Abstract
Sex differences in the incidence of respiratory diseases have been reported. Women are more susceptible to inflammatory lung disease induced by air pollution and show worse adverse pulmonary health outcomes than men. However, the mechanisms underlying these differences remain unknown. In the present study, we hypothesized that sex differences in the expression of lung inflammatory mediators affect sex-specific immune responses to environmental toxicants. We focused on the effects of ground-level ozone, a major air pollutant, in the expression and regulation of lung immunity genes. We exposed adult male and female mice to 2 ppm of ozone or filtered air (control) for 3 h. We compared mRNA levels of 84 inflammatory genes in lungs harvested 4 h postexposure using a PCR array. We also evaluated changes in lung histology and bronchoalveolar lavage fluid cell counts and protein content at 24 and 72 h postexposure. Our results revealed sex differences in lung inflammation triggered by ozone exposure and in the expression of genes involved in acute phase and inflammatory responses. Major sex differences were found in the expression of neutrophil-attracting chemokines (Ccl20, Cxcl5, and Cxcl2), the proinflammatory cytokine interleukin-6, and oxidative stress-related enzymes (Ptgs2, Nos2). In addition, the phosphorylation of STAT3, known to mediate IL-6-related immune responses, was significantly higher in ozone-exposed mice. Together, our observations suggest that a differential regulation of the lung immune response could be implicated in the observed increased susceptibility to adverse health effects from ozone observed in women vs. men.
Keywords: inflammation, oxidative stress, lung disease, STAT3, macrophage inflammatory proteins
ground-level ozone, a photochemical air pollutant and powerful oxidant, can trigger a variety of detrimental health effects, including respiratory and cardiovascular complications (32, 44). Population studies have shown that short-term exposures to ozone at concentrations within the Environmental Protection Agency standards can affect breathing and lung function, impair pulmonary innate immunity, and damage the entire respiratory epithelium (8, 38, 49, 62–64). Exposure to ozone causes complications in patients with respiratory infection, asthma, chronic obstructive pulmonary disease, cystic fibrosis, lung cancer, and cardiovascular disease, with worse effects and higher mortality in women than in men (24, 55, 59, 65). Furthermore, studies in animal models have demonstrated that ozone increases airway neutrophil recruitment, contributing to acute lung injury and hyperreactivity and promoting inflammatory lung disease (7, 15, 30, 37).
Epidemiological studies have shown correlations of high ambient ozone levels with increased hospitalizations for respiratory illnesses and mortality (11, 19, 32, 57). Ozone inhalation can trigger asthma and cause exacerbations in susceptible individuals (13, 49, 73). In this regard, clinical studies have reported sex differences in lung function and in the risk, incidence, and severity of environmental lung disease (13, 18, 21). These studies have shown that women are more susceptible and suffer worse complications of lung disease than men (5, 47, 66). Emerging data have indicated that circulating hormone levels may regulate innate immune responses and affect airway tone and inflammation during the menstrual cycle in female asthmatic patients, but the specific roles of hormones and the mechanisms involved are complex and not completely understood (20, 46, 53, 56, 60, 72). In a previous study, we have demonstrated that exposure to ozone significantly decreased survival of male and female mice after bacterial infection (53). Infected females exposed to ozone also showed more pronounced lung inflammation, lower macrophage function, and higher mortality rates than males (52). Additional work demonstrated that sex steroid hormones were responsible for the observed sex differences, indicating that both sex and air pollution may alter the effectiveness of lung host defense (20).
Alveolar macrophages and lung epithelial cells constitute the first line of defense against inhaled toxic compounds and have been suggested to initiate a cascade of inflammatory reactions upon ozone exposure. These inflammatory responses have been suggested as central processes in ozone-induced health effects (3, 16). The specific mechanisms of ozone toxicity appear to be related to oxidation of cell membranes and surfactant in the lung epithelium, resulting in lipid peroxidation, and the production of reactive oxygen species (17). The resulting oxidation products can prime resident alveolar macrophages, inducing the production of proinflammatory cytokines and chemokines that promote the recruitment of neutrophils into the lung. An excessive inflammatory response can impair the pulmonary host defense by damaging the lung epithelium, by decreasing the function of innate immunity molecules, and by reducing the phagocytic ability of resident macrophages (3, 43, 51). However, the molecular pathways that determine changes in gene expression after ozone exposure and the mechanisms behind sex differences in susceptibility and severity of pulmonary disease are still largely unknown. In this work, we investigated sex differences in the lung innate immune response, by using PCR arrays to compare the expression of inflammatory genes in the lungs of male and female mice exposed to ozone. We found sex differences in lung gene expression of cytokines, chemokines, and oxidative stress-related enzymes in filtered air-exposed animals and in response to acute ozone exposure. We also identified a potential regulatory mechanism that involves STAT3 phosphorylation. Together, our observations indicate that a differential regulation of the lung immune response could be implicated in the observed increased susceptibility to adverse health effects from ozone in women vs. men.
MATERIALS AND METHODS
Animals.
Adult male and female C57BL/6J mice (8 wk of age) were purchased from The Jackson Laboratory (stock number 000664, Bar Harbor, ME), housed and maintained in a 12:12-h light-dark cycle, with food and water available ad libitum. We chose this strain because susceptibility to ozone exposure has been shown to be strain dependent. The C57BL/6 mouse has been extensively used for ozone-induced inflammation/injury models, since this strain is highly susceptible to ozone vs. other strains (41, 42). The Pennsylvania State University College of Medicine Institutional Animal Care and Use Committee approved all procedures.
Exposure to ozone.
Male and female mice were exposed to 2 ppm of ozone or filtered air (control) for 3 h, in different chambers as described previously (25, 53, 54). Animals were euthanized under anesthesia 4 h postexposure, and lung tissue was collected for gene expression analyses. For mRNA array analysis, six adult male and six adult female mice were used per condition. For real-time PCR replication studies and Western blot experiments, 10 animals were used per group. The animal model used in this study involves a much higher concentration of ozone than would normally be found in the atmosphere [an environmental concentration of ozone above 0.3 ppm is considered extremely high and has been shown to affect pulmonary function in humans (50, 68)]. We chose this approach because previous studies have shown that higher ozone doses are required for rodents vs. humans to reach comparable amounts of ozone concentration in the distal lung (26). In addition, rodents acutely exposed to 2 ppm of ozone display comparable or lower levels of various bronchoalveolar lavage (BAL) parameters than exercising humans with considerable lower acute ozone exposures (0.4 ppm, the equivalent of an extremely high atmospheric concentration) (27).
Lung histology.
A group of male and female mice (n = 4/group) were exposed to 2 ppm of ozone or filtered air as described above. At 24 and 72 h following exposure, mice were euthanized under anesthesia, and lungs were infused through the trachea with 4% paraformaldehyde (PFA). Whole lung tissues were immersion fixed in PFA, and the right and left lung lobes were bisected in a parasagittal plane for sectioning. Tissues were processed in an automated Tissue-Tek VIP processor (Sakura Finetek USA, Torrance, CA) and paraffin embedded by using a Tissue-Tek TEC embedding station. Sections were cut at 6 μm for routine hematoxylin and eosin and Masson's trichrome staining. Images were captured with an Olympus BX51 microscope (Olympus America, Center Valley, PA) and DP71 digital camera using the CellSens Standard 1.12 imaging software. All tissues were examined by an American College of Veterinary Pathologists diplomate and two additional investigators blinded to treatments. Percentages of the areas affected were visually estimated, and the lung sections affected by inflammation and/or fibrosis were semiquantitatively scored by use of modified protocols (6, 48).
BAL analysis.
The lungs of a separate group of male and female mice exposed to ozone or filtered air (n = 6/group) were lavaged with 2.5 ml of PBS (GIBCO, catalog no. 14190-144) supplemented with 1 mM EDTA at 24 and 72 h postexposure, under standard protocols (4). The volume of recovered BAL was recorded, and the total number of cells in BAL was estimated by use of a hematocytometer. Cytospins were prepared for ∼50,000 cells per slide by using a cytocentrifuge. The cytospun cells were air dried, stained with a Hema-3 stain kit (Fisher Scientific, Pittsburgh, PA) and coverslipped. Slides were analyzed under light microscopy for the presence of macrophages, neutrophils, and lymphocytes, by three independent investigators who were blind to the treatments.
Albumin determination in BAL.
