Abstract
In contrast to the negative feedback of angiotensin II (ANG II) on juxtaglomerular renin, ANG II stimulates renin in the principal cells of the collecting duct (CD) in rats and mice via ANG II type 1 (AT1R) receptor, independently of blood pressure. In vitro data indicate that CD renin is augmented by AT1R activation through protein kinase C (PKC), but the exact mechanisms are unknown. We hypothesize that ANG II stimulates CD renin synthesis through AT1R via PKC and the subsequent activation of cAMP/PKA/CREB pathway. In M-1 cells, ANG II increased cAMP, renin mRNA (3.5-fold), prorenin, and renin proteins, as well as renin activity in culture media (2-fold). These effects were prevented by PKC inhibition with calphostin C, PKC-α dominant negative, and by PKA inhibition. Forskolin-induced increases in cAMP and renin expression were prevented by calphostin C. PKC inhibition and Ca2+ depletion impaired ANG II-mediated CREB phosphorylation and upregulation of renin. Adenylate cyclase 6 (AC) siRNA remarkably attenuated the ANG II-dependent upregulation of renin mRNA. Physiological activation of AC with vasopressin increased renin expression in M-1 cells. The results suggest that the ANG II-dependent upregulation of renin in the CD depends on PKC-α, which allows the augmentation of cAMP production and activation of PKA/CREB pathway via AC6. This study defines the intracellular signaling pathway involved in the ANG II-mediated stimulation of renin in the CD. This is a novel mechanism responsible for the regulation of local renin-angiotensin system in the distal nephron.
Keywords: prorenin, hypertension, protein kinase, calcium, adenylyl cyclase-6, gene expression, M-1 cells
in angiotensin ii - (ANG II) dependent hypertension, the intrarenal ANG II content is greater than can be explained from the levels found in plasma (30, 31). High intrarenal ANG II levels can be partially explained by enhanced ANG II type 1 receptor (AT1R)-mediated uptake of ANG II (10). Augmentation of proximal tubule angiotensinogen (AGT) synthesis and secretion is responsible for increased local intratubular ANG II generation (21). The presence of AGT in the urine indicates that AGT traverses the distal nephron segments where it may then be cleaved to ANG I, if an adequate source of renin is available.
In addition to the juxtaglomerular cells (JG), renin expression has been described in the proximal tubules, connecting tubules, and cortical and medullary collecting duct (CD) cells from the mouse, rat, and human kidney (39, 44). In contrast to JG cells, in the principal cells of the CD renin is upregulated by ANG II (36), via an AT1R-mediated mechanism (37), which is independent of blood pressure (35). In ANG II-dependent hypertensive rats with marked plasma renin activity (PRA) suppression, increased urinary levels of renin and prorenin reflect their augmented secretion by CD cells into the luminal fluid (26). In this model of experimental hypertension, intraluminal conversion of ANG I to ANG II in the CD is supported by the local presence of angiotensin-converting enzyme (ACE) protein levels (12, 13), as well as its ACE activity in CD fluid and urine (3). Collectively, the presence of ACE, AGT, and renin in the distal nephron segments contributes to elevate intratubular ANG I and ANG II generation during ANG II-dependent hypertension. Increased intrarenal ANG II may directly stimulate epithelial sodium channel (ENaC) activity in the CD, thus contributing to overall enhanced sodium reabsorption and the development of hypertension (22, 27, 34).
It is well-known that cyclic adenosine monophosphate (cAMP) signaling is the main stimulatory pathway in the regulation of renin in JG cells (19). The second messenger cAMP produced by adenylate cyclase (AC) activates protein kinase A (PKA). The free catalytic subunits of PKA translocate to the nucleus and phosphorylate transcription factor cAMP responsive element binding (CREB) protein turning on renin gene transcription (23). ANG II inhibits JG renin synthesis via AT1R through inhibition of cAMP and augmentation of intracellular Ca2+ (5, 20) levels and PKC activity (29). In contrast, in primary cultured rat inner medullary CD (IMCD) cells and mouse cortical CD cells (mpkCCDc14), ANG II increases intracellular cAMP levels (24, 25). Although recent evidence suggests that ANG II increases renin gene expression in rat IMCD via a PKC-dependent mechanism (9), the specific intracellular signaling mechanism involved in the ANG II-dependent stimulation of renin synthesis and secretion has not been fully elucidated. Furthermore, the roles of PKC on cAMP production and the activation of PKA/CREB pathway on the regulation of renin in CD cells remain unclear. In the present study, we tested the hypothesis that ANG II stimulates CD renin synthesis through AT1R-mediated PKC and subsequent activation of cAMP/PKA/CREB pathway.
METHODS
M-1 cell culture and reagents.
M-1 cells were cultured as previously described (17). ANG II was used at a concentration of 100 nmol/l and vehicle (PBS at pH 7) was used as control. Cells were harvested after 6 h of treatments either with candesartan (1 μmol/l), H89 (PKA inhibitor; 1 μmol/l), IBMX (3-isobutyl-1-methylxanthine, 1 mmol/l), or calphostin C (PKC inhibitor; 10 nmol/l). A dose-response curve for different concentrations of forskolin was done and maximal cAMP accumulation was obtained at 1 and 10 μM (Table 1). The following experiments were done at 1 μmol/l.
Table 1.
Dose-response curve for cAMP levels to different concentrations of forskolin
Data are means ± SE. Cell lysates from M-1 cells were processed after 30 min of incubation.
P < 0.05,
P < 0.01 vs. vehicle (control), n = 4–5.
Immunofluorescence studies.
M-1 cells were fixed in cold methanol blocked and stained with either rabbit anti-aquaporin-2 (cat. no. 178612, Calbiochem, San Diego, CA), rabbit anti-renin (sc H-105, Santa Cruz Biotechnology, Santa Cruz, CA), and detected with Alexa Fluor 594 conjugated to anti-rabbit IgG (Invitrogen, Carlsbad, CA). Samples were counterstained with 4′,6-diamidino-2-phenylindole (DAPI; Invitrogen). Negative controls were obtained by omission of the specific primary antibody.
Plasmids and transfection.
The expressing plasmids of PKC-α dominant negative mutant were constructed as described previously (43). M-1 were transfected with PKC-α dominant negative plasmids (Addgene plasmid 21235, Cambridge, MA) using Lipofectin (Lipofectin reagent; Invitrogen). A similar transfection protocol was followed before ANG II treatment with AC6-siRNA (cat. no. SI00165928, Qiagen, Valencia, CA).
RNA isolation and real-time quantitative PCR.
