Abstract
The disciplines of physiology and ecology are united by the shared centrality of the concept of homeostasis: the stability of a complex system via internal mechanisms of self-regulation, resilient to external perturbation. In the past decade, these fields of study have been bridged by the discovery of the lung microbiome. The respiratory tract, long considered sterile, is in fact a dynamic ecosystem of microbiota, intimately associated with the host inflammatory response, altered in disease states. If the microbiome is a “newly discovered organ,” ecology is the language we use to explain how it establishes, maintains, and loses homeostasis. In this essay, we review recent insights into the feedback mechanisms by which the lung microbiome and the host response are regulated in health and dysregulated in acute and chronic lung disease. We propose three explanatory models supported by recent studies: the adapted island model of lung biogeography, nutritional homeostasis at the host-microbiome interface, and interkingdom signaling and the community stress response.
Keywords: lung, ecology, physiology, 16S, culture-independent, microbial ecology, pulmonary, homeostasis, equilibrium
homeostasis, the stability of a system via internal mechanisms of self-regulation, resilient to external perturbation, is historically and philosophically central to the discipline of physiology. Claude Bernard's (4) nineteenth century articulation of the milieu intérieur (“the internal environment”) was inspired by his observation that the blood glucose concentration of healthy individuals remained remarkably stable whether in fasting or feasting states:
The fixity of the milieu supposes a perfection of the organism such that the external variations are at each instant compensated for and equilibrated. . . all of the vital mechanisms, however, varied they may be, have always one goal, to maintain the uniformity of the conditions of life in the internal environment. . . the stability of the internal environment is the condition for free and independent life.
Nearly a century and a half later, this “stability of the internal environment”1 remains the defining concept of respiratory physiology. On the levels of the molecule, the cell, and the organ system, our field studies how healthy lungs provide unwavering stability in oxygen delivery, pH, pulmonary and systemic arterial pressures, and countless other parameters essential for “free and independent life.” Pathophysiology, in turn, studies the disruption of homeostasis: the mechanisms by which our internal systems of self-regulation and compensation are overwhelmed or become maladaptive.
If physiology studies homeostasis within organisms, ecology studies homeostasis between organisms. Both seek to explain how it is established, sustained, and eroded.2 Ecology is replete with examples of stability of the external environment: order emerging from the dynamic complexity of organism-organism and organism-environment interactions. Across the world's oceans, the ratio of carbon to nitrogen to phosphorus in seawater is a fixed 106:16:1 (the Redfield ratio), a ratio that matches the atomic ratio of phytoplankton and remains constant despite the complex cycling of these elements between the atmosphere, the oceans, and their organisms (46). The Lotka-Volterra equations describe the periodic stability of populations in predator-prey relationships (54) [such as, famously, the moose and wolves of Michigan's Isle Royale National Park (16)]. Ecology also offers its own examples of disruption of homeostasis, either due to an external disturbance [e.g., forest fire, volcanic eruption, or introduction of an invasive alien species (41)] or via destabilizing positive feedback loops that propel explosive growth and collapse of communities (6) [e.g., the dramatic and sudden appearance of algal blooms in water systems (51)].
In the past five years, these parallel concepts, physiological and ecological homeostasis, have been bridged by the discovery of the lung microbiome. The long-held dogma that the lungs are sterile in health has been debunked by the advent of culture-independent techniques of microbial identification. Dozens of studies have confirmed that our “internal environment” is in fact the “external environment” for a diverse and dynamic community of microbiota, present in health and altered in disease (10, 15). This revolution in respiratory microbiology demands a reassessment of the concept of homeostasis within the human respiratory tract. Our new conceptual models of respiratory homeostasis must span the host-microbe divide, incorporating concepts of both physiology and ecology to explain how we, and our respiratory microbiota, maintain and lose the stability of this environment in health and illness. In this essay, we propose three models of respiratory homeostasis, all supported by recent experimental studies of the lung microbiome. We describe self-regulating systems at the host-microbiome interface that determine the number of species present and the bacterial burden in the healthy human lung; we also propose how this homeostasis is disrupted by self-propelling positive feedback loops resulting in the dysregulated inflammation and injury of pneumonia and the acute respiratory distress syndrome (ARDS).
Model 1: The Adapted Island Model of Lung Biogeography
Prior to the discovery of the lung microbiome, the microbial composition of the lung was considered a binary phenomenon: it contained either zero community members (in the “sterility” of health) or one overwhelming community member (in pneumonia). [An exception to this, ahead of its time, was the recognition of polymicrobial airway colonization in cystic fibrosis (CF) (50)]. The implausible simplicity of this binary model has been made apparent by more than 30 published studies that have tested the lungs of healthy subjects with modern molecular techniques of bacterial identification: none has demonstrated the absence of bacterial signal.
Studies of the healthy lung microbiome have been quite consistent in their key findings, across both study populations and laboratories (3, 5, 8, 27, 39, 48). The lung microbiome, as detected either by bronchoalveolar lavage (BAL) fluid or protected specimen brushings (PSB), contains a bacterial burden that is greater than procedural control specimens and less than that of the upper respiratory tract. The community members of the lung bacterial community more closely resemble those of the mouth than other potential source communities [e.g., the nose or a hematogenous source (3, 53)]; this observation holds regardless of whether the bronchoscope is inserted nasally or orally (9, 15). The most abundant genera, mirroring the mouth, are Prevotella, Veillonella, and Streptococcus. Community richness (the number of species detected in each specimen) of the lung is roughly half that of the mouth (3, 5). Intrasubject variation in lung microbiota (sampled at different sites within the lungs) is significantly less than that of intersubject variation (8): the microbiome of an individual's right middle lobe more closely resembles that of his/her left upper lobe than it does his/her neighbor's right middle lobe.
These facts, now thoroughly established, invite consideration of ecological homeostasis within the healthy respiratory tract. The microbiome of the healthy lung exhibits the hallmark of homeostasis: stability of the internal environment, order and regularity within a complex and dynamic system of population dynamics and host defenses. Some stabilizing process must explain why the community richness and abundance of the lung microbiome is consistently measured within a defined range: greater than the background signal detected in laboratory reagents, less than that of the upper respiratory tract, independent of the route of sampling (BAL or PSB, via the mouth or via the nose). In articulating these mechanisms of stability, we may draw inspiration from the principles and seminal concepts of the field of ecology.
