Abstract
Nonhemolytic variants of Haemophilus haemolyticus are difficult to differentiate from Haemophilus influenzae despite a wide difference in pathogenic potential. A previous investigation characterized a challenging set of 60 clinical strains using multiple PCRs for marker genes and described strains that could not be unequivocally identified as either species. We have analyzed the same set of strains by multilocus sequence analysis (MLSA) and near-full-length 16S rRNA gene sequencing. MLSA unambiguously allocated all study strains to either of the two species, while identification by 16S rRNA sequence was inconclusive for three strains. Notably, the two methods yielded conflicting identifications for two strains. Most of the “fuzzy species” strains were identified as H. influenzae that had undergone complete deletion of the fucose operon. Such strains, which are untypeable by the H. influenzae multilocus sequence type (MLST) scheme, have sporadically been reported and predominantly belong to a single branch of H. influenzae MLSA phylogenetic group II. We also found evidence of interspecies recombination between H. influenzae and H. haemolyticus within the 16S rRNA genes. Establishing an accurate method for rapid and inexpensive identification of H. influenzae is important for disease surveillance and treatment.
INTRODUCTION
Haemophilus influenzae is an important human pathogen involved in respiratory tract infections, such as sinusitis, acute otitis media, pneumonia, and exacerbations in chronic obstructive pulmonary disease (1–5). The majority of these infections are caused by unencapsulated H. influenzae, traditionally designated nontypeable H. influenzae (NTHi). Haemophilus haemolyticus is a close relative of H. influenzae, and the two species colonize the upper respiratory tract of humans. Although H. haemolyticus has, on rare occasion, been isolated from invasive infections (6), several lines of evidence indicate that the pathogenicity of H. haemolyticus is much reduced compared with H. influenzae. While up to 20% of presumptive H. influenzae nasopharyngeal isolates can be identified as H. haemolyticus by molecular characterization (7–9), H. haemolyticus is rarely cultured from middle ear fluid (10, 11), supporting the view that H. haemolyticus, in contrast to NTHi, is a respiratory commensal infrequently associated with otitis media. Reinvestigation of presumptive H. influenzae isolates, cultured from lower respiratory tract samples from cystic fibrosis patients (12) or from unselected clinical samples submitted to the laboratory on suspicion of lower respiratory tract infection (13), detected that <1% were misidentified strains, further supporting a minor pathogenic role for H. haemolyticus.
H. haemolyticus was traditionally identified by its hemolytic action on erythrocytes, but it has become clear that a large proportion of H. haemolyticus strains is nonhemolytic (10, 14). Such strains, which are designated as nonhemolytic H. haemolyticus, can only be differentiated from H. influenzae by DNA sequencing or extended phenotypic testing (15, 16). Although this may be a minor problem when strains are isolated from infections, it constitutes a major problem in colonization and surveillance studies.
The most reliable delineation of H. influenzae from H. haemolyticus is based on the comparison of near-full-length 16S rRNA gene sequences (17) or concatenated sequences of housekeeping gene fragments (16, 18). However, these methods are labor intensive and too expensive for routine use. A number of studies have, therefore, attempted to identify and evaluate suitable assays for rapid and inexpensive identification of H. influenzae (13, 17–23). Recently, an evaluation of 9 PCR screening assays was performed on a challenging sample of 60 nasopharyngeal carriage isolates selected to include approximately equal numbers of NTHi, H. haemolyticus, and equivocal strains (23). A drawback of the study was that the species cutoff was selected arbitrarily, while a strict definition based on partial recA and 16S rRNA gene sequences (1,142 nucleotides [nt] combined) left 11 isolates unidentified (“fuzzy species”); thus, the true performance of the PCR assays could not be calculated. In the present study, we have reinvestigated the same set of strains by near-full-length sequencing of 16S rRNA genes and multilocus sequence typing (MLST) of H. influenzae in order to unambiguously identify the isolates to species level. Unexpectedly, a significant proportion of the previously unidentified isolates were H. influenzae that had undergone complete deletion of the fucose operon.
MATERIALS AND METHODS
Bacterial strains.
The 60 strains (H01 through H60) included in the study by Binks et al. (23) were selected from a larger study investigating nasopharyngeal carriage isolates collected from otitis-prone and healthy children aged 6 to 36 months (7, 24). A total of 266 Haemophilus isolates were collected and subjected to an initial PCR screen of the 16S rRNA gene using the two primer sets that have been described as specific for either H. haemolyticus or H. influenzae (10). Twenty isolates identified as H. influenzae, 20 isolates identified as H. haemolyticus, and 20 isolates with equivocal results (bands in both or none of the PCRs or additional or aberrant bands) were then selected for further study. When the dual 16S rRNA gene PCR was repeated for the 60 strains in a different laboratory, 22 were H. influenzae, 27 were H. haemolyticus, and 11 were categorized as equivocal (23).
