Abstract
A Streptococcus suis isolate from a German hunter with streptococcal toxic shock-like syndrome (STSLS) and four additional zoonotic isolates were genotyped as mrp+ epf* (variant 1890) sly+ cps2+. All five zoonotic German strains were characterized by high multiplication in human blood samples ex vivo, but induction of only low levels of proinflammatory cytokines compared to a Chinese STSLS strain.
TEXT
Streptococcus suis is an important porcine and human pathogen causing septicemia, meningitis, and other pathologies (1, 2). More than 90% of the strains isolated from humans belong to serotype 2 (3–5), although other serotypes, such as 9 and 7, are epidemiologically also very important in pigs (6). In addition to domesticated pigs, wild boars are an important reservoir for S. suis serotype 2 strains in Germany (7). Accordingly, zoonotic cases have been described in hunters (8–12).
In August 2005, a zoonotic outbreak of S. suis diseases occurred in China, including at least 37 cases of streptococcal toxic shock-like syndrome (STSLS) (13). Multilocus sequence typing (MLST) revealed that the Chinese STSLS isolates belong to sequence type (ST) 7 within clonal complex (CC) 1 (14). An 89-kb region designated a pathogenicity island was described as a hallmark of the genome of the Chinese STSLS isolates (15) but was later identified as an integrative conjugative element present also in a similar form in S. suis isolates in Vietnam not associated with STSLS (16).
This study was initiated after a fatal case of STSLS in a German hunter. The hunter showed rapid clinical deterioration marked by hypotension (70/50 mm Hg) and multiorgan failure, including severe hepatic and renal impairment despite hospitalization in an intensive care unit. Furthermore, cardiomyopathy and coagulopathy with severe thrombocytopenia (platelets of 14/nl) as well as disseminated intravascular coagulation associated with petechiae were diagnosed (Quick value of 5% and partial thromboplastin time of >200 s). S. suis (strain BK52339) was isolated in pure culture from this patient's blood sample. The diagnosis of STSLS is in accordance with the clinical criteria defined by others (17).
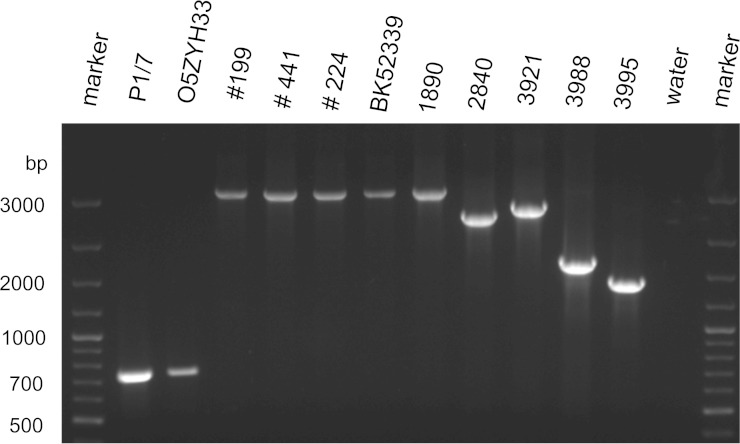
The MLST analysis (18) revealed that the German STSLS isolate BK52339, four additional German zoonotic strains, and 3 of 4 cps2+ S. suis strains from wild boars belonged to ST1 (Table 1). Differentiation of virulence-associated genes using a multiplex (MP)-PCR (19) and a PCR for detection of the 89-kb region demonstrated that BK52339 is an mrp+ sly+ cps2+ strain lacking the 89-kb region present in the genome of Chinese STSLS strains like O5ZYH33 (results not shown). The European serotype 2 strains causing problems in the pig industry belong mainly to ST1 and carry, in addition to mrp and sly, an epf gene encoding a 110-kDa extracellular factor (EF) (20). The genotypic analysis of the zoonotic German strains also included an epf PCR (19) for determination of specific variants of this highly variable gene (Fig. 1). Interestingly, the STSLS strain BK52339 as well as the other 4 German zoonotic strains generated epf amplification products of the same size as reference strain 1890 (Fig. 1) (21). This variant was also found in 2 wild boar isolates (W183.1 and W188.1) (Fig. 1) (7). In conclusion, the case of STSLS described in a hunter and the other four German zoonotic S. suis cases investigated were caused by a specific mrp+ sly+ epf* (1890) cps2+ ST1 strain. At least one of the cps2+ strains (W183.1) previously isolated from wild boars in Germany also showed this genotype.