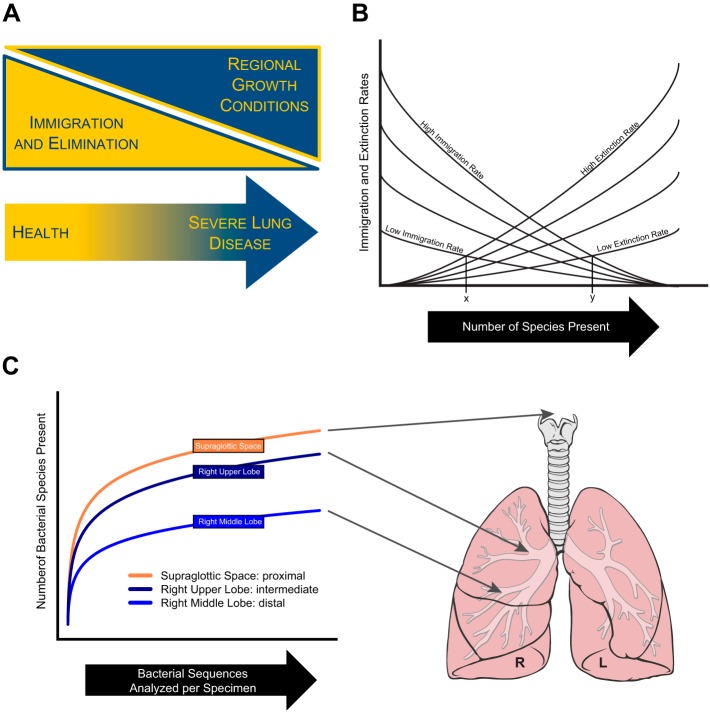
The lung microbiome, like any community (microbial or otherwise), is determined by the balance of three factors: immigration of community members, elimination/extinction of community members, and the relative reproduction rates of community members as determined by regional growth conditions (Fig. 1A). Any change in the community membership of the lung microbiome, by first principles, must be due to some combination of these three factors. Immigration to the microbiome is largely attributable to subclinical microaspiration, which has been shown to be ubiquitous among healthy subjects in multiple studies dating back to the 1920s (1, 24, 28, 45); minor contributions to immigration may be attributable to inhalation of air [which contains 104-106 bacterial cells per cubic meter (32)] and direct contiguous spread of reproducing microbial colonies. The mechanisms of microbial elimination are familiar to respiratory physiologists: cough, the mucociliary escalator, and innate and adaptive immune defenses. Finally, the ecological factors that define regional growth conditions and relative reproduction rates of lung microbiota are the physiological parameters of the lungs' internal environment: oxygen tension, pH, relative blood perfusion, relative alveolar ventilation, temperature, and the concentration and activation of host inflammatory cells. All of these physiological factors have profound in vitro and in vivo effects on the relative growth of bacteria; the growth of a given bacterial species is selectively advantaged or disadvantaged by a given constellation of oxygen, temperature, pH, etc.
Fig. 1.
The adapted island model of lung biogeography. The community of the lung microbiome is determined by 3 ecological factors (A): immigration, elimination, and the effects of regional growth conditions on the reproduction rates of community members. In health, the community is primarily determined by the balance of immigration (from microaspiration) and elimination (via cough, mucociliary clearance, and immune defenses). The number of species present at a given site in the respiratory tract in health is a steady state of dynamic equilibrium, determined by the balance of immigration and elimination factors, influenced by anatomical, functional and clinical factors (B). In an experimental validation of this model (C), proximity to the source community of the upper respiratory tract was associated with increased microbial immigration and increased community richness. A adapted from Ref. 15. B adapted from Ref. 36 with permission of John Wiley & Sons Ltd. C adapted from Ref. 8 with permission of the American Thoracic Society. Lung drawing adapted from original by Patrick J. Lynch, medical illustrator, and C. Carl Jaffe, M.D., cardiologist, via Creative Commons Attribution 2.5 license 2006 (http://goo.gl/xuJRCO).
A key initial question in the field, now convincingly and consistently answered by a variety of approaches, is to what degree the lung microbiome is determined by the dynamic turnover of immigration and elimination of microbiota, and how much by the relative reproduction rates of resident microbiota due to differential selective pressures. The answer to this question, now robustly supported by a variety of studies, study populations, and analytic approaches, is that the ecological determinants of the lung microbiome shift as patients progress from health (in which immigration and elimination are the dominant ecological factors) to severe lung disease (in which regional growth conditions are determinant) (Fig. 1A).
One revealing approach has been the application of the neutral model of biodiversity (39, 53). This model postulates that the composition of a community (in this case the lung microbiome) is determined by unbiased, “neutral” dispersal from source communities, rather than by the selective pressures of local environmental conditions. Rejection of the neutral model (that is, demonstration that community composition cannot be explained by unbiased dispersal) is evidence that a community is shaped by differences in the relative growth rates of its members. In a comprehensive analysis of the lung microbiome compared with the microbiota of 15 other body sites, Venkataraman et al. (53) found that the composition of the lung microbiome in health was best explained by neutral dispersal from the oropharynx. In a separate study, Morris et al. (39) used the neutral model to directly compare upper and lower respiratory tract microbiota in healthy volunteers. Although the authors identified some minor community members disproportionately represented in the lung compared with the mouth [e.g., a Tropheryma species that comprises around 1% of lung bacteria in health (33)], the vast majority of lung community members were found in relative abundances consistent with neutral dispersal from the mouth.
A complementary approach to this key ecological question is the study of spatial variation in the healthy human lung microbiome (8). Significant spatial variation exists within healthy lungs in physiological parameters of unambiguous importance to bacterial growth [e.g., oxygen tension, pH, temperature (11, 29, 55)]. If the healthy lung microbiome were determined by selective pressures of local growth conditions on resident bacteria, we should expect significant spatial variation in microbiota (e.g., enrichment of aerobic bacteria in the oxygen-rich apices). Yet in a recently published study of healthy volunteers, when we compared the microbiota detected at four lung sites as well as the supraglottic space (representing the source community of the upper respiratory tract), we found little evidence of spatial variation: there is no “left upper lobe microbiome” consistently distinct from that of the “right middle lobe microbiome.” Intrasubject variation in microbiota was significantly less than intersubject variation, consistent with the conclusion that the healthy human lung microbiome is relatively homogenous and largely determined by immigration from the primary source community of the mouth rather than local growth conditions.
