Abstract
The induction of allergen-specific T helper 2 (Th2) cells by lung dendritic cells (DCs) is a critical step in allergic asthma development. Airway delivery of purified allergens or microbial products can promote Th2 priming by lung DCs, but how environmentally relevant quantities and combinations of these factors affect lung DC function is unclear. Here, we investigated the ability of house dust extract (HDE), which contains a mixture of environmental adjuvants, to prime Th2 responses against an innocuous inhaled antigen. Inhalational exposure to HDE conditioned lung conventional DCs, but not monocyte-derived DCs, to induce antigen-specific Th2 differentiation. Conditioning of DCs by HDE was independent of Toll-like receptor 4 signaling, indicating that environmental endotoxin is dispensable for programming DCs to induce Th2 responses. DCs directly treated with HDE underwent maturation but were poor stimulators of Th2 differentiation. In contrast, DCs treated with bronchoalveolar lavage fluid (BALF) from HDE-exposed mice induced robust Th2 differentiation. DC conditioning by BALF was independent of the proallergic cytokines IL-25, IL-33, and thymic stromal lymphopoietin. BALF treatment of DCs resulted in upregulation of CD80 but low expression of CD40, CD86, and IL-12p40, which was associated with Th2 induction. These findings support a model whereby environmental adjuvants in house dust indirectly program DCs to prime Th2 responses by triggering the release of endogenous soluble factor(s) by airway cells. Identifying these factors could lead to novel therapeutic targets for allergic asthma.
Keywords: dendritic cells, allergic asthma, T helper 2 responses, environmental allergens, house dust
allergic asthma is a chronic lung disease characterized by allergen-induced airway inflammation and hyperresponsiveness (28). In addition to genetic susceptibility, environmental factors play a central role in asthma development (37). There is growing evidence that immunogenic agents within the living environment, including allergens and microbial products, can impact the risk for atopy and asthma (1, 11). Studies in rodents have shown that allergens and Toll-like receptor (TLR) ligands can act as adjuvants and promote sensitization to inhaled innocuous antigens, resulting in allergic airway inflammation upon subsequent antigen exposure (14, 33, 55, 57, 58). However, such studies typically involve airway delivery of either purified TLR ligands or high doses (up to 100 μg) of a single allergen and therefore are unlikely to simulate natural human exposure. For example, indoor environmental sampling suggests that humans are exposed to a complex mixture of allergens, microbial-derived products, and pollutants (1, 19, 20). Additionally, personal air samplers suggest that individuals generally inhale only nanogram quantities of allergen daily (13, 43). To address these limitations, some investigators have employed extracts of common house dust in airway sensitization models. House dust extracts (HDE) contain an environmentally relevant mixture of several indoor allergens (e.g., dust mite, cockroach, and pet dander) and microbial products (4). In contrast, commercial allergen extracts, such as those prepared from dust mites, contain large quantities of a single purified allergen. Thus airway sensitization models using HDE more accurately mimic the complex indoor environment to which individuals are normally exposed. In addition to indoor allergens, HDE also possess T helper 2 (Th2) adjuvant activity, as demonstrated by their ability to promote allergic adaptive immune responses to innocuous inhaled antigens (35, 57). An improved mechanistic understanding of how environmental adjuvants in house dust promote allergic sensitization should facilitate the development of more effective strategies for asthma prevention.
Sensitization to allergens is characterized by the development of allergen-specific Th2 cells, which mediate allergic inflammation through the production of type 2 cytokines, including IL-4, IL-5, and IL-13 (41). Th2 cell differentiation is orchestrated by signals from pulmonary dendritic cells (DCs), which capture and present inhaled antigens to naïve T cells (25). DCs express an array of innate receptors that allow them to directly sense immunogenic stimuli in the extracellular environment and initiate immune responses tailored to specific types of pathogens (42). Several pathogen-associated molecular patterns can directly stimulate DCs to produce IL-12 and IL-23, which promote the differentiation of Th1 and Th17 cells, respectively (42). However, whether Th2-promoting adjuvants can also directly program DCs to induce Th2 responses remains unclear. Several stimuli, including allergens (12, 23, 47), fungal products (26), and helminth-derived molecules (10, 50), have been reported to directly stimulate DCs to induce Th2 cells. In contrast, other studies have found that Th2 adjuvants initially activate nonhematopoietic structural cells, which secrete factors that condition DCs to prime Th2 responses. For example, dust mite allergen is reported to signal through TLR4 on airway structural cells, stimulating production of cytokines such as IL-25, IL-33, and thymic stromal lymphopoietin (TSLP), which then program lung DCs to induce Th2 differentiation (17, 24). Whether environmentally relevant adjuvants in HDE condition DCs through a direct or indirect mechanism is unknown.
Pulmonary DCs are a heterogeneous group of antigen-presenting cells that can be categorized as either conventional DCs (cDCs) or monocyte-derived DCs (moDCs) based on their developmental lineage (44). Lung cDCs are derived from Fms-like tyrosine kinase 3 ligand (FLT3L)-dependent DC precursors and are divided into two major subsets based on reciprocal expression of the integrins α-E (CD103) and α-M (CD11b) (51). Conversely, lung moDCs arise from circulating monocytes that enter the lung parenchyma under steady-state or inflammatory conditions (27, 31). Lung moDCs also express CD11b but can be distinguished from CD11bhi cDCs by expression of the complement 5a receptor 1 (C5aR1/CD88) (34) or Fc-γ receptor 1 (CD64) (39, 45). The relative contribution of cDCs and moDCs for inducing Th2 responses against inhaled allergens has been an area of debate. Although the role of CD103+ and CD11b+ cDCs in allergic airway inflammation depends on the model used, both types of DCs can induce Th2 responses against indoor allergens (33, 39, 60). Inflammatory moDCs have also been reported to induce Th2 responses against dust mite allergen (18, 39), particularly at high antigen concentrations (39). The primary DC subset responsible for inducing Th2 responses following exposure to environmentally relevant adjuvants found in HDE remains unknown.
In the present study, we investigated the mechanisms by which inhalational exposure to HDE promotes Th2 responses. We found that exposure to HDE conditioned lung cDCs, but not moDCs, to induce Th2 cells. DCs treated with bronchoalveolar fluid (BALF) from HDE-exposed mice induced robust Th2 differentiation, suggesting that HDE elicited the release of soluble factors by airway cells, which in turn conditioned DCs to prime Th2 cells. In contrast to previous studies using purified dust mite allergen, we found that the cytokines IL-25, IL-33, and TSLP were individually dispensable for conditioning DCs following HDE exposure. BALF treatment of DCs resulted in marked upregulation of CD80 but only modest induction of CD40, CD86, and IL-12p40, which was associated with Th2 induction. Overall, our findings suggest that the mechanisms for DC conditioning following exposures to high doses of purified allergens might be different than those underlying sensitization to more environmentally relevant amounts of allergens and adjuvants.
MATERIALS AND METHODS
Mice.
C57BL/6J and OT-II [C57BL/6-Tg(TcraTcrb)425Cbn/J] male mice were purchased from Jackson Laboratories (Bar Harbor, ME). Il33−/− [Il33tm1(KOMP)Vlcg] were obtained from the trans-NIH Knock-Out Mouse Project (KOMP) Repository (www.komp.org). Mice were housed in specific pathogen-free conditions at the National Institute of Environmental Health Sciences (NIEHS) and used between 6 and 12 wk of age. All experiments were approved by the Institutional Animal Care and Use Committee of the National Institute of Environmental Health Sciences.
Flow cytometric analysis.
Cells were incubated with a nonspecific binding blocking reagent cocktail of anti-mouse CD16/CD32 (2.4G2) and normal mouse and rat serum (Jackson ImmunoResearch, West Grove, PA) for 5 min. For staining of surface antigens, cells were incubated with fluorochrome [allophycocyanin (APC), APC-Cy7, Alexa Fluor 488, Alexa Fluor 647, eFluor 450, eFluor 605 NC, APC-eFluor 780, FITC, PerCP-Cy5.5, or phycoerythrin (PE)] or biotin-conjugated antibodies against mouse B220 (HIS24), CD3ε (145-2C11), CD8α (56-6.7), CD8β (53-5.8), CD11b (M1/70), CD11c (N418), CD19 (6D5), CD40 (1C10), CD49b (DX5), CD80 (16-10A1), CD86 (GL1), CD88 (20/70), CD103 (M290), Ly-6C (AL-21), Ly-6G (1A8), myosin heavy chain (MHC) class II I-Ab (AFb.120), and TER-119 (BD Biosciences, San Jose, CA; BioLegend, San Diego, CA; and eBioscience, San Diego, CA). Staining with biotinylated antibodies was followed by incubation with fluorochrome-conjugated streptavidin. Stained cells were analyzed on a five-laser LSRII (BD Biosciences) or sorted on a four-laser ARIA-II flow cytometer (BD Biosciences), and the data were analyzed using FACS Diva (BD Bioscience) and FlowJo (Treestar, Ashland, OR) software. Dead cells were excluded based on their forward and side scatter. Only single cells were analyzed.
