Abstract
We recently detected the spoligotype patterns of strains of Mycobacterium pinnipedii, a species of the Mycobacterium tuberculosis complex, in sputum samples from nine cases with pulmonary tuberculosis residing in Porto Alegre, South Brazil. Because this species is rarely encountered in humans, we further characterized these nine isolates by additional genotyping techniques, including 24-locus mycobacterial interspersed repetitive-unit–variable-number tandem-repeat (MIRU-VNTR) typing, verification of the loci TbD1, RD9, pks15/1, RDRio, and fbpC, the insertion of IS6110 at a site specific to the M. tuberculosis Latin American Mediterranean (LAM) lineage, and whole-genome sequencing. The combined analysis of these markers revealed that the isolates are in fact M. tuberculosis and more specifically belong to the LAM genotype. Most of these isolates (n = 8) were shown to be multidrug resistant (MDR), which prompted us to perform partial sequencing of the rpoA, rpoB, rpoC, katG, and inhA genes. Seven isolates (77.8%) carried the S315T mutation in katG, and one of these (11%) also presented the C(−17)T single-nucleotide polymorphism (SNP) in inhA. Interestingly, six of the MDR isolates also presented an undescribed insertion of 12 nucleotides (CCA GAA CAA CCC) in codon 516 of rpoB. No putative compensatory mutation was found in either rpoA or rpoC. This is the first report of an M. tuberculosis LAM family strain with a convergent M. pinnipedii spoligotype. These spoligotypes are observed in genotype databases at a modest frequency, highlighting that care must be taken when identifying isolates in the M. tuberculosis complex on the basis of single genetic markers.
INTRODUCTION
Tuberculosis (TB) is a disease caused by organisms belonging to the Mycobacterium tuberculosis complex (MTBC), which are known to infect humans and domestic and wild animals. The MTBC complex includes M. tuberculosis, M. africanum, M. microti, M. bovis, M. bovis bacillus Calmette-Guérin (BCG), M. caprae, M. pinnipedii, M. orygis, M. mungi, and M. suricattae (1–8). M. tuberculosis is the predominant cause of human TB worldwide, but M. africanum and M. bovis remain important agents of human disease in certain geographical regions (9). The MTBC species share identical 16S rRNA sequences, and recent studies have improved our knowledge on the genetic diversity, host range, epidemiological aspects, and differences in pathogenicity and virulence among the species of the complex (10, 11). Based on the various genotyping techniques, like spoligotyping (12), restriction fragment length polymorphism (RFLP) (13), mycobacterial interspersed repetitive-unit–variable-number tandem-repeat (MIRU-VNTR) typing (14), and whole-genome sequencing (15), M. tuberculosis strains have been subdivided into lineages and families. The most geographically widespread family worldwide (spoligotyping) is the Latin American Mediterranean (LAM) lineage, which is part of the heterogeneous Euro-American lineage, one of seven M. tuberculosis lineages (16). The LAM family strains are widespread across all five continents, with a marked incidence in South America, and they account for 17% of all strains in the SITVITWEB database (17).
In a previous work, nine drug-resistant isolates sourced from Porto Alegre (Rio Grande do Sul, Brazil) presented with the spoligotype international type 863 (SIT863), a strain type previously described by Perizzolo et al. (18). This spoligotype, according to the SITVITWEB database, is representative of M. pinnipedii and, more specifically, the PINI2 clade (17). This species was originally described as the etiological agent of TB in seals and sea lions and in some terrestrial mammals, while a single report related putative transmission to a zoo caretaker but without evolution to active disease (6, 19–21). There is a paucity of information concerning M. pinnipedii as a cause of human disease and on its pathogenicity/virulence, drug resistance, and epidemiology. The nine strains with the PINI2 spoligopattern were therefore submitted to more extensive genetic characterization and evaluation of their drug susceptibility patterns.
MATERIALS AND METHODS
Mycobacterium strains.
Nine MTBC isolates were derived from sputum samples from individuals who were diagnosed in 2006 (n = 1) and 2010 (n = 8) with pulmonary TB as part of routine diagnosis at the Hospital Sanatório Partenon, the reference center for drug-resistant TB in Porto Alegre, the capital of Rio Grande do Sul, South Brazil. The sputum samples from these patients were processed for acid-fast bacilli microscopy detection on Ziehl-Neelsen-stained slides and cultured in Lowenstein-Jensen medium. The isolates were submitted to standard bacteriological and biochemical tests for differentiation of species within the MTBC and nontuberculous mycobacteria, including biochemical testing for niacin, para-nitrobenzoic acid, and tiofeno-2-carboxylic acid hydrazine (22). In addition, they were submitted to drug susceptibility testing using the proportion method on Lowenstein-Jensen solid medium (22, 23).
Nucleic acid extraction and genotyping controls.
For genotyping, nucleic acids were extracted as described by van Soolingen et al. (24). All PCR-based genotyping reactions included negative controls (ultrapure water) and positive controls, composed of DNA from M. pinnipedii (strains 76 and 8; kindly provided from the collection of the National Institute for Public Health and the Environment-RIVM) (25), M. tuberculosis H37Rv (ATCC 27294), and two M. tuberculosis isolates of the LAM genotype from Brazil, as defined by spoligotyping and 24-locus MIRU-VNTR typing (26).
Genotyping.
All PCRs were performed on a Veriti thermal cycler (Applied Biosystems, Foster City, CA). Spoligotyping was performed using the commercially available kit from Ocimum Biosolutions (Hyderabad, India), as described by Kamerbeek et al. (12). For 24-locus MIRU-VNTR typing, amplification of loci was performed by using a commercial typing kit (Genoscreen, Lille, France) and automated MIRU-VNTR analysis, performed as previously described (27). The fragment sizes of the amplicons were analyzed on an ABI3730 DNA sequence analyzer (Applied Biosystems), and the number of copies of each locus was determined by automated assignment using the GeneMapper 4.0 software (Applied Biosystems). In the case of doubtful results, the sizes of the repeats were double checked by size estimation compared to a DNA ladder (50 and 100 bp) and the positive control (H37Rv) on agarose gel and by comparing to a reference table, as described previously (14).
Comparison of genotypes with spoligotype and MIRU-VNTR databases.