This balance between the influence of immigration and elimination and that of regional growth conditions, however, shifts dramatically in advancing lung disease (Fig. 1A). Venkataraman et al. demonstrated that the neutral model fails to explain the composition of the lung microbiome in diseased states (e.g., CF and interstitial lung disease) (53), indicating that the lung microbiota of these conditions are instead determined by selective pressure within the lung environment. The microbiota of diseased lungs [e.g., chronic obstructive pulmonary disease (18); CF (25, 56)] exhibit considerable spatial variation in microbiota, confirming both the presence of resident microbiota as well as significant spatial variation in the environmental growth conditions of diseased lungs.3 The clinically familiar phenomenon of “colonization” supports this ecological shift: culture-identified colonizers are representative community members that are well adapted to the specific environmental conditions of diseased lungs and airways.
We can now articulate the central question of ecological homeostasis in the healthy lung microbiome: how, despite its constant exposure to the external environment, is the healthy lung microbiome kept in its state of relative stability? What stabilizing process ensures the consistency of bacterial community composition, diversity, and burden observed across studies and populations? Finally, and critically, how does perturbation of this stabilizing process (either by disease or clinical intervention) influence the lung microbiome? We may draw clarification and inspiration from one of ecology's seminal models: MacArthur and Wilson's equilibrium model of island biogeography (11, 36), first proposed in 1963.
MacArthur and Wilson's model arose from two key observations regarding the species richness (the number of plant and animal species) of islands located in the tropical Pacific region of Oceania. Firstly, they noted that the number of species present on a given island was inversely correlated with its distance from New Guinea, the nearest large land mass and each island's primary source community of immigrating species. They inferred that each island's proximity to this large land mass was the primary determinant of its immigration rate. Secondly, they observed that the number of species present on an island was directly correlated with its size; they inferred from this that each island's size was the primary determinant of its extinction rate. The species richness for any given island (in the absence of local selective pressures on reproduction rates) is determined by the balance of these rates of immigration and extinction (Fig. 1B).
Reflection on Fig. 1B reveals the utility of MacArthur and Wilson's elegant conceptual model. The slope of the curve of immigration as a function of species richness is negative: with each added species there is one less potential colonist from the source community that has the potential to immigrate. The slope of the curve of elimination as a function of species richness is instead positive: with each additional species there is one more potential species that can potentially be eliminated. Changes in immigration and elimination rates alter the relative slopes of these curves and their corresponding intersections. A small island (with an accordingly high extinction rate), far from the mainland (with an accordingly low immigration rate) would be expected to have low species richness (Fig. 1B, point x). Conversely, a large island close to the mainland would be expected to have high species richness (Fig. 1B, point y). For each island there exists an equilibrium point, a dynamic steady state to which, if perturbed, the system returns. Although the species richness of a given island is stable once it has reached equilibrium, the constant turnover of newly immigrated and eliminated species ensures that the population itself is dynamic.
This model serves as a rich and useful analogy for the population dynamics of the healthy lung microbiome. Whereas the islands of Oceania are populated by immigrating species from New Guinea, the human respiratory tract is populated by continuous immigration of microbiota from the primary source community of the mouth. For any given anatomical site in the airways or alveoli, the microbial population is a function of the relative rates of immigration and elimination (Fig. 1, A and B). Although the average bacterial burden and community richness of healthy lungs is stable and consistent across studies, this stability reflects the dynamic equilibrium created by these balanced, opposing ecological forces of immigration and elimination.
This model readily permits predictions regarding how disease states and clinical interventions will alter the slopes of immigration and elimination curves, shifting the equilibrium point and influencing the lung microbiome. The slope of the immigration curve will be made steeper (more negative) by factors that accelerate microbial immigration to the lungs: close proximity to the larynx, laryngeal dysfunction, the presence of an endotracheal tube. The slope of the elimination curve will be made more shallow (less positive) by factors that decelerate microbial elimination: impaired cough reflex (as in lung transplantation or sedation), impaired mucociliary clearance (as in CF or ciliary dyskinesia), or impaired innate or adaptive immunity (as in states of immunosuppression). In two recent studies of the microbiota of healthy subjects, we have validated predictions made by this adapted island model of lung biogeography.
In the first experiment, a study of 28 healthy volunteers, we applied the model to the aerodigestive tract by comparing the microbiota of the mouth with two sites: the lungs and the stomach (3). Although the mouth is the primary microbial source community of both the lung and gastric compartments, the two sites differ markedly in the relative balance of their ecological determinants. Whereas microbial immigration via microaspiration to the lungs is modest, microbial immigration to the stomach via swallowing is high (two liters of oropharyngeal secretions are swallowed each day). Whereas microbial elimination from the lungs is active and continuous (via cough, mucociliary clearance, and host immune defenses), microbial elimination from the stomach is passive and slow (via the 3-h mechanical passage of gastric contents to the duodenum). Thus our model would predict the lungs to have the low community richness and bacterial burden of a low immigration, high extinction site (Fig. 1B, point x); in turn, the model predicts that the stomach should have the high community richness and bacterial burden of a high immigration, low extinction site (Fig. 1B, point y). These predictions were validated by the experimental results: the community richness of BAL specimens was, on average, half that of gastric specimens. The bacterial burden (as measured by 16S rRNA gene copies per 5 ml) was 10-fold higher in the stomach than in the lung; the bacterial burden of oral specimens was in turn 10-fold higher than that of the stomach. Thus, considered on the level of the organ system, the adapted island model accurately predicts the ecological features of the aerodigestive tract in health. As with all bacterial DNA-based experiments, this analysis does not distinguish between the living and the dead; the gastric pH of 2 is inhospitable to most oral bacteria.
In a second experiment, performed on 15 healthy volunteers, we tested whether the adapted island model can predict the ecological features of microbial communities sampled at various sites within the respiratory tract (8). The adapted island model would predict that in health, proximity to the source community should be the primary determinant of community features within the respiratory tree. As shown in Fig. 1C, the results were entirely consistent with the predictions of the model. The community richness of the supraglottic space (representing the source community of the upper respiratory tract) was higher than that of intrapulmonary sites, just as within MacArthur and Wilson's model the community richness of New Guinea is greater than that of its satellite islands. Within the lungs, the right upper lobe had a community richness greater than that of the right middle lobe, which is more distal from the source community by the length of the bronchus intermedius. This spatial relationship was echoed in other important ecological parameters: in community membership, relative abundance of the Firmicutes phylum, and degree of intersubject variation, the right upper lobe more closely resembled the upper respiratory tract than did sites more remote from their common microbial source community.