The remainder BAL fluid was centrifuged at 150 g for 5 min at 4°C, and supernatants were immediately frozen at −80°C. Total BAL protein determinations were performed by the BCA assay (Thermo, Rockford, IL). BAL samples (0.5 ml) were lyophilized and resuspended in 66 μl of water. Two microliters of BAL solution were loaded onto 4–15% polyacrylamide gels and analyzed by SDS-PAGE. Gels were transferred onto nitrocellulose membranes that were blocked overnight with 5% BSA solution. Membranes were incubated for 2 h at room temperature with a rat anti-albumin antibody (Cappel, MP Biomedicals, Santa Ana, CA), diluted 1:25,000 in PBS containing 0.1% Tween and 1% BSA, and for 1 h with a horseradish peroxidase-conjugated goat anti-rabbit secondary antibody (Bio-Rad, Hercules, CA), diluted 1:25,000 in PBS containing 0.1% Tween and 1% BSA. Bands were detected with enhanced chemiluminescence substrate (PerkinElmer, Waltham, MA) according to the manufacturer's instructions.
RNA purification.
Lung tissue was pulverized and homogenized in TRIzol (Life Technologies, Carlsbad, CA). RNA was extracted with the Direct-zol RNA MiniPrep (Zymo Research, Irvine, CA) and quantified by Nanodrop. RNA quality was verified with a Bioanalyzer 2100 at the Penn State Hershey Genome Sciences Core Facility.
mRNA arrays.
A total of 400 ng of purified RNA were retrotranscribed with the RT2 First Strand Kit (Qiagen, Germantown, MD). The expression of 84 genes related to inflammatory immune responses was evaluated with the Mouse Inflammatory Response and Autoimmunity PCR Array (Qiagen). A list of the array genes can be accessed at http://www.sabiosciences.com/genetable.php?pcatn=PAMM-077A. PCR Arrays were amplified with the ABI QuantStudio 12K Flex system (Life Technologies) at the Penn State Hershey Genome Sciences Core Facility. Results were analyzed online with the GeneGlobe Data Analysis Center (http://www.qiagen.com/us/products/genes%20and%20pathways/data-analysis-center-overview-page/) and normalized to a set of five endogenous controls (β-actin, β2-microglobulin, GAPDH, β-glucuronidase, and Hsp90) included in the commercial array, by the arithmetic mean method. Each array plate also contained three negative reverse transcription controls, a mouse genomic DNA contamination control, and three positive PCR controls for interassay comparisons. Heat maps were generated with the web-based program of RT2 profiler PCR Array Data Analysis (Qiagen), by using gene expression data calculated with the 2−ΔΔCT method (45). Fold changes were calculated as the normalized gene expression 2−ΔΔCT in the experimental group sample, divided by the normalized gene expression 2−ΔΔCT in the control group sample (males exposed to filtered air). Fold changes were used to express data as fold regulation vs. control, where fold regulation values greater than 1 indicate positive or upregulation and values of less than 1 indicate negative or downregulation. We arbitrarily selected a 1.5-fold regulation cutoff for our analyses. The P values were calculated based on a Student's t-test of the replicate 2−ΔΔCT values for each gene in the control group and treatment groups.
Real-time PCR.
For individual gene expression assays, total RNA was treated with the Ambion DNA-free kit (Life Technologies), and 600 ng of DNA-free RNA were retrotranscribed by use of the High Capacity cDNA reverse transcription kit (Life Technologies). The expression levels of Ccl20/MIP3α (assay no. Mm01268754), Il6/interleukin-6 (assay no. Mm00446190), Cxcl2/MIP-2α (assay no. Mm00436450), Ccl2/MCP-1 (assay no. Mm00441242), Ccl3/MIP1α (assay no. Mm00441259), Ccl5/RANTES (assay no. Mm01302427), Il1a/interleukin-1α (assay no. Mm00439620), Il1b/interleukin-1β (assay no. Mm00434228), Tnf (assay no. Mm00443260), Tlr4 (assay no. Mm00445273), Il1r1 (assay no. Mm00434237), Stat3 (assay no. Mm01219775), and Cebpb-CCAAT/enhancer-binding protein-β (assay no. Mm00843434) were measured with real-time PCR with TaqMan assays (Life Technologies) and normalized to 18s rRNA expression (Rn18s, mouse 18s rRNA TaqMan Assay, no. Mm03928990) by the relative quantification method (45). Assays were amplified at the Penn State Hershey Genome Sciences Core Facility. Results were analyzed with the QuantStudio 12K Flex Software (Applied Biosystems), by the 2−ΔΔCT method (45).
Protein extraction and Western blot.
RIPA buffer (Thermo) was used to extract protein from pulverized lung tissues, following the manufacturer's protocol. Protein concentration was determined by BCA assay (Thermo), and 20 μg were used for Western blot analysis with the following antibodies: STAT3 (AB68153), pSTAT3-Y705 (AB76315), pSTAT3-S727 (AB86430), and GAPDH (AB9485) as a loading control. All antibodies were purchased from Abcam (Cambridge, MA). For densitometric quantitation of Western blots, images were digitized by use of Bio-Rad GS800 calibrated densitometer. The relative band intensities of the Western blot results were quantified by densitometry using Bio-Rad Quantity One analysis software. Results were analyzed with GraphPad Prism 6 software.
Ingenuity Pathway Analysis.
Functional analyses of differentially expressed genes among experimental groups were performed with Ingenuity Pathway Analysis (IPA, Qiagen Redwood City, www.qiagen.com/ingenuity). This online software analyzes gene expression data in the context of known biological response and regulatory networks. We performed IPA functional analyses in genes that met the cutoff (1.5-fold up- or downregulation vs. control group) and evaluated associations with biological functions via the Ingenuity Pathways Knowledge Base. First, we compared the top canonical pathways associated with gene expression changes. For this analysis, the IPA software uses a right-tailed Fisher's exact test to obtain a P value that determines the probability of association of the dataset to each canonical pathway or function vs. random chance. Next, we obtained the most significant molecular and cellular function classifications associated with each dataset, along with the respective number of molecules and corresponding range of P values. Finally, an additional functional network analysis was performed for the top associated network functions identified by IPA. In these, genes are represented as nodes, and the biological relationships between nodes are represented by lines. All connections are supported by at least one published reference or from canonical information stored in the Ingenuity Pathways Knowledge Base. For these analyses, the software uses a Fisher's exact test to calculate a P value determining the probability that each network associated to the dataset was due to random chance alone, and it assigns a score to the pathway (a score higher than 2 has at least a 99% confidence of not being generated by random chance).
Statistical analysis.
Array data was analyzed with the manufacturer's online tool (http://www.qiagen.com/us/products/genes%20and%20pathways/data-analysis-center-overview-page/), and Student's t-test analyses were obtained for each group comparison. Real-time PCR data are presented as means ± SE. Statistical analyses for lung scoring, BAL cell counts, BAL protein determinations, real-time PCR, and Western blot experiments were performed with the GraphPad Prism 6 software. Differences among treatment groups were analyzed by one-way ANOVA followed by Tukey's post hoc analysis for lung scoring, BAL protein, and gene expression experiments, and Kruskal-Wallis one-way analysis of variance with Dunn's post hoc test for cell counts and Western blot experiments. Values of P < 0.05 were considered to be statistically significant.
RESULTS
Sex differences in ozone-induced lung inflammation.