Total RNA was extracted and transcribed to cDNA. Primers and probes used to amplify renin mRNA were as follows: Forward: 5′-AGT-ACT-ATG-GTG-AGA-TCG-GCA-TT-3′, Reverse: 5′-AGA-TTC -ACA-ACC-TCT-ATG-ACT-CCT-C-3′ and fluorogenic probe: 5′6-FAM-TTC-AAA-GTC-ATC-TTT-GAC- ACG-GGT-TCA-G- BHQ1-3. Mouse β-actin gene was used as an internal standard: Forward: 5′-ATC-ATG- AAG-TGT-GAC-GTT-GA-3′; Reverse: 5′-GAT-CTT-CAT-GGT-GCT-AGG-AGC-3′ and fluorogenic probe; 5′-6-HEX-TCT-ATG-CCA-ACA-CAG-TGC-TGT-CTG-GT-BHQ2-3′. Results are presented as a ratio between the levels of mRNA of the interest gene against β-actin.
Western blot analysis for phospho-CREB, total CREB, prorenin, and renin.
Twenty micrograms of total protein were separated and transferred to a nitrocellulose membrane (Invitrogen). Anti-phospho-CREB and total CREB were obtained from Cell Signaling (Danvers, MA). For prorenin and renin detection, a polyclonal IgG B-12 antibody was used (Santa Cruz Biotechnology). Results were expressed as the ratio between the abundance of the protein of interest and β-actin. Recombinant mouse prorenin (AnaSpec, Fremont, CA) and renin (Lee Biosolutions, St. Louis, MO) were used as standards.
Renin content in cell culture media.
Renin content in cell culture media was determined by using modified protocols from the PRA assay [GammaCoat Plasma Renin Activity 125I RIA kit (DiaSorin, Stillwater, MN)] as previously described (8).
cAMP levels and PKC activity measurements.
The cAMP levels of M-1 cells were determined with cAMP ELISA (Cayman, Ann Arbor, MI) according to the manufacturer's instructions. PKC activity was assessed using a PKC kit (ADI-EKS-420A; Enzo Life Sciences, Ann Arbor, MI) in the cell lysates and calculated as PKC activity = Average Absorbance (sample) − Average Absorbance (blank) divided by the quantity of crude protein used per assay.
Ca2+ measurements.
Cell suspensions (8 × 105 cells/ml) were loaded with Fura-2 AM (5 μM) and incubated for 30 min at room temperature and protected from light and 37°C. Then, cells were washed with PBS and suspended. A volume of 500 μl was added in a quartz cell to measure fluorescence in Fluoromax-2 spectrofluorometer (Instruments SA, Edison, NJ). Cells were preincubated for 10 min with BAPTA-AM. Measurement was done at 100 s after ANG II (100 nM) or 1 μM Thapsigargin. The [Ca2+] was calculated as: [Ca2+]i (nM) = Kd × [(R − Rmin)/Rmax − R)] × Sfb, where Kd (for Ca2+ binding to fura-2 at 37°C) = 225 nM, R = 340/380 ratio, Rmax = 340/380 ratio under Ca2+-saturating conditions, Rmin = 340/380 ratio under Ca2+-free conditions, and Sfb = ratio of baseline fluorescence (380 nm) under Ca2+-free and -bound conditions.
Statistics analyses.
For Western blot and mRNA levels, an average number of six independent experiments was performed for each treatment and represented as fold change. Data were evaluated by the Grubb test followed, when appropriate by paired and unpaired Student's t-test or by one-way ANOVA with Tukey's posttest. Significance was defined as P < 0.05. Results are expressed as means ± SE.
RESULTS
M-1 collecting duct cells express prorenin and renin.
Previous studies indicated that CD cells mainly express prorenin (9, 17). A Western blot was used to establish the protein band identity using recombinant mouse prorenin and renin. We observed a predominant band of 45 kDa, corresponding to the prorenin molecular migration pattern, and a 38-kDa band, which was consistent with renin standard (Fig. 1A). In an additional experiment, microdissected mouse renal inner medullary homogenates were loaded and probed against renin antibody and compared with M-1. As observed in Fig. 1B, the pattern was similar, according to the molecular weight standard.
Fig. 1.
A: identity of prorenin (∼45 kDa) and renin (∼40 kDa) bands was corroborated by using recombinant mouse prorenin and renin controls. B: 30 μg of M-1 cell lysate and mouse renal inner medullary tissues were loaded and probed against renin antibody showing the presence of prorenin and renin in renal medulla. C: fold change vs. control of prorenin and renin protein levels in cell lysates from M-1 cells treated at different doses of ANG II. Maximal prorenin and renin protein levels were induced at 10−7 mol/l ANG II. D: immunostaining in ANG II (10−7 mol/l) and vehicle-treated cells showing the induction of prorenin-renin in a punctuated staining pattern (red), blue: nuclei. E: renin activity in cell culture media (ng/ANG I/h/ml) after 6-h treatment *P < 0.05 vs. vehicle-treated group.
ANG II stimulates renin mRNA, prorenin-renin protein expression, and renin activity in culture media.
Figure 1C shows that both prorenin and renin protein levels were augmented by ANG II (100 nmol/l) after 6 h (prorenin: ANG II: 1.7 ± 0.3 vs. control: 1.0 ± 0.1, P < 0.05; Renin: 3.5 ± 0.4 vs. control: 1.0 ± 0.2, P < 0.05). Renin mRNA was also augmented at this concentration (renin mRNA: ANG II: 3.01 ± 0.54 vs. control: 0.8 ± 0.2, P < 0.05). All the subsequent experiments were performed at 100 nmol/l. Immunofluorescence in M-1 cells treated with ANG II during 16 h demonstrated increased prorenin-renin immunostaining in a reticular pattern (object count analysis, ANG II: 137 ± 11 vs. control: 24 ± 3, P < 0.05, Fig. 1D). ANG II treatment also increased renin activity (ANG II: 31 ± 4 vs. control: 18 ± 4 ng ANG I formed·h−1·ml−1, P < 0.05; Fig. 1E). Augmentation of renin mRNA was completely prevented by candesartan (ANG II + candesartan: 0.9 ± 0.5 vs. control: 0.8 ± 0.2, P nonsignificant).