This adapted island model of ecological homeostasis is now thus supported empirically by multiple analyses of two distinct studies of healthy volunteers. Looking forward, this model provides a conceptual framework for coherent hypothesis testing. For a given perturbation, either pathological (e.g., laryngeal dysfunction) or therapeutic (e.g., inhaled corticosteroids), we may predict the effect on the slopes of the immigration and elimination curves. The intersection of these curves, in turn, predicts the dynamic steady state for the microbiota present in given patient's respiratory tract.
Model 2: Nutritional Homeostasis at the Host-Microbiome Interface
While the adapted island model provides a useful framework for understanding the lung microbiome in health (when the community is determined by the balance of immigration and elimination), it does not incorporate any effect due to differential reproduction of community members on the composition of the microbial community. It is thus of little utility in states of advanced lung disease, when environmental pressures selectively influence the relative growth rates of community members (Fig. 1A). We thus require a conceptual model of ecological homeostasis in the lungs that can explain how the specific communities of injured airways and alveoli are established and maintained in equilibrium and how this equilibrium is abruptly disrupted in states of clinical decompensation (such as pneumonia or ARDS).
Although the relative growth of bacteria within a community may be influenced by any of a number of physiochemical factors (e.g., temperature, oxygen tension, pH), a key determinant of all reproducing bacterial communities is nutrient supply. The bacteria that comprise the lung microbiome are heterotrophic: they obtain their carbon via digestion of organic compounds.4 Bacteria differ markedly in their ability to metabolize different carbon sources, a fact we exploit clinically to identify cultured bacteria (e.g., a lactose-fermenting gram-negative rod) and manipulate the enteric microbiome (via alterations in diet) (7). Thus if we seek to understand the ecological determinants of the lung microbiome in disease, a good place to start is asking what the bacteria are eating.
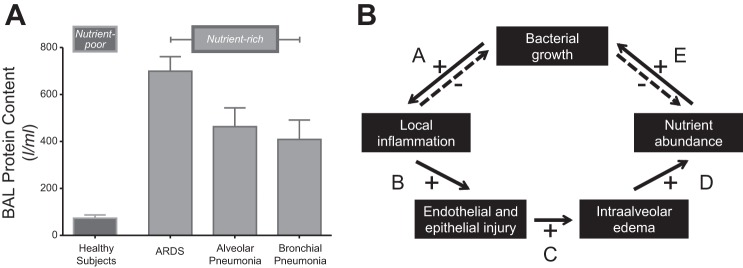
Although both are mucosa-lined luminal organs with a common embryological origin, the lungs and gut differ markedly from the perspective of microbial nutrient supply (14). Although the trachea and bronchi are, like the gut, lined with the heavily glycosylated proteins of secreted mucus, the vast majority of the lung's surface area is covered instead with a thin layer of lipid-rich surfactant. Whereas the gut lumen is replete with a constant stream of carbohydrates, proteins, and lipids, the lumen of healthy airways primarily contains inhaled and exhaled air. Thus, in health, the respiratory tract is a relatively nutrient-poor environment for most bacteria (Fig. 2A); this is one reason why local reproduction plays a minor ecological role in community composition in health (Fig. 1A).
Fig. 2.
Nutritional homeostasis at the host-microbiome interface. In health, the lungs are a nutrient-poor environment for bacteria, as evidenced by the low protein content of bronchoalveolar lavage (BAL) fluid (A). This changes in acute respiratory distress syndrome (ARDS) and pneumonia, when protein-rich edema fills the alveolar space. B: in homeostasis, the growth of a single bacterial species is inhibited by 2 negative feedback loops: increased bacterial growth provokes increased local inflammation, which kills and clears bacteria, inhibiting further bacterial growth (arrow A); and as bacteria grow they consume their available nutrient supply, inhibiting subsequent growth (arrow E). But if the provoked inflammation results in enough endothelial and epithelial injury (arrow B) to result in leak of protein- and nutrient-rich fluid into the alveolar compartment (arrow C), the growth-limiting nutrient supply is restored (arrow D) and bacterial growth is promoted (arrow E). The specific composition of available nutrients influences the relative growth rates of community members, shaping community membership. A: figure generated with data from Ref. 26. B: adapted from Ref. 11.
Yet in conditions of acute or chronic lung disease, bacterial nutrient supply surges. The airways of patients with severe CF, chronic bronchitis, bronchiectasis, and asthma are filled with the dense, glycoprotein-rich growth medium of secreted mucus. Deeper down in the respiratory ecosystem, alveoli of patients with ARDS and pneumonia are flooded with protein-rich edema from an injured alveolar-capillary barrier (26) (Fig. 2A). Thus the transition from health to respiratory illness is also a transition from bacterial nutrient scarcity to abundance, with a corresponding shift in the reproduction rates of the lung microbiome.
In Fig. 2B, we propose a conceptual model of nutritional homeostasis in the respiratory ecosystem. In health (or at least clinical stability), bacterial growth is regulated by two negative feedback loops (arrows A and E). A surge in bacterial growth provokes a local increase in alveolar inflammation, which inhibits further bacterial growth via the well-described mechanisms of microbial killing and clearance (arrow A). Additionally, the growth of a bacterial community depletes its own finite nutrient supply, which in turn inhibits further growth (arrow E).5 These tight, stabilizing negative feedback loops can be overwhelmed, however, if the provoked inflammation results in enough endothelial and epithelial injury (arrow B) to cause leak of protein-rich edema into the alveolar compartment (arrow C, Fig. 2A). This sudden abundance of organic substrate restores the community's depleted nutrient supply (arrow D), promoting further bacterial growth (arrow E). This growth, in turn, provokes further inflammation, and the positive feedback loop is perpetuated with a crescendoing intensity. This model thus provides conceptual explanations both for the homeostasis of a stable microbiome as well as the disruption of homeostasis observed in the abrupt, progressive pathologies of ARDS and pneumonia.
