Abstract
Pituitary adenylate cyclase-activating peptide (PACAP) is expressed within the gastroenteric system, where it has profound physiological effects. PACAP was shown to regulate food intake and thermogenesis centrally; however, PACAP peripheral regulation of appetite and feeding behavior is unknown. Therefore, we studied PACAP's effect on appetite and food intake control by analyzing feeding behavior and metabolic hormones in PAC1-deficient (PAC1−/−) and age-matched wild-type (WT) mice intraperitoneally injected with PACAP1–38 or PACAP1–27 before the dark phase of feeding. Food intake and feeding behavior were analyzed using the BioDAQ system. Active ghrelin, glucagon-like peptide-1 (GLP-1), leptin, peptide YY, pancreatic polypeptide, and insulin were measured following PACAP1–38 administration in fasted WT mice. PACAP1–38/PACAP1–27 injected into WT mice significantly decreased in a dose-dependent manner cumulative food intake and reduced bout and meal feeding parameters. Conversely, PACAP1–38 injected into PAC1−/− mice failed to significantly change food intake. Importantly, PACAP1–38 reduced plasma levels of active ghrelin compared with vehicle in WT mice. In PAC1−/− mice, fasting levels of active ghrelin, GLP-1, insulin, and leptin and postprandial levels of active ghrelin and insulin were significantly altered compared with levels in WT mice. Therefore, PAC1 is a novel regulator of appetite/satiety. PACAP1–38/PACAP1–27 significantly reduced appetite and food intake through PAC1. In PAC1−/− mice, the regulation of anorexigenic/orexigenic hormones was abolished, whereas active ghrelin remained elevated even postprandially. PACAP significantly reduced active ghrelin in fasting conditions. These results establish a role for PACAP via PAC1 in the peripheral regulation of appetite/satiety and suggest future studies to explore a therapeutic use of PACAP or PAC1 agonists for obesity treatment.
Keywords: pituitary adenylate cyclase-activating peptide, PAC1 receptor, appetite, ghrelin, GLP-1, leptin
pituitary adenylate cyclase-activating polypeptide (PACAP) was originally isolated from the ovine hypothalamus and is highly conserved among vertebrates (1). PACAP, because of its protein structure, particularly in the first 27 amino acids, belongs to the secretin-glucagon superfamily of hormones, which includes vasoactive intestinal peptide (VIP), gastric inhibitory peptide, glucagon-like peptide (GLP)-1 and GLP-2, growth hormone-releasing hormone, peptide histidine methionine, peptide histidine isoleucine, and exendins (34). PACAP exists in two variant forms: PACAP1–27 and the COOH-terminally extended form PACAP1–38 that originates from the same precursor and stimulates adenylate cyclase activity in pituitary cells (23, 35). PACAP functions through its PAC1 receptor and two VIP receptors, VPAC1 and VPAC2; however, PAC1 receptor is PACAP specific, exhibiting 1,000-fold greater affinity for PACAP than VIP (38). Localization studies in vertebrates have shown that PACAP and PAC1 are highly localized in the gastrointestinal (GI) tract and in the peripheral and central nervous systems (33, 42). In the GI tract, PACAP was found expressed within the enteric nervous system and gastric mucosa (33), where it regulates gastrointestinal motility and gastric acid secretion (14, 30).
Recently, PACAP has been found to play an important role in the regulation of metabolism. Centrally, PACAP was discovered to suppress appetite and feeding by intracerebroventricular (ICV) injection in vertebrate animals, such as goldfishes, chicks, rats, and mice (6, 32, 36, 37, 50, 51). PACAP injected in the hypothalamic ventromedial nucleus (VMN) reduced food intake and increased core body temperature and locomotor activity (44). Together, these data indicate a very relevant role for PACAP in the central nervous system as a mediator of energy homeostasis. Murine models with PACAP-targeted disruptions have a complex phenotype with several altered metabolic functions. PACAP knockout mice have been reported to have a greater loss of core body temperature, causing a higher mortality rate in newborn mice, thus suggesting an important role for PACAP in thermoregulation (13). However, the role of the PACAP high-affinity receptor PAC1 in appetite and energy homeostasis has not yet been clearly elucidated. PAC1-deficient (PAC1−/−) mice have been previously described to have pulmonary hypertension and heart failure (17), as well as hyperinsulinemia in fed conditions (21). The PAC1−/− mouse model has allowed us to better characterize the role of PAC1 in appetite, feeding behavior, and energy homeostasis.
Therefore, in the present study, to fully analyze the peripheral effects of PAC1 and PACAP on food intake, feeding behavior, and metabolic hormones secretion, we have investigated the effects of a PACAP intraperitoneal (IP) treatment before the start of the dark phase feeding cycle in wild-type (WT) and PAC1−/− mice. PACAP effects on food intake were studied in undisturbed mice fed ad libitum. The changes in meal patterns were analyzed using an automated food intake monitoring system. Finally, to elucidate the peripheral mechanism by which PACAP affects food intake, we measured plasma levels of orexigenic and anorexigenic hormones following PACAP injection in WT and PAC1−/− mice during fasting as well as in postprandial conditions.
MATERIALS AND METHODS
Mice.
PAC1 receptor-deficient mice and WT littermate controls were developed as previously described (21) and backcrossed for >10 generations on the C57BL/6 background. Mice were identified by ear clipping, samples were extracted for genomic DNA, and PCR was performed as previously described to establish the mouse genotype (21). All research and procedures involving mice were approved by the Department of Veterans Affairs Institutional Animal Care and Use Committee (protocol no. 05026-03).
Determination of food intake behavior.
Microstructure analysis of food intake was performed using the BioDAQ episodic food intake monitor for mice (Research Diets, New Brunswick, NJ). This system allows the continuous monitoring of meal patterns in undisturbed mice with minimal human interference as described in previously published studies (49). The system weighs the food hopper (±0.01 g) second by second and detects not eating as weight stable, and eating as weight unstable. Meals consist of one or more bouts (changes in stable weight before and after a bout) separated by an inter-meal interval (IMI ≥ 5 min). The minimum meal amount was defined as 0.02 g, and a feeding bout that occurred ≥5 min from a previous one was considered as a new meal. Therefore, food intake was considered as one meal when the feeding bout amount was ≥0.02 g and occurred within 5 min of the previous bout. Feeding parameters include cumulative food intake, bout/meal frequency, total time spent eating (time in minutes or percentages), bout/meal size, duration, and eating rate.
Food intake experiments.
PAC1−/− and WT mice (8–12 wk) were single-housed under controlled illumination (0600–1800 h), temperature (21–23°C), and humidity (40–60%). The BioDAQ cages were used for continuous monitoring of meal patterns in undisturbed mice that ingested a regular rodent diet (AIN-93M; Research Diets, New Brunswick, NJ). Mice were habituated to these cages and to feeding from the hopper after 5–7 days, as indicated by normal food intake and regular body weight gain. PACAP1–38 was administered by IP injection at concentrations of 1 nM, 10 nM, 100 nM, or 1 μM in 200 μl of saline before the start of the dark-phase feeding period (1800 h) in both PAC1−/− and WT mice. Similarly, PACAP1–27 was administered by IP injection at 1 nM, 10 nM, 100 nM, or 1 μM in 200 μl of saline before the start of the dark-phase feeding period (1800 h) in both PAC1−/− and WT mice. Simultaneously in each experiment, control groups of the same genotype mice were injected with 200 μl of saline only.
Plasma blood samples.
PACAP1–38 was administered by IP injection at a concentration of 100 nM in 200 μl of saline into both PAC1−/− and WT mice. Mice were fasted overnight before they were injected. Whole blood samples were collected 30 min postinjection. Another group of overnight fasted mice was injected with PACAP1–38, or saline as control, and then allowed to feed ad libitum for 30 min before whole blood samples were collected. Plasma samples were obtained from the whole blood collection, and a cocktail of protease inhibitors containing protease inhibitor cocktail tablets with EDTA (Roche, Indianapolis, IN), aprotinin (Pittsburgh, PA), and dipeptidyl peptidase IV (DPP-IV) inhibitor (Millipore, Billerica, MA) was added.
