenergic receptor. Proc Natl Acad Sci U S A 103:10420–10425. doi: 10.1073/pnas.0604343103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alhasan R, Njus D. 2008. The epinephrine assay for superoxide: why dopamine does not work. Anal Biochem 381:142–147. doi: 10.1016/j.ab.2008.06.030. [DOI] [PubMed] [Google Scholar]
- 29.Hughes DT, Sperandio V. 2008. Inter-kingdom signalling: communication between bacteria and their hosts. Nat Rev Microbiol 6:111–120. doi: 10.1038/nrmicro1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grim CJ, Hasan NA, Taviani E, Haley B, Chun J, Brettin TS, Bruce DC, Detter JC, Han CS, Chertkov O, Challacombe J, Huq A, Nair GB, Colwell RR. 2010. Genome sequence of hybrid Vibrio cholerae O1 MJ-1236, B-33, and CIRS101 and comparative genomics with V. cholerae. J Bacteriol 192:3524–3533. doi: 10.1128/JB.00040-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marina A, Waldburger CD, Hendrickson WA. 2005. Structure of the entire cytoplasmic portion of a sensor histidine-kinase protein. EMBO J 24:4247–4259. doi: 10.1038/sj.emboj.7600886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Villegas A, Kropinski AM. 2008. An analysis of initiation codon utilization in the domain Bacteria—concerns about the quality of bacterial genome annotation. Microbiology 154:2559–2661. doi: 10.1099/mic.0.2008/021360-0. [DOI] [PubMed] [Google Scholar]
- 33.Gao R, Stock AM. 2009. Biological insights from structures of two-component proteins. Annu Rev Microbiol 63:133–154. doi: 10.1146/annurev.micro.091208.073214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Merighi M, Septer A, Carroll-Portillo A, Bhatiya A, Porwollik S, McClelland M, Gunn J. 2009. Genome-wide analysis of the PreA/PreB (QseB/QseC) regulon of Salmonella enterica serovar Typhimurium. BMC Microbiol 9:42. doi: 10.1186/1471-2180-9-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moreira CG, Weinshenker D, Sperandio V. 2010. QseC mediates Salmonella enterica serovar Typhimurium virulence in vitro and in vivo. Infect Immun 78:914–926. doi: 10.1128/IAI.01038-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moreira CG, Sperandio V. 2012. Interplay between the QseC and QseE bacterial adrenergic sensor kinases in Salmonella enterica serovar Typhimurium pathogenesis. Infect Immun 80:4344–4353. doi: 10.1128/IAI.00803-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Karavolos MH, Winzer K, Williams P, Khan CM. 2013. Pathogen espionage: multiple bacterial adrenergic sensors eavesdrop on host communication systems. Mol Microbiol 87:455–465. doi: 10.1111/mmi.12110. [DOI] [PubMed] [Google Scholar]
- 38.Halang P, Leptihn S, Meier T, Vorburger T, Steuber J. 2013. The function of the Na+-driven flagellum of Vibrio cholerae is determined by osmolality and pH. J Bacteriol 195:4888–4899. doi: 10.1128/JB.00353-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Njoroge J, Sperandio V. 2012. Enterohemorrhagic Escherichia coli virulence regulation by two bacterial adrenergic kinases, QseC and QseE. Infect Immun 80:688–703. doi: 10.1128/IAI.05921-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Correa NE, Peng F, Klose KE. 2005. Roles of the regulatory proteins FlhF and FlhG in the Vibrio cholerae flagellar transcription hierarchy. J Bacteriol 187:6324–6332. doi: 10.1128/JB.187.18.6324-6332.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Miethke M, Skerra A. 2010. Neutrophil gelatinase-associated lipocalin expresses antimicrobial activity by interfering with l-norepinephrine-mediated bacterial iron acquisition. Antimicrob Agents Chemother 54:1580–1589. doi: 10.1128/AAC.01158-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lin PC, Türk K, Häse CC, Fritz G, Steuber J. 2007. Quinone reduction by the Na+-translocating NADH dehydrogenase promotes extracellular superoxide production in Vibrio cholerae. J Bacteriol 189:3902–3908. doi: 10.1128/JB.01651-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fry BW, Ciarlone AE. 1980. Storage at body temperature alters concentration of vasoconstrictors in local anesthetics. J Dent Res 59:1069. doi: 10.1177/00220345800590061301. [DOI] [PubMed] [Google Scholar]
- 44.Tao Z, Wang G, Goodisman J, Asefa T. 2009. Accelerated oxidation of epinephrine by silica nanoparticles. Langmuir 25:10183–10188. doi: 10.1021/la900958f. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.