Abstract
Cholecystokinin (CCK) is a peptide hormone produced in the gut and brain with beneficial effects on digestion, satiety, and insulin secretion. CCK is also expressed in pancreatic β-cells, but only in models of obesity and insulin resistance. Whole body deletion of CCK in obese mice leads to reduced β-cell mass expansion and increased apoptosis. We hypothesized that islet-derived CCK is important in protection from β-cell apoptosis. To determine the specific role of β-cell-derived CCK in β-cell mass dynamics, we generated a transgenic mouse that expresses CCK in the β-cell in the lean state (MIP-CCK). Although this transgene contains the human growth hormone minigene, we saw no expression of human growth hormone protein in transgenic islets. We examined the ability of MIP-CCK mice to maintain β-cell mass when subjected to apoptotic stress, with advanced age, and after streptozotocin treatment. Aged MIP-CCK mice have increased β-cell area. MIP-CCK mice are resistant to streptozotocin-induced diabetes and exhibit reduced β-cell apoptosis. Directed CCK overexpression in cultured β-cells also protects from cytokine-induced apoptosis. We have identified an important new paracrine/autocrine effect of CCK in protection of β-cells from apoptotic stress. Understanding the role of β-cell CCK adds to the emerging knowledge of classic gut peptides in intraislet signaling. CCK receptor agonists are being investigated as therapeutics for obesity and diabetes. While these agonists clearly have beneficial effects on body weight and insulin sensitivity in peripheral tissues, they may also directly protect β-cells from apoptosis.
Keywords: cholecystokinin, islet, β-cell, apoptosis, aging, streptozotocin
type 1 and type 2 diabetes mellitus are both diseases of reduced β-cell mass. In type 1 diabetes, autoimmune destruction of β-cells results in an absolute β-cell mass deficit. In type 2 diabetes, obesity increases insulin resistance, resulting in a relative insulin deficiency. In patients with type 2 diabetes, β-cell mass is lost via increased β-cell apoptosis (7, 16). Therefore, there has been wide interest in the development of diabetes therapeutics that preserve β-cell mass and prevent β-cell apoptosis.
Cholecystokinin (CCK) is a gastrointestinal peptide produced by duodenal I cells and secreted in response to fat and protein (31). CCK signals through two G protein-coupled receptors, the CCK1(A) receptor [CCK1(A)R] and the CCK2(B) receptor [CCK2(B)R]. In healthy and diabetic patients, CCK infusion increases insulin secretion and decreases glucose excursion after a meal, suggesting that CCK is a potential diabetes therapeutic (1, 2). CCK also increases satiety by acting centrally in the brain and therefore has also been explored as an antiobesity therapy (47). Understanding the role of CCK in regulation of β-cell mass is important in evaluating its potential efficacy as a therapeutic in diabetic and insulin resistant patients.
Surprisingly, we (33) previously found that CCK is also expressed in the pancreatic β-cell, but only in the setting of obesity. We (37) recently discovered that β-cell expression of CCK is regulated by the incretin hormone glucagon-like peptide-1 (GLP-1). We set out to understand the role of β-cell-derived CCK in β-cell mass dynamics and glucose regulation. Whole body knockout of CCK in the context of leptin-deficient obesity leads to reduced β-cell mass and increased β-cell apoptosis without affecting β-cell proliferation (33). We concluded that CCK is necessary for β-cell mass expansion and β-cell survival in obesity. Another study of whole body knockout of CCK on high-fat diet, however, found no difference in individual islet size, but overall β-cell mass and markers of proliferation or apoptosis were not measured (38). Complete loss of CCK in obese models has an overall negative effect on peripheral metabolism, making these models inadequate to isolate the specific role of β-cell-derived CCK in β-cell mass and function.
Although we had evidence that CCK was necessary to protect from β-cell apoptosis, we wanted to determine whether it was sufficient. Disparate effects on β-cell area are seen with the administration of exogenous CCK to obese mice (21, 22). However, CCK treatment also leads to reduced body weight and reduced glucose levels through peripheral actions, thereby reducing metabolic demand on the β-cell (20, 21). These studies were unable to clarify the direct role of CCK on β-cell apoptosis due to the improvements in overall metabolism compared with the control group. As CCK is a potential therapeutic for diabetes and obesity, we wanted to know more about its ability to protect from β-cell apoptosis when present in excess. Therefore, we developed a model to study the role of CCK in the β-cell without the confounder of major alterations in peripheral CCK signaling. In the present study, we developed a transgenic mouse that overexpresses CCK in β-cells to determine whether β-cell-derived CCK is sufficient to prevent β-cell apoptosis and protect β-cell mass in nonobese models of diabetes and aging.
RESEARCH DESIGN AND METHODS
Generation of MIP-CCK transgenic mice.
Mouse Cck, transcript variant 1 (631 base pairs, NM_031161.4) containing 5′- and 3′-untranslated regions (UTR) was cloned into the Gfp site of the previously described MIP-GFP-hGH expression vector on the pGEM11z backbone (18). More specifically, Cck was amplified using the following primers (forward: gcgcctcgagaacttagctggactgcagcct; reverse: gcgcctcgaggcatagcaacattaggtctggg). The final construct contained 67 bp of Cck 5′-UTR, the 348-bp open reading frame of Cck, and 216 bp of Cck 3′-UTR before the human growth hormone (hGH) minigene. Transgene DNA was microinjected into the pronuclei of C57BL/6J embryos. Six initial founder lines were identified by PCR genotyping, and three were successfully bred and phenotyped. Each line had comparable islet Cck mRNA levels and similar phenotype (weight, glucose, and insulin levels). Data presented here are from a single founder line (line 2) and their littermate wild-type controls. This line was rederived by embryo transfer and moved to a different animal facility during the course of these studies. All individual experiments were done with animals housed in a single facility (i.e., not combined with data from before or after rederivation). Mouse protocols were approved by the University of Wisconsin Animal Care and Use Committee to meet acceptable standards of humane animal care.
CCK and human growth hormone mRNA and protein measurements.
Multiple tissues, including isolated islets (46), were harvested, and RNA was isolated. Cck mRNA was quantified via quantitative PCR with Taqman primer/probe sets (Life Technologies, Foster City, CA) or using SYBR and primers for mouse Cck, Cckar, Cckbr, Tph1, Tph2, Glut2, and HGH normalized to β-actin. CCK peptide levels were measured using a radioimmunoassay (Alpco Diagnostics, Salem, NH) in islet protein lysates and in media from 200 isolated islets cultured in 2 ml of islet medium (RPMI with 8 mM glucose and 10% FBS) for 24 h. Secreted hGH was measured by enzyme-linked immunosorbent assay (ELISA) specific for human growth hormone (hGH; Roche, Indianapolis, IN) in the same islet conditioned media used to measure CCK secretion. Positive controls for hGH were run on the same ELISA, including lysate and media from INS-1 cells transfected with a hGH expression construct or a GFP control expression plasmid.
Phenotyping of MIP-CCK transgenic mice.
