Abstract
Diagnosis of cystic echinococcosis (CE) is based on the identification of the cyst(s) by imaging, using immunodiagnostic tests mainly as complementary tools in clinical settings. Among the antigens used for immunodiagnosis, previous studies described a good performance of the recombinant antigen B8/1 (rAgB) in an enzyme-linked immunosorbent assay (ELISA) format; however, in remote parts of areas where the disease is endemic, the implementation of an ELISA is difficult, so a more simple, rapid, and reliable method such as the immunochromatographic test (ICT) is required. In this study, using a set of 50 serum samples from patients with surgically confirmed CE, we compared the performance of an ICT and that of an ELISA using the rAgB. The overall sensitivities of ICT and ELISA were not statistically different (78% versus 72%; P = 0.36). The overall agreement between both tests was moderate (κ = 0.41; P < 0.01). Concordance between ICT and ELISA was substantial or almost perfect for patients with liver involvement (κ = 0.65; P < 0.001) and patients with more than one hydatid cyst (κ = 0.82; P < 0.001), respectively. Moreover, specificity analysis using a total of 88 serum samples from healthy individuals (n = 20) and patients (n = 68) with other parasitic infections revealed that ICT had a specificity of 89.8%. ICT and ELISA had similar performance for the detection of specific antibodies to E. granulosus, and ICT had a high specificity, opening the possibility of using ICT as a screening tool in rural settings.
INTRODUCTION
Cystic echinococcosis (CE) is a zoonotic disease caused by the larval stage of the dog tapeworm Echinococcus granulosus. This zoonosis has a worldwide distribution, being considered a public health problem in areas dedicated to the raising of livestock where CE is endemic (1). In these areas, risk factors such as access of dogs to contaminated viscera and close contact between infected dogs and humans facilitate and maintain the transmission of the disease (2, 3). Humans are infected by the accidental ingestion of the tapeworm eggs, which can develop to the larval stage (hydatid cyst) in any internal organ after several years. The organs more frequently involved are the liver and the lungs, representing ∼70% and ∼20% of cases, respectively (4). Diagnosis of CE is based on the identification of the hydatid cyst(s) by imaging methods (e.g., abdominal ultrasound, chest X ray, or computed tomography) (5). Immunodiagnostic tools are of use in clinical settings as a complementary diagnostic tool and have quite variable performance, which depends on the antigen or technique used and is affected by certain disease characteristics, such as cyst location and presence of cyst rupture or aggregated bacterial infection (6–9).
Among the antigens used for immunodiagnostic, hydatid cyst fluid (HCF) has been the one most widely used. Antibody-detecting assays using HCF report sensitivities between 75% and 95% with poor specificity and frequent cross-reactions (5, 9–11). More recently, synthetic peptides or recombinant antigens from the sequences of two major components of HCF (antigen B and antigen 5) have been obtained (9, 11–13). These new antigens have a better performance than their predecessors and are more reproducible across populations, improving test reliability and allowing a better test standardization (6, 9, 11, 13). Recombinant antigen B8/1(rAgB) seems to have a good diagnostic performance in an ELISA format with sensitivity between 94.6% and 95.8, and specificity between 93.9% and 100% (13, 14).
Immunochromatographic testing (ICT) has demonstrated good performance in comparison with the ELISA format for the diagnosis of alveolar echinococcosis (15, 16). ICT is a simple, rapid, and reliable method which, unlike standard ELISA methods, does not require equipment and trained personal, both of which are difficult to find in remote areas (15). This study mainly attempted to compare an ICT using rAgB and an ELISA format using the same antigen in terms of sensitivity for the diagnosis of CE.
MATERIALS AND METHODS
Serum samples.
