Abstract
The lymphatics have emerged as critical players in the progression and resolution of inflammation. The goal of this study was to identify specific microRNAs (miRNAs) that regulate lymphatic inflammatory processes. Rat mesenteric lymphatic endothelial cells (LECs) were exposed to the proinflammatory cytokine tumor necrosis factor-α for 2, 24, and 96 h, and miRNA profiling was carried out by real-time PCR arrays. Our data demonstrate a specific set of miRNAs that are differentially expressed (>1.8-fold and/or P < 0.05) in LECs in response to tumor necrosis factor-α and are involved in inflammation, angiogenesis, endothelial-mesenchymal transition, and cell proliferation and senescence. We further characterized the expression of miRNA 9 (miR-9) that was induced in LECs and in inflamed rat mesenteric lymphatics. Our results showed that miR-9 overexpression significantly repressed NF-κB expression and, thereby, suppressed inflammation but promoted LEC tube formation, as well as expression of the prolymphangiogenic molecules endothelial nitric oxide synthase and VEGF receptor type 3. LEC viability and proliferation and endothelial-mesenchymal transition were also significantly induced by miR-9. This study provides the first evidence of a distinct profile of miRNAs associated with LECs during inflammation. It also identifies the critical dual role of miR-9 in fine-tuning the balance between lymphatic inflammatory and lymphangiogenic pathways.
Keywords: lymphatic endothelial cell, microRNA, inflammation, lymphangiogenesis, signaling
the involvement of lymphatic vessels in edema resolution and immune cell trafficking and the sensitivity of these vessels to inflammatory mediators make them pivotal players in the inflammation process (65, 71). Lymphatic endothelial cells (LECs) at a site of inflammation have been shown to actively participate in and regulate inflammatory processes and host immune responses, thereby emerging as major players in progression and resolution of the inflammatory state (18, 19, 46, 47, 50, 64). Since inflammation acts as a primary trigger for pathological lymphangiogenesis, a number of proinflammatory cytokines have also been shown to function as prolymphangiogenic factors (15, 25, 49). However, it remains unclear whether lymphangiogenesis is beneficial or detrimental for the resolution of inflammation (2, 18). The lymphatic endothelium stimulated by the proinflammatory cytokine tumor necrosis factor (TNF-α) has been shown to promote the migration of leukocytes from tissue to afferent lymphatics by inducing expression of adhesion molecules such as ICAM-1, VCAM-1, and E-selectin in LECs, along with release of key chemokines, such as CCL5, CCL2, CCL20, and CCL21 (20, 21, 54, 55). The roles of TNF-α in regulating endothelial responses and tissue remodeling are typically characterized at the cellular level by rapid activation of the transcription factor NF-κB and its downstream regulation of proinflammatory genes, including cytokines, chemokines, and adhesion molecules (32). LECs have also been shown to express a number of Toll-like receptors (TLRs), including TLR1-6 and TLR9, the stimulation of which induces expression of the inflammatory cytokines IL-1β, TNF-α, and IL-6 (44). Thus LECs have emerged as an important source of inflammatory cytokines during pathogen-driven inflammation or in response to other inflammatory stimuli. LECs, in turn, respond to inflammatory cytokines by upregulating chemokines, adhesion molecules, and other cytokines, indicating that LECs are also affected by the local inflammatory milieu at sites of infection or vaccination (44). Although a large number of these molecules expressed by inflamed LECs have been described (6, 54, 55), a critical group of potential regulators of the inflammatory mechanisms, specifically the microRNAs (miRNAs), remain completely unexplored in the lymphatic system.
miRNAs are a recently recognized class of highly conserved, noncoding short (18–22 nucleotides) RNA molecules that regulate gene expression at the posttranscriptional level (27). They have been widely implicated in the regulation of endothelial dysfunction and pathologies and have assumed a particularly significant role in regulation of inflammatory mechanisms (42, 58, 67). Knockdown of a key miRNA-processing enzyme, DICER, has been shown to severely abrogate angiogenesis during development in the mouse, thereby underscoring the importance of miRNAs in vascular endothelial cell biology (29, 57). Moreover, several miRNAs have been associated with regulation of blood endothelial cell migration and proliferation, regulation of nitric oxide production, tumor angiogenesis, wound healing, and vascular inflammation, thereby directly contributing to many vascular pathologies (62). While there is a large body of literature enumerating their roles in vascular endothelial cells, very few studies have investigated the role of miRNAs in the lymphatic vasculature in the context of development and lineage specification of LECs. miRNA-31 (miR-31) has been shown to be a negative regulator of lymphatic development (43), and prospero homeobox 1 (Prox1) expression is negatively regulated by miR-181 (24), providing important evidence of mechanisms underlying lymphatic cell programming during development and neolymphangiogenesis. miR-1236 has also been shown to reduce VEGF receptor type 3 (VEGFR3) to inhibit inflammatory lymphangiogenesis (22). Hence, it is clear that our understanding of miRNA regulation of gene networks involved in various lymphatic endothelial functions, particularly those associated with inflammatory processes, is scant.
Thus, in this study, we investigated how an inflammatory stimulus modulates the expression of specific miRNAs in LECs, which would potentially contribute to a number of lymphatic pathophysiological processes. Since several lines of evidence support the link between inflammation and lymphangiogenesis, we also sought to determine if any of the dysregulated miRNAs are involved in regulation of both inflammation and lymphangiogenesis. The goals of the study were threefold: 1) to determine the changes in the expression profile of miRNAs that are associated with inflammation and the immune response using a real-time PCR array, 2) to analyze the target genes and specific pathways regulated by these miRNAs in inflamed LECs using a computational approach, and 3) to identify and validate potential miRNAs for their roles in lymphangiogenesis and inflammatory signaling.
MATERIALS AND METHODS
Cell culture and TNF-α treatments.
Rat LECs were isolated from rat mesenteric lymphatic vessel explants, and their phenotype was verified by Prox1, VEGFR3, and other lymphatic endothelial-specific markers as described elsewhere (17). Human LECs were obtained from PromoCell (Heidelberg, Germany). Cultures were grown to confluence in EGM2.MV medium (Lonza, Basel, Switzerland) as described elsewhere (11) and then maintained in low-serum (1%) medium prior to treatment. The cells were exposed to TNF-α (20 ng/ml) for 2, 24, or 96 h or maintained for corresponding durations without treatment. LECs were used at passages 3–6. TNF-α was purchased from R & D Systems (Minneapolis, MN).
miRNA and total RNA preparation.
Enriched small RNA fractions were extracted from untreated LECs or LECs exposed to TNF-α for 2, 24, or 96 h using miRNeasy and MinElute kits (Qiagen, Valencia, CA). Total RNA was also prepared using the RNeasy kit according to the manufacturer's instructions (Qiagen). The quality and quantity of RNA were determined using a spectrophotometer (model ND-1000, NanoDrop Technologies, Wilmington, DE) and an bioanalyzer system (Agilent Technologies, Santa Clara, CA).
miRNA expression profiling by real-time PCR arrays.
From each sample, 100 ng of miRNAs were reverse-transcribed using a specific RT2 miRNA first-strand cDNA synthesis kit (SABiosciences, Valencia, CA). The cDNA was mixed with RT2 SYBR Green/ROX qPCR Mastermix, and the mixture was added to a 384-well RT2 miRNA PCR array (SABiosciences, Valencia, CA), including predefined primer pairs for a set of 88 human miRNAs that are either documented or predicted to have targets involved in the regulation of immunity and inflammatory responses, in addition to a panel of 8 housekeeping genes and controls. All the real-time PCR-based experiments using the RT2 miRNA PCR array were performed in triplicate using a sequence detection system (ABI Prism 7900 HT, Applied Biosystems, Foster City, CA) according to the manufacturer's instructions. The threshold value was kept constant across arrays. Gene profiling and data analysis of miRNA expression were performed using online web-based data analysis software (SABiosciences, Frederick, MD). For normalization, miRNA expressions were compared between the treatment group and the control group at each time point. The cycle threshold (2−ΔΔCt) method was utilized to calculate the fold change. As normalization of the miRNA data is critical in elimination of the bulk of false positives, the most stably expressed genes across the arrays, including three of the manufacturer-provided housekeeping genes (U6, 4.5S, and Y1) and miR152, were used to normalize the expression data; expression of these genes was unchanged across the arrays and time points as determined by NormFinder (8).
Analysis and target prediction of microRNA.
Three independent experiments were carried out at each of the three time points for analysis of the miRNA profile. Those miRNAs that showed ≥1.8-fold difference or had a significant P value (P ≤ 0.05) (70) compared with their corresponding controls were identified as differentially expressed. The potential targets for these miRNAs were determined by bioinformatics analysis of various miRNA target prediction databases: miRWalk (13) TargetScan (http://www.targetscan.org,) MIRANDA (http://www.ebi.ac.uk,) and PicTar-Vert (http://pictar.mdc-berlin.de/).
Isolation of RNA from mesenteric lymphatic vessels of a LPS rat model.
Male Sprague-Dawley rats weighing 200 g were injected with LPS (10 mg/kg body wt ip). The rats were housed in an environmentally controlled vivarium approved by the American Association for Accreditation of Laboratory Animal Care. All animal protocols were approved by the Texas A & M University Laboratory Animal Care Committee. The animals were fasted for 24 h before each experiment, with water available ad libitum. The rats were anesthetized with Innovar-Vet (0.3 ml/kg im), which is a combination of a droperidol-fentanyl solution (droperidol at 20 mg/ml and fentanyl at 0.4 mg/ml) and diazepam (2.5 mg/kg im). For isolation of a rat mesenteric lymphatic vessel, a 6- to 7-cm-long loop of small intestine was exteriorized through a midline laparotomy as described elsewhere (66). A section of the mesentery was gently positioned over a semicircular viewing pedestal on a vessel preparation board. A mesenteric lymphatic vessel was centered over an optical window on the preparation board. The exteriorized tissues were continuously suffused with Dulbecco's phosphate-buffered saline (catalog no. 14040-133, Invitrogen, Carlsbad, CA). The lymphatic vessel (80–120 μm diameter) was isolated under a dissecting microscope, with extreme care taken not to damage the vessel, and was cleared of all the fat tissue. After isolation of the tissue, animals were euthanized with pentobarbital (200 mg/kg). The isolated vessels were rapidly snap-frozen in liquid nitrogen and then stored at −80°C until they were used for protein studies. RNA was isolated using the miRNeasy kit as described above.
