Abstract
Acute exposure to ozone (O3), an air pollutant, causes pulmonary inflammation, airway epithelial desquamation, and airway hyperresponsiveness (AHR). Pro-inflammatory cytokines—including IL-6 and ligands of chemokine (C-X-C motif) receptor 2 [keratinocyte chemoattractant (KC) and macrophage inflammatory protein (MIP)-2], TNF receptor 1 and 2 (TNF), and type I IL-1 receptor (IL-1α and IL-1β)—promote these sequelae. Human resistin, a pleiotropic hormone and cytokine, induces expression of IL-1α, IL-1β, IL-6, IL-8 (the human ortholog of murine KC and MIP-2), and TNF. Functional differences exist between human and murine resistin; yet given the aforementioned observations, we hypothesized that murine resistin promotes O3-induced lung pathology by inducing expression of the same inflammatory cytokines as human resistin. Consequently, we examined indexes of O3-induced lung pathology in wild-type and resistin-deficient mice following acute exposure to either filtered room air or O3. In wild-type mice, O3 increased bronchoalveolar lavage fluid (BALF) resistin. Furthermore, O3 increased lung tissue or BALF IL-1α, IL-6, KC, TNF, macrophages, neutrophils, and epithelial cells in wild-type and resistin-deficient mice. With the exception of KC, which was significantly greater in resistin-deficient compared with wild-type mice, no genotype-related differences in the other indexes existed following O3 exposure. O3 caused AHR to acetyl-β-methylcholine chloride (methacholine) in wild-type and resistin-deficient mice. However, genotype-related differences in airway responsiveness to methacholine were nonexistent subsequent to O3 exposure. Taken together, these data demonstrate that murine resistin is increased in the lungs of wild-type mice following acute O3 exposure but does not promote O3-induced lung pathology.
Keywords: airway responsiveness, inflammation, keratinocyte chemoattractant, methacholine, TNF
ozone (O3), a highly reactive oxidant gas, is designated as one of six criteria pollutants by the United States Environmental Protection Agency (71). In both humans and rodents, O3 is readily inhaled into the lungs where it can elicit pulmonary inflammation, airway epithelial desquamation, pulmonary vascular hyperpermeability, and airway hyperresponsiveness (AHR) (4, 19, 26, 34, 44, 48, 56, 62, 66). AHR to nonspecific bronchoconstrictors is a characteristic feature of asthma (47), and exposure to O3 causes AHR in normal and asthmatic subjects (19, 27, 38, 56). The prevalence of asthma in the U.S. is increasing, and nearly 41% percent of the U.S. population lives in areas with unhealthy levels of O3 in the ambient air such that a significant number of people with asthma are susceptible to the deleterious effects of O3 on airway responsiveness (2, 78). Thus, to ameliorate or to even prevent O3-induced AHR via the development of new therapeutic modalities, it is essential to understand the molecular mechanisms underlying O3-induced lung dysfunction.
A number of investigators, including ourselves, have demonstrated a role for pro-inflammatory cytokines [IL-1α, IL-1β, keratinocyte chemoattractant (KC), macrophage inflammatory protein (MIP)-2, osteopontin (OPN), and TNF] in the development of AHR following acute exposure to O3 (4, 14, 31, 48, 63). Specifically, antagonism of type I IL-1 receptor (IL-1R1) or genetic deficiency of OPN, chemokine (C-X-C motif) receptor 2 (CXCR2), or TNF receptor (TNFR) 1 (TNFR1) and 2 (TNFR2) significantly reduces or even abolishes the development of O3-induced AHR (4, 14, 31, 48, 63). IL-1RI is the only known signaling receptor for IL-1α and IL-1β, CXCR2 is a receptor for KC and MIP-2, and TNFR1 and TNFR2 are receptors for TNF (37, 53, 65). In addition, CXCR2, IL-1RI, and OPN promote neutrophil migration to the air spaces and/or airway epithelial desquamation following acute exposure to O3 (4, 31, 48). Although these aforementioned cytokines have been implicated in various aspects of O3-induced lung pathology, factors regulating the expression of these cytokines following acute exposure to O3 remain poorly understood.
Resistin, resistin-like molecule (RELM)-α, RELM-β, and RELM-γ are cysteine-rich pleiotropic hormones and cytokines that are encoded by a recently discovered gene family (46, 49, 68). These hormones and cytokines are differentially expressed by humans and mice such that humans express resistin and RELM-β, whereas mice express resistin, RELM-α, RELM-β, and RELM-γ (46, 49). Despite being expressed in both species, resistin is exclusively expressed by mononuclear cells in humans and by adipocytes in mice (3, 67). Consequently, because different cell types express resistin in humans and mice, disagreements have arisen as to whether the biological role of resistin is the same in both species. For example, there is clear evidence that resistin promotes insulin resistance in mice, yet its contribution to insulin resistance in humans is controversial (3, 39). Nevertheless, in both humans and mice, expression of resistin is increased in injured and inflamed tissues (9, 43, 52). Furthermore, human resistin contributes to inflammatory processes by its ability to induce the expression of IL-1α, IL-1β, IL-6, IL-8 (the human ortholog of mouse KC and MIP-2), and TNF (9, 10, 43, 64, 69). However, the contribution of murine resistin to inflammatory processes has not been well-characterized. Nevertheless, there is evidence that murine resistin promotes expression of TNF in adipocytes and exacerbates neutrophil migration to the liver in response to lipopolysaccharide (LPS)-induced hepatic injury (7, 35). Given that human and murine resistin are increased in injured and inflamed tissues and that human resistin can increase expression of cytokines that promote O3-induced lung pathology, we hypothesized that murine resistin expression is increased in the lung following acute exposure to O3 and induces expression of cytokines that mediate O3-induced lung pathology.
To test our hypotheses, wild-type C57BL/6 mice and mice genetically deficient in resistin (resistin-deficient mice) were exposed to either filtered room air (air) or O3 [2 parts/million (ppm)] for 3 h. Twenty-four hours following the cessation of exposure, expression of resistin and pro-inflammatory cytokines were measured in either lung tissue by RT-quantitative real-time PCR (qPCR) or in bronchoalveolar lavage fluid (BALF) via immunoassays. In addition, airway epithelial desquamation was assessed by histological analysis of lung tissue and enumeration of BALF epithelial cells, and airway responsiveness to aerosolized acetyl-β-methylcholine chloride (methacholine) was measured using the forced oscillation technique. Our data demonstrate that expression of resistin is increased in the lungs of wild-type mice following acute exposure to O3 but does not contribute to O3-induced lung pathology. Furthermore, to the best of our knowledge, this is the first study to demonstrate the presence of murine resistin in the airway epithelial lining fluid of mice in the absence of any inciting stimulus and following acute exposure to O3.
MATERIALS AND METHODS
Animals.
Mice with a homozygous null mutation in the gene encoding resistin (resistin-deficient mice) in a C57BL/6J genetic background were generated in a series of phases. First, mice with a heterozygous null mutation in the gene encoding resistin in a 129/SvEv−C57BL/6 genetic background were generated by replacing the coding exons of one allele of the resistin gene with the coding exons of the lacZ reporter gene that was modified to include a mouse nuclear localization signal (3). These heterozygous mice were then backcrossed into a C57BL/6J genetic background for seven generations (55). Next, male and female mice in a C57BL/6J genetic background with a heterozygous null mutation in the gene encoding resistin were mated to produce the first filial (F1) generation of resistin-deficient mice in a C57BL/6J genetic background. Because resistin-deficient mice exhibit no visible phenotypic abnormalities and are fertile (3), the descendants of the F1 generation of resistin-deficient mice in a C57BL/6J genetic background were mated to produce the resistin-deficient mice that were used in this study.
The subsequently described experiments that were performed in this study used both male and female resistin-deficient mice. Age- and gender-matched C57BL/6J mice were purchased from The Jackson Laboratory at 4−8 wk of age (Bar Harbor, ME) and used as wild-type controls. All mice used in this study were bred, generated, and/or housed within the same room within a larger multi-species, modified barrier animal care facility at The University of Texas Medical School at Houston (Houston, TX). Each room within the animal care facility was maintained at a temperature of 21.7°C and a relative humidity between 40% and 60%. Furthermore, all mice were housed in individually ventilated, microisolator cages (Tecniplast S.p.a.; Buguggiate, Varese, Italy), containing no more than five animals per cage, where they were given irradiated food (PicoLab Rodent Diet 20; LabDiet, Brentwood, MO) and sediment-filtered, autoclaved municipal water ad libitum, exposed to a 12-h:12-h light/dark cycle, and acclimated to their new environment for at least 20 days before entering the experimental protocol at 8−21 wk of age. The care and use of all animals in this study adhered to the guidelines of the National Institutes of Health (Bethesda, MD), and each of the experimental protocols used in this study was approved by the Animal Welfare Committee of The University of Texas Health Science Center at Houston (Houston, TX).
Protocol.
Three separate cohorts of wild-type and resistin-deficient were used to perform the experiments in this study. In the first cohort, each mouse was euthanized 24 h after cessation of a 3-h exposure to either air or O3 (2 ppm). Subsequently, blood, BALF, and the left lung lobe were collected from each animal. In the second cohort, mice were euthanized 24 h following cessation of exposure to air or O3 (2 ppm). Following euthanasia, blood was collected from each mouse, and the lungs of each animal were fixed in situ and then removed from the animal en bloc. In the third cohort, the mice were anesthetized 24 h after cessation of a 3-h exposure to air or O3 (2 ppm), and airway responsiveness to aerosolized methacholine was measured.
O3 exposure.
