Abstract
Background: Impaired thyroid function is a common side effect of lithium medication. Recent data indicate that lithium exposure through drinking water, although providing much lower doses than the medication, may also affect thyroid hormone levels. However, the effects in susceptible groups like pregnant women are not known.
Methods: In a population-based mother–child cohort in the Argentinean Andes (n = 194), an area with varying concentrations of lithium in the drinking water, we assessed lithium exposure repeatedly during pregnancy by measuring the concentrations in blood using inductively coupled plasma mass spectrometry. The markers of thyroid function included thyrotropin (TSH), free/total thyroxine (fT4/T4), free/total triiodothyronine (fT3/T3), thyroglobulin, and transthyretin in serum, sampled at the same time. Multiple potential confounders, including exposure to arsenic, cesium, and boron (elevated in water) as well as selenium and iodine (essential for thyroid function) were considered.
Results: The lithium concentrations in blood [median 25 μg/L (0.0036 mmol/L); range 1.9–145 μg/L (0.000027–0.021 mmol/L)] correlated significantly with those in urine and drinking water (rs = 0.84, p < 0.001, and rs = 0.40, p < 0.001, respectively). Using linear quantile regression models, we found a positive association between blood lithium (log2 transformed) and TSH concentrations, particularly in the lowest percentiles of TSH (B = 0.20 mIU/L, [95% confidence interval 0.048–0.35] at the fifth percentile). We also found inverse associations of blood lithium with transthyretin, particularly at the highest percentiles, as well as with fT3 and T3, with less obvious variation across percentiles. Unexpectedly, blood cesium concentrations (median 111 μg/L, range 2.5–711 μg/L) were also inversely associated with fT3 and T3, particularly at the highest T3 percentiles, but not with TSH or transthyretin. Arsenic and boron exposure (also through drinking water) did not show any associations with the thyroid parameters.
Conclusions: The study supports previous findings that lithium exposure through drinking water may impair thyroid function. The results regarding cesium exposure through drinking water are new. During pregnancy, impaired thyroid function may be detrimental for fetal development. The findings reinforce the need for better control of drinking water, including bottled water, as well as a health-based guideline value.
Introduction
The alkali metal lithium (Li) has long been used in the treatment of bipolar disease (1). Impairment of the thyroid function is a well-documented side effect of lithium medication (2,3). The general population may also be exposed to lithium through drinking water, including bottled water (4–7), and we recently reported that such exposure was associated with increasing thyrotropin (TSH) and decreasing free thyroxine (fT4) levels (8).
An adverse effect of lithium on the thyroid may be even more critical during pregnancy. The thyroid gland is in charge of producing hormones that are essential for pre- and postnatal growth and cognitive development (9). The fetal thyroid starts developing around the fourth week of pregnancy and becomes functional near mid gestation, which is why an appropriate maternal thyroid function is crucial for fetal development (9,10). Hypothyroidism, a condition in which the thyroid gland is incapable of producing enough thyroid hormones, has been associated with increased risk of gestational hypertension, placental abruption, preterm delivery and fetal loss (11,12), as well as lower birth weight, congenital hypothyroidism, and impaired neurological function (13–15). We recently found inverse associations between maternal exposure to lithium during pregnancy and birth size (16) and hypothesize that an impairment of the thyroid hormonal system might be an underlying mechanism. Therefore, in this study we aimed at elucidating the potential impact of lithium from drinking water on maternal thyroid function during pregnancy.
Materials and Methods
Study population
The study area included the main village San Antonio de los Cobres (about 6000 inhabitants) and the surrounding areas (about 2000 inhabitants in total): Santa Rosa de los Pastos Grandes, Tolar Grande, Salar de Pocitos, Olacapato, Cobres, Las Cuevas, El Toro, El Palomar, and Esquina de Guardia, all located at 3180–4070 meters above sea level. The drinking water concentrations of lithium, arsenic, cesium, and boron vary markedly among the villages (5). The population is mostly indigenous (16).
The details about the recruitment of this cohort have been described elsewhere (16). In brief, all pregnant women living in the Andean part of the Salta province, northern Argentina with an estimated delivery date between October 2012 and December 2013 were invited to participate in this mother–child cohort study. We recruited the pregnant women with assistance of the primary health care personnel. Out of a total of 221 pregnant women, 194 were enrolled. We excluded two women who had spontaneous abortions and 16 who lacked data on exposure or thyroid markers (Supplementary Fig. S1; Supplementary Data are available online at www.liebertpub.com/thy).
The study was approved by the regional ethical committee at Karolinska Institutet, Stockholm, Sweden, and by the Ministry of Health, Salta, Argentina. All women gave written informed consent after oral and written explanation of the study. For subjects below 18 years of age, we also obtained informed consent from the closest caregiver.
Data and sample collection
This cohort was designed to obtain repeated measures of exposure and outcomes during pregnancy. At the first visit, we collected information about age, last menstrual period, prepregnancy weight, parity, family income, education level, smoking, alcohol consumption, coca chewing, and personal and familial history of diseases (including thyroid diseases) using questionnaires. Also, at each visit we asked the women about potentially encountered health problems. We measured the maternal weight (HCG-210QM, GA.MA® professional, Italy; accurate to 100 g) at each visit and height in a standardized way. Prepregnancy body mass index was calculated as the prepregnancy body weight in kilograms divided by height in meters squared. Prepregnancy lean body mass was estimated from pre-pregnancy weight and height using the equation proposed by Watson et al. (17) and lean body mass during pregnancy was calculated by extracting the fat mass weight [in kg; calculated using bioelectrical impedance (Body Fat Monitor HBF-302, OMRON®)] from the total weight. Gestational age was calculated based on the reported date of last menstrual period, which was compared with the ultrasound-based estimate (16).
We collected blood and spot-urine samples at baseline and at each follow-up visit. Whole blood samples were collected in Trace Elements Sodium Heparin tubes (Vacuette®; Greiner Bio-One) with butterfly needles (BD Safety-Lok™, Vacutainer®, Bencton, Dickinson and Company), tested negative for trace element contamination. In addition, we fractionated serum from whole blood samples collected in Trace Elements Serum Clot Activator tubes (Vacuette ®; Greiner Bio-One) by centrifugation (3000 rpm, 10 minutes) exactly 15 minutes after blood withdrawal. Urine was collected in disposable trace element–free plastic cups and immediately transferred to 20 mL polyethylene bottles (Zinsser Analytic GMBH). Water samples were repeatedly collected in 20 mL polyethylene bottles during the study period. We collected all the biological samples at the hospital or the local health clinics during the daytime; the project logistics did not allow for sampling after overnight fasting. All samples were kept at −20°C until they were transported frozen to Karolinska Institutet, Sweden, where they were stored at −80°C until analysis. Analyses took place within two months after collection.