HDE.
HDE was prepared as previously described (46). Briefly, vacuumed dust samples were passed through a coarse sieve, weighed, and then extracted at 100 mg/ml with PBS at 4°C with mild agitation for 16 h. The samples were centrifuged to remove insoluble debris, and supernatants were sterilized by passage through a 0.22-μm filter. Endotoxin levels were assayed by a Limulus amebocyte lysate assay (Lonza, Karlsruhe, Germany), and allergens were measured using a multiplex array for indoor allergens (MARIA; Indoor Biotechnologies, Charlottesville, VA), according to the manufacturer's instructions. Titration studies revealed that airway delivery of 10 μl of HDE, which contained 4.56 ng Der f 1, 0.07 ng Fel d 1, 0.13 ng Can f 1, 0.13 ng Bla g 2, and 21.28 endotoxin units, was sufficient to sensitize mice to inhaled antigen (55).
Allergic sensitization.
Mice were anesthetized by isoflurane inhalation and given 100 μg of endotoxin-free ovalbumin (OVA) (Profos, Regensburg, Germany) with either PBS diluent alone, 10 μl of HDE, 10 ng polyinosinic-polycytidylic acid [poly(I:C)] (InvivoGen, San Diego, CA), or 120 ng Aspergillus oryzae protease (Sigma, St. Louis, MO) in a total volume of 50 μl by oropharyngeal aspiration as previously described (57, 58). In some experiments, mice received endotoxin-free OVA labeled with Alexa Fluor 647 (Invitrogen, Grand Island, NY) by oropharyngeal aspiration to measure antigen uptake by lung DCs.
Generation of FLT3L-derived cDCs.
Marrow was collected from pulverized bones, and red blood cells were lysed with 0.15 M ammonium chloride and 1 mM potassium bicarbonate. Bone marrow (BM) cells were then cultured in complete RPMI media [RPMI 1640, 10% FBS (Gemini, West Sacramento, CA), penicillin/streptomycin, and 50 ng/ml β-mercaptoethanol] supplemented with either 100–200 ng/ml recombinant human FLT3L to generate FLT3L-derived cDCs (FL-cDCs). Recombinant human FLT3L was produced by the NIEHS Protein Expression Core Facility using previously described methods (21). Media was replaced every 3–5 days, at which time fresh FLT3L was also added. Cells were harvested on days 7–9, incubated with murine CD11c microbeads (Miltenyi, Auburn, CA), and finally isolated with an automated magnet-activated cell sorter (AutoMACS) to a purity of >90% CD11c+ cells.
Isolation of lung DC subsets.
Lung DC subsets were isolated and purified by flow cytometry-based sorting to a purity of >95% as previously described (30, 32). Briefly, at 16 h following allergic sensitization, lungs were harvested, minced, and digested with Liberase TM (100 μg/ml; Roche, Indianapolis, IN), collagenase XI (250 μg/ml), hyaluronidase 1a (1 mg/ml), and DNase I (200 μg/ml; Sigma) for 1 h at 37°C. The digested tissue was passed through a 70-μm nylon strainer to obtain a single-cell suspension. DCs were enriched by discontinuous phase-density centrifugation with 16% Nycodenz (Accurate Chemical, Westbury, NY) before antibody staining. Total lung DCs were identified as CD11chiI-Ab(MHC-II)hiautofluorescent lung cells. In some experiments, lung DC subsets were isolated from naïve (unsensitized) mice.
Treatment of DCs with HDE and BALF from HDE-treated mice.
Mice were allowed to inhale 10 μl of HDE or the vehicle PBS in a total volume of 50 μl. Following euthanasia 5–6 h later, lungs were lavaged with 1 ml of media. After centrifugation to remove airway cells, BALF was sterilized by passage through a 0.22-μm filter. Purified CD11c+ FL-cDCs or sorted lung cDCs were incubated for 24 h with BALF (50% vol/vol) or with 1% vol/vol HDE. In some experiments, 10 μg/ml of neutralizing polyclonal anti-mouse TSLP (R&D Systems Minneapolis, MN) (36), neutralizing monoclonal anti-mouse IL-17E/IL-25 (Clone 50104, R&D Systems), or isotype control antibodies were added to DC cultures during BALF treatment. The HDE- or BALF-treated DCs were extensively washed before their analysis or use in T-cell coculture experiments.
Coculture of naive T cells with DCs.
Naive CD4+ T cells (CD8α−CD8β−CD11b−CD11c−CD19−CD44loB220−CD49b−I-A−Ly-6C/G−TER119−) were prepared from pools of lymph nodes and spleens of OT-II mice by negative selection using specific antibodies and an AutoMACS system (Miltenyi). Naïve CD4+ T cells (5 × 104 per well) were cultured with purified lung DCs (6.25 × 103 per well) from OVA-treated mice in 200 μl complete IMDM medium containing 10% FBS (certified; Life Technologies, Grand Island, NY), 50 μM β-mercaptoethanol, penicillin, and streptomycin in a 96-well U-bottom plate (BD Biosciences) in a CO2 incubator. In some experiments, naïve T cells were cultured with CD11c+ FL-cDCs or sorted lung DCs in the presence of 10 nM OVA323–339 peptide (New England Peptide, Gardner, MA). Proliferation was inferred from recovery of viable (Trypan blue negative) T cells after 5 days of coculture. Equal numbers of viable cells (1 × 105 cells/well) were then stimulated for 24 h with plate-bound antibodies to CD3ε (1 μg/ml) and CD28 (1 μg/ml). Cytokines in supernatants of these cultures were measured by ELISA using specific antibodies against IFN-γ (R4-6A2 and XMG1.2), IL-4 (11B11 and BVD6-24G), and IL-13 (eBio13A and eBio1316H).
cDNA amplification and analysis.
Total RNA was isolated from cells using TRIzol reagent (Life Technologies) and converted to cDNA with oligo dT and random hexamer primers using MuLV reverse transcriptase (Life Technologies). PCR amplification was performed using KiCqStart SYBR Green Primers (Sigma) specific for Il12b and Gapdh with Power SYBR Green PCR Master Mix (Life Technologies) and an Mx3000P quantitative PCR (QPCR) system (Agilent Technologies, Santa Clara, CA). The efficiency-corrected ΔCt for each gene was determined and normalized to Gapdh expression.
Statistics.
Data are expressed as means ± SD. Statistical differences between groups were calculated using a two-tailed Student's t-test, unless indicated otherwise. P < 0.05 was considered significant.
RESULTS
Lung cDCs, but not moDCs, induce Th2 differentiation following HDE exposure.
