Abstract
Adrenocortical insufficiency such as occurs in Addison's disease causes hyponatremia and renal tubular acidosis (RTA). Hyponatremia results from both aldosterone and cortisol insufficiency. RTA is due to aldosterone insufficiency. The involvement of cortisol in RTA is unclear. Here, we report a woman in her 70s who was admitted to our hospital with severe hyponatremia (106 mEq/l) and RTA. The patient exhibited low plasma cortisol levels with little response to rapid adrenocorticotropic hormone loading. In contrast, the plasma aldosterone concentration was maintained at or above the normal range. Hydrocortisone replacement greatly improved both the hyponatremia and RTA. This case suggests that both aldosterone and cortisol are involved in acid secretion from the kidney.
INTRODUCTION
Hyponatremia and metabolic acidosis are major symptoms of adrenocortical dysfunction such as occurs in Addison's disease. The hyponatremia is caused by insufficiency of both aldosterone and cortisol. Aldosterone insufficiency decreases sodium reabsorption in the collecting ducts of the kidney. Cortisol insufficiency induces the secretion of antidiuretic hormone (ADH) [1, 2], resulting in syndrome of inappropriate secretion of antidiuresis (SIAD)-like conditions [3]. The metabolic acidosis is due to insufficiency of aldosterone, which decreases acid secretion in the kidney. Here, we report a patient with severe hyponatremia and metabolic acidosis that were caused by insufficiency of cortisol but not aldosterone and were successfully treated with hydrocortisone replacement.
CASE REPORT
A woman in her 70s was transferred to our hospital from a regional hospital for treatment of severe hyponatremia. The patient was drowsy and disoriented, and her blood pressure was 118/56 mmHg. Blood chemistry indicated severe hyponatremia, low serum osmolality, high urinary osmolality and sodium concentration, detectable concentration of ADH and normal serum creatinine range (Table 1). The diameter of the inferior vena cava was 4.1/13.7 mm (inspiration/expiration). Arterial blood gas indicated metabolic acidosis with a relatively low anion gap (Table 2). Endocrinological examination showed low plasma cortisol concentration with no circadian changes (Table 3). The response of the increase in cortisol to a rapid loading of tetracosactide acetate [synthesized adrenocorticotropic hormone (ACTH)] was low. Plasma renin activity was normal, and there was a relatively high plasma aldosterone concentration (PAC). Computed tomography on brain and whole body showed no abnormal findings. Taken together, we suspected that the patient exhibited a SIAD-like condition complicated with severe renal tubular acidosis (RTA) that was caused by adrenocortical insufficiency, although the PAC remained high.
Table 1:
Blood and urine data at Day 1 on admission are shown
| Albumin (g/dl) | 3.9 |
| Serum Na (mEq/l) | 106 |
| Serum K (mEq/l) | 4.2 |
| Serum Cl (mEq/l) | 82 |
| Blood urea nitrogen (mg/dl) | 13.7 |
| Creatinine (mg/dl) | 0.67 |
| Total cholesterol (mg/dl) | 144 |
| Serum osmolality (mOsm/kg·H2O) | 207 |
| ACTH (pg/ml) | 26.1 |
| Cortisol (μg/dl) | 5.9 |
| Plasma renin activity (ng/ml/h, supine position) | 0.6 |
| PAC (pg/ml) | 177 |
| Antidiuretic hormone (pg/ml) | 2.4 |
| BNP (pg/ml) | 50.6 |
| Urine pH | 5.5 |
| Urine creatinine (mg/dl) | 131.8 |
| Urine Na (mEq/l) | 47 |
| Urine K (mEq/l) | 45 |
| Urine Cl (mEq/l) | 54 |
| Urine osmolality (mOsm/kg·H2O) | 550 |
| Urinary anion gap (= Na + K – Cl) | 38 |
Samples were taken at noon on supine position.
Table 2:
Arterial blood gas data
| Day 1 | Day 19 | |
|---|---|---|
| pH | 7.397 | 7.413 |
| paCO2 (mmHg) | 25.3 | 30.1 |
| paO2 (mmHg) | 86.7 | 104.1 |
| HCO3− (mEq/l) | 15.3 | 19.0 |
| Base excess (mEq/l) | −7.9 | −4.5 |
| Anion gap (mEq/l) | 10.3 | 10.0 |
Sample was taken at room air.