Measurement of plasma hormone levels.
Plasma levels of selected metabolic hormones were measured using a mouse metabolic hormone magnetic assay kit (Millipore) according to the manufacturer's instructions. Plasma samples were collected from both PAC1−/− and WT mice groups after either overnight fasting or, in the post prandial group, after overnight fasting followed by ad libitum access to food for 30 min before whole blood samples were collected. The hormones included in the panel were active ghrelin, GLP-1, glucagon, insulin, leptin, and peptide YY (PYY). Each sample was assayed in duplicate on a 96-well plate. Total plasma ghrelin levels were measured using an ELISA kit (Millipore). Analysis of quality control standards provided in the kit matched expectations, and the assay had an inter-assay precision of <25% and an intra-assay precision of <7%.
Statistical analysis.
To study dose-dependent effects on cumulative food intake of both PACAP1–38 and PACAP1–27, IP injected into WT or PAC1−/− mice, compared with vehicle during each hour postinjection, we used a two-way ANOVA model with dose (100 nM, 1 μM, 10 μM, or vehicle), time (hour), and a two-way interaction between dose and time. Similarly, we used a two-way ANOVA model to analyze the 4-h period of food intake. The 24-h cumulative food intake was analyzed using a one-way ANOVA model comparing dose (100 nM, 1 μM, or 10 μM) with vehicle.
The metabolic hormone parameters in PACAP1–38 IP injected into overnight-fasted WT mice were analyzed using an unpaired t-test. Metabolic hormone parameters in PAC1−/− vs. WT mice were analyzed using a two-way ANOVA model with mouse type, condition (fasted vs. postprandial), and a two-way interaction term between mouse type and condition. All analyses and graphs were conducted using GraphPad Prism 6 Software (La Jolla, CA).
RESULTS
PACAP1–38 and PACAP1–27 injected IP reduce food intake through the high-affinity PAC1 receptor.
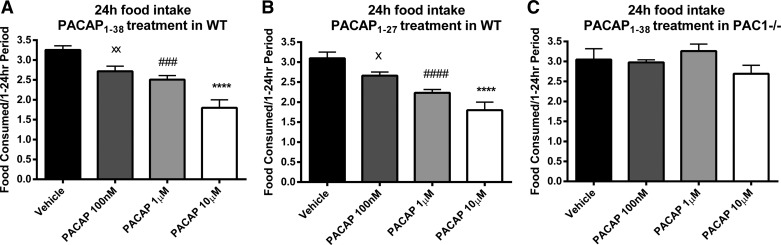
PACAP1–38 and PACAP1–27 IP injected in WT mice, at a dose of 100 nM, 1 μM, or 10 μM in 200 μl of saline, induced a dose-dependent decrease of cumulative food intake during the dark phase of feeding. The time course of PACAP effects on cumulative food intake is illustrated in Fig. 1. In WT mice, cumulative food intake after PACAP1–38 injection was significantly reduced in a dose-dependent manner, as assessed using the BioDAQ automated food intake monitoring system for mice. Both PACAP1–38 (Fig. 1A) and PACAP1–27 (Fig. 1B) significantly reduced cumulative food intake postinjection, beginning at 3 h with a 10 μM dose, at 4 h with a 1 μM dose, and at 8 h with a 100 nM dose. However, in PAC1−/− mice, no significant reduction in food intake was observed following PACAP1–38 at a dose of 100 nM, 1 μM, or 10 μM (Fig. 1C). The 24-h total food consumption was reduced following administration of PACAP1–38 at 10 μM (1.80 ± 0.20 g; P < 0.0001), 1 μM (2.51 ± 0.10 g; P = 0.0001) and 100 nM dose (2.72 ± 0.13 g; P = 0.004) compared with vehicle (3.25 ± 0.11 g; Fig. 2A). Similarly, PACAP1–27 treatment reduced the 24-h food consumption in a dose-dependent manner at 10 μM (1.79 ± 0.20 g; P < 0.0001), 1 μM (2.23 ± 0.08 g; P < 0.0001), and 100 nM (2.66 ± 0.13 g; P = 0.04) compared with vehicle (3.10 ± 0.15 g; Fig. 2B). In PAC1−/− mice, PACAP1–38 treatment at any used dose did not reduce 24-h food intake, and the 24-h food intake was nearly identical to that for vehicle in WT mice (Fig. 2C).
Fig. 1.
Pituitary adenylate cyclase-activating peptide (PACAP) variants PACAP1–38 and PACAP1–27 intraperitoneally (IP) injected at a dose of 100 nM, 1 μM, or 10 μM in 200 μl of saline induced a decrease in cumulative food intake in a dose-dependent manner during the dark phase of feeding in wild-type (WT) but not PAC1-deficient (PAC1−/−) mice. A: PACAP1–38 injection in WT mice significantly reduced cumulative food intake in a dose-dependent manner, beginning at 3 h with a 10 μM dose, at 4 h with a 1 μM dose, and at 8 h with a 100 nM dose. B: PACAP1–27 injection in WT mice significantly reduced manner cumulative food intake in a dose-dependent, beginning at 3 h with a 10 μM dose, at 4 h with a 1 μM dose, and at 8 h with a 100 nM dose. C: no significant reduction in food intake was observed following PACAP1–38 injection at 100 nM, 1 μM, or 10 μM dose in PAC1−/− mice. Data are means ± SE of 16 mice/group. **P < 0.01; ****P < 0.0001, 10 μM vs. vehicle. #P < 0.05; ##P < 0.01; ###P < 0.001; ####P < 0.0001, 1 μM vs. vehicle. ×P < 0.05; ××P < 0.01, 100 nM vs. vehicle.
Fig. 2.
Twenty-four-hour total food consumption was reduced in a dose-dependent manner following IP injection of either PACAP1–38 or PACAP1–27 before the dark phase in WT but not in PAC1−/− mice. A: PACAP1–38 injected in WT mice compared with vehicle. B: PACAP1–27 injected in WT mice compared with vehicle. C: PACAP1–38 injected in PAC1−/− mice compared with vehicle. Data are means ± SE of 16 mice/group. ****P < 0.0001, 10 μM vs. vehicle. ###P < 0.001; ####P < 0.0001, 1 μM vs. vehicle. ×P < 0.05; ××P < 0.01, 100 nM vs. vehicle.