A total of 50 serum samples from patients with either lung (n = 25) or liver (n = 25) CE that had been surgically confirmed were used to evaluate the performance of both techniques (i.e., ICT and ELISA). These serum samples were collected in previous studies by our group after informed written consent, including permission for future use of remnant samples, had been obtained. To assess specificity of ICT, a total of 88 serum samples from healthy individuals (n = 20) and patients with other parasitic infections (n = 68) were used. Other parasitic infections consisted of alveolar echinococcosis (E) caused by Echinococcus multilocularis (n = 19), clonorchiasis by Clonorchis sinensis (n = 6), cysticercosis by Taenia solium (n = 10), fascioliasis by Fasciola hepatica (n = 6), paragonimiasis by Paragonimus miyazakii (n = 4) and Paragonimus westermani (n = 5), schistosomiasis by Schistosoma haematobium (n = 9), sparganosis by Spirometra erinacei (n = 5) and taeniasis by Taenia saginata (n = 4). All infections were confirmed parasitologically, and moreover, all alveolar echinococcosis (AE) and cysticercosis cases were confirmed to be seropositive with each specific antigen.
Preparation of recombinant AgB8/1.
The recombinant 8-kDa subunit of AgB8/1 (rAgB) was expressed in a bacterial system as described previously (17) with some modifications. Briefly, a DNA fragment encoding the AgB8/1 was amplified by PCR with the primers 5′-GGGAATTCGATGATGGTTACTCGACG-3′ and 5′-TTGGATCCTTACTTTGAATCATCATCTTT-3′. The PCR products were digested with EcoRI and BamHI and cloned into the bacterial expression vector pTWIN-1 (New England BioLabs, Beverly, MA, USA) to produce a fusion protein with a chitin-binding domain and mini-inteins. The cloned plasmid was transfected into Escherichia coli ER2566, and expression of the recombinant protein was induced by addition of 0.5 mM isopropyl-β-d-thiogalactoside (IPTG) to the culture. The expressed rAgB was purified using a chitin column (New England BioLabs) according to the manufacturer's instruction. The purified rAgB did not have the fusion partner, because rAgB was released by intein activity of the fusion partner itself during purifications.
Immunochromatographic test.
The ICT kit for CE (ADAMU-CE test; ICST Co. Ltd., Saitama, Japan) was performed according to the manufacturer's instructions. In this kit, the purified rAgB (1 mg/ml) and anti-goat IgG antibody (1 mg/ml) were sprayed on a nitrocellulose membrane each in a 1-mm-width line as the test and control lines, respectively.
For assay, 10 μl of patient serum sample was mixed with 20 μl of a serum dilution buffer containing 0.1 mg/ml alkaline phosphatase-conjugated goat anti-human IgG and IgE (Dako, Tokyo, Japan). After mixing, 20 μl of mixed serum sample was applied to the sample area; within 30 s, 200 μl of the substrate solution was loaded, and the result was evaluated after 20 min.
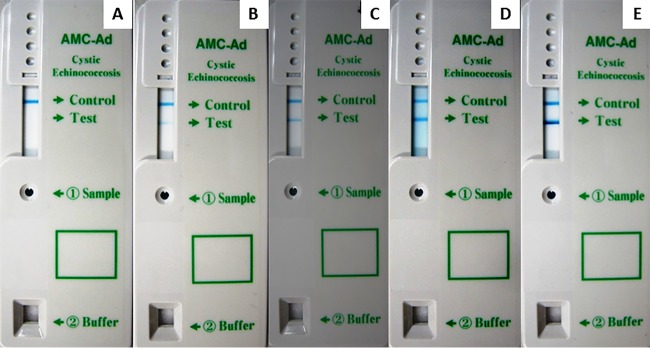
Results of ICT were independently read and classified according to band intensity by two observers; a third observer provided an additional read in the event of discordance of the other reads. A sample was considered positive if two color bands (corresponding to rAgB and anti-goat IgG antibody) appeared in the result window, whereas it was considered negative if only one band (corresponding to anti-goat IgG antibody) appeared in the result window (Fig. 1); in cases where there was no appearance of a colored anti-goat IgG antibody line, the assay was considered invalid even if a colored rAgB line appeared, and the test had to be repeated. In addition, according to the intensity of the band corresponding to rAgB, ICT results were classified into 4 categories: faint, weak, medium (equal to the control), and strong (Fig. 1 and 2).