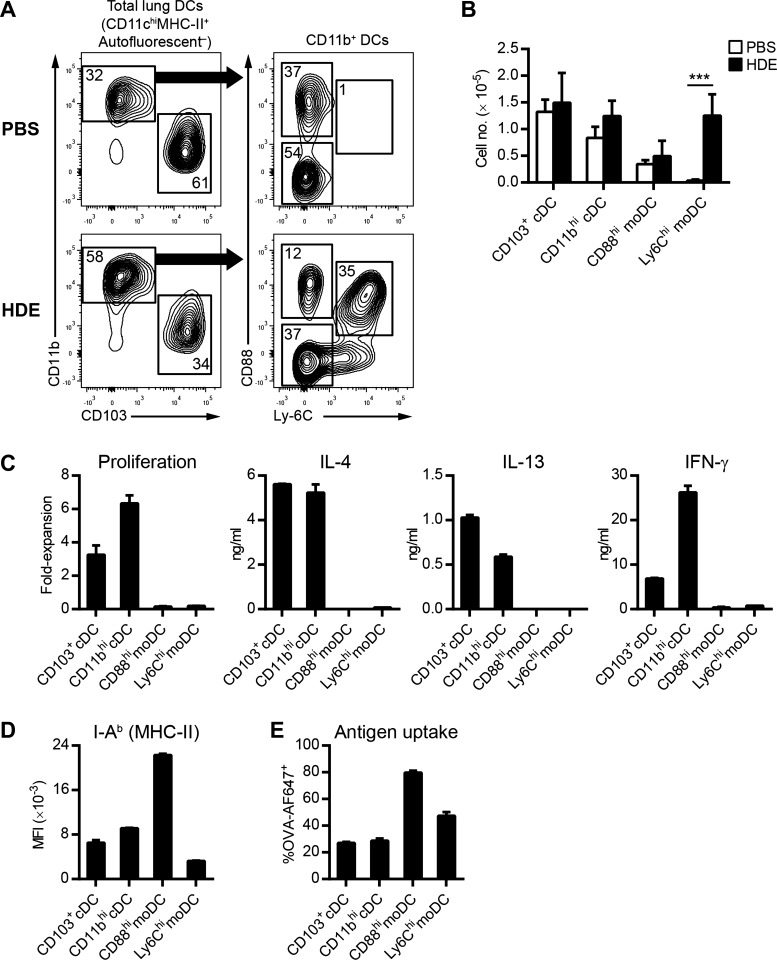
We have previously shown that airway exposure to HDE primes Th2 responses against innocuous inhaled antigens (57). Although both lung cDCs and moDCs have been reported to induce Th2 responses to purified dust mite allergen, the DC subset primarily responsible for priming Th2 responses following HDE inhalation is unknown. We first sought to characterize the DC subsets present in the lungs of HDE-exposed mice. Lung DCs can be divided into four main subsets based on their lineage and surface marker expression: CD11bloCD103hi cDCs (CD103+ cDCs), CD11bhiCD103lo cDCs (CD11bhi cDCs), CD11bhiCD88hiLy6Clo resident moDCs (CD88hi moDCs), and CD11bhiCD88hiLy6Chi inflammatory moDCs (Ly6Chi moDCs) (30, 34). Under steady-state conditions, CD103+ cDCs were the major subset of DCs present in the lungs of C57BL/6 mice, being approximately twofold more abundant than CD11b+ DCs (Fig. 1A). Further analysis of the CD11bhi fraction indicated that the majority were CD11bhi cDCs, with a smaller fraction of CD88hi moDCs (Fig. 1A). As previously reported (27, 31), Ly6Chi moDCs were absent from the lungs under steady-state conditions (Fig. 1A). Following inhalational exposure to HDE, the frequency of CD11b+ DCs increased dramatically because of an influx of Ly6Chi moDCs (Fig. 1A). The frequencies of CD103+ cDCs, CD11bhi cDCs, and CD88hi moDCs in the lungs decreased following HDE exposure (Fig. 1A). However, the absolute numbers of these DC subsets did not significantly change (Fig. 1B), indicating that the decreased frequencies were secondary to an influx of Ly6Chi moDCs and not attributable to cell death or emigration from the lungs.
Fig. 1.
Lung conventional dendritic cells (cDCs), but not monocyte-derived DCs (moDCs), induce T helper 2 (Th2) differentiation following house dust extract (HDE) exposure. A: flow cytometric analysis of lung DC subsets 16 h after airway installation of HDE or PBS. Numbers represent the frequency of cells within each gate. Data are from a single experiment, representative of 4 experiments. B: total number of individual lung DC subsets in C57BL/6 mice following HDE (solid bars) or PBS (open bars) exposure. Bars represent the means ± SD of combined data from 4 experiments. C: Th cell polarization by lung DCs. The indicated lung DC subsets were isolated from C57BL/6 mice by flow cytometry 16 h after instillation of HDE/ovalbumin (OVA) and cultured with naive CD4 T cells prepared from OT-II mice to induce Th cell differentiation. Bars represent the means ± SD of duplicate wells. Data are from 1 experiment, representative of 4 experiments. D: flow cytometric analysis of I-Ab (major histocompatibility complex II, MHC-II) expression on lung DCs following airway installation of HDE. Bars represent the mean fluorescence intensity (MFI) ± SD (n = 3 mice per group). Data are from a single experiment, representative of 2 experiments. E: flow cytometric analysis of lung DCs following airway installation of HDE and fluorescent OVA-AF647 antigen. The graph depicts the percentage of DCs that have taken up OVA-AF647 antigen. Bars represent the means ± SD (n = 3 mice per group). ***P < 0.001.
Having identified the different DC populations present in the lungs of HDE-exposed mice, we next evaluated the ability of each DC subset to prime Th2 responses. HDE was instilled into the airways of C57BL/6 mice together with endotoxin-free OVA, which alone is not allergenic and can therefore be used to test adjuvant activity of HDE. Sixteen hours later, lung DC subsets were isolated, and each subset was cultured with OVA-specific naïve CD4+ T cells to assess Th cell polarization. We found that CD103+ cDCs and CD11b+ cDCs potently induced naïve T-cell proliferation and production of IL-4 and IL-13, consistent with Th2 polarization (Fig. 1C). T cells stimulated by lung cDCs also produced IFN-γ, indicating that HDE exposure induces a mixed Th1/Th2 response. Conversely, neither CD88hi moDCs nor Ly6Chi moDCs induced significant naïve T-cell proliferation or cytokine production (Fig. 1C). Naïve T cells stimulated with either lung cDCs or moDCs did not secrete significant levels of IL-17A (data not shown), suggesting that HDE exposure does not stimulate strong Th17 responses. The inability of moDCs to stimulate naïve T cells was not due to a lack of MHC class II expression (Fig. 1D). Furthermore, both CD88hi and Ly6Chi moDCs readily acquired OVA from the airways, indicating that their inability to stimulate naïve T cells was not due to poor antigen uptake (Fig. 1E). Taken together, these data suggest that lung moDCs are intrinsically poor stimulators of naïve T cells and that lung cDCs are responsible for inducing Th2 polarization following HDE exposure.
HDE efficiently conditions lung cDCs in vivo to prime Th2 responses.
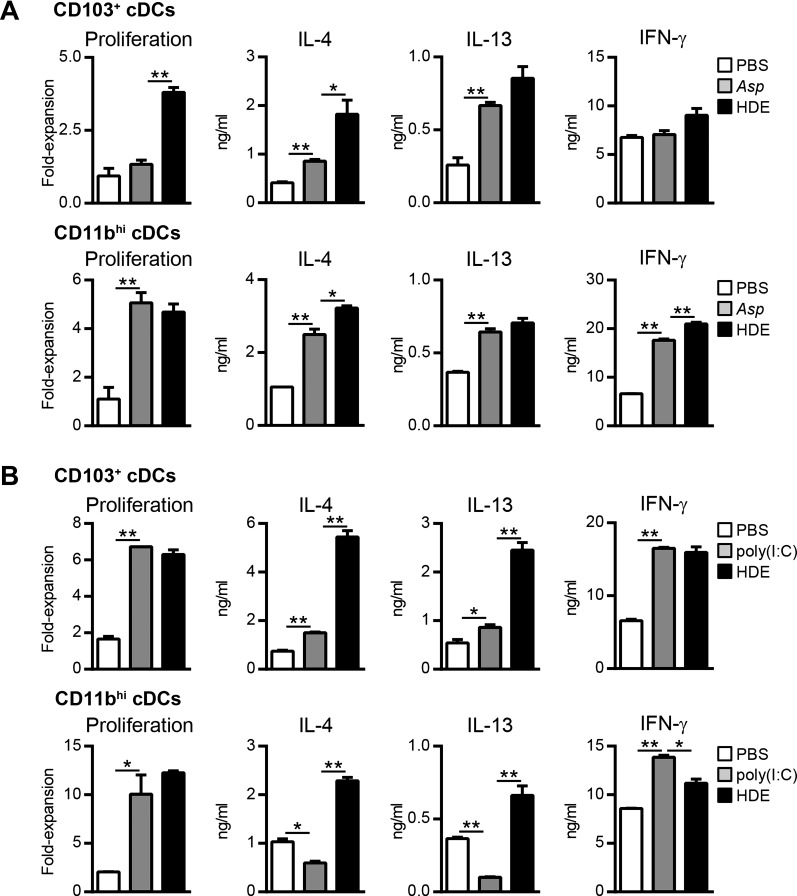
Our data suggested that environmental adjuvants in HDE conditioned lung DCs to promote Th2 responses against an innocuous inhaled antigen. We further investigated the Th2 adjuvant activity of HDE by comparing it to Aspergillus protease (Asp), which has previously been shown to promote Th2 responses against OVA when codelivered to the airways (22). As expected, CD103+ and CD11bhi cDCs from Asp/OVA-treated mice induced significantly higher levels of IL-4 and IL-13 production by OVA-specific T cells compared with cDCs from PBS/OVA-treated mice (Fig. 2A). Lung CD103+ and CD11bhi cDCs from Asp/OVA-treated mice differed in their ability to induce OVA-specific T-cell proliferation and IFN-γ production, suggesting that these DC subsets may be differentially responsive to the adjuvant activity of Asp. Importantly, lung cDCs from HDE/OVA-treated mice induced equivalent if not slightly higher IL-4 and IL-13 production by T cells compared with lung cDCs from Asp/OVA-treated mice (Fig. 2A). Thus the ability of HDE to condition lung DCs to prime Th2 responses was comparable to other known Th2 adjuvants.
Fig. 2.
