Abstract
The potassium channel Kv7.1 plays critical physiological roles in both heart and epithelial tissues. In heart, Kv7.1 and the accessory subunit KCNE1 forms the slowly activating delayed-rectifier potassium current current, which is enhanced by protein kinase A (PKA)-mediated phosphorylation. The observed current increase requires both phosphorylation of Kv7.1 and the presence of KCNE1. However, PKA also stimulates Kv7.1 currents in epithelial tissues, such as colon, where the channel does not coassemble with KCNE1. Here, we demonstrate that PKA activity significantly impacts the subcellular localization of Kv7.1 in Madin-Darby canine kidney cells. While PKA inhibition reduced the fraction of channels at the cell surface, PKA activation increased it. We show that PKA inhibition led to intracellular accumulation of Kv7.1 in late endosomes/lysosomes. By mass spectroscopy we identified eight phosphorylated residues on Kv7.1, however, none appeared to play a role in the observed response. Instead, we found that PKA acted by regulating endocytic trafficking involving the ubiquitin ligase Nedd4-2. We show that a Nedd4-2-resistant Kv7.1-mutant displayed significantly reduced intracellular accumulation upon PKA inhibition. Similar effects were observed upon siRNA knockdown of Nedd4-2. However, although Nedd4-2 is known to regulate Kv7.1 by ubiquitylation, biochemical analyses demonstrated that PKA did not influence the amount of Nedd4-2 bound to Kv7.1 or the ubiquitylation level of the channel. This suggests that PKA influences Nedd4-2-dependent Kv7.1 transport though a different molecular mechanism. In summary, we identify a novel mechanism whereby PKA can increase Kv7.1 current levels, namely by regulating Nedd4-2-dependent Kv7.1 transport.
Keywords: KCNQ1, cell signaling, basolateral targeting, epithelia, ubiquitylation
the voltage-gated potassium channel Kv7.1 (also known as KvLQT or KCNQ1) is well known for its role in the heart, where it together with its β-subunit KCNE1 constitutes the slowly activating delayed-rectifier potassium current (IKs) complex that is important for termination of the cardiac action potential (6, 29). Kv7.1 is also expressed in strial marginal and vestibular dark cells of the inner ear, where it critically contributes to secretion of potassium to the endolymph of the scala media, a process necessary for normal hearing function (25, 40). The important role of Kv7.1 in the heart and inner ear is emphasized by the association of KCNQ1 mutations with Jervell and Lange-Nielsen syndrome, an inherited disease characterized by cardiac arrhythmias and hearing loss (24, 39). Kv7.1 is also expressed in other epithelial tissues, including colon, where the channel is important for cAMP-induced chloride secretion (1, 11, 38), the kidney where the channel is involved in salt and water transport (38), and in gastric parietal cells in the stomach where it regulates gastric acid secretion (11, 26, 32).
In the heart, regulation of Kv7.1-mediated currents is crucial for adaption to adrenergic stimulation. Increased repolarizing currents lead to shorter cardiac action potentials. Macroscopically, this can be monitored as a shortening of the QT interval on the electrocardiogram (37). On the molecular level, β-adrenergic receptor stimulation leads to activation of adenylyl cyclase, which elevates intracellular cAMP levels and thereby activates protein kinase A (PKA) (41). PKA activation leads to phosphorylation of serine-27 and serine-92 in Kv7.1 and increases the slowly activating delayed-rectifier potassium current (IKs) current (20, 22). β-Adrenergic stimulation of Kv7.1 requires targeting of protein phosphatase 1 and PKA to the channel through the A-kinase-anchoring protein Yotiao (19, 22). Of note, both Yotiao and the β-subunit KCNE1 are required for the functional response observed upon PKA phosphorylation (18, 19). PKA activation of Kv7.1 also plays a role in epithelial cells. In strial marginal and vestibular dark cells from the inner ear, PKA increases IKs currents (35, 36). Furthermore, cAMP activation of Kv7.1 regulates colonic chloride secretion as forskolin enhances the Kv7.1 current, resulting in increased driving force for apical chloride secretion through the cystic fibrosis transmembrane conductance regulator (CFTR) chloride channel (27, 30). Intriguingly, Kv7.1 associates with the KCNE3 β-subunit in colon, which reportedly does not confer a functional response to PKA phosphorylation of S27 (18). This argues for the existence of additional molecular mechanisms by which PKA can enhance Kv7.1 currents.
Here, we discovered such an additional PKA-mediated regulation. We find that PKA inhibition resulted in intracellular accumulation of Kv7.1 in the polarized epithelial Madin-Darby canine kidney (MDCK) cell line. By mass spectrometry and confocal imaging we show that this effect is not a result of direct channel phosphorylation. Instead, we show that the intracellular accumulation of Kv7.1 depends on the E3 ubiquitin ligase Nedd4-2. Interestingly, we find that PKA does not alter the amount of Nedd4-2 associated with the channel nor channel ubiquitinylation levels, which suggests that PKA regulates Nedd4-2-dependent Kv7.1 trafficking indirectly. In summary, we reveal regulation of Nedd4-2-dependent endocytosis as a novel PKA-mediated stimulation of Kv7.1, which could explain how PKA enhances Kv7.1 currents in tissues such as colon, where the channel is not in complex with KCNE1.
MATERIALS AND METHODS
Chemical compounds.
N-[2-(p-bromocinnamylamino)ethyl]-5-isoquinolinesulfonamide dihydrochloride (H-89), (9S,10S,12R)-2,3,9,10,11,12-hexahydro-10-hydroxy-9-methyl-1-oxo-9,12-epoxy-1H-diindolo[1,2,3-fg:3′,2′,1′-kl]pyrrolo[3,4-i][1,6]benzodiazocine-10-carboxylic acid hexyl ester (KT-5720), 1-(5-isoquinolinesulfonyl)-2-methylpiperazine, 7β-acetoxy-8,13-epoxy-1α,6β,9α-trihydroxylabd-14-en-11-one (forskolin), leupeptin, and Pefabloc were all purchased from Sigma-Aldrich (Copenhagen, Denmark). Myristoylated protein kinase inhibitor (14–22)-amide (PKI) was from Tocris Bioscience.
DNA constructs.
The plasmids pXOOM-hKv7.1, pXOOM-hKv7.1-YA, pXOOM-hNedd4-2, pXOOM-hNedd4-2-CS, and pEGFP-N2-hKv7.1 have been described (4, 14, 15, 17). eGFP-Rab7 was a kind gift from Guiscard Seebohm. pDsRed2-ER was purchased from Clontech (Herlev, Denmark). Point mutations S6A, S6D, S27A, S27D, S27A/S92A, S27D/S92D, S92A, S92D, S407A, S407D, S409A, S409D, S468A, S468D, T470A, T470D, S484A, and S484D were introduced by mutated oligonucleotide extension (PfuTurbo Polymerase; Stratagene, La Jolla, CA) from the plasmid template, digested with DpnI (Thermo Scientific, Hvidovre, Denmark), and transformed into Escherichia coli XL1 Blue cells. All plasmids were verified by complete DNA sequencing of the cDNA insert (Macrogen, Seoul, Republic of Korea).
Transient and stable expression in MDCK cells.
MDCK (strain II) cells were grown in DMEM (Life Technologies, Nærum, Denmark) supplemented with 100 U/ml penicillin, 100 mg/ml streptomycin, and 10% FCS (Sigma-Aldrich, Brøndby, Denmark) (henceforth called full DMEM) at 37°C in a humidified atmosphere with 5% CO2.
The cells were cotransfected in suspension with 1 μg pDsRed2-ER (Clontech) and either 1 μg of wild-type or mutant pXOOM-hKv7.1 using Lipofectamine and Plus Reagent (Invitrogen, Nærum, Denmark) according to the manufacturer's protocol. During transfection, the cells were plated on glass cover slips (12 mm in diameter; Thermo Fischer Scientific, Roskilde, Denmark).
MDCK cells stably expressing pXOOM-hKv7.1, pXOOM-hKv7.1-YA, and pEGFP-N2-hKv7.1 have been described previously (2–4).