In an innovative and important recent study (44), Poroyko and colleagues used a murine model of direct lung injury to experimentally validate numerous elements of this conceptual model of nutritional homeostasis in the lung microbiome. Using intratracheal instillation of lipopolysaccharide (LPS), they reliably provoked alveolar inflammation, injury, and an influx of protein-rich edema. In the terms of our model (Fig. 2B), they bypassed the inciting event of bacterial growth (arrow A) and directly provoked its downstream consequences (arrows B–D). Consistent with the prediction of this model (arrow E), they observed a significant increase in the bacterial burden of the lungs following injury.6 Bacterial community diversity did not drop following lung injury, evidence that they did not accidentally provoke a pneumonia (12). Rather, the complex and expanding bacterial community shifted in its composition toward select members, present before injury and selectively favored by the altered growth conditions of injured lungs: Stenotrophomonas maltophilia and Ochrobactrum anthropi, both confirmed via culture. Using metabolic profiling of BAL fluid, the investigators confirmed that the injured lungs contained numerous organic substrates that are metabolized by the bacterial community members enriched following lung injury. This key observation demonstrates the plausibility of selective nutrient abundance determining the composition of the postinjury lung microbiome (Fig. 2B, arrow E). Finally, the authors “closed the loop” by validating Fig. 2B's arrow A: when a separate inflammatory exposure (IL-6) was administered to healthy mice, the coadministration of bacterial pellets obtained from the BAL of injured lungs provoked more inflammation and injury than did the bacterial pellets taken from healthy lungs. The altered lung microbiome itself potentiates further alveolar inflammation and injury. Thus, taken together, this elegant study has revealed a possible centrality to the microbiome in states of lung injury: both as its effect and as its cause.
Model 3: Interkingdom Signaling and the Community Stress Response
A key feature of homeostatic systems in physiology is signaling: molecular mechanisms by which an organism's tissues and cells communicate to other tissues and cells that the internal environment has been perturbed. Broad classes of signaling molecules correspond to their respective physiological disciplines: hormones (endocrinology, e.g., insulin's role in coordinating the response to altered blood glucose concentration), neurotransmitters (neurophysiology, e.g., the role of catecholamines in mediating the sympathetic stress response), and cytokines (immunology, e.g., TNF-α's role in the acute phase response). In our conventional models of host physiology, these molecular mediators are the “code” used to coordinate the dynamic elements of our complex internal systems. But an explosion of recent research has revealed that microbiota have cracked this code. In states of stress, microbes and their human hosts speak the same language.
We evolved into the microbes' world, not vice versa. The very structure of our eukaryotic cells is a patchwork assembly of our prokaryotic ancestors. Our cell membranes and core metabolic pathways resemble those of Bacteria (49); our mechanisms of DNA replication, transcription, and translation resemble those of Archaea (17); our mitochondrial genome specifically resembles that of Rickettsia prowazekii (2). It is thus unsurprising that microbes can recognize and respond to the molecules our cells use to communicate with each other. And, indeed, a long and growing catalogue of human signaling molecules have been shown in vitro to influence microbial growth and behavior: hormones [e.g., glucocorticoids, estrogens, and androgens (42, 58)], neurotransmitters [e.g., catecholamines and endogenous opioids (34, 57)], and cytokines [e.g., TNF-α, IL-1, IL-6, IL-8 (30, 31, 43)]. Among the many bacterial species that respond to host signaling molecules (23) are many familiar respiratory pathogens: Streptococcus pneumoniae (37), Pseudomonas aeruginosa (22), Staphylococcus aureus (40), Klebsiella pneumoniae (21), and Escherichia coli (34). Study of this “interkingdom signaling” is now its own discipline: microbial endocrinology (35, 47).
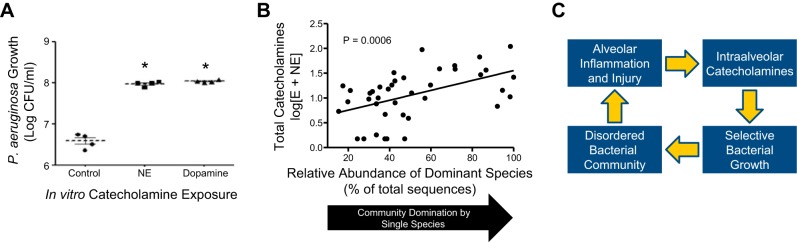
By focusing on one common respiratory pathogen (P. aeruginosa, the most common cause of ventilator-associated and hospital-associated pneumonia) and one class of signaling molecules (catecholamines, the primary neuroendocrine mediators of the host stress response), we can start to develop a conceptual model of pneumonia pathogenesis that spans the host-microbiome divide. As shown in Fig. 3A, the in vitro growth of P. aeruginosa is significantly enhanced in the presence of catecholamines (norepinephrine and dopamine) (22).7 Given that the effects of catecholamines on bacterial growth are specific to each species rather than universal among bacteria (20, 34), high catecholamine concentrations would be expected to selectively favor the growth of select members of the lung microbiome. In a recently published study of the microbiota detected in human BAL specimens (13), we observed that increased intra-alveolar catecholamine concentrations were strongly associated with collapse in community diversity and the emergence of a single dominant pathogen, the most abundant of which was P. aeruginosa (Fig. 3B).
Fig. 3.
Interkingdom signaling and the lung microbiome. Host-derived catecholamines, including norepinephrine (NE) and dopamine, promote the in vitro growth of Pseudomonas aeruginosa (A, adapted from Ref. 22 with permission of the American College of Chest Physicians). Intra-alveolar catecholamine concentrations are strongly associated with collapse of the respiratory ecosystem around a single dominant pathogen (B, adapted from Ref. 13 with permission of the American Thoracic Society). These observations suggest a positive feedback loop (C) that can propel the explosive disruption of homeostasis observed in respiratory infections with select bacteria. Similar bacterial growth promotion has been reported with other host inflammatory response molecules, including TNF-α, IL-1, IL-6, IL-8, and glucocorticoids (30, 31, 42, 43).
Taken together, these recent in vitro and in vivo observations demonstrate the plausibility of the positive feedback loop outlined in Fig. 3C. Any source of alveolar inflammation and injury (e.g., viral infection, toxic inhalation, or a large inoculum of aspirated bacteria) provokes an increase in alveolar catecholamines, which are produced by alveolar macrophages and neutrophils (19). This increase in alveolar catecholamine concentration selectively favors the growth of certain catecholamine-responsive bacteria, which alters the composition of the lung microbiome.8 In the case of complete community collapse, a single dominant species overtakes the respiratory ecosystem, both begetting more inflammation and benefiting from the surge in catecholamines provoked.