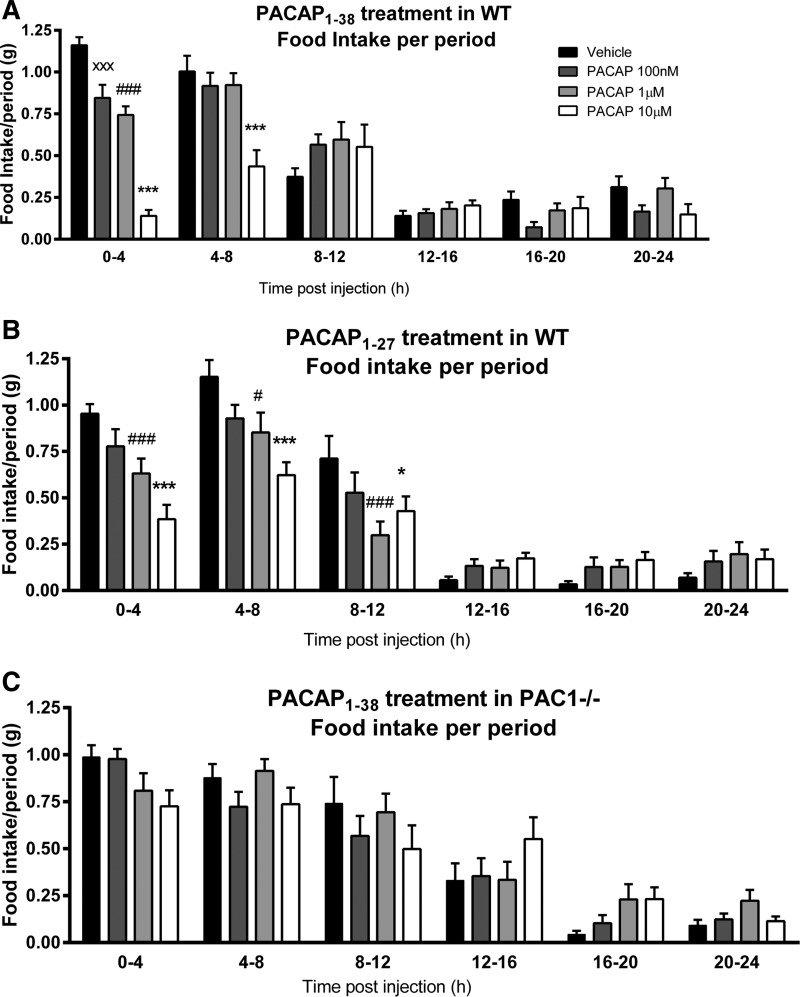
The analysis of the murine food intake data subdivided into 4-h time periods revealed a dose-dependent reduction of food intake only in PACAP1–38 or PACAP1–27 IP injected in WT but not in PAC1−/− mice. PACAP1–38 injections induced a reduction in food intake after 0–4 h postinjection at 100 nM (0.85 ± 0.08 g; P < 0.0001), 1 μM (0.92 ± 0.07 g; P < 0.0001), and 10 μM (0.14 ± 0.04 g; P = 0.005) compared with vehicle (1.16 ± 0.05 g; Fig. 3A). WT mice, injected at a higher dose of 10 μM PACAP1–38, continued to show a reduced food intake for additional 4–8 h postinjection compared with vehicle (0.44 ± 0.09 vs. 1.00 ± 0.10 g; P < 0.0001). PACAP1–27 injection induced a reduction in food intake beginning at 0–4 h postinjection at doses of 1 μM (0.63 ± 0.08 g; P < 0.0001) and 10 μM (0.38 ± 0.07 g; P = 0.03) compared with vehicle (0.95 ± 0.05 g; Fig. 3B). A PACAP1–27-induced effect of food intake reduction was also observed 4–8 h postinjection at 1 μM (0.85 ± 0.11 g; P = 0.05) and 10 μM (0.62 ± 0.07 g; P < 0.0001) compared with vehicle (1.15 ± 0.09 g). In PAC1−/− mice, IP injections of PACAP1–38 failed to cause significant alteration of food intake (Fig. 3C).
Fig. 3.
PACAP1–38 and PACAP1–27 reduced food intake in a dose-dependent manner following IP injection before the dark phase of feeding in WT but not in PAC1−/− mice. A: PACAP1–38 injection in WT mice at 100 nM, 1 μM, and 10 μM reduced food intake in a dose-dependent manner 0–4 h postinjection compared with vehicle, and PACAP1–38 injected at the higher dose of 10 μM continued to show a reduced food intake for an additional 4–8 h postinjection. B: PACAP1–27 injection in WT mice at 1 and 10 μM reduced food intake in a dose-dependent manner 0–4 h and 4–8 h postinjection compared with vehicle. C: in PAC1−/− mice, IP injections of PACAP1–38 failed to cause significant alteration of food intake. Data are means ± SE of 16 mice/group. ****P < 0.0001, 10 μM vs. vehicle. #P < 0.05; ####P < 0.0001, 1 μM vs. vehicle. ××P < 0.01, 100 nM vs. vehicle.
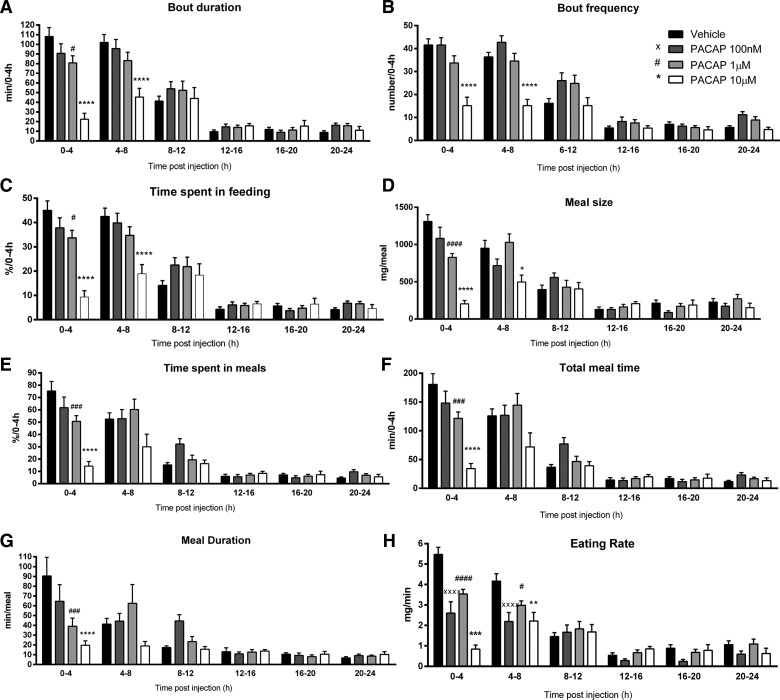
Analysis of the feeding microstructure in the studied mice, using an automated episodic feeding monitoring system, showed that PACAP1–38 altered all the examined feeding behavior parameters. PACAP1–38 significantly reduced bout duration during the first 0–4 h postinjection at 1 μM (80.87 ± 7.35 min; P = 0.01) and 10 μM (22.47 ± 6.16 min; P < 0.0001) compared with vehicle (108.10 ± 9.40 min) and also 4–8 h postinjection at a 10 μM dose (45.43 ± 9.06 min; P < 0.0001) compared with vehicle (102.03 ± 8.26 min; Fig. 4A). Similarly, PACAP1–38 significantly reduced bout frequency during the first 0–4 h postinjection at 10 μM (15.13 ± 3.72 bouts; P < 0.0001) compared with vehicle (41.56 ± 2.67 bouts) and 4–8 h postinjection (15.13 ± 2.77 bouts; P < 0.0001) compared with vehicle (36.40 ± 1.97 bouts; Fig. 4B). The percentage of time spent feeding was also significantly reduced in the first 0–4 h post PACAP1–38 injection at 1 μM (33.69 ± 3.06%; P = 0.01) and 10 μM (9.36 ± 2.57%; P < 0.0001) compared with vehicle (45.04 ± 3.92%) and also 4–8 h postinjection at 10 μM (18.92 ± 3.78%; P < 0.0001) compared with vehicle (42.52 ± 3.44%; Fig. 4C). Meal structure and pattern were therefore significantly reduced in PACAP1–38 IP injected mice. Meal size was significantly decreased in the first 0–4 h postinjection at 1 μM (823.36 ± 53.56 mg; P < 0.0001) and 10 μM (202.50 ± 47.24 mg; P < 0.0001) compared with vehicle (1,310.00 ± 90.74 mg) and 4–8 h postinjection at 10 μM (496.25 ± 92.66 mg; P = 0.01) compared with vehicle (951.20 ± 105.74 mg; Fig. 4D). The percentage of time spent in meals was significantly reduced 0–4 h postinjection at 1 μM (50.64 ± 4.69%; P = 0.0002) and 10 μM (14.26 ± 3.77%; P < 0.0001) compared with vehicle (75.24 ± 7.79%; Fig. 4E). Similarly, the total meal time was significantly reduced 0–4 h post injection at 1 μM (121.54 ± 11.25 min; P < 0.0001) and 10 μM (34.23 ± 9.04 min; P = 0.0002) compared with vehicle (180.59 ± 18.71 min; Fig. 4F). The meal duration was also significantly reduced 0–4 h postinjection at 1 μM (39.11 ± 8.21 min; P = 0.0002) and 10 μM (19.64 ± 4.58 min; P < 0.0001) compared with vehicle (90.43 ± 19.16 min; Fig. 4G). Finally, the eating rate was significantly reduced 0–4 h postinjection at 100 nM (2.61 ± 0.55 mg/min; P < 0.0001), 1 μM (3.53 ± 0.23 mg/min; P < 0.0001), and 10 μM (0.84 ± 0.20 mg/min; P < 0.0001) compared with vehicle (5.47 ± 0.35 mg/min) and also 4–8 h postinjection at 100 nM (2.19 ± 0.44 mg/min; P < 0.0001), 1 μM (2.98 ± 0.22 mg/min; P = 0.02), and 10 μM (2.22 ± 0.42 mg/min; P = 0.002) compared with vehicle (4.16 ± 0.36 mg/min; Fig. 4H).