Exposure assessment
To assess the exposure to lithium, we measured the concentrations in whole blood, and for validation, we compared the concentrations with those in urine and drinking water. We were not able to use serum lithium concentrations, as the Trace Elements Serum Clot Activator tubes were severely contaminated by lithium (18). We also measured arsenic, cesium, and boron (blood, serum, urine, and water), as they were present in the drinking water in the study area (5) and are reported to accumulate in or affect the thyroid gland (19–21). We also measured iodine in urine and selenium in serum, as these elements are essential for thyroid function (22,23).
All elements were determined using inductively coupled plasma mass spectrometry (Agilent 7700x ORS ICP-MS, Agilent Technologies), with the collision/reaction cell in no-gas mode (lithium, boron, cesium, and iodine), helium mode (arsenic), or hydrogen mode (selenium). Before analysis, the urine and water samples were diluted 1:10 with 1% nitric acid (HNO3 65% w/w, ppb-trace analysis grade, Scharlau, Scharlab S.L.). For the measurement of iodine, urine samples were diluted 1:10 with 0.1% ammonium hydroxide (NH4OH 25% w/w, Suprapur®; Merck) (24). For determination of lithium and cesium in blood and boron and selenium in serum, aliquots (0.2 mL) of the samples were diluted 1:25 with an alkali solution consisting of 1-butanol 2% (w/v), EDTA 0.05% (w/v), triton X-100 0.05% (w/v), NH4OH 1% (w/v), and internal standards (200 μg/L of Be, Ge, Rh, Lu, and Ir) (18). This method was found to provide more reliable results for blood lithium and cesium than acid digestion (18). No samples were below the limit of detection of any of the elements measured. Results for the commercially available reference materials, analyzed with the collected samples, and the limit of detection, are presented in Supplementary Table S1. Assessment of arsenic exposure was based on the sum concentration of arsenic metabolites in urine (16). To compensate for variations in the dilution of urine, we adjusted the element concentrations to the mean urinary osmolality (16).
Measurement of thyroid function
Assessment of maternal thyroid function was based on serum concentrations of TSH, total thyroxine (T4), fT4, total triiodothyronine (T3), free triiodothyronine (fT3), thyroglobulin and transthyretin, measured at the Department of Clinical Chemistry at the University Hospitals in Lund and Malmö, Sweden. In addition, anti-thyroid peroxidase (TPO) antibodies were determined in order to detect cases of autoimmune thyroid disease. TSH was analyzed using a one-step sandwich method with an electrochemiluminescence immunoassay (Roche Diagnostics), and fT4, total T4, fT3, total T3, and anti-TPO antibodies, using a two-step immunometric-competitive method with electrochemiluminescence immunoassay (Roche Diagnostics), and all measured subsequently using a Cobas® 6000/8000 analyzer system (Roche Diagnostics International Ltd.). Thyroglobulin was measured by an immunometric sandwich assay with alkaline-phosphatase as separation method and measured using an immunoassay system (IMMULITE® 2000XPi, Siemens Healthcare Diagnostics), and transthyretin was measured by an immunonephelometric technique (BN ProSpec analyzer, Siemens Healthcare Diagnostics).
Statistical analyses
Statistical analyses were performed using Stata (StataCorp. 2013. Stata Statistical Software: Release 13, StataCorp LP). Bivariate associations between exposure markers, the different markers of thyroid function, and potential covariates were initially assessed using Spearman's rank correlation (rs). We visually evaluated scatterplots of outcomes versus exposure measures and covariates and examined the associations with Lowess, running-line least squares. Differences in the various exposures and covariates across tertiles of blood lithium, and in the exposures and thyroid markers across pregnancy trimesters were evaluated using Kruskal–Wallis rank test.
We evaluated the associations between blood lithium and the thyroid markers longitudinally (1–3 measurements per woman) during pregnancy (first, second, and third trimesters), using linear quantile regression models. Quantile regression allows the examination of the associations between an exposure and the outcomes at multiple points in the distribution of the outcome, while the common linear regression analysis evaluates at the mean of the outcome only (25). We used logarithm-transformed (log2) exposure measures as those met the assumption of homogenous variances and to provide the best fit of the data. We jointly estimated the changes within the 5th, 25th, 50th, 75th, and 95th percentiles of the different thyroid markers in relation to a doubling of blood lithium, modeled as a log2-transformed variable. Estimates, confidence intervals, and p-values are based on 500 design-matrix bootstrap samples, clustered on individual identification numbers. We tested the differences between percentiles using Wald test.
Initially, we regressed the different thyroid markers against log2 blood lithium concentrations adjusting for gestational age only. We then additionally adjusted the models for covariates known to affect the thyroid function or that influenced the regression coefficients by at least 10%. Unexpectedly, blood cesium was statistically significant in several of the models between blood lithium and the thyroid parameters, and therefore, its regression coefficients are also shown in the tables. The final models presented were adjusted for gestational age (weeks), parity (tertiles), height (cm), urinary iodine (tertiles), serum selenium (tertiles), urinary arsenic (tertiles), and log2 urinary cesium (in blood lithium models) or log2 urinary lithium (in blood cesium models). Because the estimates from the initial and final models did not differ markedly, we only present the final fully adjusted models. Parity was strongly correlated with maternal age (rs = 0.79, p < 0.001) and it modified the estimates more than age; thus, we included parity, but not age, in the final models. Boron was also present in the drinking water in the study area (5) and has been suggested to impair the thyroid function in experimental studies (19). However, in our analyses serum boron was not associated with the thyroid markers and therefore not included in the final models. In a sensitivity analysis, we tested if the observed associations of blood lithium and cesium with T3 and fT3 could be influenced by illness during pregnancy, as this has been reported previously (26).
Results
Out of the 176 recruited pregnant women (Supplementary Fig. S1), we excluded five who tested positive for anti-TPO antibodies. All the remaining 171 women had at least one measurement of both exposure and thyroid markers during pregnancy (n = 27 in first, n = 82 in second, and n = 146 in third trimester; in total 255 measurements, Supplementary Fig. S1).