HDE efficiently conditions lung cDCs in vivo to induce Th2 responses. A: CD103+ cDCs (top) or CD11bhi cDCs (bottom) were prepared from the lungs of C57BL/6 mice following airway installation of OVA and either PBS diluent (open bars), Aspergillus protease (Asp, shaded bars), or HDE (solid bars) and cultured with naïve CD4+ OT-II cells to induce Th cell differentiation. Bars represent the means ± SD of duplicate wells. Data are from 1 experiment, representative of 2 experiments. B: CD103+ cDCs (top) or CD11bhi cDCs (bottom) were prepared from the lungs of C57BL/6 mice following airway installation of OVA and either PBS diluent (open bars), polyinosinic-polycytidylic acid [poly(I:C)] (shaded bars), or HDE (solid bars) and cultured with naïve CD4+ OT-II cells to induce Th cell differentiation. Bars represent the means ± SD of duplicate wells. Data are from 1 experiment, representative of 2 experiments. *P < 0.05, **P < 0.01.
We next investigated whether lung cDCs are intrinsically biased to induce Th2 responses irrespective of the type of adjuvant to which they are exposed. To address this question, we studied DCs prepared from mice that had received airway instillation of OVA together with either HDE or the viral mimetic poly(I:C), which has been reported to promote Th1 responses (49). CD103+ and CD11hi cDCs prepared from HDE/OVA- and poly(I:C)/OVA-treated mice induced equivalent OVA-specific T-cell proliferation (Fig. 2B) compared with cDCs from PBS/OVA-treated control mice, indicating that both stimuli resulted in similar DC maturation and antigen presentation. However, lung cDCs from poly(I:C)/OVA-treated mice induced lower levels of IL-4 and IL-13 by OVA-specific T cells compared with cDCs from HDE/OVA-treated mice (Fig. 2B). Lung cDCs from poly(I:C)/OVA-treated mice did induce T-cell production of IFN-γ, indicating that poly(I:C) possessed Th1 adjuvant activity (Fig. 2B). Taken together, our data indicate that the type of antigen-specific Th responses induced by lung cDCs is not intrinsically programmed but instead is adjuvant dependent.
Endotoxin in HDE is not responsible for conditioning lung cDCs to induce Th2 responses.
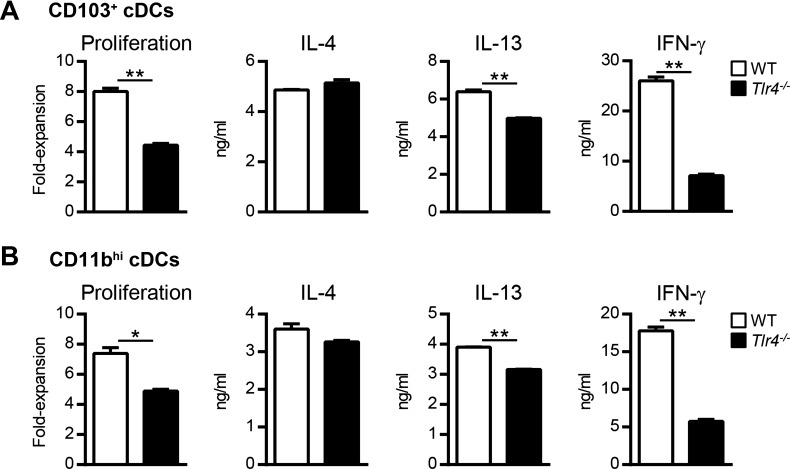
The HDE used in the current experiments has previously been reported to contain low levels of endotoxin (55), which can stimulate innate immune cells via TLR4. Because low-level endotoxin can promote Th2 responses (9, 55), we investigated whether the endotoxin residing in HDE was solely responsible for the observed conditioning of lung cDCs. HDE and OVA were instilled into the airways of wild-type or Tlr4−/− mice, which are unresponsive to endotoxin. CD103+ cDCs from Tlr4−/− mice induced slightly less T-cell proliferation than did their counterparts from wild-type mice (Fig. 3A), suggesting that in vivo TLR4 signaling is necessary for optimal T-cell stimulation. However, on a per-cell basis, T cells cultured with wild-type or Tlr4−/− CD103+ cDCs produced comparable levels of IL-4 (Fig. 3A). Tlr4−/− CD103+ cDCs also induced T cells to produce IL-13, albeit to slightly lower levels compared with wild-type DCs. Similar results were obtained with lung CD11bhi cDCs (Fig. 3B). Compared with wild-type lung cDCs, Tlr4−/− cDCs induced significantly less IFN-γ production by OVA-specific T cells (Fig. 3, A and B), suggesting that the Th1 adjuvant activity of HDE was endotoxin dependent. Together, these findings indicate that endotoxin in HDE is not solely responsible for conditioning lung cDCs to prime Th2 cells.
Fig. 3.
Toll-like receptor 4 (TLR4) signaling is not required for HDE-mediated conditioning of lung cDCs. CD103+ cDCs (A) or CD11bhi cDCs (B) were prepared from the lungs of HDE/OVA-treated wild-type (WT, open bars) or Tlr4−/− mice (solid bars) and cultured with naïve CD4+ OT-II cells to induce Th cell differentiation. Bars represent the means ± SD of duplicate wells. Data are from 1 experiment, representative of 2 experiments. *P < 0.05, **P < 0.01.
HDE exposure triggers the release of endogenous soluble factors that condition cDCs to induce Th2 responses.
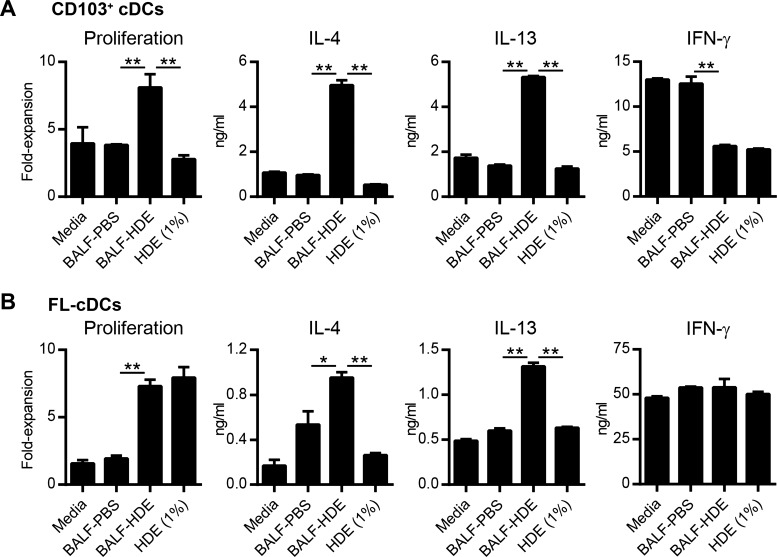
In addition to endotoxin, HDE contains a mixture of indoor allergens, including dust mite, pet dander, and cockroach allergen (55). Inhaled allergens have been reported to stimulate airway epithelial cells, which in turn secrete soluble factors that program DCs to promote Th2 responses (24). We therefore investigated whether inhalational exposure to HDE similarly triggers the release of soluble factors into the airway that condition lung DCs to prime Th2 differentiation. BALF was collected from HDE-treated mice (BALF-HDE) and added to ex vivo cultures of lung CD103+ cDCs and CD11b+ cDCs prepared from naïve mice. Very few CD11b+ cDCs remained viable after 24 h of ex vivo culture, thus precluding further analysis of this cDC subset. However, a significant proportion of CD103+ cDCs survived, and these cells were pulsed with OVA peptide and cultured with naïve OVA-specific T cells. Lung CD103+ cDCs treated with BALF-HDE induced robust T-cell proliferation and Th2 cytokine production compared with cells treated with BALF from PBS-treated mice (BALF-PBS) (Fig. 4A). BALF-HDE-treated CD103+ cDCs also induced less IFN-γ production by OVA-specific T cells compared with CD103+ cDCs treated with BALF-PBS or media alone (Fig. 4A). Although this finding suggested that inhalation of HDE indirectly conditioned DCs by triggering the release of endogenous soluble factors, it was also possible that HDE components remaining in the BALF at the time of harvest might have directly programed DCs to prime Th2 responses. To address this possibility, we directly treated lung CD103+ DCs with 1% vol/vol HDE, the maximum concentration of HDE in BALF at the time of harvest if there had been no clearance of active HDE components from the airway after instillation. These HDE-treated CD103+ cDCs did not induce significant T-cell proliferation or Th2 cytokine production (Fig. 4A), indicating that HDE did not directly condition DCs to induce Th2 responses.
Fig. 4.
HDE exposure indirectly conditions cDCs to induce Th2 responses. A: CD103+ cDCs were prepared from the lungs of untreated mice and cultured with either media alone, bronchoalveolar lavage fluid (BALF) from PBS-treated (BALF-PBS) or HDE-treated (BALF-HDE) mice, or HDE (1% vol/vol). After 24 h, DCs were washed and cultured with naïve CD4+ OT-II cells in the presence of OVA peptide to induce Th cell differentiation. Bars represent the means ± SD of duplicate wells. Data are from 1 experiment, representative of 2 experiments. B: FLT3L-derived cDCs (FL-cDCs) were treated as described in A and cultured with naïve CD4+ OT-II cells in the presence of OVA peptide to induce Th cell differentiation. Bars represent the means ± SD of duplicate wells. Data are from 1 experiment, representative of 3 experiments. *P < 0.05, **P < 0.01.
