TO THE EDITOR
Palmoplantar keratodermas (PPKs) are a group of genetically heterogeneous genodermatoses. Recently mutations in TRPV3 were identified as a cause of the rare form of PPK, Olmsted syndrome (OS; OMIM 614594; Lai-Cheong et al., 2012; Lin et al., 2012; Danso-Abeam et al., 2013; Kariminejad et al., 2014; Duchatelet et al., 2014b). OS was first reported in 1927 in an Italian American boy with painful palmoplantar keratoderma, deep fissures, pseudoainhum, curved thickened nails, and periorificial hyperkeratosis with fissuring (Olmsted, 1927). About 50 clinical cases of OS have been described, and all generally exhibit the features described by Olmsted as well as some additional features (Mevorah et al., 2005).
In this study, we report the case of six families, referred to the Pachyonychia Congenita Project for the evaluation of painful plantar keratoderma, but lacking pseudoainhum or significant periorificial keratoderma. In each case, after no mutations were identified in the PC-associated keratin genes, KRT6A, KRT6B, KRT6C, KRT16, or KRT17, and in some cases, after other candidate genes including GJB6, DSP, DSG1, KRT5, and KRT14 had been screened, we identified heterozygous missense mutations in TRPV3, thus greatly expanding the phenotypic spectrum of OS. Samples were obtained with written, informed patient consent and ethical approval by a Western Institutional Review Board that complies with principles of the Helsinki Accord.
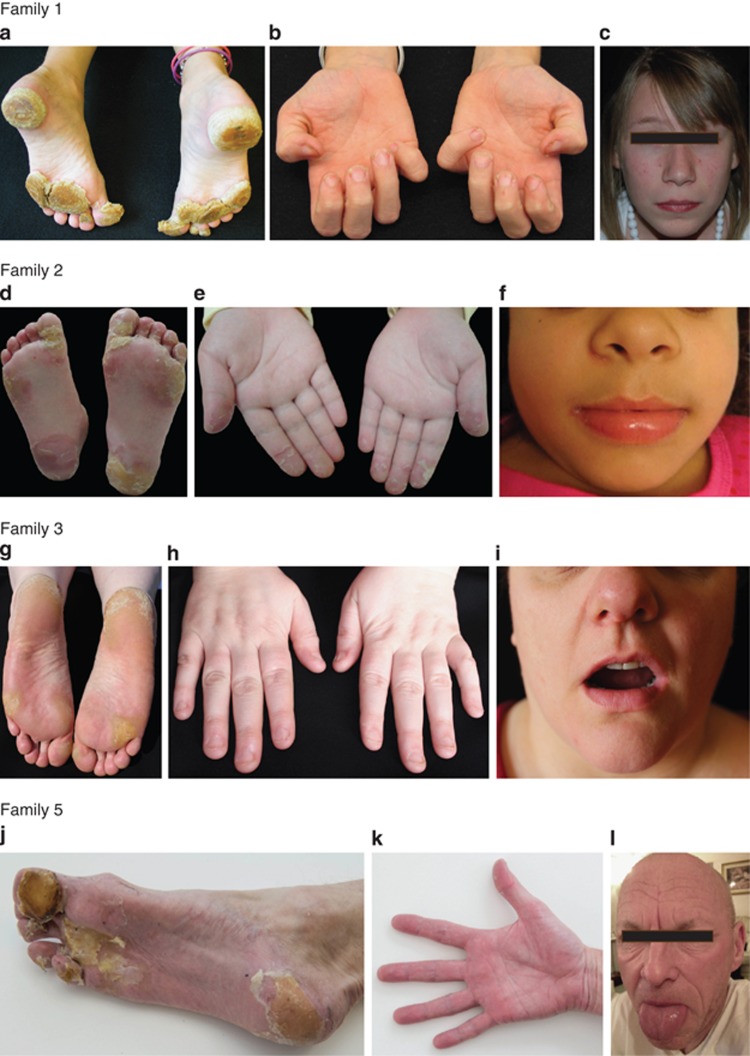
An 18-year-old girl of European ancestry from Family 1 initially noted focal callus formation on the soles at age 4. Severe, painful plantar keratoderma now necessitates periodic use of a wheelchair. She has mild keratoderma on the hands, thin nail plates with koilonychia, and fine slow-growing hair. She has no periorificial keratoderma (Figure 1a, b, c,Supplementary Figure S1 online and Supplementary Table S1 online). A likely diagnosis was PC, but no causative mutations were identified in the PC-related keratin genes nor in other candidate genes. Therefore, a whole-exome sequencing approach was performed (Supplementary Methods online), and data were analyzed for sequence variants in known keratoderma genes. A heterozygous missense mutation, p.Gly573Cys; c,1717G>T, was identified in TRPV3 and confirmed by Sanger sequencing (Supplementary Methods online) but was not present in her unaffected parents or brother. This mutation has been reported in a sporadic case of Olmsted syndrome (Lin et al., 2012).