The bacterial strains had been propagated in several laboratories prior to the present investigation. To ascertain the identity of the strains for this study, partial recA fragments were sequenced and compared with deposited sequences for these strains (23). For 6 strains, the identity could not be confirmed, and these strains were excluded from the present study (H01, fuzzy species; H08, H. haemolyticus; H09, H. haemolyticus; H22, H. influenzae, H38, Haemophilus parainfluenzae; and H50, H. haemolyticus) (categorization according to the former study [23]).
DNA sequencing.
Identification of polymorphic nucleotide positions and 16S rRNA gene sequencing were carried out as previously described (25). A 1,362-nt fragment corresponding to nucleotides 27 to 1,388 of the 16S rRNA gene in H. influenzae strain Rd was obtained from all 54 strains and used for analysis.
Fragments of the housekeeping genes adk, atpG, frdB, fucK, mdh, pgi, and recA were amplified as recommended (www.mlst.net). In the case of weak or absent bands, PCR amplification was repeated with an annealing temperature of 40°C. The fucK fragment could not be amplified from 33 of 54 strains. The remaining 6 housekeeping gene fragments were sequenced, trimmed to appropriate lengths, concatenated into a hybrid sequence of 2,712 nt, and used for multilocus sequence analysis (MLSA).
Phylogenetic reconstruction.
The 16S rRNA fragments of the 54 study isolates were compared with sequences from the type strain and 75 other reference strains of H. influenzae as well as the type strain and 35 other reference strains of H. haemolyticus (including cryptic genospecies biotype IV and other variant strains excluded from H. influenzae) (18, 26, 27). Of 166 16S rRNA sequences, 158 were unique.
The complete H. influenzae database with 1,294 sequence types was downloaded from the H. influenzae MLST website (http://pubmlst.org/hinfluenzae/) on 29 January 2014. After removal of fucK fragments and the addition of sequences from 54 study strains, the type strain, 15 other reference strains of H. influenzae, and the type strain and 35 other reference strains of H. haemolyticus (18, 27, 28), 1,268 sequences were unique.
DNA sequences were edited, assembled, and aligned using CLC Main Workbench 7 (CLC bio, Aarhus, Denmark). Phylogenetic and molecular evolutionary analyses were conducted using MEGA version 5 (29) and the neighbor-joining method. Bootstrap tests were performed with 500 (16S) or 200 (MLSA) replications.
Fucose operon analysis.
In H. influenzae, a fucose operon of approximately 10 kb is located between folD and hepA (30), while no open reading frames separate folD and hepA in the available genomes of H. haemolyticus (27). The flanking primers HI_0608_1002f and HI_0616_1708r (30) failed to amplify the region in several study strains, and new flanking primers were designed: folD.101Ra (GGTGCACGTTTACCTTG), folD.101Rb (GGAGCGCGTTTTCCTTG), hepA.53Ra (CCTAAAGCGTTTTCACTTTCAC), and hepA.53Rb (CCTAAAGTATTCTCACTTTCAC). The fucose region was amplified from all study strains by PCR (35 cycles consisting of 94°C for 15 s, 55°C for 15 s, and 68°C for 10 min) using a Kapa Long Range HotStart ReadyMix (Kapa Biosystems Ltd., London, United Kingdom) and 0.25 μM each of the four primers. PCRs targeting the other individual genes of the fucose operon, fucP, fucA, fucU, fucK, fucI, and fucR, were performed as previously described (30).
Supplementary PCR assays.
A fragment of the copper/zinc-cofactored superoxide dismutase (CuZnSOD) gene sodC was amplified by PCR using primers sodC.253f (CCAAGCTGTGATCCAAAAG) and sodC.523r (CAAGTGGAGCTGGATGATC) (numbering with reference to the position in the gene in H. parainfluenzae strain T3T1); the primers display one mismatch with the gene in H. haemolyticus and with deposited sequences of the pseudogene that is present in some strains of H. influenzae (31).