Fig. 4.
Analysis of the feeding microstructure in the studied mice, using an automated episodic feeding monitoring system, showed that PACAP1–38 injection significantly altered all the examined feeding behavior parameters compared with vehicle. A: bout duration was reduced during the first 0–4 h and 4–8 h post PACAP1–38 injection. B: bout frequency was reduced during the first 0–4 h and 4–8 h post PACAP1–38 injection. C: time spent in feeding was also reduced during the first 0–4 h and 4–8 h post PACAP1–38 injection. D: meal size was decreased in the first0-4 h and 4–8 h post PACAP1–38 injection. E: time spent in meals was reduced 0–4 h post PACAP1–38 injection. F: total meal time was reduced 0–4 h post PACAP1–38 injection. G: meal duration was also reduced 0–4 h post PACAP1–38 injection. H: eating rate was reduced 0–4 h and 4–8 h post PACAP1–38 injection. Data are means ± SE of 16 mice/group. **P < 0.01; ***P < 0.001; ****P < 0.0001, 10 μM vs. vehicle. #P < 0.05; ###P < 0.001; ####P < 0.0001, 1 μM vs. vehicle. ××××P < 0.0001, 100 nM vs. vehicle.
PACAP1–38 IP injection suppressed active ghrelin and active ghrelin/total ghrelin levels in overnight fasting WT mice.
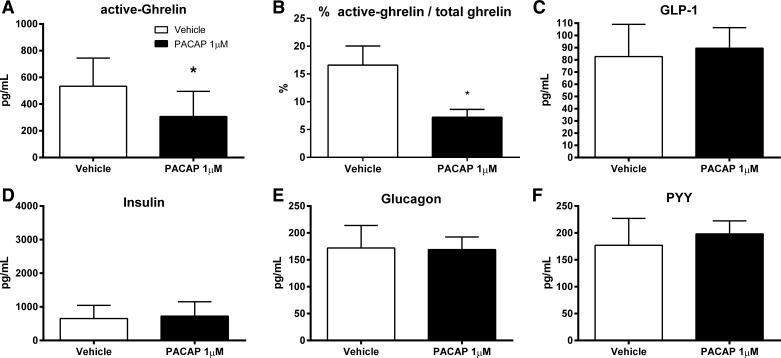
PACAP1–38 at 1 μM dose significantly reduced active ghrelin plasma levels compared with vehicle-injected controls (306.90 ± 66.78 vs. 533.40 ± 79.81 pg/ml; P = 0.04; Fig. 5A). Accordingly, the percentage of active ghrelin/total ghrelin was significantly lower following PACAP injection compared with vehicle (7.22 ± 1.41 vs. 16.59 ± 3.44%; P = 0.03; Fig. 5B). However, PACAP1–38 did not affect significantly the plasma levels of the following metabolic hormones: GLP-1 (89.49 ± 5.94 vs. 82.61 ± 9.35 pg/ml; Fig. 5C), insulin (725.10 ± 161.30 vs. 650.60 ± 139.40 pg/ml; Fig. 5D), glucagon (169.0 ± 8.32 vs. 172.0 ± 14.8 pg/ml; Fig. 5E), and PYY (198.30 ± 8.53 vs. 177.00 ± 17.66 pg/ml; Fig. 5F).
Fig. 5.
PACAP1–38 reduced plasma active ghrelin levels in fasted WT mice. Mice were fasted overnight before 1 μM of PACAP1–38 in 200 μl of saline was injected. Blood plasma was withdrawn 30 min postinjection. A: PACAP1–38 injected into fasted WT mice significantly reduced plasma active ghrelin compared with vehicle. B: percentage of ratio of plasma active ghrelin to total ghrelin. C–F: plasma levels of glucagon-like peptide-1 (GLP-1; C), insulin (D), glucagon (E), and peptide YY (PYY; F) were not significantly altered compared with vehicle. Data are means ± SE of 8 mice/group. *P < 0.05.
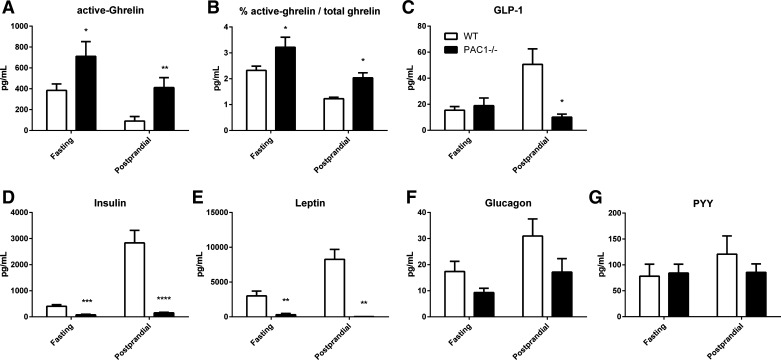
PAC1−/− mice had altered plasma levels of metabolic hormones.
PAC1−/− mice had significantly elevated levels of active ghrelin during both fasting (711.60 ± 140.90 vs. 384.60 ± 61.68 pg/ml; P = 0.04) and postprandial conditions (410.90 ± 96.02 vs. 90.28 ± 43.98 pg/ml; P = 0.006; Fig. 6A). Accordingly, the percentage of active ghrelin/total ghrelin was significantly elevated during both fasting (24.70 ± 3.62 vs. 14.62 ± 1.61%; P = 0.04) and postprandial conditions (19.21 ± 3.43 vs. 6.48 ± 3.62%; P = 0.02) in PAC1−/− mice (Fig. 6B). GLP-1 levels were significantly lower in PAC1−/− compared with WT mice during postprandial conditions (18.87 ± 5.99 vs. 50.62 ± 11.93 pg/ml; P = 0.03) but not during fasting conditions (Fig. 6C). Strikingly, plasma insulin levels were significantly lower in PAC1−/− compared with WT mice in both fasting conditions (75.92 ± 24.89 vs. 404.00 ± 65.45 pg/ml; P = 0.0002) and, more importantly, in postprandial conditions (150.00 ± 24.01 vs. 2,835.00 ± 479.60 pg/ml; P < 0.0001; Fig. 6D). Furthermore, plasma leptin levels were significantly lower in PAC1−/− compared with WT mice in both fasting (304.80 ± 176.4 vs. 3,012 ± 697.3 pg/ml; P = 0.005) and postprandial conditions (304.8 ± 176.4 vs. 3,012 ± 697.3 pg/ml; P = 0.005; Fig. 6E). Plasma levels of glucagon and PYY did not present any significant difference between PAC1−/− and WT mice during fasting or postprandial conditions (Fig. 6, F and G).
Fig. 6.
Differences in metabolic hormones in fasting and postprandial conditions between PAC1−/− and WT mice. In fasting conditions, mice were fasted overnight and plasma was withdrawn. In postprandial conditions, mice were fasted overnight and refed for 30 min before plasma was withdrawn. Plasma levels of active ghrelin (A), percentage of active ghrelin/total ghrelin (B), GLP-1 (C), insulin (D), leptin (E), glucagon (F), and PYY (G) were assessed in PAC1−/− compared with WT mice. Data are means ± SE of 8 mice/group. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001.