The spoligopatterns of the strains were compared with the SITVITWEB database (http://www.pasteur-guadeloupe.fr:8081/SITVIT_ONLINE) for defining the spoligotype international type (SIT) and distribution frequency on a global level (17). The spoligotype and 24-locus MIRU-VNTR profiles were also compared with the MIRU-VNTRplus database (http://www.miru-vntrplus.org/MIRU/index.faces#) (28). The definition of lineages was based on 24-locus MIRU-VNTR typing using best-match analysis and tree-based identification using the categorical index.
Detection of a LAM-specific IS6110 insertion.
Briefly, the LAM family was confirmed based on the verification of the presence or not of an IS6110 element at position 932204 of the H37Rv genome (GenBank accession no. NC_000962.2), as described by Sampson et al. (29).
Verification of RDRio status.
Verification of RDRio status was performed as described by Lazzarini et al. (30) using a multiplex PCR protocol. The amplified products of 1,175 bp or 530 bp in the presence or absence of the deletion, respectively, were analyzed in a 1.5% agarose gel.
Characterization of the fbpC103 SNP.
Characterization of the fbpC103 SNP was performed as described by Vasconcellos et al. (31). The amplified products of 519 bp were analyzed on 2% agarose gels after staining with ethidium bromide. Partial sequencing was performed using the BigDye Terminator kit (PE Applied Biosystems) on an ABI 3730 DNA analyzer (Programa de Desenvolvimento Technológico em Insumos para Saúde [PDTIS] DNA sequencing platform at Fiocruz [http://www.dbbm.fiocruz.br/PDTIS_Genomica/]), and the results were analyzed with SeqScape software version 3.0 (Applied Biosystems, CA, USA), as previously described (32, 33).
Detection of the insertion in pks15/1 and of RD9 and TbD1.
Six- or 7-bp insertions were detected by partial sequencing of the pks15/1 locus. The initial amplifications were performed as described by Huard et al. (33). The sequencing was performed using sequencing kits and an analyzer, as described above; the results were interpreted as previously described (32, 33). For analysis of the RD9 and TbD1 loci, we used 3-primer combinations (site specific), as described by Vasconcellos et al. (31). The 3-primer PCRs were each designed to amplify a product of one size when the target locus is intact or to produce a different band size when a known long-sequence polymorphism is present.
Sequencing of rpoA, rpoB, rpoC, katG, and inhA.
For rpoB, katG, and inhA, the PCR-based sequencing was performed as described by Ramasoota et al. (34), de Oliveira et al. (35), and Dalla Costa et al. (36), while for rpoA and rpoC, the conditions were as described by de Vos et al. (37). Sequencing and analysis of rpoA, rpoB, and rpoC genes was performed at Fiocruz-RJ, as described above, while the katG and inhA genes were sequenced and analyzed at the sequencing platform at Centro de Desenvolvimento Científico e Tecnológico (CDCT)-Fundação Estadual de Produção e Pesquisa em Saúde (FEPPS).
Whole-genome sequencing.
Three isolates (M. tuberculosis RG74, RG112, and RG621257) were submitted to paired-end sequencing (105 bp) using the Illumina HiSeq 2500 platform at the King Abdullah University of Science and Technology (KAUST), Saudi Arabia. Raw read data were mapped to the reference genome of M. tuberculosis H37Rv (GenBank accession no. NC_000962.3) using the Burrows-Wheeler Aligner tool, and variants were called using the SAMtools package (38, 39). The obtained mean fold coverages were 249.51, 251.44, and 221.94 for RG74, RG112, and RG621257, respectively. Comma-separated files containing all detected variants are available in Tables S1 to S3 in the supplemental material. A script was written to extract the nucleotide coverage at each reference genomic position, and an R script was developed to classify each strain according to the recent M. tuberculosis SNP barcode typing system (15) (see Files S1 and S2 in the supplemental material).
Nucleotide sequence accession number.
All sequencing data have been submitted to the European Nucleotide Archive (http://www.ebi.ac.uk/ena/) under study accession no. PRJEB10715.
RESULTS
Bacteriological identification.
The Ziehl-Neelsen-stained microscopic slides showed the presence of acid-fast bacilli, while the Lowenstein-Jensen cultures presented rough colonies without pigmentation after 3 to 4 weeks of incubation at 37°C. The isolates scored positive for niacin and para-nitrobenzoic acid but were nitratase negative, which is characteristic of organisms of the MTBC.
Conventional and genetic testing of drug susceptibility.
The proportion method for drug susceptibility testing on Lowenstein-Jensen solid medium demonstrated that eight isolates were multidrug resistant (MDR), while one isolate was resistant to isoniazid (INH) and another to rifampin (RIF) only; all were susceptible to ethambutol (EMB) and streptomycin (SM). The sequencing results corroborated resistance to INH by presenting the S315T mutation in katG in seven isolates, with one of these also carrying the C(−17)T SNP in inhA; one strain did not show genetic evidence for resistance to INH. Among the nine isolates that were resistant to RIF, six presented a duplication of 12 nucleotides (CCA GAA CAA CCC) located before the last nucleotide of codon 516 of rpoB, and all these also presented a substitution of GAC to GGC in codon 516, causing the amino acid change D516V, besides the insertion of the glutamine, asparagine, isoleucine, and proline (QNIP). One isolate (M. tuberculosis 100285) that did not carry the 12-nucleotide (nt) duplication presented the GAC to GTC SNP, which was responsible for the amino acid change D513V. No other mutations were observed in the studied part of rpoB in this set of isolates, including the RIF-susceptible isolate (M. tuberculosis 100056), and one isolate (RG621257) that was RIF resistant and was submitted to whole-genome sequencing presented the wild-type rpoB allele. Finally, none of the isolates presented missense mutations in the SNPs in rpoA and rpoC described by de Vos et al. (37).
Genotyping for lineage classification.