The drinking water lithium concentrations varied between 530 and 830 μg/L (n = 58) in San Antonio de los Cobres and between 5.0 and 324 μg/L (n = 83) in the surrounding villages, except for a small village 20 km east of Cobres (Aguas Blancas), where the concentrations were 958–1660 μg/L (n = 3). Overall, concentrations of both cesium (0.01–717 μg/L) and arsenic (0.82–320 μg/L) in drinking water varied in the different villages. The Spearman's rank correlation coefficients were: blood and urinary lithium, rs = 0.84 (p < 0.001); blood and water lithium (also collected at the same time), rs = 0.40 (p < 0.001); and urinary and water lithium, rs = 0.44 (p < 0.001). Blood and urinary lithium were also positively correlated with blood cesium (rs = 0.55, p < 0.001, and rs = 0.47, p < 0.001, respectively); urinary cesium (rs = 0.53, p < 0.001, and rs = 0.61, p < 0.001, respectively); and urinary arsenic (rs = 0.53, p < 0.001, and rs = 0.67, p < 0.001, respectively) but only weakly correlated with urinary iodine (rs = 0.23, p < 0.001, and rs = 0.25, p < 0.001, respectively). Blood cesium was moderately correlated with urinary cesium (rs = 0.43, p < 0.001) and water cesium (rs = 0.38, p < 0.001). To avoid collinearity in the linear quantile regression, models for blood lithium were adjusted for urinary cesium, and models for blood cesium were adjusted for urinary lithium.
Compared with the first tertile of blood lithium [median 11 μg/L (0.0016 mmol/L), range 2.1–19 (0.00030–0.0027)], the concentrations of urinary lithium, blood and urinary cesium, and urinary arsenic and iodine were significantly higher in the third tertile [median 42 μg/L (0.0060 mmol/L), range 34–145 (0.0049–0.021)] (Table 1). There was no difference in the other characteristics across the tertiles of blood lithium concentrations. More than 60% of the pregnant women had urinary iodine concentrations <150 μg/L at some point during pregnancy (Supplementary Table S2), and among the 82 women with 2–3 iodine measurements, 43 (52%) had all measurements <150 μg/L. Only two women had serum selenium concentrations <60 μg/L (Supplementary Table S2).
Table 1.
Maternal and Infant Characteristics by Tertiles of Maternal Blood Lithium
| Tertiles of maternal blood lithium concentrations | ||||
|---|---|---|---|---|
| Characteristic | Tertile 1 | Tertile 2 | Tertile 3 | p-Valuea |
| Maternal characteristics at recruitment | ||||
| Maternal age (years) | 24 (15–41) | 22 (14–39) | 26 (13–40) | 0.33 |
| Maternal education level (years) | 9 (0–15) | 10 (0–17) | 9 (0–15) | 0.54 |
| Parity (n) | 1 (0–11) | 1 (0–12) | 1 (0–8) | 0.86 |
| Height (cm) | 153 (144–169) | 152 (141–166) | 152 (134–162) | 0.17 |
| Prepregnancy weight (kg) | 53 (40–86) | 53 (43–77) | 50 (38–72) | 0.49 |
| Prepregnancy lean body mass (kg) | 38 (32–49) | 37 (34–45) | 36 (31–44) | 0.32 |
| Prepregnancy body mass index (kg/m2) | 22 (18–35) | 22 (18–35) | 23 (17–31) | 0.95 |
| History of preeclampsia [n (%), yes] | 4 (7%) | 5 (9%) | 3 (5%) | 0.75 |
| Maternal exposure in late pregnancy | ||||
| Blood lithium (μg/L) | 11 (2.1–19) | 26 (19–34) | 42 (34–145) | <0.001 |
| Blood lithium (mmol/L) | 0.0016 (0.00030–0.0027) | 0.0037 (0.0027–0.0049) | 0.0060 (0.0049–0.021) | <0.001 |
| Urinary lithium (μg/L)b | 797 (109–2403) | 1439 (671–4183) | 2522 (1078–5185) | <0.001 |
| Urinary arsenic (μg/L)b | 61 (13–1826) | 131 (31–556) | 158 (47–2450) | <0.001 |
| Blood cesium (μg/L) | 67 (2.4–576) | 112 (8.9–253) | 159 (11–711) | <0.001 |
| Urine cesium (μg/L)b | 302 (14–1748) | 461 (26–1705) | 607 (17–2248) | <0.001 |
| Serum selenium (μg/L) | 86 (62–136) | 79 (50–113) | 82 (62–137) | 0.0067 |
| Urinary iodine (μg/L)b | 92 (35–365) | 130 (44–1490) | 156 (42–659) | 0.0019 |
| Infant characteristics at birth | ||||
| Gestational age (weeks) | 39 (29–42) | 39 (32–42) | 38 (30–43) | 0.22 |
| Weight (g) | 3100 (1250–4500) | 3050 (1760–4130) | 3000 (1260–4165) | 0.64 |
| Length (cm) | 48 (40–53) | 48 (42–51) | 48 (39–51) | 0.62 |
| Head circumference (cm) | 33 (26–37) | 34 (31–40) | 34 (29–36) | 0.61 |
Maternal characteristics at recruitment, exposure data in late pregnancy (second and third trimester) and infant characteristics are shown. N = 171 (n = 57 in each tertile group). Tertiles of blood lithium are based on blood lithium concentrations in late pregnancy.
Data are presented as median (range) or percent (%).
Mean comparison based on Kruskal-Wallis rank test for continuous variables and chi-squared test for categorical.
Urinary concentrations are adjusted for the mean urinary osmolality from all urine samples (694 mOsm/kg).
As shown in Table 2, the serum concentrations of TSH and thyroglobulin increased with advancing pregnancy, while fT4, T4, fT3, and serum selenium decreased. The fraction of women with TSH levels above the reference value (28) during the first (0.1–2.5 mIU/L), second (0.2–3.0 mIU/L), and third (0.3–3.5 mIU/L) trimesters was 19%, 12%, and 13%, respectively (Supplementary Table S2). Out of all the TSH values above the reference range, 83% belonged to the middle and highest tertiles of blood lithium, and only 17% to the lowest tertile. Out of all fT3 values below the reference range, 50%, 28%, and 22% belonged to the highest, middle and lowest tertile of blood lithium, respectively. Blood lithium increased during pregnancy, while blood cesium and serum selenium tended to decrease (Table 2).
Table 2.