PCR screen for the capsule locus of H. influenzae was performed by amplification of a 760-bp fragment of bexB using primers bexB.FLF and bexB.FLR as previously described (32). H. influenzae serotype e was documented by PCR amplification using primers bexD.128R (GAAGCATCAGCACCTTGGTT) located in bexD and Hie_ecs1.366R (CGTGCGCAAACCAGCTTCAA) located in the serotype e-specific gene ecs1, encoding a putative UDP-N-acetyl-d-glucosamine 2-epimerase (33). The PCR amplifies an 895-bp fragment from H. influenzae serotype e strains; the Hie reference strain PN125 (GenBank accession number FJ939590) was used as a positive control, and the unencapsulated Haemophilus aegyptius strain NCTC 8502T was used as a negative control. PCR products were separated by 1% agarose gel electrophoresis.
RESULTS
Identification based on comparison of 16S rRNA gene sequences.
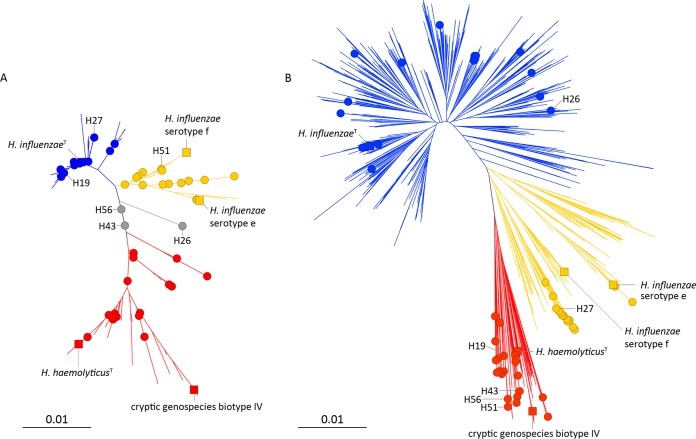
Figure 1A shows a neighbor-joining dendrogram of near-full-length (1,362 nt) 16S rRNA gene sequences comparing 54 study strains with 76 reference strains of H. influenzae and 36 reference strains of H. haemolyticus (18, 26, 27). Three major clusters can be discerned in the dendrogram: H. influenzae 16S rRNA phylogenetic group I (blue), which includes the type strain of the species, 62 other H. influenzae reference strains, and 20 study strains; H. influenzae 16S rRNA phylogenetic group II (yellow), which includes 13 H. influenzae reference strains of serotype e and f as well as other distantly related lineages and 15 study strains; and the H. haemolyticus cluster (red), which includes the type strain of the species, 35 other H. haemolyticus reference strains, and 16 study strains. Three study strains, H26, H43, and H56, take up an intermediary position in the dendrogram (gray).
FIG 1.
(A) Neighbor-joining dendrogram comparing 16S rRNA gene sequences (1,362 nucleotides) of 54 study strains (filled circles) with those of 76 reference strains of H. influenzae and 36 reference strains of H. haemolyticus and related organisms. (B) Neighbor-joining dendrogram based on concatenated sequence fragments of housekeeping genes adk, atpG, frdB, mdh, pgi, and recA (2,712 nt) of 54 study strains (filled circles) compared with 1,294 sequences from the H. influenzae MLST database and 16 additional reference strains of H. influenzae and 36 reference strains of H. haemolyticus and related organisms. H. influenzae phylogenetic group I, blue; H. influenzae phylogenetic group II, yellow; H. haemolyticus, red. Selected reference strains (solid squares): H. influenzae type strain NCTC 8143, H. influenzae serotype e, strain PN125; H. influenzae serotype f, strain HK2067; Cryptic genospecies biotype IV, strain S32F2; H. haemolyticus type strain, NCTC 10659T. Selected study strains are indicated and referred to in the text. Bars indicate 1 nt substitution per 100 residues.
The 16S rRNA gene sequences of 83 strains in H. influenzae phylogenetic group I were similar with a maximal divergence of 1.8%. The cluster was supported by a high bootstrap value of 74% (not shown). More variation was observed for 16S rRNA gene sequences of 28 strains of H. influenzae phylogenetic group II (maximal divergence of 2.4%) and 52 strains of the H. haemolyticus cluster (maximal divergence of 3.5%). The H. influenzae 16S rRNA phylogenetic group II cluster was supported by a modest bootstrap value of 7%, while the H. haemolyticus cluster was divided into several subclusters (Fig. 1A).