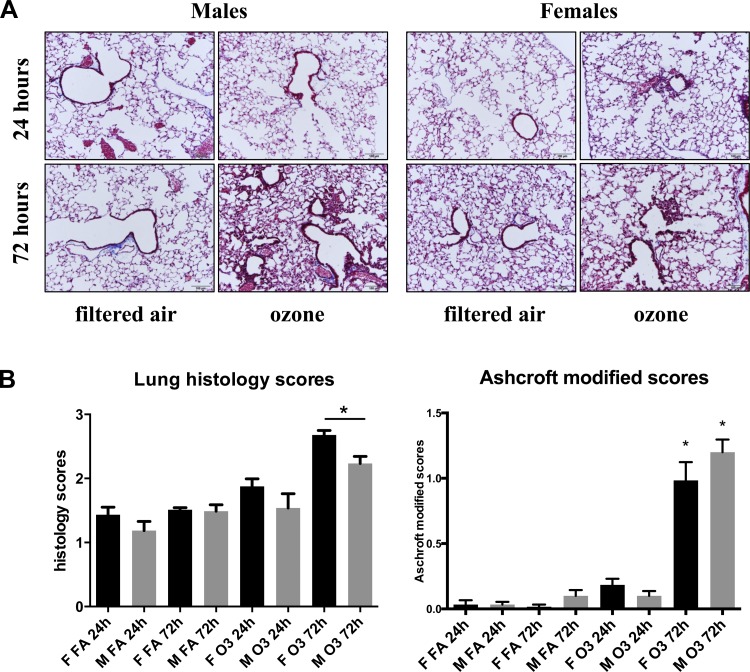
Morphological assessment of lung injury was performed by histological evaluation at 24 and 72 h following exposure to ozone (2 ppm, 3 h) or filtered air (3 h) (Fig. 1A). This analysis revealed lung lesions and mild increased inflammation in response to ozone, as indicated by epithelial thickening, type II pneumocyte hyperplasia, and moderate emphysema. Quantification of these changes indicated significant differences between animals exposed to ozone vs. filtered air at both 24 and 72 h following exposure (Fig. 1B). When lung inflammation scores were compared between male and female mice, significant differences were found at 72 h following ozone exposure, with females displaying higher scores than males (Fig. 1B, left). We also identified expansion of septal interstitia by fibroblasts in a scant collagenous matrix (nascent fibroplasia) in ∼50% of respiratory bronchioles, alveolar ducts, and adjacent alveoli of animals exposed to ozone. We performed a Masson's trichrome stain followed by a modified Ashcroft scale analysis to quantify lung fibrosis in response to ozone (Fig. 1B, right). Using the modified scores for mild fibroplasia, we found significant differences in lungs harvested 72 h after ozone exposure vs. filtered air, although no sex differences were identified.
Fig. 1.
Histological evaluation of male and female lungs following acute ozone exposure. A: Masson's trichrome staining of lung tissues obtained at 24 and 72 h postexposure to filtered air (3 h) or ozone (2 ppm, 3 h). B, left: quantification of lung inflammation by 3 independent observers expressed as means ± SE (n = 4; *P < 0.05). Scale: 0, none; 1, mild emphysema and/or epithelium thickening; 2, moderate emphysema and/or epithelium thickening; 3, moderate to severe emphysema and epithelium thickening; 4, severe emphysema and epithelium thickening with excess blood in airways; 5, degradation of epithelium with inflammatory agents in airways. Right: modified Ashcroft score for quantification of lung fibroplasia (n = 4; *P < 0.05).
Sex differences in lung vascular permeability and PMN content in response to ozone exposure.
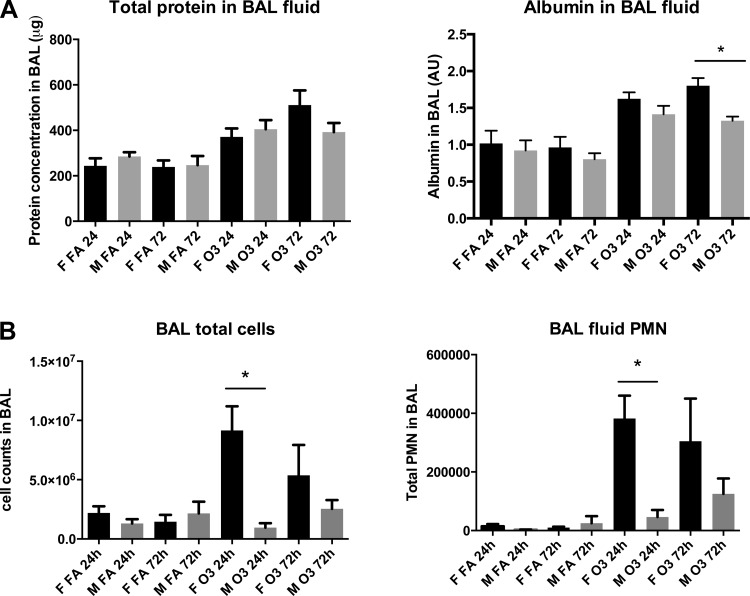
Lung injury in response to ozone was evaluated by albumin leakage into the alveolar compartment. Figure 2A shows total protein content and BAL albumin content at 24 and 72 h postexposure to ozone or filtered air. We found significantly higher BAL albumin levels in females than in males at 72 h postexposure to ozone. In addition, females displayed significantly higher total cell counts and polymorphonuclear neutrophil (PMN) number than males only at 24 h following ozone exposure (Fig. 2B). An increase in alveolar macrophage counts, as well as the presence of binucleated macrophages in BAL, was also noted in response to ozone in females at 24 and 72 h postexposure and in males at 72 h postexposure (data not shown).
Fig. 2.
Bronchoalveolar lavage (BAL) fluid total protein, albumin leakage, and cell counts. A: mouse total BAL (2.5 ml) protein levels, measured by BCA assay at 24 and 72 h postexposure to ozone or filtered air, and expressed as means ± SE. BAL albumin levels were estimated by Western blot and are expressed as arbitrary units (AU) based on densitometric analysis of 4 independent experiments (*P < 0.05, n = 6). B: mouse BAL (2.5 ml) total cell counts and polymorphonuclear neutrophil (PMN) content were measured as an index of PMN influx at 24 and 72 h postexposure to ozone or filtered air and expressed as means ± SE of 6 mice per group (*P < 0.05).
Differential inflammatory gene expression patterns in male and female mice exposed to ozone or filtered air.
We used a PCR array to evaluate the mRNA expression of 84 immune-related genes in the lungs of male and female mice exposed to 2 ppm of ozone or filtered air for 3 h. The array detected expression of 72 genes in all samples. The expression levels of the remaining 12 genes (C3, Cxcl11, Crp, Il10, Il10rb, Il9, Il22, Il17a, Itgb2, Kng1, Nr3c1, and Tollip) were outside of the detection range in all experimental groups; therefore these were excluded from the analysis. The mRNA expression of the 72 detected genes was compared among groups with the Qiagen online tool, as described in materials and methods. Cluster analysis revealed specific gene expression patterns for each experimental group, with particular groups of genes up- or downregulated in animals exposed to filtered air or in response to ozone in both sexes (Fig. 3).
Fig. 3.
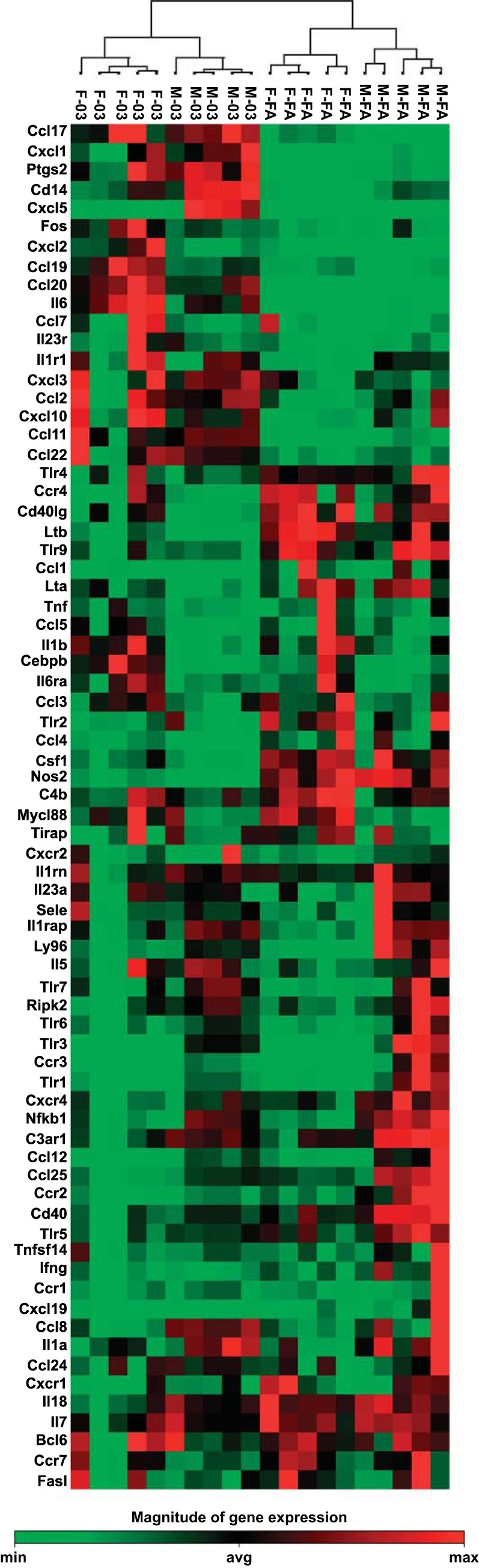
Inflammatory gene expression in males and females exposed to ozone vs. filtered air. Cluster analysis of 72 inflammatory genes in lung extracts from male and female mice exposed to 2 ppm of ozone or filtered air for 3 h (n = 5/group). Total RNA was extracted and retrotranscribed prior to amplification with the Mouse Inflammatory Response and Autoimmunity PCR Array. A complete list of genes names and accession numbers can be found at: http://www.sabiosciences.com/genetable.php?pcatn=PAMM-077A. Each column represents an individual mouse. Mice from the same group clustered together for expression patterns. Bar at bottom indicates magnitude of gene expression. M, male; F, female; FA, filtered air; O3, ozone; min, minimum; avg, average; max, maximum.