Biomarkers of Exposure and Thyroid Function and Exposure Markers Across Pregnancy
| First trimester (n = 27) | Second trimester (n = 82) | Third trimester (n = 146) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Thyroid and exposure markers | Mean ± SD | Median | [Range] | Mean ± SD | Median | [Range] | Mean ± SD | Median | [Range] | p-Valuea |
| Thyroid markers in serum | ||||||||||
| TSH (mIU/L) | 1.7 ± 1.1 | 1.4 | [0.34–4.4] | 1.9 ± 1.4 | 1.7 | [0.32–10] | 2.3 ± 1.6 | 2.1 | [0.060–17] | 0.0033 |
| fT4( pmol/L) | 16 ± 2.3 | 16 | [13–20] | 14 ± 1.8 | 14 | [11–18] | 13 ± 1.6 | 13 | [9.5–17] | <0.001 |
| T4 (nmol/L) | 160 ± 32 | 155 | [109–236] | 153 ± 25 | 147 | [87–201] | 142 ± 24 | 139 | [84–201] | 0.0011 |
| fT3 (pmol/L) | 5.1 ± 0.62 | 5.1 | [4.1–6.4] | 4.7 ± 0.45 | 4.6 | [3.6–5.7] | 4.2 ± 0.47 | 4.2 | [3–6.2] | <0.001 |
| T3 (nmol/L) | 2.6 ± 0.43 | 2.6 | [2–3.4] | 2.8 ± 0.43 | 2.8 | [1.3–3.7] | 2.7 ± 0.48 | 2.7 | [1.2–5.2] | 0.10 |
| Thyroglobulin (μg/L) | 12 ± 13 | 9.8 | [0.5–53] | 13 ± 9.0 | 11 | [0.10–49] | 16 ± 14 | 13 | [0.30–107] | 0.062 |
| Transthyretin (mg/L) | 214 ± 45 | 205 | [100–280] | 211 ± 43 | 210 | [80–340] | 205 ± 53 | 205 | [52–350] | 0.41 |
| Exposure biomarkers | ||||||||||
| Blood lithium (μg/L) | 23 ± 14 | 21 | [4.3–55] | 26 ± 19 | 23 | [2.2–145] | 29 ± 18 | 26 | [1.9–109] | 0.09 |
| Urinary lithium (μg/L) | 1372 ± 937 | 1117 | [206–3903] | 1634 ± 998 | 1398 | [143–4523] | 1714 ± 1065 | 1465 | [105–5185] | 0.13 |
| Blood cesium (μg/L) | 140 ± 73 | 132 | [11–293] | 132 ± 114 | 107 | [2.5–659] | 122 ± 98 | 111 | [3.9–711] | 0.16 |
| Urinary cesium (μg/L) | 510 ± 243 | 483 | [45–1048] | 530 ± 397 | 463 | [14–2248] | 492 ± 309 | 472 | [17–1748] | 0.79 |
| Urinary iodine (μg/L) | 154 ± 108 | 131 | [49–575] | 171 ± 188 | 121 | [43–1490] | 157 ± 121 | 115 | [35–708] | 0.87 |
| Serum selenium (μg/L) | 92 ± 8.9 | 92 | [73–115] | 91 ± 13 | 89 | [64–131] | 83 ± 15 | 81 | [50–137] | <0.001 |
Comparison across trimesters based on Kruskal-Wallis rank test.
TSH, thyrotropin; fT4, free thyroxine; T4, total thyroxine; fT3, free triiodothyronine; T3, total triiodothyronine.
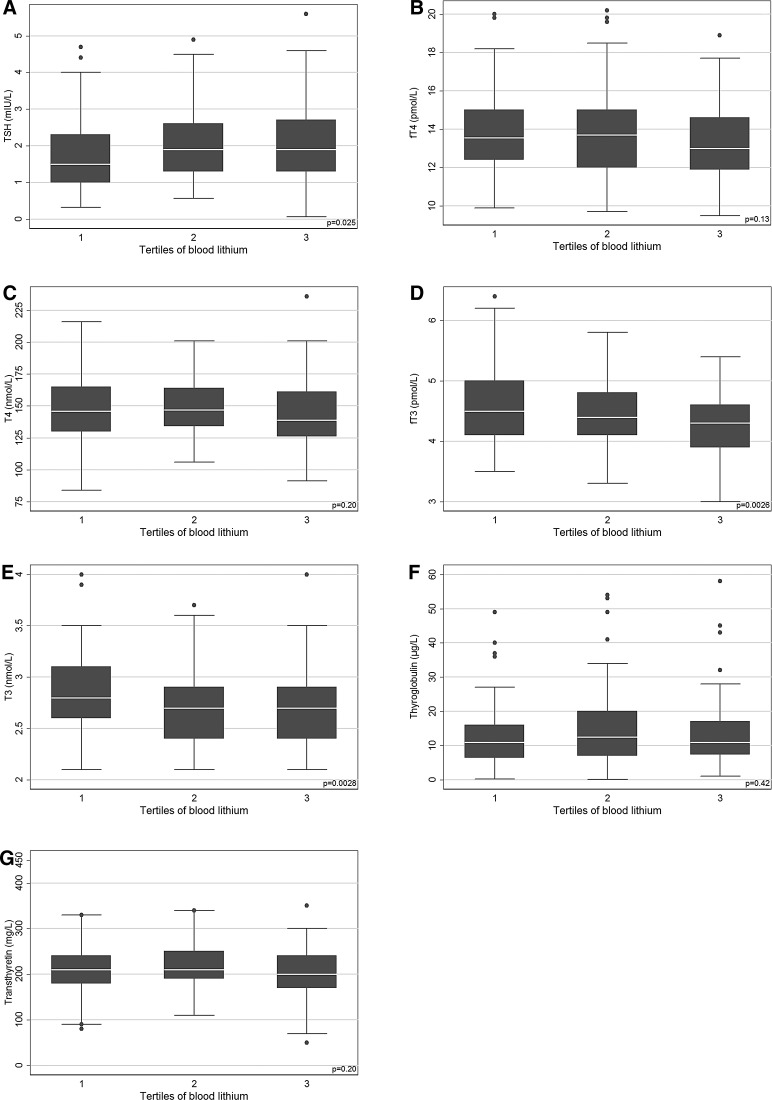
The distribution of all unadjusted thyroid markers across tertiles of blood lithium is shown in Fig. 1. TSH was higher while fT3 and total T3 were lower in the highest tertile of blood lithium compared with the lowest (Fig. 1). The multivariable adjustment of the linear quantile regression models showed only minor changes in the estimates. The regression models revealed positive associations between log2 blood lithium concentrations and TSH in serum, particularly in the lower tail of the TSH distribution (Table 3, Supplementary Fig. S2A). Each doubling of blood lithium concentrations was associated with an increase of about 0.2 mIU/L at both the fifth and twenty-fifth percentiles of TSH (Table 3). Also, the blood lithium concentrations were associated with a decrease in transthyretin, particularly in the highest percentiles (Table 3, Supplementary Fig. S2B), and with a decrease in fT3 and total T3 with less obvious variation in the estimates across outcome percentiles (Table 3, Supplementary Fig. S2C, D). Associations with fT4, total T4, and thyroglobulin were inverse, but not statistically significant (Table 3) and therefore not included in Supplementary Figure S2. Of note, gestational age was significantly associated with TSH, fT4, total T4, fT3, and transthyretin in the multivariable adjusted analyses (data not shown).