Polymorphic nucleotide positions due to intragenomic 16S rRNA gene heterogeneity of the multiple RNA operons were observed in 48/54 study strains. The average frequencies of 16S rRNA gene polymorphic nucleotide positions were 0.26% for 20 study strains of H. influenzae 16S rRNA phylogenetic group I, 0.74% for 15 study strains of H. influenzae 16S rRNA phylogenetic group II, and 1.44% for 16 study strains of the H. haemolyticus 16S rRNA cluster. Of the unclustered strains, H43 and H56 were characterized by a high divergence among their 16S rRNA gene copies (>4% polymorphic sites), and the position in the dendrogram indicates a mixture of H. influenzae and H. haemolyticus 16S rRNA genes in the genomes of these two strains. The 16S rRNA genes of strain H26 contained no polymorphic positions, and the sequence was unique; a BLAST search in GenBank showed a maximum of 1,347/1,362 identities with reference strains of H. influenzae and 1,344/1,364 identities with reference strains of H. haemolyticus.
Identification based on MLSA.
Fifty-four study strains were subjected to MLSA using the H. influenzae MLST scheme. Thirty-three strains were negative for fucK, which is typical for H. haemolyticus and related organisms excluded from H. influenzae (17, 18). The 6 other housekeeping gene fragments (adk, atpG, frdB, mdh, pgi, and recA) were concatenated into hybrid sequences of 2,712 nt. Figure 1B shows a neighbor-joining dendrogram comparing the 54 study strains with 1,294 sequence types downloaded from the MLST database and the type strains of H. influenzae and H. haemolyticus and 50 additional reference strains (18, 27, 28). An H. haemolyticus cluster (red) was clearly separate from the rest of the strains and was supported by a high bootstrap value of 86% (not shown). All of the H. haemolyticus reference strains were located in this cluster with 7 sequence types from the MLST database and 20 study strains. These 20 study strains were all negative for the fucK allele.
With an increasing number of sequence types deposited in the MLST database, it has become difficult to make a clear distinction between phylogenetic groups I and II for unencapsulated isolates (15). The continuum of H. influenzae MLSA sequences is also apparent from Fig. 1B. Annotation of phylogenetic groups in Fig. 1B is based on the location of capsulated strains, with serotype e and f strains invariably located in phylogenetic group II.
Nineteen study strains belonged to the H. influenzae MLSA phylogenetic group I cluster (blue). These strains were located on many separate branches and appear to be a random sample of typical NTHi. Fifteen study strains were located on 2 branches in the H. influenzae MLSA phylogenetic group II cluster (yellow). Two strains, H20 and H29, were almost identical with H. influenzae serotype e reference strains and were indeed shown to be serotype e as revealed by positive PCRs for capsule export protein B (bexB) and serotype e-specific epimerase genes. Sequences from 13 other strains were closely related but not identical; these 13 strains were all negative for fucK. In the MLSA tree, they were located on a branch with 9 sequence types from the MLST database (and thus positive for fucK). The clustering of 13 of 15 phylogenetic group II study strains on a single branch validates that the strains investigated are a nonrandom sample of H. influenzae isolates.
Conflicting identifications by 16S rRNA comparison versus housekeeping gene analysis.
Three study strains with ambiguous identification by 16S rRNA gene analysis (H26, H43, and H56) were clearly resolved by MLSA. Strain H26, which carried identical copies of a unique 16S rRNA gene, belonged to the core of H. influenzae by MLSA. Strains H43 and H56, with 56 and 55 polymorphic positions in their 16S rRNA genes, respectively, were clearly located in the H. haemolyticus cluster by MLSA. Furthermore, conflicting identification using the 2 methods was obtained for 3 other strains (H19, H27, and H51). H19 belonged to H. influenzae phylogenetic group I by 16S rRNA and to H. haemolyticus by MLSA, H27 belonged to H. influenzae 16S rRNA phylogenetic group I but to H. influenzae phylogenetic group II by MLSA, and H51 belonged to H. influenzae phylogenetic group II by 16S rRNA and to H. haemolyticus by MLSA (Fig. 1A and B).
With respect to the decisive distinction between H. influenzae and H. haemolyticus, the distribution of marker genes hpd, iga, and sodC in the 6 strains supported the identification obtained by MLSA (the expected genotype of H. influenzae was positive for hpd and iga and negative for sodC; the expected genotype of H. haemolyticus was negative for hpd and iga and positive for sodC).
Complete deletion of the fucose operon.
In H. influenzae, a fucose operon of approximately 10 kb is located between the folate dehydrogenase folD and the ATP-dependent helicase hepA (30), while no open reading frames separate the genes in the available genomes of H. haemolyticus (27). We used a long range PCR to amplify the entire fucose operon in the 54 study strains. All 21 fucK-positive strains (19 MLSA phylogenetic group I strains and 2 H. influenzae serotype e strains) gave rise to amplicons of approximately 10 kb, while all 33 fucK-negative strains (13 strains from a single branch in MLSA phylogenetic group II and 20 H. haemolyticus) gave rise to amplicons of approximately 700 nt (not shown).