Glucose and insulin were measured in serum from retroorbital bleeds, using the glucose oxidase method and an insulin ELISA, as previously described (26). Intraperitoneal glucose tolerance tests (IPGTT) were performed on 12-wk and 46- to 63-wk-old animals after a 4-h fast with 2 mg/g body wt of sterile dextrose. Intraperitoneal insulin tolerance tests (IPITT) were performed on 12-wk and 24- to 28-wk-old animals after a 4-h fast with 0.75 U/kg body wt of sterile insulin. Total pancreatic insulin content was measured in 8-wk-old animals as previously described (3). Pancreas sections from 8-wk-old animals were immunostained for cleaved caspase-3 (Cell Signaling Technology, Danvers, MA), Ki67 (Cell Signaling), insulin (Dako, Carpenteria, CA), and nuclei (DAPI). β-Cell mass, islet area, and %Ki67-positive β-cells were quantified using ImageJ software (National Institutes of Health, Bethesda, MD). Sections from three different positions within the pancreas were stained, and an average of 50 islets was quantified from each of four animals per genotype. Pancreas sections from 52- to 65-wk-old animals were immunostained for insulin (Sigma-Aldrich, St. Louis, MO), and DAPI and fractional islet area and islet size were measured using ImageJ software. Twenty low-power fields were quantified from each of seven animals per genotype. Pancreatic sections from 52- to 65-wk-old animals were stained with hematoxylin and eosin and scored by blinded reviewers for the severity of fibrosis and inflammation on a scale of 1 to 3 (3 being most severe).
Glucose-stimulated insulin secretion.
Single-islet insulin secretion assays were performed as described in Ref. 50. A total of 14–30 isolated islets were individually placed in 96-well v-bottom plates and incubated for 24 h in regular islet media. Islets were washed and incubated in Krebs buffer containing 1.7 mM glucose for 45 min. The islets were then incubated in Krebs buffer containing low (1.7 mM) or high (16.7 mM) glucose for 60 min. Media were harvested, and islets were rinsed and lysed. Insulin ELISA was performed on the media and islet lysate. Percent insulin secretion was calculated [media insulin/(media insulin + islet lysate insulin)].
Streptozotocin experiments.
Streptozotocin (STZ; 50 mg/kg) was administered within 15 min of preparation via intraperitoneal injection daily for 5 consecutive days to male mice 10–13 wk of age. One cohort of animals was euthanized on day 8 for assessment of β-cell apoptosis via terminal deoxynucleotidyl-transferase (dUTP) nick-end labeling (TUNEL) and proliferation with Ki67 staining. Another cohort was followed for 30 days with tail vein glucometer readings.
Adenovirus construction.
Ad-GFP was purchased from Viraquest (North Liberty, IA). Ad-CCK-GFP viral vector plasmid was constructed by cloning mouse Cck cDNA into a bicistronic vector that expresses both CCK and GFP protein from one mRNA via an internal ribosomal entry site. Ad-CCK-GFP was then constructed by cotransfection of the bicistronic expression plasmid with pJM17 (the adenoviral genome backbone) into 293 cells, as previously described (4). The adenovirus was negative for E1A contamination (32).
Cell culture experiments.
Mouse (MIN6-B1) and rat (INS-1) insulinoma cells were cultured as previously described (36). INS-1 cells were treated with Ad-CCK-GFP or Ad-GFP adenovirus at a multiplicity of infection equal to 10. Forty-eight hours after infection, cells received overnight treatment with 10 ng/ml human IL-1β (Roche Molecular Biochemicals, Indianapolis, IN) and 50 ng/ml rat TNFα (R&D Systems, Minneapolis, MN). Cell death and apoptosis were assessed via the CytoTox-Glo and Caspase-Glo 3/7 assays (Promega, Madison, WI).
Statistical analysis.
Pairwise comparisons were performed with an unpaired Student's two-tailed t-test. AUC was calculated with the trapezoidal method on individual animals, starting at the baseline value where these differed between groups. Two-way ANOVA with Bonferroni posttest was performed to identify differences between genotypes in ITT or in GTT glucose or insulin values. GraphPad Prism was used for statistical analyses. Error bars represent SE.
RESULTS
Generation of MIP-CCK transgenic mice.
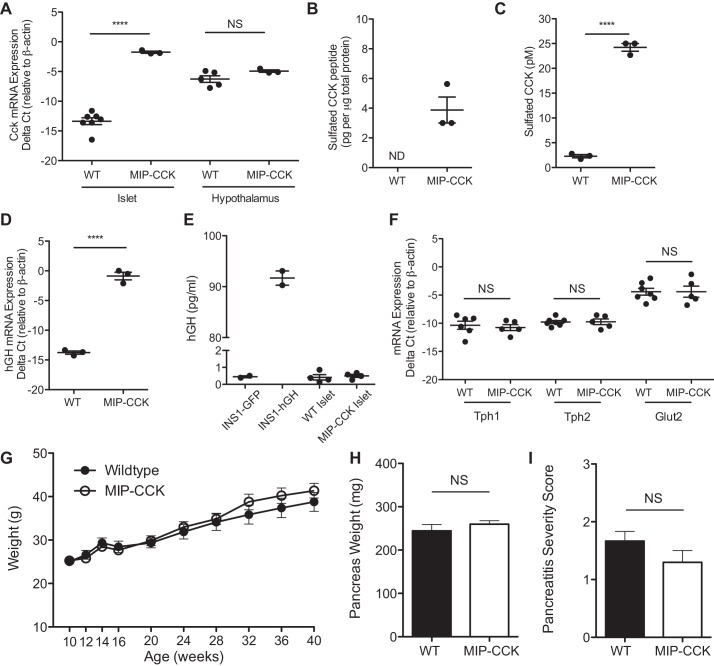
We generated a transgenic mouse using the mouse insulin 1 gene promoter (MIP) to drive CCK overexpression specifically in the β-cell (MIP-CCK). Cck expression was dramatically increased in isolated islets from 10-wk-old transgenic mice (Fig. 1A). Since CCK is an abundant neuropeptide and has pleiotropic effects as a neuromodulator (reviewed in Ref. 11), it was important that CCK overexpression be confined to β-cells. Cck expression was not increased in the hypothalamus in MIP-CCK mice (Fig. 1A), consistent with known expression patterns of this transgene construct (18). We additionally verified that Cck expression was not changed in whole brain, liver, intestine, striated muscle, spleen, kidney, gallbladder, heart, lung, or adipose tissue (data not shown). To confirm protein expression and proper posttranslational processing, we measured sulfated CCK peptide by RIA. CCK peptides must be O-sulfated at a carboxy-terminal tyrosine residue to bind to the CCK1(A)R. Sulfated CCK peptide was undetectable in islets from lean wild-type mice. In islets from MIP-CCK mice, sulfated CCK peptide was found at 4 pg/μg of total protein (Fig. 1B), compared with our previous finding of 3 pg/μg total protein in islets from ob/ob mice (33). Sulfated CCK is also secreted into the media from isolated MIP-CCK islets (Fig. 1C). The MIP-CCK mice are therefore a model of β-cell-derived CCK overexpression, with β-cell CCK expression levels similar to those found in obese models.
Fig. 1.
MIP-CCK mice (transgenic mice expressing CCK in β-cells in the lean state) demonstrate islet-specific overexpression of CCK and do not produce human growth hormone (hGH) protein. A: Cck mRNA expression is increased in MIP-CCK islets and unchanged in hypothalamus compared with wild type (WT). B: islet CCK protein expression is present in MIP-CCK islet lysate. C: secreted CCK peptide is found in conditioned medium from MIP-CCK islets. D: spliced hGH mRNA is expressed in MIP-CCK islets. E: there is no secretion of hGH into medium from isolated MIP-CCK islets incubated in 8 mM glucose for 24 h. A positive control of INS-1 cells transfected with a hGH expression plasmid (INS-1 hGH) shows significant secretion of hGH into the medium. F: there is no change in Tph1, Tph2, or Glut2 expression in MIP-CCK islets. G: body weight does not differ between wild-type (●) and MIP-CCK (○) male mice (n = 6–15, P = 0.36). H: pancreas weight does not differ in 8-wk-old animals (n = 7–8, P = 0.37. I: there is no evidence of increased fibrosis or inflammation in aged pancreata from MIP-CCK mice. Sections were qualitatively graded on a scale of 1–3 (1 = mild, 3 = severe) for level of inflammation and fibrosis (ages 57–65 wks, n = 5–6, P = 0.83). (NS, not significant; ND, not detected. ****P < 0.0001, ***P < 0.001).