To confirm that BALF from HDE-treated mice can condition DCs to promote Th2 differentiation, we performed similar experiments using cDCs generated in vitro from BM precursors in the presence of recombinant FLT3L (FL-cDCs). Similar to lung CD103+ cDCs, FL-cDCs treated with BALF-HDE induced robust T-cell proliferation and Th2 differentiation (Fig. 4B). Although FL-cDCs treated directly with HDE did stimulate T-cell proliferation, they failed to induce significant Th2 cytokine production (Fig. 4B). In contrast to lung cDCs, FL-cDCs induced comparable IFN-γ production by OVA-specific T cells, regardless of the treatment conditions (Fig. 4B). Taken together, these findings suggest that HDE exposure triggers cells lining the airways to release soluble factors that condition cDCs to direct Th2 differentiation.
HDE-mediated conditioning of cDCs does not involve IL-25, IL-33, and TSLP.
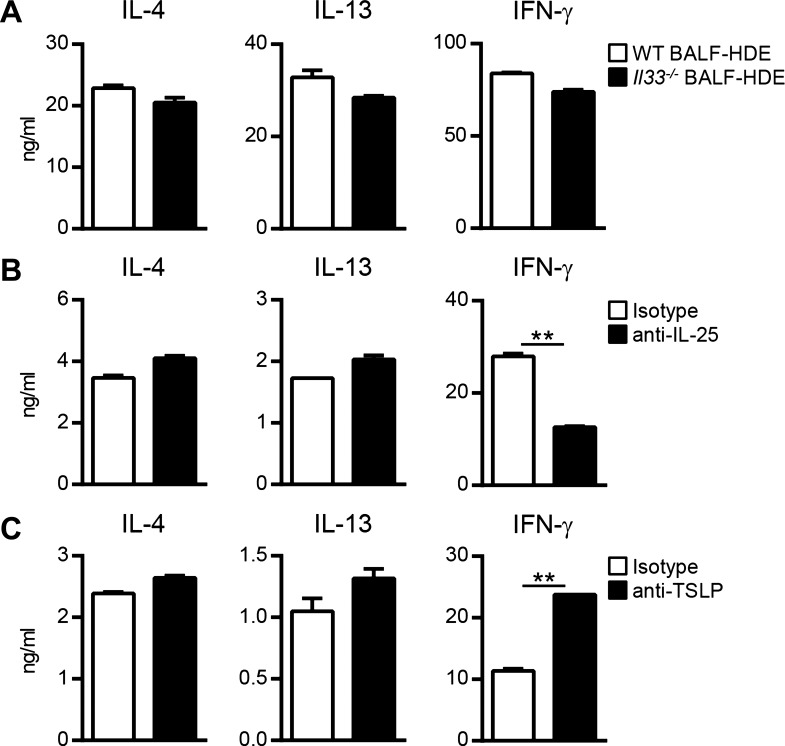
Because BALF-HDE similarly conditioned FL-cDCs and lung CD103+ cDCs to prime Th2 responses, our subsequent mechanistic studies focused on FL-cDCs because of their greater viability during in vitro culture. Allergens and microbial products found in house dust can stimulate airway cells to produce the proallergic cytokines IL-25, IL-33, and TSLP (24, 57), which have been reported to act on DCs and promote Th2 differentiation (5, 6). However, others have reported that these cytokines are either dispensable or not directly involved with conditioning DCs to induce Th2 responses against inhaled allergens (8, 16). We therefore investigated the role of IL-25, IL-33, and TSLP in DC conditioning following HDE exposure. To determine whether IL-33 is required for cDC conditioning, we compared the activities of BALF from HDE-treated Il33−/− or wild-type mice. FL-cDCs treated with BALF-HDE from Il33−/− mice were fully capable of priming Th2 differentiation, indicating that IL-33 is not required for BALF-mediated conditioning of cDCs (Fig. 5A). To determine the requirement of IL-25 and TSLP, FL-cDCs were stimulated for 24 h with BALF-HDE in the presence of neutralizing cytokine-specific or isotype control antibodies. Neutralization of either IL-25 or TSLP did not inhibit the ability of BALF-HDE to condition cDCs to induce IL-4 and IL-13 production by OVA-specific T cells (Fig. 5, B–C). Neutralization of IL-25 resulted in decreased IFN-γ production by T cells stimulated with BALF-HDE-conditioned DCs (Fig. 5B). In contrast, neutralization of TSLP resulted in higher IFN-γ production by T cells stimulated with BALF-HDE-conditioned DCs (Fig. 5C), which is consistent with prior reports that TSLP inhibits the ability of DCs to induce IFN-γ production by T cells (52). Taken together, these results suggest that the canonical Th2-promoting cytokines IL-25, IL-33, and TSLP are dispensable for conditioning DCs to induce Th2 responses following HDE exposure.
Fig. 5.
HDE-mediated conditioning of cDCs does not involve IL-25, IL-33, or thymic stromal lymphopoietin (TSLP). A: FL-cDCs prepared from C57BL/6 mice were treated with BALF-HDE from WT (open bars) or Il33−/− mice (solid bars) for 24 h and then cultured with naïve CD4+ OT-II cells in the presence of OVA peptide to induce Th cell differentiation. Bars represent the means ± SD of duplicate wells. Data are from 1 experiment, representative of 2 experiments. B and C: FL-cDCs were treated with BALF-HDE in the presence of neutralizing anti-IL-25 (B, solid bars), anti-TSLP (C, solid bars), or isotype control antibodies (B and C, open bars) for 24 h and then cultured with naïve CD4+ OT-II cells in the presence of OVA peptide to induce Th cell differentiation. Bars represent the means ± SD of duplicate wells. Data are from 1 experiment, representative of 2 experiments. **P < 0.01.
Activation of cDCs with BALF-HDE results in upregulation of CD80 but low expression of CD40, CD86, and IL-12p40.
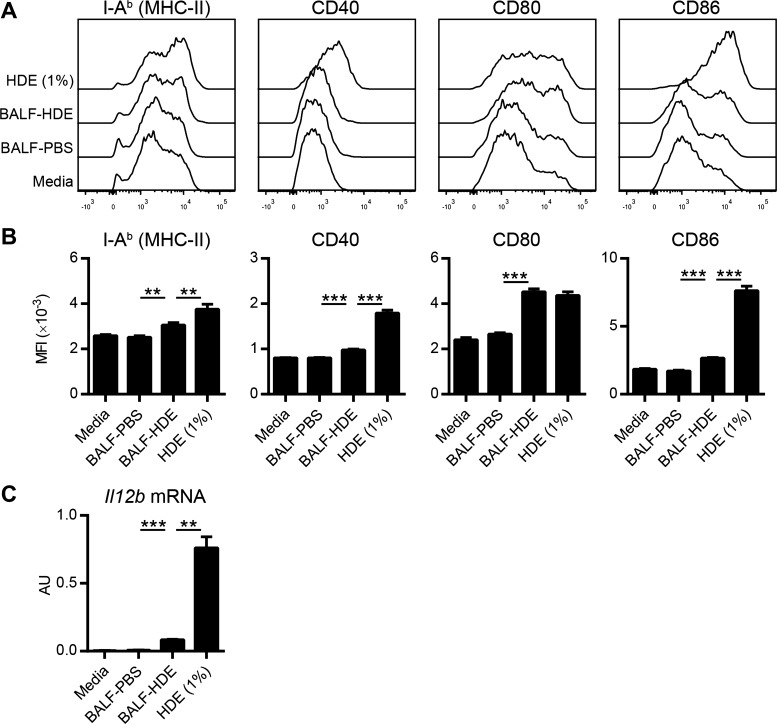
During antigen presentation, the levels of MHC class II and costimulatory molecules displayed by DCs dictate the strength of T-cell receptor signaling, which is an important determinant of Th cell polarization (38). IL-12 production by DCs is also critical for promoting Th1 responses and inhibiting Th2 differentiation (42). We therefore compared expression of these molecules in cDCs treated with either HDE or BALF-HDE. MHC class II molecule (I-Ab) expression was modestly increased by both treatments although more so by direct HDE treatment than by BALF-HDE (Fig. 6, A and B). BALF-HDE treatment of cDCs resulted in strong upregulation of CD80 but only modest induction of CD40 and CD86 compared with DCs treated directly with HDE (Fig. 6, A and B). Furthermore, direct treatment with HDE induced marked expression of IL-12p40 mRNA by cDCs, whereas BALF-HDE did not (Fig. 6C). These data suggest that soluble factors within BALF-HDE lead to a DC activation state characterized by upregulation of CD80 but low expression of CD40, CD86, and IL-12p40, which may favor Th2 differentiation.