Figure 1.
Clinical features. Proband of Family 1: (a) severe calluses on the weight-bearing areas of the soles (at age 9), (b) thin nail plates with koilonychia (age 9), and (c) no perioral keratoderma and fine hair, age 12. Proband of Family 2: (d) calluses on the soles of the feet, (e) blisters and peeling of skin on the fingertips, and (f) lack of perioral hyperkeratosis. Proband of Family 3: (g) calluses on the soles, (h) koilonychia of nails, and (i) no perioral lesions. Proband of Family 5: (j) hyperkeratosis on the soles, (k) no palmar hyperkeratosis, and (l) sparse fragile hair, mild lateral perioral fissuring, and no oral leukokeratosis. Images are published with patients' consent.
TRPV3 was considered a candidate gene for five additional families in which no mutations were identified in the PC-associated keratin genes or in other candidate genes. Exons and intron/exon boundaries of TRPV3 were amplified by PCR for Sanger sequencing (Supplementary Methods online).
A 7-year-old Brazilian girl in Family 2 presented with easily peeling hyperkeratosis on her toes and feet at about 18 months of age, and these have evolved into painful, focal hyperkeratosis. She has erythema and hyperkeratosis of the distal fingers and subungual hyperkeratosis. She has had transient periorificial hyperkeratosis and her nail plates are normal (Figure 1d, e, f,Table 1, Supplementary Figure S1 online). A previously unreported heterozygous missense mutation, p.Gly568Val; c.1703G>T, was identified (Supplementary Figure S2 online); this mutation was not detected in either of her unaffected parents. Amino acid, p.Gly568 is highly conserved across several species. This mutation is not in the dbSNP database or the NHLBI Exome Variant Server (http://evs.gs.washington.edu/EVS/).
Table 1. Clinical findings in patients with mutations in TRPV3.
| Report | Inheritance | TRPV3 mutation | Plantar keratoderma | Palmar keratoderma | Pseudoainhum | Periorificial keratoderma | Hair |
|---|---|---|---|---|---|---|---|
| Olmsted's patient | Diffuse-S | Diffuse-S | Present | Present | Dry | ||
| Lin et al., 2012 | AD | p.Gly573Ser (4)1 | M(1)2; Mod(1); S(2) | M(1)2; Mod(1); S(2) | Present | M(1)2; Mod(1); S(2) | Alopecia - M(1); Mod (1); S (2) |
| Lin et al., 2012 | AD | p.Gly573Cys (1) | M | M | Absent | M | Alopecia - M |
| Lin et al., 2012 | AD | p.Trp692Gly (1) | Mod | Mod | Present | Mod | Alopecia - M |
| Lai-Cheong et al., 2012 | AD | p.Gly573Ser (1) | Diffuse-S | Diffuse-S | Present | M | Fine-dry |
| Danso-Abeam et al., 2013 | AD | p.Gly573Ala (1) | Diffuse-S | Diffuse-S | Absent | S | Alopecia-S |
| Duchatelet et al., 2014b | AD | p.Leu673Phe (1) | Diffuse-S | Diffuse-S | Absent | Absent | Fine, dry |
| Duchatelet et al., 2014a | AR | p.Gly568Cys; p.Gln216_Gly262del (1) | Diffuse-S (1); Focal-Mod(1) | NR | Absent | Absent | Fine-dry |
| Eytan et al., 2014 | AR | p.Trp521Ser (1) | Diffuse-S | Diffuse-S | Absent | Present | Sparse |
| Kariminejad et al., 2014 | AD | p.Trp692Cys (1) | Diffuse-S | Diffuse-S | Present | Present (Mod) | Sparse; fragile |
| He et al., 2015 | AD | p.Gln580Pro (1)2 | Focal-Mod | Focal-Mod | Absent | Absent | Normal |
| Family 1 | AD | p.Gly573Cys (1) | Focal-S | Focal-M | Absent | Absent | Fine |
| Family 2 | AD | p.Gly568Val (1) | Focal-Mod | Focal-M | Absent | M | Normal |
| Family 3 | AD | p.Gly568Asp (2)2 | Focal-Mod | M | Absent | M | Fine |
| Family 4 | AD | p.Gly568Asp (1) | Focal-Mod | Focal-M | Absent | NR | Normal |
| Family 5 | AD | p.Gly573Ser (1) | Focal-Mod | M/transient | Absent | M/transient | Fragile, sparse |
| Family 6 | AD | p.Gly573Ser (1) | Focal-Mod | M | Absent | Absent | Normal |
| Report | Follicular keratosis | Erythema | Hyperhidrosis | Nails | Lesional itch | Lesional pain | Leukokeratosis |
|---|---|---|---|---|---|---|---|
