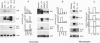Abstract
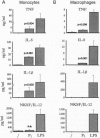
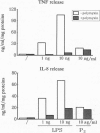
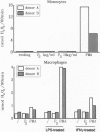
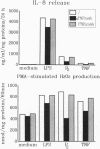
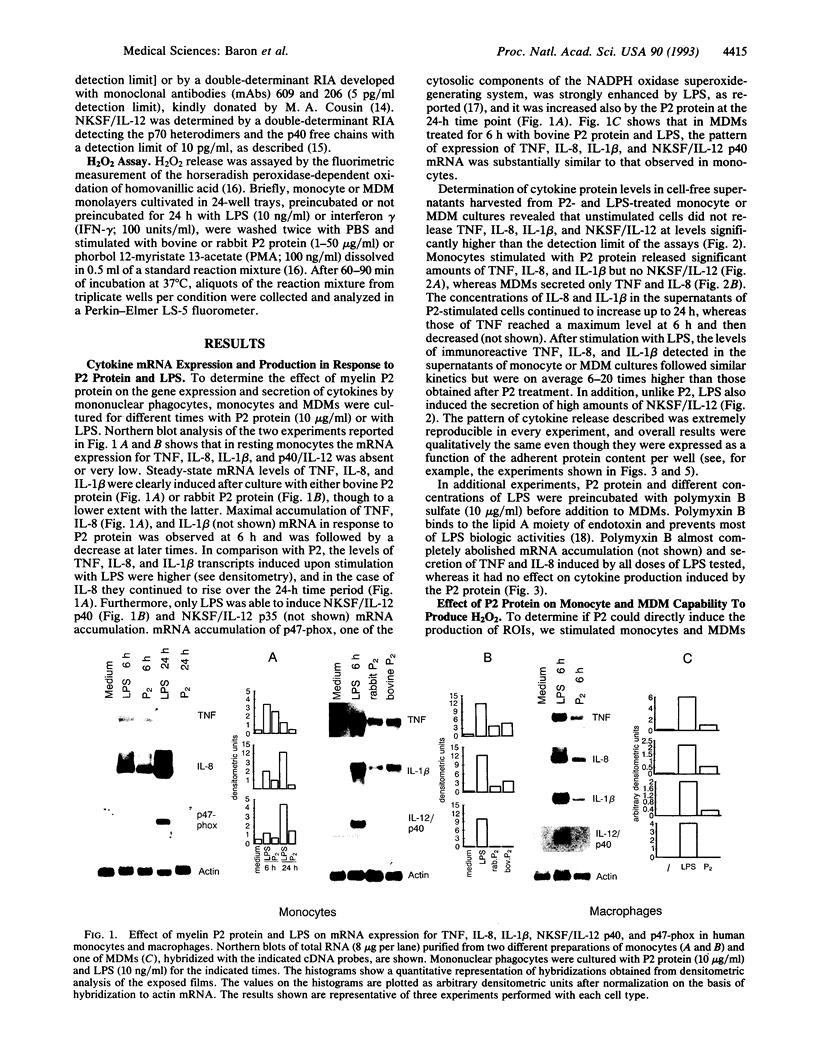
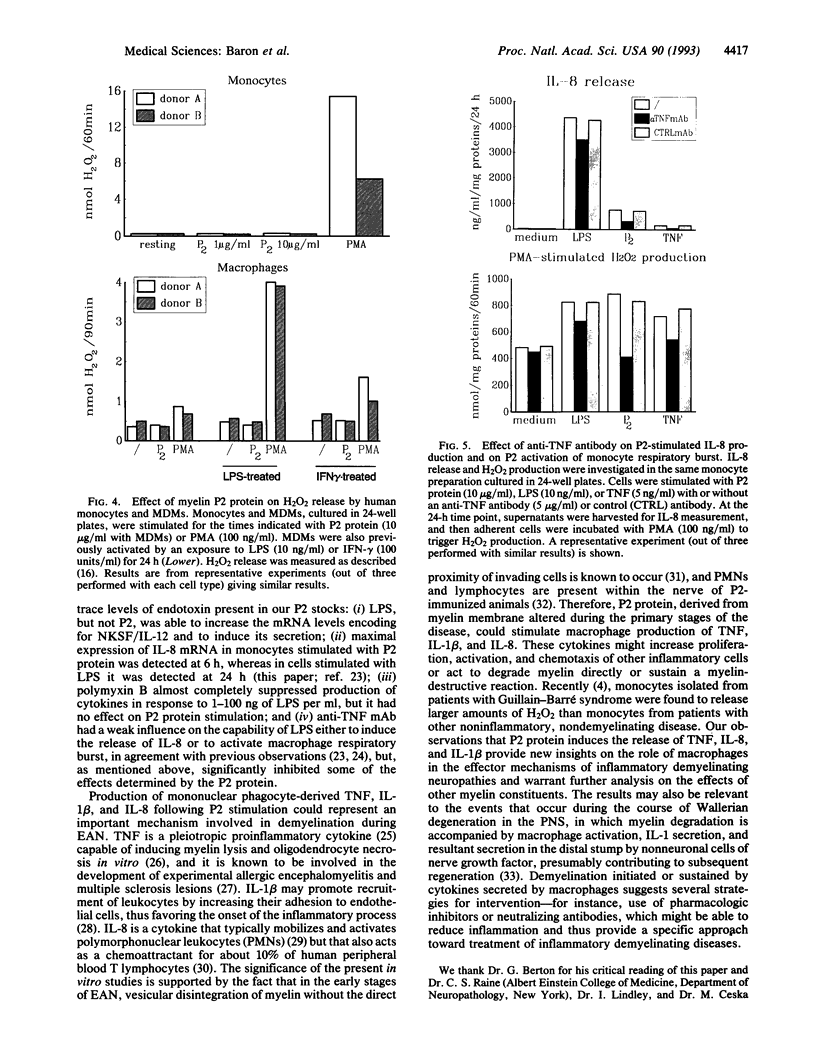
In this study we examined the effect of myelin P2 protein on some proinflammatory functions exerted by human mononuclear phagocytes. Northern blot analysis demonstrated that P2 protein selectively induced in monocytes and macrophages mRNA accumulation of tumor necrosis factor (TNF), interleukin 1 beta (IL-1 beta), and interleukin 8 (IL-8) in a time-dependent manner. Natural killer stimulating factor (IL-12) mRNA and protein secretion was strongly induced by lipopolysaccharide but not by P2 protein. Supernatants harvested from P2-stimulated monocytes contained significant amounts of TNF, IL-1 beta, and IL-8, whereas those from macrophages contained only TNF and IL-8. The effect of the P2 protein on TNF and IL-8 mRNA accumulation and secretion was not affected by polymyxin B, which, on the other hand, almost completely abolished the effect of lipopolysaccharide. Finally, P2 protein did not directly trigger hydrogen peroxide release but, through the induced release of TNF, potentiated monocyte respiratory burst capability. Since P2 protein is the antigen responsible for the induction of experimental allergic neuritis, these findings identify a potential mechanism involved in the inflammatory reaction and myelin damage during experimental allergic neuritis.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baggiolini M., Clark-Lewis I. Interleukin-8, a chemotactic and inflammatory cytokine. FEBS Lett. 1992 Jul 27;307(1):97–101. doi: 10.1016/0014-5793(92)80909-z. [DOI] [PubMed] [Google Scholar]
- Ballin R. H., Thomas P. K. Electron microscope observations on demyelination and remyelination in experimental allergic neuritis. I. Demyelination. J Neurol Sci. 1969 Jan-Feb;8(1):1–18. doi: 10.1016/0022-510x(69)90037-9. [DOI] [PubMed] [Google Scholar]