Cytokine analysis in LECs and lymphatic vessels.
RNA was isolated from LECs treated with TNF-α and from mesenteric lymphatic vessels as described above. Primers specific to IL-1β, IL-6, monocyte chemoattractant protein 1 (MCP-1), c-fos, and macrophage inflammatory protein 2 (MIP-2) were designed such that they flanked two exonic regions. RNA was converted to first-strand cDNA using the SuperScript III RT first-strand cDNA synthesis kit (Life Technologies, Gaithersburg, MD). Real-time PCR was carried out using SYBR Green PCR master mix (Life Technologies) as described below. 60S ribosomal protein L19 (RPL19) was used as an internal loading control. The difference in the relative expression of cytokines between untreated and TNF-α-treated LECs or LPS-treated animals was calculated using the 2−ΔΔCt method.
miRNA in vitro and in vivo analysis.
Quantitative RT-PCR (qRT-PCR) assays were performed using a SYBR Green miRNA assay kit (Life Technologies) for the mature miRNA (miR-9-1) and the target-specific stem-loop reverse-transcription primer. The forward primer sequences for hsa-miR-9-1, rno-miR-9-1, and RNU6 were purchased from Ambion (Life Technologies) and used for PCR validation. qRT-PCR was performed using SYBR Green Supermix and a sequence detection system (ABI Prism 7900 HT, Applied Biosystems) according to the manufacturer's instructions. LECs were exposed to TNF-α (20 ng/ml) for 24 h, and miRNA was isolated as described above. The difference in the relative expression of miRNA-9 between untreated and TNF-α-treated LECs was calculated using the 2−ΔΔCt method. The difference in relative expression of miR-9 between LPS-treated and normal rats was calculated similarly.
miRNA mimic and inhibitor transfection.
Human LECs were grown to ∼70% confluence and then transfected with 100–500 nM miR-9 mimic or inhibitor (Life Technologies) using Lipofectamine 2000 (Life Technologies) for 24–48 h according to the manufacturer's instructions. For controls, cells were mock-treated or transfected with control mimic or inhibitor sequences. Transfections were performed using Opti-MEM medium (Invitrogen). The cells were grown to 70% confluence and then transfected in 500 μl of antibiotic-free medium. The transfection medium was replaced after 6 h, and the cells were allowed to recover and then maintained in complete EGM2.MV medium (Lonza). The cells were closely monitored for death or toxicity. To verify the degree of mimic overexpression and inhibitor-mediated downregulation of miR-9 in LECs, cells were treated with control miRNAs, miR-9 mimic, and inhibitor, and RNA was isolated as described above. Real-time quantification of miR-9 was also carried out in these cells as described above. All experiments were performed in triplicates.
LEC tube formation assay.
The ability of LECs transfected with miR-9 mimics or inhibitors to form capillary networks was evaluated by endothelial tube formation assay in the absence or presence of TNF-α (20 ng/ml) as described elsewhere with some modifications (38). After 96-well plates were precoated with 40 μl of Matrigel per well, they were allowed to polymerize for 1 h at 37°C. LECs were trypsinized at 48 h posttransfection, and 2 × 104 cells were seeded into each well in 250 μl of EGM2.MV medium (Lonza). The cells were allowed to form networks for 16–24 h. For fluorescence visualization, the cells were incubated with 2 mM calcein-AM (Invitrogen) for 30 min at 37°C. As the fluorescent dye calcein-AM readily permeates live intact cells, visualization of tube formation is facilitated. Cells transfected with a scrambled miRNA were used as a control. Images were acquired by an inverted fluorescence microscope (Olympus, Tokyo, Japan). Quantitative analysis of network structure was performed with ImageJ software (http://rsbweb.nih.gov/ij/) by counting the number of intersections in the network and measuring the total length of the structures. Results are plotted as means ± SE.
Western blots and immunofluorescence.
Western blot analysis was carried out as previously described (7). Briefly, LECs were directly lysed using 1× SDS buffer, and proteins were separated on a 4–20% SDS-polyacrylamide gel. Proteins were transferred onto a nylon membrane and then probed with corresponding primary antibodies. The antibodies were as follows: phosphorylated Akt (1:1,000 dilution), total Akt (1:1,000 dilution), vascular endothelial (VE)-cadherin (1:1,000 dilution), neural (N)-cadherin (1:1,000 dilution), phosphorylated IκB (1:2,000 dilution), total IκB (1:1,000 dilution), phosphorylated NF-κB (1:1,000 dilution), total NF-κB (1:2,000 dilution), β-catenin (1:1,000 dilution), VEGFR3 (1:1,000 dilution), endothelial nitric oxide synthase (eNOS; 1:1,000 dilution), and phosphorylated eNOS (1:1,000 dilution). The blots were then probed with the corresponding secondary antibodies and developed with West Dura extended-duration substrate. For loading control, the blots were probed with β-actin (1:1,000 dilution) antibody. Densitometry analyses on the resulting bands were performed using Quantity One Multi-Analyst software (Bio-Rad, Hercules, CA). For quantification, experiments were repeated three or four times for each sample, and means ± SE were calculated.
Immunofluorescence experiments were carried out using cultured LECs. Briefly, the LECs were grown to ∼70% confluence and treated with TNF-α (20 ng/ml) for 24 h or transfected with miR-9 mimics or inhibitors, or control miRNA sequences were plated onto coverslips and grown to ∼70% confluence. Cells were then fixed with 2% paraformaldehyde, permeabilized with ice-cold methanol, and exposed to NF-κB primary antibody for 1 h. Normal mouse serum was used in place of corresponding primary antibody as a control. After incubation with secondary antibody (conjugated to a fluorescent dye) for 1 h in darkness followed by several stringent washes, the coverslips were mounted onto glass slides and allowed to partially air-dry. Coverslips were mounted using ProLong antifade solution containing 4′,6′-diaminido-2-phenylindole and allowed to cure overnight. The secondary antibody was goat anti-mouse Alexa Fluor 488. Maximum projections of series sections with step size of 0.5-μm thickness were imaged using the Leica AOBS SP2 confocal microscope with a N plan ×40 dry objective with 1.15 numerical aperture.
Luciferase assays.
Luciferase assays were carried out as described elsewhere (5) with some modifications. LECs were plated in 96-well plates in complete medium and, after 24 h, cotransfected with 100 ng of full-length NF-κB1 3′-untranslated region (UTR) RenSP GoClone luciferase reporter vectors encoding the miR-9-1 binding seed region (SwitchGear Genomics, Carlsbad, CA) and 50 nM miR-9 mimic (Life Technologies). The pLightSwitch_3′-UTR reporter vector (3,910 bp) has a Renilla luminescent reporter gene, called RenSP, multiple cloning sites, constitutive promoter, ampicillin site, and an Ori sequence (SwitchGear Genomics). Cotransfections were carried out using DharmaFECT Duo transfection reagent (GE Dharmacon, Lafayette, CO). Nontargeting control miRNA and empty GoClone reporter vector with no 3′-UTR were used as controls. After 48 h, cells were lysed, and luciferase activities were determined using the LightSwitch luciferase assay system according to the manufacturer's instructions (SwitchGear Genomics). Each well was read for 2 s, and the enzymatic activity was quantified using a luminometer (SpectraMax Gemini, Molecular Devices, Sunnyvale, CA). RenSP luciferase activity is expressed as change relative to control miRNA, which was set at 100.
Bromodeoxyuridine cell proliferation assay.
The bromodeoxyuridine (BrdU) incorporation assay, which measures cell proliferation, was performed using the BrdU cell proliferation ELISA kit (Roche Diagnostics, Indianapolis, IN) according to the manufacturer's instructions as previously described (61). Briefly, LECs were cultured in 96-well plates, transfected with miR-9 mimics, inhibitors, or control miRNAs, and grown in the absence or presence of TNF-α (20 ng/ml) for 48 h. BrdU labeling solution was added, and the cells were incubated for 2 h. Absorbance was measured in an ELISA reader at 450 nm with a reference wavelength of 690 nm. All experiments were carried out in triplicates. The relative absorbance values were quantified and plotted.
XTT cell viability assay.
LECs were transfected with 100 nM miRNA control, miR-9 mimics, or inhibitors and grown in the absence or presence of TNF-α. After 48 h, cell viability was determined using TACS XTT cell viability assay (R & D Systems, Minneapolis, MN) according to the manufacturer's instructions as described previously (23). Triplicate wells were assayed for cell viability in each treatment group.
Statistical analysis.
Analysis of miRNA expression data across the miRNA arrays was performed using SABiosciences online PCR array data analysis web portal (http://pcrdataanalysis.sabiosciences.com/pcr/arrayanalysis.php). Values are means ± SE. Statistical analyses were done using a Student's t-test or one- or two-way ANOVA as appropriate. P < 0.05 was regarded as statistically significant.
RESULTS
TNF-α promotes an inflammatory pathway in the lymphatics.
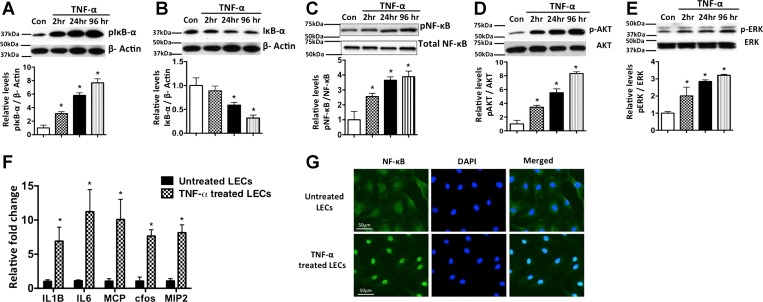
We first sought to evaluate the role of TNF-α in mediating inflammation in LECs. As shown in Fig. 1, A and B, treatment with TNF-α resulted in a significant increase in phosphorylated IκB at 2, 24, and 96 h and a corresponding decrease in total IκB. Phosphorylated NF-κB was also increased in the TNF-α-treated cells. TNF-α modulated the activation of the inflammatory pathways phosphorylated Akt and phosphorylated ERK in a time-dependent manner, with maximum activation at 96 h posttreatment (Fig. 1, D and E). Furthermore, TNF-α significantly induced the expression of key inflammatory cytokines, such as IL-1β, IL-6, MCP-1, c-fos, and MIP-2, in LECs, indicating the activation of several inflammatory signaling pathways (Fig. 1F). As shown in Fig. 1G, a marked nuclear translocation of NF-κB in TNF-α-treated LECs was found, demonstrating the activation of NF-κB. Positive 4′,6′-diaminido-2-phenylindole staining, along with NF-κB, clearly demonstrates the nuclear localization of NF-κB in response to TNF-α compared with the untreated control LECs.