We also performed PCR targeting the other genes of the fucose operon, fucP, fucA, fucU, fucI, and fucR. Products of the expected size were obtained with all fucK-positive study strains, while all fucK-negative strains were negative for all genes of the fucose operon. Thus, the fucose operon was not translocated to other parts of the genome by genomic rearrangements in fucK-negative strains.
Assessment of PCR assays for delineation of H. influenzae.
Unambiguous allocation to species level by MLSA was used to calculate the true performance of PCR screening results previously reported for the study collection (23). The best discrimination of H. influenzae from H. haemolyticus was obtained with hpd 1, hpd 3, and iga, which were positive for 32 of 34 H. influenzae strains and negative for 20 of 20 H. haemolyticus strains (Table 1).
TABLE 1.
Genotypes of 54 Haemophilus carriage isolates defined by PCR assays
| Speciesa | No. (%) of isolates positive for: |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| fucKb | sodCb | ompP2c | ompP6c | ompP6 (hrm)c | lgtCc | hpd 1c | hpd 3c | igac | |
| H. influenzae | 21 (62) | 9 (27) | 27 (79) | 34 (100) | 34 (100) | 28 (82) | 32 (94) | 32 (94) | 32 (94) |
| H. haemolyticus | 0 (0) | 20 (100) | 2 (10) | 7 (35) | 6 (30) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
As defined be MLSA (H. influenzae, n = 34; H. haemolyticus, n = 20).
This study.
PCR results from reference 23.
DISCUSSION
It has become clear that widely used identification methods are not able to differentiate strains of H. influenzae reliably from H. haemolyticus. This has important implications for the clinical laboratory because accurate species identification is paramount for treatment and disease surveillance.
Several recent studies have explored the use of PCR screens for the distinction of these species (13, 18–23). The major challenge in the evaluation of these studies is the lack of a universal delineation of H. influenzae. Various collections of strains have been investigated, and different tests are used to define H. influenzae; thus, isolates that are included in the species in one investigation may be excluded from the species in another. We subjected a challenging collection of carriage strains to MLSA and 16S rRNA gene sequencing in order to clarify the distribution of marker genes in borderline or fuzzy strains. Unambiguous identification to species level enabled accurate evaluation of PCR assays and confirmed the superiority of PCR assays hpd 1, hpd 3, and iga. However, the characterization also revealed a selected study collection, with 13 of 34 H. influenzae strains located on a particular branch in H. influenzae MLSA phylogenetic group II. The entire fucose operon consisting of 6 genes was absent from the chromosome of these 13 strains. Fermentation of fucose is considered a stable trait of H. influenzae, and the fucK gene is part of the MLST scheme (34). Consequently, strains lacking fucK cannot be assigned a sequence type by the MLST scheme. fucK-negative strains of H. influenzae appear to be rare, and prior to this study, only 17 such isolates were reported (12, 28, 30). Interestingly, 13 of the 17 previously reported isolates are located on the same branch in phylogenetic group II as the 13 isolates from the present study. Given the biased nature of the present study sample, the performance of the PCR assays will differ when applied to other collections. We found 32 of 34 H. influenzae strains to be positive for the hpd gene, which is similar to other studies (17, 35). The absence of hpd in strains of H. influenzae has recently been confirmed by whole-genome sequencing (36).
Unexpectedly, conflicting identifications were obtained for 2 of 54 study strains. These strains clustered with reference strains of H. influenzae by 16S rRNA sequencing and with H. haemolyticus by MLSA. The presence of the H. haemolyticus marker gene sodC and the absence of the H. influenzae marker genes hpd and iga supported the identification arising from MLSA. Thus, the strains contain 16S rRNA gene copies representative of H. influenzae while 9 protein coding genes, located on separate regions of the chromosome, are compatible with H. haemolyticus. We hypothesize that interspecies transfer of 16S rRNA genes has resulted in the development of such hybrid genomes. A mixture of H. influenzae and H. haemolyticus 16S rRNA genes in the genomes of strains H43 and H56 is indicated by their position in the dendrogram (Fig. 1A), and such mixtures have previously been documented for other strains of H. haemolyticus by sequencing of individual RNA operons (25). However, an exclusive presence of 16S rRNA genes from a different species, to our knowledge, has not been reported before; if corroborated, this would constitute a significant challenge to current hierarchic taxonomy that depends on 16S rRNA comparisons (37).