Sex differences in lung gene expression of mice exposed to filtered air.
Table 1 shows the relative expression levels of significant inflammatory genes in the lungs of female mice vs. male mice exposed to filtered air. We used a cutoff of 1.5 for differences in fold regulation. We found that the expression of 4 genes (Cxcl2, Myd88, C4b, and Ccl19) was significantly upregulated, and the expression of 20 additional genes (Tlr3, Ccr3, Tlr1, Cxcl5, Ccl12, Tlr6, Il23a, Ccr2, Ly96, Ccl11, Il1a, Nfkb1, Il1rap, Cd14, Cxcr4, Ripk2, Ccl25, C3ar1, Cd40, and Ptgs2) was significantly downregulated in females vs. males (Table 1).
Table 1.
Relative gene expression in females vs. males exposed to filtered air
| Gene Symbol | Fold Regulation | P Value |
|---|---|---|
| Cxcl2 | 3.77 | 0.038 |
| Myd88 | 2.63 | 5.3E-05 |
| C4b | 1.73 | 0.023 |
| Ccl19 | 1.68 | 0.029 |
| Il1r1 | −1.19 | 0.045 |
| Cxcr2 | −1.49 | 0.013 |
| Ptgs2 | −1.50 | 0.005 |
| Cd40 | −1.50 | 0.004 |
| C3ar1 | −1.55 | 0.015 |
| Ccl25 | −1.62 | 0.024 |
| Ripk2 | −1.72 | 0.034 |
| Cxcr4 | −1.73 | 0.006 |
| Cd14 | −1.80 | 0.014 |
| Il1rap | −1.81 | 0.030 |
| Nfkb1 | −1.82 | 3.1E-05 |
| Il1a | −2.09 | 0.021 |
| Ccl11 | −2.19 | 0.042 |
| Ly96 | −2.23 | 0.012 |
| Ccr2 | −2.35 | 0.005 |
| Il23a | −2.81 | 0.044 |
| Tlr6 | −2.84 | 0.042 |
| Ccl12 | −2.95 | 0.027 |
| Cxcl5 | −3.00 | 0.048 |
| Tlr1 | −3.22 | 0.019 |
| Ccr3 | −3.39 | 0.031 |
| Tlr3 | −3.65 | 0.014 |
Differential effects of ozone in gene expression of male and female lungs.
We next evaluated the effects of ozone exposure vs. filtered air in lung inflammatory gene expression in male (Table 2) and female mice (Table 3). We found that 12 genes were significantly overexpressed at least 1.5-fold in males exposed to ozone vs. males exposed to filtered air (Ccl20, Cxcl5, Il6, Cxcl1, Cxcl2, Ccl11, Ccl17, Ptgs2, Cd14, Ccl19, Cxcl3, and Ccl22). In females, we found that 11 genes (Ccl20, Il6, Cxcl5, Cxcl2, Ccl11, Ptgs2, Cxcl10, Ccl19, Cd14, Fos, and Ccl17) were significantly upregulated in response to ozone vs. filtered air. In males exposed to ozone, 14 genes (Nos2, Ltb, Ccr2, Ifng, Lta, Ccl4, Cd40lg, Tlr9, Ccr4, Cd40, Csf1, Cxcr4, Ccl25, and Tlr5) were significantly downregulated (Table 2), and in females exposed to ozone, only 10 genes were significantly downregulated (Nos2, Ccr2, Ltb, Ccl25, Il18, Tlr9, Cd40, Tlr2, Csf1, and Cd40lg) (Table 3).
Table 2.
Relative gene expression in males exposed to ozone vs. filtered air
| Gene Symbol | Fold Regulation | P Value |
|---|---|---|
| Ccl20 | 77.68 | 2.2E-04 |
| Cxcl5 | 52.82 | 0.002 |
| Il6 | 14.32 | 9.9E-04 |
| Cxcl1 | 9.64 | 0.001 |
| Cxcl2 | 8.94 | 3.8E-04 |
| Ccl11 | 4.28 | 6.0E-06 |
| Ccl17 | 4.17 | 9.0E-06 |
| Ptgs2 | 3.43 | 4.7E-05 |
| Cd14 | 2.62 | 0.002 |
| Ccl19 | 2.58 | 6.9E-04 |
| Cxcl3 | 2.53 | 0.002 |
| Ccl22 | 1.88 | 2.2E-04 |
| Tlr5 | −1.57 | 0.014 |
| Ccl25 | −1.76 | 0.032 |
| Cxcr4 | −1.78 | 0.019 |
| Csf1 | −1.83 | 0.009 |
| Cd40 | −1.90 | 4.2E-04 |
| Ccr4 | −2.06 | 0.025 |
| Tlr9 | −2.07 | 0.010 |
| Cd40lg | −2.18 | 0.005 |
| Ccl4 | −2.85 | 0.029 |
| Lta | −2.97 | 0.007 |
| Ifng | −3.10 | 0.019 |
| Ccr2 | −3.12 | 0.004 |
| Ltb | −3.44 | 0.002 |
| Nos2 | −7.00 | 5.0E-06 |
Table 3.
Relative gene expression in females exposed to ozone vs. filtered air
| Gene Symbol | Fold Regulation | P Value |
|---|---|---|
| Ccl20 | 395.30 | 1.2E-05 |
| Il6 | 48.09 | 5.3E-05 |
| Cxcl5 | 32.53 | 9.0E-06 |
| Cxcl2 | 16.40 | 0.008 |
| Ccl11 | 7.26 | 0.009 |
| Ptgs2 | 3.82 | 0.009 |
| Cxcl10 | 3.73 | 0.014 |
| Ccl19 | 2.82 | 9.0E-04 |
| Cd14 | 2.73 | 0.005 |
| Fos | 2.61 | 0.004 |
| Ccl17 | 2.33 | 0.016 |
| Il1r1 | 1.47 | 0.043 |
| Cd40lg | −1.69 | 0.022 |
| Csf1 | −1.70 | 0.002 |
| Tlr2 | −1.75 | 0.002 |
| Cd40 | −1.84 | 0.033 |
| Tlr9 | −2.41 | 1.9E-02 |
| Il18 | −2.51 | 8.9E-03 |
| Ccl25 | −2.63 | 0.005 |
| Ltb | −2.82 | 1.4E-04 |
| Ccr2 | −2.94 | 0.027 |
| Nos2 | −3.48 | 1.1E-04 |
Differences in gene expression levels in males and females exposed to ozone.
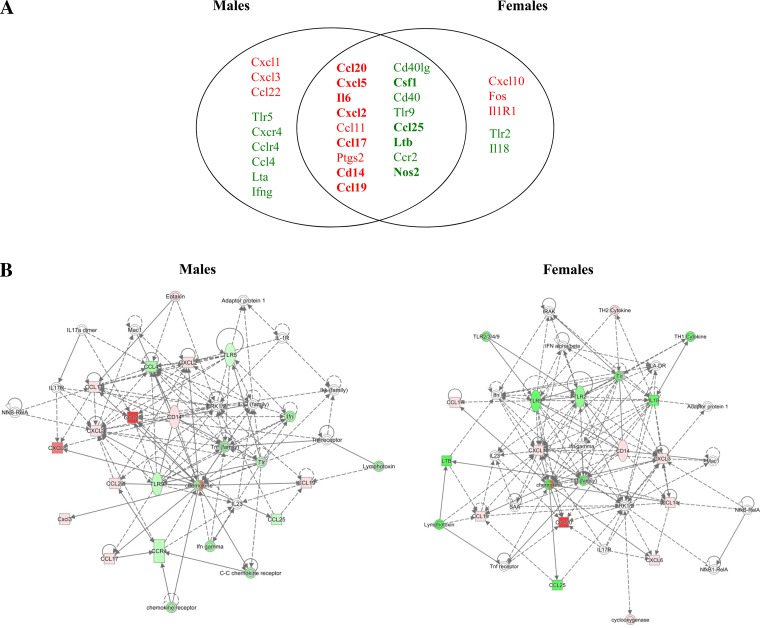
The genes that were up- and downregulated in response to ozone were similar in males and females. However, of the genes overexpressed in response to ozone, female mice exhibited higher expression levels of Ccl20, Il6, Cxcl2, Cxcl10, and Fos compared with males. Meanwhile, of the genes underexpressed in response to ozone, male mice exhibited significantly lower expression of Nos2, Lta, and Ifng compared with females (Tables 2 and 3).