TABLE 1.
Clinical background, sequence type, and profile of virulence-associated genes of S. suis strains investigated in this study
| S. suis strain | Source |
cps | MLST |
Profile of virulence-associated genes | ||
|---|---|---|---|---|---|---|
| Clinical background | Reference or study | Sequence type | Clonal complex | |||
| BK52339 | Human (hunter): STSLS | This study | cps2 | 1 | 1 | mrp+ epf* (1890) sly+ |
| MAC 724 | Human: sudden death | 7 | cps2 | 1 | 1 | mrp+ epf* (1890) sly+a |
| 199 | Human (hunter): meningitis | 7;11 | cps2 | 1 | 1 | mrp+ epf* (1890) sly+a |
| 441 | Human: septicemia | cps2 | 1 | 1 | mrp+ epf* (1890) sly+ | |
| 224 | Human: endocarditis | 24 | cps2 | 1 | 1 | mrp+ epf* (1890) sly+ |
| O5ZYH33 | Human: STSLS | 15;25 | cps2 | 7 | 1 | mrp+ epf+ sly+ |
| W57.2 | Wild boar carrier | 7 | cps2 | 1 | 1 | mrp+ epf* (3004) sly+a |
| W59.2 | Wild boar carrier | 7 | cps2 | 1 | 1 | mrp+ epf* (3004) sly+a |
| W183.1 | Wild boar carrier | 7 | cps2 | 1 | 1 | mrp+ epf* (1890) sly+a |
| W188.1 | Wild boar carrier | 7 | cps2 | 156 | 1 | mrp+ epf* (1890) sly+a |
| P1/7 | Pig: meningitis | 26 | cps2 | 1b | 1 | mrp+ epf+ sly+ |
| T15 | Pig: pneumonia | 27 | cps2 | |||
| B2441/96 | Pig: pneumonia | cps2 | 28c | 27c | ||
| A3286/94 | Pig: meningitis | cps9 | 99c | 16 (87)c | mrp* sly+ | |
| B2663/96 | Pig | cps9 | mrp* sly+ | |||
| B2795/96 | Pig: pneumonia | cps7 | 29 | 27d | ||
| 451 | Pig: meningitis | cps7 | ||||
FIG 1.
Differentiation of epf* variants by PCR (19) revealed that the German STSLS strain BK52339 carries the 1890 epf* variant, which was also found in other German zoonotic isolates (199, 441, and 224). Strains 1890, 2840, 3921,3988, and 3995 were included for determination of epf variants as in previous studies (7, 21).
To phenotypically characterize the German zoonotic isolates, we determined their survival in human blood samples ex vivo in comparison to that of various other strains by inoculating 1 ml of freshly drawn heparinized blood with approximately 1.5 × 105 CFU. Importantly, all 5 human zoonotic strains from Germany that we investigated, including the STSLS strain from the hunter, at least tripled the mean specific bacterial load during 2 h of incubation at 37°C in human blood samples (Fig. 2A). In contrast, the Chinese STSLS strain O5ZYH33 and the CC27 strain B2441/96 were efficiently killed (mean survival factor [SF], 0.05 [SD, 1.18] and mean SF, 0.14 [SD 0.21], respectively). The survival factors of the four cps2+ strains isolated from wild boars were significantly lower than those of the German zoonotic strains (analysis of variance [ANOVA], P < 0.05), although the wild boar strain W57.2 doubled its number during 2 h of incubation in human blood samples (mean SF, 2.1 [SD, 1.18]). In conclusion, the STSLS isolate BK52339 and the other 4 German zoonotic strains investigated share a specific profile of virulence-associated factors [mrp+ sly+ epf* (1890) cps2+] and the ability to proliferate efficiently in human blood samples ex vivo in contrast to the Chinese STSLS strain O5ZYH33, the CC27 strain B2441/96, and the two cps7 and two cps9 strains. However, the zoonotic strains of other clonal complexes are negative for epf but also belong to serotype 2, which indicates that the capsule is a major zoonotic determinant (5). Of note, transmission electron microscopy using lysine-ruthenium red (LRR) staining as described previously (22) confirmed that the two STSLS isolates, O5ZYH33 and BK52339, are both encapsulated, which makes it unlikely that reduced encapsulation is the explanation for the lower survival in human blood samples of the former (Fig. 2).
FIG 2.