Fig. 1.
TNF-α-mediated inflammatory signaling in lymphatic endothelium. A–E: representative Western blots of protein samples from lymphatic endothelial cells (LECs) exposed to TNF-α (20 ng/ml) for 2, 24, and 96 h and probed with phosphorylated (p-) and total forms of IκB, NF-κB, Akt, and ERK. Experiments were carried out in triplicates. β-Actin was used as endogenous housekeeping control. Quantitative values obtained from each experiment are shown below each blot. *P < 0.05 vs. control (Con). F: induction of inflammatory cytokines in inflamed lymphatics. TNF-α induces key proinflammatory cytokines such as IL-1β, IL-6, monocyte chemoattractant protein 1 (MCP-1), c-fos, and macrophage inflammatory protein 2 (MIP-2), in LECs. LECs were treated for 24 h with TNF-α (20 ng/ml). Real-time quantification was carried out, and fold change relative to untreated control was calculated. Experiments were done in triplicates. Values are means ± SE. *P < 0.05. G: immunofluorescence image of activated NF-κB translocation into nucleus in LECs; 4′,6′-diaminido-2-phenylindole (DAPI) was used for nuclear staining. Magnification ×40. Scale bar = 50 μm.
TNF-α mediates differential and temporal expression of miRNAs associated with the inflammatory response in lymphatic endothelium.
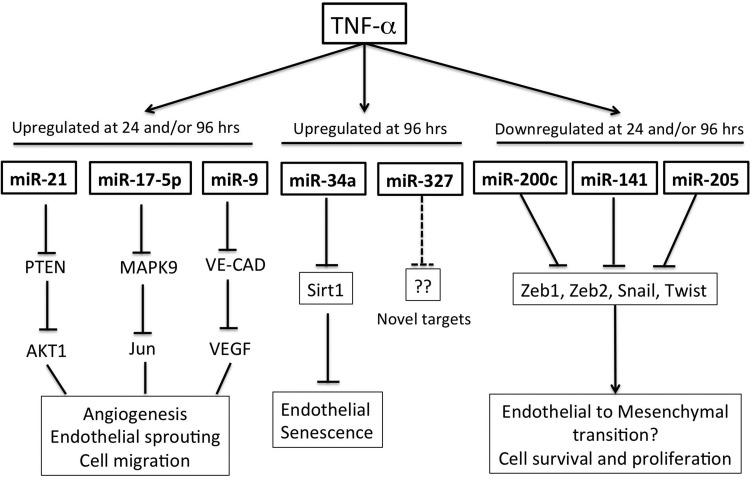
To determine how TNF-α regulates miRNAs associated with various inflammatory processes within the LECs, using an “inflammatory and autoimmune response real-time RT-PCR-based miRNA array,” we analyzed the expression patterns of 88 different miRNAs in LECs after stimulation with TNF-α for 2, 24, or 96 h. Upon quantification of the real-time PCR data obtained from three independent experiments, only 30 of 88 miRNAs analyzed showed a ≥1.8-fold change and/or had a significant P value (P < 0.05) over the different time points analyzed (Table 1). Of these, only one miRNA was upregulated at 2 h of TNF-α exposure, while five miRNAs were downregulated. At 24 h, the number of induced miRNAs increased to 11, whereas 3 miRNAs were downregulated. Several miRNAs showed differential expression at 96 h: a total of 12 were upregulated and 7 were downregulated (Table 1). We found several overlapping miRNAs at each of the time points analyzed, whereas a number of them were unique to a specific time point (Table 1). Of these, miR-144 and miR-205 were downregulated at 2 and 96 h and miR-136 was downregulated at 24 and 96 h, while miR-20a, miR-203, miR-20b-5p, miR-21, miR-325-3p, miR-9, and miR-19a were upregulated at 24 and 96 h. miRNA target analysis (see materials and methods) shows that the identified target genes of miRNAs could be broadly classified as involved in angiogenesis, inflammation, epithelial-mesenchymal transition (EMT)/endothelial-mesenchymal transition (EndMT), and cell proliferation and senescence (Table 2, Fig. 2). No information was available in the literature for miR-878, miR-327, miR-760-5p, miR-325-3p, and miR-448 with respect to their roles in endothelial cell function. Table 2 summarizes the potential functional relevance, expression in other endothelial cells, and some selected targets of these miRNAs relevant to endothelial cell function.
Table 1.
miRNA signature in inflamed LECs at various time points
| 2 h |
24 h |
96 h |
||||||
|---|---|---|---|---|---|---|---|---|
| miRNA | Fold Change | P Value | miRNA | Fold Change | P Value | miRNA | Fold Change | P Value |
| miR-144 | −1.78 | 0.0208* | miR-760-5p | −1.98 | 0.1438 | miR-291a-3p | −1.84 | 0.5236 |
| miR-539 | −2.11 | 0.2257 | miR-136 | −3.41 | 0.0810 | miR-327 | −1.99 | 0.7706 |
| miR-323 | −2.56 | 0.8966 | miR-141 | −2.56 | 0.2903 | miR-495 | −1.91 | 0.4155 |
| miR 205 | −2.13 | 0.3140 | miR-17-5p | 1.98 | 0.0260* | miR-136 | −3.47 | 0.1238 |
| miR-878 | −2.32 | 0.1848 | miR-203 | 2.8 | 0.0161* | miR-144 | −2.06 | 0.3750 |
| miR-200c | 3.423 | 0.7734 | miR-20a | 2.03 | 0.0188* | miR-145 | −2.19 | 0.4110 |
| miR-20b-5p | 2.38 | 0.0248* | miR-205 | −2.20 | 0.0008* | |||
| miR-21 | 1.95 | 0.0070* | miR-203 | 2.08 | 0.1141 | |||
| miR-325-3p | 2.79 | 0.0113* | miR-34a | 2.27 | 0.0044* | |||
| miR-9 | 1.89 | 0.0240* | miR-20a | 1.80 | 0.3234 | |||
| miR-27a | 1.35 | 0.0400* | miR-20b-5p | 1.80 | 0.3193 | |||
| miR-322 | 1.63 | 0.0129* | miR-21 | 1.81 | 0.4062 | |||
| miR-878 | 1.89 | 0.1755 | miR-325-3p | 2.49 | 0.1674 | |||
| miR-19a | 1.87 | 0.1256 | miR-497 | 3.19 | 0.0803 | |||
| miR-9 | 2.04 | 0.1287 | ||||||
| miR-34c | 1.79 | 0.2294 | ||||||
| miR-384-5p | 2.69 | 0.1393 | ||||||
| miR-19a | 1.86 | 0.4900 | ||||||
| miR-19b | 1.91 | 0.4372 | ||||||
LECs, lymphatic endothelial cells.
Statistically significant (P < 0.05).
Table 2.
Pathways activated by the differentially expressed miRNAs and validated targets
| miRNAs | Time Point Expressed | Involvement in Pathways (Relevant to ECs/LECs) | Expression in Other ECs | Selected Specific Validated Targets |
|---|---|---|---|---|
| miR-200c | 2 h | EMT/EndMT, apoptosis, senescence | HUVEC, HMEC | ZEB1, ZEB2 |
| miR-136 | 24 h, 96 h | EMT/EndMT, apoptosis | BCL2 | |
| miR-205 | 96 h | EMT/EndMT | HAEC, HUVEC | ZEB1/2, ERB3, VEGFA |
| miR-141 | 24 h | EMT/EndMT, cell proliferation, migration | HUVEC | PTEN, ZEB1, ZEB2 |
| miR-9 | 24 h, 96 h | EMT/EndMT, inflammation, angiogenesis | HUVEC, HPAEC | NF-KB1, E-CAD, STAT3, CXCR4, MCPIP1 |
| miR-21 | 24 h, 96 h | EMT/EndMT, angiogenesis | HUVEC | PTEN, PDCD4 |
| miR-27a | 24 h | Angiogenesis | HUVEC | SPROUTY2, SEMA6 |
| miR-20a | 2 h, 24 h, 96 h | Angiogenesis | HUVEC | EPH2, EPHB4, VEGF |
| miR-20b-5p | 24 h, 96 h | Angiogenesis | HUVEC, HBMEC | VEGF |
| miR-17-5p | 24 h | Angiogenesis, cell proliferation, apoptosis | HUVEC, HPAEC | E2F1, TIMP1, MAPK9 |
| miR-19b | 96 h | Angiogenesis, inflammation | HUVEC, HBMEC | SOCS1, RNF11 |
| miR-145 | 96 h | Proliferation, differentiation, angiogenesis, apoptosis | HUVEC | p70S6K1, VEGF |
| miR-322 | 24 h | Angiogenesis, oxidative stress | HUVEC | CUL2, FGF2 |
| miR-144 | 2 h, 96 h | Oxidative stress response | HUVEC | NRF2 |
| miR-34a | 96 h | Cell senescence, proliferation | HUVEC | SIRT1 |
| miR-34c | 96 h | Cell senescence, EMT | HUVEC | Notch1, BCL2 |
| miR-497 | 96 h | Apoptosis | BCL2 | |
| miR-384-5p | 96 h | Apoptosis | PIK3CD | |
| miR-291a-3p | 96 h | Embryonic stem cell cycle regulator | MMVEC | Unknown |
| miR 397 | 96 h | Unknown | Unknown | |
| miR-325-3p | 24 h, 96 h | Unknown | Unknown | |
| miR-327 | 96 h | Unknown | MMVEC | Unknown |
| miR-760-5p | 24 h | Unknown | Unknown | |
| miR 448 | 2 h | Unknown | Unknown | |
| miR-878 | 24 h | Unknown | Unknown |
ECs, endothelial cells; EMT, epithelial-mesenchymal transition; EndMT, endothelial-mesenchymal transition; HMEC, human microvascular ECs; HUVEC, human umbilical vein ECs; HPAEC, human pulmonary artery ECs; HBMEC, human brain microvascular ECs; MMVEC, myocardial microvascular ECs.