Calcium switch experiment.
MDCK cells stably expressing pXOOM-hKv7.1 or pXOOM-hKv7.1-YA were plated on glass cover slips, and the calcium switch experiment was performed as previously described (4). Briefly, cells grown to confluence in low-calcium medium (calcium concentration <5 μM) were induced to polarize by changing to a medium containing 1.8 mM calcium (full DMEM).
Nedd4-2 knockdown in MDCK cells.
Double-stranded small-interfering RNA (siRNA) targeting canine Nedd4-2 (5′-GGGAAGAGAAGGUGGACAA-3′) and nontargeting control siRNA (5′-CCAUCCUGAUGUCGCAAUA-3′) (Eurogentec, Liége, Belgium) were transfected (20 nM) into MDCK cells stably expressing Kv7.1 using siLentFect (Bio-Rad, Copenhagen, Denmark) according to the manufacturer's protocol. Enhanced green fluorescent protein (eGFP-pcDNA3) was added to the transfection as a marker for siRNA-transfected cells. The cells were plated on glass cover slips and allowed to grow for 2 days to reach confluence and polarize. To inhibit PKA the cells were incubated for 90 min with 20 μM H-89 and fixed, and, subsequently, membrane and intracellular Kv7.1 signals were quantified in eGFP-positive cells. Membrane-to-intracellular ratios were calculated, and a Student's t-test was performed to test for statistical significance between Nedd4-2-targeting siRNA- and nontargeting siRNA-transfected cells.
Cell lysates.
MDCK cells stably expressing pEGFP-N2-hKv7.1 or hKv7.1 were grown to confluence, allowed to polarize in T75 flasks (Nunc; Thermo Fisher Scientific), and thereafter solubilized in solubilization buffer (50 mM Tris, pH 7.4, 10 mM NaCl, 10 mM KCl, 10 mM NaF, 1% Triton X-100, 0.5% sodium deoxycholate, 8 μM leupeptin, 0.4 μM Pefabloc, 1 mM orthovanadate, 5 mM sodium fluoride, and 5 mM β-glycerophosphate) for 3 h. The samples were centrifuged at 20,627 g at 4°C for 10 min, and the supernatant was collected. The protein concentrations were measured using the DC protein Assay (Bio-Rad Laboratories) according to the manufacturer's instructions and calibrated to 1.0–1.5 μg/μl.
Immunoprecipitations.
MDCK-Kv7.1 lysate (750 μg) was precleared with Dynabeads-Protein G (Life Technologies) for 1 h at 4°C. The precleared lysate was subsequently incubated with 3 μg of rabbit anti-Kv7.1 antibody (Alomone Labs), or, as a control, 3 μg normal rabbit IgG (Santa Cruz Biotechnology) for 1 h at 4°C.
Dynabeads-Protein G was added, and incubation continued for 1 h. After being washed, proteins bound to the Dynabeads were eluted by heating for 10 min at 75°C in SDS sample buffer. One-third to one-half was loaded per lane.
Western blotting.
Proteins were separated on either 4–20% gradient RunBlue SDS-PAGE (Expedeon) or 4–15% gradient mini-Protean TGX (Bio-Rad) polyacrylamide gels using the Bio-Rad Laboratories minigel system (Bio-Rad). Proteins were transferred to a nonfluorescent Immobilon PVDF membrane (45 μM; Millipore) in 25 mM Tris base, 200 mM glycine, 20% methanol, and 1% SDS using a minitransblot system (Hercules). After transfer, the membranes were blocked for 1 h at room temperature (RT) in blocking buffer consisting of Odyssey blocking buffer (LI-COR Biosciences) diluted 1:1 in PBS. The membranes were incubated overnight at 4°C in blocking buffer containing primary antibodies. After being washed with PBS, the membranes were incubated for 45 min with fluorescently conjugated secondary antibodies diluted in blocking buffer. Bound antibody was detected by the Odyssey CLx Imaging System (LI-COR Biosciences) using 800- and 700-nm channels.
Immunoprecipitation for mass spectrometry and in-gel digest.
Kv7.1-GFP channels were immunoprecipitated from 1.5 mg of protein lysate using agarose beads coupled to a GFP-binder protein (Chromotek). After being washed, bound proteins were eluted with 1× standard Laemmli buffer containing 100 mM dithiothreitol (70°C, 3 min) and separated by SDS-PAGE (4–15% Bis-Tris gels; Bio-Rad). Proteins were fixed in the gel (40 ml water, 50 ml acetonitrile, and 10 ml acetic acid, 10 min) and visualized with colloidal Coomassie staining (Invitrogen). By comparing the fixed gel with the Western blot, the lane corresponding to monomeric Kv7.1 channel was excised, minced, and destained (50% 25 mM ammonium bicarbonate, 50% acetonitrile) in a thermomixer [3 × 20 min, 800 revolutions/min (rpm), RT]. Subsequent steps were performed as described in detail (21). Gel dices were dehydrated (acetonitrile, 10 min, 800 rpm), disulfide bonds were reduced (10 mM dithiothreitol in 25 mM ammonium bicarbonate, 45 min, RT, 800 rpm), and cysteines were alkylated (55 mM chloroacetamide in 25 mM ammonium bicarbonate, 30 min, 24°C in darkness, 800 rpm). The gel plugs were washed in 25 mM ammonium bicarbonate and dehydrated in acetonitrile. Proteins were digested by trypsin [50 μl 12.5 ng/μl sequencing grade trypsin (Promega, Nacta, Sweden) in 25 mM ammonium bicarbonate for 1 h, followed by addition of 100 μl 25 mM ammonium bicarbonate, left overnight at 37°C]. Trypsin activity was quenched by acidification of the mixture with trifluoroacetic acid to pH ∼2, and peptides were extracted from the gel plugs with 30% acetonitrile in 3% trifluoroacetic acid (30 min, 800 rpm) followed by 80% acetonitrile in 0.5% acetic acid (30 min, 800 rpm) and finally in 100% acetonitrile. The organic solvents were removed by evaporation in a vacuum centrifuge. Finally, the extracted peptides were purified on STAGE-tips containing two C18 filters.
Mass spectrometry, LC-MS/MS.
The peptides were eluted in 96-well microtiter plates with 2 × 10 μl 40% acetonitrile in 0.5% acetic acid, and the acetonitrile was subsequently evaporated using a vacuum. The peptide mixtures were acidified with 0.1% trifluoroacetic acid in 2% acetonitrile and analyzed by on-line nanoflow LC-MS/MS. Peptide separation was performed by reversed-phase C18 HPLC on an Easy nLC system (Thermo Fisher Scientific) loading 5-μl samples with a constant flow of 750 nl/min on 15-cm-long analytical columns, packed in-house with 3-μm C18 beads, and eluting peptides using a 135-min segmented gradient of increasing (5–80%) buffer (80% acetonitrile in 0.5% acetic acid) at a constant flow of 250 nl/min. The HPLC effluent was electrosprayed into an LTQ Orbitrap Velos mass spectrometer (Thermo Fisher Scientific), and the peptide mixture was analyzed by full-scan MS spectra [mass-to-charge ratio (m/z) 300-2,000, resolution 30,000] in the Orbitrap analyzer after accumulation of 1,000,000 ions in the Orbitrap within a maximum fill time of 1,000 ms with the lock mass option enabled to improve mass accuracy. For every full scan the most intense peptide ions were sequentially isolated (up to 10 for every full scan) and fragmented by higher-energy collisional dissociation (HCD) in the octopole collision cell, and fragments were recorded by the Orbitrap mass analyzer after accumulation of 50,000 ions with a maximum fill time of 250 ms and using a normalized collision energy of 40%.
Mass spectrometry data analysis.