In conclusion, the present investigation underscores the limitations of marker genes for unambiguous separation of H. influenzae and H. haemolyticus. Recombination between the two species may not be rare (38, 39) and may even involve rRNA genes. Comparative whole-genome analysis of H. influenzae and H. haemolyticus is warranted to elucidate the genomic differences between the two species (36, 40). Accurate species identification may necessitate the use of MLSA or at least detection of multiple marker genes.
REFERENCES
- 1.Agrawal A, Murphy TF. 2011. Haemophilus influenzae infections in the H. influenzae type b conjugate vaccine era. J Clin Microbiol 49:3728–3732. doi: 10.1128/JCM.05476-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brook I, Foote PA, Hausfeld JN. 2006. Frequency of recovery of pathogens causing acute maxillary sinusitis in adults before and after introduction of vaccination of children with the 7-valent pneumococcal vaccine. J Med Microbiol 55:943–946. doi: 10.1099/jmm.0.46346-0. [DOI] [PubMed] [Google Scholar]
- 3.Coker TR, Chan LS, Newberry SJ, Limbos MA, Suttorp MJ, Shekelle PG, Takata GS. 2010. Diagnosis, microbial epidemiology, and antibiotic treatment of acute otitis media in children: a systematic review. JAMA 304:2161–2169. doi: 10.1001/jama.2010.1651. [DOI] [PubMed] [Google Scholar]
- 4.Sethi S, Murphy TF. 2008. Infection in the pathogenesis and course of chronic obstructive pulmonary disease. N Engl J Med 359:2355–2365. doi: 10.1056/NEJMra0800353. [DOI] [PubMed] [Google Scholar]
- 5.Leibovitz E, Jacobs MR, Dagan R. 2004. Haemophilus influenzae: a significant pathogen in acute otitis media. Pediatr Infect Dis J 23:1142–1152. [PubMed] [Google Scholar]
- 6.Anderson R, Wang X, Briere EC, Katz LS, Cohn AC, Clark TA, Messonnier NE, Mayer LW. 2012. Haemophilus haemolyticus isolates causing clinical disease. J Clin Microbiol 50:2462–2465. doi: 10.1128/JCM.06575-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kirkham LA, Wiertsema SP, Mowe EN, Bowman JM, Riley TV, Richmond PC. 2010. Nasopharyngeal carriage of Haemophilus haemolyticus in otitis-prone and healthy children. J Clin Microbiol 48:2557–2559. doi: 10.1128/JCM.00069-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pickering J, Smith-Vaughan H, Beissbarth J, Bowman JM, Wiertsema S, Riley TV, Leach AJ, Richmond P, Lehmann D, Kirkham LA. 2014. Diversity of nontypeable Haemophilus influenzae strains colonizing Australian Aboriginal and non-Aboriginal children. J Clin Microbiol 52:1352–1357. doi: 10.1128/JCM.03448-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hare KM, Binks MJ, Grimwood K, Chang AB, Leach AJ, Smith-Vaughan H. 2012. Culture and PCR detection of Haemophilus influenzae and Haemophilus haemolyticus in Australian Indigenous children with bronchiectasis. J Clin Microbiol 50:2444–2445. doi: 10.1128/JCM.00566-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murphy TF, Brauer AL, Sethi S, Kilian M, Cai X, Lesse AJ. 2007. Haemophilus haemolyticus: a human respiratory tract commensal to be distinguished from Haemophilus influenzae. J Infect Dis 195:81–89. doi: 10.1086/509824. [DOI] [PubMed] [Google Scholar]
- 11.Hariadi NI, Zhang L, Patel M, Sandstedt SA, Davis GS, Marrs CF, Gilsdorf JR. 2015. Comparative profile of heme acquisition genes in disease-causing and colonizing nontypeable Haemophilus influenzae and Haemophilus haemolyticus. J Clin Microbiol 53: 2132–2137. doi: 10.1128/JCM.00345-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fenger MG, Ridderberg W, Olesen HV, Norskov-Lauritsen N. 2012. Low occurrence of ‘non-haemolytic Haemophilus haemolyticus’ misidentified as Haemophilus influenzae in cystic fibrosis respiratory specimens, and frequent recurrence of persistent H. influenzae clones despite antimicrobial treatment. Int J Med Microbiol 302:315–319. doi: 10.1016/j.ijmm.