FIG 1.
Examples of ICTs with negative results and different intensities of positive bands. (A) Negative result; (B) positive faint-intensity result; (C) positive weak-intensity result; (D) positive medium-intensity (equal to control) result; (E) positive strong-intensity result.
FIG 2.
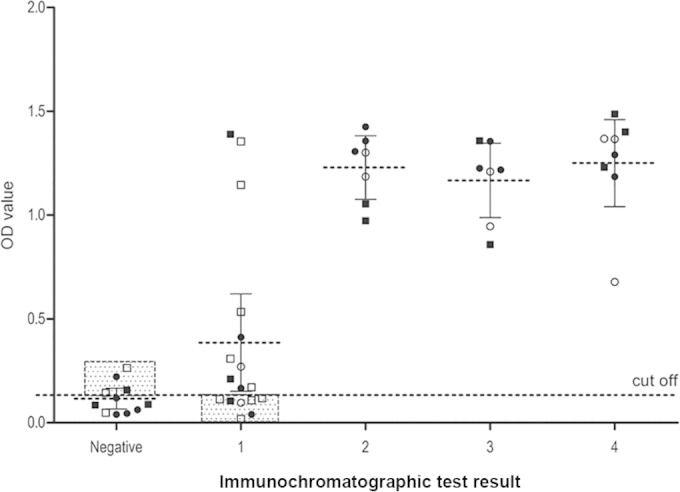
ICT results by organ and presence of cyst complication according to band intensity and ELISA result. 1, faint; 2, weak; 3, medium (equal to the control); 4, strong. Circles represent liver involvement, and squares represent lung involvement. Filled symbols represent the presence of cyst complication. Discordant pairs are framed and shaded.
Enzyme-linked immunosorbent assay.
ELISA was carried out in flat-bottom 96-well microplates (Nunc, Maxisorp, Roskilde, Denmark) as previously described (18). The microplates were coated with 100 ng/ml of rAgB diluted in phosphate-buffered saline (PBS) and incubated at 4°C overnight. Excess antigen was removed by washing with PBS. Blocking was performed with blocking buffer (1% casein in 20 mM Tris-HCl [pH 7.6] containing 150 mM NaCl), and the plates were incubated at 37°C for 1 h. The plates were washed twice with PBS containing 0.05% Tween 20 (PBST); 100 μl of diluted sera (1/100 dilution in blocking buffer) was added, and plates were incubated at 37°C for 1.5 h. After five washes with PBST, 100 μl of protein G-peroxidase conjugate (1/4,000 dilution in blocking buffer; Invitrogen, Camarillo, CA, USA) was added to each well, and the plates were incubated at 37°C for 1.5 h. Plates were washed six times with PBST and once with PBS and incubated with 100 μl of substrate solution (0.4 mM 2,2-azino-di[3-ethyl-benzthiazoline-6-sulfonate] in 0.2 M citric acid buffer [pH 4.7]) at 25°C for 30 min. The color reaction was stopped by application of 1% SDS. The optical density at 405 nm (OD405) was determined using an ELISA plate reader (Immuno Mini NJ2300; Nalge Nunc International, Tokyo, Japan). The cutoff point was set as the mean OD plus 3 standard deviations (SD) for 28 negative-control samples (from healthy individuals).
Statistical analysis.
The overall sensitivities of ICT and ELISA were compared using a McNemar test; additionally, the association between a positive result in either the ELISA or the ICT and the characteristics of the disease (e.g., number of cysts, organ involved, and presence of cyst complication) was evaluated by single (SLR) and multiple (MLR) logistic regression analyses. Agreement between ICT result and ELISA was calculated and analyzed using the kappa test. We considered differences with a confidence interval of 95% or higher statistically significant (P ≤ 0.05).