The acquired data were processed using MaxQuant software (version 1.0.14.7) (Max-Planck Institute of Biochemistry, Department of Proteomics and Signal Transduction) by which the precursor MS signal intensities were determined, and HCD MS/MS spectra were deisotoped and filtered such that only the 10 most abundant fragments per 100-m/z range were retained. Proteins were identified using the Mascot search algorithm (www.matrixscience.com) by searching all MS/MS spectra against a concatenated forward/reversed version of a canine and human protein database supplemented with the Kv7.1 and Kv7.1-GFP amino acid sequences and frequently observed contaminants. The HCD-MS/MS spectra were searched with fixed modification of carbamidomethylcysteine, and we allowed for variable modifications of oxidation (M), acetylation (protein N-term), Gln → pyro-Glu, and phosphorylation (STY). Search parameters were set to an initial precursor ion tolerance of 7 parts/min, MS/MS tolerance at 0.02 Da, and requiring strict tryptic specificity with a maximum of two missed cleavages.
Topology of Kv7.1.
The topology figure (Fig. 5A) was created using the protein sequence for hKv7.1 (uniprot entry: P51787) in the TMRPres2D software (33). The topology figure was thereafter modified in Photoshop CS5.
Fig. 5.
Summary of proteomics results for Kv7.1. A: topological representation of the Kv7.1 channel, including the amino acid sequence. The amino acid sequences highlighted in blue were covered in the mass spectrometry experiments, and the identified phosphorylation sites are highlighted in red circles. As expected, all identified phosphorylation sites localize to the cytosolic parts of the channel. B: table summarizing the proteomics evaluation of the identified Kv7.1 phosphorylation sites. The table contains information on the amino acid position of the modified residue, the probability of localizing the phosphorylation site to that specific residue, likely kinase motifs, Mascot and PTM scores for the respective peptide identifications, the charge state, and the mass-to-charge ratio for the identified peptide covering the phosphorylation site as well as the intensity of the peptide identification. Ac, Acetylation; ph, phosphorylation; ox, oxidation.
Antibodies.
The antibodies used in this study were as follows: goat polyclonal anti-Kv7.1 (immunocytochemistry, 1:100, C-20; Santa Cruz Biotechnology, Heidelberg, Germany), rabbit anti-Kv7.1 (Western blots, 1:1,000, APC-022; Alomone Labs), rabbit anti-Nedd4-2 (Western blot, 1:1,000, no. 4023S; Cell Signaling Technology), rabbit anti-Nedd4-2 (S448) (Western blot, 1:1,000, no. 8063S; Cell Signaling Technology), mouse anti-pCREB (S133) (1:100, 10E9; Merck Millipore), mouse anti-mono- and polyubiquitinylated conjugates (1:250, FK2; Enzo Life Sciences), rabbit anti-calnexin (1:3,000; Enzo Life Sciences), mouse anti-actin (1:15,000, Merck Millipore), and mouse anti-LAMP2 (1:100, AC17; Acris antibodies, Herford, Germany).
Primary antibodies were detected using Alexa Fluor 488- and Alexa Fluor 555-conjugated secondary antibodies. Alexa Flour-conjugated phalloidins were used to identify actin filaments and 4′,6-diamidino-2-phenylindole to stain the nucleus. All were purchased from Life Technologies. IRDye 800CW- and IRDye 680RD-conjugated secondary antibodies (1:10,000; LI-COR Biosciences) were used in Western blots.
Immunofluorescence.
After fixation for 30 min in 4% paraformaldehyde, cells were permeabilized in 0.2% fish skin gelatin in PBS supplemented with 0.1% Triton X-100. Primary antibodies were applied for 1 h followed by secondary antibodies for 45 min. The cover slips were mounted in Prolong Gold (Life Technologies).
Confocal microscopy and imaging.
Laser scanning confocal microscopy was performed with the Leica TCS SP2 system or the Zeiss LSM 780 (or LSM710) confocal systems. Images were acquired using a ×63 water immersion objective, numeric aperture (NA) 1.2 (Leica TCS SP2) or a ×63 oil immersion objective, NA 1.4 (Zeiss LSM 780 and Zeiss LSM 710 confocal system), both with a pinhole size of 1 airy units and a pixel format of 1,024 × 1,024. Line averaging was used to reduce noise. For double- and triple-labeling experiments, sequential scanning was employed. The acquired images were treated using Adobe Photoshop or Zen 2009 from Zeiss. Z-stacks for three-dimensional (3D) reconstructions were obtained on the Zeiss LSM 780 confocal system with the above-mentioned configurations. The 3D software Imaris 7.2 from Bitplane was used to transform the acquired z-stacks into 3D representations. Cells in 3D are illustrated with the normal shading option.
Quantifications.
Quantification of confocal signals was performed as previously described (2). Briefly, total cell fluorescence intensities and intracellular fluorescent signals were measured with the Zen2010 confocal software from Zeiss using the submembranous actin cytoskeleton to define the localization of the plasma membrane. Background fluorescence signals were obtained in the nucleus. Signals originating from the plasma membrane were obtained by subtracting the intracellular signal from the total cell fluorescent signal. The surface-associated signal from cells before treatment (0 h) was set to 100%, and the signals from other time points were calculated as a percentage of the signals from 0 h. The data analysis was carried out using Student's t-test. Data are presented as means ± SE.
Quantification of Western blot data was carried out using Image Studio Version 3.1 (LI-COR Biosciences).
RESULTS
Inhibition of PKA leads to internalization and possibly lysosomal degradation of Kv7.1.
Because Kv7.1 currents in epithelia from, e.g., trachea and colon are known to increase in response to cAMP, we wondered whether PKA activity has an effect on Kv7.1 cell surface expression. To test this hypothesis, we treated MDCK cells with the PKA inhibitor H-89 for 3–5 h. As shown, exogenously expressed human Kv7.1 channels were localized to the basolateral membrane of polarized MDCK cells [time (t) = 0 and 5 h, Fig. 1, A and B] as previously reported (4, 15). However, in cells treated with 20 μM H-89, Kv7.1 accumulated in intracellular vesicular structures (t = 3 and 5 h H-89, Fig. 1, A and B). A similar response was observed when applying 5 and 10 μM H-89 (data not shown). Quantification revealed that PKA inhibition significantly reduced the fraction of surface-expressed channels to 56 ± 4% at t = 3 h, P < 0.001 and 53 ± 4% at t = 5 h, P < 0.001, compared with control (Fig. 1C). To examine how efficiently H-89 inhibited PKA activity in our experimental setup, we analyzed the amount of cAMP-response element-binding protein (CREB)-Ser133 (pCREB), the archetypical PKA substrate in cells exposed to 20 μM H-89 or 50 μM forskolin (to activate PKA). Immunocytochemistry demonstrated a reduction in nuclear pCREB signals in cells exposed to H-89 compared with control, while a substantial increase in pCREB signals was observed in cells treated with forskolin (Fig. 1D). These observations were confirmed by Western blots of lysates from control MDCK cells and cells treated for 3 h with 20 μM H-89 or for 1 h with 50 μM forskolin (Fig. 1E). Quantifications of seven Western blots revealed that H-89 deceased the abundance of pCREB around 50% (52.1 ± 7.8% compared with control, Fig. 1F). Conversely, forskolin significantly increased pCREB abundance threefold (289.2 ± 26.9%, Fig. 1G) compared with control (Fig. 1F).
Fig. 1.