Recently, several reports have described expression of human growth hormone (hGH) protein from the hGH minigene, which is included in many murine transgene constructs to increase transgene expression (3, 6, 45). As our MIP-CCK construct contained the same hGH minigene, we needed to examine whether we also had expression of hGH protein. We did find high expression levels of hGH mRNA by using primers that would detect only a processed hGH mRNA with intron 2 spliced out (Fig. 1D); however, we did not see any hGH protein secreted from MIP-CCK islets (Fig. 1E). We used INS-1 cells transfected with a hGH expression plasmid as a positive control. Conditioned medium from islets of another transgenic mouse line containing the hGH minigene was also run on the same plate and had levels of secreted hGH of ∼7 pg/ml, serving as an additional positive control (3). We also examined whole islet lysate and measured secretion into the medium after 1 h in 20 mM glucose and again found no hGH protein from MIP-CCK islets (data not shown). Secreted hGH is purported to signal through the prolactin receptor and lead to increased expression of serotonin synthesis genes Tph1 and Tph2 (6). We saw no increase in expression of Tph1 or Tph2 in MIP-CCK islets (Fig. 1F). We also saw no decline in Glut2 expression, which has been hypothesized to explain the resistance to STZ seen in hGH-expressing mice. Taken together, we see no evidence of hGH protein expression or activation of downstream signaling pathways in MIP-CCK mice despite containing the hGH minigene and producing hGH mRNA.
MIP-CCK mice do not have systemic effects from islet-derived CCK.
Although circulating CCK can ameliorate weight gain and induce satiety (19), MIP-CCK mice gain weight normally over their lifespan (Fig. 1G). We also saw no difference in pancreatic weight in 8-wk-old animals (Fig. 1H). To determine whether β-cell CCK secretion significantly increased CCK levels in peripheral circulation, we attempted to measure serum CCK levels. However, after the necessary serum extraction steps and resultant sample loss, both fed and fasted CCK levels were at or below the limit of detection of the RIA. Comparatively, mice given exogenous CCK had measurable serum CCK on the same assay (data not shown). Therefore, β-cell-specific CCK expression did not lead to pharmacological elevations in serum CCK. CCK administration can cause pancreatitis in mouse models (52). Because we were increasing CCK expression in the islet, we wanted to ensure that the surrounding exocrine pancreas was not adversely affected. We scored pancreatic sections from mice over 1 yr of age for inflammation and fibrosis and found no evidence of pancreatitis in MIP-CCK animals (Fig. 1I). Together, these findings suggest there is minimal extraislet CCK signaling in the MIP-CCK model.
Young, lean MIP-CCK mice have normal glucose homeostasis and β-cell mass.
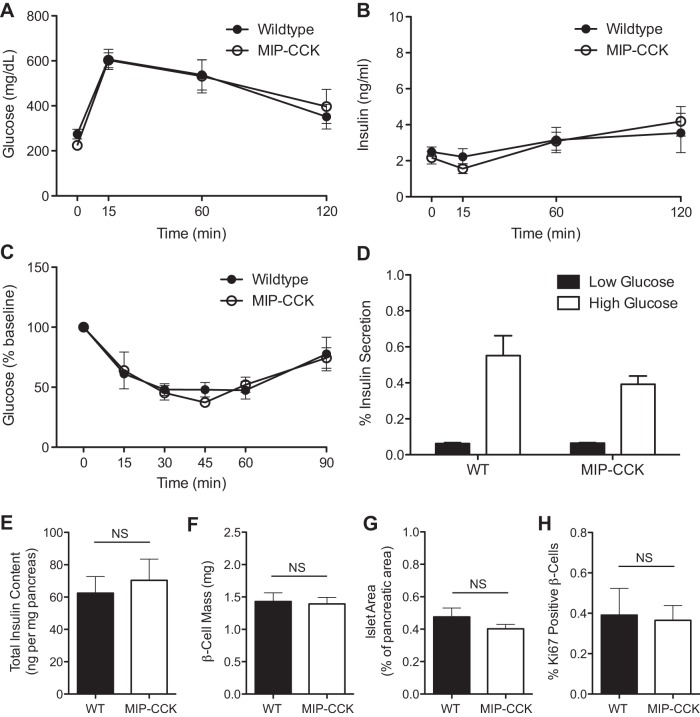
We hypothesized that the primary effect of β-cell-specific CCK overexpression would be protection from β-cell apoptosis. Since β-cell apoptosis is a rare event in lean, unstressed mice, we did not expect to see an effect on glucose homeostasis or β-cell mass in these animals. In fact, glucose tolerance and glucose-stimulated insulin levels did not differ from wild-type mice at 8 wk of age (Fig. 2, A and B). Fasted glucose and insulin also did not differ from controls (time 0 values in Fig. 2, A and B). There was also no difference in insulin sensitivity, measured with an insulin tolerance test (ITT) at 12 wk of age (Fig. 2C). As circulating CCK can directly stimulate insulin secretion, we wanted to determine whether islets from MIP-CCK animals were hypersecreting insulin due to increased levels of local CCK. We found no difference in insulin secretion at low (1.7 mM) or high (16.7 mM) glucose (Fig. 2D). We also found no difference in total pancreas insulin content (Fig. 2E), β-cell mass (Fig. 2F), or fractional islet area (Fig. 2G) in 8-wk-old mice. Because there have been variable reports on the ability of CCK to stimulate β-cell proliferation (9, 29, 32), we looked to see if MIP-CCK mice had increased β-cell proliferation. We found no difference in the number of Ki67-positive β-cells in MIP-CCK mice (Fig. 2H). We did not identify any apoptotic β-cells (using cleaved caspase-3 staining) in either wild-type or MIP-CCK islets at this young age, consistent with a very low rate of apoptosis. Therefore, overexpression of CCK in the β-cell did not affect β-cell growth or development and led to normal glucose homeostasis and insulin secretion in young mice.
Fig. 2.
Young, lean MIP-CCK mice have normal glucose homeostasis and β-cell mass. IP-GTT was performed on male mice aged 12 wk. A and B: glucose (P = 0.95) and insulin (P = 0.94) values were similar at all time points (n = 4). There were also no differences in fasting glucose or insulin values (time 0 min). IPTT was performed on male mice aged 12 wk. C: insulin sensitivity was not different from wild type (n = 4, P = 0.93). D: there was no difference in glucose-stimulated insulin secretory capacity at low (P = 0.81) or high (P = 0.23) glucose in isolated islets from 8-wk-old animals. Filled bars, low glucose (1.7 mM); open bars, high glucose (16.7 mM) (n = 4). E: total pancreatic insulin content did not differ from wild type in 8-wk-old animals (n = 7–8, P = 0.64). F and G: quantitative analysis from pancreatic sections demonstrated normal β-cell mass (P = 0.83) and fractional islet area (P = 0.28) in MIP-CCK mice (n = 4). H: no change in percent Ki67-positive β-cells was observed (n = 4, P = 0.87).