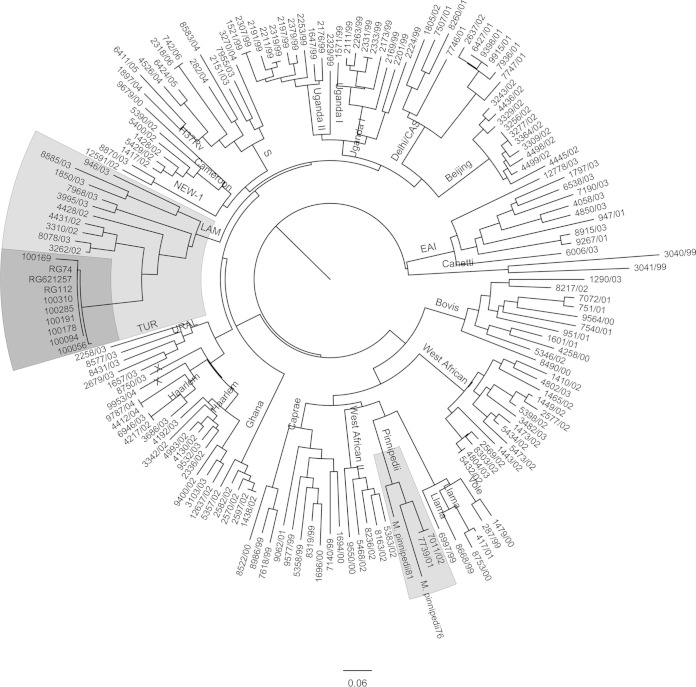
All nine isolates presented SIT863, which is characteristic of M. pinnipedii (Fig. 1). Upon 24-locus MIRU-VNTR typing, eight isolates presented the same MIRU pattern, while one isolate presented five fewer copies of MIRU21 (see Table S4 in the supplemental material). In a comparison of these MIRU patterns to those present in the SITVIT2 database, they were clearly different from the patterns that are characteristic of M. pinnipedii. Using the neighbor-joining-based phylogenetic tree building tool of MIRU-VNTRplus, the patterns of these isolates were organized within the MIRU patterns characteristic of LAM9 (Fig. 2). In addition, when constructing a neighbor-joining tree together with 24-locus MIRU patterns from a recent sample set from Brazil (26), the MIRU patterns were closest to those of LAM strains (Fig. 2). Upon analysis of the presence of other M. tuberculosis markers characteristic of LAM, their LAM nature was confirmed, and all were TbD1 negative and RD9 positive while not presenting the RDRio genotype.
FIG 1.
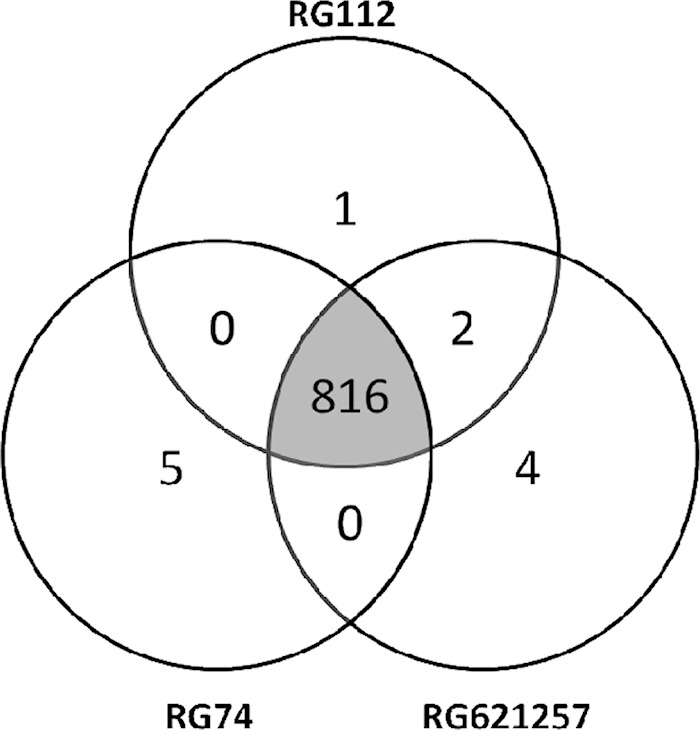
Venn diagram depicting the number of SNPs shared by the three M. tuberculosis clinical isolates subjected to WGS.
FIG 2.
Phylogenetic tree positioning the MIRUs of the isolates in MIRU-VNTRplus. CAS, Central Asian; EAI, Eastern African and Indian; TUR, Turkey.
Whole-genome sequencing-based typing.
The raw sequence reads for RG74, RG112, and RG621257 were aligned to the H37Rv reference sequence, and 869, 877, and 881 SNPs were identified, respectively. Upon excluding SNPs present in proline-glutamate (PE)/proline-proline-glutamate (PPE) genes and other repeat-rich regions, the three isolates shared 816 SNPs, each strain bearing between one and five unique SNPs (Fig. 1). In addition, 72 (RG74), 74 (RG112), and 87 (RG621257) short indels (<100 bp) were present. According to the 62-SNP barcoding scheme recently proposed by Coll et al. (15), the three strains were classified as belonging to lineage 4.3.3, part of the Euro-American lineage (lineage 4), confirming their LAM classification. Whole-genome sequencing confirmed the mutations observed by conventional sequencing of the hot spot region of rpoB, including the 12-nt insertion at genomic position 761123 (WGS codon 435). The isolate without the insertion did not present an SNP in the rest of rpoB (see Table S4 in the supplemental material). In addition, the absence of a mutation in rpoA and rpoC was confirmed, except for the presence of a synonymous SNP (GCC to GCG) at position 763370 (amino acid position 542).
DISCUSSION
M. pinnipedii, formally known as the “seal bacillus,” was described in 2003 on the basis of a characterization of isolates of M. tuberculosis-like organisms obtained from seals (6) and being the causative agent of TB in this mammal host. Later, the involvement of M. pinnipedii in TB transmission to humans was observed in a European zoo, where sea lion keepers had been infected but did not develop active TB during the time of the study (20). This M. pinnipedii isolate sourced from the animals was susceptible to the main antituberculosis drugs, isoniazid and rifampin, and streptomycin, ethambutol, and para-aminosalicylic acid (6).