RESULTS
The overall sensitivities of ICT and ELISA were not statistically different (78% versus 72%; P = 0.36), with minor variations in terms of the organ involved, the number of cysts, and the presence of cyst complications (“complicated cyst” refers to any cyst that could be either broken or infected). SLR and MLR showed no association between cysts' characteristics and a positive result in either test (Table 1).
TABLE 1.
Sensitivity of ELISA and ICT according to cyst characteristica
| CE characteristic | ICT |
ELISA |
||||
|---|---|---|---|---|---|---|
| No. positive (%) | SLR OR (95% CI) | MLR OR (95% CI) | No. positive (%) | SLR OR (95% CI) | MLR OR (95% CI) | |
| Involved organ | ||||||
| Lung (n = 25) | 19 (76.0) | 1 (ref) | 1 (ref) | 17 (68.0) | 1 (ref) | 1 (ref) |
| Liver (n = 25) | 20 (80.0) | 1.26 (0.32–4.83) | 1.26 (0.32–4.83) | 19 (76.0) | 1.49 (0.43–5.17) | 1.56 (0.43–5.66) |
| No. of cysts | ||||||
| Single (n = 33) | 26 (78.8) | 1 (ref) | 1 (ref) | 22 (66.7) | 1 (ref) | 1 (ref) |
| Multiple (n = 17) | 13 (76.5) | 0.87 (0.21–3.53) | 0.82 (0.20–3.43) | 14 (82.4) | 2.33 (0.55–9.87) | 2.08 (0.47–9.17) |
| Presence of complication | ||||||
| Uncomplicated (n = 24) | 18 (75.0) | 1 (ref) | 1 (ref) | 15 (62.5) | 1 (ref) | 1 (ref) |
| Complicated (n = 26) | 21 (80.8) | 1.40 (0.36–5.36) | 1.45 (0.37–5.70) | 21 (80.8) | 2.52 (0.70–9.04) | 2.28 (0.61–8.45) |
SLR, single logistic regression; MLR, multiple logistic regression (adjusting each variable by the other listed variables); OR, odds ratio; CI, confidence interval; ref, reference.
The concordance between the results of both tests was evaluated by the kappa test (Table 2). Overall agreement between both tests was moderate (κ = 0.41; P < 0.01). However, a substantial and an almost perfect agreement between ICT and ELISA were found for patients with liver involvement (κ = 0.65; P < 0.001) and for patients with more than one hydatid cyst (κ = 0.82; P < 0.001) (Fig. 2).
TABLE 2.
Agreement between ELISA and ICT according to CE characteristic
| CE characteristic | No. (%) of: |
Kappa (P) | |
|---|---|---|---|
| Concordant pairs | Discordant pairs | ||
| Organ | |||
| Liver | 22 (88.0) | 3 (12.0) | 0.65 (<0.001) |
| Lung | 17 (68.0) | 8 (32.0) | 0.21 (0.14) |
| No. of cysts | |||
| Single | 23 (69.7) | 10 (30.3) | 0.25 (0.07) |
| Multiple | 16 (94.1) | 1 (5.9) | 0.82 (<0.001) |
| Presence of complication | |||
| Uncomplicated | 17 (70.8) | 7 (29.2) | 0.33 (0.04) |
| Complicated | 22 (84.6) | 4 (15.4) | 0.50 (<0.05) |
Most of the disagreement occurred in patients with uncomplicated lung cysts. Thus, we explored the characteristics of the disease according to the intensity of the ICT reaction. Interestingly, all uncomplicated lung cysts which were positive by ICT had been read as faint, with no weak, moderate, or strong reactions in this group (Table 3).
TABLE 3.