| Olmsted's patient | NR | Lesion border; dorsal hands | Hands | Thickened | NR | Present | NR |
| Lin et al., 2012 | Scalp | Lesion border | NR | NR | S (4)2 | Present | NR |
| Lin et al., 2012 | Scalp | Lesion border | NR | NR | S | Present | NR |
| Lin et al., 2012 | NR | Lesion border | NR | NR | S | Present | NR |
| Lai-Cheong et al., 2012 | NR | NR | NR | Dystrophy | No | “Functional impairment” | NR |
| Danso-Abeam et al., 2013 | NR | Lesion border | NR | Dystrophy | S | S | NR |
| Duchatelet et al., 2014b | NR | Erythromelalgia | Present | Thin, brittle | S | S | NR |
| Duchatelet et al., 2014a | M | Erythromelalgia | Present | Normal | S | S | NR |
| Eytan et al., 2014 | NR | Diffuse, palms and soles | NR | NR | Present | Present | Present |
| Kariminejad et al., 2014 | NR | NR | NR | Dystrophic/absent | S | S | NR |
| He et al., 2015 | NR | NR | NR | NR | NR | NR | NR |
| Family 1 | M | Lesion border | Hands and feet | Koilonychia; thin plates | Absent | S | Absent |
| Family 2 | Absent | Lesion border; distal digits (hands) | Feet | Normal | Absent | S | Absent |
| Family 3 | M | Lesion border | Feet | Koilonychia; thin plates; onychoschizia | S | S | Present |
| Family 4 | NR | Lesion border | NR | Onychoschizia | NR | S | Absent |
| Family 5 | M | Lesion border; dorsal feet | Feet | Koilonychia; thin plates; onychoschizia | Absent | S | Absent |
| Family 6 | NR | Lesion border | No | Normal | NR | S | Absent |
Abbreviations: M, mild; Mod, moderate; NR, not reported; S, severe.
Individual families.
Individuals.
The proband from Family 3, a 38-year-old European female, developed calluses on her feet at the age of 8–9 years. She now has severe plantar pain and difficulty in walking (Figure 1g, h, i, Table 1,Supplementary Figure S1 online). She has thin nail plates with koilonychia. Her father, two paternal uncles and grandmother were similarly affected showing autosomal dominant inheritance of the disorder. A previously unreported heterozygous missense mutation, p.Gly568Asp;c.1703G>A (Supplementary Figure S2 online), was identified in the proband and in one affected paternal uncle; it was not present in an unaffected paternal uncle nor in her mother, (her father is deceased). This mutation is not listed in dbSNP or the NHLBI Exome Variant Server.
Interestingly, we found the same mutation, p.Gly568Asp, in Family 4 from South America. The first sign of a skin abnormality in the 25-year-old proband was peeling skin on her feet at age 4 years. Painful, focal keratoses formed on her feet and to a lesser extent on her hands. She has mild periungual hyperkeratosis on her fingers and toes, onychoschizia and longitudinal overcurvature of several toenails. She has no periorificial hyperkeratosis, and her hair is normal (Table 1). No clinical information or DNA samples were available from her parents or from other family members.
A 55-year-old man of European ancestry from Family 5 presented with painful calluses on the soles of his feet. Focal hyperkeratoses with thin surrounding rim of erythema started on his heels as a child when he started to walk and spread to the soles of his feet. He has thin nail plates with koilonychia and sparse, fragile hair. He develops severe hyperhidrosis accompanied by burning pain in the feet and bright erythema on the dorsal hands and feet in response to extremes of temperature. He has no palmar hyperkeratosis, but has had transient perioral and periauricular hyperkeratosis (Figure 1j, k, l, Table 1,Supplementary Figure S1 online). He believes that etretinate and acitretin have significantly improved his quality of life. His father was also affected. A heterozygous missense mutation, p.Gly573Ser; c.1717G>A, the most commonly reported mutation to date in TRPV3, was identified in this individual.