In the spolDB4 database, the spoligotype profile obtained for the isolates under analysis in the present study corresponded to SIT863, initially described as characteristic of the M. tuberculosis U family (40) and later on redefined and presented by SITVITWEB as PINI2 (17). Isolates with the genotype SIT863 were reported in three earlier studies carried out in Porto Alegre, the first reported by Cafrune et al. (41), who observed six human isolates between 2005 and 2007. Subsequently, Perizzolo et al. (18) identified two isolates between 2004 and 2006, while Khuleis et al. (42) reported five additional isolates in a study carried out between 2007 and 2008. Outside Brazil, there have been reports of a single case in the United States and two more in Venezuela (43, 44). At SITVITWEB, four cases were reported, and three of these were isolated in Pelotas (Rio Grande do Sul, Brazil) but without information about the isolation data; the other case was isolated in the United States in 2003. These four SIT863 strains were classified as PINI2 based on revised rules for PINI, PINI1, and PINI2 (unpublished data). Accordingly, PINI2 (to which SIT863 belongs) is characterized by the obligate presence of spacer 25 only; therefore, the SIT863 pattern (presence of spacers 25 to 28) is compatible with the PINI2 definition and does not match any other signature in our updated SITVIT2 database. A review of the SITVIT2 database showed the presence of a total of 159 isolates with spoligotypes characteristic of M. pinnipedii (PINI1, n = 33; PINI2, n = 105; PINI-like, n = 7) but with the same number of isolates with the SIT863 pattern as in the earlier version of the database (data not shown). These data suggest that human infection rates for M. pinnipedii might be higher than has been suggested in earlier studies (20).
However, the misclassification of lineages of M. tuberculosis by spoligotyping has been reported, and it is now widely accepted that this genotyping method does not present the same level of discrimination of M. tuberculosis isolates as that with MIRU-VNTR typing (45). Convergent evolution has been described as relatively common when using the direct repeat (DR) region as a genetic marker, and incorrect classification of lineages by spoligotyping was recently evidenced in Brazil (26) and more frequently when the deletion of large blocks of spacers occurs, such as causing misclassification of M. tuberculosis of the Beijing type (46). Therefore, our observation of human isolates with the SIT863 spoligotype characteristic of PINI2, together with the lack of notification of M. pinnipedii as a cause of human TB, lead us to believe that we might be dealing with cases of misclassification.
It has been shown that MIRU-VNTR typing is a robust procedure for strain typing and phylogenetic classification (26, 27, 47), being either in a 9-, 12-, 15-, or 24-allele format in a comparison of large databases (MIRU-VNTRplus and SITVIT2) and 24-locus MIRU-VNTR typing as a single procedure; it is capable of reliable identification and discrimination of M. tuberculosis strains (25). The pattern we observed in the nine isolates was clearly different from that obtained with the two M. pinnipedii control strains, as can be observed in Fig. 2, and different from those characteristic of M. pinnipedii in SITVITWEB (256324222321, 216424222322, 256424222321, and 226424253522). In addition, the construction of a neighbor-joining tree using MIRU-VNTRplus demonstrated that our MIRU-VNTR profile was close to that of LAM genotypes (Fig. 2), and this was confirmed when we compared the genotype with that obtained in a recent study in Rio de Janeiro (data not shown).
The LAM lineage of the isolates was confirmed by the additional genotyping procedures specific for LAM strains, including the presence of IS6110 at a particular site of the genome (48). Another marker is present in the polyketide synthase (pks)15/1 locus, reported to be polymorphic among members of the MTBC (49). This genetic region has an intact open reading frame in Indo-Oceanic, East Asian, and East African-Indian lineages. However, the Euro-American lineage contains a typical 7-bp deletion in the pks15/1 gene, while other MTBC species, such as M. pinnipedii, contain a 6-bp deletion (31, 33, 49–51). Presently, a 7-bp insertion instead of the 6-bp insert specific for M. pinnipedii was observed. A third marker, the fbpC SNP, which changed nucleotide G to A at codon 103 (E103N), differentiates LAM strains from non-LAM strains (52); this also confirmed the LAM nature of the present isolates. Finally, we verified the absence of TbD1, which is characteristic of M. tuberculosis, and the presence of RD9, which is characteristic of M. africanum and MTBC species that infect mostly animals (53). Interestingly, the phenotype of the isolates also corroborated them being M. tuberculosis and not M. pinnipedii, since all were niacin positive, and M. pinnipedii is niacin negative.
While all M. pinnipedii isolates reported so far are susceptible to antibiotics, seven of the nine isolates were MDR; INH resistance was due to S315T in katG in six cases, while one isolate presented S315T and an additional T-to-G transition at position −17 of inhA. Interestingly, in seven of the nine isolates, a duplication of 12 nucleotides was observed in codon 516, which was reported earlier in Porto Alegre by Perizzolo et al. (18), causing the insertion of four amino acids. Because isolate RG621257 did not present a mutant rpoB allele, we aimed to discover if mutations in the rest of the genome and not present in RG74 and RG112 might explain resistance to RIF. We observed some SNPs in genes associated with efflux pumps and impermeability of cell membranes (data not shown), and whether these mutations are directly associated with resistance to RIF is under investigation.
Because of the size of this insertion and its possible putative influence on the structure of the beta polymerase unit (unpublished data), we expected to observe compensatory mutations in either rpoA or rpoC, as described recently in M. tuberculosis strains (37, 54, 55). Surprisingly, we did not observe any non-synonymous mutations in the rpoA and rpoC regions covered by our sequencing approach, and this might mean that (i) the duplication does not interfere with the biological function of the polymerase, which seems unlikely due to the size of the duplication; (ii) unknown and undescribed compensatory mutations are present in other regions of the genome; or (iii) this is a recent evolutionary event for which no compensatory mutation has yet been acquired and is fixed in the population that contains the clinical isolate. Interestingly, in a RIF-resistant isolate of M. smegmatis, an insertion of six amino acids was observed at codons 434 and 435 of rpoB, which led to growth attenuation (56).
To classify our strains to the lineage level, confirm their LAM nature also based on the SNP barcode (15), and better understand their similarity on a genetic level, three strains were submitted to whole-genome sequencing. The observed genetic distances between the three isolates whose genomes have been sequenced also point toward recent transmission of this strain. The three isolates were found to be within 5 to 11 SNPs in distance, which strongly indicates an epidemiological link between the patients, as reported by Walker et al. (56); the strongest link was between isolates RG112 and RG621257, with a genome difference of only 5 SNPs. The remaining isolate (RG74), which presented differences of 8 or 11 SNPs from the other strains, falls into the indeterminate interval (6 to 12 SNPs) (56) for the delineation of TB outbreaks using WGS data. This demonstrated that independent of the lineage nature of these isolates, they are part of an active transmission chain of MDR-TB in Porto Alegre over a period of several years.