Fig. 2.
Schematic representation of specific microRNAs (miRNAs) that are regulated by TNF-α treatment in lymphatics and some of their key functions in endothelial cells. Specific targets of the miRNAs regulating a particular physiological response are shown below each miRNA. PTEN, phosphatase and tensin homolog; VE-CAD, vascular endothelial cadherin; ZEB1, zinc finger E-box-binding homeobox 1; Sirt1, sirtuin.
From our screening and analysis for identification of a miRNA that could have a role in modulating lymphatic inflammation and could potentially be involved in the process of lymphangiogenesis, miR-9, which was found to be significantly induced in LECs by TNF-α at 24 and 96 h, stood out. miR-9 has been implicated in inflammatory processes and has been shown to promote endothelial cell motility and angiogenesis in response to inflammatory stimuli (5, 72). Hence, miR-9 was selected for further functional analysis, as we hypothesized that it may play an important role in fine-tuning the TNF-α-induced inflammatory reactions in LECs and may also have a role in lymphangiogenesis.
miR-9 is induced in LECs in vitro and in inflamed lymphatic vessels in vivo.
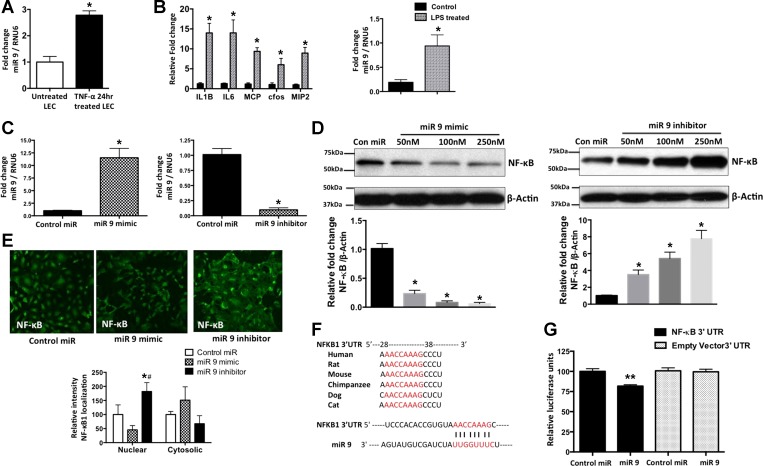
To further validate the data that we obtained from real-time PCR-based miRNA arrays, miR-9 in LECs treated with TNF-α for 24 h was quantified. miR-9 showed a significant induction in response to TNF-α (2.781 ± 0.0950 and 1.000 ± 0.1240 in TNF-α-treated and untreated LECs, respectively, P = 0.0003, n = 3; Fig. 3A). TNF-α is induced by LPS, which also significantly affects lymphatic function (5, 26). To assess whether the relative levels of miR-9 are increased in vivo in the lymphatic tissues under systemic inflammatory conditions as induced by LPS, we determined the expression of miR-9 in mesenteric lymphatic vessels isolated from normal rats and rats treated intraperitoneally with LPS for 24 h. Figure 3B shows that LPS causes a significant induction in the relative levels of IL-1β, IL-6, MCP-1, c-fos, and MIP-2 in mesenteric lymphatic vessels, suggesting widespread inflammatory signaling in the mesentery of LPS-injected rats. qRT-PCR data show that miR-9 is significantly induced (0.19 ± 0.03 and 0.94 ± 0.13 in untreated and LPS-treated vessels, respectively, P = 0.0051, n = 3) in mesenteric lymphatic vessels from 24-h LPS-treated animals (Fig. 3B). Thus our data show that miR-9 is regulated in the lymphatics in response to inflammatory stimuli in the mesenteric bed.
Fig. 3.
miRNA-9 (miR-9) is expressed in vitro and in vivo in LECs and targets NF-κB. A: quantification of data from real-time RT-PCR with mature miR-9-1 primers in LECs exposed to TNF-α (20 ng/ml) for 24 h. B: induction of inflammatory cytokines and miR-9 in inflamed lymphatics. Rats were injected with LPS (10 mg/kg ip) for 24 h, and mesenteric lymphatics were isolated. RNA was isolated from mesenteric lymphatic vessels. Levels of various cytokines and miR-9 were quantified in real time, and fold change relative to untreated control rats was calculated. RPL19 and RNU6 were used as endogenous controls for analysis of cytokines and miR-9, respectively. Experiments were done in triplicates. Values are means ± SE. *P < 0.05. C: quantification of miR-9 mimics and inhibitor transfection efficiency. Real-time PCR was carried out with mature miR-9-1 primers in LECs after transfection with miR-9 mimics or inhibitors for 48 h. Data represent fold change of miR-9 relative to RNU6, which was used as an endogenous control. Values are means ± SE (n = 3). *P < 0.05. D: representative Western blots of samples from LECs transfected with different concentrations (50–250 nM) of miR-9 mimics or inhibitors, as well as a control miRNA sequence. β-Actin was used as a loading control. Blots represent results from 3 experiments. Ratio of NF-κB to β-actin was calculated and is shown as fold change below each blot. *P < 0.05 vs. control. E: immunofluorescence images of NF-κB expression in LECs transfected with 100 nM miR-9 mimic, miR-9 inhibitor, and a control miR sequence for 48 h. Relative nuclear and cytoplasmic signal intensity in each cell was quantified and is shown below images. Values are means ± SE. *P < 0.05 vs. control miR. #P < 0.05 vs. miR-9 mimic. Magnification ×20. F: cross-species sequence alignment and conservation of the putative miR-9 binding site located in the 3′-untranslated region (UTR; nucleotide 29–35) of the NF-κB1 transcript. G: luciferase reporter assay in LECs. Control or miR-9 mimics (100 nM) were cotransfected with the wild-type 3′-UTR of NF-κB1 or with the empty 3′-UTR vector backbone. Renilla luciferase signal was analyzed by luminometer and quantified, and data are plotted. Values are means ± SE from 3 independent experiments. **P < 0.01.
miR-9 inhibits NF-κB signaling in LECs but promotes lymphatic tube formation.
miR-9 decreases NF-κB expression in immune cells, such as monocytes and neutrophils (5), and miRNA target analysis databases showed a conserved miR-9 seed sequence in the 3′-UTR of the NF-κB1 gene. Hence, we examined the NF-κB protein levels in LECs transfected with increasing concentrations of miR-9 mimic and miR-9 inhibitor. First, we determined the efficiency of induction or inhibition of relative levels of endogenous miR-9 upon application of mimics or inhibitors, respectively, by quantitative real-time PCR assays. RNU6 was used as an endogenous housekeeping control. miR-9 levels were significantly induced by 11.33-fold after mimic treatment (1.013 ± 0.05772 and 11.53 ± 1.072 in control miR- and miR-9 mimic-treated LECs, respectively, P = 0.0006, n = 3) and reduced by 10.25-fold after inhibitor treatment (1.013 ± 0.05772 and 0.09879 ± 0.01876 in control miR- and miR-9 inhibitor-treated LECs, respectively, P = 0.0001, n = 3; Fig. 3C). Consistent with these findings, miR-9 overexpression significantly inhibited NF-κB in a concentration-dependent manner (0.23 ± 0.03 and 1.015 ± 0.05 in 50 nM miR-9-treated LECs and controls, respectively, P = 0.0002, n = 3; 0.082 ± 0.015 in 100 nM miR-9-treated LECs, P < 0.0001, n = 3; and 0.0620 ± 0.012 in 250 nM miR-9-treated LECs, P < 0.0001, n = 3), while inhibition of endogenous miR-9 increased the relative levels of NF-κB (3.523 ± 0.3044 and 1.030 ± 0.03 in 50 nM miR-9-treated LECs and control, respectively, P = 0.0012, n = 3; 5.430 ± 0.43 in 100 nM miR-9-treated LECs, P = 0.0005, n = 3; and 7.780 ± 0.56 in 250 nM miR-9-treated LECs, P = 0.0003, n = 3; Fig. 3D). Immunofluorescence data show an increased level of NF-κB, as well as NF-κB nuclear translocation, in miR-9 inhibitor-treated LECs compared with cells treated with miR-9 mimics (Fig. 3E).
To further determine if NF-κB1 is a direct target of miR-9 in LECs, luciferase assays were carried out with miR-9 mimic and NF-κB1 3′-UTR constructs. Luciferase activity was significantly decreased in cells cotransfected with the NF-κB1 3′-UTR reporter vector and miR-9 mimic compared with cells cotransfected with the control miR and NF-κB1 3′-UTR (100.0 ± 1.925 vs. 81.74 ± 0.9894, P = 0.0011, n = 3). However, no significant change in luciferase activity was detected in cells cotransfected with the empty 3′-UTR reporter vector and either the miR-9 mimic or control miR sequences (Fig. 3G). A graphical representation of the seed sequence binding region of miR-9 and NF-κB1 3′-UTR and its conservation across species is shown in Fig. 3F.