- Berton G., Dusi S., Serra M. C., Bellavite P., Rossi F. Studies on the NADPH oxidase of phagocytes. Production of a monoclonal antibody which blocks the enzymatic activity of pig neutrophil NADPH oxidase. J Biol Chem. 1989 Apr 5;264(10):5564–5568. [PubMed] [Google Scholar]
- Beutler B., Cerami A. The biology of cachectin/TNF--a primary mediator of the host response. Annu Rev Immunol. 1989;7:625–655. doi: 10.1146/annurev.iy.07.040189.003205. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cassatella M. A., Bazzoni F., Amezaga M. A., Rossi F. Studies on the gene expression of several NADPH oxidase components. Biochem Soc Trans. 1991 Feb;19(1):63–67. doi: 10.1042/bst0190063. [DOI] [PubMed] [Google Scholar]
- Cassatella M. A., Bazzoni F., Flynn R. M., Dusi S., Trinchieri G., Rossi F. Molecular basis of interferon-gamma and lipopolysaccharide enhancement of phagocyte respiratory burst capability. Studies on the gene expression of several NADPH oxidase components. J Biol Chem. 1990 Nov 25;265(33):20241–20246. [PubMed] [Google Scholar]
- Cassatella M. A., Hartman L., Perussia B., Trinchieri G. Tumor necrosis factor and immune interferon synergistically induce cytochrome b-245 heavy-chain gene expression and nicotinamide-adenine dinucleotide phosphate hydrogenase oxidase in human leukemic myeloid cells. J Clin Invest. 1989 May;83(5):1570–1579. doi: 10.1172/JCI114054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Andrea A., Rengaraju M., Valiante N. M., Chehimi J., Kubin M., Aste M., Chan S. H., Kobayashi M., Young D., Nickbarg E. Production of natural killer cell stimulatory factor (interleukin 12) by peripheral blood mononuclear cells. J Exp Med. 1992 Nov 1;176(5):1387–1398. doi: 10.1084/jem.176.5.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeForge L. E., Kenney J. S., Jones M. L., Warren J. S., Remick D. G. Biphasic production of IL-8 in lipopolysaccharide (LPS)-stimulated human whole blood. Separation of LPS- and cytokine-stimulated components using anti-tumor necrosis factor and anti-IL-1 antibodies. J Immunol. 1992 Apr 1;148(7):2133–2141. [PubMed] [Google Scholar]
- Dinarello C. A. Biology of interleukin 1. FASEB J. 1988 Feb;2(2):108–115. [PubMed] [Google Scholar]