Earlier outbreaks of MDR strains have been reported in the city of Porto Alegre, including a LAM5 strain reported by Perizzolo et al. (18) and a recent MDR-TB LAM2 strain (57), both being of the RDRio genotype; the nine isolates described here, however, were not of the RDRio genotype.
This work further reinforces the extreme care needed to be taken when using databases for comparisons of genotype identifications of local M. tuberculosis isolates, and it highlights the need to use multiple markers for correct species and/or lineage assignation of isolates with infrequent spoligotypes.
Supplementary Material
ACKNOWLEDGMENTS
Miguel Viveiros was supported by “Ciências em Fronteiras/Professor Visitante Especial” (no. 88881.064961/2014-01) (Jose R. Lapa e Silva/UFRJ coordinator) by CAPES/MEC/Brazil. This study was partially supported by a research grant from the European Society of Clinical Microbiology and Infectious Diseases, for which we would like to acknowledge the Study Group for Mycobacterial Infections; the Fundação para a Ciência e Tecnologia, Portugal (project reference no. PTDC/SAU-EPI/122400/2010); a postdoctoral fellowship (SFRH/BPD/95406/2013) awarded to João Perdigão; and by CNPq (441499/2014-7 MCTI/CNPQ/Universal 14/2014).
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JCM.02012-15.
REFERENCES
- 1.van Soolingen D, van der Zanden AG, de Haas PE, Noordhoek GT, Kiers A, Foudraine NA, Portaels F, Kolk AH, Kremer K, van Embden JD. 1998. Diagnosis of Mycobacterium microti infections among humans by using novel genetic markers. J Clin Microbiol 36:1840–1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Niemann S, Richter E, Rüsch-Gerdes S. 1999. Biochemical and genetic evidence for the transfer of Mycobacterium tuberculosis subsp. caprae Aranaz et al. to the species Mycobacterium bovis Karlson and Lessel 1970 (approved lists 1980) as Mycobacterium bovis subsp. caprae comb. nov. Int J Syst Evol Microbiol 52:433–436. doi: 10.1099/00207713-52-2-433. [DOI] [PubMed] [Google Scholar]
- 3.Garnier T, Eiglmeier K, Camus JC, Medina N, Mansoor H, Pryor M, Duthoy S, Grondin S, Lacroix C, Monsempe C, Simon S, Harris B, Atkin R, Doggett J, Mayes R, Keating L, Wheeler PR, Parkhill J, Barrell BG, Cole ST, Gordon SV, Hewinson RG. 2003. The complete genome sequence of Mycobacterium bovis. Proc Natl Acad Sci U S A 100:7877–7882. doi: 10.1073/pnas.1130426100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cousins DV, Williams SN, Reuter R, Forshaw D, Chadwick B, Coughran D, Collins P, Gales N. 1993. Tuberculosis in wild seals and characterization of the seal bacillus. Aust Vet J 70:92–97. doi: 10.1111/j.1751-0813.1993.tb03284.x. [DOI] [PubMed] [Google Scholar]
- 5.Frota CC, Hunt DM, Buxton RS, Rickman L, Hinds J, Kremer K, van Soolingen D, Colston MJ. 2004. Genome structure in the vole bacillus, Mycobacterium microti, a member of the Mycobacterium tuberculosis complex with a low virulence for humans. Microbiology 150:1519–1527. doi: 10.1099/mic.0.26660-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cousins DV, Bastida R, Cataldi A, Quse V, Redrobe S, Dow S, Duignan P, Murray A, Dupont C, Ahmed N, Collins DM, Butler WR, Dawson D, Rodríguez D, Loureiro J, Romano MI, Alito A, Zumarraga M, Bernardelli A. 2003. Tuberculosis in seals caused by a novel member of the Mycobacterium tuberculosis complex: Mycobacterium pinnipedii sp. nov. Int J Syst Evol Microbiol. 53:1305–1314. doi: 10.1099/ijs.0.02401-0. [DOI] [PubMed] [Google Scholar]
- 7.van Ingen J, Rahim Z, Mulder A, Boeree MJ, Simeone R, Brosch R, van Soolingen D. 2012. Characterization of Mycobacterium orygis as M. tuberculosis complex subspecies. Emerg Infect Dis 18:653–655. doi: 10.3201/eid1804.110888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parsons SD, Drewe JA, Gey van Pittius NC, Warren RM, van Helden PD. 2013. Novel cause of tuberculosis in meerkats, South Africa. Emerg Infect Dis 19:2004–2007. doi: 10.3201/eid1912.130268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Jong BC, Antonio M, Gagneux S. 2010. Mycobacterium africanum–review of an important cause of human tuberculosis in West Africa. PLoS Negl Trop Dis 4:e744. doi: 10.1371/journal.pntd.0000744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Van Soolingen D. 2001. Molecular epidemiology of tuberculosis and other mycobacterial infections: main methodologies and achievements. J Intern Med 249:1–26. doi: 10.1046/j.1365-2796.2001.00772.x. [DOI] [PubMed] [Google Scholar]
- 11.Gagneux S. 2013. Genetic diversity in Mycobacterium tuberculosis. Curr Top Microbiol Immunol 374:1–25. doi: 10.1007/82.2013.329. [DOI] [PubMed] [Google Scholar]
- 12.Kamerbeek J, Schouls L, Kolk A, van Agterveld M, van Soolingen D, Kuijper S, Bunschoten A, Molhuizen H, Shaw R, Goyal M, van Embden J. 1997. Simultaneous detection and strain differentiation of Mycobacterium tuberculosis for diagnosis and epidemiology. J Clin Microbiol 35:907–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Embden JD, Cave MD, Crawford JT, Dale JW, Eisenach KD, Gicquel B, Hermans P, Martin C, McAdam R, Shinnick TM, Small PM. 1993. Strain identification of Mycobacterium tuberculosis by DNA fingerprinting: recommendations for a standardized methodology. J Clin Microbiol 31:406–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Supply P, Mazars E, Lesjean S, Vincent V, Gicquel B, Locht C. 2000. Variable human minisatellite-like regions in the Mycobacterium tuberculosis genome. Mol Microbiol 36:762–771. doi: 10.1046/j.1365-2958.2000.01905.x. [DOI] [PubMed] [Google Scholar]