Kv7.1 accumulates intracellularly upon inhibition of protein kinase A (PKA). Madin-Darby canine kidney (MDCK) cells stably expressing Kv7.1 were subjected to a calcium switch for 24 h followed by treatment with 20 μM H-89 for 3–5 h. A: confocal scans of MDCK-Kv7.1 cells before (0 h) and 3 (3 h H-89) and 5 (5 h H-89) h after addition of H-89. Untreated cells are presented as a control (5 h). A staining of the submembranous actin cytoskeleton was used to visualize the localization of the cell surface, and the nucleus was counterstained with DAPI. As illustrated, Kv7.1 is expressed in the basolateral membrane before treatment (0 h) and in the untreated control cells (5 h) but appears in vesicular structures after incubation with H-89. Scale bar = 10 μm. B: 3-dimensinal (3D) representation of Kv7.1-expressing MDCK cells before and 5 h after addition of H-89. Scale bars = 10 μm. C: quantification of the membrane-associated fraction of Kv7.1 fluorescent signals before and after H-89 incubation. The membrane-associated fluorescent signals obtained after H-89 treatment (3 h H-89 and 5 h H-89) are displayed as a percentage of the membrane-associated signal before treatment (0 h), which was set to 100%. A significant (***P < 0.001) reduction in the percentage of Kv7.1 fluorescence at the cell surface was observed after both 3 and 5 h of H-89 treatment. Fluorescent signals were quantified from 3 individual experiments as described in materials and methods. Data are expressed as mean values for the 3 experiments ± SE. D: staining for S133-phosphorylated CREB (pCREB) in control MDCK cells or cells exposed to 20 μM H-89 for 3 h or 50 μM forskolin for 1 h. Scale bar = 10 μm. E: representative Western blot showing the effects of 20 μM H-89 (3 h exposure) and 50 μM forskolin (1 h exposure) on the abundance of pCREB (top) in polarized Kv7.1-expressing MDCK cells. Calnexin staining was included as a loading control (bottom). F: quantitative analysis of the pooled data from 7 Western blots. The pCREB bands were normalized to the corresponding calnexin bands. H-89 and forskolin signals are displayed as a percentage of the pCREB signal in control cells, which was set to 100%. ns, Not significant. ***P < 0.001 using 1-way ANOVA/Tukey's post hoc test.
Fig. 1—Continued.
Because H-89 is known to have off-target effects, we further validated that the observed accumulation of Kv7.1 in intracellular vesicles was due to PKA inhibition. With the use of a different PKA inhibitor, KT-5720, a similar response was observed (Fig. 2A). We furthermore tested the effect of myristoylated PKI on Kv7.1 localization. For this purpose, MDCK-Kv7.1 cells were serum-starved for 5 h before addition of 10 μM PKI for 1 h. Similar to our observations with H-89 and KT-5720, PKI treatment resulted in the appearance of intracellular Kv7.1 vesicular structures (Fig. 2B), thereby supporting that the redistribution of Kv7.1 is PKA mediated.
Fig. 2.
Kv7.1 accumulates intracellularly upon exposure to PKA inhibitors KT-5720 and myristoylated protein kinase A inhibitor 14–22 amide. A: MDCK cells stably expressing Kv7.1 were subjected to a calcium switch for 24 h (control) followed by treatment with 10 μM KT-5720 (3 h 10 μM KT-5720) for 3 h. Staining of the submembranous actin cytoskeleton was used to visualize the localization of the cell surface, and the nucleus was counterstained with DAPI. As illustrated, Kv7.1 is expressed in the basolateral membrane before treatment (control), but is primarily localized in vesicular structures after incubation with KT-5720. Scale bar = 10 μm. B: MDCK-Kv7.1 cells were serum starved for 5 h followed by treatment with 10 μM myristoylated PKA inhibitor (14–22)-amide (PKI) for 1 h. Phalloidin staining of the actin cytoskeleton indicates the outline of the cells. Whereas control cells displayed Kv7.1 expression primarily associated by the plasma membrane, PKI-treated cells showed appearance of Kv7.1 in intracellular vesicles. Scale bar = 10 μM.
To address the specificity of the PKA response, we tested the impact of H-89 and KT-5720 on the localization of the highly related Kv7.4 potassium channel. MDCK cells were transfected with Kv7.4 and allowed to polarize. In accordance with previous reports, immunocytochemistry revealed that Kv7.4 localized to the basolateral membrane of polarized MDCK similarly to Kv7.1 (10 and Fig. 3). However, PKA inhibition had no effect on the membrane expression of Kv7.4 (Fig. 3), demonstrating that the intracellular accumulation induced by PKA inhibition is specific for Kv7.1.
Fig. 3.
Intracellular accumulation in response to PKA inhibition is specific for Kv7.1. MDCK cells were transfected with Kv7.4 and allowed to polarize before being subjected to either 20 μM H-89 or 10 μM KT-5720 treatments for 3 h. As demonstrated, inhibition of PKA (with either inhibitor) did not affect the localization of Kv7.4, since the channel remained associated with the basolateral membrane like observed in control cells (0 h). DAPI and phalloidin were used to label the nucleus and the actin cytoskeleton, respectively. Scale bar = 10 μm.
Activation of endogenous PKA promotes Kv7.1 surface expression in polarized MDCK cells.
We next investigated if we could impact Kv7.1 surface expression levels by further activating PKA instead of inhibiting it. To this end, we treated polarized MDCK cells expressing Kv7.1 with 50 μM forskolin for 1 h. We kept the incubation time with forskolin short, since there are reports that forskolin can cause activation of serum- and glucocorticoid-inducible kinase 1, a known regulator of Kv7.1, when applied for 2 h and more (3, 13). As illustrated in Fig. 4, forskolin treatment led to a small but significant (P < 0.05) increase in the fraction of membrane-expressed channels, thereby further supporting that PKA influences the surface localization of Kv7.1 in polarized MDCK cells.
Fig. 4.
PKA activation promotes Kv7.1 surface expression in polarized MDCK cells. MDCK cells stably expressing Kv7.1 were grown to confluence and allowed to polarize. The cells were then treated with 50 μM forskolin for 1 h. Cells were fixed before and after treatment and stained for Kv7.1 (A). DAPI and phalloidin were used to label the nucleus and the actin cytoskeleton, respectively. Scale bar = 10 μm. B: quantification of membrane-expressed Kv7.1 channels in untreated (control) and forskolin-treated cells. The 1-h forskolin treatment resulted in a significant (*P < 0.05) increase in membrane-expressed channels. 29 > N > 24 for each condition.
Identified Kv7.1 phosphorylation sites cannot explain channel redistribution caused by PKA inhibition.
To address the molecular mechanism by which PKA inhibition leads to a reduction in Kv7.1 surface expression, we first analyzed the phosphorylation state of basolaterally localized Kv7.1 channels by mass spectroscopic analysis. For purification purposes, we used a GFP-tagged version of Kv7.1, which we verified to localize similarly to WT-Kv7.1 (data not shown). We identified 81 peptides matching the Kv7.1 amino acid sequence, resulting in a sequence coverage of 58% (Fig. 5A). From the identified peptides, we were able to localize seven phosphorylation sites to specific amino acids (S6, S27, S92, S407, S409, S468, and S484). One phosphorylation site, T470, was identified with ambiguity in the localization, since this particular site was not identified in a fragment spectrum in absence of S468 (Fig. 5).
We next generated the corresponding alanine and aspartate mutants to mimic the dephosphorylated or constitutively phosphorylated state, respectively. The Kv7.1 mutants were expressed in MDCK cells, along with the ER marker DsRed-ER, and the localization of Kv7.1 was analyzed. None of the identified serine sites appeared to have an effect on the steady-state localization of Kv7.1, since all mutant proteins localized to the basolateral membrane similarly to the wild-type channel (Fig. 6). In contrast, the T470D mutant was partly intracellular, colocalizing with the ER marker, whereas T470A localized to the basolateral membrane, implicating this residue in Kv7.1 surface expression (Fig. 7A). However, when exposed to H-89, Kv7.1-T470A still accumulated in vesicular structures similar to the wild type, which suggests that T470 is not involved in the redistribution of Kv7.1 observed upon PKA inhibition (Fig. 7B). Thus none of the identified phosphorylation sites appeared to play a role in H-89-induced Kv7.1 redistribution.
Fig. 6.
Mutation of phosphorylated serines does not affect subcellular localization of Kv7.1. Alanine (A) and aspartate (B) mutants of the identified serine phosphorylation sites were coexpressed with the ER marker pDsRed2-ER in MDCK cells. The cells were stained for Kv7.1, and horizontal and vertical confocal scans were obtained. No effects on localization were observed for any of the mutants, since all alanine and aspartate mutants were expressed in the basolateral membrane similarly to the wild-type (WT) channel. Scale bar = 10 μm.
Fig. 7.