Mutation p.Gly573Ser, was also found in a 7-year-old girl of European ancestry (Family 6), who developed thickening of the skin on her heels at about 4 years of age (Table 1). She has severe plantar pain and now uses crutches to aid her mobility. Her parents are unaffected.
The genetic basis of autosomal dominant OS was recently elucidated (Lin et al., 2012) when heterozygous missense mutation, p.Gly573Ser, was identified in TRPV3 in a Chinese family. Mutations in TRPV3 were subsequently identified in five additional Chinese families. All developed symptoms before 1 year of age, had varying severity of palmoplantar hyperkeratosis, periorificial hyperkeratosis, alopecia, and severe lesional pain and itch. All but one had constricting digital bands. Several heterozygous mutations have been reported at codons 573; p.Gly573Ser (Lai-Cheong et al., 2012), p.Gly573Ala (Danso-Abeam et al., 2013), and p.Gly573Cys (Lin et al., 2012) and two mutations at codons 692; p.Trp692Gly (Lin et al., 2012) and p.Trp692Cys (Kariminejad et al., 2014). The heterozygous missense mutation p.Leu673Phe was found in a patient with OS and erythromelalgia (Duchatelet et al., 2014b). Homozygous missense and compound heterozygous mutations in TRPV3 have been shown to result in recessive OS with (Duchatelet et al., 2014a) or without erythromelalgia (Eytan et al., 2014). Recently, the heterozygous missense mutation p.Gln580Pro was identified in a family with focal palmoplantar keratoderma (He et al., 2015), more reminiscent of the cases described here.
TRPV3 belongs to the family of transient receptor potential (TRP) cation channels and is widely expressed in keratinocytes and hair follicles (Peier et al., 2002 Nilius et al., 2013) as well as in other tissues including the brain, spinal cord, sensory neurons, and the cornea. Mutations in TRPV3 causing autosomal dominant OS were shown to be gain-of-function mutations resulting in increased TRPV3 activity (Lin et al., 2012). In this study, two, to our knowledge previously unreported, mutations were identified at codon 568. Interestingly another amino acid substitution at this position, p.Gly568Cys, was recently reported in combination with a splice site mutation, exhibiting autosomal recessive inheritance in this case (Duchatelet et al., 2014a). TRPV3 forms a tetrameric complex, each subunit consists of six transmembrane domains (S1–S6) and a cytoplasmic amino and carboxy termini (Supplementary Figure S2 online). p.Gly568 is within the linker region between S4 and S5, near the boundary of S4. It is predicted that substitution of this glycine is less damaging than substitutions further within the S4–S5 linker such as p.Gly573 (Duchatelet et al., 2014a). In silico prediction tools (PolyPhen and Mutation Taster) predict all three variants at codon 568, p.Gly568Asp, p.Gly568Val (this study), and p.Gly568Cys (Duchatelet et al., 2014a) to be damaging. In our families, no other mutations were identified in TRPV3, and in Family 2, the parents were wild-type for p.Gly568 indicating a de novo mutation, p.Gly568Val, in the proband. Mutation p.Gly568Asp was shown to be dominantly inherited in Family 3; the mutation was identified in the proband and an affected paternal uncle (affected father is deceased). However, in the family reported with p.Gly568Cys in combination with a splice site mutation (Duchatelet et al., 2014a) the unaffected father was heterozygous for p.Gly568Cys and the clinical phenotype of the two affected brothers was significantly different. Overall, these findings suggest that environmental factors/modifier genes may also be involved in determining the phenotypic variability.
TRPV3 is involved in many cellular and physiological processes. Recently, Cheng et al. (2010) demonstrated the important role of TRPV3 in regulating EGFR signaling in hair and skin barrier function using a TRPV3 knockout mouse model that developed a wavy hair coat and curly whiskers in addition to a red, dry scaly skin at birth, reminiscent of mice with a defective skin barrier.
Although reported as a thermosensitive cation channel, activated at 30–33 °C, this thermosensory role is unclear (Nilius and Biro, 2013). Interestingly, coexistence of erythromelalgia with OS has been reported (Duchatelet et al., 2014a, b), and one of our patients has findings compatible with erythromelalgia. Many OS patients report hyperhidrosis (including four of ours).