- 15.Coll F, McNerney R, Guerra-Assunção JA, Glynn JR, Perdigão J, Viveiros M, Portugal I, Pain A, Martin N, Clark TG. 2014. A robust SNP barcode for typing Mycobacterium tuberculosis complex strains. Nat Commun 5:4812. doi: 10.1038/ncomms5812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gagneux S, DeRiemer K, Van T, Kato-Maeda M, de Jong BC, Narayanan S, Nicol M, Niemann S, Kremer K, Gutierrez MC, Hilty M, Hopewell PC, Small PM. 2006. Variable host-pathogen compatibility in Mycobacterium tuberculosis. Proc Natl Acad Sci U S A 103:2869–2873. doi: 10.1073/pnas.0511240103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Demay C, Liens B, Burguière T, Hill V, Couvin D, Millet J, Mokrousov I, Sola C, Zozio T, Rastogi N. 2012. SITVITWEB–a publicly available international multimarker database for studying Mycobacterium tuberculosis genetic diversity and molecular epidemiology. Infect Genet Evol 12:755–766. doi: 10.1016/j.meegid.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 18.Perizzolo PF, Dalla Costa ER, Ribeiro AW, Spies FS, Ribeiro MO, Dias CF, Unis G, Almeida da Silva P, Gomes HM, Suffys PN, Rossetti ML. 2012. Characteristics of multidrug-resistant Mycobacterium tuberculosis in southern Brazil. Tuberculosis (Edinb) 92:56–59. doi: 10.1016/j.tube.2011.09.008. [DOI] [PubMed] [Google Scholar]
- 19.Moser I, Prodinger WM, Hotzel H, Greenwald R, Lyashchenko KP, Bakker D, Gomis D, Seidler T, Ellenberger C, Hetzel U, Wuennemann K, Moisson P. 2008. Mycobacterium pinnipedii: transmission from South American sea lion (Otaria byronia) to Bactrian camel (Camelus bactrianus bactrianus) and Malayan tapirs (Tapirus indicus). Vet Microbiol 127:399–406. doi: 10.1016/j.vetmic.2007.08.028. [DOI] [PubMed] [Google Scholar]
- 20.Kiers A, Klarenbeek A, Mendelts B, Van Soolingen D, Koëter G. 2008. Transmission of Mycobacterium pinnipedii to humans in a zoo with marine mammals. Int J Tuberc Lung Dis 12:1469–1473. [PubMed] [Google Scholar]
- 21.Jurczynski K, Scharpegge J, Ley-Zaporozhan J, Ley S, Cracknell J, Lyashchenko K, Greenwald R, Schenk JP. 2011. Computed tomographic examination of South American sea lions (Otaria flavescens) with suspected Mycobacterium pinnipedii infection. Vet Rec 169:608. doi: 10.1136/vr.100234. [DOI] [PubMed] [Google Scholar]
- 22.Ministério da Saúde Brasil. 2011. Manual de recomendações para o controle da tuberculose no Brasil, Secretaria de Vigilância em Saúde/Programa Nacional de Controle da Tuberculose, Ministério da Saúde Brasil, Brasília, Brazil: http://portalsaude.saude.gov.br/images/pdf/2015/junho/30/MANUAL-DE-RECOMENDACOES-PARA-O-CONTROLE-DA-TUBERCULOSE-NO-BRASIL.pdf. [Google Scholar]
- 23.Canetti G, Fox W, Khomenko A, Mahler HT, Menon MK, Mitchison DA, Rist N, Šmelev NA. 1969. Advances in techniques of testing mycobacterial drug sensitivity, and the use of sensitivity tests in tuberculosis control programmes. Bull World Health Organ 41:21–43. [PMC free article] [PubMed] [Google Scholar]
- 24.van Soolingen D, de Haas PE, Hermans PW, van Embden JD. 1994. DNA fingerprinting of Mycobacterium tuberculosis. Methods Enzymol 235:196–205. doi: 10.1016/0076-6879(94)35141-4. [DOI] [PubMed] [Google Scholar]
- 25.Kremer K, van Soolingen D, Frothingham R, Haas WH, Hermans PW, Martín C, Palittapongarnpim P, Plikaytis BB, Riley LW, Yakrus MA, Musser JM, van Embden JD. 1999. Comparison of methods based on different molecular epidemiological markers for typing of Mycobacterium tuberculosis complex strains: interlaboratory study of discriminatory power and reproducibility. J Clin Microbiol 37:2607–2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vasconcellos SE, Acosta CC, Gomes LL, Conceição EC, Lima KV, de Araujo MI, Leite Mde L, Tannure F, Caldas PC, Gomes HM, Santos AR, Gomgnimbou MK, Sola C, Couvin D, Rastogi N, Boechat N, Suffys PN. 2014. Strain classification of Mycobacterium tuberculosis isolates in Brazil based on genotypes obtained by spoligotyping, mycobacterial interspersed repetitive unit typing and the presence of large sequence and single nucleotide polymorphism. PLoS One 9:e107747. doi: 10.1371/journal.pone.0107747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Supply P, Gaudin C, Raze D. 2014. Optimization of standard 24-locus variable-number tandem-repeat typing of Mycobacterium tuberculosis isolates: a multicenter perspective. J Clin Microbiol 52:3518–3519. doi: 10.1128/JCM.01790-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weniger T, Krawczyk J, Supply P, Niemann S, Harmsen D. 2010. MIRU-VNTRplus: a Web tool for polyphasic genotyping of Mycobacterium tuberculosis complex bacteria. Nucleic Acids Res 38:W326–W331. doi: 10.1093/nar/gkq351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sampson SL, Warren RM, Richardson M, van der Spuy GD, van Helden PD. 1999. Disruption of coding regions by IS6110 insertion in Mycobacterium tuberculosis. Tuber Lung Dis 79:349–359. doi: 10.1054/tuld.1999.0218. [DOI] [PubMed] [Google Scholar]