Mimicked phosphorylation of T470 results in reduced membrane expression of Kv7.1, but Kv7.1-T470A is still endocytosed in response to PKA inhibition. Kv7.1-T470A and Kv7.1-T470D mutants were coexpressed with DsRed2-ER in MDCK cells, which were allowed to polarize before fixation. A: Kv7.1-T470A is expressed in the basolateral membrane similar to wild-type Kv7.1. In contrast, Kv7.1-T470D is primarily localized to the ER as indicated by colocalization with the ER marker. A small fraction of Kv7.1-T470D can be detected in the basolateral membrane. DAPI was used to stain the nucleus. Scale bar = 10 μm. B: MDCK cells expressing Kv7.1-T470A were treated with 20 μM H-89 for 3 h. Illustrated are confocal scans of Kv7.1-T470A-expressing MDCK cells before (0 h) and 3 h after addition of H-89 (3 h H-89). Untreated cells are presented as a control (3 h). As illustrated, Kv7.1-T470A responds to H-89 treatment by accumulating in intracellular vesicles. Scale bar = 10 μm.
PKA inhibition results in endocytosis of Kv7.1.
To understand how PKA signaling causes redistribution of Kv7.1 in polarized MDCK, we next addressed the fate of Kv7.1 upon H-89 treatment. We costained H-89-treated Kv7.1-expressing MDCK cells for Kv7.1 and a number of compartmental markers. We observed that intracellular vesicles containing Kv7.1 displayed a close association with the late endosome/lysosome marker LAMP2 (Fig. 8A).
Fig. 8.
Kv7.1 codistributes with late endosomal/lysosomal markers in response to H-89 treatment. A: confocal scans of MDCK-Kv7.1 cells treated with 20 μM H-89 for 3 h and colabeled with the late endosomal/lysosomal marker LAMP-2. Scale bar = 10 (top) and 5 (bottom) μm. B: MDCK cells were cotransfected with hKv7.1 and green fluorescent protein (GFP)-Rab7. After polarization, the transfected cells were exposed to 20 μM H-89 for 3 h, and the cells were colabeled for Kv7.1 and GFP. Kv7.1 showed no significant overlap with Rab7 under control conditions but became closely associated with this lysosomal marker after exposure to H-89. Scale bar = 10 (top) and 5 (bottom) μm.
To further confirm the close association of Kv7.1 with late endosomes/lysosomes upon H-89 treatment, we cotransfected MDCK cells with Kv7.1 and GFP-Rab7, a key regulator of lysosome biogenesis and a marker of late endosomes/lysosomes (7). We first verified that overexpression of GFP-Rab7 did not alter the subcellular distribution of Kv7.1, which was indeed the case, since the channel remained associated with the basolateral plasma membrane (Fig. 8B). In addition, no association between Kv7.1 and GFP-Rab7 was detected under control conditions. However, upon H-89-treatment, Kv7.1 labeling became closely associated with GFP-Rab7. Most Kv7.1-containing vesicles either colocalized with GFP-Rab7 or were surrounded by GFP-Rab7 labeling (Fig. 8B). These observations would suggest that PKA inhibition led to endocytosis and subsequent lysosomal degradation of Kv7.1.
The Nedd4-2-resistant Kv7.1-YA mutant is not affected by PKA inhibition.
Our data suggested that PKA regulates endocytic transport of Kv7.1. A major player in Kv7.1 endocytosis and turnover appears to be the ubiquitinylation enzyme Nedd4-2 (14), and interestingly PKA can inhibit Nedd4-2 by direct phosphorylation (28). To investigate if the observed intracellular accumulation of Kv7.1 involves Nedd4-2, we subjected polarized MDCK cells stably expressing a Nedd4-2 interaction-deficient Kv7.1 mutant (Kv7.1-YA) to treatment with H-89 for 3–5 h. The Kv7.1-YA mutant has a Y to A mutation in the Nedd4-2 interaction domain (LPxY), which inhibits its interaction with Nedd4-2 (14). In contrast to wild-type channels, Kv7.1-YA mutant channels remained strongly associated with the basolateral membrane throughout the treatment with no detectable intracellular accumulation (Fig. 9A). Quantification of the surface-associated Kv7.1 fluorescent signals confirmed that the fraction of Kv7.1-YA channels expressed at the cell surface was unaffected by PKA inhibition (Fig. 9B). This strongly indicates that the intracellular accumulation of Kv7.1 initiated by PKA inhibition involves Nedd4-2.
Fig. 9.
Kv7.1-YA localization is unaffected by PKA inhibition. MDCK cells stably expressing the Kv7.1-YA mutant were treated with 20 μM H-89 for 3–5 h. A: confocal scans of MDCK-Kv7.1-YA cells before (0 h) and 3 (3 h H-89) and 5 (5 h H-89) h after addition of H-89. As illustrated, Kv7.1-YA localization is unaffected by H-89 treatment, since the channel remains localized to the basolateral membrane after addition of the PKA inhibitor (3 h H-89 and 5 h H-89). Scale bar = 10 μm. B: quantification of the membrane-associated fraction of Kv7.1-YA fluorescence before and after PKA inhibition. The membrane-associated fluorescent signals determined after H-89 treatment (3 h H-89 and 5 h) are displayed as a percentage of membrane-associated signal from the cells before treatment (0 h), which was set to 100%, were not statistically significantly different from the control. Fluorescent signals were quantified from 3 individual experiments as described in materials and methods. Data are expressed as mean values for the 3 experiments ± SE.
siRNA knockdown of Nedd4-2 protects Kv7.1 from internalization upon PKA inhibition.
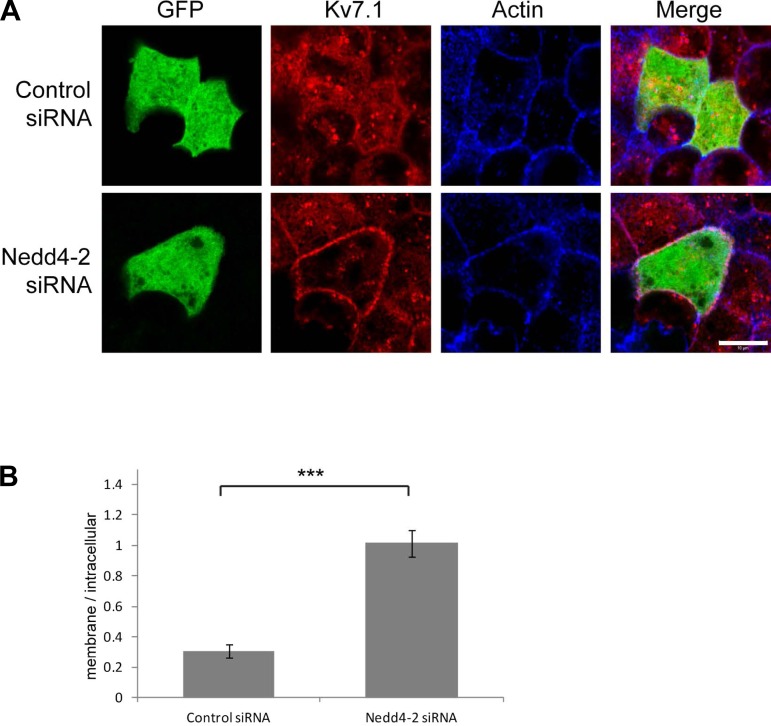
To further confirm the role of Nedd4-2, we reduced Nedd4-2 expression using canine-specific siRNA oligos known to efficiently downregulate Nedd4-2 in MDCK cells (38a). We cotransfected siRNA targeting Nedd4-2 or nonspecific control siRNA together with eGFP, which was used as a marker for transfected cells, and treated the transfected cells with H-89 for 90 min. Knockdown of Nedd4-2 did not alter Kv7.1 localization under control conditions, since the channel was still basolaterally expressed (data not shown). However, as demonstrated in Fig. 10, it significantly protected Kv7.1 from intracellular accumulation upon PKA inhibition, since more channels were surface expressed in cells transfected with Nedd4-2-specific siRNA than in cells transfected with nonspecific control siRNA.