Comparative analysis of survival of S. suis strains isolated from humans in Germany (■) to other cps2, cps9, and cps7 strains in human blood samples ex vivo (A) and confirmation of capsule expression by LRR staining and transmission electron microscopy in the strains indicated (B). The German STSLS isolate BK52339 showed a high survival factor in human blood samples ex vivo comparable to the survival factors of other cps2 strains of CC1 but significantly higher than the survival factors of the STSLS Chinese isolate O5ZYH33 and the CC27 strain B2441/96. The survival factor of each strain was determined 7 times in independent experiments with blood samples from different German volunteers not exposed to pigs occupationally. Strains 10, MAC724, W183.1, W188.1, A3286/94, B2663/93, B2795/96, and 451 were not included in every experiment but at least in 5 of the 7. The specific bacterial contents (CFU/ml) were determined through serial platings after 0 min and 120 min of incubation at 37°C. The survival factor represents the ratio of the CFU after 2 h to the CFU at time zero. Bars and error bars represent mean values and standard deviations, respectively. Analysis of variance (ANOVA) was conducted for statistics. Survival factors significantly different from those of strain BK52339 are indicated by asterisks (P < 0.05). Bars in panel B, 200 nm.
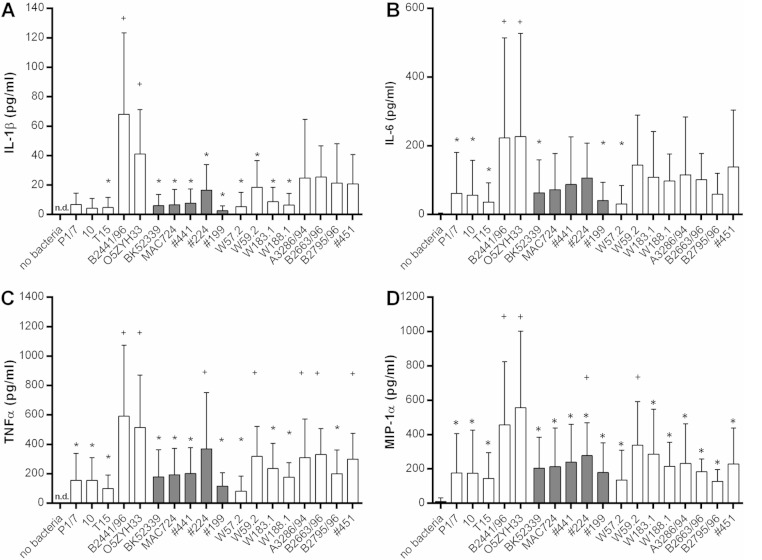
A cytokine storm has been discussed as a distinct feature of the pathogenesis of STSLS caused by S. suis as patients with S. suis-associated STSLS showed high serum concentrations of interleukin-6 (IL-6), tumor necrosis factor-alpha (TNF-α), and IL-1β (17). Furthermore, infections of mice demonstrated that a Chinese ST7 STSLS strain induced higher systemic levels of these proinflammatory cytokines than the virulent European mrp+ epf+ sly+ cps2+ strain P1/7 (23). Accordingly, we hypothesized that induction of cytokines in human blood samples ex vivo might show associations with the different clinical backgrounds of our strains under investigation. The cytokine concentrations were determined using human cytometric bead array flex sets (BD Biosciences, San Jose, CA, USA) with an Accuri C6 flow cytometer (BD Biosciences). As shown in Fig. 3 infection of human blood samples with the Chinese strain O5ZYH33 for 2 h led to levels of IL-1β (mean 41 pg/dl; SD, 30 pg/dl), IL-6 (mean, 227 pg/dl; SD, 300 pg/dl), TNF-α (mean, 515 pg/dl; SD, 356 pg/dl), and macrophage inflammatory protein 1 alpha (MIP-1α) (mean, 557 pg/dl; SD, 444 pg/dl) that were significantly higher than the concentrations recorded after incubation with the STSLS isolate BK52399 for IL-1β (mean, 6 pg/dl; SD, 8 pg/dl), IL-6 (mean, 63 pg/dl; SD, 95 pg/dl), TNF-α, (mean, 178 pg/dl; SD, 185 pg/dl), and MIP-1α, (mean, 204 pg/dl; SD, 180 pg/dl). In general, the levels of these proinflammatory cytokines were rather low in blood samples incubated with any of the German zoonotic strains. Significant differences among the German zoonotic strains in cytokine induction were not observed with the exception of a significantly higher level of TNF-α in strain 224-infected than in strain 199-infected blood samples. None of the other S. suis strains tested, including the isolates from wild boars and the two cps7 and two cps9 strains, induced IL-1β, IL-6, and MIP-1α concentrations significantly different from those for the German zoonotic strains with the clear exception of the CC27 strain B2441/96 inducing IL-1β, IL-6, TNF-α, and MIP-1α levels similar to those for the Chinese STSLS strain O5ZYH33 (Fig. 3). In conclusion, the German zoonotic strains exhibited prominent growth in human blood samples but, in comparison to the STSLS Chinese strain O5ZYH33 and the CC27 strain B2441/96, only low induction of proinflammatory cytokines such as IL-1β, IL-6, TNF-α, and MIP-1α within 2 h of ex vivo infection of human blood samples.