Results of ICT using the RAgB8/1with sera from CE patients
| CE organ involved | CE type | No. of cysts | No. (%) of results that were: |
||||
|---|---|---|---|---|---|---|---|
| Negative | Faint | Weak | Medium | Strong | |||
| Lung | Uncomplicated | Single (n = 9) | 2 (22.2) | 7 (77.8) | 0 | 0 | 0 |
| Multiple (n = 3) | 1 (33.3) | 2 (66.7) | 0 | 0 | 0 | ||
| Complicated | Single (n = 7) | 2 (28.6) | 2 (28.6) | 2 (28.6) | 1 (14.2) | 0 | |
| Multiple (n = 6) | 1 (16.6) | 1 (16.6) | 0 | 1 (16.6) | 3 (50.0) | ||
| Liver | Uncomplicated | Single (n = 9) | 2 (22.2) | 2 (22.2) | 1 (11.1) | 1 (11.1) | 3 (33.3) |
| Multiple (n = 3) | 1 (33.3) | 0 | 1 (33.3) | 1 (33.3) | 0 | ||
| Complicated | Single (n = 8) | 1 (12.5) | 2 (25.0) | 0 | 3 (37.5) | 2 (25.0) | |
| Multiple (n = 5) | 1 (20.0) | 1 (20.0) | 3 (60.0) | 0 | 0 | ||
The specificity of ICT was evaluated using sera from healthy individuals and other parasitic infections. All samples were negative by ICT except nine (47.4%) sera from AE patients. Specificities of ICT with and without AE sera were 89.8% and 100.0%, respectively.
DISCUSSION
We present here the results of an exploratory study comparing the sensitivity of a rapid test (ICT) using the recombinant antigen B8/1 (rAgB) with a standard ELISA using the same antigen, in paired serum samples from patients with lung or liver CE. The sensitivities of both tests were similar, with values in the range reported by previous studies using other antigens and techniques (6–8).
The sensitivity of standard ELISA using rAgB in our study is lower than the sensitivity reported by Mohammadzadeh et al. (94.6%); (13) but very similar to that reported by Li et al. (77.6%) (18). The performance of immunodiagnostic tools is affected by some disease characteristics, including cyst location, presence of cyst complication, and number of cystic lesions (6–8). The higher sensitivity of ELISA in the series reported by Mohammadzadeh et al. (13) could be associated with higher proportions of individuals with large cysts, surgical cases, multiple cysts, or complicated lesions; unfortunately, no information is provided on the characteristics of the cases evaluated. On the other hand, the ELISA sensitivity among liver cases found in our series (80%) is almost the same that reported by Li et al., who used a series of liver CE cases.
The overall agreement observed between ICT and ELISA was moderate (κ = 0.41; P < 0.001). This value of kappa is lower than those found by previous studies that transferred a recombinant antigen (rEm18) of another Echinococcus species, Echinococcus multilocularis, from an ELISA to an ICT format (15, 16). Agreement could have been affected by the type of antibody detected by each test: the ELISA evaluated only a specific IgG response, whereas ICT evaluated both IgG and IgE responses. There are differences in Ig subtype responses: the IgG response in CE is stronger than the IgE response, although there is a higher production of anti-IgE among liver CE patients (19, 20). In our case, the discrepancies between tests are mainly attributable to a sizable proportion of subjects with a faint reaction in the ICT (7/11 [63%]), which is mainly observed in subjects with lung CE (5/7 [71%]). In addition, most faint reactions occurred in lung infection cases, suggesting that these were not false-positive results, which would have been similarly frequent or even more frequent in patients with liver disease, which tends to have a stronger response. The concentrations of rAgB used in the elaboration of each test (much higher in the ICT) could also have contributed to the observed differences in case detection.
Nine (47.4%) of 19 AE patients' sera showed a positive result by ICT, and this result was concordant with the previous reports showing that 40 to 85% of AE patient sera reacted with rAgB by ELISA (17, 18); however, since these previous studies were performed in arctic/subarctic areas where AE and CE are coendemic, this cross-reaction will not be an issue if we use the ICT in Peru, which is located in a subtropical area where infection by E. multilocularis has not been reported. In addition, in our study, sera from healthy subjects and patients with other parasitic infections except AE showed a negative result in the ICT, which indicated that this ICT was a highly specific test.