Aged MIP-CCK mice have increased β-cell area.
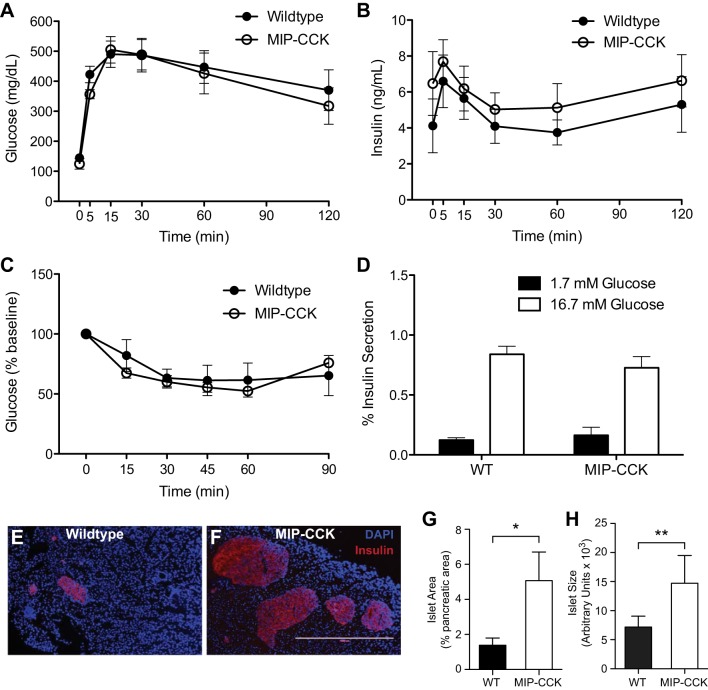
With age, increased insulin resistance and β-cell stress can lead to the accumulation of β-cell apoptosis (14, 39). We therefore decided to study the effect of CCK overexpression in aged animals. We performed an IP-GTT on animals of ∼1 yr of age and found no difference in glucose tolerance or in fasted glucose values (Fig. 3A). There was no statistically significant increase in insulin during GTT in the aged MIP-CCK mice, nor was there a significant difference in fasted insulin levels (P = 0.35; Fig. 3B). We measured insulin sensitivity with an ITT at ∼26 wk of age and again found normal insulin sensitivity (Fig. 3C). Fasting glucose levels during the ITT also did not differ between groups (data not shown). We again found no differences in either low (1.7 mM) or high (16.7 mM) glucose-stimulated insulin secretion from isolated islets at ∼28 wk of age (Fig. 3D). Together, these data demonstrate that β-cell CCK does not affect peripheral glucose homeostasis or insulin sensitivity in aged mice and does not have any significant effect on insulin secretion.
Fig. 3.
Aged MIP-CCK mice have increased β-cell area. IPGTT was performed on male mice aged 46–63 wk. A and B: glucose (P = 0.75) and insulin (P = 0.37) values were similar at all time points (n = 7–9). C: IPITT in male mice aged 24–28 wk shows that MIP-CCK insulin sensitivity is not different from wild type (n = 4–6, P = 0.76). D: there was no difference in glucose-stimulated insulin secretory capacity at low (P = 0.51) or high (P = 0.34) glucose in isolated islets from 26- to 30-wk-old animals. Filled bars, low glucose (1.7 mM); open bars, high glucose (16.7 mM) (n = 4–6). E and F: wild-type and MIP-CCK pancreata from 52- to 65-wk-old mice were immunostained for insulin (DAPI, blue; insulin, red). G and H: quantitative analysis from pancreatic sections demonstrated increased fractional islet area and average islet area in MIP-CCK mice (n = 7). *P < 0.05, **P < 0.01.
To determine whether β-cell CCK expression had a direct effect on β-cell mass in the aged mouse, we measured fractional islet area at ∼1 yr of age. MIP-CCK mice had 3.7-fold increased fractional islet area (Fig. 3G) and 2-fold larger islet size (Fig. 3H). We again wanted to determine whether this was due to increased proliferation or decreased apoptosis. However, we found essentially no Ki67-positive cells in islets from either wild-type or MIP-CCK mice at 1 yr of age. We were similarly unable to detect any difference in apoptosis in aged pancreata, because apoptotic cells were very rare. This is consistent with previous reports of extremely low β-cell turnover in aged mice (30, 49, 51). Because we saw no difference in β-cell mass and no increase in β-cell proliferation in young MIP-CCK mice, we propose that the increased islet area in older MIP-CCK mice may be due to protection from the rare apoptotic events that accumulate over time.
STZ treatment of MIP-CCK mice.
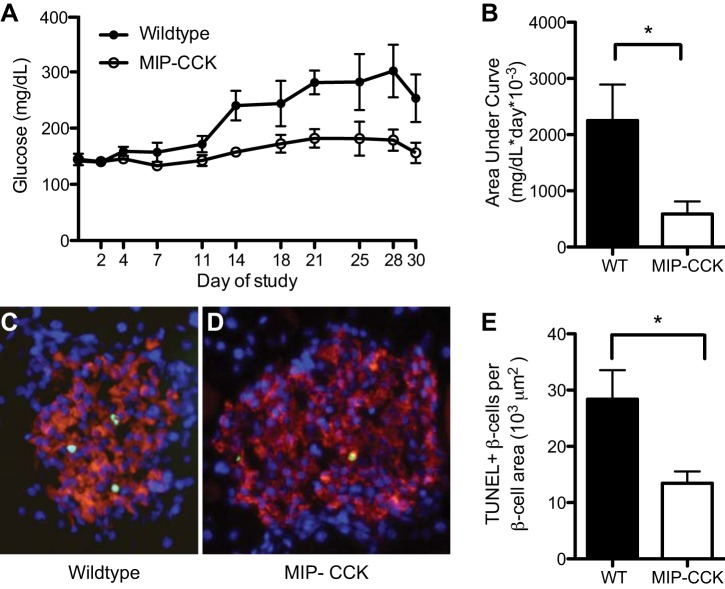
To directly study the effect of β-cell CCK expression on apoptosis, we treated 10- to 13-wk-old animals with multiple low-dose STZ to induce β-cell apoptosis. Wild-type mice developed sustained hyperglycemia by day 14 after STZ, whereas MIP-CCK mice remained euglycemic (Fig. 4A). Using AUC analysis, we confirmed that glucose was significantly reduced in MIP-CCK mice compared with wild-type controls (Fig. 4B). A second cohort of mice was euthanized on day 8, a time when maximal apoptosis was occurring in response to STZ (42). β-Cell apoptosis was reduced twofold in MIP-CCK mice after STZ treatment, as measured by TUNEL-positive β-cells per β-cell area (Fig. 4, C–E). Notably, we saw no difference in β-cell size between MIP-CCK and wild-type islets on these sections (data not shown, P = 0.86), so normalization to β-cell area was appropriate. We also examined numerous sections for Ki67-positive cells at this time point but ultimately found only two Ki67-positive cells in wild-type vs. three Ki67-positive cells in MIP-CCK mice out of over 400 islets per genotype (n = 4). Therefore, the proliferative rate on day 8 after low-dose STZ remained low and there was no clear increase in β-cell proliferation in MIP-CCK mice. In summary, β-cell CCK expression preserves β-cell area with aging and protects from STZ-induced β-cell apoptosis and diabetes.
Fig. 4.