Fig. 10.
Small-interfering RNA (siRNA) knockdown of Nedd4-2 protects Kv7.1 from internalization upon PKA inhibition. A: MDCK cells stably expressing Kv7.1 were transiently transfected with siRNA targeting Nedd4-2 or with noncoding control siRNA. Enhanced GFP (eGFP) was cotransfected to identify transfected cells. Two days after transfection, the cells were treated with 10 μM H-89 for 90 min. As shown, Kv7.1 was internalized in cells expressing control siRNA but not in cells expressing siRNA targeting Nedd-2 (Nedd4-2 siRNA). Scale bar, 10 μm. B: the ratios between membrane and intracellular Kv7.1 signals in cells expressing control siRNA and siRNA targeting Nedd4-2. The ratio was significantly higher in cells expressing Nedd4-2 siRNA than in cells expressing the control siRNA. ***P < 0.001.
PKA inhibition does not alter Nedd4-2 binding to Kv7.1 nor Kv7.1 ubiquitinylation levels.
It is known that PKA can inhibit Nedd4-2 by direct phosphorylation at three sites known to promote dissociation of Nedd4-2 from target proteins thereby preventing target ubiquitination (23). We therefore speculated that PKA might promote Kv7.1 surface expression by this mechanism. To examine this possibility, we first examined the phosphorylation level of Nedd4-2 at one of these sites, serine-468 in human Nedd4-2, in control MDCK-Kv7.1 lysates compared with lysates from cells treated with 20 μM H-89 for 3 h or 50 μM forskolin for 1 h. Surprisingly, we did not observe consistent changes in the phorphorylation level at this site, which was confirmed by quantification (Fig. 11, A and B).
Fig. 11.
Kv7.1 redistribution is not caused by increased Nedd4-2 binding or ubiquitinylation. A: representative Western blot showing the effects of 20 μM H-89 (3 h exposure) and 50 μM forskolin (1 h exposure) on the abundance of S468-phosphorylated Nedd4-2 (top) in polarized Kv7.1-expressing MDCK cells. Actin staining was included as a loading control (bottom). B: quantitative analysis of the pooled data from 8 such experiments. The pNedd4-2 bands were normalized to the corresponding actin bands. pNedd4-2 signals in the H-89- and forskolin-treated samples are displayed as a percentage of the pNedd4-2 signal in control cells, which was set to 100%. ns, Differences are not significant using 1-way ANOVA/Tukey's post hoc test. C: representative Western blot showing the specificity of the Kv7.1 immunoprecipitation reaction. Lysates from control, H-89-, or forskolin-treated Kv7.1-expressing MDCK cells were subject to immunoprecipitation using either normal rabbit IgG (ctrl IgG) or anti-Kv7.1 IgG. Immunoprecipitated material was analyzed by Western blot probed with anti-Kv7.1 antibodies. Kv7.1 was precipitated using anti-Kv7.1 antibody, but not using normal rabbit IgG. Arrows point to homomeric, dimeric, trimeric and tetrameric bands of Kv7.1. D: representative blots showing the amount of Nedd4-2 coimmunoprecipitating with Kv7.1 in H-89- and forskolin-treated lysates (top) in polarized Kv7.1-expressing MDCK cells. Panel on bottom shows the amount of Kv7.1 immunoprecipitated from the same samples. E: quantitative analysis of the pooled data from 5 immunoprecipitations. The Nedd4-2 bands were normalized to the amount of Kv7.1 precipitated from the same samples. Nedd4-2 signal intensities in the H-89- and forskolin-treated samples are displayed as a percentage of the amount of Nedd4-2 coprecipitating under control conditions, which was set to 100%. F: representative ubiquitin blot (top) showing the amount of ubiquitin in the Kv7.1 immunoprecipitate under control conditions or after treatment with H-89 or forskolin in polarized Kv7.1-expressing MDCK cells. Panel on bottom shows the amount of Kv7.1 immunoprecipitated from the same samples. G: quantitative analysis of the pooled ubiquitin data from 5 immunoprecipitations. The ubiquitin signal was quantified as band intensity from 70 to 100 kDa and normalized to the amount of Kv7.1 precipitated from the same samples. Ubiquitin signals in H-89- and forskolin-treated samples are displayed as a percentage of the signal observed under control conditions, which was set to 100%.
Although phosphorylation at S468 appeared unaffected by PKA inhibition/activation under our experimental conditions, PKA could still be speculated to regulate the interaction between Kv7.1 and Nedd4-2. We therefore examined the amount of Nedd4-2 coimmunoprecipitating with Kv7.1 under control conditions or in MDCK cells treated with H-89 or forskolin. We first verified the specificity of our Kv7.1 immunoprecipitation reaction. As shown, we were able to efficiently pull down Kv7.1 from MDCK-Kv7.1 lysates using a Kv7.1-specific antibody, but not when using control IgG (Fig. 11C). Furthermore, endogenous Nedd4-2 was specifically and efficiently coimmunoprecipitated, confirming the strong interaction between these two proteins (Fig. 11D). Supportive of the phosphorylation data, however, no consistent changes in the amount of Nedd4-2 bound to Kv7.1 were observed upon H-89 or forskolin treatments, which was confirmed by quantification (Fig. 11E). In support of this, we furthermore did not see changes in the ubiquitinylation level of Kv7.1 when manipulating PKA activity (Fig. 11, F and G). Altogether these observations suggest that the intracellular accumulation of Kv7.1 observed in response to PKA inhibition is not caused by altered Nedd4-2 phosphorylation regulating interaction with Kv7.1 and channel ubiquitinylation.
DISCUSSION
In this study, we demonstrate for the first time that PKA can promote Kv7.1 currents by two different mechanisms. In addition to the well-described effect on current conduction (22), we show that PKA regulates the subcellular localization of this important voltage-gated channel. We found that increased PKA activity induced by 1 h forskolin treatment resulted in a significant increase in the fraction of Kv7.1 channels expressed in the basolateral membrane. In contrast, 3–5 h of PKA inhibition by H-89 resulted in intracellular accumulation of Kv7.1 channels, which was reflected by a 40–50% decrease in the fraction of membrane-associated Kv7.1. This effect was confirmed using two other PKA inhibitors, KT-5720 and PKI, and was specific for Kv7.1, since basolaterally localized Kv7.4 channels remained surface localized upon the same treatment. Hypothesizing a direct effect of PKA on the phosphorylation state of Kv7.1, we identified eight phosphorylation sites (7 serines, 1 threonine) using high-resolution tandem mass spectroscopy. Two of these sites, S27 and S92, have previously been demonstrated to be directly phosphorylated in response to PKA activation (20, 22). Two additional sites, S468 and T470, have been suggested as PKA phosphorylation sites, yet direct phosphorylation was not addressed (42). Position S484 is furthermore predicted to be a PKA phosphorylation site by the GPS2.1 phosphorylation prediction software (http://gps.biocuckoo.org/online.php). Analysis of the identified sites revealed that mutation of the identified serine residues was without effect on the localization pattern of Kv7.1, whereas T470 had a significant impact on channel localization. The observation that the T470D mutant was partly retained in the ER while the corresponding alanine mutant trafficked to the surface membrane could indicate that dephosphorylation of the site is necessary for efficient ER exit. However, because the corresponding alanine mutant displayed wild-type localization and responded to PKA inhibition, this site does not appear to be involved in the intracellular accumulation caused by PKA inhibition. In summary, we did not find any evidence for a trafficking effect by PKA-mediated channel phosphorylation.