FIG 3.
The Chinese S. suis STSLS strain O5ZYH33 and the CC27 strain B2441/96 elicited significantly higher titers of proinflammatory cytokines in 2 h of infection of human blood samples ex vivo than the STSLS isolate BK52399 of the hunter and other zoonotic strains from Germany (■). Bars show the mean concentrations of IL-1β (A), IL-6 (B), TNF-α (C), and MIP-1α (D) in heparin plasma collected after 2 h of ex vivo infection with the indicated S. suis strains of 7 independent experiments (see Fig. 2; no bacteria refers to the mock control; n.d., not detectable). Strains 10, MAC724, W183.1, W188.1, A3286/94, B2663/96, B2795/96, and 451 were not included in every experiment but at least in 5 of the 7. Significant differences from the values for strain O5ZYH33 are indicated by asterisks (P < 0.05). The mean values of strain B2441/96 were also significantly increased from the values marked with asterisks in addition to the TNF-α concentrations of strains 451, A3286/94, and W59.2. Significant differences among the German zoonotic strains were not recorded, except for a significant higher induction of TNF-α in strain 224-infected in comparison to 199-infected blood samples. Significant differences from the control lacking bacteria are indicated by plus signs (P < 0.05).
ACKNOWLEDGMENTS
S. suis strains O5ZYH33, 199, MAC 724, 441, 10, and T15 were kindly provided by Jiaqi Tang (Research Institute for Medicine of Nanjing Command, Nanjing, China), Ingo Sobottka (Institute for Infection Medicine, University Hospital Hamburg Eppendorf, Germany), Rudolf Lütticken (German National Reference Center for Streptococci, University Hospital RWTH Aachen, Germany), Barbara Spellerberg (Institute of Medical Microbiology and Hygiene, Ulm University, Germany), and Hilde Smith and Henk Wisselink (both DLO-Lelystadt, The Netherlands), respectively.
This study was financially supported by the German Federal Ministry for Research and Education (BMBF) within the Helmholtz-CAS-Joint Research Group ZooStrep (HCJRG-116). C.H. is supported by SFB 1021 from the Deutsche Forschungsgemeinschaft. The Hessian State Laboratory is supported by the Hessian Ministry for the Environment, Climate Change, Agriculture and Consumer Protection.