Proinflammatory cytokine and chemokine expression measured by real-time PCR.
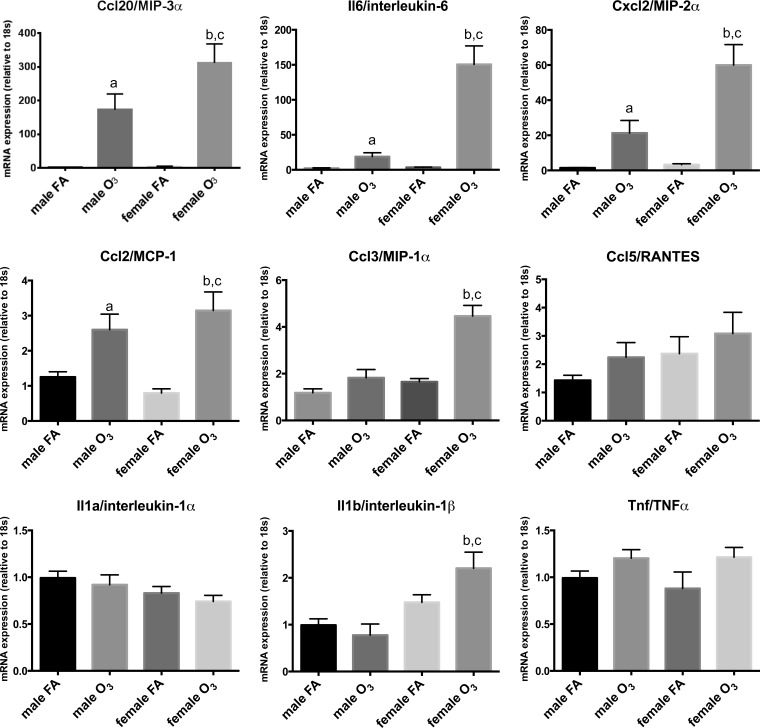
Because most of the genes affected by ozone exposure in males and females belonged to the family of proinflammatory cytokines (Fig. 3), and to validate gene expression data by an independent method, a selected set of genes was additionally evaluated by real-time RT-PCR in a larger sample size (n = 10/experimental group). As shown in Fig. 4, differential gene expression analysis by real-time RT-PCR of Ccl20, Il6, Cxcl2, Ccl2, Ccl3, Ccl5, Il1a, Il1b, and Tnf in lungs of male and female mice largely matched the data obtained by PCR arrays, albeit at a much higher sensitivity level, showing significant differences in ozone exposed females vs. males for Ccl20 (MIP-3α), Il6 (IL-6), Cxcl2 (MIP-2α), Ccl2 (MCP-1), and Ccl3 (MIP-1α). This suggests that the mRNA array technique, although accurately detecting trends in altered gene expression profiles, may not be suitable to accurately quantify gene expression levels. Moreover, the use of real-time PCR TaqMan assays also allowed for the detection of significant differences among groups that were not evident in the arrays. For example, significant differences were found for Il1b expression, with females showing higher levels in response to ozone vs. females exposed to filtered air, and in females exposed to ozone vs. males exposed to ozone (Fig. 4).
Fig. 4.
Relative mRNA expression of select set of genes analyzed via real-time PCR. Expression levels of selected cytokines (Il6, Il1a, Il1b, Tnf) and chemokines (Ccl20, Cxcl2, Ccl2, Ccl3, Ccl5) in lung extracts of male and female mice exposed to ozone (O3) or filtered air (FA). Ozone-treated females expressed significantly higher levels of Ccl20, Il6, Cxcl2, and Ccl3 than ozone-treated males (a, significant difference vs. male FA group; b, significant difference vs. female FA group; c, significant difference vs. male O3 group). Results are expressed as means ± SE. Significant differences were analyzed by ANOVA (P < 0.05, n = 10/group).
Pattern recognition receptor and transcription factor expression in response to ozone.
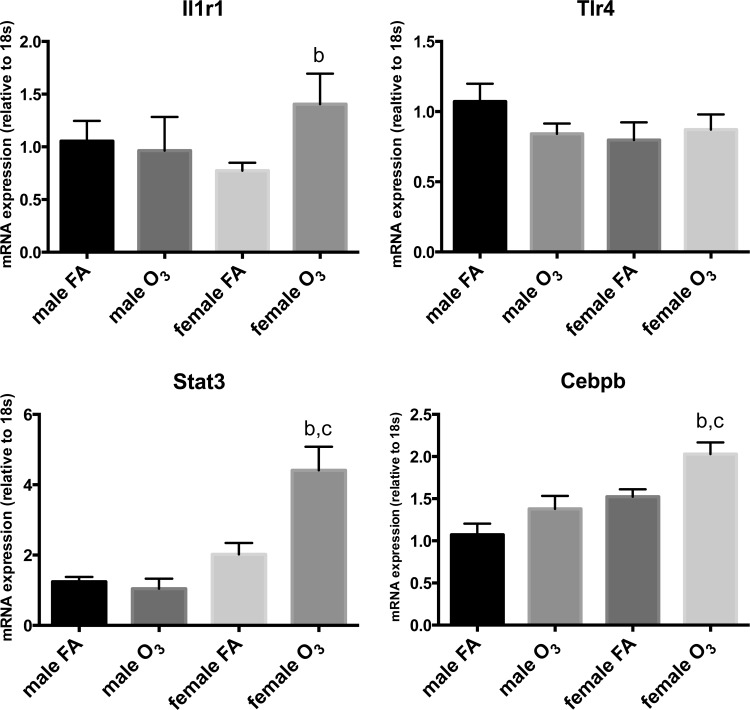
We next evaluated whether ozone affected the expression of the Il1r1 and Tlr4 receptors, known to mediate immune responses associated with Il1a, Il1b, and Il6 (34, 71). Figure 5 shows that the lungs of females exposed to ozone expressed significantly higher levels of Il1r1 than females exposed to filtered air; however, no differences were found for Tlr4 between these groups. In addition, the expression of the transcription factors Stat3 and Cebpb, also related to acute inflammatory responses, was significantly higher in females exposed to ozone vs. females exposed to filtered air, but not in males exposed to ozone compared with males exposed to filtered air (Fig. 5).
Fig. 5.
Relative mRNA expression of select group of receptors and transcription factors via real-time PCR. Expression levels of selected immune related receptors (Il1r1, Tlr4) and transcription factors (Stat3, Cebpb) in lung extracts of male and female mice exposed to ozone (O3) or filtered air (FA). Ozone-treated females expressed significantly higher levels of transcription factors Stat3 and Cebpb than ozone-treated males (b, significant difference vs. female FA group; c, significant difference vs. male O3 group). Results are expressed as means ± SE. Significant differences were analyzed by ANOVA (P < 0.05, n = 10/group).