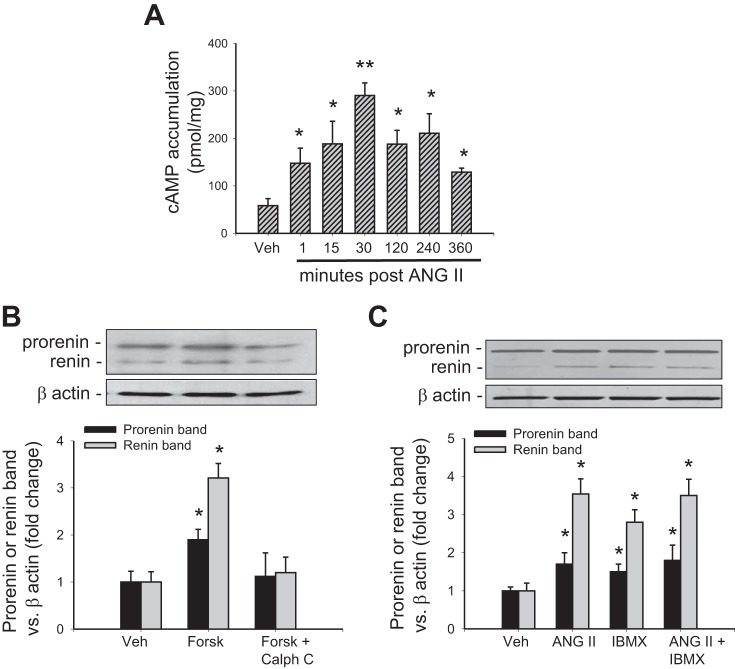
ANG II-mediated increase in intracellular cAMP levels depends on PKC activity.
Figure 2A shows that ANG II significantly increased cAMP levels compared with controls, starting at 1 min and reaching a peak at 30 min (290 ± 26 pmol/mg, P < 0.05). Then, we tested whether ANG II-dependent cAMP accumulation or forskolin-mediated increases in intracellular cAMP depend on PKC activity. Table 1 shows a cAMP dose-response curve against different concentrations of forskolin and Table 2 summarizes cAMP levels among different treatments at 30 min of incubation. ANG II-dependent increases in cAMP were completely suppressed by calphostin C and candesartan (AT1 receptor blocker). We next used a phosphodiesterase inhibitor (IBMX). As expected, IBMX increases cAMP; however, no further increases were observed in ANG II + IBMX group. To determine whether cAMP levels are correlated with prorenin and renin protein levels, we performed a Western blot. As shown in Fig. 2B, forskolin significantly increased prorenin band (1.89 ± 0.22 vs. control: 1.00 ± 0.23, P < 0.05) and renin band (3.21 ± 0.31 vs. control: 1.00 ± 0.20, P < 0.05). This effect was suppressed by calphostin C. Either ANG II or IBMX enhanced prorenin (1.7 ± 0.3- and 1.5 ± 0.2-fold, respectively) and renin (3.5 ± 0.4- and 2.8 ± 0.3-fold, respectively); however, ANG II + IBMX did not cause further increase (1.8 ± 0.4- and 3.5 ± 0.4-fold, respectively) compared with ANG II alone or IBMX alone (Fig. 2C). Regardless, the apparent cAMP accumulation observed in ANG II+forskolin, prorenin and renin levels was not different from ANG II or forskolin alone (data not shown). Because ANG II and IBMX increase cAMP levels to a similar extent and the combination of both drugs does not produce additive effects, we further assessed the possibility that ANG II may act via inhibition of cAMP degradation, using a combined treatment of IBMX and forskolin. This combined treatment elicited an additive effect on cAMP levels compared with foskolin or IBMX alone (Table 2).
Fig. 2.
Renin expression and cAMP accumulation induced by ANG II depend on protein kinase C (PKC) activity. A: time course of ANG II treatment and cAMP levels in M-1 cells. *P < 0.05 vs. vehicle-treated group. **P < 0.01 vs. vehicle-treated group. B: forskolin-dependent augmentation of prorenin and renin levels was prevented by calphostin C, a PKC inhibitor. C: 3-isobutyl-1-methylxanthine (IBMX) upregulates prorenin and renin; however, there is no additive effect between IBMX and ANG II. *P < 0.05 vs. vehicle-treated group.
Table 2.
Summary of cAMP levels
| ANG II + | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Vehicle | ANG II | Cand | Cal C | IBMX | H89 | Forsk | Cal C | IBMX | IBMX + Forsk | H89 | Forsk | Forsk + Cal C | |
| cAMP, pmol/mg | 54 ± 18 | 290 ± 26* | 67 ± 26 | 87 ± 32 | 305 ± 33* | 59 ± 18 | 400 ± 42† | 44 ± 9 | 227 ± 11* | 875 ± 22‡ | 49 ± 10 | 381 ± 40† | 75 ± 21 |
Data are means ± SE. Summary of cAMP levels in response to ANG II (30 min), ANG II + AT1 receptor blocker candesartan (Cand), ANG II + calphostin C (PKC inhibitor), forskolin (adenylate cyclase activator), IBMX (phosphatase inhibitor), IBMX+forskolin, PKA inhibitor H89, and or forskolin + calphostin C. Inhibitors were added 20 min before ANG II or forskolin treatments.
P < 0.05,
P < 0.01,
P < 0.001 vs. vehicle (control), n = 4–5.
PKA inhibition prevented the ANG II-dependent upregulation of renin.
To further confirm that PKA is involved in the AT1R-mediated signaling pathway responsible for renin upregulation, we treated the M-1 cells with the PKA inhibitor H89 20 min before ANG II. H89 blunted the ANG II-mediated upregulation of renin mRNA (Fig. 3A), prorenin and renin protein (Fig. 3B). The same effect was observed for renin activity in cell culture media (Fig. 3C).
Fig. 3.
Inhibition of PKA activity blunted the ANG II-dependent renin upregulation in M-1 collecting duct (CD) cells. Renin mRNA (A) and prorenin-renin protein levels (B) were induced by ANG II; however, H89 (PKA inhibitor) prevented this effect. C: similar effect was seen when we analyzed renin activity in cell culture media. *P < 0.05 vs. vehicle-treated group.