ICT using rAgB is a rapid and simple test that demonstrated a good sensitivity for the detection of specific antibodies to E. granulosus. Further analysis of the stability of ICT and a large-scale evaluation might be necessary to evaluate its utility in mass screening programs in areas of endemicity as a primary screening tool.
ACKNOWLEDGMENTS
Partial support from FIC-NIH training grant TW001140 is acknowledged. H.H.G. is supported by a Wellcome Trust Senior International Research Fellowship in Public Health and Tropical Medicine. Production of immunochromatographic test kits for both CE (ADAMU-CE) and AE (ADAMU-AE) was financially supported by the Translational Research Project (2007-2011) by the Ministry of Education, Japan. The serological studies were supported by a Grant-in-Aid for Scientific Research from Japan Society for the Promotion of Science (21256003, 24256002) to A.I.
REFERENCES
- 1.Brunetti E, and White AC Jr. 2012. Cestode infestations: hydatid disease and cysticercosis. Infect Dis Clin North Am 26:421–435. doi: 10.1016/j.idc.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 2.Wang Z, Wang X, Liu X. 2008. Echinococcosis in China, a review of the epidemiology of Echinococcus spp. Ecohealth 5:115–126. doi: 10.1007/s10393-008-0174-0. [DOI] [PubMed] [Google Scholar]
- 3.Yang YR, Sun T, Li Z, Zhang J, Teng J, Liu X, Liu R, Zhao R, Jones MK, Wang Y, Wen H, Feng X, Zhao Q, Zhao Y, Shi D, Bartholomot B, Vuitton DA, Pleydell D, Giraudoux P, Ito A, Danson MF, Boufana B, Craig PS, Williams GM, McManus DP. 2006. Community surveys and risk factor analysis of human alveolar and cystic echinococcosis in Ningxia Hui Autonomous Region, China. Bull World Health Organ 84:714–721. doi: 10.2471/BLT.05.025718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.da Silva AM. 2010. Human echinococcosis: a neglected disease. Gastroenterol Res Pract 2010:583297. doi: 10.1155/2010/583297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brunetti E, Kern P, Vuitton DA, Writing Panel for the WHO-IWGE . 2010. Expert consensus for the diagnosis and treatment of cystic and alveolar echinococcosis in humans. Acta Trop 114:1–16. doi: 10.1016/j.actatropica.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 6.Hernandez-Gonzalez A, Santivanez S, Garcia HH, Rodriguez S, Munoz S, Ramos G, Orduna A, Siles-Lucas M. 2012. Improved serodiagnosis of cystic echinococcosis using the new recombinant 2B2t antigen. PLoS Negl Trop Dis 6:e1714. doi: 10.1371/journal.pntd.0001714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Santivanez SJ, Arias P, Portocarrero M, Rodriguez S, Gonzalez AE, Gilman RH, Gavidia CM, Garcia HH. 2012. Serological diagnosis of lung cystic hydatid disease using the synthetic p176 peptide. Clin Vaccine Immunol 19:944–947. doi: 10.1128/CVI.05540-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Santivanez SJ, Sotomayor AE, Vasquez JC, Somocurcio JG, Rodriguez S, Gonzalez AE, Gilman RH, Garcia HH. 2008. Absence of brain involvement and factors related to positive serology in a prospective series of 61 cases with pulmonary hydatid disease. Am J Trop Med Hyg 79:84–88. [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang W, Wen H, Li J, Lin R, McManus DP. 2012 Immunology and immunodiagnosis of cystic echinococcosis: an update. Clin Dev Immunol 2012:101895. doi: 10.1155/2012/101895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barnes TS, Deplazes P, Gottstein B, Jenkins DJ, Mathis A, Siles-Lucas M, Torgerson PR, Ziadinov I, Heath DD. 2012. Challenges for diagnosis and control of cystic hydatid disease. Acta Trop 123:1–7. doi: 10.1016/j.actatropica.2012.02.066. [DOI] [PubMed] [Google Scholar]