Mice were exposed to O3 in the following manner. Conscious mice were individually placed into one of eight cells of a custom-designed, stainless steel wire mesh cage (Marlin Steel Wire Products, Baltimore, MD). The dimensions of each cell in the wire mesh cage were 10.5 cm (length) × 16.5 cm (width) × 16 cm (height). Once the lid of the wire mesh cage was closed and fastened, the cage was placed inside a 75.5 l powder-coated aluminum exposure chamber with a Plexiglas door (Teague Enterprises, Woodland, CA). All mice were exposed to O3 within this chamber. O3 was generated by passing dry medical air through a Sander Certizon 25 ozoniser (Erwin Sander Elektroapparatebau, Uetze-Eltze, Germany) that was subsequently mixed with activated charcoal-filtered room air in the chamber. Air containing O3 was delivered to the chamber at a rate of 2.95 l/min via a stainless steel thermal O3 mass flow controller (Model GFC; Aalborg, Orangeburg, NY). At a rate of 1.0 l/min, the atmosphere within the chamber was drawn continuously through a sampling port and delivered to an O3 analyzer (Model IN-2000-L2-LC; IN USA, Norwood, MA), which determined the O3 concentration within the chamber atmosphere via ultraviolet photometry. For exposure to air, the experimental protocol was identical to the O3 exposure except that the ozoniser was not powered on in this instance. For all experiments in this study, the temperature and relative humidity inside of the exposure chamber were 21−24°C and 28−51%, respectively, and air within the chamber was renewed at a rate of 9−14 changes per hour. Finally, although the concentration of O3 (2 ppm) used in these studies exceeds the current U.S. National Ambient Air Quality Standards for O3 of 0.075 ppm (71), we previously justified the use of this O3 concentration in our experiments (4).
Blood collection and isolation of serum.
Twenty-four hours following cessation of exposure to air or O3, each mouse within the first cohort was euthanized with an intraperitoneal injection of pentobarbital sodium (200 mg/kg; Vortech Pharmaceuticals, Dearborn, MI). Once each animal was deeply anesthetized, which was confirmed by the loss of pedal withdrawal and ocular reflexes, the mouse was placed in the supine position, and a median thoracotomy was performed to expose the heart and lungs in situ. Afterward, the right ventricle of the heart was punctured with a 25-gauge needle attached to a 1-ml syringe (Becton, Dickinson and Company, Franklin Lakes, NJ), and blood was slowly withdrawn to prevent the heart from collapsing, and thus maximize the amount of blood collected. Blood retrieved from each animal was immediately placed into a Microtainer tube containing serum separator additive (Becton, Dickinson and Company) and was allowed to clot at room temperature for at least 30 min. Afterward, the blood was fractionated by centrifugation (15,000 g for 2 min) at 4°C to isolate serum from the nonfluid elements of the blood. The serum was then retrieved from the Microtainer tube and stored at −20°C until needed.
BAL.
After blood was obtained from the heart of each animal in the first cohort, a tracheotomy was performed on each mouse to perform a BAL. To that end, the trachea and the larynx were exposed in situ by the surgical excision of overlying fur, skin, and fascia. Next, a small incision on the ventral surface of the trachea directly inferior to the larynx was made using microscissors to facilitate the insertion of a 20-gauge fluorinated ethylene propylene polymer catheter (Becton, Dickinson and Company) into the incision, and thus the lumen of the trachea. The catheter was then attached to a 1-ml syringe containing 1 ml of lavage buffer (PBS containing 0.6 mM of ethylenediaminetetraacetic acid). Once the distal end of the catheter was securely in place within the lumen of the trachea, the lungs were gently lavaged four times. The first lavage was with 1.0 ml of lavage buffer, and the second lavage was with the resulting lavagate instilled into the lungs again, subsequently retrieved a second time, and then stored on ice. This process was repeated again with a separate aliquot (1.0 ml) of lavage buffer, and the resulting lavagate from the second retrieval was pooled with the first lavagate, which was already stored on ice. The pooled lavagate was centrifuged at 600 g for 10 min at 4°C. Following centrifugation, the BALF supernatant was carefully collected and stored at −80°C until needed. The remaining cell pellet was resuspended in 1 ml of Hanks′ balanced salt solution (HyClone Laboratories, Logan, UT), and the total number of cells within this cell suspension was enumerated using a hemacytometer (Hausser Scientific, Horsham, PA). To perform a differential cell analysis on the cells within the BALF, an aliquot of the cell suspension containing 25,000 cells was spun at 800 rpm for 10 min at room temperature using a CytoZEN cytology centrifuge (Hettich Instruments, Beverly, MA). The slides were then air-dried and stained with the Hema 3 stain set (Fisher Diagnostics, Middletown, VA). The cells on each slide were examined at total magnification of 400× using bright-field microscopy. Cells were classified as eosinophils, lymphocytes, macrophages, neutrophils, or respiratory epithelial cells according to standard morphological features (20, 24). In particular, respiratory epithelial cells were identified by the presence of cilia. Cells that could not be clearly identified as one of the aforementioned cell types were categorized as indeterminate cells. At least 300 cells per slide were counted during the differential cell analysis.
Immunoassays and protein quantification.
According to the manufacturer's instructions, a number of commercially available immunoassays were used to quantify the concentration of BALF and/or serum hormones and cytokines. Specifically, Quantikine enzyme-linked immunosorbent assays (R&D Systems, Minneapolis, MN) were used to determine the concentration of BALF and/or serum chemerin, OPN, and resistin; a Bio-Plex multiplex immunoassay (Bio-Rad Laboratories, Hercules, CA) was used to quantify the concentration of BALF granulocyte-colony stimulating factor (G-CSF), IL-17A, and TNF; and a MILLIPLEX MAP multiplex immunoassay (EMD Millipore, Billerica, MA) was used to determine the concentration of BALF interferon gamma (IFN-γ), IL-4, IL-5, IL-6, IL-12 p70, and KC. As per the manufacturer's instructions, the Bio-Rad Protein Assay (Bio-Rad Laboratories) that is based on the method of Bradford (11) was used to spectroscopically quantify the total concentration of BALF protein. Solutions of BSA (Sigma-Aldrich, St. Louis, MO), ranging between 1.25 and 20 μg/ml, were used to construct a standard curve for the determination of protein in the experimental samples.
Lung histology and immunohistochemistry.
Twenty-four hours following cessation of exposure to either air or O3, each mouse within the second cohort was euthanized with pentobarbital sodium (200 mg/kg ip). Following euthanasia, as described in the above text, blood was collected from the heart of each animal and serum isolated from the blood and stored at −20°C. Subsequently, paraffin-embedded lung sections were prepared from formalin-fixed lungs of these mice as previously described (15). Afterward, these sections were either stained with hematoxylin and eosin to assess peribronchiolar inflammation and bronchiolar epithelium injury or subjected to immunohistochemical staining to detect the presence of resistin in lung tissue.
Inflated portions of hematoxylin- and eosin-stained lung sections were blindly examined under bright-field microscopy by a veterinary pathologist to determine peribronchiolar inflammation and bronchiolar epithelial injury scores. The peribronchiolar inflammation score is the product of the severity and prevalence of bronchiolar inflammation. Severity was assigned a numerical value based on the thickness of the inflammatory cell infiltrates surrounding the bronchioles (0 = no cells; 1 = 1−3 cells thick; 2 = 4−6 cells thick; 3 = 7−10 cells thick; 4 = greater than 10 cells thick). Prevalence was assigned a numerical value according to the percentage of airways in each section encompassed by inflammatory cells (0 = no bronchioles; 1 = <25% of the bronchioles; 2 = 25−50% of the bronchioles; 3 = 51−75% of the bronchioles; 4 = >75% of bronchioles in the section). The bronchiolar epithelium injury score was determined by assigning a numerical value based on the extent of segmental epithelial ulcerations and epithelial desquamation (0 = no significant lesions; 1 = <25% of the bronchioles; 2 = 25−50% of the bronchioles; 3 = 51−75% of the bronchioles; 4 = >75% of the bronchioles).
To determine whether resistin was expressed in lung tissue of wild-type mice following exposure to air or O3, formalin-fixed and paraffin-embedded lung sections from wild-type mice were subjected to immunohistochemical analysis. First, sections underwent deparaffinization and rehydration, respectively, by immersing the sections in xylene and ethanol gradients. Once immersion in the final ethanol gradient was complete, the sections were rinsed with water and immediately submerged in sub-boiling sodium citrate buffer (pH = 6.0) for 20 min to stimulate antigen retrieval. Next, the sections were placed in a solution of 3% hydrogen peroxide for 15 min to suppress endogenous peroxidase activity. The sections were then blocked with 1% normal goat serum (Vector Laboratories, Burlingame, CA) followed by avidin and biotin (Vector Laboratories) to prevent nonspecific binding of the primary antibody and high-background staining, respectively. Afterward, the sections were incubated overnight at 4°C with the primary antibody [anti-mouse resistin polyclonal antibody (1 μg/ml; R&D Systems)]. The following day the sections were incubated with a biotinylated secondary antibody [goat immunoglobulin (Ig) G; Vector Laboratories] for 30 min, an avidin DH:biotinylated enzyme complex (Vector Laboratories) for 45 min, and then 3,3′-diaminobenzidine tetrahydrochloride (DAB; Vector Laboratories) until the sections began to appear brown. Commencing with the conclusion of antigen retrieval in sodium citrate buffer and terminating after incubation in DAB, each section was rinsed with PBS before proceeding to the next step of the immunohistochemistry protocol. Following the final wash in PBS after incubation in DAB, the sections were counterstained with Harris hematoxylin (Fisher Scientific, Pittsburgh, PA) for no more than 30 s, rinsed in water, dehydrated, overlaid with a glass coverslip, and then blindly examined via bright-field microscopy by a veterinary pathologist. Finally, to confirm the specificity of the primary antibody, two experiments that served as negative controls were executed in a similar manner. First, a separate lung section from a wild-type mouse was incubated with a polyclonal goat IgG (1 μg/ml; R&D Systems), an isotype control, in place of the primary antibody. Second, a lung section from a resistin-deficient mouse was incubated with the primary antibody (1 μg/ml).
Measurement of respiratory system responsiveness to methacholine.
Twenty-four hours following cessation of exposure to air or O3, each mouse within the third cohort was anesthetized with pentobarbital sodium (50 mg/kg ip; Oak Pharmaceuticals, Lake Forest, IL) and xylazine hydrochloride (7 mg/kg ip; Vedco, St. Joseph, MO). Once the animal was deeply anesthetized, confirmed by loss of pedal withdrawal and ocular reflexes, the mouse was placed in the supine position. Next, a horizontal incision in the middle of the anterior aspect of the neck was made with surgical scissors to expose the hypodermis. The hypodermis was then gently dissected with surgical forceps to expose the trachea and larynx in situ. Microscissors were then used to make a small incision on the ventral tracheal surface directly inferior to the larynx so that an 18-gauge tubing adapter (Becton, Dickinson and Company) could be inserted and subsequently secured in place within the lumen of the trachea. With the chest wall intact, the mice were then attached to a specialized ventilator (flexiVent; SCIREQ Scientific Respiratory Equipment, Montréal, Québec, Canada) via the tubing adaptor and ventilated with a tidal volume of 0.3 ml at a frequency of 150 breaths per minute (2.5 Hz). The flexiVent is equipped with a computer-controlled piston pump that is capable of delivering tidal volumes to the animal in a sinusoidal flow pattern. To prevent atelectasis at the end of expiration, a positive end-expiratory pressure of 3 cm H2O was applied to the lungs by placing the expiratory line under water.