DISCUSSION
This study addresses the peripheral role of PACAP and its high-affinity receptor PAC1 in appetite control, feeding behavior, and metabolic hormone regulation. Our data show that both PACAP1–38 and PACAP1–27, IP injected in WT mice before the beginning of the dark phase, produced a significant long-lasting and dose-dependent decrease in food intake and meal patterns for 24 h postinjection. Although PACAP binds to different receptors, its peripheral anorexigenic effects appear to be mediated primarily by PAC1 receptor activation. In fact, PACAP1–38 IP injected in PAC1−/− mice failed to induce a decrease of food intake during the dark phase of feeding. Notably, our data support the hypothesis that the mechanisms underlying PACAP/PAC1 suppression of appetite and feeding behavior are linked to the inhibition of active ghrelin release and the regulation of GLP-1, insulin, and leptin hormone secretion.
The hypothalamus, GI tract, and peripheral organs such as the liver, pancreas, and adipose tissue are parts of an integrated system that regulates appetite and food intake. In the central nervous system (CNS), the greatest density of PACAP and PAC1 occurs in the hypothalamus supraoptic nuclei, VMN, and periventricular nuclei (PVN), areas known to play a critical role in appetite regulation and energy homeostasis, thus supporting the hypothesis that PACAP/PAC1 pathway is central in the regulation of metabolism and energy balance (18, 44, 47, 54). In the CNS, several studies demonstrated that ICV injected PACAP suppresses appetite and food intake in mice and rats (6, 31, 36, 37, 44) as well as in chicks (50) and goldfishes (32). In the CNS, the hypothalamus has two sites, the PVN and the VMN, which regulate energy homeostasis (28, 52). PACAP and PAC1 receptor mRNA is highly expressed in the hypothalamus, specifically in the arcuate nucleus (ARC), VMN, and dorsomedial nuclei (16, 17). It also has been reported that in the CNS, PACAP and PAC1 mRNA expression are significantly increased by excessive feeding (31). In the VMN, an area of the hypothalamus that upon stimulation enhances satiety and promotes metabolism through the sympathetic nervous system, PACAP mRNA expression decreases during fasting conditions, but it is increased following a high-fat diet (52, 59). The expression of both PACAP and its PAC1 receptor in the hypothalamus suggests that they play a crucial role in the central regulation of feeding behavior via the hypothalamic melanocortin system (39). The central physiological effects of PACAP are mediated through its PAC1 receptor, as confirmed by the use of the PAC1-specific antagonist PACAP6–38, which blocks the inhibitory central effects of PACAP on food intake (37, 39, 44, 45). Furthermore, these enhancing satiety effects were replicated by using the PAC1 receptor-specific agonist maxadilan (38), which was shown to significantly suppress food intake in mice (60). Previous studies showed that a single 1-μg dose of PACAP, injected centrally into the VMN before the nocturnal phase of feeding, produced a significant body weight loss 24 h postinjection (18). Similarly, when PACAP was administered centrally in the posterior region of the stria terminalis bed nucleus, weight loss was observed for 24 h postinjection in a dose-dependent manner (24). Recently, PACAP and thyrotropin-releasing hormone (TRH) were shown to be crucial in the ARC to the excitatory drive of the agouti-related peptide-expressing neurons that control hunger (27).
In the GI tract, PACAP and its PAC1 receptor have been shown to play major physiological effects in the GI tract. PACAP and PAC1 immunoreactivity was localized in the myenteric ganglia and nerve fibers in the longitudinal smooth muscle layers of the esophagus, stomach, and small and large intestines (3, 26, 33, 46). In this study, we demonstrate that peripherally administered PACAP affects food intake up to 24 h.
Our results demonstrate that PACAP is a novel peripheral nutrient sensor that regulates food intake and satiety through its high-affinity PAC1 receptor, which inhibits active ghrelin levels. Previously, PACAP and PAC1 were found expressed on gastric nerves (33), and PAC1, localized on gastric enterochromaffin-like (ECL) and parietal cells in the gastric mucosa, was shown to regulate acid secretion (30, 61) through somatostatin release, activation of somatostatin receptor 2 (41), and stimulation of pepsinogen release from chief cells (7). In this study, we demonstrated that PACAP IP administration inhibits active ghrelin secretion in overnight-fasted mice. Additionally, we found in PAC1−/− mice a dysregulation in active ghrelin secretion during both fasting and postprandial conditions. In PAC1−/− mice, ghrelin levels remained elevated even after feeding. In the GI tract, ghrelin is predominantly found in the stomach, where it is mainly produced by the PD/1 endocrine cells of the gastric oxyntic mucosa and, by binding to the GHS-R1a receptor, stimulates food intake (25, 58). In the gastrointestinal tract, the primary role of ghrelin is to stimulate food intake and modulate energy expenditure (58). In both rodents and in humans, plasma ghrelin levels are elevated during fasting and decreased after feeding (54). There are two known forms of ghrelin: a 28-amino acid peptide with an n-octanoylated serine in position 3, acyl-ghrelin (active ghrelin), and a des-acylated [des-(Gln14)] ghrelin. Desacyl ghrelin is considered the inactive form of ghrelin because it does not activate the ghrelin receptor (GHS-R1a) and does not induce the same endocrine effects of acyl ghrelin (19). Ghrelin has also been detected in the small intestine, hypothalamus, pituitary gland, pancreas, heart, and adipose tissue (11, 19, 25, 55, 57). Currently, the mechanisms regulating ghrelin secretion are not fully understood; however, peptide hormones such as oxytocin and dopamine have been reported to regulate in vitro ghrelin secretion (20). Dopamine, which is substantially produced in the GI tract, was demonstrated to stimulate ghrelin secretion via its D1A receptors (20). Although PACAP and PAC1 expression has not yet been identified on PD/1 cells of the gastric fundus, PAC1 receptors have been characterized on ECL cells (61), which have been shown to secrete ghrelin (48). Further knowledge on ghrelin secretion processes is currently limited due to the difficulty of pure gastric mucosa cells isolation techniques (22).