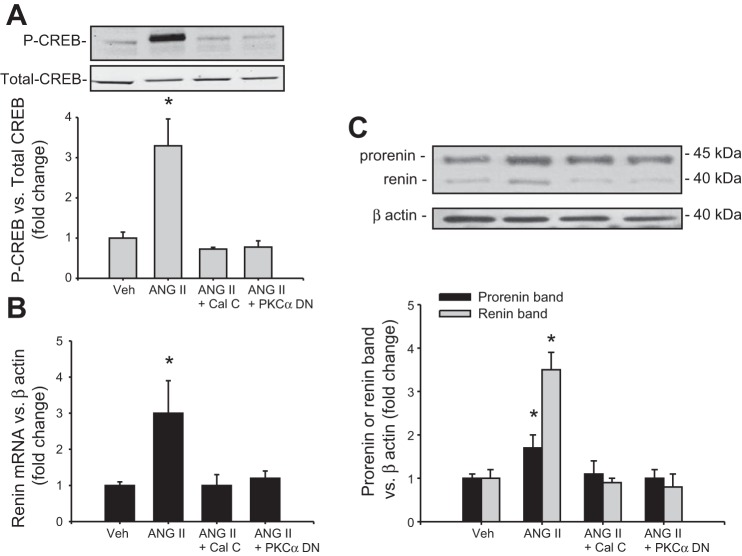
PKC-α dominant negative prevented CREB phosphorylation and renin upregulation in response to ANG II treatment.
To further confirm the role of PKC on renin expression in CD, we performed a Western blot analysis of renin and CREB in M-1 cells treated with ANG II or ANG II plus calphostin C. Because PKC-α is a predominant isoform in the CD, we tested whether this isoform is involved in the AT1R downstream pathway. As shown in Fig. 4A, CREB phosphorylation was augmented after 30 min of ANG II treatment (3.2 ± 0.6 vs. 1.00 ± 0.14, P < 0.05). This effect was blunted by calphostin C, an inhibitor of classic and novel PKC isoforms. Because PKC-α is the dominant isoform present in the CD, we tested the effect of a PKC-α dominant negative on CREB phosphorylation and renin expression. Transfections with PKC-α suppressed ANG II-mediated CREB phosphorylation. Both treatments also prevented the effects of ANG II on renin mRNA and prorenin and renin protein levels (Fig. 4, B-C).
Fig. 4.
Inhibition of PKC activity suppressed the ANG II-dependent phosphorylation of CREB and upregulation of renin synthesis in M-1 cells. Cells were either treated with calphostin C, a broad spectrum PKC inhibitor, or transfected with a PKC-α dominant negative (DN; 48 h before treatment). A: CREB phosphorylation mediated by ANG II treatment was blunted by PKC inhibition or PKC-α DN. Renin mRNA (B) and prorenin and renin protein levels (C) were not augmented in M-1 cells previously treated with ANG II + calphostin C or transfected with PKC-α DN. *P < 0.05 vs. vehicle-treated group.
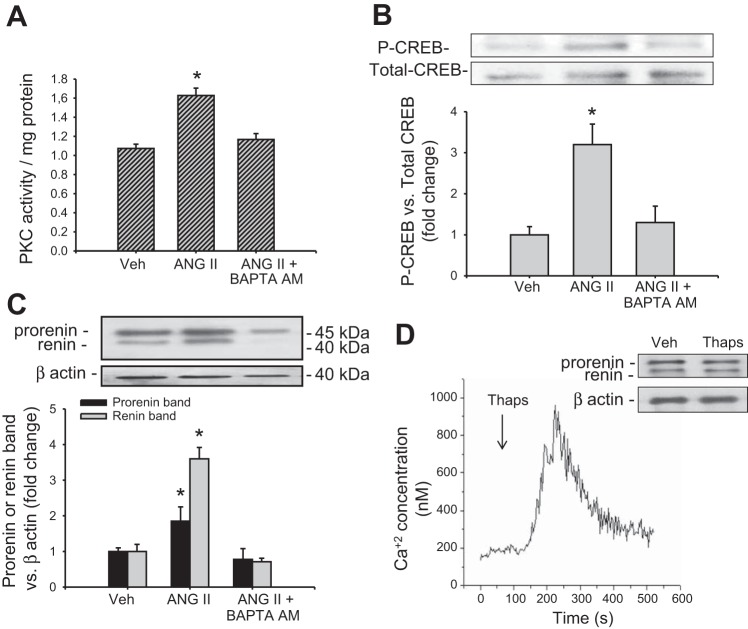
Intracellular Ca2+ is involved in the ANG II-dependent upregulation prorenin and renin.
To further assess the involvement of a Ca2+-dependent PKC, M-1 cells were treated with BAPTA-AM, an intracellular Ca2+ chelator. BAPTA-AM suppressed the ANG II-mediated increase in intracellular Ca2+ (not shown), PKC activity (Fig. 5A), CREB phosphorylation (Fig. 5B), and prorenin and renin protein levels (Fig. 5C). Thapsigargin, a noncompetitive inhibitor of the sarco/endoplasmic reticulum Ca2+-ATPase (SERCA), increased intracellular Ca2+ levels; however, it did not alter prorenin and renin protein levels (Fig. 5D).
Fig. 5.
ANG II-dependent upregulation of prorenin and renin depends on intracellular Ca2+ availability. M-1 cells were preincubated with BAPTA-AM (see methods) and treated with ANG II. Depletion of intracellular Ca2+ suppressed the increased PKC activity (A), CREB phosphorylation (B), and prorenin renin upregulation (C) mediated by ANG II. D: thapsigargin (Thaps) raises intracellular Ca2+; however, it did not change prorenin and renin protein levels. *P < 0.05 vs. vehicle-treated group.
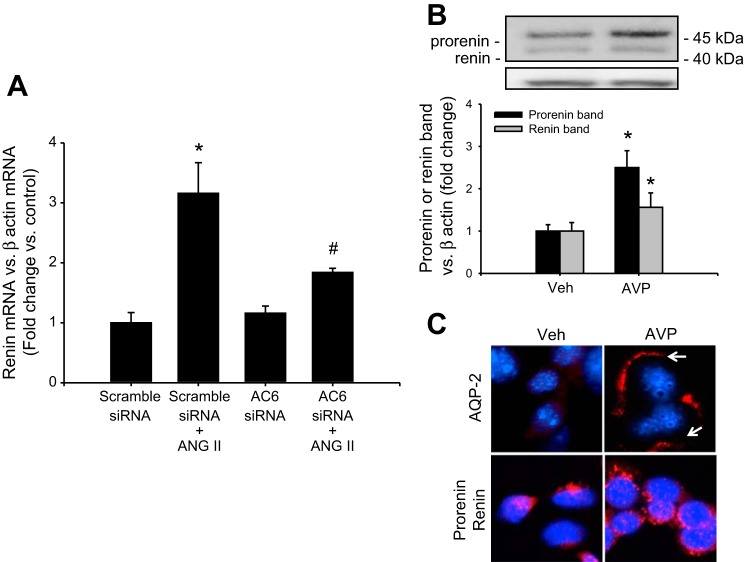
ANG II-dependent renin mRNA upregulation depends on AC6.