With use of the forced oscillation technique (5, 25, 51, 59), the dynamic resistance and compliance of the entire respiratory system (RRS and CRS, respectively) was derived from the equation of motion of a single-compartment linear model of respiratory system mechanics (73). For the experiments performed in this study, RRS and CRS were determined by perturbing ventilation with 2.5 Hz sinusoidal forcing functions, which were 1.25 s in duration. The flexiVent was used to both ventilate the lungs and deliver sinusoidal forcing functions. Furthermore, by the calibration of the flexiVent with the tubing adaptor in place, any opposition to the flow of gas caused by the tubing adaptor was removed from all measurements (5). Thus the data reported here only represent changes in the mechanical properties of the respiratory system. By tracing the movement of the computer-controlled piston pump during the execution of a sinusoidal forcing function, the flexiVent can accurately measure the volume of gas delivered to the animal (V) and, subsequently, calculate the flow of gas at the airway opening (V̇ao) (51). In addition, the flexiVent measures pressure at the airway opening (Pao) via a pressure transducer. Consequently, RRS, CRS, and P0, a constant that corrects for errors in estimating functional residual capacity (6, 41), can be derived by multiple linear regression when Pao, V̇ao, and V are fit to the equation of motion of a single-compartment linear model of respiratory system mechanics (73): Pao = RRSV̇ao + (1/CRS)V + P0.
Dose-response curves to methacholine (Sigma-Aldrich) were generated as follows. First, ventilation was paused for 6 s, and a pressure of 30 cm H2O was applied to the system to inflate the lungs to capacity to standardize lung volume history. Afterward, ventilation was resumed for at least 6 s, and then a 2.5-Hz sinusoidal forcing function was applied, while ventilation was paused for 1.25 s, to determine RRS and CRS. This entire procedure was repeated five more times to ensure reproducible baseline RRS and CRS values. Next, 100 μl of PBS was aerosolized with an Aeroneb Lab Micropump Nebulizer (Aerogen Limited, Galway, Ireland) and delivered to the lungs of the animal through the inspiratory line of the flexiVent and the tubing adaptor. The aerosol was delivered for 10 s followed by sinusoidal forcing functions, as described above, which perturbed ventilation every eighth breath to determine RRS and CRS. After a total of 15 sinusoidal forcing functions were applied and RRS and CRS measurements were made, the perturbations ceased and ventilation resumed uninterrupted. To re-establish baseline conditions following the administration of PBS, we standardized lung volume history by again inflating the lungs to capacity by applying a pressure of 30 cm H2O to the system. Afterward, we measured RRS and CRS. This entire procedure was repeated at least two more times to ensure that RRS and CRS returned to baseline. Once RRS and CRS returned to baseline, 100 μl of methacholine dissolved in PBS was delivered to the animal in the same manner as described for aerosolized PBS. Methacholine was administered in approximate half-logarithm intervals between 0.1 and 100 mg/ml. After the delivery of each dose of methacholine, 15 sinusoidal forcing functions were applied every eighth breath as described previously, and RRS and CRS determined. Following completion of RRS and CRS measurements after the administration of each dose of methacholine, baseline conditions were re-established as described above, and then the next dose of methacholine was delivered to the animal. For each animal, the mean RRS and CRS values for PBS and each dose of methacholine were determined by averaging the five highest measurements of RRS or the five lowest measurements of CRS in response to PBS or each dose of methacholine. Finally, to establish the goodness of fit of the equation of motion of a single-compartment linear model of respiratory system mechanics to our data, the flexiVent software (Version 5.3) calculates a coefficient of determination. Any measurement with a coefficient of determination less than 0.9 was excluded from the study.
Total RNA extraction, complementary DNA (cDNA) synthesis, and RT-qPCR.
After a BAL was performed on each animal in the first cohort, the left lung lobe was excised from the animal, snap-frozen in liquid nitrogen, and stored at −80°C until needed. To initiate RNA extraction and subsequent RNA isolation from the left lung lobe, the frozen lung was placed in 1−3 ml of TRIzol® Reagent (Life Technologies, Grand Island, NY) and homogenized at full speed using a rotor-stator homogenizer. RNA was then isolated from the resulting homogenate, treated with PureLink DNase (Invitrogen, Carlsbad, CA), and purified using the PureLink RNA Mini Kit (Life Technologies) according to the manufacturer's instructions. The concentration and integrity of RNA within each sample was determined with the Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA). cDNA was synthesized from 1 μg of total RNA using the iScript cDNA Synthesis Kit (Bio-Rad Laboratories, Hercules, CA). qPCR was performed using the LightCycler 96 System (Roche Diagnostics, Indianapolis, IN) and the KAPA SYBR FAST qPCR Kit (Kapa Biosystems, Wilmington, MA) as per the instructions of the manufacturers.
We determined the relative copy number of IL-1α (Il1a), IL-1β (Il1b), and TNF (Tnf) mRNA in the left lung lobe by expressing the mRNA copy number of Il1a, Il1b, and Tnf relative to the mRNA copy number of ribosomal protein S29 (Rps29), a reference gene (17). Intron-spanning primer pairs for each gene and the expected size of each cDNA amplicon are found in Table 1. The primer sequences used in this study for Il1a and Il1b were previously reported by Gabellec et al. (21) while the primer sequences for Rps29 and Tnf were designed with Primer-BLAST and Primer3Plus, respectively (72, 76). With the use of the primer pairs found in Table 1, cDNA from air- or O3-exposed resistin-deficient mice was amplified by PCR, purified, cloned using a TOPO TA Cloning Kit (Invitrogen), and sequenced (Macrogen, Rockville, MD). The resulting plasmid DNA that contained the cDNA sequence of our aforementioned genes was subjected to a maxipreparation (Invitrogen), linearized with HindIII (New England Biolabs, Ipswich, MA), and then used to construct standard curves for RT-qPCR that contained copy numbers ranging from 101 to 109. The copy numbers of Il1a, Il1b, Tnf, and Rps29 in our experimental samples were extrapolated from their respective standard curves, and the Il1a, Il1b, and Tnf mRNA relative copy number was determined as described in the above text. There was no effect of genotype or exposure on Rps29 mRNA copy number (data not shown). Finally, for each pair of primers, melting curve analysis yielded a single peak consistent with one PCR product.
Table 1.
Primer sequences used for RT-quantitative real-time PCR
| Gene | Gene Symbol | RefSeq Accession Number | Sense Primer | Antisense Primer | Amplicon Size, bp |
|---|---|---|---|---|---|
| IL-1α | Il1a | NM_010554.4 | 5′-CAAACTGATGAAGCTCGTCA-3′ | 5′-TCTCCTTGAGCGCTCACGAA-3′ | 225 |
| IL-1β | Il1b | NM_008361.3 | 5′-CTGTGTCTTTCCCGTGGACC-3′ | 5′-CAGCTCATATGGGTCCGACA-3′ | 200 |
| Ribosomal Protein S29 | Rps29 | NM_009093.2 | 5′-CGTCTGAAGGCAAGATGGGTC-3′ | 5′-CATTCAAGGTCGCTTAGTCCA-3′ | 198 |
| TNF | Tnf | NM_013693.3 | 5′-ATGGCCTCCCTCTCATCAGT-3′ | 5′-GGGGCTCTGAGGAGTAGACA-3′ | 599 |
Statistical analyses of data.
The effect of exposure (air or O3) and genotype (wild-type or resistin-deficient) on BALF, serum, histology, and lung tissue parameters, body mass, and baseline respiratory system mechanics were assessed by a two-way ANOVA for normally distributed data or by a Kruskal-Wallis one-way ANOVA for nonnormally distributed data. Depending on whether the data were normally or nonnormally distributed, the Fisher's least significant difference test or the Wilcoxon signed-rank test, respectively, was used as a follow-up to determine the significance of differences between the individual groups. Pre- and post-exposure body masses were compared using a Student's t-test for paired samples. Statistical analysis of the repeated measures comprising the methacholine dose-response curves was completed using the area under the curve analysis with respect to increased response compared with the response following PBS administration. Area under the curve analysis was performed using R (Version 2.15.3) (57), whereas Stata 12 was used for all other statistical analyses (StataCorp, College Station, TX). The results are expressed as means ± SE. A P value less than 0.05 was considered significant.
RESULTS
Effect of O3 and resistin deficiency on BALF and serum resistin.
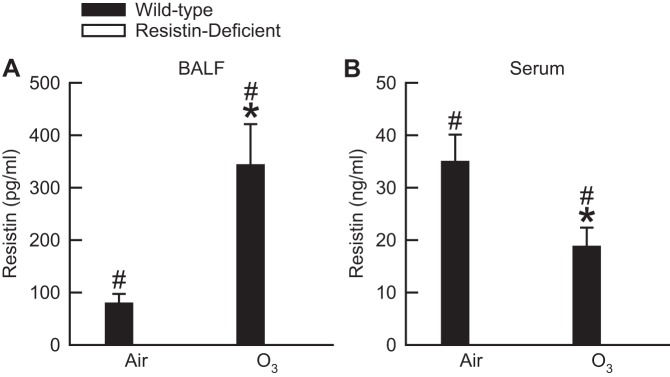
Twenty-four hours following cessation of exposure to air, resistin was detectable in BALF and serum of wild-type mice (Fig. 1). When compared with air-exposed wild-type mice, O3 caused a significant fourfold increase in BALF resistin, whereas it caused a significant twofold reduction in serum resistin. We did attempt to measure resistin in BALF and serum of resistin-deficient mice exposed to air or O3. However, regardless of exposure, resistin was not detectable in either BALF or serum of resistin-deficient mice when using the immunoassay described in materials and methods (Fig. 1).
Fig. 1.