FIG. 1.
Box plots of maternal blood lithium concentrations and thyrotropin (TSH) (A); free thyroxine (fT4) (B); total T4 (C); free triiodothyronine (fT3) (D); total T3 (E); thyroglobulin (F); and transthyretin (G). Box layers describe the 75th, 50th, and 25th percentiles. Extreme outliers are not shown.
Table 3.
Quantile Regression Models of Associations of Maternal Blood Concentrations of Lithium and Cesium (μg/L; log2-Transformed) with Markers of Thyroid Function During Pregnancy
| Exposure biomarkers in blood | |||||
|---|---|---|---|---|---|
| Thyroid marker | Percentile | log2 lithium B [95% CI]a | p−Value | log2 cesium B [95% CI]b | p-Value |
| TSH (mIU/L) | 5th | 0.20 [0.048, 0.35] | 0.010 | 0.050 [−0.052, 0.15] | 0.34 |
| 25th | 0.17 [0.017, 0.32] | 0.029 | 0.032 [−0.074, 0.14] | 0.56 | |
| 50th | 0.13 [−0.056, 0.31] | 0.17 | 0.041 [−0.12, 0.20] | 0.62 | |
| 75th | 0.032 [−0.23, 0.29] | 0.81 | 0.028 [−0.20, 0.26] | 0.81 | |
| 95th | 0.049 [−0.51, 0.61] | 0.86 | 0.15 [−0.22, 0.52] | 0.43 | |
| Free T4 (pmol/L) | 5th | −0.086 [−0.39, 0.22] | 0.58 | −0.27 [−0.37, 0.31] | 0.88 |
| 25th | −0.071 [−0.41, 0.27] | 0.68 | 0.098 [−0.12, 0.31] | 0.38 | |
| 50th | 0.022 [−0.33, 0.38] | 0.90 | 0.11 [−0.088, 0.30] | 0.29 | |
| 75th | −0.053 [−0.52, 0.42] | 0.83 | −0.14 [−0.44, 0.15] | 0.35 | |
| 95th | 0.29 [−0.17, 0.76] | 0.21 | −0.023 [−0.34, 0.29] | 0.89 | |
| Total T4 (nmol/L) | 5th | 3.5 [−4.4, 11] | 0.38 | −0.81 [−6.2, 4.5] | 0.77 |
| 25th | −0.96 [−5.1, 3.2] | 0.65 | 1.2 [−1.9, 4.2] | 0.46 | |
| 50th | −2.9 [−7.5, 1.7] | 0.21 | −0.71 [−4.1, 2.7] | 0.68 | |
| 75th | −1.4 [−6.5, 3.8] | 0.61 | −0.45 [−4.0, 3.1] | 0.81 | |
| 95th | 2.2 [−6.8, 11] | 0.63 | −0.21 [−6.4, 6.0] | 0.95 | |
| Free T3 (pmol/L) | 5th | −0.12 [−0.26, 0.024] | 0.10 | −0.054 [−0.17, 0.060] | 0.35 |
| 25th | −0.031 [−0.14, 0.073] | 0.56 | 0.027 [−0.053, 0.11] | 0.51 | |
| 50th | −0.086 [−0.18, 0.008] | 0.073 | −0.016 [−0.096, 0.065] | 0.70 | |
| 75th | −0.090 [−0.21, 0.028] | 0.13 | −0.051 [−0.13, 0.026] | 0.19 | |
| 95th | −0.15 [−0.35, 0.049] | 0.14 | −0.13 [−0.25, −0.0040] | 0.043 | |
| Total T3 (nmol/L) | 5th | −0.023 [−0.22, 0.17] | 0.82 | −0.026 [−0.13, 0.082] | 0.63 |
| 25th | −0.089 [−0.17, −0.0051] | 0.038 | −0.029 [−0.11, 0.053] | 0.49 | |
| 50th | −0.061 [−0.14, 0.015] | 0.12 | −0.048 [−0.11, 0.015] | 0.14 | |
| 75th | −0.090 [−0.20, 0.022] | 0.12 | −0.072 [−0.14, −0.0026] | 0.042 | |
| 95th | −0.22 [−0.51, 0.070] | 0.14 | −0.17 [−0.31, −0.023] | 0.023 | |
| Thyroglobulin (μg/L) | 5th | −0.40 [−2.0, 1.2] | 0.63 | 0.25 [−0.64, 1.1] | 0.56 |
| 25th | 0.16 [−0.98, 1.3] | 0.79 | 0.21 [−0.84, 1.3] | 0.69 | |
| 50th | −0.13 [−2.2, 2.0] | 0.90 | 0.64 [−0.88, 2.2] | 0.41 | |
| 75th | 0.25 [−3.3, 3.8] | 0.89 | 0.52 [−1.2, 2.3] | 0.56 | |
| 95th | −2.1 [−9.1, 4.9] | 0.55 | 2.4 [−2.6, 7.4] | 0.35 | |
| Transthyretin (mg/L) | 5th | 3.0 [−15, 21] | 0.74 | 8.9 [−4.0, 22] | 0.18 |
| 25th | −5.5 [−15, 3.9] | 0.26 | 0.69 [−6.0, 7.4] | 0.84 | |
| 50th | −6.2 [−16, 3.5] | 0.21 | −0.42 [−6.8, 6.0] | 0.90 | |
| 75th | −11 [−22, −0.073] | 0.049 | −1.8 [−10, 6.3] | 0.66 | |
| 95th | −17 [−38, 3.8] | 0.11 | −2.7 [−15, 9.9] | 0.68 | |
n = 171; observations = 255
Adjusted for gestational age (weeks), height (cm), parity (tertiles), urinary arsenic (tertiles), urinary iodine (tertiles), serum selenium (tertiles), and log2 urinary cesium.
Adjusted for gestational age (weeks), height (cm), parity (tertiles), urinary arsenic (tertiles), urinary iodine (tertiles), serum selenium (tertiles), and log2 urinary lithium.
p-Value test for trend across percentiles.