Table 3:
Circadian change and response to ACTH loading of cortisol
| Circadian change of cortisol | ||||
| Time | 7:00 | 12:00 | 16:00 | 23:00 |
| Cortisol (μg/dl) | 2.7 | 3.5 | 3.0 | 2.1 |
| ACTH (pg/ml) | 22.3 | 16.7 | 16.5 | 22.0 |
| Rapid ACTH loading | ||||
| Time after the loading (h) | 0 | 1 | 2 | |
| Cortisol (μg/dl) | 3.3 | 10.5 | 13.8 | |
After 250 μg tetracosactide acetate was injected intravenously, the cortisol concentration of cortisol was measured at the indicated time.
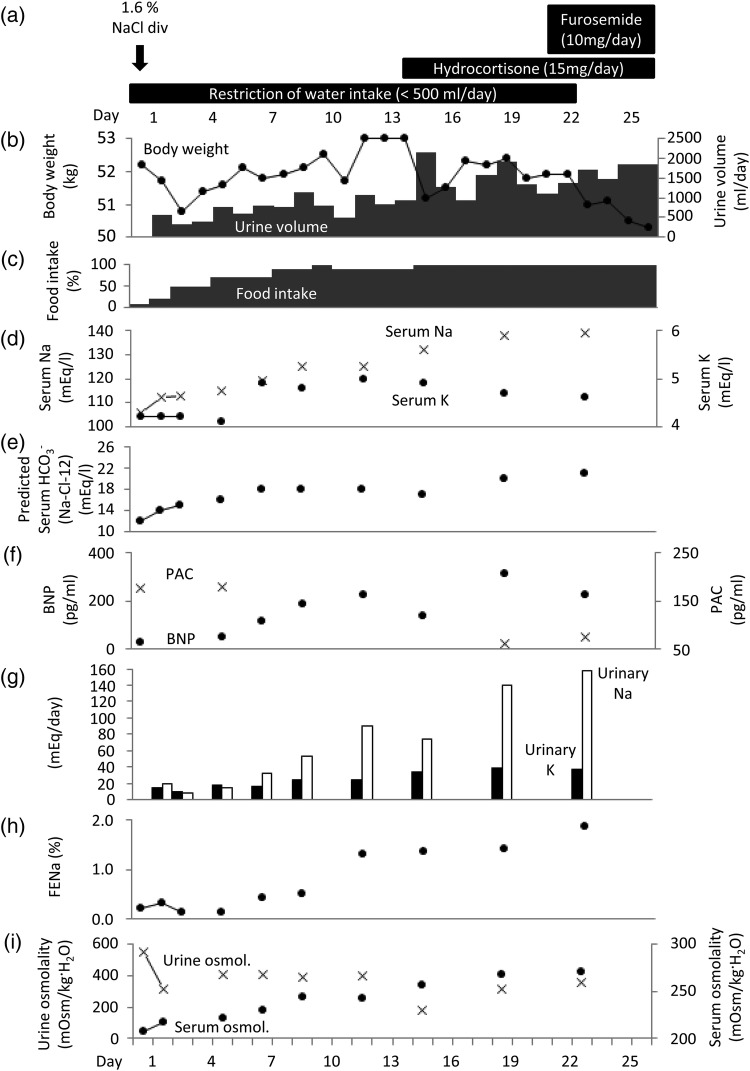
We began fluid restriction (<500 ml/day) and intravenous drip infusion of a 1.6% NaCl solution. The patient returned to consciousness when the sodium concentration reached 111 mEq/l (Fig. 1a). The serum sodium concentration and predicted HCO3− concentration (HCO3− = Na-Cl-12) increased to 125 and 18 mEq/l, respectively, at Day 9 after admission. The serum potassium concentration gradually increased from 4.2 at Day 1 to 5.0 mEq/l at Day 12 as her diet intake recovered. Because the hyponatremia did not further improve, we prescribed hydrocortisone at 10 mg in the morning and 5 mg in the evening on Day 14.
Figure 1:
Change in physical, blood and urine data over the time course. Body weight and urine volume (b), food intake (c), serum sodium and potassium concentrations (d), predicted HCO3− concentration (e), PAC and BNP (f), urinary sodium and potassium (g), fractional excretion of sodium (h), and urine and serum osmolality (i) are plotted. Details of intravenous drip infusion and medications are indicated (a).
On the first day of treatment, urine output increased up to 2000 ml/day and remained at 1500 ml/day thereafter. At Day 19, the serum sodium concentration increased to 138 mEq/l. The arterial HCO3− concentration was 19.0 mEq/l (Table 2). The predicted HCO3− concentration increased to 20 mEq/l (Fig. 1e). The serum potassium concentration decreased (Fig. 1d). The PAC decreased to a normal range (Fig. 1f). Because the plasma brain natriuretic peptide (BNP) level increased (Fig. 1f), we were concerned about heart failure due to volume overload by dietary intake. We therefore prescribed 10 mg/day of furosemide (Fig. 1a). The predicted HCO3− concentration was finally increased to 22 mEq/l, and the patient was discharged.