REFERENCES
- 1.Gottschalk M. 2011. Streptococcosis, p 841–855. In Zimmerman JJ, Kariker LA, Ramirez A, Schwartz KJ, Stevenson GW (ed), Diseases of swine, 10th ed Wiley-Blackwell, Oxford, United Kingdom. [Google Scholar]
- 2.Gottschalk M, Xu J, Calzas C, Segura M. 2010. Streptococcus suis: a new emerging or an old neglected zoonotic pathogen? Future Microbiol 5:371–391. doi: 10.2217/fmb.10.2. [DOI] [PubMed] [Google Scholar]
- 3.Mai NT, Hoa NT, Nga TV, Linh ID, Chau TT, Sinh DX, Phu NH, Chuong LV, Diep TS, Campbell J, Nghia HD, Minh TN, Chau NV, de Jong MD, Chinh NT, Hien TT, Farrar J, Schultsz C. 2008. Streptococcus suis meningitis in adults in Vietnam. Clin Infect Dis 46:659–667. doi: 10.1086/527385. [DOI] [PubMed] [Google Scholar]
- 4.Arends JP, Zanen HC. 1988. Meningitis caused by Streptococcus suis in humans. Rev Infect Dis 1988 10:131–137. [DOI] [PubMed] [Google Scholar]
- 5.Schultsz C, Jansen E, Keijzers W, Rothkamp A, Duim B, Wagenaar JA, van der Ende A. 2012. Differences in the population structure of invasive Streptococcus suis strains isolated from pigs and from humans in The Netherlands. PLoS One 7:e33854. doi: 10.1371/journal.pone.0033854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wisselink HJ, Smith HE, Stockhofe-Zurwieden N, Peperkamp K, Vecht U. 2000. Distribution of capsular types and production of muramidase-released protein (MRP) and extracellular factor (EF) of Streptococcus suis strains isolated from diseased pigs in seven European countries. Vet Microbiol 74:237–248. doi: 10.1016/S0378-1135(00)00188-7. [DOI] [PubMed] [Google Scholar]
- 7.Baums CG, Verkühlen GJ, Rehm T, Silva LM, Beyerbach M, Pohlmeyer K, Valentin-Weigand P. 2007. Prevalence of Streptococcus suis genotypes in wild boars of Northwestern Germany. Appl Environ Microbiol 73:711–717. doi: 10.1128/AEM.01800-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bonmarchand G, Massari P, Humbert G, Leroy J, Morel A, Lemeland JF, Vannier P. 1985. Group-R streptococci- wild boars as a 2nd reservoir. Scand J Infect Dis 17:121–122. doi: 10.3109/00365548509070431. [DOI] [PubMed] [Google Scholar]
- 9.Grebe T, Bergenthal D, Fahr AM, Scheja HW. 1997. Meningitis caused by Streptococcus suis type 2 in an adult. Dtsch Med Wochenschr 122:1244–1247. (In German.) doi: 10.1055/s-2008-1047754. [DOI] [PubMed] [Google Scholar]
- 10.Halaby T, Hoitsma E, Hupperts R, Spanjaard L, Luirink M, Jacobs J. 2000. Streptococcus suis meningitis, a poacher's risk. Eur J Clin Microbiol Infect Dis 19:943–5. doi: 10.1007/PL00011230. [DOI] [PubMed] [Google Scholar]
- 11.Rosenkranz M, Elsner HA, Sturenburg HJ, Weiller C, Rother J, Sobottka I. 2003. Streptococcus suis meningitis and septicemia contracted from a wild boar in Germany. J Neurol 250:869–870. doi: 10.1007/s00415-003-1103-3. [DOI] [PubMed] [Google Scholar]
- 12.Pedroli S, Kobisch M, Beauchet O, Chaussinand JP, Lucht F. 2003. Streptococcus suis bacteremia. Presse Med 32:599–601. (In French.) [PubMed] [Google Scholar]
- 13.Tang J, Wang C, Feng Y, Yang W, Song H, Chen Z, Yu H, Pan X, Zhou X, Wang H, Wu B, Wang H, Zhao H, Lin Y, Yue J, Wu Z, He X, Gao F, Khan AH, Wang J, Zhao GP, Wang Y, Wang X, Chen Z, Gao GF. 2006. Streptococcal toxic shock syndrome caused by Streptococcus suis serotype 2. PLoS Med 3:e151. doi: 10.1371/journal.pmed.0030151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ye C, Zhu X, Jing H, Du H, Segura M, Zheng H, Kan B, Wang L, Bai X, Zhou Y, Cui Z, Zhang S, Jin D, Sun N, Luo X, Zhang J, Gong Z, Wang X, Wang L, Sun H, Li Z, Sun Q, Liu H, Dong B, Ke C, Yuan H, Wang H, Tian K, Wang Y, Gottschalk M, Xu J. 2006. Streptococcus suis sequence type 7 outbreak, Sichuan, China. Emerg Infect Dis 12:1203−1208. doi: 10.3201/eid1708.060232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen C, Tang J, Dong W, Wang C, Feng Y, Wang J, Zheng F, Pan X, Liu D, Li M, Song Y, Zhu X, Sun H, Feng T., Guo Z, Ju A, Ge J, Dong Y, Sun W, Jiang Y, Wang J, Yan J, Yang H, Wang X, Gao GF, Yang R, Wang J, Yu J. 2007. A glimpse of streptococcal toxic shock syndrome from comparative genomics of S. suis 2 Chinese isolates. PLoS One 2:e315. doi: 