- 30.Lazzarini LCO, Huard RC, Boechat NL, Gomes HM, Oelemann MC, Kurepina N, Shashkina E, Mello FC, Gibson AL, Virginio MJ, Marsico AG, Butler WR, Kreiswirth BN, Suffys PN, Lapa e Silva JR, Ho JL. 2007. Discovery of a novel Mycobacterium tuberculosis lineage that is a major cause of tuberculosis in Rio de Janeiro, Brazil. J Clin Microbiol 45:3891–3902. doi: 10.1128/JCM.01394-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vasconcellos SE, Huard RC, Niemann S, Kremer K, Santos AR, Suffys PN, Ho JL. 2010. Distinct genotypic profiles of the two major clades of Mycobacterium africanum. BMC Infect Dis 10:80. doi: 10.1186/1471-2334-10-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huard RC, Lazzarini LCO, Butler W, van Soolingen D, Ho JL. 2003. PCR-based method to differentiate the subspecies of the Mycobacterium tuberculosis complex on the basis of genomic deletions. J Clin Microbiol 41:1637–1650. doi: 10.1128/JCM.41.4.1637-1650.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huard RC, Fabre M, de Haas P, Lazzarini LCO, van Soolingen D, Cousins D, Ho JL. 2006. Novel genetic polymorphisms that further delineate the phylogeny of the Mycobacterium tuberculosis complex. J Bacteriol 188:4271–4287. doi: 10.1128/JB.01783-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ramasoota P, Pitaksajjakul P, Phatihattakorn W, Pransujarit V, Boonyasopun J. 2006. Mutations in the rpoB gene of rifampicin-resistant Mycobacterium tuberculosis strains from Thailand and its evolutionary implication. Southeast Asian J Trop Med Public Health 37:136–147. [PubMed] [Google Scholar]
- 35.de Oliveira MM, da Silva Rocha A, Cardoso Oelemann M, Gomes HM, Fonseca L, Werneck-Barreto AM, Valim AM, Rossetti ML, Rossau R, Mijs W, Vanderborght B, Suffys P. 2003. Rapid detection of resistance against rifampicin in isolates of Mycobacterium tuberculosis from Brazilian patients using a reverse-phase hybridization assay. J Microbiol Methods 53:335–342. [DOI] [PubMed] [Google Scholar]
- 36.Dalla Costa ER, Ribeiro MO, Silva MSN, Arnold LS, Rostirolla DC, Cafrune PI, Espinoza RC, Palaci M, Telles MA, Ritacco V, Suffys PN, Lopes ML, Campelo CL, Miranda SS, Kremer K, da Silva PEA, Fonseca LS, Ho JL, Kritski AL, Rossetti MLR. 2009. Correlations of mutations in katG, oxyR-ahpC and inhA genes and in vitro susceptibility in Mycobacterium tuberculosis clinical strains segregated by spoligotype families from tuberculosis prevalent countries in South America. BMC Microbiol 9:39. doi: 10.1186/1471-2180-9-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.de Vos M, Müller B, Borrell S, Black PA, van Helden PD, Warren RM, Gagneux S, Victor TC. 2012. Putative compensatory mutations in the rpoC gene of rifampicin-resistant Mycobacterium tuberculosis are associated with ongoing transmission. Antimicrob Agents Chemother 57:827–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li H, Durbin R. 2009. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 25:1754–1760. doi: 10.1093/bioinformatics/btp698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, Marth G, Abecasis G, Durbin R, 1000 Genome Project Data Processing Subgroup . 2009. The Sequence Alignment/Map format and SAMtools. Bioinformatics 25:2078–2079. doi: 10.1093/bioinformatics/btp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brudey K, Driscoll JR, Rigouts L, Prodinger WM, Gori A, Al-Hajoj SA, Allix C, Aristimuño L, Arora J, Baumanis V, Binder L, Cafrune P, Cataldi A, Cheong S, Diel R, Ellermeier C, Evans JT, Fauville-Dufaux M, Ferdinand S, Garcia de Viedma D, Garzelli C, Gazzola L, Gomes HM, Guttierez MC, Hawkey PM, van Helden PD, Kadival GV, Kreiswirth BN, Kremer K, Kubin M, Kulkarni SP, Liens B, Lillebaek T, Ho ML, Martin C, Martin C, Mokrousov I, Narvskaïa O, Ngeow YF, Naumann L, Niemann S, Parwati I, Rahim Z, Rasolofo-Razanamparany V, Rasolonavalona T, Rossetti ML, Rüsch-Gerdes S, Sajduda A, Samper S, Shemyakin IG, et al. . 2006. Mycobacterium tuberculosis complex genetic diversity: mining the fourth international spoligotyping database (SpolDB4) for classification, population genetics and epidemiology. BMC Microbiol 6:23. doi: 10.1186/1471-2180-6-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cafrune PI, Possuelo LG, Ribeiro AW, Ribeiro MO, Unis G, Jarczewski CA, Rossetti ML, Zaha A. 2009. Prospective study applying spoligotyping directly to DNA from sputum samples of patients suspected of having tuberculosis. Can J Microbiol 55:895–900. doi: 10.1139/W09-033. [DOI] [PubMed] [Google Scholar]
- 42.Kuhleis D, Ribeiro AW, Costa ER, Cafrune PI, Schmid KB, Costa LL, Ribeiro MO, Zaha A, Rossetti ML. 2012. Tuberculosis in a southern Brazilian prison. Mem Inst Oswaldo Cruz 107:909–915. doi: 10.1590/S0074-02762012000700012. [DOI] [PubMed] [Google Scholar]
- 43.Borsuk S, Dellagostin MM, Madeira Sde G, Lima C, Boffo M, Mattos I, Almeida da Silva PE, Dellagostin OA. 2005. Molecular characterization of Mycobacterium tuberculosis isolates in a region of Brazil with a high incidence of tuberculosis. Microbes Infect 7:1338–1344. doi: 10.1016/j.micinf.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 44.Sequera CM, Delgado SV, Araque MW, Torrealba OM, Núñez MR, da Mato JO, Abadia PE, Takiff H, de Waard J. 2008. Mycobacterium tuberculosis: espoligotipos en el Estado Carabobo, Venezuela. Rev Chi Infect 25:362–367. doi.org/10.4067/S0716-10182008000500009. [PubMed] [Google Scholar]