The concentration of resistin in bronchoalveolar lavage fluid (BALF; A) and serum (B) of wild-type C57BL/6 mice and mice genetically deficient in resistin (resistin-deficient mice) 24 h following cessation of a 3-h exposure to either filtered room air (air) or ozone [O3; 2 parts/million (ppm)]. Resistin was not detectable in BALF or serum of resistin-deficient mice exposed to air or O3 when using the immunoassay described in materials and methods. Each value is expressed as means ± SE; n = 7 to 8 mice in each group. *P < 0.05 compared with genotype-matched mice exposed to air; #P < 0.05 compared with resistin-deficient mice with an identical exposure.
Immunolocalization of resistin in the lungs of wild-type mice.
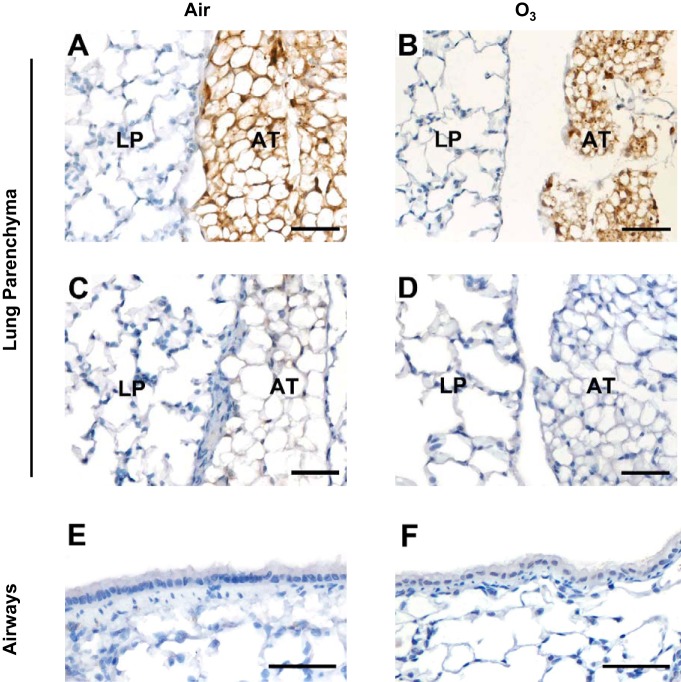
Immunohistochemistry was used to examine resistin expression in lung tissue obtained from wild-type mice exposed to air or O3. Figure 2 contains representative light photomicrographs of formalin-fixed, paraffin embedded lung sections from air- or O3-exposed wild-type or resistin-deficient mice. The lung parenchyma of wild-type mice exposed to either air or O3 showed no immunoreactivity to the anti-mouse resistin antibody (Fig. 2, A and B). However, regardless of exposure, adipose tissue adjacent to the lungs was strongly positive for resistin (Fig. 2, A and B). The anti-mouse resistin antibody failed to elicit any immunoreactivity in either the lung parenchyma or adipose tissue of an air-exposed resistin-deficient mouse (Fig. 2C). Similarly, a polyclonal goat IgG, an isotype control, resulted in no immunoreactivity in either the lung parenchyma or adipose tissue of a wild-type mouse exposed to O3 (Fig. 2D). Finally, no evidence of immunoreactivity to the anti-mouse resistin-antibody was present in the airways (bronchioles) of wild-type mice exposed to air or O3 (Fig. 2, E and F).
Fig. 2.
Representative light photomicrographs demonstrating either the presence or absence of resistin immunolocalization within either the lung parenchyma (LP) and adjacent adipose tissue (AT) (A−D) or airways (bronchioles) (E and F) of wild-type C57BL/6 (A, B, D, E, and F) or resistin-deficient mice (C) 24 h following cessation of a 3-h exposure to either filtered room air (air) or ozone (O3; 2 ppm). Tissue sections in A, B, C, E, and F were incubated with an anti-mouse resistin-antibody while the tissue section in D was incubated with a polyclonal goat immunoglobulin (IgG), an isotype control. The lungs were fixed in situ with 10% phosphate-buffered formalin 24 h following the cessation of exposure to air or O3. In A−F, the images have been magnified with a 40× objective lens while each of the scale bars in A−F represents 50 μm; n = 6 mice in each group.
Effect of O3 and resistin deficiency on pulmonary inflammation.
Previous data demonstrate that human resistin can increase the expression of cytokines (IL-1α, IL-1β, IL-6, IL-8, and TNF) that promote pulmonary inflammation, airway epithelial desquamation, and/or AHR following acute exposure to O3 (9, 10, 14, 28, 43, 48, 63, 64, 69). Thus we examined the impact of resistin deficiency on the expression of these and other cytokines (KC and OPN) in our mice, as well as the migration of macrophages and neutrophils to the air spaces, since all of these factors contribute to the pulmonary toxicity induced by acute O3 exposure (4, 14, 30–32, 48, 50, 63). In addition, we examined the effect of resistin deficiency on expression of cytokines (chemerin, G-CSF, IFN-γ, IL-4, IL-5, IL-12 p70, and IL-17A) that have the potential to modulate pulmonary pathology in response to acute O3 exposure based on their actions in other models of lung disease (23, 58, 60, 61, 79).
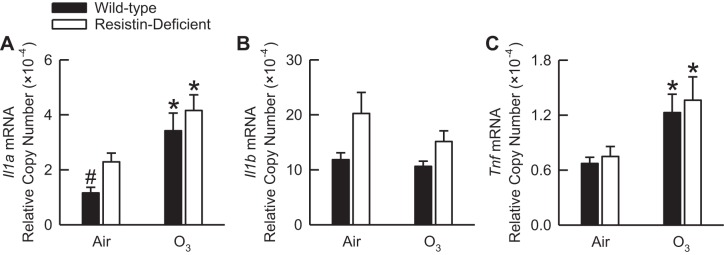
We previously reported that IL-1α and IL-1β protein were not detectable in BALF from wild-type mice that were subjected to the same O3 exposure regimen used in this study (30). Furthermore, regardless of exposure, TNF protein was undetectable in BALF of wild-type or resistin-deficient mice (data not shown). Thus we determined the relative copy number of mRNA of each of these cytokines in the lung tissue of air- or O3-exposed wild-type and resistin-deficient mice by RT-qPCR. Following exposure to air, Il1a, Il1b, and Tnf mRNA relative copy numbers were greater in resistin-deficient as compared with wild-type mice (Fig. 3). However, only the Il1a mRNA relative copy number was significantly greater in air-exposed resistin-deficient as compared with air-exposed wild-type mice (Fig. 3A). O3 significantly increased Il1a and Tnf mRNA in wild-type and resistin-deficient mice. However, there were no genotype-related differences in Il1a and Tnf mRNA following O3 exposure (Fig. 3, A and C). In both genotypes, Il1b mRNA was unaffected by O3 (Fig. 3B).
Fig. 3.
The IL-1α (Il1a; A), IL-1β (Il1b; B), and tumor necrosis factor (Tnf; C) messenger ribonucleic acid (mRNA) relative copy number in the left lung lobe tissue of wild-type C57BL/6 mice and mice genetically deficient in resistin (resistin-deficient mice) 24 h following cessation of a 3-h exposure to either filtered room air (air) or ozone (O3; 2 ppm). The Il1a, Il1b, and Tnf mRNA copy number was expressed relative to the mRNA copy number of ribosomal protein S29. Each value is expressed as means ± SE; n = 7 to 8 mice in each group. *P < 0.05 compared with genotype-matched mice exposed to air; #P < 0.05 compared with resistin-deficient mice with an identical exposure.
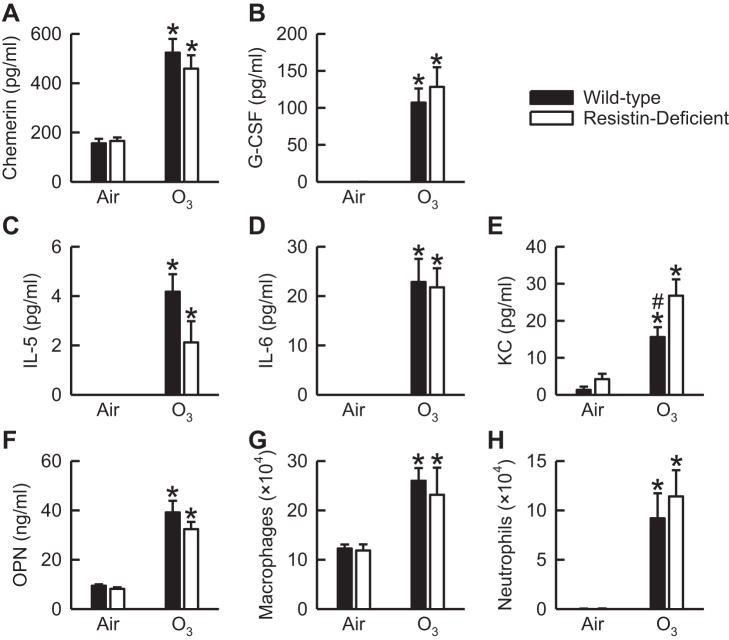
Following exposure to air, chemerin, G-CSF, KC, OPN, macrophages, and neutrophils were present in BALF of wild-type and/or resistin-deficient mice (Fig. 4, A, B, and E–H). However, no genotype-related differences in these cytokines and cells existed following air exposure. We did measure IL-5 and IL-6 in BALF of air-exposed mice of both genotypes, yet IL-5 and IL-6 were not detectable using the immunoassay described in materials and methods (Fig. 4, C and D). All of the aforementioned BALF cytokines and cells were significantly increased by O3. However, with the exception of BALF KC (Fig. 4E), which was significantly greater in O3-exposed resistin-deficient as compared with O3-exposed wild-type mice, no significant genotype-related differences existed in any of these other outcome indicators following O3 exposure. As seen in Fig. 4C, BALF IL-5 was reduced in O3-exposed resistin-deficient as compared with O3-exposed wild-type mice, yet this difference was not statistically significant (P = 0.06). Regardless of exposure, IFN-γ, IL-4, IL-12 p70, and IL-17A were not detectable in BALF of either wild-type or resistin-deficient mice (data not shown). Finally, we found no evidence of peribronchiolar inflammation in the hematoxylin- and eosin-stained lung sections of air- or O3-exposed wild-type and resistin-deficient mice (data not shown).
Fig. 4.