MIP-CCK mice are resistant to STZ-induced diabetes. A and B: glucose after STZ treatment and AUC above a glucose value of 150 mg/dl demonstrate that MIP-CCK mice are resistant to STZ-induced hyperglycemia (n = 7–9). C and D: wild-type and MIP-CCK pancreata harvested on day 8 were immunostained (DAPI, blue; insulin, red; TUNEL, green). E: quantitative analysis demonstrated reduced β-cell apoptosis in MIP-CCK mice (n = 5). *P < 0.05.
CCK protects β-cells from apoptosis in vitro.
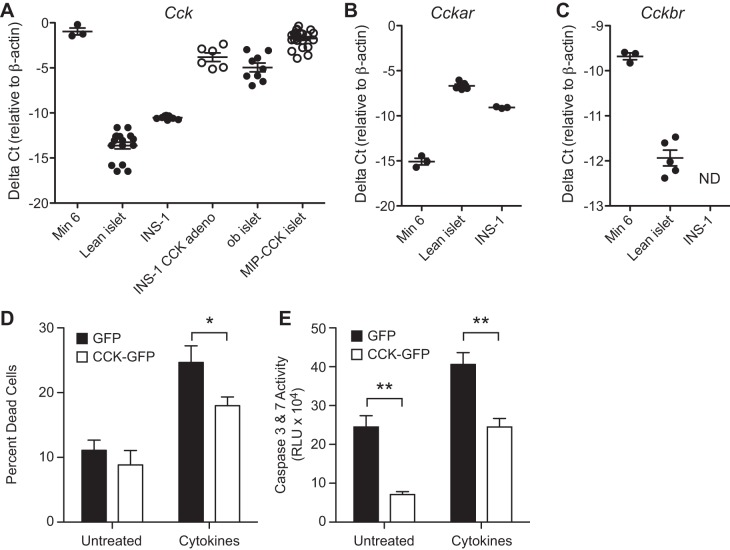
Although our in vivo model suggests a direct effect of β-cell CCK in protection from β-cell apoptosis, intraislet signaling or alterations in whole animal physiology could be playing an unanticipated role. We therefore wanted to confirm a direct antiapoptotic effect of CCK overexpression in isolated β-cells. We previously demonstrated that Cck knockdown in MIN6 cells causes increased susceptibility to cytokine-induced cell death (33). Similar to islets from obese mice, MIN6 cells express high amounts of Cck mRNA and reduced Cckar mRNA levels (Fig. 5, A–C). Thus, MIN6 cells model islets from ob/ob mice, and decreased Cck expression increases the susceptibility of MIN6 cells to apoptosis (33). INS-1 cells are more similar to lean mouse islets with low Cck, high Cckar, and low Cckbr expression (Fig. 5, A–C). Therefore, we used INS-1 cells to investigate whether increased Cck expression could directly protect β-cells from apoptosis.
Fig. 5.
CCK can directly protect islets from apoptosis. A: Cck mRNA expression levels differ in MIN6 and INS-1 cell lines. INS-1 cells infected with CCK-GFP adenovirus (INS-1 CCK adeno) have upregulated Cck expression similar to the upregulation in ob/ob islet or MIP-CCK islet vs. lean islet. B and C: Cckar and Cckbr mRNA expression is shown in cell lines and lean mouse islet. There was a reduction in cytokine-induced cell death (D) and caspase-3 and -7 activity (E) in INS-1 cells treated with Ad-CCK-GFP. *P < 0.05, **P < 0.01.
Using an adenovirus (Ad-CCK-GFP), we increased Cck mRNA expression in INS-1 cells 200-fold (Fig. 5A). This level of overexpression is similar to that observed in ob/ob islet or MIP-CCK islet (Fig. 5A). We then induced cell death with cytokine treatment. CCK overexpression decreased cytokine-induced cell death by 27% (Fig. 5D). To confirm that reduced cell death was due to reduced apoptosis rather than necrosis, we repeated the above experiment and assayed for caspase-3 and -7 activity. Cck overexpression reduced baseline caspase activity by 71% and cytokine-induced caspase activation by 40% (Fig. 5E). In conclusion, β-cell CCK expression is sufficient to protect β-cells from apoptosis in vivo and in vitro.
DISCUSSION
CCK is a peptide hormone that has potential use as a therapeutic for diabetes and obesity due to its known roles in the gut/brain axis. In a previous study (34), we demonstrated that CCK expression is dramatically upregulated in islets in response to obesity or insulin resistance. This is similar to a growing body of evidence that islets begin producing gut peptides, like glucagon-like peptide-1 (GLP-1), under conditions of obesity or stress (12, 17, 27, 37, 41). The role of these islet-derived gut peptides in the regulation of β-cell mass and function remains unclear. However, we have recently shown that GLP-1 stimulates β-cell production and secretion of CCK (37). In this study, we wanted to look more directly at the role of CCK produced in the β-cell to determine whether it was sufficient to protect against β-cell apoptosis. Lean mice produce little to no CCK in the islet [(34) and Fig. 1A]. Therefore, we generated a transgenic mouse (MIP-CCK) to express CCK in the β-cells of lean mice. These mice have islet CCK concentrations similar to those found in obese mice [Fig. 1B and (34)]. We challenged these mice with stressors and found that β-cell-derived CCK could preserve β-cell area and protect against apoptosis.
MIP-CCK mice contain the hGH minigene enhancer in the transgene construct, yet they do not produce hGH protein (Fig. 1E). Therefore, our findings represent direct effects of CCK expression, not unanticipated effects of hGH. There is no evidence of activation of downstream signaling pathways involved in serotonin synthesis (Fig. 1F), as has been described in other transgenic mice that produce hGH protein (6). A reduction in Glut2 expression was specifically hypothesized to explain the resistance to STZ in hGH-producing transgenic animals (6). Since MIP-CCK mice have no reduction in Glut2 (Fig. 1F), the protection from STZ can be attributed to a direct effect from CCK overexpression rather than effects of hGH. Notably, we (3) recently found hGH mRNA and protein expression from a similar transgenic construct derived from the MIP-GFP-hGH construct. It is unclear why there is no translation of hGH from the MIP-CCK-hGH transgene despite the production of spliced hGH mRNA (Fig. 1D). The presence of 216 bp of 3′-UTR after the Cck stop codon may be enough to ensure complete ribosomal detachment before the hGH start codon. Alternatively, the intervening sequences in other constructs may contain an internal ribosome entry site not found in this construct. Genomic insertional effects may also contribute. Ultimately, this suggests that every transgenic line containing the hGH minigene should be tested for production of hGH protein, not simply mRNA.
Age is a clear risk factor for the development of type 2 diabetes (8). With aging, the β-cell has limited regenerative capacity and increased susceptibility to β-cell apoptosis (30, 51). Therefore, the aged animal represents a model to study the physiological impact of β-cell mass regulation over time. β-Cell expression of CCK results in increased β-cell area and islet size in MIP-CCK mice over 1 yr of age (Fig. 3). We did not find any differences in β-cell mass or replication in young animals, suggesting that CCK overexpression does not affect β-cell development or proliferation. Our cumulative work suggests that CCK does not play a role in promoting β-cell proliferation in mice (32, 34). The increased β-cell area in older mice may represent preservation of β-cell mass due to reduced accumulated apoptotic events over time.