10.1371/journal.pone.0000315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holden MT, Hauser H, Sanders M, Ngo TH, Cherevach I, Cronin Goodhead AI, Mungall K, Quail MA, Price C, Rabbinowitsch E, Sharp S, Croucher NJ, Chieu TB, Mai NT, Diep TS, Chinh NT, Kehoe M, Leigh JA, Ward PN, Dowson CG, Whatmore AM, Chanter N, Iversen P, Gottschalk M, Slater JD, Smith HE, Spratt BG, Xu J, Ye C, Bentley S, Barrell BG, Schultsz C, Maskell DJ, Parkhill J. 2009. Rapid evolution of virulence and drug resistance in the emerging zoonotic pathogen Streptococcus suis. PLoS One 4:e6072. doi: 10.1371/journal.pone.0006072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ye C, Zheng H, Zhang J, Jing H, Wang L, Xiong Y, Wang W, Zhou Z, Sun Q, Luo X, Du H, Gottschalk M., Xu J. 2009. Clinical, experimental, and genomic differences between intermediately pathogenic, highly pathogenic, and epidemic Streptococcus suis. J Infect Dis 199:97–107. doi: 10.1086/594370. [DOI] [PubMed] [Google Scholar]
- 18.King SJ, Leigh JA, Heath PJ, Luque I, Tarradas C, Dowson CG, Whatmore AM. 2002. Development of a multilocus sequence typing scheme for the pig pathogen Streptococcus suis: Identification of virulent clones and potential capsular serotype exchange. J Clin Microbiol 40:3671–3680. doi: 10.1128/JCM.40.10.3671-3680.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Silva LM, Baums CG, Rehm T, Wisselink HJ, Goethe R, Valentin-Weigand P. 2006. Virulence-associated gene profiling of Streptococcus suis isolates by PCR. Vet Microbiol 115:117–127. doi: 10.1016/j.vetmic.2005.12.013. [DOI] [PubMed] [Google Scholar]
- 20.Vecht U, Wisselink HJ, Jellema ML, Smith HE. 1991. Identification of 2 proteins associated with virulence of Streptococcus suis type 2. Infect Immun 59:3156–3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smith HE, Reek FH, Vecht U, Gielkens ALJ, Smits MA. 1993. Repeats in an extracellular protein of weakly pathogenic strains of Streptococcus suis type-2 are absent in pathogenic strains. Infect Immun 61:3318–3326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hammerschmidt S, Wolff S, Hocke A, Rosseau S, Muller E, Rohde M. 2005. Illustration of pneumococcal polysaccharide capsule during adherence and invasion of epithelial cells. Infect Immun 73:4653–4667. doi: 10.1128/IAI.73.8.4653-4667.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lachance C, Gottschalk M, Gerber PP, Lemire P, Xu J, Segura M. 2013. Exacerbated type II interferon response drives hypervirulence and toxic shock by an emergent epidemic strain of Streptococcus suis. Infect Immun 81:1928–1939. doi: 10.1128/IAI.01317-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heidt MC, Mohamed W, Hain T, Vogt PR, Chakraborty T, Domann E. 2005. Human infective endocarditis caused by Streptococcus suis serotype 2. J Clin Microbiol 43:4898–4901. doi: 10.1128/JCM.43.9.4898-4901.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bi Y, Li J, Yang L, Zhang S, Li Y, Jia X, Sun L, Yin Y, Qin C, Wang B, Gao GF, Liu W. 2014. Assessment of the pathogenesis of Streptococcus suis type 2 infection in piglets for understanding streptococcal toxic shock-like syndrome, meningitis, and sequelae. Vet Microbiol 173:299–309. doi: 10.1016/j.vetmic.2014.08.010. [DOI] [PubMed] [Google Scholar]
- 26.Jacobs AAC, Loeffen PLW, van den Berg AJG, Storm PK. 1994. Identification, purification, and characterization of a thiol-activated hemolysin (suilysin) of Streptococcus suis. Infect Immun 62:1742–1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vecht U, Arends JP, van der Molen EJ, van Leengoed LA. 1989. Differences in virulence between two strains of Streptococcus suis type II after experimentally induced infection of newborn germ-free pigs. Am J Vet Res 50:1037–1043. [PubMed] [Google Scholar]
- 28.Rehm T, Baums CG, Strommenger B, Beyerbach M, Valentin-Weigand P, Goethe R. 2007. Amplified fragment length polymorphism of Streptococcus suis strains correlates with their profile of virulence-associated genes and clinical background. J Med Microbiol 56:102–109. doi: 10.1099/jmm.0.46616-0. [DOI] [PubMed] [Google Scholar]