Instead, we reveal that the molecular mechanism by which PKA inhibition leads to redistribution of surface-expressed Kv7.1 channels involves regulation of endocytic transport dependent on the E3 ubiquitin ligase Nedd4-2, which is known to play a role in endocytosis and possibly lysosomal degradation of several ion channels (5, 28). Employing a Nedd4-2-resistant Kv7.1 mutant, we could prevent channel endocytosis caused by PKA inhibition. We confirmed these findings by an alternative approach employing siRNA knockdown of Nedd4-2 in MDCK cells, which protected Kv7.1 from internalization upon PKA inhibition. In agreement with a Nedd4-2-mediated effect, the related channel Kv7.4, which does not contain the PY motif, remained surface expressed in response to PKA inhibition. Overall, our data indicate that PKA counteracts Nedd4-2-dependent Kv7.1 endocytosis. However, intriguingly, our biochemical data suggest that PKA does not regulate Kv7.1 endosomal trafficking by inhibiting channel interaction with Nedd4-2 or reducing channel ubiquitinylation levels as previously reported for epithelial sodium channel (ENaC) (31). This could suggest that, even though Nedd4-2 is involved in the PKA-dependent Kv7.1 redistribution, Nedd4-2 is not the primary PKA target responsible for the observed effect on Kv7.1 localization.
Our studies suggest that PKA can regulate the activity of Kv7.1 in two different ways: by direct phosphorylation resulting in increased channel conductance (22) and by regulating the endocytic transport of the channels. Interestingly, ENaC and CFTR that are both regulated by Nedd4-2 also seem to be regulated by such a PKA-mediated dual response. PKA activation leads to increased surface expression of both ENaC and CFTR (12, 31). In addition, the open probability of ENaC and the activity of CFTR are increased in response to activation of PKA (9, 34). It is tempting to speculate that direct phosphorylation by PKA in combination with a decreased channel turnover by PKA could be a general regulatory mechanism for ion channels regulated by Nedd4-2. Although we have performed our studies in the MDCK cell line, such a dual increase of Kv7.1 current in response to PKA activation could well be of physiological relevance in the heart, where Nedd4-2 is highly expressed (16). The same response might also be important in epithelial cells of the inner ear where Kv7.1 is important for potassium secretion, a process that is enhanced by cAMP stimulation. It could furthermore also play a role in the colon where cAMP activation of Kv7.1-KCNE3 complexes is important for chloride secretion. The precise mechanism behind cAMP-induced chloride secretion is still somewhat obscure. Kv7.1-KCNE3 seems to be at least partially responsible for the increase in chloride secretion observed upon cAMP stimulation, but it has been demonstrated that KCNE3, in contrast to KCNE1 and KCNE2, does not confer a functional response to PKA phosphorylation of S27 in Kv7.1 (18). This argues for the existence of alternative mechanisms by which PKA can stimulate Kv7.1-KCNE3 complexes. Such a mechanism could be PKA-stimulated increases in the surface expression of Kv7.1. Further studies are required to establish the precise molecular target for PKA in this connection and the physiological role of this pathway.
GRANTS
This work was supported by The Danish National Research Foundation, The Velux Foundation, The Danish Council for Independent Research Medical Sciences (grant no. 12–127262 to S.-P. Olesen), The Novo Nordisk Foundation (to S.-P. Olesen), the Aase og Ejnar Danielsens Fond (to H. B. Rasmussen), and The Danish Heart Foundation (to M. N. Andersen). The Novo Nordisk Foundation Center for Protein Research is supported financially by the Novo Nordisk Foundation (Grant agreement NNF14CC0001).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: M.N.A., A.L., N.S., J.V.O., and H.B.R. conception and design of research; M.N.A., A.L., L.L.H., A.B.S., and H.B.R. performed experiments; M.N.A., A.L., L.L.H., A.B.S., N.S., J.V.O., and H.B.R. analyzed data; M.N.A., A.L., L.L.H., A.B.S., N.S., S.-P.O., J.V.O., and H.B.R. interpreted results of experiments; M.N.A., A.L., L.L.H., A.B.S., and H.B.R. prepared figures; M.N.A. drafted manuscript; M.N.A., A.L., L.L.H., A.B.S., N.S., S.-P.O., and H.B.R. edited and revised manuscript; M.N.A., A.L., L.L.H., A.B.S., N.S., S.-P.O., J.V.O., and H.B.R. approved final version of manuscript.
ACKNOWLEDGMENTS
We acknowledge the Core Facility for Integrated Microscopy, Faculty of Health and Medical Sciences, University of Copenhagen.
REFERENCES
- 1.Alzamora R, O'Mahony F, Ko WH, Yip TWN, Carter D, Irnaten M, Harvey BJ. Berberine reduces cAMP-induced chloride secretion in T84 human colonic carcinoma cells through inhibition of basolateral KCNQ1 channels. Front Physiol 2: 33, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andersen MN, Krzystanek K, Jespersen T, Olesen SP, Rasmussen HB. AMP-activated protein kinase downregulates Kv7.1 cell surface expression. Traffic 13: 143–156, 2012. [DOI] [PubMed] [Google Scholar]
- 3.Andersen MN, Krzystanek K, Petersen F, Bomholtz SH, Olesen SP, Abriel H, Jespersen T, Rasmussen HB. A phosphoinositide 3-kinase (PI3K)-serum- and glucocorticoid-inducible kinase 1 (SGK1) pathway promotes Kv7.1 channel surface expression by inhibiting Nedd4-2 protein. J Biol Chem 288: 36841–36854, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andersen MN, Olesen SP, Rasmussen HB. Kv7.1 surface expression is regulated by epithelial cell polarization. Am J Physiol Cell Physiol 300: C814–C824, 2011. [DOI] [PubMed] [Google Scholar]
- 5.Andersen MN, Rasmussen HB. AMPK: a regulator of ion channels. Commun Integr Biol 5: 480–484, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barhanin J, Lesage F, Guillemare E, Fink M, Lazdunski M, Romey G. K(V)LQT1 and lsK (minK) proteins associate to form the I(Ks) cardiac potassium current. Nature 384: 78–80, 1996. [DOI] [PubMed] [Google Scholar]
- 7.Bucci C, Thomsen P, Nicoziani P, McCarthy J, van Deurs B. Rab7: a key to lysosome biogenesis. Mol Biol Cell 11: 467–480, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheng SH, Rich DP, Marshall J, Gregory RJ, Welsh MJ, Smith AE. Phosphorylation of the R domain by cAMP-dependent protein kinase regulates the CFTR chloride channel. Cell 66: 1027–1036, 1991. [DOI] [PubMed] [Google Scholar]
- 10.David JP, Andersen MN, Olesen SP, Rasmussen HB, Schmitt N. Trafficking of the IKs -complex in MDCK cells: site of subunit assembly and determinants of polarized localization. Traffic 14: 399–411, 2013. [DOI] [PubMed] [Google Scholar]
- 11.Dedek K, Waldegger S. Colocalization of KCNQ1/KCNE channel subunits in the mouse gastrointestinal tract. Pflugers Arch 442: 896–902, 2001. [DOI] [PubMed] [Google Scholar]
- 12.Holleran JP, Zeng J, Frizzell RA, Watkins SC. Regulated recycling of mutant CFTR is partially restored by pharmacological treatment. J Cell Sci 126: 2692–2703, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ismail NAS, Baines DL, Wilson SM. The phosphorylation of endogenous Nedd4-2 In Na(+)-absorbing human airway epithelial cells. Eur J Pharmacol 732: 32–42, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jespersen T, Membrez M, Nicolas CS, Pitard B, Staub O, Olesen SP, Baró I, Abriel H. The KCNQ1 potassium channel is down-regulated by ubiquitylating enzymes of the Nedd4/Nedd4-like family. Cardiovasc Res 74: 64–74, 2007. [DOI] [PubMed] [Google Scholar]