Fig. 6.
Activation of cDCs with BALF-HDE results in upregulation of CD80 but low expression of CD40, CD86, and IL-12p40. A–C: FL-cDCs were treated for 24 h with either media, HDE (1% vol/vol), BALF-PBS, or BALF-HDE. A: representative histograms of I-Ab (MHC II) and costimulatory molecule display on FL-cDCs following the indicated treatments. B: MFI of I-Ab or costimulatory molecule expression as determined by flow cytometry. Bars represent the means ± SD of triplicate samples. Data are from 1 experiment, representative of 3 experiments. C: Il12b mRNA expression in treated FL-cDCs was measured by qPCR and normalized to Gapdh expression. Bars represent the means ± SD of duplicate samples. Data are from 1 experiment, representative of 2 experiments. **P < 0.01, ***P < 0.001. AU, arbitrary units.
DISCUSSION
Studies investigating Th2 induction by inhaled allergens have generally involved the delivery of high-dose purified allergen (e.g., dust mite allergen) directly to the airways. Although such models have been helpful for investigating potential mechanisms underlying allergic asthma, they are unlikely to mimic normal human exposures to natural levels of allergens and other immunogenic factors in the living environment. To more accurately simulate exposure to the living environment, we treated mice with sterile HDE, which contains environmentally relevant quantities of indoor allergens and microbial products. In this airway sensitization model, mice are exposed to roughly 5 ng of dust mite allergen, which is consistent with the estimated amount of dust mite allergen that humans inhale during a night of sleep (13). In contrast, studies employing purified dust mite extracts have involved airway delivery of several orders of magnitude higher amounts of allergen (up to 100 μg) (17). Using our environmentally relevant model, we demonstrate that HDE conditions lung cDCs to prime Th2 responses against an innocuous inhaled antigen. In contrast to studies using high doses of purified dust mite allergen, DC conditioning by HDE was independent of TLR4 activity. Similar to models using high-dose allergen challenge, HDE stimulated airway-lining cells to release soluble factors that instructed DCs to promote Th2 responses. However, HDE-mediated conditioning of DCs was independent of the canonical proallergic cytokines IL-25, IL-33, and TSLP, suggesting that other soluble factors are responsible for programming DCs to induce Th2 responses.
Following airway exposure to HDE, we found that lung DCs (but not moDCs) stimulate naïve T cells and instruct them to differentiate into Th2 cells. This observation extends our previous studies demonstrating that CD103+ cDCs stimulate Th2 responses to OVA, dust mite, and cockroach allergens. When resolved from moDCs using differential display of CD88, CD11b+ cDCs also induce Th2 differentiation, in agreement with other reports that CD11b+ cDCs can prime Th2 responses (39, 60). Whether CD103+ cDCs can generally induce Th2 responses is unclear, as some studies reported that CD103+ cDCs are not potent in this regard. Differences in lung DC isolation techniques or antigen dose might account for this apparent discrepancy. It is noteworthy, however, that human lung CD141+ DCs, which are considered the correlate of murine lung CD103+ cDCs, preferentially induce Th2 responses (59). Ultimately, the development of models that allow more specific deletion of lung CD103+ and CD11b+ cDCs without causing inflammation will be necessary to fully delineate the roles of these DC subsets in allergic airway inflammation.
In the present study, we found that CD88hi and Ly6Chi moDCs were unable to prime naïve T cells despite readily acquiring antigen from the airways. Additionally, we previously reported that lung moDCs lack Ccr7 expression and thus do not migrate to lymph nodes (30, 31), which is the primary site for antigen presentation to naïve T cells. Taken together, these findings indicate that lung moDCs are unlikely to be involved with the initiation of allergen-specific Th2 responses. This is consistent with a recent report that moDCs are not required for allergic airway inflammation to dust mite (60). However, other studies have shown moDCs to be necessary for inducing Th2 responses following inhalational exposure to diesel particles or high levels of dust mite allergen (18, 40). The reason for these discordant findings is unclear but may be related to incomplete separation of moDCs and cDCs or model-specific mechanistic differences. By secreting proinflammatory cytokines and presenting antigen to effector T cells, it is possible that moDCs are involved with perpetuating rather than initiating Th2 responses within the lungs. Our findings also bear on the broader question of whether CD88hi and Ly6Chi moDCs are DCs. These cells express CD11c and MHC-II molecules, which are traditionally considered markers for DCs (29), but can also be displayed by macrophages (2). The ability to transport antigen to lymph nodes and efficiently stimulate naïve T cells is also considered a key functional attribute of DCs (3). Given that lung moDCs lack these functional characteristics, it may be more appropriate to refer to them as monocyte-derived cells rather than DCs. This would be in line with a recent proposal of classifying mononuclear phagocytes based on their ontogeny (15). Adopting a standardized nomenclature for the different antigen-presenting cell populations in the lungs will be important for accurately communicating research findings.
How DCs are instructed to promote Th2 responses following allergen exposure remains largely unknown. DCs express several innate immune receptors that have been implicated in allergen recognition, including TLR, Nod-like receptors, and C-type lectin receptors (21). However, innate immune receptors are also expressed by other cells along the respiratory tract, including airway epithelial cells and alveolar macrophages (17, 53). There is mounting evidence that cross talk between DCs and airway-lining cells is required for priming Th2 responses against allergens (24). Consistent with this model, we found that HDE indirectly conditioned DCs to induce Th2 responses. Although DCs directly exposed to HDE underwent maturation, they were poor inducers of Th2 responses. Conversely, DCs treated with BALF from HDE-exposed mice were programmed to efficiently prime Th2 cell differentiation. Treatment of DCs with BALF-HDE resulted in significant upregulation of CD80 but not CD40, CD86, or IL-12p40, which likely favors induction of Th2 cells. DCs expressing high levels of CD80, but not CD86, have previously been shown to promote Th2 differentiation (7). Furthermore, low IL-12 production by DCs is associated with Th2 induction (42). Taken together, these findings strongly suggest that HDE triggers airway-lining cells to release soluble factors, which in turn induce a DC activation state that promotes Th2 differentiation.
Identifying the molecules that program DCs to induce Th2 responses is critical to understand allergic sensitization through the airway and is the focus of considerable investigation. Previous studies have shown that airway instillation of dust mite allergen triggers airway epithelial cells to release the proallergic cytokines IL-25, IL-33, and TSLP, which have been implicated in conditioning DCs to induce Th2 responses (24). For example, TSLP-treated human and murine DCs prime naïve T cells to differentiate into Th2 cells (5, 48). IL-33-activated DCs have also been reported to prime Th2 responses in vitro and to exacerbate allergic lung inflammation in vivo following adoptive transfer (6). IL-25 has been shown to enhance Th2 priming by TSLP-activated DCs, but whether IL-25 is directly responsible for conditioning DCs is unclear (54). Our finding that IL-25, IL-33, and TSLP are dispensable for HDE-mediated conditioning of DCs to prime Th2 cells suggests that low levels of allergen and microbial products present in HDE might induce allergic sensitization through mechanisms distinct from those described in high-dose allergen sensitization models. Our findings also imply that therapeutic targeting of IL-25, IL-33, or TSLP individually may not be sufficient to prevent sensitization to aeroallergens under normal environmental exposure conditions. It is possible that HDE-mediated conditioning of lung DCs is dependent on other known inflammatory cytokines, such as granulocyte macrophage colony-stimulating factor (56), or on a previously unidentified secreted protein. Alternatively, HDE may trigger airway epithelial cells to release endogenous damage-associated molecules, such as uric acid or ATP, which have been shown to promote Th2 induction by DCs (24). A comprehensive analysis of the molecules that differ between BALF-PBS and BALF-HDE will be informative, and this is the focus of ongoing research.
In conclusion, our findings demonstrate that inhalational exposure to environmental adjuvants present in house dust can program pulmonary cDCs to preferentially promote Th2 responses against innocuous antigens. Our results support a model in which immunogenic factors in the living environment trigger airway cells to release soluble mediators, which in turn condition cDCs to prime Th2 differentiation. Identifying the factors responsible for DC conditioning may lead to novel therapeutic targets for asthma and other allergic diseases of the respiratory tract.