Although several studies have shown the close association of inflammation and lymphangiogenesis (19, 46, 47, 64), the role of the proinflammatory cytokine TNF-α in modulating lymphangiogenesis remains controversial (4, 6, 22, 48). Since miR-9 was upregulated in the TNF-α-treated LECs and it also directly targets NF-κB, we assessed the effects of miR-9 in LECs on lymphatic tube formation. LECs transfected with a control miRNA oligonucleotide or a miR-9 mimic or inhibitor were subjected to Matrigel assay (see materials and methods). Compared with the control miR, overexpression of miR-9 in LECs, grown under two different conditions (EGM2.MV and EBM2 + 3% FBS), led to a significant increase in the ability of LECs to form tubelike networks with uninterrupted branch points under both culture conditions (21.33 ± 2.028 and 59.67 ± 2.728 in control and mimic-treated LECs, respectively, P = 0.0004, n = 3; Fig. 4, A and B). miR-9 inhibitor significantly reduced tube formation (21.33 ± 2.028 and 11.67 ± 1.764 in control and inhibitor-treated LECs, respectively, P = 0.0228, n = 3). We also carried out tube formation assays after modulating the levels of miR-21, another miRNA that was induced in the TNF-α-treated LECs (Table 1, Fig. 4, A and B). miR-21 mimics caused a significant reduction in LEC tube formation, but the miR-21 antagomirs did not have a significant effect (Fig. 4, A and B). However, TNF-α, alone or in the presence of miR-9 or miR-21 mimics or inhibitors, did not promote lymphatic tube formation (Fig. 4C).
Fig. 4.
miR-9 promotes LEC tube formation and migration. A–C: LEC tube formation was assayed in complete medium (EGM2.MV), medium supplemented with 3% serum (EBM2 + 3% FBS), and medium supplemented with 3% serum in the presence of TNF-α (20 ng/ml). Cells were stained with calcein-AM for 20 min and imaged with an inverted fluorescence microscope. Representative images are from 3 independent experiments. Scale bars = 100 μm. D: quantification of branch points and tube lengths as indicated by arrows in A and B. Values are means ± SE; n = 3. *P < 0.05.
miR-9 regulates key players in the lymphangiogenesis and EndMT pathways.
Since VEGFR3 is a key determinant of lymphangiogenesis and has been shown to be important for growth and survival signals in LECs, we examined the effects of miR-9 on VEGFR3 expression. Overexpression of miR-9 significantly increased the expression of VEGFR3 in LECs, whereas miR-9 inhibitors decreased the relative levels of VEGFR3 (Fig. 5A). TNF-α decreased VEGFR3 expression in a time-dependent manner, with a significant decrease by 2 h and a ∼75% decrease by 24 h (Fig. 5B).
Fig. 5.
Effects of miR-9 and TNF-α on VEGF receptor type 3 (VEGFR3) expression in lymphatics. A: representative Western blot of VEGFR3 in LECs transfected with 200 nM miR-9 mimic or inhibitor or a control miR sequence. β-Actin was used as a loading control. B: representative Western blot of VEGFR3 in LECs treated with TNF-α (20 ng/ml). Blots were done in triplicates. Results were quantified and are shown below images. Values are means ± SE. *P < 0.05 vs. control.
To further delineate the molecular mechanisms regulating the miR-9-mediated increase in VEGFR3 expression and subsequent LEC tube formation, we investigated the effects of miR-9 on the expression patterns of VE-cadherin, N-cadherin, eNOS, and phosphorylated eNOS. These molecules have been reported in various studies to regulate VEGFR3 expression and/or lymphangiogenesis and are also implicated in EMT or EndMT (31, 35, 39). As shown in Fig. 6A, miR-9 markedly decreased the expression of VE-cadherin while increasing the levels of N-cadherin. In addition, miR-9 mimics increased the levels of eNOS and phosphorylated eNOS protein levels in LECs. However, the miR-9 inhibitor did not have a significant effect on expression of these proteins (Fig. 6A). Concurrently, TNF-α also significantly increased expression of β-catenin, N-cadherin, and zinc finger E-box-binding homeobox 1 (ZEB1), key molecules involved in the initiation and progression of the EndMT pathways, while decreasing the levels of VE-cadherin, at 2 and 24 h in LECs. No significant change in expression of the housekeeping control β-actin was observed (Fig. 6B). As EndMT mechanisms are associated with an increase in cell viability and proliferation, we carried out these assays in the absence or presence of miR-9, as well as with and without TNF-α. miR-9 mimics significantly increased proliferation of LECs as assessed by BrdU assay (P = 0.0143, n = 3), whereas miR inhibitors induced a decrease, although it was not significant, in proliferation. TNF-α also significantly increased proliferation (P = 0.0356, n = 3; Fig. 6C). Furthermore, miR-9 significantly increased LEC viability by ∼50% (P = 0.02, n = 3) as assessed by XTT assay (Fig. 6D). TNF-α also had similar effects on LEC viability (P = 0.0125, n = 3). Furthermore, our data show that miR-9 inhibitor significantly reduced TNF-α-induced LEC proliferation and viability. However, miR-9 mimics had no significant effect on LEC proliferation in the presence of TNF-α (Fig. 6, C and D).
Fig. 6.
miR-9 and TNF-α-mediated signaling and endothelial-mesenchymal transition (EndMT) in LECs. A: representative Western blots showing expression of VE-cadherin, N-cadherin, endothelial NO synthase (eNOS), and phosphorylated eNOS in LECs transfected with control miR, miR-9 mimics, and inhibitors. Values from 3 independent experiments were quantified and are shown below each blot. B: representative Western blots of protein samples from LECs exposed to TNF-α (20 ng/ml) for 2, 24, and 96 h showing corresponding levels of β-catenin, ZEB1, VE-cadherin, and N-cadherin. Results from experiments carried out in triplicates were quantified and are shown below each blot. Values are means ± SE. *P < 0.05. C: bromodeoxyuridine (BrdU) assay for cell proliferation. LECs were treated with miR-9 mimics or inhibitors (100 nM) in the absence or presence of TNF-α (20 ng/ml) for 2 h, and relative absorbance was measured and plotted. Experiments were done in triplicates. D: XTT assay for cell viability. LECs were treated as described in C, and XTT was added for 4 h. Relative absorbance was measured and plotted. All experiments were done in triplicates. Values are means ± SE. *P < 0.05 vs. control miR. #P < 0.05 vs. miR-9 mimic.
DISCUSSION
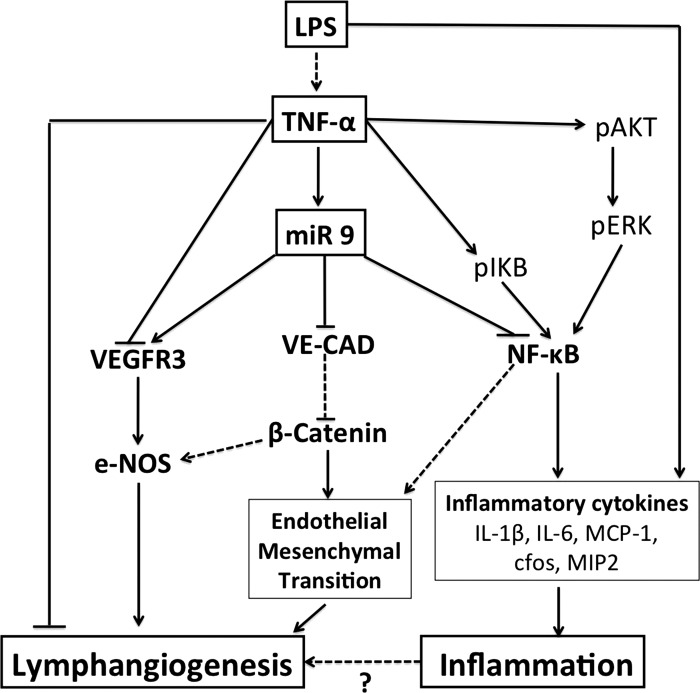
This study provides the first evidence of a specific subset of miRNAs differentially expressed in LECs exposed to proinflammatory stimuli that are associated with TNF-α-mediated inflammatory pathways. The dysregulated miRNAs have been found to be broadly involved in regulation of pathways underlying inflammation (miR-9 and miR-21), angiogenesis (miR-20a, miR-20b-5p, miR-21, miR-9, miR-145, miR-27a, miR-17-5p, miR-322, and miR-19b), EMT/EndMT (miR-141, miR-200c, miR-136, miR-21, and miR-9), and cell senescence (miR-34a and miR-34c) and proliferation (miR-203, miR-141, and miR-17-5p). Furthermore, the target analyses also revealed a group of miRNAs (miR-291a-3p, miR-397, miR-325-3p, miR-327, miR-760-5p, miR-448, and miR-878) that have no documented role in endothelial biology, with some having no validated target until now, suggesting that these miRNAs might have novel roles in the regulation of lymphatic endothelial function. In addition, this study identifies miR-9 as a potential regulator of TNF-α-mediated inflammatory mechanisms, and while increased miR-9 targets key inflammatory genes such as NF-κB, it augments lymphangiogenesis and EndMT pathways in LECs (Fig. 7). Thus our data demonstrate a set of regulatory miRNAs involved in different cellular pathways that may potentially modulate inflammation and lymphangiogenic signaling in the lymphatics.
Fig. 7.
Schematic representation of miR-9 as a modulator of lymphatic inflammation and lymphangiogenesis. In LECs, LPS stimulates TNF-α. Inflammatory stimuli, such as TNF-α and LPS, induce expression of miR-9. Many of the effects of TNFα and LPS are mediated by activation of NF-κB and, in turn, its induction of downstream inflammatory factors. TNF-α also activates the Akt/ERK pathway, which plays a role in phosphorylation of IκB and activation of NF-κB. miR-9 regulates lymphatic inflammation by repressing NF-κB. On the other hand, miR-9 expression inhibits expression of VE-cadherin and activates eNOS in LECs. Decreased VE-cadherin expression activates β-catenin and has been shown to induce epithelial-mesenchymal transition. We predict that miR-9 also activates endothelial-mesenchymal transition by a similar mechanism by activation of β-catenin. miR-9 also acts as a prolymphangiogenic molecule and induces expression of VEGFR3, thereby promoting lymphangiogenesis. TNF-α induces miR-9; however, it decreases VEGFR3 expression and increases activation of NF-κB; subsequently, TNF-α allows inflammation to proceed. Solid arrows indicate mechanisms that have been directly assessed in this study; dashed arrows indicate previously published findings.