Whereas negative feedback loops promote self-regulation and stability, positive feedback loops promote dysregulation and instability via amplification of signal. The feedback loop in Fig. 3C may explain a fundamental feature of bacterial pneumonia: its uniformly acute onset. As clinicians know well, bacterial pneumonia arises abruptly over hours rather than over days or weeks. The chest X-ray of a ventilated patient may turn from clear to consolidated overnight, even in the absence of gross aspiration. This explosive suddenness bears the hallmarks of a self-amplifying positive feedback loop such as outlined in Fig. 3C.
Catecholamines are only one class of host stress signals that may participate in this form of interkingdom feedback loop. Numerous other host inflammatory signals with in vitro potential to promote bacterial growth and virulence are also elevated in the alveoli of patients with pneumonia [e.g., TNF-α, IL-1, and IL-6 (38)]. Furthermore, this conceptual model is likely not specific to the pathogenesis of pneumonia: a similar but distinct milieu of inflammatory molecules fill the alveoli of patients with ARDS, likely influencing the microbiota of that disease.
Exogenous catecholamines and adrenergic agonists are among the most commonly administered medications in patients with critical illness and respiratory disease: systemically as vasopressors (norepinephrine, epinephrine, dopamine), as antihypertensives (clonidine) and as sedatives (dexmedetomidine), and via the airways as bronchodilators. Catecholamine antagonists are also ubiquitous in clinical practice (e.g., beta-blockers in cardiovascular disease). No study to date has evaluated whether exogenous catecholamines independently influence the lung microbiome. The mechanisms by which catecholamines and cytokines affect the growth and virulence of bacteria are largely unknown (35) and may represent an enormous untapped reservoir of targets for prevention and treatment of respiratory infection. The notion that “protective” lung microbiota may participate in stabilizing feedback loops with the host immune interface is attractive but to date has not been demonstrated.
Conclusion
The disciplines of physiology and ecology converge conceptually with the concept of homeostasis; they converge biologically at the interface of the host and its microbiome. We here propose three conceptual models by which the complex system of the lung microbiome establishes and maintains homeostasis in health, and loses it in states of clinical decompensation. These models provide theoretical frameworks for the next generation of physiological investigations into respiratory health and illness.
GRANTS
Funding was provided by National Institutes of Health Grants T32HL00774921 (R. P. Dickson), UL1TR000433 (R. P. Dickson), U01HL098961 (G. B. Huffnagle), and R01HL114447 (G. B. Huffnagle). Support was provided by the Host Microbiome Initiative of the University of Michigan (R. P. Dickson), the Michigan Institute for Clinical & Health Research (R. P. Dickson), and the University of Michigan Center for Integrative Research in Critical Care (R. P. Dickson). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author (s).
AUTHOR CONTRIBUTIONS
R.P.D. conception and design of research; R.P.D. prepared figures; R.P.D. drafted manuscript; R.P.D., J.R.E.-D., and G.B.H. edited and revised manuscript; R.P.D., J.R.E.-D., and G.B.H. approved final version of manuscript.
Footnotes
Another intellectual legacy of the nineteenth century that studies homeostasis is the field of economics, though economists prefer the terms static and dynamic equilibrium. But that is a topic for another essay.
This spatial variation in microbial growth conditions of advanced lung disease will be of no surprise to clinicians familiar with the spatial heterogeneity of CT scan findings within the lungs of patients across disease states (e.g., apical emphysema, the bibasilar parenchymal changes of idiopathic pulmonary fibrosis, the patchwork consolidation of ARDS).
The study of how bacteria maintain a stability of function (e.g., metabolism) despite perturbations to their external environment is its own discipline: microbial physiology. Bacteria, in turn, harbor their own ecosystems of phages, the most abundant biological entity on earth (52). There is thus a host-microbiome interface within our host-microbiome interface.
In the language of ecology, the population regulation of the lung microbiome is density dependent, constrained by the small carrying capacity afforded by the alveoli.
This observation itself is remarkable: the “sterile” exposure of LPS instillation caused a fivefold surge in the burden of alveolar bacteria. How many of LPS's presumed direct effects on in vivo lung biology are in fact secondary to dysregulation of the respiratory ecosystem?
Intriguingly, this growth enhancement is environment dependent: P. aeruginosa responds to catecholamines when its culture medium is nutrient poor, but not in conditions of nutrient abundance (20, 23). This is thus an instance of bacteria altering their own behavior in response to environmental perturbations: microbial physiology.
It is suspicious that the Proteobacteria phylum (home to numerous familiar gram-negative respiratory pathogens) is disproportionately represented both among catecholamine-responsive bacterial species as well as among the bacteria enriched in the microbiota of inflamed lungs (15).