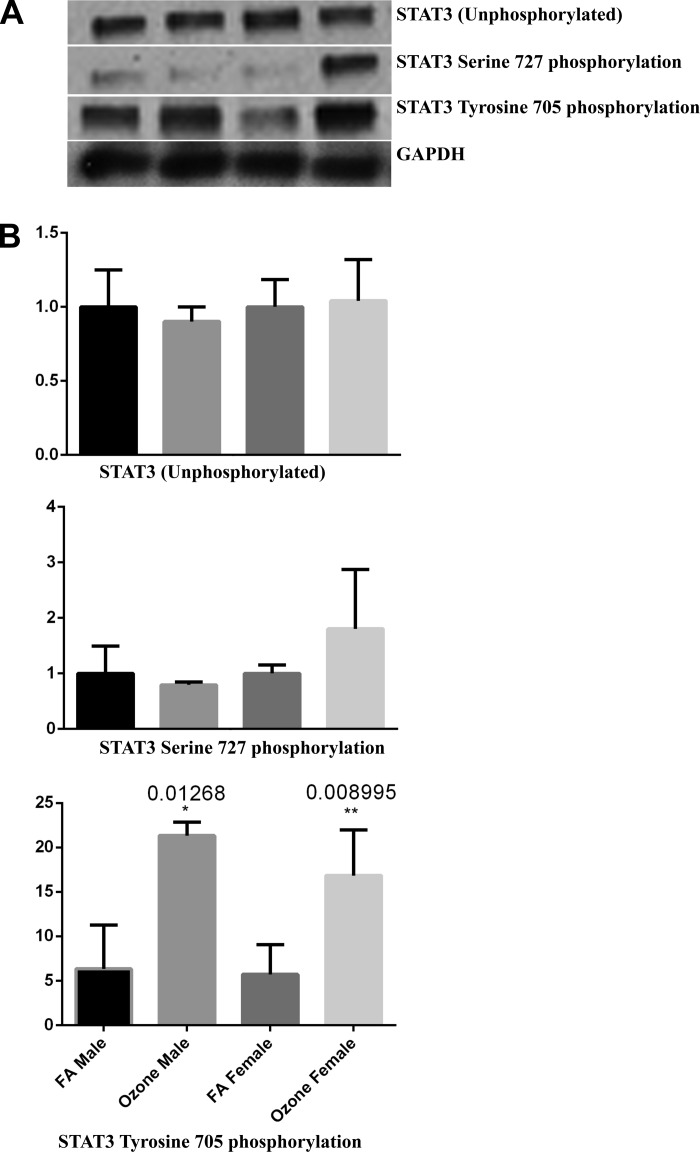
Phosphorylation of STAT3 as a potential mechanism for IL-6 induced inflammation.
Finally, since inflammatory responses associated with IL-6 have been shown to involve activation of STAT3 and related intracellular pathways (12, 23, 28), and, since females expressed higher levels of Stat3 mRNA in response to ozone (Fig. 5), we next evaluated whether sex differences existed in STAT3 protein expression and activation in response to ozone. We chose two STAT3 phosphorylation sites, serine 727 (S727), known to regulate STAT3 function both positively and negatively, and tyrosine 705 (Y705), known to mediate STAT3 dimerization, nuclear translocation, and DNA binding (1). We found no differences in STAT3 protein expression and no statistically significant difference in the phosphorylation at S727 measured by Western blot 4 h postexposure, although a trend toward increased phosphorylation was observed, but not statistically tested. However, phosphorylation of STAT3 at Y705 was significantly higher in animals exposed to ozone vs. filtered air in both sexes (Fig. 6).
Fig. 6.
Ozone exposure induces STAT3 Y705 phosphorylation. A: Western blot analyses of total STAT3, phosphorylated STAT3 at S727, and phosphorylated STAT3 at Y705 in mouse whole lung homogenates. B: expression levels were quantified by densitometric analysis and normalized to GAPDH. Results are expressed relative to the filtered air-exposed control group levels. Significant differences between groups were determined by Kruskal-Wallis ANOVA on ranks with Dunn's post hoc test. *P < 0.05; **P < 0.01 vs. control (n = 6/group).
DISCUSSION
Innate immunity plays a critical role against infection and oxidative damage from inhaled air pollutants. Exposure to ozone results in a rapid influx of inflammatory cells into the lower respiratory tract, promoting oxidative stress and airway hyperreactivity, while increasing the risk of respiratory infection (3, 39). Although sex differences have been reported in the susceptibility and severity of a number of environmental inflammatory lung diseases, the specific mechanisms underlying these differences have not been clearly defined. In this work, we used an acute ozone exposure model to evaluate sex differences in the inflammatory response at the gene expression level. First, we characterized the model by identifying sex differences in lung histology and BAL measurements of lung injury and inflammation. Next, we evaluated whether changes in gene expression correlated with these changes. We found sex differences in lung morphology, as well as BAL cell counts and protein content in response to ozone, with females showing increased damage vs. males. We also found that expression of inflammatory mediators varies with sex under basal conditions and following exposure to ozone, indicating a potential sexual dimorphism in the mechanisms associated with the inflammatory response to this air pollutant.
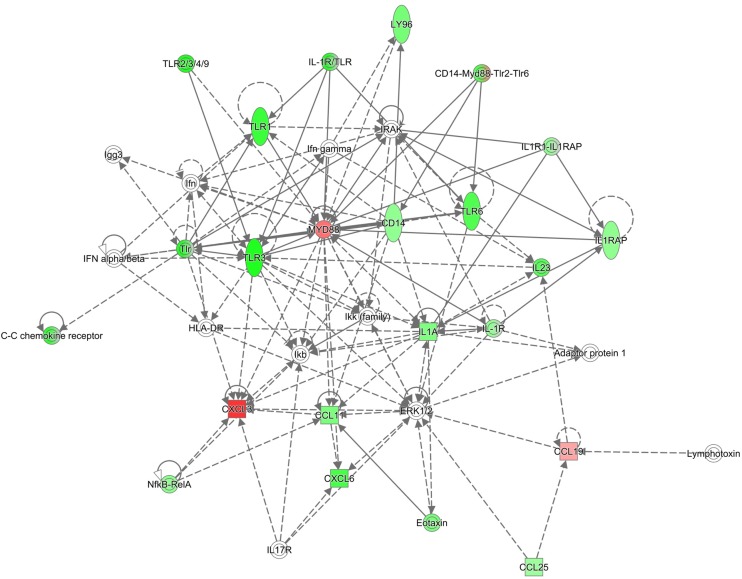
Numerous studies have reported significant differences in lung function, innate immune responses to bacterial infection, and lung disease pathogenesis in mice breathing clean air, with males typically showing weaker immune responses than females (14, 69, 75). In our gene expression array data in lung tissue obtained from mice exposed to filtered air, we found differences in cytokine and chemokine expression between males and females. Interestingly, the mRNA levels of inflammatory mediators in the lungs of females was significantly lower for almost 30% of the genes analyzed (20/72). The major gene families affected included Toll-like receptors (Tlr1, Tlr3, Tlr6), cytokines (Il1a, Il23a), chemokines (Cxcl5, Ccl12, Ccl11, Ccl25), cytokine and chemokine receptors (Ccr3, Ccr2, Cxcr4, Il1rap), as well as other immune mediators, enzymes, receptors and transcription factors (C3ar1, Cd14, Cd40, Ripk2, Ptgs2, Nfkb, Ly96). In contrast, the expression of only 5% of the genes studied (4/72) was significantly higher in females vs. males exposed to filtered air. These groups of genes are involved in macrophage activation (Cxcl2, Ccl19), Toll-like-receptor activation (Myd88), and modulation of interleukin-6 responses (C4b). Further analysis performed with IPA revealed four main canonical pathways affected in females vs. males exposed to filtered air. These are related to immune cell adhesion and movement, as well as pattern recognition receptor functions (Table 4A). Furthermore, the top molecular functions associated with differentially expressed genes in males and females were also linked to cell movement and proliferation, which are crucial events in the lung inflammatory response (Table 4B), and the top associated network functions included inflammatory response, cell-to-cell signaling and interaction, and cellular movement pathways (Table 4C). The functional relationship plot shown in Fig. 7 indicates that many of these genes, whose expression differed more than 1.5-fold in females vs. males, have been reported to have direct (solid lines) or indirect (dashed lines) associations, indicating that these may belong to common pathways that are activated in response to ozone.
Table 4.