In this work we support the hypothesis that PACAP and PAC1 are able to modulate appetite and feeding behavior through the regulation of metabolic hormone pathways. Appetite and feeding behavior are controlled by a series of hormonal and neural signals, which are generated in the GI tract to provide feedback to the CNS on the availability of nutrients. PACAP has been found in vivo to release insulin (9) and glucagon (59) from the pancreas, glucocorticoids from the adrenal cortex (40), growth hormone from the pituitary gland (50), and catecholamine from the adrenal medulla (15). PAC1−/− mice have a dysregulation in insulin secretion, as confirmed in the current study by plasma insulin levels measured in fasting and postprandial conditions (21). It has been reported that PAC1 receptors are expressed on pancreatic β-cells (9, 21) and stimulate insulin secretion; therefore, they can have a protective role in type 1 diabetes. Our results demonstrate that in the PAC1−/− mice, there is a reduction in insulin responsiveness to meal ingestion, thus confirming previously reported studies that showed PACAP insulinotropic effect. In PAC1−/− there was no increase in postprandial plasma insulin levels, in such a way as to confirm that in physiological conditions, PAC1 receptor is responsible for the control of insulin secretion after food intake (8, 10, 21). No significant differences were noted between the studied WT and PAC1−/− mice groups in food intake at baseline. Thus we cannot conclude whether endogenous PACAP has effects on appetite. We also found that PAC1 is involved in the regulation of leptin, which was abolished in PAC1−/− mice during both fasting and postprandial conditions. Leptin exerts its effects on food intake and metabolic rate by acting on hypothalamic neurons (18). PACAP is an important mediator of the leptin effects in the CNS, since PACAP mRNA was significantly reduced in fasting leptin knockout ob/ob mice but increased following an ICV injection of leptin (18). Furthermore, in PACAP knockout mice (PACAP−/−), an ICV injection of leptin had no significant effect in modulating feeding behavior (53). PAC1 has been shown to be an important player in leptin-induced anorexia, body weight loss, and body temperature modulation (18, 53). Leptin is mainly synthesized by the adipose tissue that expresses PAC1 receptors (56). We showed that exogenous PACAP affected primarily ghrelin plasma levels, whereas in the PAC1−/− mouse there was a decrease in leptin, elevated ghrelin, and reduced GLP-1 and basal insulin levels; PACAP IP treatment reduced only serum ghrelin levels. These results suggest that PACAP may act at other receptors such as VPAC1 or VPAC2, especially in its high-affinity PAC1 receptor-deficient mice. Therefore, future studies targeting the PACAP/PAC1 signaling pathways to block the hypertensive effect of leptin while preserving its metabolic and anorexigenic effects may be a potential novel therapeutic target for treating both obesity and hypertension. Ghrelin, physiologically released during fasting conditions, is known to be the most potent orexigenic hormone. Consequently, on the basis of our data documenting PACAP-induced suppression of ghrelin plasma levels even following fasting conditions, we support the hypothesis that this is the main mechanism underlying PACAP anorexigenic effects. Characterization of PACAP and PAC1 roles in energy homeostasis has provided insights into the widespread and complex physiological role of PACAP. Several studies using PACAP−/− mice have demonstrated that lack of PACAP results in abnormalities of metabolic homeostasis. PACAP is crucial for thermoregulation, because PACAP−/− mice had a greater loss of core body temperature due to insufficient norepinephrine stimulation of brown adipose tissue (13). Also, PACAP−/− mice had an altered thyroid hormone axis leading to lower mRNA levels of TRH and brown adipose tissue type 2 deiodinase (1). Physiologically, PACAP−/− mice have been reported to be leaner than their littermates on a regular chow diet at 21°C due to decreased adiposity; however, this difference was eliminated at 28°C (1, 12). Furthermore, PACAP−/− mice had higher concentrations of serum triglycerides and cholesterol (44). PACAP injections into the VMN of WT mice increased core body temperature and spontaneous locomotor activity (44) as well as brown adipose uncoupling protein 1 mRNA expression, thus confirming PACAP's role in thermogenesis. In vitro, PACAP was able to stimulate adipogenesis in NIH 3T3-L1 preadipocyte cell line by increasing cAMP production and phosphorylation of MAPK (ERK1/2) (4). Therefore, PACAP can mediate brown fat tissue adipogenesis, possibly through its PAC1 receptor, given that PAC1 mRNA and receptors expression were significantly upregulated following PACAP stimulation (4).
In conclusion, our results suggest that peripheral PACAP is a potential novel appetite suppressant and regulator of orexigenic/anorexigenic hormones. The central and peripheral PACAP/PAC1 regulation of appetite/satiety and feeding behavior further supports the emerging concept of the GI tract as an endocrine organ with an essential sensing and signaling role toward the physiological body energy homeostasis. Previously, in prior studies, PACAP administered intravenously was able to cross the blood-brain barrier (5). Therefore, although in our studies we did not specifically investigate whether this would occur following IP injection, we cannot completely exclude a central effect of PACAP. Most importantly, the effects of PAC1 receptor activation that lead to appetite suppression, through inhibition of active ghrelin and regulation of leptin and insulin secretion, point to a novel potential approach for treating obesity and insulin resistance in the future by using PAC1 agonists. The long-lasting satiety-inducing effect of PACAP via PAC1 observed in our study is of particular interest for future clinical studies. PACAP has been reported to have poor metabolic stability and a half-life of minutes in the blood circulation, where it is catabolized by DEPP-IV (29, 62). Future studies should explore the potential use of PACAP in combination with DPP-IV inhibitors, which are already utilized in clinical trials for appetite disorders and obesity treatments (2) to enhance the anorexigenic and metabolic effects of PACAP. With all findings taken together, the current report supports evidence that the PACAP/PAC1 pathway is a potential regulator of appetite/satiety, body metabolism, and energy expenditure even in the periphery, thus establishing a rationale for a potential use of PACAP/PAC1 agonists as novel pharmacological agents in the treatment of appetite disorders as well as obesity and its associated pathologies.
The discovery that PACAP via PAC1 not only induces satiety but also regulates energy expenditure suggests that PACAP analogs may be promising therapeutic agents for obesity treatment and need to be further explored. In summary, PACAP administered peripherally suppresses ghrelin plasma levels and appetite in such a way as to enhance satiety.
GRANTS
This work was supported by two Department of Veterans Affairs Merit Review Awards (to P. Germano and J. R. Pisegna), the Department of Veterans Affairs Merit Review Shared Equipment Evaluation Program (to P. Germano), and National Institute of Diabetes and Digestive and Kidney Diseases Grant P30 DK-41301 (to CURE: Digestive Diseases Research Center, Models of Gastrointestinal Function and Disease Core).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.P.V., D.G., J.R.P., and P.G. conception and design of research; J.P.V., D.G., L.L., S.O., R.S., J.R.P., and P.G. performed experiments; J.P.V., D.G., L.L., S.O., R.S., J.N., W.P., J.R.P., and P.G. analyzed data; J.P.V., D.G., L.L., S.O., R.S., J.N., J.R.P., and P.G. interpreted results of experiments; J.P.V., D.G., L.L., S.O., J.N., W.P., J.R.P., and P.G. prepared figures; J.P.V., D.G., R.S., J.R.P., and P.G. drafted manuscript; J.P.V., D.G., L.L., S.O., R.S., J.N., W.P., J.R.P., and P.G. edited and revised manuscript; J.P.V., D.G., L.L., S.O., R.S., J.N., W.P., J.R.P., and P.G. approved final version of manuscript.