Because CCK increases insulin secretion in mice, humans, and isolated rat islets (2, 13, 19, 21, 24), we considered that islet CCK production could increase insulin secretion. However, we find no increase in basal or glucose-stimulated insulin secretion in isolated islets from young (Fig. 2D) or aged (Fig. 3D) MIP-CCK mice. In previous studies, CCK concentrations of 100 pM to 100 nM were used to document enhanced insulin secretion (13, 15, 21, 44). Although we cannot estimate the concentration of CCK within the islet microenvironment in vivo in MIP-CCK mice, the amount of CCK in medium from isolated MIP-CCK islets was only ∼20 pM after 24 h (Fig. 1C), suggesting subtherapeutic concentrations for enhanced insulin secretion in isolated islets ex vivo incubated for only 1 h in secretion medium. Although we saw no significant difference in insulin levels during glucose tolerance tests, whether locally expressed CCK can subtly enhance insulin secretion in vivo remains unknown.
We show that β-cell CCK protects against acute apoptotic stressors such as STZ (Fig. 4) and cytokines (Fig. 5). These studies directly implicate islet CCK expression in the protection from β-cell apoptosis. Similar antiapoptotic effects are described for other incretins, such as GLP-1 and glucose-dependent insulinotropic polypeptide (GIP) (35, 40). The downstream signaling of the GLP-1, GIP, and CCK1(A) receptors converge on the pro-survival signaling of protein kinase A (11, 31). A recent paper found CCK1(A)R signaling via β-arrestin, extracellular signal-regulated protein kinase (ERK), the 90-kDa ribosomal S6 kinase (p90RSK), and Bad regulates the β-cell antiapoptotic effects of CCK in high-glucose conditions (44). The expression of CCK receptors in the pancreatic islet has been controversial, with several studies finding disparate results in different species and using different antibodies (5, 11, 23, 25, 28, 43, 48). There are many challenges with antibodies raised against G protein-coupled receptors, and sensitivity and specificity problems can lead to uncertainty in the results (10). We have clearly demonstrated CCK1(A)R mRNA expression in pancreatic islets [Fig. 5 and (37)], and blockade of the CCK1(A)R prevents downstream signaling and CCK-stimulated insulin secretion in mouse islets (44). Therefore, the CCK1(A)R is very likely to be the mediator of CCK signaling in the β-cell. Future studies will continue to explore the specific signaling pathways that mediate the protection from β-cell apoptosis by CCK.
Irwin and colleagues (21, 22) have studied subacute CCK treatment in mice on a high-fat diet. Their results have been conflicting; CCK treatment led to no difference in the fractional percent islet area in one study (21), whereas they did see increased β-cell area with CCK treatment in a second study (22). We estimate that peak serum CCK concentrations in those studies were ∼300 nM (based on 25 nmol/kg dose and blood volume of ∼80 ml/kg), pharmacological levels much higher than in our MIP-CCK mice (Fig. 1C). These higher serum CCK levels after injection resulted in reduced body weight and improved insulin sensitivity, reducing metabolic demand for β-cell mass expansion compared with the more obese and insulin-resistant control groups (19, 21, 22). In fact, increased β-cell turnover was observed, with increases in both proliferation and apoptosis markers (21), suggesting secondary effects on the β-cell. In contrast, MIP-CCK mice had normal weight gain and exocrine pancreatic histology (Fig. 1, G–I), suggesting limited peripheral CCK signaling. Our previous and current work demonstrates no direct effect of CCK on β-cell proliferation (32, 33), and our current data support a direct effect of CCK on the islet to protect from β-cell apoptosis. Our findings in MIP-CCK animals differ from subacute injection studies due to chronic CCK exposure over the entire lifespan and our ability to isolate the effect on the islet without confounding changes in peripheral metabolism.
In summary, we have demonstrated that β-cell-specific CCK overexpression is sufficient to preserve β-cell area and prevent β-cell apoptosis and STZ-induced diabetes. This is a unique insight into the role of β-cell-derived CCK, which has not been largely studied. Since CCK has multiple positive effects on glucose homeostasis at the β-cell and the periphery, it is an attractive potential therapeutic for type 2 diabetes.
GRANTS
J. A. Lavine was supported by the Medical Scientist Training Program (T32 GM-08692). C. R. Kibbe is funded by National Institute on Aging (5T32 AG-000213). M. Baan was supported by the National Institutes of Health training grants T32 RR-023916 and T32 OD-010423. D. B. Davis has received funding from the National Institute of Diabetes and Digestive and Kidney Diseases (DK-083442), US Department of Veterans Affairs (1I01BX001880), the University of Wisconsin, and the Wisconsin Partnership Program.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: J.A.L., C.R.K., and D.B.D. conception and design of research; J.A.L., C.R.K., M.B., S.S., H.M.U., K.A.E., L.M.M., K.A.S., D.P.E., and D.B.D. performed experiments; J.A.L., C.R.K., M.B., S.S., H.M.U., K.A.E., L.M.M., K.A.S., D.P.E., and D.B.D. analyzed data; J.A.L., C.R.K., M.B., S.S., H.M.U., K.A.E., L.M.M., K.A.S., D.P.E., and D.B.D. interpreted results of experiments; J.A.L., C.R.K., and D.B.D. prepared figures; J.A.L., C.R.K., and D.B.D. drafted manuscript; J.A.L., C.R.K., M.B., H.M.U., and D.B.D. edited and revised manuscript; J.A.L., C.R.K., M.B., S.S., H.M.U., K.A.E., L.M.M., K.A.S., D.P.E., and D.B.D. approved final version of manuscript.
ACKNOWLEDGMENTS
We acknowledge generous assistance with blastocyst injection of the MIP-CCK transgenic animals from Dr. Phillip Scherer at the University of Texas-Southwestern. We also gratefully acknowledge support from Dr. Alan Attie at the University of Wisconsin-Madison for initial development of the MIP-CCK animals and provision of the hGH expression construct. We acknowledge technical assistance from Kathryn Scheuler, Joshua Suhonen, and Mohammed Ahmed. We thank Danielle Fontaine, Justin Bushkofsky, Amelia Linnemann, and Alan Attie for critical review of this manuscript.
S. Sirinvaravong's current affiliation: University of South Carolina, Department of Medicine, Division of Endocrinology, Diabetes, and Metabolism. L. M. Meske's current affiliation: Department of Surgery, University of Wisconsin-Madison. K. A. Sacotte's current affiliation: Northwestern University Medical School. D. P. Erhardt's current affiliation: University of Utah, Department of Medicine.
This work was performed using facilities and resources from the William S. Middleton Memorial Veterans Hospital. This work does not represent the views of the Department of Veterans Affairs or the United States Government.