CI, confidence interval.
Cesium concentrations were inversely associated with fT3 and T3, particularly at the higher percentiles (Table 3, Supplementary Fig. S2E, F). For each doubling of blood cesium, the 95th percentile of fT3 and total T3 decreased by 0.13 pmol/L, and 0.17 nmol/L, respectively (Table 3).
In a sensitivity analysis, we tested if the observed associations of blood lithium and cesium with T3 and fT3 could be influenced by illness during pregnancy. Different types of infections (i.e., cold, vaginal infection, urinary tract infection, and fever) were the most frequently reported illnesses. When we grouped all infections, neither fT3 nor T3 was different. In those having infection, mean fT3 was 4.46 pmol/L (range 4.31–4.60), and in women without any infection 4.43 (4.34–4.5; p = 0.67); while mean T3 was 2.77 nmol/L (range 2.65–2.87) in women with infection and 2.69 (range 2.62–2.76) in women without infection (p = 0.24). When we adjusted the statistical models of fT3 and T3 in relation to blood lithium or cesium for this variable (infections yes/no), the estimates remained essentially the same and the variable “infections” was not statistically significant in any of the models. The frequency of most other illnesses encountered was generally low—for example, nausea/abdominal pain (first trimester n = 2, second trimester n = 2, third trimester n = 5)—and for this reason we could not test this statistically. However, women having any illnesses other than infection had similar fT3 and T3 as women without such illnesses.
Discussion
This study provides the first indication that exposure to lithium and cesium through drinking water may adversely affect thyroid function during pregnancy. In particular, increasing blood lithium concentrations were associated with higher serum TSH, but also lower fT3, total T3 and transthyretin levels. The quantile regression models indicate that the women in the lowest percentiles of TSH and highest percentiles of T3 are more susceptible to lithium. An unexpected secondary finding is the inverse association between cesium, which also occurred at highly varying concentrations in the drinking water, and both fT3 and T3.
One previous study from Buenos Aires, on the East coast of Argentina, presumably with a very low water lithium concentration, reported median TSH concentrations of 0.95, 1.5 and 1.6 mIU/L in the first, second, and third trimesters, respectively, (27) which is similar to the observed concentrations in our study, particularly in the first lithium tertile, (1.3, 1.7, and 1.8 mIU/L, respectively). Our results on lithium exposure and thyroid function are in agreement with previous studies, although most of those concerned lithium medication, which results in much higher blood lithium concentrations (therapeutic range 0.8–1.2 mmol/L). A recent meta-analysis and a retrospective cohort study of clinical studies on lithium therapy showed that lithium therapy is associated with an increased risk of hypothyroidism (2,3). In support of a low-dose effect at the population level, we previously observed a positive association between lithium exposure through drinking water and TSH in nonpregnant women in the same Andean area (8). The potential health consequences of the indicated effects of lithium on thyroid markers, including the potential relation to the previously found lithium-related shorter birth length in the present cohort (16), need to be elucidated. In particular, the children need to be followed-up, as lithium readily crosses the placenta (28) and thyroid hormones are known to play a critical role in fetal growth and development (9,10), including cognitive function (14,15).
Suggested mechanisms for the effects of lithium medication on thyroid function include inhibition of iodine uptake, inhibition of iodotyrosine coupling, alteration in thyroglobulin structure, and inhibition of thyroxine secretion (29,30), most of which would fit with the present findings. The results relating to T3 and fT3 could conceivably be due to an inhibitory action of lithium on the conversion of T4 to T3 (31). Lithium is known to accumulate in the thyroid gland and also to cross the blood–brain barrier and accumulate in the hypothalamus (32,33). Therefore, lithium may impair the thyroid homeostasis both at the pituitary level, directly in the thyroid follicles, and via disruption of the thyroid hormone transport or conversion in the bloodstream. If confirmed in other studies, the found inverse associations between blood lithium and transthyretin might be a novel mechanism of low-level lithium exposure. Transthyretin is one of the proteins responsible for transporting T4 in the body and the most abundant T4 transporter in the cerebrospinal fluid (34). Importantly, transthyretin is produced in placental trophoblastic cells and transports thyroid hormones to the fetus (35). Thus, a decrease in transthyretin might lead to lower fetal thyroid hormone levels (36).
Cesium is also known to accumulate in the thyroid gland, as shown for the radioactive form (21). Still, we found only one previous study evaluating exposure to stable cesium (and multiple other elements) and thyroid function, based on the NHANES 2007–2008 data. It showed an inverse association between urinary cesium concentrations and TSH, but no associations with fT3 or T3 (37). However, the cesium concentrations were extremely low (median 4.6 μg/L, interquartile range 3.4–6.1 μg/L), and the results cannot be compared with those of the present study with two orders of magnitude higher urinary concentrations.
About half of the studied women were found to be iodine deficient, which might increase susceptibility to lithium toxicity. Due to the lack of power, this was not tested in the present study. Although iodine deficiency–related goiter is prevalent in the province of Salta (38,39), the prevalence of goiter among school-aged children decreased after the introduction of iodinated table salt; from 68% in 1975, to 32% in 1981 (38), and to 6.3% by 2008 (40). Our present findings of low iodine concentrations in the Andean part of Salta province raise the need for a thorough follow-up. We found very low iodine concentrations in both the drinking water (5–30 μg/L) and the water from a couple of the salt lakes (salars; 100–400 μg/L). Thus, the use of the salt from the many salars as table salt would provide little of the required iodine.
The exposure to both lithium and cesium had a marked spatial variation in the study area. Similarly high drinking water concentrations of lithium have been found in a few other countries (7,41), but obviously, more intense water monitoring is needed worldwide. A number of brands of bottled water with lithium concentrations above 1 mg/L have been reported (7), but a large number of brands remain to be analyzed for lithium and cesium. Although the drinking water most likely was the main source of exposure in the present study, the correlation with lithium and cesium in blood or urine was only moderate. This suggests a variation in the water consumption, both directly and through food and drinks, e.g. the commonly eaten soups (∼1100 μg lithium/L), especially during the cold winter period. Probably, the food as such (mainly cereals, vegetables, and some meat) contributed little to the exposure as very few crops are grown in the arid Puna region and samples of meat from lama animals, grazing in the surrounding Puna, were found to contain only about 40 μg/kg of lithium. Inhalation of dust might however have contributed some because of the prevailing windy climate. Nevertheless, the variations emphasize the need for repeated measurements of individual exposure biomarkers for reliable assessment of the internal dose.