- 11.Tawfeek GM, Elwakil HS, El-Hoseiny L, Thabet HS, Sarhan RM, Awad NS, Anwar WA. 2011. Comparative analysis of the diagnostic performance of crude sheep hydatid cyst fluid, purified antigen B and its subunit (12 Kda), assessed by ELISA, in the diagnosis of human cystic echinococcosis. Parasitol Res 108:371–376. doi: 10.1007/s00436-010-2074-9. [DOI] [PubMed] [Google Scholar]
- 12.Carmena D, Benito A, Eraso E. 2007. Recent advances in the immunodiagnosis of human cystic echinococcosis. Enferm Infecc Microbiol Clin 25:263–269. (In Spanish.) doi: 10.1157/13100468. [DOI] [PubMed] [Google Scholar]
- 13.Mohammadzadeh T, Sako Y, Sadjjadi SM, Sarkari B, Ito A. 2012. Comparison of the usefulness of hydatid cyst fluid, native antigen B and recombinant antigen B8/1 for serological diagnosis of cystic echinococcosis. Trans R Soc Trop Med Hyg 106:371–375. doi: 10.1016/j.trstmh.2012.01.012. [DOI] [PubMed] [Google Scholar]
- 14.Tamarozzi F, Sako Y, Ito A, Piccoli L, Grisolia A, Itoh S, Gatti S, Meroni V, Genco F, Brunetti E. 2013. Recombinant AgB8/1 ELISA test vs commercially available IgG ELISA test in the diagnosis of cystic echinococcosis. Parasite Immunol 35:433–440. doi: 10.1111/pim.12050. [DOI] [PubMed] [Google Scholar]
- 15.Sako Y, Fukuda K, Kobayashi Y, Ito A. 2009. Development of an immunochromatographic test to detect antibodies against recombinant Em18 for diagnosis of alveolar echinococcosis. J Clin Microbiol 47:252–254. doi: 10.1128/JCM.01476-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sako Y, Tappe D, Fukuda K, Kobayashi Y, Itoh S, Frosch M, Gruner B, Kern P, Ito A. 2011. Immunochromatographic test with recombinant Em18 antigen for the follow-up study of alveolar echinococcosis. Clin Vaccine Immunol 18:1302–1305. doi: 10.1128/CVI.05156-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mamuti W, Yamasaki H, Sako Y, Nakao M, Xiao N, Nakaya K, Sato N, Vuitton DA, Piarroux R, Lightowlers MW, Craig PS, Ito A. 2004. Molecular cloning, expression, and serological evaluation of an 8-kilodalton subunit of antigen B from Echinococcus multilocularis. J Clin Microbiol 42:1082–1088. doi: 10.1128/JCM.42.3.1082-1088.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li T, Ito A, Chen X, Sako Y, Qiu J, Xiao N, Qiu D, Nakao M, Yanagida T, Craig PS. 2010. Specific IgG responses to recombinant antigen B and em18 in cystic and alveolar echinococcosis in China. Clin Vaccine Immunol 17:470–475. doi: 10.1128/CVI.00466-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Force L, Torres JM, Carrillo A, Busca J. 1992. Evaluation of eight serological tests in the diagnosis of human echinococcosis and follow-up. Clin Infect Dis 15:473–480. doi: 10.1093/clind/15.3.473. [DOI] [PubMed] [Google Scholar]
- 20.Sbihi Y, Rmiqui A, Rodriguez-Cabezas MN, Orduna A, Rodriguez-Torres A, Osuna A. 2001. Comparative sensitivity of six serological tests and diagnostic value of ELISA using purified antigen in hydatidosis. J Clin Lab Anal 15:14–18. doi:. [DOI] [PMC free article] [PubMed] [Google Scholar]