REFERENCES
- 1.Adams BA, Gray SL, Isaac ER, Bianco AC, Vidal-Puig AJ, Sherwood NM. Feeding and metabolism in mice lacking pituitary adenylate cyclase-activating polypeptide. Endocrinology 149: 1571–1580, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahrén B, Hughes TE. Inhibition of dipeptidyl peptidase-4 augments insulin secretion in response to exogenously administered glucagon-like peptide-1, glucose-dependent insulinotropic polypeptide, pituitary adenylate cyclase-activating polypeptide, and gastrin-releasing peptide in mice. Endocrinology 146: 2055–2059, 2005. [DOI] [PubMed] [Google Scholar]
- 3.Arimura A, Somogyvári-Vigh A, Miyata A, Mizuno K, Coy DH, Kitada C. Tissue distribution of PACAP as determined by RIA: highly abundant in the rat brain and testes. Endocrinology 129: 2787–2789, 1991. [DOI] [PubMed] [Google Scholar]
- 4.Arsenijevic T, Gregoire F, Chiadak J, Courtequisse E, Bolaky N, Perret J, Delporte C. Pituitary adenylate cyclase activating peptide (PACAP) participates in adipogenesis by activating ERK signaling pathway. PLoS One 8: e72607, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Banks WA, Kastin AJ, Komaki G, Arimura A. Passage of pituitary adenylate cyclase activating polypeptide1-27 and pituitary adenylate cyclase activating polypeptide1-38 across the blood-brain barrier. J Pharmacol Exp Ther 267: 690–696, 1993. [PubMed] [Google Scholar]
- 6.Chance WT, Thompson H, Thomas I, Fischer JE. Anorectic and neurochemical effects of pituitary adenylate cyclase activating polypeptide in rats. Peptides 16: 1511–1516, 1995. [DOI] [PubMed] [Google Scholar]
- 7.Felley CP, Qian JM, Mantey S, Pradhan T, Jensen RT. Chief cells possess a receptor with high affinity for PACAP and VIP that stimulates pepsinogen release. Am J Physiol Gastrointest Liver Physiol 263: G901–G907, 1992. [DOI] [PubMed] [Google Scholar]
- 8.Filipsson K, Pacini G, Scheurink AJ, Ahrén B. PACAP stimulates insulin secretion but inhibits insulin sensitivity in mice. Am J Physiol Endocrinol Metab 274: E834–E842, 1998. [DOI] [PubMed] [Google Scholar]
- 9.Filipsson K, Sundler F, Hannibal J, Ahrén B. PACAP and PACAP receptors in insulin producing tissues: localization and effects. Regul Pept 74: 167–175, 1998. [DOI] [PubMed] [Google Scholar]
- 10.Fridolf T, Sundler F, Ahrén B. Pituitary adenylate cyclase-activating polypeptide (PACAP): occurrence in rodent pancreas and effects on insulin and glucagon secretion in the mouse. Cell Tissue Res 269: 275–279, 1992. [DOI] [PubMed] [Google Scholar]
- 11.Gnanapavan S, Kola B, Bustin SA, Morris DG, McGee P, Fairclough P, Bhattacharya S, Carpenter R, Grossman AB, Korbonits M. The tissue distribution of the mRNA of ghrelin and subtypes of its receptor, GHS-R, in humans. J Clin Endocrinol Metab 87: 2988, 2002. [DOI] [PubMed] [Google Scholar]
- 12.Gray SL, Cummings KJ, Jirik FR, Sherwood NM. Targeted disruption of the pituitary adenylate cyclase-activating polypeptide gene results in early postnatal death associated with dysfunction of lipid and carbohydrate metabolism. Mol Endocrinol 15: 1739–1747, 2001. [DOI] [PubMed] [Google Scholar]
- 13.Gray SL, Yamaguchi N, Vencová P, Sherwood NM. Temperature-sensitive phenotype in mice lacking pituitary adenylate cyclase-activating polypeptide. Endocrinology 143: 3946–3954, 2002. [DOI] [PubMed] [Google Scholar]
- 14.Grider JR, Makhlouf GM. Colonic peristaltic reflex: identification of vasoactive intestinal peptide as mediator of descending relaxation. Am J Physiol Gastrointest Liver Physiol 251: G40–G45, 1986. [DOI] [PubMed] [Google Scholar]
- 15.Hamelink C, Tjurmina O, Damadzic R, Young WS, Weihe E, Lee HW, Eiden LE. Pituitary adenylate cyclase-activating polypeptide is a sympathoadrenal neurotransmitter involved in catecholamine regulation and glucohomeostasis. Proc Natl Acad Sci USA 99: 461–466, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hannibal J. Pituitary adenylate cyclase-activating peptide in the rat central nervous system: an immunohistochemical and in situ hybridization study. J Comp Neurol 453: 389–417, 2002. [DOI] [PubMed] [Google Scholar]
- 17.Hashimoto H, Nogi H, Mori K, Ohishi H, Shigemoto R, Yamamoto K, Matsuda T, Mizuno N, Nagata S, Baba A. Distribution of the mRNA for a pituitary adenylate cyclase-activating polypeptide receptor in the rat brain: an in situ hybridization study. J Comp Neurol 371: 567–577, 1996. [DOI] [PubMed] [Google Scholar]
- 18.Hawke Z, Ivanov TR, Bechtold DA, Dhillon H, Lowell BB, Luckman SM. PACAP neurons in the hypothalamic ventromedial nucleus are targets of central leptin signaling. J Neurosci 29: 14828–14835, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hosoda H, Kojima M, Matsuo H, Kangawa K. Ghrelin and des-acyl ghrelin: two major forms of rat ghrelin peptide in gastrointestinal tissue. Biochem Biophys Res Commun 279: 909–913, 2000. [DOI] [PubMed] [Google Scholar]
- 20.Iwakura H, Ariyasu H, Hosoda H, Yamada G, Hosoda K, Nakao K, Kangawa K, Akamizu T. Oxytocin and dopamine stimulate ghrelin secretion by the ghrelin-producing cell line, MGN3-1 in vitro. Endocrinology 152: 2619–2625, 2011. [DOI] [PubMed] [Google Scholar]
- 21.Jamen F, Persson K, Bertrand G, Rodriguez-Henche N, Puech R, Bockaert J, Ahrén B, Brabet P. PAC1 receptor-deficient mice display impaired insulinotropic response to glucose and reduced glucose tolerance. J Clin Invest 105: 1307–1315, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kageyama H, Kitamura Y, Hosono T, Kintaka Y, Seki M, Takenoya F, Hori Y, Nonaka N, Arata S, Shioda S. Visualization of ghrelin-producing neurons in the hypothalamic arcuate nucleus using ghrelin-EGFP transgenic mice. Regul Pept 145: 116–121, 2008. [DOI] [PubMed] [Google Scholar]
- 23.Kimura C, Ohkubo S, Ogi K, Hosoya M, Itoh Y, Onda H, Miyata A, Jiang L, Dahl RR, Stibbs HH, Arimura A, Fujino M. A novel peptide which stimulates adenylate cyclase: molecular cloning and characterization of the ovine and human cDNAs. Biochem Biophys Res Commun 166: 81–89, 1990. [DOI] [PubMed] [Google Scholar]
- 24.Kocho-Schellenberg M, Lezak KR, Harris OM, Roelke E, Gick N, Choi I, Edwards S, Wasserman E, Toufexis DJ, Braas KM, May V, Hammack SE. PACAP in the BNST produces anorexia and weight loss in male and female rats. Neuropsychopharmacology 39: 1614–1623, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature 402: 656–660, 1999. [DOI] [PubMed] [Google Scholar]
- 26.Köves K, Arimura A, Vigh S, Somogyvári-Vigh A, Miller J. Immunohistochemical localization of PACAP in the ovine digestive system. Peptides 14: 449–455, 1993. [DOI] [PubMed] [Google Scholar]
- 27.Krashes MJ, Shah BP, Madara JC, Olson DP, Strochlic DE, Garfield AS, Vong L, Pei H, Watabe-Uchida M, Uchida N, Liberles SD, Lowell BB. An excitatory paraventricular nucleus to AgRP neuron circuit that drives hunger. Nature 507: 238–242, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leibowitz SF, Hammer NJ, Chang K. Hypothalamic paraventricular nucleus lesions produce overeating and obesity in the rat. Physiol Behav 27: 1031–1040, 1981. [DOI] [PubMed] [Google Scholar]
- 29.Li M, Maderdrut JL, Lertora JJ, Batuman V. Intravenous infusion of pituitary adenylate cyclase-activating polypeptide (PACAP) in a patient with multiple myeloma and myeloma kidney: a case study. Peptides 28: 1891–1895, 2007. [DOI] [PubMed] [Google Scholar]
- 30.Lu Y, Germano P, Ohning GV, Vu JP, Pisegna JR. PAC1 deficiency in a murine model induces gastric mucosa hypertrophy and higher basal gastric acid output. J Mol Neurosci 43: 76–84, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Matsuda K, Maruyama K, Miura T, Uchiyama M, Shioda S. Anorexigenic action of pituitary adenylate cyclase-activating polypeptide (PACAP) in the goldfish: feeding-induced changes in the expression of mRNAs for PACAP and its receptors in the brain, and locomotor response to central injection. Neurosci Lett 386: 9–13, 2005. [DOI] [PubMed] [Google Scholar]