AC6 is the most abundant isoform in the CD (4, 14, 40, 41). As shown in Fig. 6A, AC6-siRNA partially suppressed the effect of ANG II on renin upregulation (scramble-siRNA: 1.00 ± 0.18; ANG II: 3.12 ± 0.55; AC6-siRNA: 1.12 ± 0.15; ANG II + AC6-siRNA: 1.78 ± 0.12), indicating that AC6 is involved, at least in part, in the downstream pathway mediated by the activation of AT1R.
Fig. 6.
Adenylate cyclase (AC) activity is responsible for renin upregulation. AC6 is the most prominent isoform in CD cells. A: as observed in the plot, AC6 RNAi transfection partially suppresses the upregulation of renin mRNA mediated by ANG II, suggesting that AC6 is responsible for cAMP production after ANG II treatment. B: because the most prominent activator of the AC in the CD is vasopressin, (AVP) we investigate whether AVP treatment increases prorenin and renin protein levels. A representative Western blot showing the augmentation of prorenin and renin after 6 h of AVP treatment. C: compared with vehicle-treated cells, AVP causes increased aquaporin (AQP)-2 immunostaining and apical localization (arrows), which was correlated with the enhanced prorenin-renin staining in a granular pattern. *P < 0.05 vs. vehicle-treated group. #P < 0.05 vs. ANG II-treated group.
Physiological stimulation of cAMP/PKA/CREB pathway with vasopressin causes the upregulation of prorenin and renin in M-1 cells.
We further used vasopressin treatment as a maneuver to physiologically increase intracellular cAMP. We evaluated whether V2 receptor-dependent activation of cAMP/PKA/CREB pathway stimulates renin expression in M-1 cells. As shown in Fig. 6B, vasopressin (10−7 mol/l) significantly increased prorenin band (2.51 ± 0.41 vs. control: 1.00 ± 0.15, P < 0.05) and renin band (1.56 ± 0.31 vs. control: 1.00 ± 0.20, P < 0.05); no stimulation was observed at lower concentrations in M-1 cells (data not shown). Prorenin-renin immunostaining in punctuated pattern parallels with the increased intensity and plasma membrane localization of aquaporin (AQP)-2 (Fig. 6C).
DISCUSSION
The present study unravels the intracellular pathway by which ANG II stimulates renin synthesis and secretion by the principal cells in the CD. The potent stimulatory effect of ANG II on renin expression in the principal cells involves the activation of AT1R and downstream signaling via PKC and cAMP/PKA/CREB pathways. The functional importance of the findings reported in the present study was confirmed by the demonstration that ANG II mediated increased renin activity in the cell culture media of M-1 cells. Importantly, we demonstrated that the stimulation of cAMP production depends on the activation of Ca2+-dependent PKC-α.
Renin is primarily synthesized by the JG cells; however, its production has also been found in the principal cells of the CD. Augmented renin synthesis in the CD of animal models of hypertension (9, 17, 35) and diabetes (17) suggests that distal tubular renin plays a critical role in the regulation of intratubular RAS activity (46). In the JG cells, the augmentation of intracellular cAMP levels constitutes the central stimulus for renin gene expression (19, 23). In these cells, the initial activation of a G protein-coupled receptor stimulates cAMP generation, PKA activation, and CREB phosphorylation, leading to enhanced renin gene expression (7, 19). In contrast, pharmacological increases in intracellular Ca2+ concentration by thapsigargin inhibit renin gene expression in a time- and concentration-dependent manner and this effect is prevented by BAPTA-AM (20). Ortiz-Capisano et al. (32) demonstrated that intracellular cAMP content was increased markedly with BAPTA-AM in JG cells. The activity of AC is responsible for cAMP generation and the Ca2+-inhibited AC5 and AC6 isoforms are present in JG cells (7, 32). Thus, it is proposed that Ca2+ decreases the generation of cAMP by the Ca2+-inhibited isoforms of AC, which results in the reduction of cAMP content and renin synthesis in JG cells. As part of the negative feedback, ANG II-dependent PKC activation downregulates the expression of renin in JG cells (29). Phorbol 12-myristate13-acetate (PMA), a PKC activator, augments PKC activity and decreases renin expression in JG cells (29). In contrast to the inhibitory effect that ANG II exerts on renin in JG cells, in the present study, we demonstrated that ANG II increases prorenin and renin synthesis and release by the CD cells (9, 17, 36). In vitro studies using a CD cell line and primary cultures of IMCD cells showed that ANG II also upregulates renin synthesis (17) and that AT1R antagonists blunted this effect (37). Although commercially available AT1R antibodies have been suggested to be nonspecific for AT1R (16), there is immunohistochemical and functional data suggesting basolateral and apical membrane distribution of the AT1R in the CD (15, 34). Then, AT1R may be involved in intratubular ANG II stimulation by both apical and basolateral sides (Fig. 7). Further studies using permeable supports are necessary to explore this possibility and subcellular distribution of prorenin or renin in response to ANG II.
Fig. 7.
Proposed intracellular mechanism involved in the ANG II-mediated augmentation of renin synthesis and secretion in the collecting duct. Activation of AT1R leads to the activation of PKC-α, which is a calcium-dependent PKC. Our data demonstrated that in CD cells the inhibition of PKC activity blunted cAMP accumulation. Then, it is probable that PKC-α potentiates receptor-mediated (GPCR) activation of AC6 or the inhibition of phosphodiestareses (PDE) all of which causes the increased PKA activity, CREB phosphorylation turning on renin gene expression. Alternatively, some reports suggested that PKC might directly mediate CREB phosphorylation. Prorenin and renin can be secreted to the lumen as shown previously by our enhancing ANG II formation. Prorenin binds to the (pro)renin receptor [(P)RR] is able to cleave angiotensinogen (AGT) coming from proximal tubules. Due to the presence of angiotensin-converting enzyme (ACE) in distal nephrons, newly formed intratubular ANG II may activate luminal AT1R enhancing the activity of epithelial sodium channel (ENaC) and promoting sodium entry. CREB, cAMP-responsive element binding protein; DAG, diacyl glycerol; IP3, inositol trisphosphate; PIP2, phosphatidylinositol 4,5-bisphosphate.