- Fontaine S., Damais C., Lando D., Cousin M. A. Monoclonal antibodies to human interleukin-1 beta and their use in a sensitive two-site enzyme linked immunosorbent assay. Lymphokine Res. 1989 Summer;8(2):129–139. [PubMed] [Google Scholar]
- Hartung H. P., Schäfer B., Heininger K., Stoll G., Toyka K. V. The role of macrophages and eicosanoids in the pathogenesis of experimental allergic neuritis. Serial clinical, electrophysiological, biochemical and morphological observations. Brain. 1988 Oct;111(Pt 5):1039–1059. doi: 10.1093/brain/111.5.1039. [DOI] [PubMed] [Google Scholar]
- Hartung H. P., Schäfer B., Heininger K., Toyka K. V. Suppression of experimental autoimmune neuritis by the oxygen radical scavengers superoxide dismutase and catalase. Ann Neurol. 1988 May;23(5):453–460. doi: 10.1002/ana.410230505. [DOI] [PubMed] [Google Scholar]
- Kadlubowski M., Hughes R. A. Identification of the neuritogen for experimental allergic neuritis. Nature. 1979 Jan 11;277(5692):140–141. doi: 10.1038/277140a0. [DOI] [PubMed] [Google Scholar]
- Lampert P. W. Mechanism of demyelination in experimental allergic neuritis. Electron microscopic studies. Lab Invest. 1969 Feb;20(2):127–138. [PubMed] [Google Scholar]
- Larsen C. G., Anderson A. O., Appella E., Oppenheim J. J., Matsushima K. The neutrophil-activating protein (NAP-1) is also chemotactic for T lymphocytes. Science. 1989 Mar 17;243(4897):1464–1466. doi: 10.1126/science.2648569. [DOI] [PubMed] [Google Scholar]
- Levy R., Malech H. L. Effect of 1,25-dihydroxyvitamin D3, lipopolysaccharide, or lipoteichoic acid on the expression of NADPH oxidase components in cultured human monocytes. J Immunol. 1991 Nov 1;147(9):3066–3071. [PubMed] [Google Scholar]
- Lindholm D., Heumann R., Meyer M., Thoenen H. Interleukin-1 regulates synthesis of nerve growth factor in non-neuronal cells of rat sciatic nerve. Nature. 1987 Dec 17;330(6149):658–659. doi: 10.1038/330658a0. [DOI] [PubMed] [Google Scholar]
- Matsushima K., Morishita K., Yoshimura T., Lavu S., Kobayashi Y., Lew W., Appella E., Kung H. F., Leonard E. J., Oppenheim J. J. Molecular cloning of a human monocyte-derived neutrophil chemotactic factor (MDNCF) and the induction of MDNCF mRNA by interleukin 1 and tumor necrosis factor. J Exp Med. 1988 Jun 1;167(6):1883–1893. doi: 10.1084/jem.167.6.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison D. C., Jacobs D. M. Binding of polymyxin B to the lipid A portion of bacterial lipopolysaccharides. Immunochemistry. 1976 Oct;13(10):813–818. doi: 10.1016/0019-2791(76)90181-6. [DOI] [PubMed] [Google Scholar]