Comparison of miRNA expression between LECs and blood endothelial cells and within diverse blood vascular endothelial cells has been characterized (41, 43). However, the role of inflammation-associated miRNAs was not specifically investigated. This is the first report of the expression patterns (either induction or downregulation) of 30 miRNAs in the lymphatic endothelium in response to an inflammatory stimulus. Furthermore, our characterization of the functional role of miR-9 provides essential data that would enable us to identify other miRNAs involved in the inflammatory signaling mechanisms in the lymphatics. We demonstrate the expression and regulation of a distinct subset of miRNAs in inflamed lymphatics that participate in various lymphatic physiological functions. The expression of these miRNAs reported in other types of endothelial cells is summarized in Table 2. We first discuss how several of the miRNAs identified in this study correlate to various signaling mechanisms, specifically to cell proliferation, angiogenesis, and EndMT, which identify underlying regulatory mechanisms in the responses of LECs under inflammatory stimuli.
miR-21 and the miR-17-92 cluster.
In our analysis, we found a fairly large group of miRNAs that have been shown to be associated with angiogenesis. This is not surprising, as several of the key molecular regulators of angiogenesis, such as VEGFs, are also common to lymphangiogenesis during inflammation (1). miR-21, which has been shown to be both anti- and proangiogenic (33, 52), is significantly upregulated in LECs in response to TNF-α (Table 1). Results of the latter study (52) are consistent with our findings that miR-21 mimics reduced tube formation in LECs (Fig. 4, A and B), suggesting an antilymphangiogenic role. Several other miRNAs identified in this study (miR-17-5p, miR-19a, miR-19b-1 and miR-20a) belong to the miR-17-92 cluster, which exhibits a cell-intrinsic antiangiogenic effect in endothelial cells and also regulates inflammation (12, 45).
miR-141, miR-136, miR-205, and miR-200c in EndMT of LECs.
Another cluster of miRNAs that we identified were involved in EndMT/EMT mechanisms (Table 2, Fig. 2). Evidence from recent studies suggests that EndMT is an important contributor to cardiac and vascular development, as well as to pathophysiological vascular remodeling and tissue remodeling (3). The prosurvival protein ZEB1 has been identified as a direct target of miR-200c and miR-141, and ZEB1 is a key molecule involved in the processes of EMT and EndMT that directly suppress E-cadherin (34, 40). Downregulation of miR-141 and miR-205 has been implicated in EMT progression, whereas upregulation of miR-21 and miR-9 has been linked to EndMT and EMT, respectively (30, 37). Similar expression patterns for these miRNAs (Table 1, Fig. 2) suggest that TNF-α would also induce EndMT in the LECs by activation and inhibition of specific miRNAs. Our data support this conclusion, as TNF-α induces ZEB1 expression, decreases VE-cadherin expression, and increases N-cadherin expression in a time-dependent manner (Fig. 6), which is one of the main characteristics of EMT/EndMT progression.
Role of miR-9 in lymphangiogenesis and in modulation of lymphatic inflammation.
Our study is the first report on the dual role of miR-9 in modulation of lymphatic inflammation and lymphangiogenic mechanisms. Although miR-9 showed a level of induction similar to that of several other miRNAs that were identified, its functional significance is profound. Our data clearly demonstrate that miR-9 directly targets NF-κB, a key regulator of inflammatory responses and increased LEC proliferation, viability, and tube formation, while also activating EndMT mechanisms. We also evaluated the effects of another miRNA induced by TNF-α, miR-21, on LEC tube formation. However, only miR-9 mimics showed a robust increase in tube formation, while miR-21 did not (Fig. 4, A and B).
Recent studies have also underscored the importance of miR-9 in mediating inflammatory responses. miR-9 potentiates LPS-mediated release of proinflammatory cytokines, targets the proinflammatory MCP-1-induced protein 1 (MCPIP1), and also promotes cancer metastasis through an EMT-like mechanism (39, 69, 72). miR-9 is encoded by three distinct genomic loci, miR-9-1, miR-9-2, and miR-9-3, that give rise to mature miR-9 species with identical sequences. We identified miR-9-1 to be significantly induced in response to TNF-α in LECs at 24 and 96 h. Previously, TNF-α and LPS were shown to upregulate miR-9 in monocytes and neutrophils (5). Our real-time miRNA array data were further verified by independent real-time primer assays for miR-9-1 in LECs (Fig. 3A). In addition, we found a significantly increased level of miR-9 expression in mesenteric lymphatic vessels from LPS-treated animals, indicating that this regulatory pathway was also active in the lymphatics in vivo (Table 1, Fig. 3B). miR-9 was found to inhibit NF-κB and significantly activate VEGFR3 in LECs (Figs. 3 and 5). Our luciferase assays demonstrated that NF-κB1 is a direct target of miR-9 in LECs (Fig. 3G) and are consistent with results from a previous study that shows similar levels of luciferase activity in monocytes when miR-9 binds to the NF-κB1 seed region (5). It is interesting to note that TNF-α-induced miR-9 negatively regulates NF-κB in LECs, while TNF-α itself stimulates NF-κB activation in LECs (Fig. 1). It has been proposed that as NF-κB is a key regulator of inflammation, its levels are likely to be a strictly controlled and time-regulated event for the proper progression of the inflammatory response. Thus it is conceivable that the same inflammatory stimuli activate miRNA with opposing functions to maintain the balance between inflammation progression and resolution. This hypothesis is supported by our data (Table 1) showing that TNF-α also induces miR-19, which is known to positively regulate NF-κB (16).
Saban et al. (51) showed that NF-κB is persistently expressed in the lymphatic endothelium. The induction of VEGFR3 under these conditions is at first somewhat paradoxical, as NF-κB has also been shown to be a positive regulator of VEGFR3 (15). Since miR-9 directly inhibits NF-κB, as demonstrated by our Western blot and luciferase assays, it is possible that miR-9 activates other positive regulators of VEGFR3-mediated lymphangiogenesis, such as the Notch family of proteins or neuropilin (56, 68), in the absence of NF-κB signaling. However, this warrants further investigation. Similar to our findings, it has been shown that VEGFR3 mRNA and protein are significantly decreased in inflamed lymphatics during acute skin inflammation in mice, which is consistent with our finding that TNF-α decreased VEGFR3 expression in LECs (Fig. 5B). The inflammatory cytokine IL-1β has also been shown to inhibit lymphatic tube formation in vitro by induction of miR-1236, which negatively targets VEGFR3 and, thereby, lymphangiogenesis (22). Although several studies have shown that TNF-α does not promote LEC tube formation and suppresses lymphangiogenesis in murine and human arthritic joints (6, 22, 48), there is conflicting evidence about its in vivo roles (4). This would also explain why even though TNF-α promotes EndMT-associated genes, such as β-catenin, ZEB1, N-cadherin, and VE-cadherin in LECs (Fig. 6B), as well as LEC proliferation (Fig. 6C), it does not promote LEC tube formation (Fig. 4C). This inherent paradox could be explained by the fact that it simultaneously induces a set of miRNAs with pro- and antilymphangiogenic functions, which may be mitigating the effect of miR-9 in response to TNF-α (Table 2). VEGFR3 has been shown to induce eNOS, a major lymphangiogenic molecule (10, 31). Here, we have shown that miR-9 mimic significantly upregulates expression of VEGFR3, eNOS, and phosphorylated eNOS levels in LECs (Figs. 5 and 6A). miR-9 also targets the CXCR4/CXCL12 signaling axis, which is implicated in the progression of several inflammatory diseases and also potentiates lymphangiogenesis by a VEGFR3/VEGF-C-independent mechanism (36, 60, 73). As we found VEGFR3 to be induced by miR-9, we can speculate that miR-9 targets CXCR4 in LECs to preferentially activate the VEGFR3-mediated lymphangiogenic pathways in response to inflammatory stimuli. Furthermore, our data demonstrate that miR-9 overexpression significantly induced LEC proliferation and viability, whereas inhibition of endogenous miR-9 dampened the effect, showing that miR-9 affects LEC proliferation, as well as tube formation (Figs. 4 and 6). These mechanisms are critical first steps for EndMT and/or lymphangiogenic processes. While miR-9 inhibitor decreases the TNF-α-induced proliferation, miR-9 mimics could not overcome the TNF-α-mediated inhibition of this process. Also, interestingly, miR-9 overexpression increases VEGFR3 expression, while TNF-α (which significantly induces miR-9) decreases VEGFR3 expression (Fig. 5). Thus these data could be mechanistically explained by a time-dependent feedback mechanism involving NF-κB and miR-9, where TNF itself stimulates NF-κB and miR-9, which is induced by TNF-α, negatively regulates it. Lending credence to this hypothesis, a recent study has shown that NF-κB activation also induces miR-9 in immune cells, possibly as a feedback mechanism to limit inflammation (5). Thus a model of inflammation is proposed wherein TNF-α increases NF-κB and, subsequently, inflammatory activation depresses VEGFR3 and allows inflammation to proceed. On the other hand, miR-9 maintains the balance of lymphangiogenesis, as well as inflammation, as it increases VEGFR3 and eNOS expression and induces LEC proliferation and tube formation while inhibiting NF-κB-mediated inflammatory processes (Fig. 7).
Taken together, our results demonstrate for the first time that inflamed LECs express a specific profile of miRNAs that regulate several critical pathways. A number of the identified miRNAs have not been studied in endothelial cells and, to the best of our knowledge, have no validated targets. Further experiments are warranted for functional characterization and validation of the predicted targets of these identified miRNAs. Furthermore, our data provide the first evidence that miR-9 inhibits NF-κB-mediated inflammatory mechanisms while activating VEGFR3-mediated lymphangiogenic mechanisms. This implication has tremendous therapeutic potential. Differential expression of miRNAs may contribute to several lymphatic inflammation-related pathologies. miRNA profiles and their specific gene targets show promise as specific biomarkers of lymphatic inflammation and can be used as therapeutic targets.
GRANTS
This work was supported by National Institutes of Health Grants RO1 DK-99221 (to M. Muthuchamy), HL-089784 (to M. J. Davis), and RO1 HL-96552 (to D. C. Zawieja).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.C., D.C.Z., M.J.D., and M.M. developed the concept and designed the research; S.C. performed the experiments; S.C. and M.M. analyzed the data; S.C. and M.M. interpreted the results of the experiments; S.C. and M.M. prepared the figures; S.C. and M.M. drafted the manuscript; S.C., D.C.Z., M.J.D., and M.M. edited and revised the manuscript; S.C., D.C.Z., M.J.D., and M.M. approved the final version of the manuscript.