The concentration of chemerin (A), granulocyte-colony stimulating factor (G-CSF; B), IL-5 (C), IL-6 (D), keratinocyte chemoattractant (KC; E), and osteopontin (OPN; F) as well as the number of macrophages (G) and neutrophils (H) in bronchoalveolar lavage fluid (BALF) of wild-type C57BL/6 mice and mice genetically deficient in resistin (resistin-deficient mice) 24 h following cessation of a 3-h exposure to either filtered room air (air) or ozone (O3; 2 ppm). IL-5 and IL-6 were not detectable in BALF of wild-type and resistin-deficient mice exposed to air when using the immunoassay described in materials and methods. Each value is expressed as means ± SE; n = 6−8 mice in each group. *P < 0.05 compared with genotype-matched mice exposed to air; #P < 0.05 compared with resistin-deficient mice with an identical exposure.
Effect of O3 and resistin deficiency on pulmonary injury.
Hyperpermeability of the alveolar-capillary membrane, demonstrated by an increase in BALF protein, and airway epithelial desquamation are two features of pulmonary injury induced by O3 (1, 12). CXCR2, the receptor for KC, and IL-1RI contribute to airway epithelial desquamation while human resistin promotes pulmonary vascular hyperpermeability (29, 31, 48). Given the observations that resistin deficiency enhanced O3-induced increases in BALF KC (Fig. 4E) and that BALF resistin was increased by O3 exposure (Fig. 1A), we examined the impact of resistin deficiency on airway epithelial desquamation and pulmonary vascular hyperpermeability.
We semi-quantitatively assessed the degree of bronchiolar epithelium injury in hematoxylin- and eosin-stained lung sections that were prepared from formalin-fixed and paraffin-embedded lungs obtained from wild-type and resistin-deficient mice exposed to air or O3 (Fig. 5, A−E). In air-exposed mice, regardless of genotype, no significant lesions were present in the lungs (Fig. 5, A, B, and E). In wild-type and resistin-deficient mice, O3 caused significant injury to the bronchiolar epithelium (Fig. 5, C–E), characterized by the presence of detached bronchiolar epithelial cells that were degenerate and necrotic. In addition, portions of the bronchiolar epithelial mucosa in both wild-type and resistin-deficient mice exposed to O3 were eroded and ulcerated. However, there were no differences in injury scores between wild-type and resistin-deficient mice following exposure to O3 (Fig. 5E). Consistent with our semi-quantitative histologic analyses, the number of BALF epithelial cells was significantly increased by O3 in both genotypes, yet there were no genotype-related differences in the number of BALF epithelial cells following air or O3 exposure (Fig. 5F). Similar observations were made for BALF protein (Fig. 5G).
Fig. 5.
A−D: representative light photomicrographs of hematoxylin- and eosin-stained lung sections, the bronchiolar epithelium injury score (E), the number of bronchoalveolar lavage fluid (BALF) epithelial cells (F), and the concentration of BALF protein (G) from wild-type C57BL/6 mice and mice genetically deficient in resistin (resistin-deficient mice) 24 h following cessation of a 3-h exposure to either filtered room air (air) or ozone (O3; 2 ppm). A and B are lung sections from air-exposed wild-type and resistin-deficient mice, respectively. C and D are lung sections from O3-exposed wild-type and resistin-deficient mice, respectively. The black arrows in A and B are directed at bronchiolar epithelial cells that appear normal and are attached to the basement membrane while the blue arrows in C and D are directed at detached bronchiolar epithelial cells that are degenerate and necrotic. The blue arrowheads in C and D are directed at eroded and ulcerated segments of the bronchiolar epithelial mucosa. The lungs were fixed in situ with 10% phosphate-buffered formalin 24 h following cessation of exposure to air or O3. In A−D, the images have been magnified with a 40× objective lens while each of the scale bars in A−D represents 50 μm. In E−G, each value is expressed as means ± SE; n = 6−8 mice for each group. *P < 0.05 compared with genotype-matched mice exposed to air.
Effect of O3 exposure and resistin deficiency on body mass and respiratory system responsiveness to methacholine.
Before exposure to air or O3, there were no differences in the body masses of wild-type and resistin-deficient mice (Table 2). Exposure to air caused a small but significant increase in the body masses of wild-type and resistin-deficient mice while exposure to O3 caused a significant decrease in the body masses of wild-type and resistin-deficient mice (Table 2). Finally, both O3-exposed wild-type and resistin-deficient mice weighed significantly less than genotype-matched air-exposed controls (Table 2).
Table 2.
Body mass and baseline respiratory system mechanics of wild-type and resistin-deficient mice exposed to filtered room air or ozone
| Body Mass, g |
Respiratory System Mechanics |
|||
|---|---|---|---|---|
| Genotype and Exposure | Preexposure | Postexposure | Total respiratory system resistance, cm H2O/ml/s | Total respiratory system compliance, ml/cm H2O |
| Air | ||||
| Wild-type | 26.1 ± 2.6 | 26.5 ± 2.5† | 0.58 ± 0.03 | 0.042 ± 0.002 |
| Resistin-Deficient | 25.8 ± 1.9 | 26.0 ± 1.9† | 0.59 ± 0.04 | 0.038 ± 0.002 |
| O3 | ||||
| Wild-type | 25.2 ± 1.9 | 23.1 ± 1.5†‡ | 0.60 ± 0.02 | 0.039 ± 0.001 |
| Resistin-Deficient | 25.7 ± 1.5 | 23.4 ± 1.2†‡ | 0.72 ± 0.05 | 0.034 ± 0.002 |
Values are means ± SE for 6 mice in each group. The preexposure body mass measurements were made immediately before exposure to filtered room air (air) or ozone [O3; 2 parts per million (ppm)] for 3 h, whereas the postexposure body mass measurements were made in the same animals 24 h following the cessation of a 3-h exposure to air or O3 (2 ppm). As described in materials and methods, the respiratory system mechanics measurements were made after the administration of PBS that occurred 24 h following cessation of a 3-h exposure to either air or O3 (2 ppm).
P < 0.05 compared with preexposure body mass;
P < 0.05 compared with postexposure genotype-matched mice exposed to air.
In Table 2, we also report baseline values of RRS and CRS obtained from wild-type and resistin-deficient mice 24 h following cessation of exposure to air or O3. These baseline values were obtained from the mice after the administration of PBS as described in materials and methods. No genotype-related differences existed in baseline RRS or CRS following exposure to air. As compared with genotype-matched, air-exposed controls, O3 had no effect on baseline RRS or CRS in either wild-type or resistin-deficient mice. Furthermore, following exposure to O3, no genotype-related differences in baseline RRS or CRS existed.
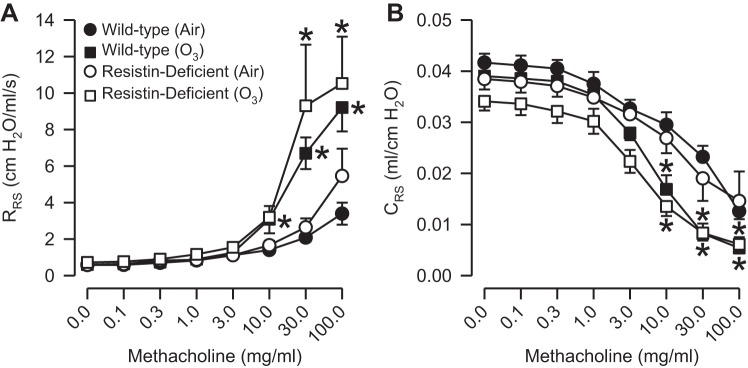
In air-exposed mice, regardless of genotype, methacholine induced significant increases in RRS and significant decreases in CRS when compared with the measurements of RRS and CRS obtained following PBS administration (Fig. 6, A and B). There were no genotype-related differences in responses to methacholine for RRS or CRS following air exposure. Similar to our observation in air-exposed mice, methacholine caused a significant increase in RRS and a significant decrease in CRS in O3-exposed mice that was independent of genotype (Fig. 6, A and B). However, responses to methacholine for RRS and CRS were significantly increased and decreased, respectively, when compared with those of genotype-matched, air-exposed controls. Furthermore, there were no genotype-related differences in response to methacholine for either RRS or CRS following exposure to O3.
Fig. 6.
Responses to aerosolized acetyl-β-methylcholine chloride (methacholine) for total respiratory resistance (RRS; A) and compliance (CRS; B) in wild-type C57BL/6 mice and mice genetically deficient in resistin (resistin-deficient mice) 24 h following cessation of a 3-h exposure to either filtered room air (air) or ozone (O3; 2 ppm). Each value is expressed as means ± SE; n = 6 mice for each group. *P < 0.05 compared with genotyped-matched mice exposed to air.
DISCUSSION
Expression of resistin, a cysteine-rich pleiotropic hormone and cytokine, is increased at sites of tissue injury and inflammation in both humans and mice (9, 43, 52). Human resistin can induce expression of pro-inflammatory cytokines (IL-1α, IL-1β, IL-6, IL-8, and TNF) that contribute to the pulmonary pathology resulting from acute exposure to O3 (9, 10, 14, 28, 43, 48, 63, 64, 69). However, the ability of murine resistin to induce expression of these cytokines or their mouse orthologs is not known. In this study, we demonstrate that murine resistin is present in BALF in the absence of any inciting stimulus and following acute exposure to O3 (Fig. 1A). Nevertheless, murine resistin does not contribute to any aspect of O3-induced pulmonary pathology, including neutrophil migration to the air spaces (Fig. 4H), airway epithelial desquamation (Fig. 5), or AHR (Fig. 6).
Resistin was present in BALF of wild-type mice exposed to air (Fig. 1A), and to the best of our knowledge this is the first study demonstrating the presence of resistin in the epithelial lining fluid of the lung in the absence of any inciting stimulus. However, resistin was not expressed in lung tissue from air-exposed wild-type mice (Fig. 2, A and E). Taken together, these data suggest that BALF resistin is derived from cells of a nonpulmonary origin. Resistin is robustly expressed almost exclusively in the adipose tissue of mice (67), and consistent with this observation we demonstrate that adipose tissue adjacent to the lungs of air-exposed wild-type mice was strongly positive for resistin (Fig. 2A). Furthermore, resistin was detectable in the serum of these same animals (Fig. 1B). Thus our data suggest that resistin is synthesized by adipocytes, secreted into the blood, and diffused into the air spaces, potentially across the alveolar-capillary barrier. This is a plausible scenario given that serum proteins are found in BALF obtained from rodents exposed to air (1).