ANG II-mediated upregulation of prorenin and renin protein levels is prevented by PKC inhibition (9). We previously showed that the PKC activator PMA mimicked the effect of ANG II in rat primary cultured IMCD cells (9). In these cells and mpkCCDc14 cells, ANG II treatment increases intracellular cAMP levels (24, 25), an effect that is prevented by PKC inhibition with staurosporin (24). The PKC enzymes are a family of at least 12 isoforms with different structures, substrate requirements, and expression (47). They are divided into three groups: conventional PKCs that are Ca2+-dependent and activated by DAG, novel PKCs that are Ca2+-independent and activated by DAG, and atypical PKCs that are also Ca2+-independent and do not require DAG activation (47). In this study, we demonstrate that the ANG II stimulation of CD renin occurs via a Ca2+-dependent PKC and that increases in intracellular Ca2+ are not enough to enhance prorenin or renin expression. PKC-α is the most abundant isoform present in renal CD cells (33). We used a PKC-α dominant negative, which expresses mutated nonfunctional PKC-α, competing with the normal isoforms to specifically suppress PKC-α activity. The decreases in renin transcript levels and prorenin and renin protein abundances in response to PKC pharmacological inhibition and PKC-α dominant negative indicate that the PKC-α isoform is involved in the stimulation of cAMP/PKA/CREB pathway responsible for renin synthesis in M-1 cells. In IMCD cells, ANG II treatment increased AQP-2 expression through CREB activation (24, 25). Furthermore, the marked augmentation of CREB phosphorylation after ANG II treatment suggests that ANG II stimulates renin gene via the activation of CREB in M-1 cells. In PKC-α knockout mice, the phosphorylated CREB levels are significantly decreased compared with that of wild-type (45). It is possible that in M-1 cells, PKC either phosphorylates and activates CREB directly (45) or by increasing the AC6 activity (1, 2, 18).
ANG II-mediated effect on CD renin synthesis also depends on PKA activation because H89 completely suppressed this effect. This is consistent with the robust increase in intracellular cAMP and CREB phosphorylation after ANG II treatment. By raising intracellular cAMP levels with forskolin, we were able to increase renin mRNA (∼9-fold) and prorenin and renin protein abundances (∼3-fold) levels. Interestingly, the upregulation of prorenin and renin induced by forskolin was dependent on PKC activity. Supporting our results, Beazely et al. (1) showed that PMA also potentiated cAMP accumulation in cells expressing endogenous AC6. The finding that ANG II-dependent upregulation of renin depends on increases in cAMP in CD cells further emphasizes the central stimulatory role of cAMP in regulation on renin expression. Intracellular cAMP levels are determined by the balance between the generation via AC and the degradation by phosphodiesterases. It is likely that either the ANG II-mediated augmentation of intracellular Ca2+ or PKC activity both inhibit cAMP degradation by impeding the action of PDE (Fig. 7). Thus, we used IBMX, a phosphodiesterase inhibitor, to distinguish between effects of ANG II on cAMP formation or degradation. The IBMX treatment increased cAMP levels; however, the combination of IBMX with ANG II caused a slight but not significant increase in cAMP compared with each treatment alone (Table 2). This treatment did not show further increases in prorenin or renin abundances (Fig. 2C). To further examine this possibility, we performed experiments combining IBMX plus forskolin. As shown in Table 2, IBMX plus forskolin treatment remarkably increased cAMP levels. Another possibility for the modulatory effect of ANG II on AC could involve phosphorylation of another component of the system, including Gs and/or Gi protein by PKC activation (42). In fact, it has been suggested that PKC can directly phosphorylate CREB (28).
As a maneuver to increase AC activity in M-1 cells, we used vasopressin, a known physiological stimuli for cAMP-mediated increase for AQP-2 abundance in plasma membrane. Vasopressin increased prorenin and renin abundance, plasma membrane AQP-2, and cAMP levels (198 ± 30 vs. 38 ± 12 pg/mg, P < 0.05). The upregulation of AQP-2 would parallel the enhancements of renin expression, water reabsorption, and intratubular ANG II formation. These data agreed with our results demonstrating that silencing of AC6 leads to a reduction in the ANG II-dependent stimulation of renin synthesis, thus indicating that AC6 is required for the ANG II signaling in CD cells. Future studies using AC6 knockout mice will provide further inputs on the role of AC6 in the ANG II-mediating increases in cAMP and CD renin.
Mice overexpressing renin in the CD exhibit high blood pressure (38). Upregulation of renin synthesis and secretion in ANG II-induced hypertensive rats (26) and mice (11) indicate that CD renin plays an important role in modulating blood pressure and further support the concept that CD renin contributes to the generation of intratubular ANG II and stimulation of distal sodium transport (34, 48). Furthermore, the presence of the (pro)renin receptor in distal segments also suggests that prorenin and renin may be anchored by this receptor in neighboring cells, thus activating intracellular signaling pathways that have been related to tissue damage (8). Therefore, the development of renin inhibitors with better penetration into the distal tubular segments (6) could be of clinical relevance for a better control of hypertension and its related consequences.
In conclusion, the present study defines the intracellular signaling mechanism by which ANG II/AT1R stimulates renin in the CD. We demonstrate that in these cells, a Ca2+-dependent PKC is required for the ANG II-induced stimulation of renin synthesis and secretion via augmentation of cAMP mediated by AC6, as well as PKA activation, and CREB phosphorylation, which turns on the renin gene. This is a novel pathophysiological mechanism that regulates the local RAS in the distal nephron.
GRANTS
The present study was funded by the National Institute of General Medical Sciences of the National Institutes of Health (NIH; CoBRE P30GM103337), the NIH-National Institute of Diabetes and Digestive and Kidney Diseases (DK104375-01), the LA-CaTS (U54-GM104940), and the Tulane School of Medicine Pilot Funds for M. C. Prieto; the FONDECYT grant from Chile (No. 11121217) for A. A. Gonzalez; and the National Council for Scientific and Technological Development (CNPq) PostDoctoral Fellowship from Brazil for L. S. Lara.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: A.A.G., L.L., and M.C.P. conception and design of research; A.A.G., L.L., L.S.L., C.R.B., C.I.-G., N.S.-P., V.R.G., and D.M.S. performed experiments; A.A.G., L.L., L.S.L., C.R.B., C.I.-G., D.M.S., and M.C.P. analyzed data; A.A.G., L.L., L.S.L., C.I.-G., and M.C.P. interpreted results of experiments; A.A.G. prepared figures; A.A.G. and M.C.P. drafted manuscript; A.A.G. and M.C.P. edited and revised manuscript; A.A.G. and M.C.P. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank the Molecular Core of the Tulane Renal Hypertension Center of Excellence and Dr. Ryosuke Satou for technical support.