ACKNOWLEDGMENTS
The authors thank Aparna Krishnan and Yang Lee for technical help and literature survey.
REFERENCES
- 1.Adams RH, Alitalo K. Molecular regulation of angiogenesis and lymphangiogenesis. Nat Rev Mol Cell Biol 8: 464–478, 2007. [DOI] [PubMed] [Google Scholar]
- 2.Alexander JS, Chaitanya GV, Grisham MB, Boktor M. Emerging roles of lymphatics in inflammatory bowel disease. Ann NY Acad Sci 1207 Suppl 1: E75–E85, 2010. [DOI] [PubMed] [Google Scholar]
- 3.Arciniegas E, Frid MG, Douglas IS, Stenmark KR. Perspectives on endothelial-to-mesenchymal transition: potential contribution to vascular remodeling in chronic pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 293: L1–L8, 2007. [DOI] [PubMed] [Google Scholar]
- 4.Baluk P, Yao LC, Feng J, Romano T, Jung SS, Schreiter JL, Yan L, Shealy DJ, McDonald DM. TNF-α drives remodeling of blood vessels and lymphatics in sustained airway inflammation in mice. J Clin Invest 119: 2954–2964, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bazzoni F, Rossato M, Fabbri M, Gaudiosi D, Mirolo M, Mori L, Tamassia N, Mantovani A, Cassatella MA, Locati M. Induction and regulatory function of miR-9 in human monocytes and neutrophils exposed to proinflammatory signals. Proc Natl Acad Sci USA 106: 5282–5287, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chaitanya GV, Franks SE, Cromer W, Wells SR, Bienkowska M, Jennings MH, Ruddell A, Ando T, Wang Y, Gu Y, Sapp M, Mathis JM, Jordan PA, Minagar A, Alexander JS. Differential cytokine responses in human and mouse lymphatic endothelial cells to cytokines in vitro. Lymphat Res Biol 8: 155–164, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chakraborty S, Nepiyushchikh Z, Davis MJ, Zawieja DC, Muthuchamy M. Substance P activates both contractile and inflammatory pathways in lymphatics through the neurokinin receptors NK1R and NK3R. Microcirculation 18: 24–35, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen SY, Wang Y, Telen MJ, Chi JT. The genomic analysis of erythrocyte microRNA expression in sickle cell diseases. PLos One 3: e2360, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cloonan N, Brown MK, Steptoe AL, Wani S, Chan WL, Forrest AR, Kolle G, Gabrielli B, Grimmond SM. The miR-17-5p microRNA is a key regulator of the G1/S phase cell cycle transition. Genome Biol 9: R127, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coso S, Zeng Y, Opeskin K, Williams ED. Vascular endothelial growth factor receptor-3 directly interacts with phosphatidylinositol 3-kinase to regulate lymphangiogenesis. PLos One 7: e39558, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dellinger MT, Brekken RA. Phosphorylation of Akt and ERK1/2 is required for VEGF-A/VEGFR2-induced proliferation and migration of lymphatic endothelium. PLos One 6: e28947, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Doebele C, Bonauer A, Fischer A, Scholz A, Reiss Y, Urbich C, Hofmann WK, Zeiher AM, Dimmeler S. Members of the microRNA-17-92 cluster exhibit a cell-intrinsic antiangiogenic function in endothelial cells. Blood 115: 4944–4950, 2010. [DOI] [PubMed] [Google Scholar]
- 13.Dweep H, Sticht C, Pandey P, Gretz N. miRWalk-database: prediction of possible miRNA binding sites by “walking” the genes of three genomes. J Biomed Inform 44: 839–847, 2011. [DOI] [PubMed] [Google Scholar]
- 14.El-Chemaly S, Malide D, Zudaire E, Ikeda Y, Weinberg BA, Pacheco-Rodriguez G, Rosas IO, Aparicio M, Ren P, MacDonald SD, Wu HP, Nathan SD, Cuttitta F, McCoy JP, Gochuico BR, Moss J. Abnormal lymphangiogenesis in idiopathic pulmonary fibrosis with insights into cellular and molecular mechanisms. Proc Natl Acad Sci USA 106: 3958–3963, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Flister MJ, Wilber A, Hall KL, Iwata C, Miyazono K, Nisato RE, Pepper MS, Zawieja DC, Ran S. Inflammation induces lymphangiogenesis through up-regulation of VEGFR-3 mediated by NF-κB and Prox1. Blood 115: 418–429, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gantier MP, Stunden HJ, McCoy CE, Behlke MA, Wang D, Kaparakis-Liaskos M, Sarvestani ST, Yang YH, Xu D, Corr SC, Morand EF, Williams BR. A miR-19 regulon that controls NF-κB signaling. Nucleic Acids Res 40: 8048–8058, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hayes H, Kossmann E, Wilson E, Meininger C, Zawieja D. Development and characterization of endothelial cells from rat microlymphatics. Lymphat Res Biol 1: 101–119, 2003. [DOI] [PubMed] [Google Scholar]
- 18.Huggenberger R, Siddiqui SS, Brander D, Ullmann S, Zimmermann K, Antsiferova M, Werner S, Alitalo K, Detmar M. An important role of lymphatic vessel activation in limiting acute inflammation. Blood 117: 4667–4678, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ji RC. Lymphatic endothelial cells, inflammatory lymphangiogenesis, and prospective players. Curr Med Chem 14: 2359–2368, 2007. [DOI] [PubMed] [Google Scholar]
- 20.Johnson LA, Clasper S, Holt AP, Lalor PF, Baban D, Jackson DG. An inflammation-induced mechanism for leukocyte transmigration across lymphatic vessel endothelium. J Exp Med 203: 2763–2777, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johnson LA, Jackson DG. Inflammation-induced secretion of CCL21 in lymphatic endothelium is a key regulator of integrin-mediated dendritic cell transmigration. Int Immunol 22: 839–849, 2010. [DOI] [PubMed] [Google Scholar]
- 22.Jones D, Li Y, He Y, Xu Z, Chen H, Min W. Mirtron microRNA-1236 inhibits VEGFR-3 signaling during inflammatory lymphangiogenesis. Arterioscler Thromb Vasc Biol 32: 633–642, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kano M, Seki N, Kikkawa N, Fujimura L, Hoshino I, Akutsu Y, Chiyomaru T, Enokida H, Nakagawa M, Matsubara H. miR-145, miR-133a and miR-133b: tumor-suppressive miRNAs target FSCN1 in esophageal squamous cell carcinoma. Int J Cancer 127: 2804–2814, 2010. [DOI] [PubMed] [Google Scholar]
- 24.Kazenwadel J, Michael MZ, Harvey NL. Prox1 expression is negatively regulated by miR-181 in endothelial cells. Blood 116: 2395–2401, 2010. [DOI] [PubMed] [Google Scholar]
- 25.Kim H, Kataru RP, Koh GY. Regulation and implications of inflammatory lymphangiogenesis. Trends Immunol 33: 350–356, 2012. [DOI] [PubMed] [Google Scholar]
- 26.Kim KE, Koh YJ, Jeon BH, Jang C, Han J, Kataru RP, Schwendener RA, Kim JM, Koh GY. Role of CD11b+ macrophages in intraperitoneal lipopolysaccharide-induced aberrant lymphangiogenesis and lymphatic function in the diaphragm. Am J Pathol 175: 1733–1745, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim VN. MicroRNA biogenesis: coordinated cropping and dicing. Nat Rev Mol Cell Biol 6: 376–385, 2005. [DOI] [PubMed] [Google Scholar]
- 28.Kovacic JC, Mercader N, Torres M, Boehm M, Fuster V. Epithelial-to-mesenchymal and endothelial-to-mesenchymal transition: from cardiovascular development to disease. Circulation 125: 1795–1808, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kuehbacher A, Urbich C, Zeiher AM, Dimmeler S. Role of Dicer and Drosha for endothelial microRNA expression and angiogenesis. Circ Res 101: 59–68, 2007. [DOI] [PubMed] [Google Scholar]
- 30.Kumarswamy R, Volkmann I, Jazbutyte V, Dangwal S, Park DH, Thum T. Transforming growth factor-β-induced endothelial-to-mesenchymal transition is partly mediated by microRNA-21. Arterioscler Thromb Vasc Biol 32: 361–369, 2012. [DOI] [PubMed] [Google Scholar]
- 31.Lahdenranta J, Hagendoorn J, Padera TP, Hoshida T, Nelson G, Kashiwagi S, Jain RK, Fukumura D. Endothelial nitric oxide synthase mediates lymphangiogenesis and lymphatic metastasis. Cancer Res 69: 2801–2808, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lawrence T. The nuclear factor NF-κB pathway in inflammation. Cold Spring Harb Perspect Biol 1: a001651, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu LZ, Li C, Chen Q, Jing Y, Carpenter R, Jiang Y, Kung HF, Lai L, Jiang BH. MiR-21 induced angiogenesis through AKT and ERK activation and HIF-1α expression. PLos One 6: e19139, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu Y, El-Naggar S, Darling DS, Higashi Y, Dean DC. Zeb1 links epithelial-mesenchymal transition and cellular senescence. Development 135: 579–588, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lohela M, Bry M, Tammela T, Alitalo K. VEGFs and receptors involved in angiogenesis versus lymphangiogenesis. Curr Opin Cell Biol 21: 154–165, 2009. [DOI] [PubMed] [Google Scholar]
- 36.Lu J, Luo H, Liu X, Peng Y, Zhang B, Wang L, Xu X, Peng X, Li G, Tian W, He ML, Kung H, Li XP. miR-9 targets CXCR4 and functions as a potential tumor suppressor in nasopharyngeal carcinoma. Carcinogenesis 35: 554–563, 2014. [DOI] [PubMed] [Google Scholar]
- 37.Lu MH, Huang CC, Pan MR, Chen HH, Hung WC. Prospero homeobox 1 promotes epithelial-mesenchymal transition in colon cancer cells by inhibiting E-cadherin via miR-9. Clin Cancer Res 18: 6416–6425, 2012. [DOI] [PubMed] [Google Scholar]