Exposure of wild-type mice to O3 significantly increased BALF resistin (Fig. 1A). Similar to that of air-exposed wild-type mice, resistin was not expressed in the lung tissue of wild-type mice exposed to O3 (Fig. 2, B and F). However, adipose tissue adjacent to the lungs of O3-exposed wild-type exhibited robust expression of resistin (Fig. 2B). Consequently, it is probable that the resistin in the BALF of O3-exposed mice is derived from adipocytes in the same manner as described above for air-exposed animals. Furthermore, permeability of the alveolar-capillary barrier is increased in O3-exposed wild-type mice, a phenomenon that is confirmed by a significant increase in BALF protein (Fig. 5G). Because resistin is not expressed in lung tissue following O3 exposure (Fig. 2, B and F), increased permeability of the alveolar-capillary barrier could allow more resistin to diffuse into the air spaces of O3-exposed animals. Concomitantly, serum resistin decreases following O3 exposure (Fig. 1B). The reason for this phenomenon is unclear. However, due to increased permeability of the alveolar-capillary barrier by O3, resistin may diffuse into the air spaces from the blood more rapidly than it can be replenished by adipocytes, leading to a transient decrease in serum resistin. Alternatively, resistin decreases during times of fasting (3), and mice decrease food and water consumption during and following exposure to O3 (40, 70), which is consistent with the significant weight loss observed in O3-exposed mice (Table 2). Thus O3-exposed mice are likely in a semi-fasted state that contributes to a decrease in serum resistin. Consistent with this latter hypothesis, we previously reported that serum leptin, which undergoes a marked decline in fasted animals, is reduced to similar levels in air-exposed fasted mice and O3-exposed mice given free access to food (33).
With the exception of KC (Fig. 4E), we found no effect of resistin deficiency on lung tissue or BALF cytokine expression or the migration of inflammatory cells (macrophages and neutrophils) to the air spaces. Indeed, we were surprised that we found no effect of resistin deficiency on IL-1α, IL-1β, IL-6, and TNF expression given that human resistin has previously been shown to induce the expression of these cytokines (9, 10, 43, 64, 69). To the best of our knowledge, there is only one other study that has examined the contribution of resistin to the development of lung pathology in mice. Jiang et al. (29) reported that humanized resistin mice, which express human resistin in mononuclear cells but are genetically deficient in murine resistin, demonstrate increased pulmonary TNF expression, neutrophil migration to the lungs, and increased pulmonary vascular hyperpermeability in response to the intratracheal administration of LPS. The ability of human resistin to induce the aforementioned pathological features in humanized resistin mice administered LPS is consistent with its well-known pro-inflammatory properties (9, 10, 43, 64, 69). However, the reason Jiang and colleagues demonstrate a role for resistin in the development of pulmonary pathology while we do not is unclear, but there are several potential reasons for this discrepancy. First, the contribution of resistin to the development of lung disease may be stimulus specific, an observation which is consistently demonstrated for other cytokines, including IL-6 and IL-11 (13, 32, 42, 74). Second, in the study of Jiang and colleagues (29), levels of BALF resistin in mice administered LPS were almost two hundred times greater than those observed in BALF of O3-exposed mice. Consequently, resistin may not be sufficiently elevated in the lungs of O3-exposed mice to initiate pathological effects. Third, although not yet verified in mice, human resistin binds to toll-like receptor 4 (TLR4) while mouse resistin binds to an isoform of decorin (ΔDCN) that has not been verified to bind human resistin (8, 16, 69). In mice, TLR4 has been shown to be essential for the development of O3-induced pulmonary inflammation and O3-induced AHR (22, 75). Also in mice, ΔDCN is expressed on adipose stromal cells in the lung (16), and adipose stromal cells have been shown to be beneficial in ameliorating lung pathology in several mouse models of lung disease, including LPS-induced acute lung injury (18, 77). Thus, at certain concentrations, mouse resistin may bind both TLR4 and ΔDCN in response to O3-induced lung injury but have opposing effects on the ensuing pathology such that no net effect is observed. Alternatively, the amino acid sequences of mouse and human resistin are 59% homologous (68). Therefore, species-related differences in the amino acid sequence of human and mouse resistin may impede the binding of mouse resistin to TLR4 and thus provide an explanation for the failure of murine resistin to elicit inflammation in our model.
We have also considered two additional possibilities as to the reason we failed to observe any effect of resistin deficiency on O3-induced pulmonary pathology. First, Kleeberger et al. (36) have previously demonstrated that pulmonary responses in mice exposed to O3 acutely (2 ppm for 3 h) as compared with subacutely (0.3 ppm for 48 h) are under the control of different genetic loci. Consistent with these observations, we previously reported that both IL-1RI and IL-6 minimally contribute to pulmonary inflammation following acute O3 exposure (2 ppm for 3 h), whereas both IL-1RI and IL-6 play a more prominent role in the development of pulmonary inflammation following subacute O3 exposure (0.3 ppm for 72 h) (30, 32). Consequently, it is certainly plausible that resistin contributes to pulmonary inflammation following subacute as compared with acute O3 exposure. Second, we have also considered the possibility that unlike human resistin, murine resistin is not pro-inflammatory. For example, RELM-α, a member of the same gene family as resistin, is not expressed in humans but is expressed in mice (46). Interestingly, murine RELM-α and human resistin demonstrate a very similar pattern of expression (46). Thus, in future experiments, it may be more appropriate to study the role of RELM-α in murine pulmonary responses to O3 to delineate and translate a role for human resistin in O3-induced lung pathology.
No genotype-related differences in IL-1α, IL-1β, TNF, or OPN expression existed following O3 exposure. Because each of these cytokines have been implicated in the development of O3-induced AHR (4, 14, 30, 31, 48, 63), we were not surprised that increases in airway responsiveness to methacholine induced by O3 were not different between the genotypes (Fig. 6). We previously demonstrated that the receptor for KC, CXCR2, contributes to O3-induced AHR and O3-induced neutrophil migration to the air spaces (31). Curiously, BALF KC was the only outcome indicator that was significantly different between wild-type and resistin-deficient mice following exposure to O3 (Fig. 4E). Despite BALF KC being greater in O3-exposed resistin-deficient as compared with O3-exposed wild-type mice, we observed no genotype-related differences in O3-induced neutrophil migration to the lungs or O3-induced AHR (Figs. 4H and 6). This observation may result from the fact that the differences in BALF KC between the genotypes was not sufficient enough to lead to any pathophysiological changes.
In conclusion, we demonstrate that murine resistin is elevated in BALF obtained from mice acutely exposed to O3. However, in contrast with the pro-inflammatory properties demonstrated by human resistin (9, 10, 43, 64, 69), our data demonstrate that murine resistin does not contribute to several sequelae induced by inflammatory cytokines following acute exposure to O3, including neutrophil migration to the air spaces, airway epithelial desquamation, and AHR. Because of differences in the cellular origin and differences between the amino acid sequences of human and murine resistin (67), it is premature to conclude from our data in mice that resistin will also be a noncontributory factor to the development of O3-induced pulmonary pathology in humans. Therefore, to delineate the potential contribution of resistin to O3-induced pulmonary pathology in humans, it may be warranted to repeat the experiments described here with humanized resistin mice or mice genetically deficient in RELM-α (45, 54).
GRANTS
The research reported in this publication was supported, in part, by National Institutes of Health (NIH) National Institute of Environmental Health Sciences award number R03ES022378 (to R. A. Johnston), NIH National Institute of Allergy and Infectious Diseases award number R03AI107432 (to R. A. Johnston), and NIH National Institute of Diabetes and Digestive and Kidney Diseases award number P01DK049210 (to M. A. Lazar).
DISCLAIMERS
The content contained within this publication is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
S.S.R., F.M., T.W., C.L.A., C.Y.S., K.J.C., A.L.A., and R.A.J. performed experiments; S.S.R., J.B.R., F.M., K.R.C., R.E.P., C.S.B., T.W., C.L.A., C.Y.S., K.J.C., A.L.A., M.R.B., J.L.A., I.U.H., and R.A.J. analyzed data; S.S.R., J.B.R., K.R.C., R.E.P., C.S.B., T.W., C.L.A., M.R.B., J.L.A., I.U.H., and R.A.J. interpreted results of experiments; S.S.R. and R.A.J. drafted manuscript; S.S.R., J.B.R., I.U.H., and R.A.J. edited and revised manuscript; S.S.R., J.B.R., F.M., K.R.C., R.E.P., C.S.B., T.W., C.L.A., C.Y.S., K.J.C., A.L.A., M.R.B., J.L.A., I.U.H., and R.A.J. approved final version of manuscript; R.E.P. and R.A.J. prepared figures; M.R.B., J.L.A., I.U.H., and R.A.J. conception and design of research.
ACKNOWLEDGMENTS
We thank Mitchell A. Lazar, of the Perelman School of Medicine at the University of Pennsylvania in Philadelphia, PA, for providing us with breeding pairs of resistin-deficient mice.