REFERENCES
- 1.Amberson JB. A clinical consideration of abscesses and cavities of the lung. Bull Johns Hopkins Hosp 94: 227–237, 1954. [PubMed] [Google Scholar]
- 2.Andersson SGE, Zomorodipour A, Andersson JO, Sicheritz-Ponten T, Alsmark UCM, Podowski RM, Naslund AK, Eriksson AS, Winkler HH, Kurland CG. The genome sequence of Rickettsia prowazekii and the origin of mitochondria. Nature 396: 133–140, 1998. [DOI] [PubMed] [Google Scholar]
- 3.Bassis CM, Erb-Downward JR, Dickson RP, Freeman CM, Schmidt TM, Young VB, Beck JM, Curtis JL, Huffnagle GB. Analysis of the upper respiratory tract microbiotas as the source of the lung and gastric microbiotas in healthy individuals. mBio 6: 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bernard C. Leçons sur les pheínomeÌnes de la vie communs aux animaux et veígeítaux. Paris: Baillere, 1878. [Google Scholar]
- 5.Charlson ES, Bittinger K, Haas AR, Fitzgerald AS, Frank I, Yadav A, Bushman FD, Collman RG. Topographical continuity of bacterial populations in the healthy human respiratory tract. Am J Respir Crit Care Med 184: 957–963, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crespi BJ. Vicious circles: positive feedback in major evolutionary and ecological transitions. Trends Ecol Evol 19: 627–633, 2004. [DOI] [PubMed] [Google Scholar]
- 7.David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE, Ling AV, Devlin AS, Varma Y, Fischbach MA, Biddinger SB, Dutton RJ, Turnbaugh PJ. Diet rapidly and reproducibly alters the human gut microbiome. Nature 505: 559–563, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dickson RP, Erb-Downward JR, Freeman CM, McCloskey L, Beck JM, Huffnagle GB, Curtis JL. Spatial variation in the healthy human lung microbiome and the adapted island model of lung biogeography. Ann Am Thorac Soc 12: 821–830, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dickson RP, Erb-Downward JR, Freeman CM, Walker N, Scales BS, Beck JM, Martinez FJ, Curtis JL, Lama VN, Huffnagle GB. Changes in the lung microbiome following lung transplantation include the emergence of two distinct Pseudomonas species with distinct clinical associations. PLoS One 9: e97214, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dickson RP, Erb-Downward JR, Huffnagle GB. The role of the bacterial microbiome in lung disease. Expert Rev Respir Med 7: 245–257, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dickson RP, Erb-Downward JR, Huffnagle GB. Towards an ecology of the lung: new conceptual models of pulmonary microbiology and pneumonia pathogenesis. Lancet Respir Med 2: 238–246, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dickson RP, Erb-Downward JR, Prescott HC, Martinez FJ, Curtis JL, Lama VN, Huffnagle GB. Analysis of culture-dependent versus culture-independent techniques for identification of bacteria in clinically obtained bronchoalveolar lavage fluid. J Clin Microbiol 52: 3605–3613, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dickson RP, Erb-Downward JR, Prescott HC, Martinez FJ, Curtis JL, Lama VN, Huffnagle GB. Intraalveolar catecholamines and the human lung microbiome. Am J Respir Crit Care Med 192: 257–259, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dickson RP, Huffnagle GB. The lung microbiome: new principles for respiratory bacteriology in health and disease. PLoS Pathog 11: e1004923, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dickson RP, Martinez FJ, Huffnagle GB. The role of the microbiome in exacerbations of chronic lung diseases. Lancet 384: 691–702, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dixon KR, Cornwell GW. A mathematical model for predator and prey populations. Res Popul Ecol 12: 127–136, 1970. [Google Scholar]
- 17.Edgell DR, Doolittle WF. Archaea and the origin (s) of DNA replication proteins. Cell 89: 995–998, 1997. [DOI] [PubMed] [Google Scholar]
- 18.Erb-Downward JR, Thompson DL, Han MK, Freeman CM, McCloskey L, Schmidt LA, Young VB, Toews GB, Curtis JL, Sundaram B, Martinez FJ, Huffnagle GB. Analysis of the lung microbiome in the “healthy” smoker and in COPD. PLoS One 6: e16384, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Flierl MA, Rittirsch D, Nadeau BA, Chen AJ, Sarma JV, Zetoune FS, McGuire SR, List RP, Day DE, Hoesel LM, Gao H, Van Rooijen N, Huber-Lang MS, Neubig RR, Ward PA. Phagocyte-derived catecholamines enhance acute inflammatory injury. Nature 449: 721–725, 2007. [DOI] [PubMed] [Google Scholar]
- 20.Freestone P. Communication between bacteria and their hosts. Scientifica (Cairo) 2013: 15, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Freestone PP, Haigh RD, Williams PH, Lyte M. Stimulation of bacterial growth by heat-stable, norepinephrine-induced autoinducers. FEMS Microbiol Lett 172: 53–60, 1999. [DOI] [PubMed] [Google Scholar]
- 22.Freestone PP, Hirst RA, Sandrini SM, Sharaff F, Fry H, Hyman S, O'Callaghan C. Pseudomonas aeruginosa-catecholamine inotrope interactions: a contributory factor in the development of ventilator-associated pneumonia? Chest 142: 1200–1210, 2012. [DOI] [PubMed] [Google Scholar]
- 23.Freestone PP, Sandrini SM, Haigh RD, Lyte M. Microbial endocrinology: how stress influences susceptibility to infection. Trends Microbiol 16: 55–64, 2008. [DOI] [PubMed] [Google Scholar]
- 24.Gleeson K, Eggli DF, Maxwell SL. Quantitative aspiration during sleep in normal subjects. Chest 111: 1266–1272, 1997. [DOI] [PubMed] [Google Scholar]
- 25.Goddard AF, Staudinger BJ, Dowd SE, Joshi-Datar A, Wolcott RD, Aitken ML, Fligner CL, Singh PK. Direct sampling of cystic fibrosis lungs indicates that DNA-based analyses of upper-airway specimens can misrepresent lung microbiota. Proc Natl Acad Sci USA 109: 13769–13774, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Günther A, Siebert C, Schmidt R, Ziegler S, Grimminger F, Yabut M, Temmesfeld B, Walmrath D, Morr H, Seeger W. Surfactant alterations in severe pneumonia, acute respiratory distress syndrome, and cardiogenic lung edema. Am J Respir Crit Care Med 153: 176–184, 1996. [DOI] [PubMed] [Google Scholar]
- 27.Hilty M, Burke C, Pedro H, Cardenas P, Bush A, Bossley C, Davies J, Ervine A, Poulter L, Pachter L, Moffatt MF, Cookson WO. Disordered microbial communities in asthmatic airways. PLoS One 5: e8578, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huxley EJ, Viroslav J, Gray WR, Pierce AK. Pharyngeal aspiration in normal adults and patients with depressed consciousness. Am J Med 64: 564–568, 1978. [DOI] [PubMed] [Google Scholar]