- 38.Luo Y, Zhou H, Liu L, Shen T, Chen W, Xu B, Han X, Zhang F, Scott RS, Alexander JS, Alam A, Huang S. The fungicide ciclopirox inhibits lymphatic endothelial cell tube formation by suppressing VEGFR-3-mediated ERK signaling pathway. Oncogene 30: 2098–2107, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ma L, Young J, Prabhala H, Pan E, Mestdagh P, Muth D, Teruya-Feldstein J, Reinhardt F, Onder TT, Valastyan S, Westermann F, Speleman F, Vandesompele J, Weinberg RA. miR-9, a MYC/MYCN-activated microRNA, regulates E-cadherin and cancer metastasis. Nat Cell Biol 12: 247–256, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Magenta A, Cencioni C, Fasanaro P, Zaccagnini G, Greco S, Sarra-Ferraris G, Antonini A, Martelli F, Capogrossi MC. miR-200c is upregulated by oxidative stress and induces endothelial cell apoptosis and senescence via ZEB1 inhibition. Cell Death Differ 18: 1628–1639, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McCall MN, Kent OA, Yu J, Fox-Talbot K, Zaiman AL, Halushka MK. MicroRNA profiling of diverse endothelial cell types. BMC Med Genomics 4: 78, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.O'Connell RM, Rao DS, Baltimore D. microRNA regulation of inflammatory responses. Annu Rev Immunol 30: 295–312, 2012. [DOI] [PubMed] [Google Scholar]
- 43.Pedrioli DM, Karpanen T, Dabouras V, Jurisic G, van de Hoek G, Shin JW, Marino D, Kalin RE, Leidel S, Cinelli P, Schulte-Merker S, Brandli AW, Detmar M. miR-31 functions as a negative regulator of lymphatic vascular lineage-specific differentiation in vitro and vascular development in vivo. Mol Cell Biol 30: 3620–3634, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pegu A, Qin S, Fallert Junecko BA, Nisato RE, Pepper MS, Reinhart TA. Human lymphatic endothelial cells express multiple functional TLRs. J Immunol 180: 3399–3405, 2008. [DOI] [PubMed] [Google Scholar]
- 45.Philippe L, Alsaleh G, Bahram S, Pfeffer S, Georgel P. The miR-17 approximately 92 cluster: a key player in the control of inflammation during rheumatoid arthritis. Front Immunol 4: 70, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pober JS, Sessa WC. Evolving functions of endothelial cells in inflammation. Nat Rev Immunol 7: 803–815, 2007. [DOI] [PubMed] [Google Scholar]
- 47.Podgrabinska S, Kamalu O, Mayer L, Shimaoka M, Snoeck H, Randolph GJ, Skobe M. Inflamed lymphatic endothelium suppresses dendritic cell maturation and function via Mac-1/ICAM-1-dependent mechanism. J Immunol 183: 1767–1779, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Polzer K, Baeten D, Soleiman A, Distler J, Gerlag DM, Tak PP, Schett G, Zwerina J. Tumour necrosis factor blockade increases lymphangiogenesis in murine and human arthritic joints. Ann Rheum Dis 67: 1610–1616, 2008. [DOI] [PubMed] [Google Scholar]
- 49.Ran S, Montgomery KE. Macrophage-mediated lymphangiogenesis: the emerging role of macrophages as lymphatic endothelial progenitors. Cancers (Basel) 4: 618–657, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Randolph GJ, Angeli V, Swartz MA. Dendritic-cell trafficking to lymph nodes through lymphatic vessels. Nat Rev Immunol 5: 617–628, 2005. [DOI] [PubMed] [Google Scholar]
- 51.Saban MR, Towner R, Smith N, Abbott A, Neeman M, Davis CA, Simpson C, Maier J, Memet S, Wu XR, Saban R. Lymphatic vessel density and function in experimental bladder cancer. BMC Cancer 7: 219, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sabatel C, Malvaux L, Bovy N, Deroanne C, Lambert V, Gonzalez ML, Colige A, Rakic JM, Noel A, Martial JA, Struman I. MicroRNA-21 exhibits antiangiogenic function by targeting RhoB expression in endothelial cells. PLos One 6: e16979, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sakamoto I, Ito Y, Mizuno M, Suzuki Y, Sawai A, Tanaka A, Maruyama S, Takei Y, Yuzawa Y, Matsuo S. Lymphatic vessels develop during tubulointerstitial fibrosis. Kidney Int 75: 828–838, 2009. [DOI] [PubMed] [Google Scholar]
- 54.Sawa Y, Sugimoto Y, Ueki T, Ishikawa H, Sato A, Nagato T, Yoshida S. Effects of TNF-α on leukocyte adhesion molecule expressions in cultured human lymphatic endothelium. J Histochem Cytochem 55: 721–733, 2007. [DOI] [PubMed] [Google Scholar]
- 55.Sawa Y, Tsuruga E. The expression of E-selectin and chemokines in the cultured human lymphatic endothelium with lipopolysaccharides. J Anat 212: 654–663, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shawber CJ, Funahashi Y, Francisco E, Vorontchikhina M, Kitamura Y, Stowell SA, Borisenko V, Feirt N, Podgrabinska S, Shiraishi K, Chawengsaksophak K, Rossant J, Accili D, Skobe M, Kitajewski J. Notch alters VEGF responsiveness in human and murine endothelial cells by direct regulation of VEGFR-3 expression. J Clin Invest 117: 3369–3382, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Suarez Y, Fernandez-Hernando C, Pober JS, Sessa WC. Dicer dependent microRNAs regulate gene expression and functions in human endothelial cells. Circ Res 100: 1164–1173, 2007. [DOI] [PubMed] [Google Scholar]
- 58.Suarez Y, Sessa WC. MicroRNAs as novel regulators of angiogenesis. Circ Res 104: 442–454, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Suzuki Y, Ito Y, Mizuno M, Kinashi H, Sawai A, Noda Y, Mizuno T, Shimizu H, Fujita Y, Matsui K, Maruyama S, Imai E, Matsuo S, Takei Y. Transforming growth factor-β induces vascular endothelial growth factor-C expression leading to lymphangiogenesis in rat unilateral ureteral obstruction. Kidney Int 81: 865–879, 2012. [DOI] [PubMed] [Google Scholar]
- 60.Tamamura H, Fujii N. The therapeutic potential of CXCR4 antagonists in the treatment of HIV infection, cancer metastasis and rheumatoid arthritis. Expert Opin Ther Targets 9: 1267–1282, 2005. [DOI] [PubMed] [Google Scholar]
- 61.Tsuchiya S, Fujiwara T, Sato F, Shimada Y, Tanaka E, Sakai Y, Shimizu K, Tsujimoto G. MicroRNA-210 regulates cancer cell proliferation through targeting fibroblast growth factor receptor-like 1 (FGFRL1). J Biol Chem 286: 420–428, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Urbich C, Kuehbacher A, Dimmeler S. Role of microRNAs in vascular diseases, inflammation, and angiogenesis. Cardiovasc Res 79: 581–588, 2008. [DOI] [PubMed] [Google Scholar]
- 63.Vass DG, Shrestha B, Haylor J, Hughes J, Marson L. Inflammatory lymphangiogenesis in a rat transplant model of interstitial fibrosis and tubular atrophy. Transplant Int 25: 792–800, 2012. [DOI] [PubMed] [Google Scholar]
- 64.Vigl B, Aebischer D, Nitschke M, Iolyeva M, Rothlin T, Antsiferova O, Halin C. Tissue inflammation modulates gene expression of lymphatic endothelial cells and dendritic cell migration in a stimulus-dependent manner. Blood 118: 205–215, 2011. [DOI] [PubMed] [Google Scholar]
- 65.von der Weid PY, Muthuchamy M. Regulatory mechanisms in lymphatic vessel contraction under normal and inflammatory conditions. Pathophysiology 17: 263–276, 2010. [DOI] [PubMed] [Google Scholar]
- 66.Wang W, Nepiyushchikh Z, Zawieja DC, Chakraborty S, Zawieja SD, Gashev AA, Davis MJ, Muthuchamy M. Inhibition of myosin light chain phosphorylation decreases rat mesenteric lymphatic contractile activity. Am J Physiol Heart Circ Physiol 297: H726–H734, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wu F, Yang Z, Li G. Role of specific microRNAs for endothelial function and angiogenesis. Biochem Biophys Res Commun 386: 549–553, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Xu Y, Yuan L, Mak J, Pardanaud L, Caunt M, Kasman I, Larrivee B, Del Toro R, Suchting S, Medvinsky A, Silva J, Yang J, Thomas JL, Koch AW, Alitalo K, Eichmann A, Bagri A. Neuropilin-2 mediates VEGF-C-induced lymphatic sprouting together with VEGFR3. J Cell Biol 188: 115–130, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yao H, Ma R, Yang L, Hu G, Chen X, Duan M, Kook Y, Niu F, Liao K, Fu M, Hu G, Kolattukudy P, Buch S. MiR-9 promotes microglial activation by targeting MCPIP1. Nat Commun 5: 4386, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yin K, Hacia JG, Zhong Z, Paine ML. Genome-wide analysis of miRNA and mRNA transcriptomes during amelogenesis. BMC Genomics 15: 998, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zgraggen S, Ochsenbein AM, Detmar M. An important role of blood and lymphatic vessels in inflammation and allergy. J Allergy (Cairo) 2013: 672381, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhuang G, Wu X, Jiang Z, Kasman I, Yao J, Guan Y, Oeh J, Modrusan Z, Bais C, Sampath D, Ferrara N. Tumour-secreted miR-9 promotes endothelial cell migration and angiogenesis by activating the JAK-STAT pathway. EMBO J 31: 3513–3523, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhuo W, Jia L, Song N, Lu XA, Ding Y, Wang X, Song X, Fu Y, Luo Y. The CXCL12-CXCR4 chemokine pathway: a novel axis regulates lymphangiogenesis. Clin Cancer Res 18: 5387–5398, 2012. [DOI] [PubMed] [Google Scholar]