REFERENCES
- 1.Alpert SM, Schwartz BB, Lee SD, Lewis TR. Alveolar protein accumulation. A sensitive indicator of low level oxidant toxicity. Arch Intern Med 128: 69–73, 1971. [DOI] [PubMed] [Google Scholar]
- 2.American Lung Association. American Lung Association State of the Air 2015. Chicago, IL, 2015. [Google Scholar]
- 3.Banerjee RR, Rangwala SM, Shapiro JS, Rich AS, Rhoades B, Qi Y, Wang J, Rajala MW, Pocai A, Scherer PE, Steppan CM, Ahima RS, Obici S, Rossetti L, Lazar MA. Regulation of fasted blood glucose by resistin. Science 303: 1195–1198, 2004. [DOI] [PubMed] [Google Scholar]
- 4.Barreno RX, Richards JB, Schneider DJ, Cromar KR, Nadas AJ, Hernandez CB, Hallberg LM, Price RE, Hashmi SS, Blackburn MR, Haque IU, Johnston RA. Endogenous osteopontin promotes ozone-induced neutrophil recruitment to the lungs and airway hyperresponsiveness to methacholine. Am J Physiol Lung Cell Mol Physiol 305: L118–L129, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bates JH, Schuessler TF, Dolman C, Eidelman DH. Temporal dynamics of acute isovolume bronchoconstriction in the rat. J Appl Physiol 82: 55–62, 1997. [DOI] [PubMed] [Google Scholar]
- 6.Bates JH, Shardonofsky F, Stewart DE. The low-frequency dependence of respiratory system resistance and elastance in normal dogs. Respir Physiol 78: 369–382, 1989. [DOI] [PubMed] [Google Scholar]
- 7.Beier JI, Guo L, von Montfort C, Kaiser JP, Joshi-Barve S, Arteel GE. New role of resistin in lipopolysaccharide-induced liver damage in mice. J Pharmacol Exp Ther 325: 801–808, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Benomar Y, Gertler A, De Lacy P, Crepin D, Ould Hamouda H, Riffault L, Taouis M. Central resistin overexposure induces insulin resistance through Toll-like receptor 4. Diabetes 62: 102–114, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bertolani C, Sancho-Bru P, Failli P, Bataller R, Aleffi S, DeFranco R, Mazzinghi B, Romagnani P, Milani S, Gines P, Colmenero J, Parola M, Gelmini S, Tarquini R, Laffi G, Pinzani M, Marra F. Resistin as an intrahepatic cytokine: overexpression during chronic injury and induction of proinflammatory actions in hepatic stellate cells. Am J Pathol 169: 2042–2053, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bokarewa M, Nagaev I, Dahlberg L, Smith U, Tarkowski A. Resistin, an adipokine with potent proinflammatory properties. J Immunol 174: 5789–5795, 2005. [DOI] [PubMed] [Google Scholar]
- 11.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254, 1976. [DOI] [PubMed] [Google Scholar]
- 12.Castleman WL, Dungworth DL, Schwartz LW, Tyler WS. Acute respiratory bronchiolitis: an ultrastructural and autoradiographic study of epithelial cell injury and renewal in rhesus monkeys exposed to ozone. Am J Pathol 98: 811–840, 1980. [PMC free article] [PubMed] [Google Scholar]
- 13.Cenci E, Mencacci A, Casagrande A, Mosci P, Bistoni F, Romani L. Impaired antifungal effector activity but not inflammatory cell recruitment in interleukin-6-deficient mice with invasive pulmonary aspergillosis. J Infect Dis 184: 610–617, 2001. [DOI] [PubMed] [Google Scholar]
- 14.Cho HY, Zhang LY, Kleeberger SR. Ozone-induced lung inflammation and hyperreactivity are mediated via TNF-α receptors. Am J Physiol Lung Cell Mol Physiol 280: L537–L546, 2001. [DOI] [PubMed] [Google Scholar]
- 15.Dahm PH, Richards JB, Karmouty-Quintana H, Cromar KR, Sur S, Price RE, Malik F, Spencer CY, Barreno RX, Hashmi SS, Blackburn MR, Haque IU, Johnston RA. Effect of antigen sensitization and challenge on oscillatory mechanics of the lung and pulmonary inflammation in obese carboxypeptidase E-deficient mice. Am J Physiol Regul Integr Comp Physiol 307: R621–R633, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Daquinag AC, Zhang Y, Amaya-Manzanares F, Simmons PJ, Kolonin MG. An isoform of decorin is a resistin receptor on the surface of adipose progenitor cells. Cell Stem Cell 9: 74–86, 2011. [DOI] [PubMed] [Google Scholar]
- 17.de Jonge HJ, Fehrmann RS, de Bont ES, Hofstra RM, Gerbens F, Kamps WA, de Vries EG, van der Zee AG, te Meerman GJ, ter Elst A. Evidence based selection of housekeeping genes. PLoS One 2: e898, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feizpour A, Boskabady MH, Ghorbani A. Adipose-derived stromal cell therapy affects lung inflammation and tracheal responsiveness in guinea pig model of COPD. PLoS One 9: e108974, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Foster WM, Brown RH, Macri K, Mitchell CS. Bronchial reactivity of healthy subjects: 18–20 h postexposure to ozone. J Appl Physiol 89: 1804–1810, 2000. [DOI] [PubMed] [Google Scholar]
- 20.Fredrickson TN, Harris AW. Atlas of Mouse Hematopathology. Singapore: Harwood Academic Publishers, 2000. [Google Scholar]
- 21.Gabellec MM, Griffais R, Fillion G, Haour F. Expression of interleukin 1 alpha, interleukin 1 beta and interleukin 1 receptor antagonist mRNA in mouse brain: regulation by bacterial lipopolysaccharide (LPS) treatment. Brain Res Mol Brain Res 31: 122–130, 1995. [DOI] [PubMed] [Google Scholar]
- 22.Garantziotis S, Li Z, Potts EN, Lindsey JY, Stober VP, Polosukhin VV, Blackwell TS, Schwartz DA, Foster WM, Hollingsworth JW. TLR4 is necessary for hyaluronan-mediated airway hyperresponsiveness after ozone inhalation. Am J Respir Crit Care Med 181: 666–675, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 23.Gregory AD, Hogue LA, Ferkol TW, Link DC. Regulation of systemic and local neutrophil responses by G-CSF during pulmonary Pseudomonas aeruginosa infection. Blood 109: 3235–3243, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Griesenbach U, Munkonge FM, Sumner-Jones S, Holder E, Smith SN, Boyd AC, Gill DR, Hyde SC, Porteous D, Alton EW; UK Cystic fibrosis gene therapy consortium. Assessment of CFTR function after gene transfer in vitro and in vivo. Methods Mol Biol 433: 229–242, 2008. [DOI] [PubMed] [Google Scholar]
- 25.Hantos Z, Daroczy B, Suki B, Nagy S, Fredberg JJ. Input impedance and peripheral inhomogeneity of dog lungs. J Appl Physiol 72: 168–178, 1992. [DOI] [PubMed] [Google Scholar]
- 26.Hatch GE, Slade R, Harris LP, McDonnell WF, Devlin RB, Koren HS, Costa DL, McKee J. Ozone dose and effect in humans and rats. A comparison using oxygen-18 labeling and bronchoalveolar lavage. Am J Respir Crit Care Med 150: 676–683, 1994. [DOI] [PubMed] [Google Scholar]
- 27.Hiltermann TJ, Stolk J, Hiemstra PS, Fokkens PH, Rombout PJ, Sont JK, Sterk PJ, Dijkman JH. Effect of ozone exposure on maximal airway narrowing in non-asthmatic and asthmatic subjects. Clin Sci 89: 619–624, 1995. [DOI] [PubMed] [Google Scholar]
- 28.Holz O, Khalilieh S, Ludwig-Sengpiel A, Watz H, Stryszak P, Soni P, Tsai M, Sadeh J, Magnussen H. SCH527123, a novel CXCR2 antagonist, inhibits ozone-induced neutrophilia in healthy subjects. Eur Respir J 35: 564–570, 2010. [DOI] [PubMed] [Google Scholar]
- 29.Jiang S, Park DW, Tadie JM, Gregoire M, Deshane J, Pittet JF, Abraham E, Zmijewski JW. Human resistin promotes neutrophil proinflammatory activation and neutrophil extracellular trap formation and increases severity of acute lung injury. J Immunol 192: 4795–4803, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Johnston RA, Mizgerd JP, Flynt L, Quinton LJ, Williams ES, Shore SA. Type I interleukin-1 receptor is required for pulmonary responses to subacute ozone exposure in mice. Am J Respir Cell Mol Biol 37: 477–484, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Johnston RA, Mizgerd JP, Shore SA. CXCR2 is essential for maximal neutrophil recruitment and methacholine responsiveness after ozone exposure. Am J Physiol Lung Cell Mol Physiol 288: L61–L67, 2005. [DOI] [PubMed] [Google Scholar]
- 32.Johnston RA, Schwartzman IN, Flynt L, Shore SA. Role of interleukin-6 in murine airway responses to ozone. Am J Physiol Lung Cell Mol Physiol 288: L390–L397, 2005. [DOI] [PubMed] [Google Scholar]
- 33.Johnston RA, Theman TA, Terry RD, Williams ES, Shore SA. Pulmonary responses to acute ozone exposure in fasted mice: effect of leptin administration. J Appl Physiol 102: 149–156, 2007. [DOI] [PubMed] [Google Scholar]
- 34.Kaneko T, Ikeda H, Fu L, Nishiyama H, Okubo T. Platelet-activating factor mediates the ozone-induced increase in airway microvascular leakage in guinea pigs. Eur J Pharmacol 292: 251–255, 1995. [DOI] [PubMed] [Google Scholar]
- 35.Kim SJ, Nian C, McIntosh CH. Resistin knockout mice exhibit impaired adipocyte glucose-dependent insulinotropic polypeptide receptor (GIPR) expression. Diabetes 62: 471–477, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kleeberger SR, Levitt RC, Zhang LY. Susceptibility to ozone-induced inflammation. II. Separate loci control responses to acute and subacute exposures. Am J Physiol Lung Cell Mol Physiol 264: L21–L26, 1993. [DOI] [PubMed] [Google Scholar]
- 37.Konrad FM, Reutershan J. CXCR2 in acute lung injury. Mediators Inflamm 2012: 740987, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kreit JW, Gross KB, Moore TB, Lorenzen TJ, D′Arcy J, Eschenbacher WL. Ozone-induced changes in pulmonary function and bronchial responsiveness in asthmatics. J Appl Physiol 66: 217–222, 1989. [DOI] [PubMed] [Google Scholar]
- 39.Kusminski CM, McTernan PG, Kumar S. Role of resistin in obesity, insulin resistance and Type II diabetes. Clin Sci 109: 243–256, 2005. [DOI] [PubMed] [Google Scholar]