- 15.Jespersen T, Rasmussen HB, Grunnet M, Jensen HS, Angelo K, Dupuis DS, Vogel LK, Jorgensen NK, Klaerke DA, Olesen SP. Basolateral localisation of KCNQ1 potassium channels in MDCK cells: molecular identification of an N-terminal targeting motif. J Cell Sci 117: 4517–4526, 2004. [DOI] [PubMed] [Google Scholar]
- 16.Kamynina E, Debonneville C, Bens M, Vandewalle A, Staub O. A novel mouse Nedd4 protein suppresses the activity of the epithelial Na+ channel. FASEB J 15: 204–214, 2001. [DOI] [PubMed] [Google Scholar]
- 17.Krzystanek K, Rasmussen HB, Grunnet M, Staub O, Olesen SP, Abriel H, Jespersen T. Deubiquitylating enzyme USP2 counteracts Nedd4-2-mediated downregulation of KCNQ1 potassium channels. Heart Rhythm 9: 440–448, 2012. [DOI] [PubMed] [Google Scholar]
- 18.Kurokawa J, Bankston JR, Kaihara A, Chen L, Furukawa T, Kass RS. KCNE variants reveal a critical role of the beta subunit carboxyl terminus in PKA-dependent regulation of the IKs potassium channel. Channels (Austin) 3: 16–24, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kurokawa J, Chen L, Kass RS. Requirement of subunit expression for cAMP-mediated regulation of a heart potassium channel. Proc Natl Acad Sci USA 100: 2122–2127, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lundby A, Andersen MN, Steffensen AB, Horn H, Kelstrup CD, Francavilla C, Jensen LJ, Schmitt N, Thomsen MB, Olsen JV. In vivo phosphoproteomics analysis reveals the cardiac targets of β-adrenergic receptor signaling. Sci Signal 6: rs11, 2013. [DOI] [PubMed] [Google Scholar]
- 21.Lundby A, Olsen JV. GeLCMS for in-depth protein characterization and advanced analysis of proteomes. Methods Mol Biol 753: 143–155, 2011. [DOI] [PubMed] [Google Scholar]
- 22.Marx SO, Kurokawa J, Reiken S, Motoike H, D'Armiento J, Marks AR, Kass RS. Requirement of a macromolecular signaling complex for beta adrenergic receptor modulation of the KCNQ1-KCNE1 potassium channel. Science 295: 496–499, 2002. [DOI] [PubMed] [Google Scholar]
- 23.Nagaki K, Yamamura H, Shimada S, Saito T, Hisanaga S, Taoka M, Isobe T, Ichimura T. 14-3-3 Mediates phosphorylation-dependent inhibition of the interaction between the ubiquitin E3 ligase Nedd4-2 and epithelial Na+ channels. Biochemistry 45: 6733–6740, 2006. [DOI] [PubMed] [Google Scholar]
- 24.Neyroud N, Tesson F, Denjoy I, Leibovici M, Donger C, Barhanin J, Fauré S, Gary F, Coumel P, Petit C, Schwartz K, Guicheney P. A novel mutation in the potassium channel gene KVLQT1 causes the Jervell and Lange-Nielsen cardioauditory syndrome. Nat Genet 15: 186–189, 1997. [DOI] [PubMed] [Google Scholar]
- 25.Nicolas M, Demêmes D, Martin A, Kupershmidt S, Barhanin J. KCNQ1/KCNE1 potassium channels in mammalian vestibular dark cells. Hear Res 153: 132–145, 2001. [DOI] [PubMed] [Google Scholar]
- 26.Pan Q, Ma J, Zhou Q, Li J, Tang Y, Liu Y, Yang Y, Xiao J, Peng L, Li P, Liang D, Zhang H, Chen YH. KCNQ1 loss-of-function mutation impairs gastric acid secretion in mice. Mol Biol Rep 37: 1329–1333, 2010. [DOI] [PubMed] [Google Scholar]
- 27.Preston P, Wartosch L, Günzel D, Fromm M, Kongsuphol P, Ousingsawat J, Kunzelmann K, Barhanin J, Warth R, Jentsch TJ. Disruption of the K+ channel beta-subunit KCNE3 reveals an important role in intestinal and tracheal Cl- transport. J Biol Chem 285: 7165–7175, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rotin D, Staub O. Role of the ubiquitin system in regulating ion transport. Pflugers Arch 461: 1–21, 2011. [DOI] [PubMed] [Google Scholar]
- 29.Sanguinetti MC, Curran ME, Zou A, Shen J, Spector PS, Atkinson DL, Keating MT. Coassembly of K(V)LQT1 and minK (IsK) proteins to form cardiac I(Ks) potassium channel. Nature 384: 80–83, 1996. [DOI] [PubMed] [Google Scholar]
- 30.Schroeder BC, Waldegger S, Fehr S, Bleich M, Warth R, Greger R, Jentsch TJ. A constitutively open potassium channel formed by KCNQ1 and KCNE3. Nature 403: 196–199, 2000. [DOI] [PubMed] [Google Scholar]
- 31.Snyder PM, Olson DR, Kabra R, Zhou R, Steines JC. cAMP and serum and glucocorticoid-inducible kinase (SGK) regulate the epithelial Na(+) channel through convergent phosphorylation of Nedd4-2. J Biol Chem 279: 45753–45758, 2004. [DOI] [PubMed] [Google Scholar]
- 32.Song P, Groos S, Riederer B, Feng Z, Krabbenhöft A, Smolka A, Seidler U. KCNQ1 is the luminal K+ recycling channel during stimulation of gastric acid secretion. J Physiol (Lond) 587: 3955–3965, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Spyropoulos IC, Liakopoulos TD, Bagos PG, Hamodrakas SJ. TMRPres2D: high quality visual representation of transmembrane protein models. Bioinformatics 20: 3258–3260, 2004. [DOI] [PubMed] [Google Scholar]
- 34.Stutts MJ, Rossier BC, Boucher RC. Cystic fibrosis transmembrane conductance regulator inverts protein kinase A-mediated regulation of epithelial sodium channel single channel kinetics. J Biol Chem 272: 14037–14040, 1997. [DOI] [PubMed] [Google Scholar]
- 35.Sunose H, Liu J, Marcus DC. cAMP increases K+ secretion via activation of apical IsK/KvLQT1 channels in strial marginal cells. Hear Res 114: 107–116, 1997. [DOI] [PubMed] [Google Scholar]
- 36.Sunose H, Liu J, Shen Z, Marcus DC. cAMP increases apical IsK channel current and K+ secretion in vestibular dark cells. J Membr Biol 156: 25–35, 1997. [DOI] [PubMed] [Google Scholar]
- 37.Terrenoire C, Clancy CE, Cormier JW, Sampson KJ, Kass RS. Autonomic control of cardiac action potentials: role of potassium channel kinetics in response to sympathetic stimulation. Circ Res 96: e25–e34, 2005. [DOI] [PubMed] [Google Scholar]
- 38.Vallon V, Grahammer F, Volkl H, Sandu CD, Richter K, Rexhepaj R, Gerlach U, Rong Q, Pfeifer K, Lang F. KCNQ1-dependent transport in renal and gastrointestinal epithelia. Proc Natl Acad Sci USA 102: 17864–17869, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38a.Van Campenhout CA, Eitelhuber A, Gloeckner CJ, Giallonardo P, Gegg M, Oller H, Grant SGN, Krappmann D, Ueffing M, Lickert H. Dlg3 trafficking and apical tight junction formation is regulated by nedd4 and nedd4-2 e3 ubiquitin ligases. Dev Cell 21: 479–491, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang Q, Curran ME, Splawski I, Burn TC, Millholland JM, VanRaay TJ, Shen J, Timothy KW, Vincent GM, de Jager T, Schwartz PJ, Toubin JA, Moss AJ, Atkinson DL, Landes GM, Connors TD, Keating MT. Positional cloning of a novel potassium channel gene: KVLQT1 mutations cause cardiac arrhythmias. Nat Genet 12: 17–23, 1996. [DOI] [PubMed] [Google Scholar]
- 40.Wangemann P, Liu J, Marcus DC. Ion transport mechanisms responsible for K+ secretion and the transepithelial voltage across marginal cells of stria vascularis in vitro. Hear Res 84: 19–29, 1995. [DOI] [PubMed] [Google Scholar]
- 41.Xiang YK. Compartmentalization of beta-adrenergic signals in cardiomyocytes. Circ Res 109: 231–244, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang T, Kanki H, Roden DM. Phosphorylation of the IKs channel complex inhibits drug block: novel mechanism underlying variable antiarrhythmic drug actions. Circulation 108: 132–134, 2003. [DOI] [PubMed] [Google Scholar]