- 45.Gagneux S, Deriemer K, Van T, Kato-Maeda M, de Jong BC, Narayanan S, Nicol M, Niemann S, Kremer K, Gutierrez MC, Hilty M, Hopewell PC, Small PM. 2006. Variable host-pathogen compatibility in Mycobacterium tuberculosis. Proc Natl Acad Sci U S A 103:2869–2873. doi: 10.1073/pnas.0511240103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fenner L, Malla B, Ninet B, Dubuis O, Stucki D, Borrel Sonia Borrel Huna T, Bodmer T, Egger M, Gagneux S. 2011. ‘‘Pseudo-Beijing’’: evidence for convergent evolution in the direct repeat region of Mycobacterium tuberculosis. PLoS One 6:e24737. doi: 10.1371/journal.pone.0024737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Oelemann MC, Diel R, Vatin V, Haas W, Rüsch-Gerdes S, Locht C, Niemann S, Supply P. 2007. Assessment of an optimized mycobacterial interspersed repetitive-unit–variable-number tandem-repeat typing system combined with spoligotyping for population-based molecular epidemiology studies of tuberculosis. J Clin Microbiol 45:691–697. doi: 10.1128/JCM.01393-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gagneux S, Small PM. 2007. Global phylogeography of Mycobacterium tuberculosis and implications for tuberculosis product development. Lancet Infect Dis 7:328–337. doi: 10.1016/S1473-3099(07)70108-1. [DOI] [PubMed] [Google Scholar]
- 49.Constant P, Perez E, Malaga W, Lanéelle MA, Saurel O, Daffé M, Guilhot C. 2002. Role of the pks15/1 gene in the biosynthesis of phenolglycolipids in the Mycobacterium tuberculosis complex. Evidence that all strains synthesize glycosylated p-hydroxybenzoic methyl esters and that strain devoid of phenolglycolipids harbor a frameshift mutation in the pks15/1 gene. J Biol Chem 277:38148–38158. doi: 10.1074/jbc.M206538200. [DOI] [PubMed] [Google Scholar]
- 50.Reed MB, Domenech P, Manca C, Su H, Barczak AK, Kreiswirth BN, Kaplan G, Barry CE III. 2004. A glycolipid of hypervirulent tuberculosis strains that inhibits the innate immune response. Nature 431:84–87. doi: 10.1038/nature02837. [DOI] [PubMed] [Google Scholar]
- 51.Gibson AL, Huard RC, Gey van Pittius NC, Lazzarini LC, Driscoll J, Kurepina N, Zozio T, Sola C, Spindola SM, Kritski AL, Fitzgerald D, Kremer K, Mardassi H, Chitale P, Brinkworth J, Garcia de Viedma D, Gicquel B, Pape JW, van Soolingen D, Kreiswirth BN, Warren RM, van Helden PD, Rastogi N, Suffys PN, Lapa e Silva J, Ho JL. 2008. Application of sensitive and specific molecular methods to uncover global dissemination of the major RDRio sublineage of the Latin American-Mediterranean Mycobacterium tuberculosis spoligotype family. J Clin Microbiol 46:1259–1267. doi: 10.1128/JCM.02231-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Comas I, Borrell S, Roetzer A, Rose G, Malla B, Kato-Maeda M, Galagan J, Niemann S, Gagneux S. 2012. Whole-genome sequencing of rifampicin-resistant Mycobacterium tuberculosis strains identifies compensatory mutations in RNA polymerase genes. Nat Genet 44:106–110. doi: 10.1038/ng.1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Brosch R, Gordon SV, Marmiesse M, Brodin P, Buchrieser C, Eiglmeier K, Garnier T, Gutierrez C, Hewinson G, Kremer K, Parsons LM, Pym AS, Samper S, van Soolingen D, Cole ST. 2002. A new evolutionary scenario for the Mycobacterium tuberculosis complex. Proc Natl Acad Sci U S A 99:3684–3689. doi: 10.1073/pnas.052548299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Perdigão J, Silva H, Machado D, Macedo R, Maltez F, Silva C, Jordao L, Couto I, Mallard K, Coll F, Hill-Cawthorne GA, McNerney R, Pain A, Clark TG, Viveiros M, Portugal I. 2014. Unraveling Mycobacterium tuberculosis genomic diversity and evolution in Lisbon, Portugal, a highly drug resistant setting. BMC Genomics 15:991. doi: 10.1186/1471-2164-15-991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Malshetty V, Kurthkoti K, China A, Mallick B, Yamunadevi S, Sang PB, Srinivasan N, Nagaraja V, Varshney U. 2010. Novel insertion and deletion mutants of RpoB that render Mycobacterium smegmatis RNA polymerase resistant to rifampicin-mediated inhibition of transcription. Microbiology 156:1565–1573. doi: 10.1099/mic.0.036970-0. [DOI] [PubMed] [Google Scholar]
- 56.Walker TM, Ip CL, Harrell RH, Evans JT, Kapatai G, Dedicoat MJ, Eyre DW, Wilson DJ, Hawkey PM, Crook DW, Parkhill J, Harris D, Walker AS, Bowden R, Monk P, Smith EG, Peto TE. 2013. Whole-genome sequencing to delineate Mycobacterium tuberculosis outbreaks: a retrospective observational study. Lancet Infect Dis 13:137–146. doi: 10.1016/S1473-3099(12)70277-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dalla Costa ER, Lazzarini LC, Perizzolo PF, Díaz CA, Spies FS, Costa LL, Ribeiro AW, Barroco C, Schuh SJ, da Silva Pereira MA, Dias CF, Gomes HM, Unis G, Zaha A, Almeida da Silva PE, Suffys PN, Rossetti ML. 2013. Mycobacterium tuberculosis of the RDRio genotype is the predominant cause of tuberculosis and associated with multidrug resistance in Porto Alegre City, South Brazil. J Clin Microbiol 51:1071–1077. doi: 10.1128/JCM.01511-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.