- 40.Last JA, Gohil K, Mathrani VC, Kenyon NJ. Systemic responses to inhaled ozone in mice: Cachexia and down-regulation of liver xenobiotic metabolizing genes. Toxicol Appl Pharmacol 208: 117–126, 2005. [DOI] [PubMed] [Google Scholar]
- 41.Lauzon AM, Bates JH. Estimation of time-varying respiratory mechanical parameters by recursive least squares. J Appl Physiol 71: 1159–1165, 1991. [DOI] [PubMed] [Google Scholar]
- 42.Lee CG, Hartl D, Matsuura H, Dunlop FM, Scotney PD, Fabri LJ, Nash AD, Chen NY, Tang CY, Chen Q, Homer RJ, Baca M, Elias JA. Endogenous IL-11 signaling is essential in Th2- and IL-13-induced inflammation and mucus production. Am J Respir Cell Mol Biol 39: 739–746, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee JH, Ort T, Ma K, Picha K, Carton J, Marsters PA, Lohmander LS, Baribaud F, Song XY, Blake S. Resistin is elevated following traumatic joint injury and causes matrix degradation and release of inflammatory cytokines from articular cartilage in vitro. Osteoarthritis Cartilage 17: 613–620, 2009. [DOI] [PubMed] [Google Scholar]
- 44.Mudway IS, Kelly FJ. Ozone and the lung: a sensitive issue. Mol Aspects Med 21: 1–48, 2000. [DOI] [PubMed] [Google Scholar]
- 45.Munitz A, Waddell A, Seidu L, Cole ET, Ahrens R, Hogan SP, Rothenberg ME. Resistin-like molecule alpha enhances myeloid cell activation and promotes colitis. J Allergy Clin Immunol 122: 1200–1207, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nair MG, Guild KJ, Artis D. Novel effector molecules in type 2 inflammation: lessons drawn from helminth infection and allergy. J Immunol 177: 1393–1399, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.O′Byrne PM, Inman MD. Airway hyperresponsiveness. Chest 123: 411S–416S, 2003. [DOI] [PubMed] [Google Scholar]
- 48.Park JW, Taube C, Swasey C, Kodama T, Joetham A, Balhorn A, Takeda K, Miyahara N, Allen CB, Dakhama A, Kim SH, Dinarello CA, Gelfand EW. Interleukin-1 receptor antagonist attenuates airway hyperresponsiveness following exposure to ozone. Am J Respir Cell Mol Biol 30: 830–836, 2004. [DOI] [PubMed] [Google Scholar]
- 49.Patel SD, Rajala MW, Rossetti L, Scherer PE, Shapiro L. Disulfide-dependent multimeric assembly of resistin family hormones. Science 304: 1154–1158, 2004. [DOI] [PubMed] [Google Scholar]
- 50.Pendino KJ, Meidhof TM, Heck DE, Laskin JD, Laskin DL. Inhibition of macrophages with gadolinium chloride abrogates ozone-induced pulmonary injury and inflammatory mediator production. Am J Respir Cell Mol Biol 13: 125–132, 1995. [DOI] [PubMed] [Google Scholar]
- 51.Pillow JJ, Korfhagen TR, Ikegami M, Sly PD. Overexpression of TGF-alpha increases lung tissue hysteresivity in transgenic mice. J Appl Physiol 91: 2730–2734, 2001. [DOI] [PubMed] [Google Scholar]
- 52.Pini M, Gove ME, Sennello JA, van Baal JW, Chan L, Fantuzzi G. Role and regulation of adipokines during zymosan-induced peritoneal inflammation in mice. Endocrinology 149: 4080–4085, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Probert L. TNF and its receptors in the CNS: the essential, the desirable and the deleterious effects. Neuroscience 302: 2–22, 2015. [DOI] [PubMed] [Google Scholar]
- 54.Qatanani M, Szwergold NR, Greaves DR, Ahima RS, Lazar MA. Macrophage-derived human resistin exacerbates adipose tissue inflammation and insulin resistance in mice. J Clin Invest 119: 531–539, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Qi Y, Nie Z, Lee YS, Singhal NS, Scherer PE, Lazar MA, Ahima RS. Loss of resistin improves glucose homeostasis in leptin deficiency. Diabetes 55: 3083–3090, 2006. [DOI] [PubMed] [Google Scholar]
- 56.Que LG, Stiles JV, Sundy JS, Foster WM. Pulmonary function, bronchial reactivity, and epithelial permeability are response phenotypes to ozone and develop differentially in healthy humans. J Appl Physiol 111: 679–687, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.R Development Core Team. R: a language and environment for statistical computing. (Online) R Foundation for Statistical Computing; http://www.R-project.org/ [15 Sept. 2015]. [Google Scholar]
- 58.Ruwanpura SM, McLeod L, Brooks GD, Bozinovski S, Vlahos R, Longano A, Bardin PG, Anderson GP, Jenkins BJ. IL-6/Stat3-driven pulmonary inflammation, but not emphysema, is dependent on interleukin-17A in mice. Respirology 19: 419–427, 2014. [DOI] [PubMed] [Google Scholar]
- 59.Schuessler TF, Bates JH. A computer-controlled research ventilator for small animals: design and evaluation. IEEE Trans Biomed Eng 42: 860–866, 1995. [DOI] [PubMed] [Google Scholar]
- 60.Schwarze J, Cieslewicz G, Joetham A, Ikemura T, Makela MJ, Dakhama A, Shultz LD, Lamers MC, Gelfand EW. Critical roles for interleukin-4 and interleukin-5 during respiratory syncytial virus infection in the development of airway hyperresponsiveness after airway sensitization. Am J Respir Crit Care Med 162: 380–386, 2000. [DOI] [PubMed] [Google Scholar]
- 61.Schwarze J, Hamelmann E, Cieslewicz G, Tomkinson A, Joetham A, Bradley K, Gelfand EW. Local treatment with IL-12 is an effective inhibitor of airway hyperresponsiveness and lung eosinophilia after airway challenge in sensitized mice. J Allergy Clin Immunol 102: 86–93, 1998. [DOI] [PubMed] [Google Scholar]
- 62.Seltzer J, Bigby BG, Stulbarg M, Holtzman MJ, Nadel JA, Ueki IF, Leikauf GD, Goetzl EJ, Boushey HA. O3-induced change in bronchial reactivity to methacholine and airway inflammation in humans. J Appl Physiol 60: 1321–1326, 1986. [DOI] [PubMed] [Google Scholar]
- 63.Shore SA, Schwartzman IN, Le Blanc B, Murthy GG, Doerschuk CM. Tumor necrosis factor receptor 2 contributes to ozone-induced airway hyperresponsiveness in mice. Am J Respir Crit Care Med 164: 602–607, 2001. [DOI] [PubMed] [Google Scholar]
- 64.Silswal N, Singh AK, Aruna B, Mukhopadhyay S, Ghosh S, Ehtesham NZ. Human resistin stimulates the pro-inflammatory cytokines TNF-alpha and IL-12 in macrophages by NF-kappaB-dependent pathway. Biochem Biophys Res Commun 334: 1092–1101, 2005. [DOI] [PubMed] [Google Scholar]
- 65.Sims JE. IL-1 and IL-18 receptors, and their extended family. Curr Opin Immunol 14: 117–122, 2002. [DOI] [PubMed] [Google Scholar]
- 66.Slade R, Watkinson WP, Hatch GE. Mouse strain differences in ozone dosimetry and body temperature changes. Am J Physiol Lung Cell Mol Physiol 272: L73–L77, 1997. [DOI] [PubMed] [Google Scholar]
- 67.Steppan CM, Bailey ST, Bhat S, Brown EJ, Banerjee RR, Wright CM, Patel HR, Ahima RS, Lazar MA. The hormone resistin links obesity to diabetes. Nature 409: 307–312, 2001. [DOI] [PubMed] [Google Scholar]
- 68.Steppan CM, Lazar MA. The current biology of resistin. J Inter Med 255: 439–447, 2004. [DOI] [PubMed] [Google Scholar]
- 69.Tarkowski A, Bjersing J, Shestakov A, Bokarewa MI. Resistin competes with lipopolysaccharide for binding to toll-like receptor 4. J Cell Mol Med 14: 1419–1431, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Umezu T, Suzuki AK, Miura T, Koizumi A. Effects of ozone and nitrogen dioxide on drinking and eating behaviors in mice. Environ Res 61: 51–67, 1993. [DOI] [PubMed] [Google Scholar]
- 71.United States Environmental Protection Agency. National Ambient Air Quality Standards (NAAQS). (Online) http://www.epa.gov/ttn/naaqs/criteria.html [15 Sept. 2015].
- 72.Untergasser A, Nijveen H, Rao X, Bisseling T, Geurts R, Leunissen JA. Primer3Plus, an enhanced web interface to Primer3. Nucleic Acids Res 35: W71–W74, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wald A, Jason D, Murphy TW, Mazzia VD. A computers system for respiratory parameters. Comput Biomed Res 2: 411–429, 1969. [DOI] [PubMed] [Google Scholar]
- 74.Waxman AB, Einarsson O, Seres T, Knickelbein RG, Warshaw JB, Johnston R, Homer RJ, Elias JA. Targeted lung expression of interleukin-11 enhances murine tolerance of 100% oxygen and diminishes hyperoxia-induced DNA fragmentation. J Clin Invest 101: 1970–1982, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Williams AS, Leung SY, Nath P, Khorasani NM, Bhavsar P, Issa R, Mitchell JA, Adcock IM, Chung KF. Role of TLR2, TLR4, and MyD88 in murine ozone-induced airway hyperresponsiveness and neutrophilia. J Appl Physiol 103: 1189–1195, 2007. [DOI] [PubMed] [Google Scholar]
- 76.Ye J, Coulouris G, Zaretskaya I, Cutcutache I, Rozen S, Madden TL. Primer-BLAST: a tool to design target-specific primers for polymerase chain reaction. BMC Bioinformatics 13: 134, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhang S, Danchuk SD, Bonvillain RW, Xu B, Scruggs BA, Strong AL, Semon JA, Gimble JM, Betancourt AM, Sullivan DE, Bunnell BA. Interleukin 6 mediates the therapeutic effects of adipose-derived stromal/stem cells in lipopolysaccharide-induced acute lung injury. Stem Cells 32: 1616–1628, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhang X, Morrison-Carpenter T, Holt JB, Callahan DB. Trends in adult current asthma prevalence and contributing risk factors in the United States by state: 2000–2009. BMC Public Health 13: 1156, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhao L, Yang W, Yang X, Lin Y, Lv J, Dou X, Luo Q, Dong J, Chen Z, Chu Y, He R. Chemerin suppresses murine allergic asthma by inhibiting CCL2 production and subsequent airway recruitment of inflammatory dendritic cells. Allergy 69: 763–774, 2014. [DOI] [PubMed] [Google Scholar]