GRANTS
This work was supported by the Intramural Research Program of the National Institutes of Health, the National Institute of Environmental Health Sciences (ZIA ES102025-09), and the National Center for Advancing Translational Sciences, National Institutes of Health (1KL2TR001109).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: T.P.M., S.Y.T., D.N.C., and H.N. conception and design of research; T.P.M., K.N., G.S.W., and H.N. performed experiments; T.P.M., K.N., and G.S.W. analyzed data; T.P.M., D.N.C., and H.N. interpreted results of experiments; T.P.M. prepared figures; T.P.M. drafted manuscript; T.P.M., S.Y.T., D.N.C., and H.N. edited and revised manuscript; T.P.M., D.N.C., and H.N. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Maria Sifre and Carl Bortner for help with cell sorting by flow cytometry, the NIEHS Protein Expression Core Facility for producing recombinant FLT3L, Ligon Perrow for support with animal experiments, and Michael Fessler and Steven Kleeberger (NIEHS) for critical reading of the manuscript.
REFERENCES
- 1.Ahluwalia SK, Matsui EC. The indoor environment and its effects on childhood asthma. Curr Opin Allergy Clin Immunol 11: 137–143, 2011. [DOI] [PubMed] [Google Scholar]
- 2.Bain CC, Mowat AM. The monocyte-macrophage axis in the intestine. Cell Immunol 291: 41–48, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature 392: 245–252, 1998. [DOI] [PubMed] [Google Scholar]
- 4.Batzer G, Lam DP, Paulus P, Boasen J, Ng N, Horner AA. Using house dust extracts to understand the immunostimulatory activities of living environments. Immunobiology 212: 491–498, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bell BD, Kitajima M, Larson RP, Stoklasek TA, Dang K, Sakamoto K, Wagner KU, Kaplan DH, Reizis B, Hennighausen L, Ziegler SF. The transcription factor STAT5 is critical in dendritic cells for the development of TH2 but not TH1 responses. Nat Immunol 14: 364–371, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Besnard AG, Togbe D, Guillou N, Erard F, Quesniaux V, Ryffel B. IL-33-activated dendritic cells are critical for allergic airway inflammation. Eur J Immunol 41: 1675–1686, 2011. [DOI] [PubMed] [Google Scholar]
- 7.Cheng X, Wang C, Qian G, Zhu B. CD80, but not CD86 were up-regulated on the spleen-derived dendritic cells from OVA-sensitized and challenged BALB/c mice. Immunol Lett 89: 31–38, 2003. [DOI] [PubMed] [Google Scholar]
- 8.Chu DK, Llop-Guevara A, Walker TD, Flader K, Goncharova S, Boudreau JE, Moore CL, Seunghyun In T, Waserman S, Coyle AJ, Kolbeck R, Humbles AA, Jordana M. IL-33, but not thymic stromal lymphopoietin or IL-25, is central to mite and peanut allergic sensitization. J Allergy Clin Immunol 131: 187–200; e181–e188, 2013. [DOI] [PubMed] [Google Scholar]
- 9.Eisenbarth SC, Piggott DA, Huleatt JW, Visintin I, Herrick CA, Bottomly K. Lipopolysaccharide-enhanced, toll-like receptor 4-dependent T helper cell type 2 responses to inhaled antigen. J Exp Med 196: 1645–1651, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Everts B, Perona-Wright G, Smits HH, Hokke CH, van der Ham AJ, Fitzsimmons CM, Doenhoff MJ, van der Bosch J, Mohrs K, Haas H, Mohrs M, Yazdanbakhsh M, Schramm G. Omega-1, a glycoprotein secreted by Schistosoma mansoni eggs, drives Th2 responses. J Exp Med 206: 1673–1680, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gereda JE, Leung DY, Thatayatikom A, Streib JE, Price MR, Klinnert MD, Liu AH. Relation between house-dust endotoxin exposure, type 1 T-cell development, and allergen sensitisation in infants at high risk of asthma. Lancet 355: 1680–1683, 2000. [DOI] [PubMed] [Google Scholar]
- 12.Ghaemmaghami AM, Gough L, Sewell HF, Shakib F. The proteolytic activity of the major dust mite allergen Der p 1 conditions dendritic cells to produce less interleukin-12: allergen-induced Th2 bias determined at the dendritic cell level. Clin Exp Allergy 32: 1468–1475, 2002. [DOI] [PubMed] [Google Scholar]
- 13.Gore RB, Hadi EA, Craven M, Smillie FI, O'Meara TJ, Tovey ER, Woodcock A, Custovic A. Personal exposure to house dust mite allergen in bed: nasal air sampling and reservoir allergen levels. Clin Exp Allergy 32: 856–859, 2002. [DOI] [PubMed] [Google Scholar]
- 14.Gough L, Sewell HF, Shakib F. The proteolytic activity of the major dust mite allergen Der p 1 enhances the IgE antibody response to a bystander antigen. Clin Exp Allergy 31: 1594–1598, 2001. [DOI] [PubMed] [Google Scholar]
- 15.Guilliams M, Ginhoux F, Jakubzick C, Naik SH, Onai N, Schraml BU, Segura E, Tussiwand R, Yona S. Dendritic cells, monocytes and macrophages: a unified nomenclature based on ontogeny. Nat Rev Immunol 14: 571–578, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Halim TY, Steer CA, Matha L, Gold MJ, Martinez-Gonzalez I, McNagny KM, McKenzie AN, Takei F. Group 2 innate lymphoid cells are critical for the initiation of adaptive T helper 2 cell-mediated allergic lung inflammation. Immunity 40: 425–435, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hammad H, Chieppa M, Perros F, Willart MA, Germain RN, Lambrecht BN. House dust mite allergen induces asthma via Toll-like receptor 4 triggering of airway structural cells. Nat Med 15: 410–416, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hammad H, Plantinga M, Deswarte K, Pouliot P, Willart MA, Kool M, Muskens F, Lambrecht BN. Inflammatory dendritic cells–not basophils–are necessary and sufficient for induction of Th2 immunity to inhaled house dust mite allergen. J Exp Med 207: 2097–2111, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Horner AA. Toll-like receptor ligands and atopy: a coin with at least two sides. J Allergy Clin Immunol 117: 1133–1140, 2006. [DOI] [PubMed] [Google Scholar]
- 20.Huss K, Adkinson NF Jr, Eggleston PA, Dawson C, Van Natta ML, Hamilton RG. House dust mite and cockroach exposure are strong risk factors for positive allergy skin test responses in the Childhood Asthma Management Program. J Allergy Clin Immunol 107: 48–54, 2001. [DOI] [PubMed] [Google Scholar]
- 21.Iwasaki A, Medzhitov R. Control of adaptive immunity by the innate immune system. Nat Immunol 16: 343–353, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kheradmand F, Kiss A, Xu J, Lee SH, Kolattukudy PE, Corry DB. A protease-activated pathway underlying Th cell type 2 activation and allergic lung disease. J Immunol 169: 5904–5911, 2002. [DOI] [PubMed] [Google Scholar]
- 23.Kobayashi T, Iijima K, Radhakrishnan S, Mehta V, Vassallo R, Lawrence CB, Cyong JC, Pease LR, Oguchi K, Kita H. Asthma-related environmental fungus, Alternaria, activates dendritic cells and produces potent Th2 adjuvant activity. J Immunol 182: 2502–2510, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lambrecht BN, Hammad H. Allergens and the airway epithelium response: gateway to allergic sensitization. J Allergy Clin Immunol 134: 499–507, 2014. [DOI] [PubMed] [Google Scholar]
- 25.Lambrecht BN, Hammad H. Lung dendritic cells in respiratory viral infection and asthma: from protection to immunopathology. Annu Rev Immunol 30: 243–270, 2012. [DOI] [PubMed] [Google Scholar]
- 26.Lamhamedi-Cherradi SE, Martin RE, Ito T, Kheradmand F, Corry DB, Liu YJ, Moyle M. Fungal proteases induce Th2 polarization through limited dendritic cell maturation and reduced production of IL-12. J Immunol 180: 6000–6009, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lin KL, Suzuki Y, Nakano H, Ramsburg E, Gunn MD. CCR2+ monocyte-derived dendritic cells and exudate macrophages produce influenza-induced pulmonary immune pathology and mortality. J Immunol 180: 2562–2572, 2008. [DOI] [PubMed] [Google Scholar]
- 28.Martinez FD, Vercelli D. Asthma. Lancet 382: 1360–1372, 2013. [DOI] [PubMed] [Google Scholar]