REFERENCES
- 1.Ahren B, Holst JJ, Efendic S. Antidiabetogenic action of cholecystokinin-8 in type 2 diabetes. J Clin Endocrinol Metab 85: 1043–1048, 2000. [DOI] [PubMed] [Google Scholar]
- 2.Ahren B, Pettersson M, Uvnäs-Moberg K, Gutniak M, Efendic S. Effects of cholecystokinin (CCK)-8, CCK-33, and gastric inhibitory polypeptide (GIP) on basal and meal-stimulated pancreatic hormone secretion in man. Diabetes Res Clin Pract 13: 153–161, 1991. [DOI] [PubMed] [Google Scholar]
- 3.Baan M, Kibbe CR, Bushkofsky JR, Harris TW, Sherman DS, Davis DB. Transgenic expression of the human growth hormone minigene promotes pancreatic beta cell proliferation. Am J Physiol Regul Integr Comp Physiol. 2015. Jul 22:ajpregu.00244.2015. doi: 10.1152/ajpregu.00244.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Becker TC, Noel RJ, Coats WS, Gomez-Foix AM, Alam T, Gerard RD, Newgard CB. Use of recombinant adenovirus for metabolic engineering of mammalian cells. Methods Cell Biol 43: 161–189, 1994. [DOI] [PubMed] [Google Scholar]
- 5.Bourassa J, Lainé J, Kruse ML, Gagnon MC, Calvo É, Morisset J. Ontogeny and species differences in the pancreatic expression and localization of the CCK(A) receptors. Biochem Biophys Res Commun 260: 820–828, 1999. [DOI] [PubMed] [Google Scholar]
- 6.Brouwers B, de Faudeur G, Osipovich AB, Goyvaerts L, Lemaire K, Boesmans L, Cauwelier EJG, Granvik M, Pruniau VPEG, Van Lommel L, Van Schoors J, Stancill JS, Smolders I, Goffin V, Binart N, In't Veld P, Declercq J, Magnuson MA, Creemers JWM, Schuit F, Schraenen A. Impaired islet function in commonly used transgenic mouse lines due to human growth hormone minigene expression. Cell Metab 20: 979–990, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, Butler PC. Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes 52: 102–110, 2003. [DOI] [PubMed] [Google Scholar]
- 8.Centers for Disease Control, and Prevention. National Diabetes Statistics Report: Estimates of Diabetes and Its Burden in the United States, 2014. Atlanta, GA: US Department of Health and Human Services, 2014. [Google Scholar]
- 9.Chen S, Turner S, Tsang E, Stark J, Turner H, Mahsut A, Keifer K, Goldfinger M, Hellerstein MK. Measurement of pancreatic islet cell proliferation by heavy water labeling. Am J Physiol Endocrinol Metab 293: E1459–E1464, 2007. [DOI] [PubMed] [Google Scholar]
- 10.Drucker DJ. Incretin action in the pancreas: potential promise, possible perils, and pathological pitfalls. Diabetes 62: 3316–3323, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dufresne M. Cholecystokinin and Gastrin Receptors. Physiol Rev 86: 805–847, 2006. [DOI] [PubMed] [Google Scholar]
- 12.Ellingsgaard H, Hauselmann I, Schuler B, Habib AM, Baggio LL, Meier DT, Eppler E, Bouzakri K, Wueest S, Muller YD, Hansen AMK, Reinecke M, Konrad D, Gassmann M, Reimann F, Halban PA, Gromada J, Drucker DJ, Gribble FM, Ehses JA, Donath MY. Interleukin-6 enhances insulin secretion by increasing glucagon-like peptide-1 secretion from L cells and alpha cells. Nat Med 17: 1481–1489, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fridolf T, Karlsson S, Ahren B. Role of extracellular Na+ on CCK-8-induced insulin secretion. Biochem Biophys Res Commun 192: 1162–1168, 1993. [DOI] [PubMed] [Google Scholar]
- 14.Gu Z, Du Y, Liu Y, Ma L, Li L, Gong Y, Tian H, Li C. Effect of aging on islet beta-cell function and its mechanisms in Wistar rats. Age (Dordr) 34: 1393–1403, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guenifi A, Ahren B, Abdel-Halim SM. Differential effects of glucagon-like peptide-1 (7–36)amide versus cholecystokinin on arginine-induced islet hormone release in vivo and in vitro. Pancreas 22: 58–64, 2001. [DOI] [PubMed] [Google Scholar]
- 16.Hanley SC, Austin E, Assouline-Thomas B, Kapeluto J, Blaichman J, Moosavi M, Petropavlovskaia M, Rosenberg L. β-Cell mass dynamics and islet cell plasticity in human type 2 diabetes. Endocrinology 151: 1462–1472, 2010. [DOI] [PubMed] [Google Scholar]
- 17.Hansen AMK, Bödvarsdottir TB, Nordestgaard DNE, Heller RS, Gotfredsen CF, Maedler K, Fels JJ, Holst JJ, Karlsen AE. Upregulation of alpha cell glucagon-like peptide 1 (GLP-1) in Psammomys obesus—an adaptive response to hyperglycaemia? Diabetologia 54: 1379–1387, 2011. [DOI] [PubMed] [Google Scholar]
- 18.Hara M, Wang X, Kawamura T, Bindokas VP, Dizon RF, Alcoser SY, Magnuson MA, Bell GI. Transgenic mice with green fluorescent protein-labeled pancreatic β-cells. Am J Physiol Endocrinol Metab 284: E177–E183, 2003. [DOI] [PubMed] [Google Scholar]
- 19.Irwin N, Frizelle P, Montgomery IA, Moffett RC, O'Harte FPM, Flatt PR. Beneficial effects of the novel cholecystokinin agonist (pGlu-Gln)-CCK-8 in mouse models of obesity/diabetes. Diabetologia 55: 2747–2758, 2012. [DOI] [PubMed] [Google Scholar]
- 20.Irwin N, Frizelle P, O'Harte FPM, Flatt PR. Metabolic effects of activation of CCK receptor signaling pathways by twice-daily administration of the enzyme-resistant CCK-8 analog, (pGlu-Gln)-CCK-8, in normal mice. J Endocrinol 216: 53–59, 2013. [DOI] [PubMed] [Google Scholar]
- 21.Irwin N, Montgomery IA, Moffett RC, Flatt PR. Chemical cholecystokinin receptor activation protects against obesity-diabetes in high fat fed mice and has sustainable beneficial effects in genetic ob/ob mice. Biochem Pharmacol 85: 81–91, 2013. [DOI] [PubMed] [Google Scholar]
- 22.Irwin N, Pathak V, Flatt PR. A Novel CCK-8/GLP-1 hybrid peptide exhibiting prominent insulinotropic, glucose-lowering, and satiety actions with significant therapeutic potential in high-fat-fed mice. Diabetes 64: 2996–3009, 2015. [DOI] [PubMed] [Google Scholar]
- 23.Kageyama H, Kita T, Horie S, Takenoya F, Funahashi H, Kato S, Hirayama M, Young Lee E, Sakurai J, Inoue S, Shioda S. Immunohistochemical analysis of cholecystokinin A receptor distribution in the rat pancreas. Regul Pept 126: 137–143, 2005. [DOI] [PubMed] [Google Scholar]
- 24.Karlsson S, Ahren B. CCK-8-stimulated insulin secretion in vivo is mediated by CCKA receptors. Eur J Pharmacol 213: 145–146, 1992. [DOI] [PubMed] [Google Scholar]
- 25.Karlsson S, Sundler F, Ahren B. CCK receptor subtype in insulin-producing cells: a combined functional and in situ hybridization study in rat islets and a rat insulinoma cell line. Regul Pept 78: 95–103, 1998. [DOI] [PubMed] [Google Scholar]