- 29.Ingenito EP, Solway J, McFadden ER Jr, Pichurko B, Bowman HF, Michaels D, Drazen JM. Indirect assessment of mucosal surface temperatures in the airways: theory and tests. J Appl Physiol 63: 2075–2083, 1987. [DOI] [PubMed] [Google Scholar]
- 30.Kanangat S, Meduri GU, Tolley EA, Patterson DR, Meduri CU, Pak C, Griffin JP, Bronze MS, Schaberg DR. Effects of cytokines and endotoxin on the intracellular growth of bacteria. Infect Immun 67: 2834–2840, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kaza SK, McClean S, Callaghan M. IL-8 released from human lung epithelial cells induced by cystic fibrosis pathogens Burkholderia cepacia complex affects the growth and intracellular survival of bacteria. Int J Med Microbiol 301: 26–33, 2011. [DOI] [PubMed] [Google Scholar]
- 32.Lighthart B. Mini-review of the concentration variations found in the alfresco atmospheric bacterial populations. Aerobiologia 16: 7–16, 2000. [Google Scholar]
- 33.Lozupone C, Cota-Gomez A, Palmer BE, Linderman DJ, Charlson ES, Sodergren E, Mitreva M, Abubucker S, Martin J, Yao G, Campbell TB, Flores SC, Ackerman G, Stombaugh J, Ursell L, Beck JM, Curtis JL, Young VB, Lynch SV, Huang L, Weinstock GM, Knox KS, Twigg H, Morris A, Ghedin E, Bushman FD, Collman RG, Knight R, Fontenot AP. Widespread colonization of the lung by Tropheryma whipplei in HIV infection. Am J Respir Crit Care Med 187: 1110–1117, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lyte M, Ernst S. Catecholamine induced growth of gram negative bacteria. Life Sci 50: 203–212, 1992. [DOI] [PubMed] [Google Scholar]
- 35.Lyte M, Freestone PP. Microbial Endocrinology: Interkingdom Signaling in Infectious Disease and Health. New York: Springer Science+Business Media, 2010. [Google Scholar]
- 36.MacArthur RH, Wilson EO. An equilibrium theory of insular zoogeography. Evolution 373–387, 1963. [Google Scholar]
- 37.Marks LR, Davidson BA, Knight PR, Hakansson AP. Interkingdom signaling induces Streptococcus pneumoniae biofilm dispersion and transition from asymptomatic colonization to disease. mBio 4: 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Monton C, Torres A, El-Ebiary M, Filella X, Xaubet A, de la Bellacasa JP. Cytokine expression in severe pneumonia: a bronchoalveolar lavage study. Crit Care Med 27: 1745–1753, 1999. [DOI] [PubMed] [Google Scholar]
- 39.Morris A, Beck JM, Schloss PD, Campbell TB, Crothers K, Curtis JL, Flores SC, Fontenot AP, Ghedin E, Huang L, Jablonski K, Kleerup E, Lynch SV, Sodergren E, Twigg H, Young VB, Bassis CM, Venkataraman A, Schmidt TM, Weinstock GM. Comparison of the respiratory microbiome in healthy nonsmokers and smokers. Am J Respir Crit Care Med 187: 1067–1075, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.O'Donnell PM, Aviles H, Lyte M, Sonnenfeld G. Enhancement of in vitro growth of pathogenic bacteria by norepinephrine: importance of inoculum density and role of transferrin. Appl Environ Microbiol 72: 5097–5099, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pickett STA, White PS. The Ecology of Natural Disturbance and Patch Dynamics. San Diego: Academic, 1985. [Google Scholar]
- 42.Plotkin BJ, Roose RJ, Erikson Q, Viselli SM. Effect of androgens and glucocorticoids on microbial growth and antimicrobial susceptibility. Curr Microbiol 47: 514–520, 2003. [DOI] [PubMed] [Google Scholar]
- 43.Porat R, Clark BD, Wolff SM, Dinarello CA. Enhancement of growth of virulent strains of Escherichia coli by interleukin-1. Science 254: 430–432, 1991. [DOI] [PubMed] [Google Scholar]
- 44.Poroyko V, Meng F, Meliton A, Afonyushkin T, Ulanov A, Semenyuk E, Latif O, Tesic V, Birukova AA, Birukov KG. Alterations of lung microbiota in a mouse model of LPS-induced lung injury. Am J Physiol Lung Cell Mol Physiol 309: L76–L83, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Quinn LH, Meyer OO. The relationship of sinusitis and bronchiectasis. Arch Otolaryngol 10: 152–165, 1929. [Google Scholar]
- 46.Redfield AC. On the proportions of organic derivatives in sea water and their relation to the composition of plankton. In: James Johnstone Memorial Volume. Liverpool, UK: University Press of Liverpool, 1934. [Google Scholar]
- 47.Sandrini S, Aldriwesh M, Alruways M, Freestone P. Microbial endocrinology: host-bacteria communication within the gut microbiome. J Endocrinol 225: R21–R34, 2015. [DOI] [PubMed] [Google Scholar]
- 48.Segal LN, Alekseyenko AV, Clemente JC, Kulkarni R, Wu B, Chen H, Berger KI, Goldring RM, Rom WN, Blaser MJ, Weiden MD. Enrichment of lung microbiome with supraglottic taxa is associated with increased pulmonary inflammation. Microbiome 1: 19, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shapiro JA. Evolution: A View From the 21st Century. Upper Saddle River, NJ: FT Press Science, 2011. [Google Scholar]
- 50.Sibley CD, Rabin H, Surette MG. Cystic fibrosis: a polymicrobial infectious disease. Future Microbiol 1: 53–61, 2006. [DOI] [PubMed] [Google Scholar]
- 51.Sunda WG, Graneli E, Gobler CJ. Positive feedback and the development and persistence of ecosystem disruptive algal blooms. J Phycol 42: 963–974, 2006. [Google Scholar]
- 52.Thurber RV. Current insights into phage biodiversity and biogeography. Curr Opin Microbiol 12: 582–587, 2009. [DOI] [PubMed] [Google Scholar]
- 53.Venkataraman A, Bassis CM, Beck JM, Young VB, Curtis JL, Huffnagle GB, Schmidt TM. Application of a neutral community model to assess structuring of the human lung microbiome. mBio 6: 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Volterra V. Fluctuations in the abundance of a species considered mathematically. Nature 118: 558–560, 1926. [Google Scholar]
- 55.West JB. Regional differences in the lung. Chest 74: 426–437, 1978. [DOI] [PubMed] [Google Scholar]
- 56.Willner D, Haynes MR, Furlan M, Schmieder R, Lim YW, Rainey PB, Rohwer F, Conrad D. Spatial distribution of microbial communities in the cystic fibrosis lung. ISME J 6: 471–474, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zaborina O, Lepine F, Xiao G, Valuckaite V, Chen Y, Li T, Ciancio M, Zaborin A, Petroff E, Turner JR, Rahme LG, Chang E, Alverdy JC. Dynorphin activates quorum sensing quinolone signaling in Pseudomonas aeruginosa. PLoS Pathog 3: e35, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang X, Essmann M, Burt ET, Larsen B. Estrogen effects on Candida albicans: a potential virulence-regulating mechanism. J Infect Dis 181: 1441–1446, 2000. [DOI] [PubMed] [Google Scholar]