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 13.Norskov-Lauritsen N. 2009. Detection of cryptic genospecies misidentified as Haemophilus influenzae in routine clinical samples by assessment of marker genes fucK, hap, and sodC. J Clin Microbiol 47:2590–2592. doi: 10.1128/JCM.00013-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mukundan D, Ecevit Z, Patel M, Marrs CF, Gilsdorf JR. 2007. Pharyngeal colonization dynamics of Haemophilus influenzae and Haemophilus haemolyticus in healthy adult carriers. J Clin Microbiol 45:3207–3217. doi: 10.1128/JCM.00492-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Norskov-Lauritsen N. 2014. Classification, identification, and clinical significance of Haemophilus and Aggregatibacter species with host specificity for humans. Clin Microbiol Rev 27:214–240. doi: 10.1128/CMR.00103-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McCrea KW, Xie J, LaCross N, Patel M, Mukundan D, Murphy TF, Marrs CF, Gilsdorf JR. 2008. Relationships of nontypeable Haemophilus influenzae strains to hemolytic and nonhemolytic Haemophilus haemolyticus strains. J Clin Microbiol 46:406–416. doi: 10.1128/JCM.01832-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Theodore MJ, Anderson RD, Wang X, Katz LS, Vuong JT, Bell ME, Juni BA, Lowther SA, Lynfield R, MacNeil JR, Mayer LW. 2012. Evaluation of new biomarker genes for differentiating Haemophilus influenzae from Haemophilus haemolyticus. J Clin Microbiol 50:1422–1424. doi: 10.1128/JCM.06702-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Norskov-Lauritsen N, Overballe MD, Kilian M. 2009. Delineation of the species Haemophilus influenzae by phenotype, multilocus sequence phylogeny, and detection of marker genes. J Bacteriol 191:822–831. doi: 10.1128/JB.00782-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McCrea KW, Xie J, Marrs CF, Gilsdorf JR. 2010. Prevalence of genetic differences in phosphorylcholine expression between nontypeable Haemophilus influenzae and Haemophilus haemolyticus. BMC Microbiol 10:286. doi: 10.1186/1471-2180-10-286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pickering J, Binks MJ, Beissbarth J, Hare KM, Kirkham LA, Smith-Vaughan H. 2014. A PCR-high-resolution melt assay for rapid differentiation of nontypeable Haemophilus influenzae and Haemophilus haemolyticus. J Clin Microbiol 52:663–667. doi: 10.1128/JCM.02191-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pickering J, Richmond PC, Kirkham LA. 2014. Molecular tools for differentiation of non-typeable Haemophilus influenzae from Haemophilus haemolyticus. Front Microbiol 5:664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fung WW, O'Dwyer CA, Sinha S, Brauer AL, Murphy TF, Kroll JS, Langford PR. 2006. Presence of copper- and zinc-containing superoxide dismutase in commensal Haemophilus haemolyticus isolates can be used as a marker to discriminate them from nontypeable H. influenzae isolates. J Clin Microbiol 44:4222–4226. doi: 10.1128/JCM.01376-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Binks MJ, Temple B, Kirkham LA, Wiertsema SP, Dunne EM, Richmond PC, Marsh RL, Leach AJ, Smith-Vaughan HC. 2012. Molecular surveillance of true nontypeable Haemophilus influenzae: an evaluation of PCR screening assays. PLoS One 7:e34083. doi: 10.1371/journal.pone.0034083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wiertsema SP, Kirkham LA, Corscadden KJ, Mowe EN, Bowman JM, Jacoby P, Francis R, Vijayasekaran S, Coates HL, Riley TV, Richmond P. 2011. Predominance of nontypeable Haemophilus influenzae in children with otitis media following introduction of a 3+0 pneumococcal conjugate vaccine schedule. Vaccine 29:5163–5170. doi: 10.1016/j.vaccine.2011.05.035. [DOI] [PubMed] [Google Scholar]
- 25.Norskov-Lauritsen N. 2011. Increased level of intragenomic 16S rRNA gene heterogeneity in commensal strains closely related to Haemophilus influenzae. Microbiology 157:1050–1055. doi: 10.1099/mic.0.047233-0. [DOI] [PubMed] [Google Scholar]
- 26.Sacchi CT, Alber D, Dull P, Mothershed EA, Whitney AM, Barnett GA, Popovic T, Mayer LW. 2005. High level of sequence diversity in the 16S rRNA genes of Haemophilus influenzae isolates is useful for molecular subtyping. J Clin Microbiol 43:3734–3742. doi: 10.1128/JCM.43.8.3734-3742.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jordan IK, Conley AB, Antonov IV, Arthur RA, Cook ED, Cooper GP, Jones BL, Knipe KM, Lee KJ, Liu X, Mitchell GJ, Pande PR, Petit RA, Qin S, Rajan VN, Sarda S, Sebastian A, Tang S, Thapliyal R, Varghese NJ, Ye T, Katz LS, Wang X, Rowe L, Frace M, Mayer LW. 2011. Genome sequences for five strains of the emerging pathogen Haemophilus haemolyticus. J Bacteriol 193:5879–5880. doi: 10.1128/JB.05863-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shuel ML, Karlowsky KE, Law DK, Tsang RS. 2011. Nonencapsulated or nontypeable Haemophilus influenzae are more likely than their encapsulated or serotypeable counterparts to have mutations in their fucose operon. Can J Microbiol 57:982–986. doi: 10.1139/w11-017. [DOI] [PubMed] [Google Scholar]