Sex differences in mice exposed to filtered air
| A. Sex differences in top canonical pathways in mice exposed to filtered air | ||
|---|---|---|
| Canonical Pathways | P Value | |
| LXR/RXR activation | 8.44E-15 | |
| Toll-like receptor signaling | 1.47E-14 | |
| TREM1 signaling | 1.64E-14 | |
| Granulocyte adhesion and diapedesis | 2.75E-13 | |
| B. Top molecular and cellular functions in female vs. male mice exposed to filtered air | ||
|---|---|---|
| Molecular and Cellular Functions | P Value | No. of Molecules |
| Cell-to-cell signaling and interaction | 2.00E-05–4.92E-26 | 24 |
| Cellular movement | 2.00E-05–4.92E-26 | 21 |
| Cellular function and maintenance | 1.79E-05–7.90E-23 | 23 |
| Cellular development | 1.59E-05–8.51E-16 | 22 |
| Cellular growth and proliferation | 1.59E-05–8.51E-16 | 21 |
| C. Top associated network functions in females vs. males exposed to filtered air | ||
|---|---|---|
| Associated Network Functions | Score | |
| Inflammatory response, lipid metabolism, small molecule biochemistry | 33 | |
| Hematological system development and function, tissue morphology, inflammatory response | 14 | |
| Cardiovascular system development and function, cellular movement, hematological system development and function | 8 | |
| Inflammatory response, cell signaling, protein synthesis | 2 | |
Fig. 7.
Ingenuity Pathway Analysis for inflammatory gene expression in females vs. males exposed to filtered air. Diagram of biological networks of selected genes whose expression were up- and downregulated at least 1.5-fold in the lungs of females vs. males exposed to filtered air (n = 6/group). The diagram shows reported direct (solid lines) and indirect (dashed lines) interactions for these genes. Each gene or group of gene is represented as a node in green (downregulated) or red (upregulated). Network analysis was performed with Ingenuity Pathway Analysis (see materials and methods for details).
In humans and rodents, inhalation of ozone is associated with an inflammatory response characterized by accumulation of macrophages in the lower airways. Activation of alveolar macrophages results in the release of proinflammatory mediators that contribute to neutrophil recruitment, oxidative stress, and lung injury (29). In our mice exposed to ozone, we identified a differential (quantitative and qualitative) gene expression response in the lungs of males vs. females (Fig. 3), although a similar number of genes were affected in both groups (Tables 2 and 3). Major differences between sexes involved cytokine and chemokine expression (Ccl20, Il6, Cxcl2, Cxcl10, Cxcl9, Ifng, Lta, and Ccl3), as well as expression of nuclear proteins and enzymes (Fos, Nos2). IPA analysis of the major canonical pathways altered in response to ozone revealed similar results for males and females (Tables 5A and 6A). We found that, although both males and females showed differential expression of genes related to adaptive immune T and B cell signaling, only females showed significant changes in genes related to innate and adaptive immunity communications (Tables 5B and 6B). The results from IPA top network analysis indicated that females had a higher number of genes from the Inflammatory Response, Cell-to-Cell Signaling and Interaction, and Cellular Movement networks, whereas more genes affected in males belonged to Hematological Disease, Immunological Disease, and Infectious Disease pathways (Tables 5C and 6C). The network association analysis for this dataset also identified similar pathways altered by ozone in males and females, with cytokine and chemokine expression and intracellular signaling kinase pathways as central nodes (Fig. 8). Taken together, these results indicate that the sexual dimorphism observed in the immune response to ozone may be associated with differential activation of intracellular mechanisms and gene regulatory networks in the lung cells of males vs. females. Our results are particularly relevant to women's health, since epidemiological studies show a higher prevalence of environmental lung diseases affecting adaptive immunity in women, and accumulating evidence indicates that circulating levels of female sex hormones may be important physiological modulators of lung function and immunity in female asthmatic patients (13, 56, 60). With the rise in the burden of asthma and other inflammatory lung diseases in women worldwide, it is essential that we increase our understanding of the biological roles of sex hormones in modulating airway inflammation and immunity (2, 9, 10). Future studies in ovariectomized mice may shed light in the specific roles of female sex hormones and the mechanisms associated with their receptors in the control of innate immune responses in the lung.
Table 5.
Effects of ozone exposure in male mice
| A. Top canonical pathways differentially expressed in males in response to ozone | ||
|---|---|---|
| Canonical Pathways | P Value | |
| Granulocyte adhesion and diapedesis | 1.14E-18 | |
| Agranulocyte adhesion and diapedesis | 2.54E-18 | |
| Altered T cell and B cell signaling in rheumatoid arthritis | 1.04E-15 | |
| B. Top molecular and cellular functions affected by ozone exposure in male mice | ||
|---|---|---|
| Molecular and Cellular Functions | P Value | No. of Molecules |
| Cellular movement | 1.55E-07–3.27E-33 | 26 |
| Cell-to-cell signaling and interaction | 1.51E-07–1.96E-31 | 25 |
| Cellular function and maintenance | 8.31E-08–2.38E-22 | 26 |
| Cellular development | 1.11E-07–9.89E-20 | 22 |
| Cellular growth and proliferation | 1.11E-07–9.89E-20 | 23 |
| C. Top associated network functions affected by ozone exposure in male mice | ||
|---|---|---|
| Associated Network Functions | Score | |
| Cell-to-cell signaling and interaction, cellular movement, immune cell trafficking | 39 | |
| Hematological disease, immunological disease, infectious disease | 8 | |
| Cancer, organismal injury and abnormalities, increased levels of AST | 7 | |
| Dermatological diseases and conditions, cellular movement, hematological system development and function | 3 | |
| Cell-to-cell signaling and interaction, cellular growth and proliferation, hematological system development and function | 3 | |
Table 6.
Effects of ozone exposure in female mice
| A. Top canonical pathways differentially expressed in females in response to ozone | ||
|---|---|---|
| Canonical Pathways | P Value | |
| Altered T cell and B cell signaling in rheumatoid arthritis | 1.74E-14 | |
| Granulocyte adhesion and diapedesis | 6.32E-14 | |
| Agranulocyte adhesion and diapedesis | 1.15E-13 | |
| Communication between innate and adaptive immune cells | 3.16E-12 | |
| B. Top molecular and cellular functions affected by ozone exposure in female mice | ||
|---|---|---|
| Molecular and Cellular Functions | P Value | No. of Molecules |
| Cellular movement | 4.29E-07–2.85E-28 | 21 |
| Cell-to-cell signaling and interaction | 4.05E-07–8.34E-27 | 21 |
| Cellular growth and proliferation | 3.79E-07–2.43E-20 | 20 |
| Cellular function and maintenance | 4.29E-07–3.31E-20 | 21 |
| Cellular development | 4.29E-07–4.19E-19 | 20 |
| C. Top associated network functions affected by ozone exposure in female mice | ||
|---|---|---|
| Associated Network Functions | ||
| Cell-to-cell signaling and interaction, cellular movement, immune cell trafficking | 34 | |
| Inflammatory response, cell-to-cell signaling and interaction, cellular movement | 6 | |
| Skeletal and muscular system development and function, hematological disease, immunological disease | 5 | |
| Cardiovascular system development and function, organ morphology, skeletal and muscular system development and function | 3 | |
| Cancer, cell cycle, cellular development | 2 | |
Fig. 8.
Comparison of gene networks affected by ozone exposure in males and females. A: Venn diagram showing genes with significant changes in expression in response to ozone exposure in lungs of male and female mice. The intersection shows genes affected in both sexes in response to ozone (green = downregulated, red = upregulated). Genes with significant differences in expression (P < 0.05) between males and females are shown in bold. B: diagram of biological networks of selected genes whose expression were up- and downregulated at least 1.5-fold in the lungs of animals exposed to ozone vs. filtered air (n = 6/group). Left, changes in male mice; right, genes affected in female mice. Both diagrams show reported direct (solid lines) and indirect (dashed lines) gene interactions. Each gene or group of gene is represented as a node in green (downregulated) or red (upregulated). Network analysis was performed with Ingenuity Pathway Analysis (see materials and methods for details).
Although the response to ozone in males and females in both the array and pathway analyses was highly similar, we found that the intensity of the response clearly differed between sexes (Fig. 3, and Tables 2 and 3). Quantitative real-time PCR in a subset of selected genes confirmed these gene expression patterns. Our results showed that the Ccl20/MIP-3α, Il6, and Cxcl2/MIP-2α mRNAs were the most affected by ozone inhalation in both males and females but had significantly higher expression in females than in males (Fig. 4). Previous studies in lung cells have reported increased levels of CCL20 upon exposure to air particulate matter (61) and have proposed a role for this inflammatory mediator in the transition from innate to adaptive immunity and in recruitment of dendritic cells into the lung in response to LPS (67). The macrophage inflammatory protein and chemoattractant CXCL2 and CCL2, respectively, were also reported as major contributors to the immune response to various oxidants (33), and increased IL-6 levels have been reported in BAL obtained from animals exposed to ozone, although no sex differences were previously described (70). The specific cell types that secrete CCL20, CXCL2, and IL-6, as well as the mechanisms by which these and other inflammatory cytokines activate and promote infiltration of immune cells to the airways, are still not completely identified.