In this study, heterozygous missense mutations were identified in TRPV3 in six families, (two previously unreported and two recurrent mutations) with painful, palmoplantar keratoderma. Clinically, none were as severe as typical OS (Table 1).
The cases we have described expand the phenotypic spectrum of Olmsted syndrome caused by mutations in TRPV3. Mutations in TRPV3 should be considered as a cause of painful PPK even in the absence of periorificial hyperkeratosis and pseudoainhum as described by Olmsted. In contrast and to avoid confusion, painful PPKs caused by mutations in genes other than TRPV3 probably should not be referred to as Olmsted syndrome.
Acknowledgments
We thank all the patients and families involved in this study and Dr Antonella Tosti, Miami, FL, USA and Dr Sherri Bale, GeneDx, MD, USA for referring patients. We also thank Professor Maurice van Steensel and Dr Eli Sprecher for valuable comments and discussions and to Holly Evans of PC Project for all her help with data preparation. FJDS and NJW are supported by grants from the Pachyonychia Congenita Project (to FJDS, www.pachyonychia.org) and Tenovus Scotland (to FJDS). The Centre for Dermatology and Genetic Medicine at the University of Dundee is supported by a Wellcome Trust Strategic Award (098439/Z/12/Z to WHIM).
The authors state no conflict of interest.
Footnotes
Supplementary material is linked to the online version of the paper at http://www.nature.com/jid
Supplementary Material
References
- Cheng X, Jin J, Hu L et al. (2010) TRP channel regulates EGFR signaling in hair morphogenesis and skin barrier formation. Cell 141:331–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danso-Abeam D, Zhang J, Dooley J et al. (2013) Olmsted syndrome: exploration of the immunological phenotype. Orphanet J Rare Dis 8:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchatelet S, Guibbal L, de Veer S et al. (2014. a) Olmsted syndrome with erythromelalgia caused by recessive TRPV3 mutations. Br J Dermatol 171:675–678 [DOI] [PubMed] [Google Scholar]
- Duchatelet S, Pruvost S, de Veer S et al. (2014. b) A new TRPV3 missense mutation in a patient with Olmsted syndrome and erythromelalgia. JAMA Dermatol 150:303–306 [DOI] [PubMed] [Google Scholar]
- Eytan O, Fuchs-Telem D, Mevorach B et al. (2014) Olmsted syndrome caused by a homozygous recessive mutation in TRPV3. J Invest Dermatol 136:1752–1754 [DOI] [PubMed] [Google Scholar]
- He Y, Zeng K, Zhang X et al. (2015) A Gain of Function Mutation in TRPV3 causes focal palmoplantar keratoderma in a Chinese family. J Invest Dermatol 135:907–909 [DOI] [PubMed] [Google Scholar]
- Kariminejad A, Barzegar M, Abdollahimajd F et al. (2014) Olmsted syndrome in an Iranian boy with a new de novo mutation in TRPV3. Clin Exp Dermatol 39:492–495 [DOI] [PubMed] [Google Scholar]
- Lai-Cheong JE, Sethuraman G, Ramam M et al. (2012) Recurrent heterozygous missense mutation, p.Gly573Ser, in the TRPV3 gene in an Indian boy with sporadic Olmsted syndrome. Br J Dermatol 167:440–442 [DOI] [PubMed] [Google Scholar]
- Lin Z, Chen Q, Lee M et al. (2012) Exome sequencing reveals mutations in TRPV3 as a cause of Olmsted syndrome. Am J Hum Genet 90:558–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mevorah B, Goldberg I, Sprecher E et al. (2005) Olmsted syndrome: mutilating palmoplantar keratoderma with periorificial keratotic plaques. J Am Acad Dermatol 53:S266–S272 [DOI] [PubMed] [Google Scholar]
- Nilius B, Biro T. (2013) TRPV3: a 'more than skinny' channel. Exp Dermatol 22:447–452 [DOI] [PubMed] [Google Scholar]
- Nilius B, Biro T, Owsianik G. (2013) TRPV3: time to decipher a poorly understood family member!. J Physiol 592:295–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olmsted HC. (1927) Keratodermia palmaris et plantaris congenitalis: report of a case showing associated lesions of unusual location. Am J Dis Child 33:757–764 [Google Scholar]
- Peier AM, Reeve AJ, Andersson DA et al. (2002) A heat-sensitive TRP channel expressed in keratinocytes. Science 296:2046–2049 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.