- 29.Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. 2011. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28:2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ridderberg W, Fenger MG, Norskov-Lauritsen N. 2010. Haemophilus influenzae may be untypable by the multilocus sequence typing scheme due to a complete deletion of the fucose operon. J Med Microbiol 59:740–742. doi: 10.1099/jmm.0.018424-0. [DOI] [PubMed] [Google Scholar]
- 31.McCrea KW, Wang ML, Xie J, Sandstedt SA, Davis GS, Lee JH, Marrs CF, Gilsdorf JR. 2010. Prevalence of the sodC gene in nontypeable Haemophilus influenzae and Haemophilus haemolyticus by microarray-based hybridization. J Clin Microbiol 48:714–719. doi: 10.1128/JCM.01416-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Davis GS, Sandstedt SA, Patel M, Marrs CF, Gilsdorf JR. 2011. Use of bexB to detect the capsule locus in Haemophilus influenzae. J Clin Microbiol 49:2594–2601. doi: 10.1128/JCM.02509-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Giufre M, Cardines R, Mastrantonio P, Cerquetti M. 2010. Genetic characterization of the capsulation locus of Haemophilus influenzae serotype e. J Clin Microbiol 48:1404–1407. doi: 10.1128/JCM.01721-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meats E, Feil EJ, Stringer S, Cody AJ, Goldstein R, Kroll JS, Popovic T, Spratt BG. 2003. Characterization of encapsulated and noncapsulated Haemophilus influenzae and determination of phylogenetic relationships by multilocus sequence typing. J Clin Microbiol 41:1623–1636. doi: 10.1128/JCM.41.4.1623-1636.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smith-Vaughan HC, Chang AB, Sarovich DS, Marsh RL, Grimwood K, Leach AJ, Morris PS, Price EP. 2014. Absence of an important vaccine and diagnostic target in carriage- and disease-related nontypeable Haemophilus influenzae. Clin Vaccine Immunol 21:250–252. doi: 10.1128/CVI.00632-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Price EP, Sarovich DS, Nosworthy E, Beissbarth J, Marsh RL, Pickering J, Kirkham LA, Keil AD, Chang AB, Smith-Vaughan HC. 2015. Haemophilus influenzae: using comparative genomics to accurately identify a highly recombinogenic human pathogen. BMC Genomics 16:641. doi: 10.1186/s12864-015-1857-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ludwig W, Klenk H-P. 2001. Overview: a phylogenetic backbone and taxonomic framework for procaryotic systematics, p. 49–65. In Boone DR, Castenholz RW, Garrity GM (ed), Bergey's manual of systematic bacteriology, 2nd ed, vol 1 Springer, New York, NY. [Google Scholar]
- 38.Witherden EA, Bajanca-Lavado MP, Tristram SG, Nunes A. 2014. Role of inter-species recombination of the ftsI gene in the dissemination of altered penicillin-binding-protein-3-mediated resistance in Haemophilus influenzae and Haemophilus haemolyticus. J Antimicrob Chemother 69:1501–1509. doi: 10.1093/jac/dku022. [DOI] [PubMed] [Google Scholar]
- 39.Sondergaard A, Witherden EA, Norskov-Lauritsen N, Tristram SG. 2015. Interspecies transfer of the penicillin-binding protein 3-encoding gene ftsI between Haemophilus influenzae and Haemophilus haemolyticus can confer reduced susceptibility to beta-lactam antimicrobial agents. Antimicrob Agents Chemother 59:4339–4342. doi: 10.1128/AAC.04854-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.De Chiara M, Hood D, Muzzi A, Pickard DJ, Perkins T, Pizza M, Dougan G, Rappuoli R, Moxon ER, Soriani M, Donati C. 2014. Genome sequencing of disease and carriage isolates of nontypeable Haemophilus influenzae identifies discrete population structure. Proc Natl Acad Sci U S A 111:5439–5444. doi: 10.1073/pnas.1403353111. [DOI] [PMC free article] [PubMed] [Google Scholar]