- Perussia B., Chan S. H., D'Andrea A., Tsuji K., Santoli D., Pospisil M., Young D., Wolf S. F., Trinchieri G. Natural killer (NK) cell stimulatory factor or IL-12 has differential effects on the proliferation of TCR-alpha beta+, TCR-gamma delta+ T lymphocytes, and NK cells. J Immunol. 1992 Dec 1;149(11):3495–3502. [PubMed] [Google Scholar]
- Ponzin D., Menegus A. M., Kirschner G., Nunzi M. G., Fiori M. G., Raine C. S. Effects of gangliosides on the expression of autoimmune demyelination in the peripheral nervous system. Ann Neurol. 1991 Nov;30(5):678–685. doi: 10.1002/ana.410300508. [DOI] [PubMed] [Google Scholar]
- Ropper A. H. The Guillain-Barré syndrome. N Engl J Med. 1992 Apr 23;326(17):1130–1136. doi: 10.1056/NEJM199204233261706. [DOI] [PubMed] [Google Scholar]
- Rosen J. L., Brown M. J., Hickey W. F., Rostami A. Early myelin lesions in experimental allergic neuritis. Muscle Nerve. 1990 Jul;13(7):629–636. doi: 10.1002/mus.880130712. [DOI] [PubMed] [Google Scholar]
- Selmaj K. W., Raine C. S. Tumor necrosis factor mediates myelin and oligodendrocyte damage in vitro. Ann Neurol. 1988 Apr;23(4):339–346. doi: 10.1002/ana.410230405. [DOI] [PubMed] [Google Scholar]
- Shannon K. Genetic alterations in leukemia: events on a grand scale. Blood. 1992 Jul 1;80(1):1–2. [PubMed] [Google Scholar]
- Szefler S. J., Norton C. E., Ball B., Gross J. M., Aida Y., Pabst M. J. IFN-gamma and LPS overcome glucocorticoid inhibition of priming for superoxide release in human monocytes. Evidence that secretion of IL-1 and tumor necrosis factor-alpha is not essential for monocyte priming. J Immunol. 1989 Jun 1;142(11):3985–3992. [PubMed] [Google Scholar]
- Valletta E. A., Berton G. Desensitization of macrophage oxygen metabolism on immobilized ligands: different effect of immunoglobulin G and complement. J Immunol. 1987 Jun 15;138(12):4366–4373. [PubMed] [Google Scholar]