- 29.Metlay JP, Witmer-Pack MD, Agger R, Crowley MT, Lawless D, Steinman RM. The distinct leukocyte integrins of mouse spleen dendritic cells as identified with new hamster monoclonal antibodies. J Exp Med 171: 1753–1771, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moran TP, Nakano H, Kondilis-Mangum HD, Wade PA, Cook DN. Epigenetic control of Ccr7 expression in distinct lineages of lung dendritic cells. J Immunol 193: 4904–4913, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nakano H, Burgents JE, Nakano K, Whitehead GS, Cheong C, Bortner CD, Cook DN. Migratory properties of pulmonary dendritic cells are determined by their developmental lineage. Mucosal Immunol 6: 678–691, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nakano H, Cook DN. Pulmonary antigen presenting cells: isolation, purification, and culture. Methods Mol Biol 1032: 19–29, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nakano H, Free ME, Whitehead GS, Maruoka S, Wilson RH, Nakano K, Cook DN. Pulmonary CD103(+) dendritic cells prime Th2 responses to inhaled allergens. Mucosal Immunol 5: 53–65, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nakano H, Moran TP, Nakano K, Gerrish KE, Bortner CD, Cook DN. Complement receptor C5aR1/CD88 and dipeptidyl peptidase-4/CD26 define distinct hematopoietic lineages of dendritic cells. J Immunol 194: 3808–3819, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ng N, Lam D, Paulus P, Batzer G, Horner AA. House dust extracts have both TH2 adjuvant and tolerogenic activities. J Allergy Clin Immunol 117: 1074–1081, 2006. [DOI] [PubMed] [Google Scholar]
- 36.Ohba T, Haro H, Ando T, Koyama K, Hatsushika K, Suenaga F, Ohnuma Y, Nakamura Y, Katoh R, Ogawa H, Hamada Y, Nakao A. A potential role of thymic stromal lymphopoietin in the recruitment of macrophages to mouse intervertebral disc cells via monocyte chemotactic protein 1 induction: implications for herniated discs. Arthritis Rheum 58: 3510–3519, 2008. [DOI] [PubMed] [Google Scholar]
- 37.Panettieri RA Jr, Covar R, Grant E, Hillyer EV, Bacharier L. Natural history of asthma: persistence versus progression-does the beginning predict the end? J Allergy Clin Immunol 121: 607–613, 2008. [DOI] [PubMed] [Google Scholar]
- 38.Paul WE, Zhu J. How are T(H)2-type immune responses initiated and amplified? Nat Rev Immunol 10: 225–235, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Plantinga M, Guilliams M, Vanheerswynghels M, Deswarte K, Branco-Madeira F, Toussaint W, Vanhoutte L, Neyt K, Killeen N, Malissen B, Hammad H, Lambrecht BN. Conventional and monocyte-derived CD11b(+) dendritic cells initiate and maintain T helper 2 cell-mediated immunity to house dust mite allergen. Immunity 38: 322–335, 2013. [DOI] [PubMed] [Google Scholar]
- 40.Provoost S, Maes T, Joos GF, Tournoy KG. Monocyte-derived dendritic cell recruitment and allergic T(H)2 responses after exposure to diesel particles are CCR2 dependent. J Allergy Clin Immunol 129: 483–491, 2012. [DOI] [PubMed] [Google Scholar]
- 41.Pulendran B, Artis D. New paradigms in type 2 immunity. Science 337: 431–435, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pulendran B, Tang H, Manicassamy S. Programming dendritic cells to induce T(H)2 and tolerogenic responses. Nat Immunol 11: 647–655, 2010. [DOI] [PubMed] [Google Scholar]
- 43.Sakaguchi M, Inouye S, Sasaki R, Hashimoto M, Kobayashi C, Yasueda H. Measurement of airborne mite allergen exposure in individual subjects. J Allergy Clin Immunol 97: 1040–1044, 1996. [DOI] [PubMed] [Google Scholar]
- 44.Satpathy AT, Wu X, Albring JC, Murphy KM. Re(de)fining the dendritic cell lineage. Nat Immunol 13: 1145–1154, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schlitzer A, McGovern N, Teo P, Zelante T, Atarashi K, Low D, Ho AW, See P, Shin A, Wasan PS, Hoeffel G, Malleret B, Heiseke A, Chew S, Jardine L, Purvis HA, Hilkens CM, Tam J, Poidinger M, Stanley ER, Krug AB, Renia L, Sivasankar B, Ng LG, Collin M, Ricciardi-Castagnoli P, Honda K, Haniffa M, Ginhoux F. IRF4 transcription factor-dependent CD11b+ dendritic cells in human and mouse control mucosal IL-17 cytokine responses. Immunity 38: 970–983, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sever ML, Arbes SJ Jr, Gore JC, Santangelo RG, Vaughn B, Mitchell H, Schal C, Zeldin DC. Cockroach allergen reduction by cockroach control alone in low-income urban homes: a randomized control trial. J Allergy Clin Immunol 120: 849–855, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shreffler WG, Castro RR, Kucuk ZY, Charlop-Powers Z, Grishina G, Yoo S, Burks AW, Sampson HA. The major glycoprotein allergen from Arachis hypogaea, Ara h 1, is a ligand of dendritic cell-specific ICAM-grabbing nonintegrin and acts as a Th2 adjuvant in vitro. J Immunol 177: 3677–3685, 2006. [DOI] [PubMed] [Google Scholar]
- 48.Soumelis V, Reche PA, Kanzler H, Yuan W, Edward G, Homey B, Gilliet M, Ho S, Antonenko S, Lauerma A, Smith K, Gorman D, Zurawski S, Abrams J, Menon S, McClanahan T, de Waal-Malefyt Rd R, Bazan F, Kastelein RA, Liu YJ. Human epithelial cells trigger dendritic cell mediated allergic inflammation by producing TSLP. Nat Immunol 3: 673–680, 2002. [DOI] [PubMed] [Google Scholar]
- 49.Stahl-Hennig C, Eisenblatter M, Jasny E, Rzehak T, Tenner-Racz K, Trumpfheller C, Salazar AM, Uberla K, Nieto K, Kleinschmidt J, Schulte R, Gissmann L, Muller M, Sacher A, Racz P, Steinman RM, Uguccioni M, Ignatius R. Synthetic double-stranded RNAs are adjuvants for the induction of T helper 1 and humoral immune responses to human papillomavirus in rhesus macaques. PLoS Pathog 5: e1000373, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Steinfelder S, Andersen JF, Cannons JL, Feng CG, Joshi M, Dwyer D, Caspar P, Schwartzberg PL, Sher A, Jankovic D. The major component in Schistosome eggs responsible for conditioning dendritic cells for Th2 polarization is a T2 ribonuclease (omega-1). J Exp Med 206: 1681–1690, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sung SS, Fu SM, Rose CE Jr, Gaskin F, Ju ST, Beaty SR. A major lung CD103 (alphaE)-beta7 integrin-positive epithelial dendritic cell population expressing Langerin and tight junction proteins. J Immunol 176: 2161–2172, 2006. [DOI] [PubMed] [Google Scholar]
- 52.Taylor BC, Zaph C, Troy AE, Du Y, Guild KJ, Comeau MR, Artis D. TSLP regulates intestinal immunity and inflammation in mouse models of helminth infection and colitis. J Exp Med 206: 655–667, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Taylor PR, Martinez-Pomares L, Stacey M, Lin HH, Brown GD, Gordon S. Macrophage receptors and immune recognition. Annu Rev Immunol 23: 901–944, 2005. [DOI] [PubMed] [Google Scholar]
- 54.Wang YH, Angkasekwinai P, Lu N, Voo KS, Arima K, Hanabuchi S, Hippe A, Corrigan CJ, Dong C, Homey B, Yao Z, Ying S, Huston DP, Liu YJ. IL-25 augments type 2 immune responses by enhancing the expansion and functions of TSLP-DC-activated Th2 memory cells. J Exp Med 204: 1837–1847, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Whitehead GS, Thomas SY, Cook DN. Modulation of distinct asthmatic phenotypes in mice by dose-dependent inhalation of microbial products. Environ Health Perspect 122: 34–42, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Willart MA, Deswarte K, Pouliot P, Braun H, Beyaert R, Lambrecht BN, Hammad H. Interleukin-1alpha controls allergic sensitization to inhaled house dust mite via the epithelial release of GM-CSF and IL-33. J Exp Med 209: 1505–1517, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wilson RH, Maruoka S, Whitehead GS, Foley JF, Flake GP, Sever ML, Zeldin DC, Kraft M, Garantziotis S, Nakano H, Cook DN. The Toll-like receptor 5 ligand flagellin promotes asthma by priming allergic responses to indoor allergens. Nat Med 18: 1705–1710, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wilson RH, Whitehead GS, Nakano H, Free ME, Kolls JK, Cook DN. Allergic sensitization through the airway primes Th17-dependent neutrophilia and airway hyperresponsiveness. Am J Respir Crit Care Med 180: 720–730, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yu CI, Becker C, Metang P, Marches F, Wang Y, Toshiyuki H, Banchereau J, Merad M, Palucka AK. Human CD141+ dendritic cells induce CD4+ T cells to produce type 2 cytokines. J Immunol 193: 4335–4343, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhou Q, Ho AW, Schlitzer A, Tang Y, Wong KH, Wong FH, Chua YL, Angeli V, Mortellaro A, Ginhoux F, Kemeny DM. GM-CSF-licensed CD11b+ lung dendritic cells orchestrate Th2 immunity to Blomia tropicalis. J Immunol 193: 496–509, 2014. [DOI] [PubMed] [Google Scholar]