DISCUSSION
Adrenocortical insufficiency causes hyponatremia and Type IV RTA due to insufficiency of cortisol (glucocorticoid) and aldosterone (mineralocorticoid). An interesting point in the present case is that the patient exhibited low cortisol but not aldosterone concentrations, although she exhibited both hyponatremia and metabolic acidosis. Both cortisol and aldosterone are key factors that regulate serum sodium concentration. Aldosterone maintains sodium concentration by activating epithelial Na channels in the collecting duct of the kidney. The regulatory mechanism of sodium concentration by cortisol has not been sufficiently investigated. Some studies suggest that cortisol insufficiency induces ADH secretion, resulting in SIAD-like conditions [1, 2, 4].
Interestingly, administration of hydrocortisone improved the acidosis, suggesting that the glucocorticoid action of hydrocortisone should improve RTA in the present case. We were unable to classify the present case as Type IV RTA because the PAC remained high and the serum potassium concentration was not high enough to diagnose hyperkalemia. Because the metabolic acidosis was normalized with respiratory compensation, hyperkalemia may not have occurred. In addition, after the treatment of acidosis with hydrocortisone, urinary pH was still low (5.5), suggesting the absence of urinary loss of HCO3−. That result would deny the possibility of Type I and II RTA. These findings suggest that RTA in the present case was caused by cortisol insufficiency, which would not be applicable to the current classification of RTA.
Cortisol and aldosterone bind to the mineralocorticoid receptor (MR) [5]. 11-Beta hydroxysteroid dehydrogenase 2 deactivates cortisol, resulting in the predominant action of aldosterone in the collecting ducts [6]. However, because the cortisol plasma concentration is 1000 times higher than that of aldosterone, cortisol might still have an effect on acid secretion in the collecting duct. A more likely interpretation is the insufficiency of ammoniagenesis by cortisol in proximal tubules [7]. Ammonia (NH3) is produced in the proximal tubules and secreted from the collecting ducts with H+. Therefore, we propose the following mechanisms regarding metabolic acidosis in the present case: (i) cortisol insufficiency induces metabolic acidosis due to insufficient acid secretion and (ii) metabolic acidosis induces mild potassium concentration increase [8].
In summary, we report a case that exhibited severe hyponatremia due to insufficiency of cortisol, but not aldosterone. Replacement of hydrocortisone improved not only hyponatremia but also RTA, suggesting the involvement of cortisol in maintaining sodium concentration and acid secretion by the kidney.
CONFLICT OF INTEREST STATEMENT
None declared.
REFERENCES
- 1.Olchovsky D, Ezra D, Vered I, Hadani M, Shimon I. Symptomatic hyponatremia as a presenting sign of hypothalamic-pituitary disease: a syndrome of inappropriate secretion of antidiuretic hormone (SIADH)-like glucocorticosteroid responsive condition. J Endocrinol Invest 2005;28:151–156. [DOI] [PubMed] [Google Scholar]
- 2.Yatagai T, Kusaka I, Nakamura T, Nagasaka S, Honda K, Ishibashi S et al. Close association of severe hyponatremia with exaggerated release of arginine vasopressin in elderly subjects with secondary adrenal insufficiency. Eur J Endocrinol 2003;148:221–226. [DOI] [PubMed] [Google Scholar]
- 3.Ellison DH, Berl T. The syndrome of inappropriate antidiuresis. N Engl J Med 2007;356:2064–2072. [DOI] [PubMed] [Google Scholar]
- 4.Raff H. Glucocorticoid inhibition of neurohypophysial vasopressin secretion. Am J Physiol 1987;252:R635–R644. [DOI] [PubMed] [Google Scholar]
- 5.Funder JW. Glucocorticoid and mineralocorticoid receptors: biology and clinical relevance. Annu Rev Med 1997;48:231–240. [DOI] [PubMed] [Google Scholar]
- 6.Náray-Fejes-Tóth A, Rusvai E, Fejes-Tóth G. Minealocorticoid receptors and 11 beta-steroid dehydrogenase activity in renal principal and intercalated cells. Am J Physiol 1994;266:F76–F80. [DOI] [PubMed] [Google Scholar]
- 7.Walbourne TC, Givens G, Joshi S. Renal ammoniagenic response to chronic acid loading: role of glucocorticoids. Am J Physiol 1988;254:F134–F138. [DOI] [PubMed] [Google Scholar]
- 8.Lee Hamm L, Hering-Smith KS, Nakhoul NL. Acid-base and potassium homeostasis. Semin Nephrol. 2013;33:257–264. [DOI] [PubMed] [Google Scholar]