- 26.Keller MP, Choi Y, Wang P, Davis DB, Rabaglia ME, Oler AT, Stapleton DS, Argmann C, Schueler KL, Edwards S, Steinberg HA, Chaibub Neto E, Kleinhanz R, Turner S, Hellerstein MK, Schadt EE, Yandell BS, Kendziorski C, Attie AD. A gene expression network model of type 2 diabetes links cell cycle regulation in islets with diabetes susceptibility. Genome Res 18: 706–716, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kilimnik G, Kim A, Steiner DF, Friedman TC, Hara M. Intraislet production of GLP-1 by activation of prohormone convertase 1/3 in pancreatic α-cells in mouse models of ß-cell regeneration. Islets 2: 149–155, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Konno K, Takahashi-Iwanaga H, Uchigashima M, Miyasaka K, Funakoshi A, Watanabe M, Iwanaga T. Cellular and subcellular localization of cholecystokinin (CCK)-1 receptors in the pancreas, gallbladder, and stomach of mice. Histochem Cell Biol 143: 301–312, 2015. [DOI] [PubMed] [Google Scholar]
- 29.Kuntz E, Pinget M, Damgé P. Cholecystokinin octapeptide: a potential growth factor for pancreatic beta cells in diabetic rats. JOP 5: 464–475, 2004. [PubMed] [Google Scholar]
- 30.Kushner JA. The role of aging upon β cell turnover. J Clin Invest 123: 990–995, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lavine JA, Attie AD. Gastrointestinal hormones and the regulation of β-cell mass. Ann NY Acad Sci 1212: 41–58, 2010. [DOI] [PubMed] [Google Scholar]
- 32.Lavine JA, Raess PW, Davis DB, Rabaglia ME, Presley BK, Keller MP, Beinfeld MC, Kopin AS, Newgard CB, Attie AD. Contamination with E1A-positive wild-type adenovirus accounts for species-specific stimulation of islet cell proliferation by CCK: a cautionary note. Mol Endocrinol 24: 464–467, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lavine JA, Raess PW, Stapleton DS, Rabaglia ME, Suhonen JI, Schueler KL, Koltes JE, Dawson JA, Yandell BS, Samuelson LC, Beinfeld MC, Davis DB, Hellerstein MK, Keller MP, Attie AD. Cholecystokinin is up-regulated in obese mouse islets and expands beta-cell mass by increasing beta-cell survival. Endocrinology 151: 3577–3588, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lavine JA, Raess PW, Stapleton DS, Rabaglia ME, Suhonen JI, Schueler KL, Koltes JE, Dawson JA, Yandell BS, Samuelson LC, Beinfeld MC, Davis DB, Hellerstein MK, Keller MP, Attie AD. Cholecystokinin is up-regulated in obese mouse islets and expands beta-cell mass by increasing beta-cell survival. Endocrinology 151: 3577–3588, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li Y, Hansotia T, Yusta B, Ris F, Halban PA, Drucker DJ. Glucagon-like peptide-1 receptor signaling modulates beta cell apoptosis. J Biol Chem 278: 471–478, 2003. [DOI] [PubMed] [Google Scholar]
- 36.Lilla V, Webb G, Rickenbach K, Maturana A, Steiner DF, Halban PA, Irminger JC. Differential gene expression in well-regulated and dysregulated pancreatic beta-cell (MIN6) sublines. Endocrinology 144: 1368–1379, 2003. [DOI] [PubMed] [Google Scholar]
- 37.Linnemann AK, Neuman JC, Battiola TJ, Wisinski JA, Kimple ME, Davis DB. Glucagon-like peptide-1 regulates cholecystokinin production in β-cells to protect from apoptosis. Mol Endocrinol 29: 978–987, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lo CM, Obici S, Dong HH, Haas M, Lou D, Kim DH, Liu M, D'Alessio D, Woods SC, Tso P. Impaired insulin secretion and enhanced insulin sensitivity in cholecystokinin-deficient mice. Diabetes 60: 2000–2007, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maedler K, Schumann DM, Schulthess F, Oberholzer J, Bosco D, Berney T, Donath MY. Aging correlates with decreased beta-cell proliferative capacity and enhanced sensitivity to apoptosis: a potential role for Fas and pancreatic duodenal homeobox-1. Diabetes 55: 2455–2462, 2006. [DOI] [PubMed] [Google Scholar]
- 40.Maida A, Hansotia T, Longuet C, Seino Y, Drucker DJ. Differential importance of glucose-dependent insulinotropic polypeptide vs glucagon-like peptide 1 receptor signaling for beta cell survival in mice. Gastroenterology 137: 2146–2157, 2009. [DOI] [PubMed] [Google Scholar]
- 41.Marchetti P, Lupi R, Bugliani M, Kirkpatrick CL, Sebastiani G, Grieco FA, Del Guerra S, D'Aleo V, Piro S, Marselli L, Boggi U, Filipponi F, Tinti L, Salvini L, Wollheim CB, Purrello F, Dotta F. A local glucagon-like peptide 1 (GLP-1) system in human pancreatic islets. Diabetologia 55: 3262–3272, 2012. [DOI] [PubMed] [Google Scholar]
- 42.Mellado-Gil J, Rosa TC, Demirci C, Gonzalez-Pertusa JA, Velazquez-Garcia S, Ernst S, Valle S, Vasavada RC, Stewart AF, Alonso LC, Garcia-Ocana A. Disruption of hepatocyte growth factor/c-Met signaling enhances pancreatic beta-cell death and accelerates the onset of diabetes. Diabetes 60: 525–536, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Morisset J, Julien S, Lainé J. Localization of cholecystokinin receptor subtypes in the endocine pancreas. J Histochem Cytochem 51: 1501–1513, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ning SL, Zheng WS, Su J, Liang N, Li H, Zhang D, Dong JH, Zhang ZK, Cui M, Hu QX, Chen CC, Liu CH, Wang C, Pang Q, Chen Y, Yu X, Sun JP. Different downstream signaling of CCKAR regulates distinct functions of CCK in pancreatic β cells. Br J Pharmacol. 2015. Aug 6. doi: 10.1111/bph.13271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Oropeza D, Jouvet N, Budry L, Campbell JE, Bouyakdan K, Lacombe J, Perron G, Bergeron V, Neuman JC, Brar HK, Fenske RJ, Meunier C, Sczelecki S, Kimple ME, Drucker DJ, Screaton RA, Poitout V, Ferron M, Alquier T, Estall JL. Phenotypic characterization of MIP-CreERT1Lphi mice with transgene-driven islet expression of human growth hormone. Diabetes. 2015. Jul 7. pii: db150272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rabaglia ME, Gray-Keller MP, Frey BL, Shortreed MR, Smith LM, Attie AD. α-Ketoisocaproate-induced hypersecretion of insulin by islets from diabetes-susceptible mice. Am J Physiol Endocrinol Metab 289: E218–E224, 2005. [DOI] [PubMed] [Google Scholar]
- 47.Rodgers RJ, Tschop MH, Wilding JPH. Anti-obesity drugs: past, present and future. Disease Models & Mechanisms 5: 621–626, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schweiger M, Erhard MH, Amselgruber WM. Cell-specific localization of the cholecystokininA receptor in the porcine pancreas. Anat Histol Embryol 29: 357–361, 2000. [DOI] [PubMed] [Google Scholar]
- 49.Teta M, Long SY, Wartschow LM, Rankin MM, kushner JA. Very slow turnover of beta-cells in aged adult mice. Diabetes 54: 2557–2567, 2005. [DOI] [PubMed] [Google Scholar]
- 50.Truchan NA, Brar HK, Gallagher SJ, Neuman JC, Kimple ME. Validation of a single-islet microplate assay to measure mouse and human islet insulin secretion. Islets. In press, 2015. doi: 10.1080/19382014.2015.1076607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tschen SI, Dhawan S, Gurlo T, Bhushan A. Age-dependent decline in beta-cell proliferation restricts the capacity of beta-cell regeneration in mice. Diabetes 58: 1312–1320, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Willemer S, Elsasser HP, Adler G. Hormone-induced pancreatitis. Eur Surg Res 24, Suppl 1: 29–39, 1992. [DOI] [PubMed] [Google Scholar]