REFERENCES
- 1.Beazely MA, Alan JK, Watts VJ. Protein kinase C and epidermal growth factor stimulation of Raf1 potentiates adenylyl cyclase type 6 activation in intact cells. Mol Pharmacol 67: 250–259, 2005. [DOI] [PubMed] [Google Scholar]
- 2.Beazely MA, Watts VJ. Galphaq-coupled receptor signaling enhances adenylate cyclase type 6 activation. Biochem Pharmacol 70: 113–120, 2005. [DOI] [PubMed] [Google Scholar]
- 3.Casarini DE, Boim MA, Stella RCR, Krieger-Azzolini MH, Krieger JE, Schor N. Angiotensin I-converting enzyme activity in tubular fluid along the rat nephron. Am J Physiol Renal Physiol 272: F405–F409, 1997. [DOI] [PubMed] [Google Scholar]
- 4.Defer N, Best-Belpomme M, Hanoune J. Tissue specificity and physiological relevance of various isoforms of adenylyl cyclase. Am J Physiol Renal Physiol 279: F400–F416, 2000. [DOI] [PubMed] [Google Scholar]
- 5.Dellabruna R, Pinet F, Corvol P, Kurtz A. Opposite regulation of renin gene-expression by cyclic-AMP and calcium in isolated mouse juxtaglomerular cells. Kidney Int 47: 1266–1273, 1995. [DOI] [PubMed] [Google Scholar]
- 6.Feldman DL, Jin L, Xuan H, Persohn E, Zhou W, Schuetz H, Park JK, Muller DN, Luft FC. The direct renin inhibitor aliskiren localizes and persists in rat kidneys. Am J Physiol Renal Physiol 305: F1593–F1602, 2013. [DOI] [PubMed] [Google Scholar]
- 7.Germain S, Bonnet F, Philippe J, Corvol P, Pinet F. Molecular regulation of human renin gene expression: cell-specific role of intron A. Hypertension 30: 17, 1997. [Google Scholar]
- 8.Gonzalez AA, Lara LS, Luffman C, Seth DM, Prieto MC. Soluble form of the (Pro) renin receptor is augmented in the collecting duct and urine of chronic angiotensin II-dependent hypertensive rats. Hypertension 57: 859–864, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gonzalez AA, Liu L, Lara LS, Seth DM, Navar LG, Prieto MC. Angiotensin II stimulates renin in inner medullary collecting duct cells via protein kinase C and independent of epithelial sodium channel and mineralocorticoid receptor activity. Hypertension 57: 594–599, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gonzalez-Villalobos R, Klassen RB, Allen PL, Navar LG, Hammond TG. Megalin binds and internalizes angiotensin II. Am J Physiol Renal Physiol 288: F420–F427, 2005. [DOI] [PubMed] [Google Scholar]
- 11.Gonzalez-Villalobos RA, Satou R, Ohashi N, Semprun-Prieto LC, Katsurada A, Kim C, Upchurch GM, Prieto MC, Kobori H, Navar LG. Intrarenal mouse renin-angiotensin system during ANG II-induced hypertension and ACE inhibition. Am J Physiol Renal Physiol 298: F150–F157, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gonzalez-Villalobos RA, Satou R, Seth DM, Semprun-Prieto LC, Katsurada A, Kobori H, Navar LG. Angiotensin-converting enzyme-derived angiotensin II formation during angiotensin II-induced hypertension. Hypertension 53: 351–355, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gonzalez-Villalobos RA, Seth DM, Satou R, Horton H, Ohashi N, Miyata K, Katsurada A, Tran DV, Kobori H, Navar LG. Intrarenal angiotensin II and angiotensinogen augmentation in chronic angiotensin II-infused mice. Am J Physiol Renal Physiol 295: F772–F779, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grunberger C, Obermayer B, Klar J, Kurtz A, Schweda F. The calcium paradoxon of renin release–calcium suppresses renin exocytosis by inhibition of calcium-dependent adenylate cyclases AC5 and AC6. Circ Res 99: 1197–1206, 2006. [DOI] [PubMed] [Google Scholar]
- 15.Harrison-Bernard LM, Navar LG, Ho MM, Vinson GP, El-Dahr SS. Immunohistochemical localization of ANG II AT1 receptor in adult rat kidney using a monoclonal antibody. Am J Physiol Renal Physiol 273: F170–F177, 1997. [DOI] [PubMed] [Google Scholar]
- 16.Herrera M, Sparks MA, Fonso-Pecchio AR, Harrison-Bernard LM, Coffman TM. Lack of specificity of commercial antibodies leads to misidentification of angiotensin type 1 receptor protein. Hypertension 61: 253–258, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kang JJ, Toma I, Sipos A, Meer EJ, Vargas SL, Peti-Peterdi J. The collecting duct is the major source of prorenin in diabetes. Hypertension 51: 1597–1604, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kawabe J, Ebina T, Toya Y, Oka N, Schwencke C, Duzic E, Ishikawa Y. Regulation of type V adenylyl cyclase by PMA-sensitive and -insensitive protein kinase C isoenzymes in intact cells. FEBS Lett 384: 273–276, 1996. [DOI] [PubMed] [Google Scholar]
- 19.Klar J, Sandner P, Muller MW, Kurtz A. Cyclic AMP stimulates renin gene transcription in juxtaglomerular cells. Pflügers Arch 444: 335–344, 2002. [DOI] [PubMed] [Google Scholar]
- 20.Klar J, Sigl M, Obermayer B, Schweda F, Kramer BK, Kurtz A. Calcium inhibits renin gene expression by transcriptional and posttranscriptional mechanisms. Hypertension 46: 1340–1346, 2005. [DOI] [PubMed] [Google Scholar]
- 21.Kobori H, Harrison-Bernard LM, Navar LG. Expression of angiotensinogen mRNA and protein in angiotensin II-dependent hypertension. J Am Soc Nephrol 12: 431–439, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Komlosi P, Fuson AL, Fintha A, Peti-Peterdi J, Rosivall L, Warnock DG, Bell PD. Angiotensin I conversion to angiotensin II stimulates cortical collecting duct sodium transport. Hypertension 42: 195–199, 2003. [DOI] [PubMed] [Google Scholar]
- 23.Kurtz A, Wagner C. Cellular control of renin secretion. J Exp Biol 202: 219–225, 1999. [DOI] [PubMed] [Google Scholar]
- 24.Lee YJ, Song IK, Jang KJ, Nielsen J, Frokiær J, Nielsen S, Kwon TH. Increased AQP2 targeting in primary cultured IMCD cells in response to angiotensin II through AT(1) receptor. Am J Physiol Renal Physiol 292: F340–F350, 2007. [DOI] [PubMed] [Google Scholar]