The strengths of our study include the population-based prospective design, covering most of the Andean part of Salta province, with wide ranges of exposure to lithium and cesium. Another strength is the use of multiple biomarkers of thyroid function, collected longitudinally across pregnancy, along with the measurements of the exposure biomarkers. One of the limitations of our study is the lack of samples for all women at all time points. Also, the potential impact of the low atmospheric oxygen concentration at the high altitudes on thyroid function (42) was not assessed. However, all women in the study were living at high altitude (all at more than 3,000 meters above sea level) and 56% of the women had lived in the area since birth. Moreover, we did not have the possibility to measure thyroxine-binding globulin (TBG), which might have had an impact on the T3 levels due to its increase in early pregnancy (43). However, in any case, an impact of elevated TBG on T3 would rather decrease the possibilities to detect associations with lithium and cesium. Another limitation is the fairly small cohort. Indeed, the findings emphasize the need for larger studies also in other populations.
In conclusion, this study indicates that elevated environmental lithium and cesium exposure may adversely affect the maternal thyroid function during pregnancy. Due to the important role of thyroid hormones in child development, in particular neurodevelopment, a follow up of early-life lithium-exposed children is warranted. Equally important is a follow-up of the iodine status and to make sure that the pregnant women and children, in particular, have adequate iodine intake. Our findings are of public health relevance and they reinforce the need for more drinking water measurements worldwide and also guideline values for drinking water, including bottled water.
Supplementary Material
Acknowledgments
We thank the participant mothers as well as the local physicians Dr. Graciela Colque, Dr. Luis Lima, Dr. Alicia Soriano, and Dr. Wilfredo Medrano. We are also thankful to all the community health workers, Dr. Margareta Langeén, and Anna Karin Bernhardsson for assisting in the recruitment and sampling of the study individuals, and to Margaretha Grandér, Helena Nordqvist, and Ying Lu for technical assistance with trace element analyses. We also thank Dr. Maria Kippler for commenting on the manuscript.
This research was supported by grants from the Swedish Research Council Formas (Grant number: 210-2011-960) and Karolinska Institutet.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Grandjean EM, Aubry JM. 2009. Lithium: updated human knowledge using an evidence-based approach. Part I: clinical efficacy in bipolar disorder. CNS Drugs 23:225–240 [DOI] [PubMed] [Google Scholar]
- 2.McKnight RF, Adida M, Budge K, Stockton S, Goodwin GM, Geddes JR. 2012. Lithium toxicity profile: a systematic review and meta-analysis. Lancet 379:721–728 [DOI] [PubMed] [Google Scholar]
- 3.Shine B, McKnight RF, Leaver L, Geddes JR. 2015. Long-term effects of lithium on renal, thyroid, and parathyroid function: a retrospective analysis of laboratory data. Lancet 386:461–468 [DOI] [PubMed] [Google Scholar]
- 4.Bluml V, Regier MD, Hlavin G, Rockett IR, Konig F, Vyssoki B, Bschor T, Kapusta ND. 2013. Lithium in the public water supply and suicide mortality in Texas. J Psychiatr Res 47:407–411 [DOI] [PubMed] [Google Scholar]
- 5.Concha G, Broberg K, Grander M, Cardozo A, Palm B, Vahter M. 2010. High-level exposure to lithium, boron, cesium, and arsenic via drinking water in the Andes of northern Argentina. Environl Sci Technol 44:6875–6880 [DOI] [PubMed] [Google Scholar]
- 6.Kabacs N, Memon A, Obinwa T, Stochl J, Perez J. 2011. Lithium in drinking water and suicide rates across the East of England. Br J Psychiatry 198:406–407 [DOI] [PubMed] [Google Scholar]
- 7.Reimann C, Birke M. 2010. Geochemistry of European Bottled Water. Borntraeger Science Publishers, Stuttgart, Germany [Google Scholar]
- 8.Broberg K, Concha G, Engstrom K, Lindvall M, Grander M, Vahter M. 2011. Lithium in drinking water and thyroid function. Environ Health Perspect 119:827–830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.World Health Organization (WHO) 2012 Possible developmental early effects of endocrine disruptors on child health. WHO, Geneva, Switzerland [Google Scholar]
- 10.Polak M. 2014. Human fetal thyroid function. Endocr Dev 26:17–25 [DOI] [PubMed] [Google Scholar]
- 11.Allan WC, Haddow JE, Palomaki GE, Williams JR, Mitchell ML, Hermos RJ, Faix JD, Klein RZ. 2000. Maternal thyroid deficiency and pregnancy complications: implications for population screening. J Med Screen 7:127–130 [DOI] [PubMed] [Google Scholar]
- 12.Casey B. 2005. Environmental contaminants and maternal thyroid function. Am J Obstet Gynecol 193:1889–1890 [DOI] [PubMed] [Google Scholar]
- 13.Chen LM, Du WJ, Dai J, Zhang Q, Si GX, Yang H, Ye EL, Chen QS, Yu LC, Zhang C, Lu XM. 2014. Effects of subclinical hypothyroidism on maternal and perinatal outcomes during pregnancy: a single-center cohort study of a Chinese population. PLoS One 9:e109364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haddow JE, Palomaki GE, Allan WC, Williams JR, Knight GJ, Gagnon J, O'Heir CE, Mitchell ML, Hermos RJ, Waisbren SE, Faix JD, Klein RZ. 1999. Maternal thyroid deficiency during pregnancy and subsequent neuropsychological development of the child. N Engl J Med 341:549–555 [DOI] [PubMed] [Google Scholar]
- 15.Zimmermann MB. 2012. The effects of iodine deficiency in pregnancy and infancy. Paediatr Perinat Epidemiol 26:108–117 [DOI] [PubMed] [Google Scholar]
- 16.Harari F, Langeen M, Casimiro E, Bottai M, Palm B, Nordqvist H, Vahter M. 2015. Environmental exposure to lithium during pregnancy and fetal size: a longitudinal study in the Argentinean Andes. Environ Int 77:48–54 [DOI] [PubMed] [Google Scholar]