- 32.Matsuda K, Maruyama K, Nakamachi T, Miura T, Shioda S. Effects of pituitary adenylate cyclase-activating polypeptide and vasoactive intestinal polypeptide on food intake and locomotor activity in the goldfish, Carassius auratus. Ann NY Acad Sci 1070: 417–421, 2006. [DOI] [PubMed] [Google Scholar]
- 33.Miampamba M, Germano PM, Arli S, Wong HH, Scott D, Taché Y, Pisegna JR. Expression of pituitary adenylate cyclase-activating polypeptide and PACAP type 1 receptor in the rat gastric and colonic myenteric neurons. Regul Pept 105: 145–154, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miyata A, Arimura A, Dahl RR, Minamino N, Uehara A, Jiang L, Culler MD, Coy DH. Isolation of a novel 38 residue-hypothalamic polypeptide which stimulates adenylate cyclase in pituitary cells. Biochem Biophys Res Commun 164: 567–574, 1989. [DOI] [PubMed] [Google Scholar]
- 35.Miyata A, Jiang L, Dahl RD, Kitada C, Kubo K, Fujino M, Minamino N, Arimura A. Isolation of a neuropeptide corresponding to the N-terminal 27 residues of the pituitary adenylate cyclase activating polypeptide with 38 residues (PACAP38). Biochem Biophys Res Commun 170: 643–648, 1990. [DOI] [PubMed] [Google Scholar]
- 36.Mizuno Y, Kondo K, Terashima Y, Arima H, Murase T, Oiso Y. Anorectic effect of pituitary adenylate cyclase activating polypeptide (PACAP) in rats: lack of evidence for involvement of hypothalamic neuropeptide gene expression. J Neuroendocrinol 10: 611–616, 1998. [DOI] [PubMed] [Google Scholar]
- 37.Morley JE, Horowitz M, Morley PM, Flood JF. Pituitary adenylate cyclase activating polypeptide (PACAP) reduces food intake in mice. Peptides 13: 1133–1135, 1992. [DOI] [PubMed] [Google Scholar]
- 38.Moro O, Lerner EA. Maxadilan, the vasodilator from sand flies, is a specific pituitary adenylate cyclase activating peptide type I receptor agonist. J Biol Chem 272: 966–970, 1997. [DOI] [PubMed] [Google Scholar]
- 39.Mounien L, Do Rego JC, Bizet P, Boutelet I, Gourcerol G, Fournier A, Brabet P, Costentin J, Vaudry H, Jégou S. Pituitary adenylate cyclase-activating polypeptide inhibits food intake in mice through activation of the hypothalamic melanocortin system. Neuropsychopharmacology 34: 424–435, 2009. [DOI] [PubMed] [Google Scholar]
- 40.Nussdorfer GG, Malendowicz LK. Role of VIP, PACAP, and related peptides in the regulation of the hypothalamo-pituitary-adrenal axis. Peptides 19: 1443–1467, 1998. [DOI] [PubMed] [Google Scholar]
- 41.Piqueras L, Taché Y, Martínez V. Peripheral PACAP inhibits gastric acid secretion through somatostatin release in mice. Br J Pharmacol 142: 67–78, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pisegna JR, Oh DS. Pituitary adenylate cyclase-activating polypeptide: a novel peptide with protean implications. Curr Opin Endocrinol Diabetes Obes 14: 58–62, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pisegna JR, Wank SA. Molecular cloning and functional expression of the pituitary adenylate cyclase-activating polypeptide type I receptor. Proc Natl Acad Sci USA 90: 6345–6349, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Resch JM, Boisvert JP, Hourigan AE, Mueller CR, Yi SS, Choi S. Stimulation of the hypothalamic ventromedial nuclei by pituitary adenylate cyclase-activating polypeptide induces hypophagia and thermogenesis. Am J Physiol Regul Integr Comp Physiol 301: R1625–R1634, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Resch JM, Maunze B, Gerhardt AK, Magnuson SK, Phillips KA, Choi S. Intrahypothalamic pituitary adenylate cyclase-activating polypeptide regulates energy balance via site-specific actions on feeding and metabolism. Am J Physiol Endocrinol Metab 305: E1452–E1463, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schulz S, Röcken C, Mawrin C, Weise W, Höllt V, Schulz S. Immunocytochemical identification of VPAC1, VPAC2, and PAC1 receptors in normal and neoplastic human tissues with subtype-specific antibodies. Clin Cancer Res 10: 8235–8242, 2004. [DOI] [PubMed] [Google Scholar]
- 47.Segal JP, Stallings NR, Lee CE, Zhao L, Socci N, Viale A, Harris TM, Soares MB, Childs G, Elmquist JK, Parker KL, Friedman JM. Use of laser-capture microdissection for the identification of marker genes for the ventromedial hypothalamic nucleus. J Neurosci 25: 4181–4188, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Srivastava A, Kamath A, Barry SA, Dayal Y. Ghrelin expression in hyperplastic and neoplastic proliferations of the enterochromaffin-like (ECL) cells. Endocr Pathol 15: 47–54, 2004. [DOI] [PubMed] [Google Scholar]
- 49.Stengel A, Goebel M, Wang L, Rivier J, Kobelt P, Mönnikes H, Taché Y. Activation of brain somatostatin(2) receptors stimulates feeding in mice: analysis of food intake microstructure. Physiol Behav 101: 614–622, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tachibana T, Saito S, Tomonaga S, Takagi T, Saito ES, Boswell T, Furuse M. Intracerebroventricular injection of vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide inhibits feeding in chicks. Neurosci Lett 339: 203–206, 2003. [DOI] [PubMed] [Google Scholar]
- 51.Tachibana T, Tomonaga S, Oikawa D, Saito S, Takagi T, Saito ES, Boswell T, Furuse M. Pituitary adenylate cyclase activating polypeptide and vasoactive intestinal peptide inhibit feeding in the chick brain by different mechanisms. Neurosci Lett 348: 25–28, 2003. [DOI] [PubMed] [Google Scholar]
- 52.Takaki A, Aou S, Oomura Y, Okada E, Hori T. Feeding suppression elicited by electrical and chemical stimulations of monkey hypothalamus. Am J Physiol Regul Integr Comp Physiol 262: R586–R594, 1992. [DOI] [PubMed] [Google Scholar]
- 53.Tanida M, Hayata A, Shintani N, Yamamoto N, Kurata Y, Shibamoto T, Morgan DA, Rahmouni K, Hashimoto H. Central PACAP mediates the sympathetic effects of leptin in a tissue-specific manner. Neuroscience 238: 297–304, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vaudry D, Gonzalez BJ, Basille M, Yon L, Fournier A, Vaudry H. Pituitary adenylate cyclase-activating polypeptide and its receptors: from structure to functions. Pharmacol Rev 52: 269–324, 2000. [PubMed] [Google Scholar]
- 55.Vu JP, Wang HS, Germano PM, Pisegna JR. Ghrelin in neuroendocrine tumors. Peptides 32: 2340–2347, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wei Y, Mojsov S. Tissue specific expression of different human receptor types for pituitary adenylate cyclase activating polypeptide and vasoactive intestinal polypeptide: implications for their role in human physiology. J Neuroendocrinol 8: 811–817, 1996. [DOI] [PubMed] [Google Scholar]
- 57.Wierup N, Svensson H, Mulder H, Sundler F. The ghrelin cell: a novel developmentally regulated islet cell in the human pancreas. Regul Pept 107: 63–69, 2002. [DOI] [PubMed] [Google Scholar]
- 58.Yang J, Brown MS, Liang G, Grishin NV, Goldstein JL. Identification of the acyltransferase that octanoylates ghrelin, an appetite-stimulating peptide hormone. Cell 132: 387–96, 2008. [DOI] [PubMed] [Google Scholar]
- 59.Yokota C, Kawai K, Ohashi S, Watanabe Y, Suzuki S, Yamashita K. Stimulatory effects of pituitary adenylate cyclase-activating polypeptide (PACAP) on insulin and glucagon release from the isolated perfused rat pancreas. Acta Endocrinol (Copenh) 129: 473–479, 1993. [DOI] [PubMed] [Google Scholar]
- 60.Yu R, Yi T, Xie S, Hong A. Long-term administration of maxadilan improves glucose tolerance and insulin sensitivity in mice. Peptides 29: 1347–1353, 2008. [DOI] [PubMed] [Google Scholar]
- 61.Zeng N, Athmann C, Kang T, Lyu RM, Walsh JH, Ohning GV, Sachs G, Pisegna JR. PACAP type I receptor activation regulates ECL cells and gastric acid secretion. J Clin Invest 104: 1383–1391, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhu L, Tamvakopoulos C, Xie D, Dragovic J, Shen X, Fenyk-Melody JE, Schmidt K, Bagchi A, Griffin PR, Thornberry NA, Sinha Roy R. The role of dipeptidyl peptidase IV in the cleavage of glucagon family peptides: in vivo metabolism of pituitary adenylate cyclase activating polypeptide-(1–38). J Biol Chem 278: 22418–23, 2003. [DOI] [PubMed] [Google Scholar]