- 25.Li CL, Wang WD, Rivard CJ, Lanaspa MA, Summer S, Schrier RW. Molecular mechanisms of angiotensin II stimulation on aquaporin-2 expression and trafficking. Am J Physiol Renal Physiol 300: F1255–F1261, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu L, Gonzalez AA, McCormack M, Seth DM, Kobori H, Navar LG, Prieto MC. Increased renin excretion is associated with augmented urinary angiotensin II levels in chronic angiotensin II-infused hypertensive rats. Am J Physiol Renal Physiol 301: F1195–F1201, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mamenko M, Zaika O, Prieto MC, Jensen VB, Doris PA, Navar LG, Pochynyuk O. Chronic angiotensin II infusion drives extensive aldosterone-independent epithelial Na+ channel activation. Hypertension 62: 1111–1122, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martin F, Mora L, Laorden M, Milanes M. Protein kinase C phosphorylates the cAMP response element binding protein in the hypothalamic paraventricular nucleus during morphine withdrawal. Br J Pharmacol 163: 857–875, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Muller MW, Todorov V, Kramer BK, Kurtz A. Angiotensin II inhibits renin gene transcription via the protein kinase C pathway. Pflügers Arch 444: 499–505, 2002. [DOI] [PubMed] [Google Scholar]
- 30.Navar LG, Lewis L, Hymel A, Braam B, Mitchell KD. Tubular fluid concentrations and kidney contents of angiotensins I and II in anesthetized rats. J Am Soc Nephrol 5: 1153–1158, 1994. [DOI] [PubMed] [Google Scholar]
- 31.Navar LG, Von Thun AM, Zou L, El-Dahr SS, Mitchell KD. Enhancement of intrarenal angiotensin II levels in 2 kidney 1 clip and angiotensin II induced hypertension. Blood Press Suppl 2: 88–92, 1995. [PubMed] [Google Scholar]
- 32.Ortiz Capisano MC, Ortiz PA, Garvin JL, Beierwaltes WH. Calcium-inhibitable adenylate cyclase 5 mediates renin release from isolated juxtaglomerular (JG) cells induced by decreased intracellular calcium. Hypertension 48: E73, 2006. [Google Scholar]
- 33.Ostlund E, Mendez CF, Jacobsson G, Fryckstedt J, Meister B, Aperia A. Expression of protein kinase C isoforms in renal tissue. Kidney Int 47: 766–773, 1995. [DOI] [PubMed] [Google Scholar]
- 34.Peti-Peterdi J, Warnock DG, Bell PD. Angiotensin II directly stimulates ENaC activity in the cortical collecting duct via AT(1) receptors. J Am Soc Nephrol 13: 1131–1135, 2002. [DOI] [PubMed] [Google Scholar]
- 35.Prieto-Carrasquero MC, Botros FT, Pagan J, Kobori H, Seth DM, Casarini DE, Navar LG. Collecting duct renin is upregulated in both kidneys of 2-kidney, 1-clip goldblatt hypertensive rats. Hypertension 51: 1590–1596, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Prieto-Carrasquero MC, Harrison-Bernard LM, Kobori H, Ozawa Y, Hering-Smith KS, Hamm LL, Navar LG. Enhancement of collecting duct renin in angiotensin II-dependent hypertensive rats. Hypertension 44: 223–229, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Prieto-Carrasquero MC, Kobori H, Ozawa Y, Gutierrez A, Seth D, Navar LG. AT1 receptor-mediated enhancement of collecting duct renin in angiotensin II-dependent hypertensive rats. Am J Physiol Renal Physiol 289: F632–F637, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ramkumar N, Kohan DE. Role of collecting duct renin in blood pressure regulation. Am J Physiol Regul Integr Comp Physiol 305: R92–R94, 2013. [DOI] [PubMed] [Google Scholar]
- 39.Rohrwasser A, Morgan T, Dillon HF, Zhao L, Callaway CW, Hillas E, Zhang S, Cheng T, Inagami T, Ward K, Terreros DA, Lalouel JM. Elements of a paracrine tubular renin-angiotensin system along the entire nephron. Hypertension 34: 1265–1274, 1999. [DOI] [PubMed] [Google Scholar]
- 40.Roos KP, Bugaj V, Mironova E, Stockand JD, Ramkumar N, Rees S, Kohan DE. Adenylyl cyclase VI mediates vasopressin-stimulated ENaC activity. J Am Soc Nephrol 24: 218–227, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Roos KP, Strait KA, Raphael KL, Blount MA, Kohan DE. Collecting duct-specific knockout of adenylyl cyclase type VI causes a urinary concentration defect in mice. Am J Physiol Renal Physiol 302: F78–F84, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rozengurt E, Murray M, Zachary I, Collins M. Protein kinase C activation enhances cAMP accumulation in Swiss 3T3 cells: inhibition by pertussis toxin. Proc Natl Acad Sci USA 84: 2282–2286, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Soh JW, Weinstein IB. Roles of specific isoforms of protein kinase C in the transcriptional control of cyclin D1 and related genes. J Biol Chem 278: 34709–34716, 2003. [DOI] [PubMed] [Google Scholar]
- 44.Taugner R, Hackenthal E, Inagami T, Nobiling R, Poulsen K. Vascular and tubular renin in the kidneys of mice. Histochemistry 75: 473–484, 1982. [DOI] [PubMed] [Google Scholar]
- 45.Thai TL, Blount MA, Klein JD, Sands JM. Lack of protein kinase C-α leads to impaired urine concentrating ability and decreased aquaporin-2 in angiotensin II-induced hypertension. Am J Physiol Renal Physiol 303: F37–F44, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.van den HM, Batenburg WW, Jainandunsing S, Garrelds IM, van Gool JM, Feelders RA, van den Meiracker AH, Danser AH. Urinary renin, but not angiotensinogen or aldosterone, reflects the renal renin-angiotensin-aldosterone system activity and the efficacy of renin-angiotensin-aldosterone system blockade in the kidney. J Hypertens 29: 2147–2155, 2011. [DOI] [PubMed] [Google Scholar]
- 47.Way KJ, Chou E, King GL. Identification of PKC-isoform-specific biological actions using pharmacological approaches. Trends Pharmacol Sci 21: 181–187, 2000. [DOI] [PubMed] [Google Scholar]
- 48.Zhao D, Seth DM, Navar LG. Enhanced distal nephron sodium reabsorption in chronic angiotensin II-infused mice. Hypertension 54: 120–126, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]