- 17.Watson PE, Watson ID, Batt RD. 1980. Total body water volumes for adult males and females estimated from simple anthropometric measurements. Am J Clin Nutr 33:27–39 [DOI] [PubMed] [Google Scholar]
- 18.Lu Y, Kippler M, Harari F, Grander M, Palm B, Nordqvist H, Vahter M. 2015. Alkali dilution of blood samples for high throughput ICP-MS analysis-comparison with acid digestion. Clin Biochem 48:140–147 [DOI] [PubMed] [Google Scholar]
- 19.Armstrong TA, Spears JW, Lloyd KE. 2001. Inflammatory response, growth, and thyroid hormone concentrations are affected by long-term boron supplementation in gilts. J Anim Sci 79:1549–1556 [DOI] [PubMed] [Google Scholar]
- 20.Lindgren A, Vahter M, Dencker L. 1982. Autoradiographic studies on the distribution of arsenic in mice and hamsters administered 74As-arsenite or -arsenate. Acta Pharmacol Toxicol (Copenh) 51:253–265 [DOI] [PubMed] [Google Scholar]
- 21.Nelson A, Ullberg S, Kristoffersson H, Ronnback C. 1961. Distribution of radiocesium in mice. An autoradiographic study. Acta radiol 55:374–384 [DOI] [PubMed] [Google Scholar]
- 22.Kohrle J. 2013. Selenium and the thyroid. Curr Opin Endocrinol Diabetes Obes 20:441–448 [DOI] [PubMed] [Google Scholar]
- 23.World Health Organization, UNICEF, International Council for the Control of Iodine Deficiency Disorders 2007 Assessment of Iodine Deficiency Disorders and Monitoring Their Elimination: A Guide for Programme Managers. Third edition. WHO, Geneva [Google Scholar]
- 24.Rydbeck F, Bottai M, Tofail F, Persson LA, Kippler M. 2014. Urinary iodine concentrations of pregnant women in rural Bangladesh: a longitudinal study. J Expo Sci Environ Epidemiol 24:504–509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bottai M, Frongillo EA, Sui X, O'Neill JR, McKeown RE, Burns TL, Liese AD, Blair SN, Pate RR. 2014. Use of quantile regression to investigate the longitudinal association between physical activity and body mass index. Obesity (Silver Spring) 22:E149–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boelen A, Kwakkel J, Fliers E. 2011. Beyond low plasma T3: local thyroid hormone metabolism during inflammation and infection. Endocr Rev 32:670–693 [DOI] [PubMed] [Google Scholar]
- 27.Rodríguez MF, Silva MC, Lutzky C, Ilera V, Zunino A, Scalise C, Pinto G, López M, Méndez V, Chiesa A, Gauna A. 2014. Thyroid disorders and iodine deficiency in a pregnant population. Revista Argentina de Salud Pública 5:11–16 [Google Scholar]
- 28.Harari F, Ronco AM, Concha G, Llanos M, Grander M, Castro F, Palm B, Nermell B, Vahter M. 2012. Early-life exposure to lithium and boron from drinking water. Reprod Toxicol 34:552–560 [DOI] [PubMed] [Google Scholar]
- 29.Berens SC, Bernstein RS, Robbins J, Wolff J. 1970. Antithyroid effects of lithium. J Clin Invest 49:1357–1367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Burrow GN, Burke WR, Himmelhoch JM, Spencer RP, Hershman JM. 1971. Effect of lithium on thyroid function. J Clin Endocrinol Metab 32:647–652 [DOI] [PubMed] [Google Scholar]
- 31.Terao T, Oga T, Nozaki S, Ohta A, Otsubo Y, Yamamoto S, Zamami M, Okada M. 1995. Possible inhibitory effect of lithium on peripheral conversion of thyroxine to triiodothyronine: a prospective study. Int Clin Psychopharmacol 10:103–105 [DOI] [PubMed] [Google Scholar]
- 32.Berens SC, Wolff J, Murphy DL. 1970. Lithium concentration by the thyroid. Endocrinology 87:1085–1087 [DOI] [PubMed] [Google Scholar]
- 33.Mukherjee BP, Bailey PT, Pradhan SN. 1976. Temporal and regional differences in brain concentrations of lithium in rats. Psychopharmacology (Berl) 48:119–121 [DOI] [PubMed] [Google Scholar]
- 34.Schreiber G, Southwell BR, Richardson SJ. 1995. Hormone delivery systems to the brain-transthyretin. Exp Clin Endocrinol Diabetes 103:75–80 [DOI] [PubMed] [Google Scholar]
- 35.McKinnon B, Li H, Richard K, Mortimer R. 2005. Synthesis of thyroid hormone binding proteins transthyretin and albumin by human trophoblast. J Clin Endocrinol Metab 90:6714–6720 [DOI] [PubMed] [Google Scholar]
- 36.Darnerud PO, Morse D, Klasson-Wehler E, Brouwer A. 1996. Binding of a 3,3', 4,4'-tetrachlorobiphenyl (CB-77) metabolite to fetal transthyretin and effects on fetal thyroid hormone levels in mice. Toxicology 106:105–114 [DOI] [PubMed] [Google Scholar]
- 37.Yorita Christensen KL. 2013. Metals in blood and urine, and thyroid function among adults in the United States 2007–2008. Int J Hyg Environ Health 216:624–632 [DOI] [PubMed] [Google Scholar]
- 38.Morón C, Nordera JV, Pérez Somigliana MC, Katz R, De Galvez BC, Virgili E. 1984. Eliminación del Bocio Endémico en Escolares del Valle de Lerma, Salta, Argentina. Bol Oficina Sanit Panam 97:471–477 [PubMed] [Google Scholar]
- 39.Salvaneschi JP, García JRAR. 2009. El bocio endémico en la República Argentina. Antecedentes, extensión y magnitud de la endemia, antes y después del empleo de la sal enriquecida con yodo: Primera Parte. Rev Argent Endocrinol Metab 46:48–57 [Google Scholar]
- 40.Méndez V, Chiesa A, Prieto L, Bergadá R, Gruñeiro-Papendieck L. 2008. Surveillance of iodine deficiency in Salta Capital. Rev Argent Endocrinol Metab 45:206–213 [Google Scholar]
- 41.Helbich M, Bluml V, Leitner M, Kapusta ND. 2013. Does altitude moderate the impact of lithium on suicide? A spatial analysis of Austria. Geospat Health 7:209–218 [DOI] [PubMed] [Google Scholar]
- 42.Sarne D. 2000. Effects of the Environment, Chemicals, and Drugs on Thyroid Function. [Updated 2010 Dec 21] In: De Groot LJ, Beck-Peccoz P, Chrousos G, Dungan K, Grossman A, Hershman JM, Koch C, McLachlan R, New M, Rebar R, Singer F, Vinik A, Weickert MO. (eds). Endotext [Internet]. MDText.com, Inc., South Dartmouth, MA [Google Scholar]
- 43.Lockitch G. 1993. Handbook of Diagnostic Biochemistry and Hematology in Normal Pregnancy. CRC Press, Boca Raton [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.