Studies in Tlr4 and Il1r1 knockout mice have indicated that both type I interleukin receptor and Toll-like receptor 4 are required for macrophage activation and overall lung immune responses to ozone (17, 34). In addition, IL1R1, the only signaling receptor for interleukin-1 alpha and beta, has been identified as a major contributor to the IL-6 induction in response to ozone (34). Our real-time PCR data revealed a significantly higher expression of Il1r1, but not Tlr4, in females exposed to ozone, indicating that the sex differences observed in Il6 expression in the acute response to ozone may be associated with Il1r1 at the time point studied (Fig. 5).
Interleukin-6 is a pleiotropic cytokine required for neutrophil recruitment, known to regulate both innate and adaptive responses (22, 35). Binding of IL-6 to its receptor can induce activation of multiple intracellular signal transducers (28, 40). The sequential downstream pathways associated with these complexes include phosphorylation, dimerization, and nuclear translocation of the transcription factor STAT3, resulting in differential modulation of gene expression (36). Since Stat3 was not included in the original gene array, we evaluated its expression by real-time PCR, and we identified a significant overexpression in females exposed to ozone, consistent with our previous Il6 and Il1r1 expression data. In addition, since the transcription factor CCAAT/enhancer-binding protein beta (Cebpb) has been recently recognized as a modulator of acute lung injury (74), and associated with induction of IL-1 and IL-6 in response to LPS (31, 58), we also verified its expression by real-time PCR (Fig. 5) and found a significant increase in the lungs of females exposed to ozone vs. males and filtered air-exposed animals. Together, these results show that molecular pathways associated with IL-1 and IL-6 induction may represent major mechanisms implicated in the differential immune response to ozone in males and females. Since activation of STAT3 has been previously reported as a major mediator of ozone-induced acute lung injury (23), we next evaluated whether sex differences existed in STAT3 phosphorylation lung extracts. Interestingly, despite significant changes in Stat3 mRNA levels, no differences were found in total STAT3 protein levels or phosphorylation at amino acid S727. However, phosphorylation of STAT3 at amino acid Y705 was significantly higher in both males and females in response to ozone (Fig. 6), indicating that STAT3 phosphorylation may mediate overall immune responses to ozone in both males and female mice but may not be responsible for the sex differences observed.
In summary, exposure to ozone has numerous negative effects on lung health and innate pulmonary host defense (30, 32, 44). Our studies in mice show significant differences in proinflammatory cytokine and chemokine expression and associated gene expression and functional networks between males and females exposed to both filtered air and ozone. Although our study has several limitations, including the use of whole lung tissue instead of selected cell types, and the use of female mice in all stages of the estrous cycle instead of animals with controlled hormone levels, the information obtained supports the hypothesis of differential gene expression networks, and potentially associated signaling pathways, as mediators of sex differences in the lung inflammatory response to ozone. Understanding the regulatory roles of the differentially expressed genes in response to environmental insults may provide the foundation for the identification of sex-specific therapeutic targets for acute lung inflammation and injury.
GRANTS
N. Cabello is a scholar of the PSU INTREPID program R25HL103166 “Introduction of a Novel Tiered Research Experience Promoting Inclusion and Diversity.” P. Silveyra's research is supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development of the National Institutes of Health under BIRCWH award number K12HD055882 “Career Development Program in Women's Health Research at Penn State.” The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
N.C., V.M., Z.C.C., and P.S. conception and design of research; N.C., V.M., U.S., S.L.D., Z.C.C., N.A.E., T.K.C., C.R.C., and P.S. performed experiments; N.C., V.M., U.S., N.A.E., and P.S. analyzed data; N.C., V.M., U.S., S.L.D., Z.C.C., T.K.C., C.R.C., and P.S. interpreted results of experiments; N.C., V.M., and P.S. prepared figures; N.C., V.M., U.S., S.L.D., Z.C.C., N.A.E., T.K.C., C.R.C., and P.S. edited and revised manuscript; N.C., V.M., U.S., S.L.D., Z.C.C., N.A.E., T.K.C., C.R.C., and P.S. approved final version of manuscript; P.S. drafted manuscript.
ACKNOWLEDGMENTS
The authors thank the Pennsylvania State University College of Medicine Genome Sciences Core Facility for Bioanalyzer analysis and real-time PCR equipment. The authors also thank Dr. Joanna Floros and her staff for assistance with ozone exposure experiments.
REFERENCES
- 1.Aggarwal BB, Kunnumakkara AB, Harikumar KB, Gupta SR, Tharakan ST, Koca C, Dey S, Sung B. Signal transducer and activator of transcription-3, inflammation, and cancer: how intimate is the relationship? Ann NY Acad Sci 1171: 59–76, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akinbami LJ, Moorman JE, Bailey C, Zahran HS, King M, Johnson CA, Liu X. Trends in asthma prevalence, health care use, and mortality in the United States, 2001–2010. NCHS Data Brief: 1–8, 2012. [PubMed] [Google Scholar]
- 3.Alexis NE, Lay JC, Hazucha M, Harris B, Hernandez ML, Bromberg PA, Kehrl H, Diaz-Sanchez D, Kim C, Devlin RB, Peden DB. Low-level ozone exposure induces airways inflammation and modifies cell surface phenotypes in healthy humans. Inhal Toxicol 22: 593–600, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ali M, Umstead TM, Haque R, Mikerov AN, Freeman WM, Floros J, Phelps DS. Differences in the BAL proteome after Klebsiella pneumoniae infection in wild type and SP-A−/− mice. Proteome Sci 8: 34, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.American Lung Association. Taking Her Breath Away: the Rise of COPD in Women, 2013 (Online). Chicago, IL: American Lung Association, 2013. http://www.lung.org/assets/documents/publications/lung-disease-data/rise-of-copd-in-women-full.pdf. [Google Scholar]
- 6.Ashcroft T, Simpson JM, Timbrell V. Simple method of estimating severity of pulmonary fibrosis on a numerical scale. J Clin Pathol 41: 467–470, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bassett D, Elbon-Copp C, Otterbein S, Barraclough-Mitchell H, Delorme M, Yang H. Inflammatory cell availability affects ozone-induced lung damage. J Toxicol Environ Health A 64: 547–565, 2001. [DOI] [PubMed] [Google Scholar]
- 8.Bernstein JA, Alexis N, Barnes C, Bernstein IL, Bernstein JA, Nel A, Peden D, Diaz-Sanchez D, Tarlo SM, Williams PB. Health effects of air pollution. J Allergy Clin Immunol 114: 1116–1123, 2004. [DOI] [PubMed] [Google Scholar]
- 9.Braman SS. The global burden of asthma. Chest 130: 4S–12S, 2006. [DOI] [PubMed] [Google Scholar]
- 10.Brozek G, Lawson J, Szumilas D, Zejda J. Increasing prevalence of asthma, respiratory symptoms, and allergic diseases: four repeated surveys from 1993–2014. Respir Med 109: 982–990, 2015. [DOI] [PubMed] [Google Scholar]
- 11.Burnett RT, Smith-Doiron M, Stieb D, Raizenne ME, Brook JR, Dales RE, Leech JA, Cakmak S, Krewski D. Association between ozone and hospitalization for acute respiratory diseases in children less than 2 years of age. Am J Epidemiol 153: 444–452, 2001. [DOI] [PubMed] [Google Scholar]
- 12.Camporeale A, Marino F, Papageorgiou A, Carai P, Fornero S, Fletcher S, Page BD, Gunning P, Forni M, Chiarle R, Morello M, Jensen O, Levi R, Heymans S, Poli V. STAT3 activity is necessary and sufficient for the development of immune-mediated myocarditis in mice and promotes progression to dilated cardiomyopathy. EMBO Mol Med 5: 572–590, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Caracta CF. Gender differences in pulmonary disease. Mt Sinai J Med 70: 215–224, 2003. [PubMed] [Google Scholar]
- 14.Carey MA, Card JW, Voltz JW, Germolec DR, Korach KS, Zeldin DC. The impact of sex and sex hormones on lung physiology and disease: lessons from animal studies. Am J Physiol Lung Cell Mol Physiol 293: L272–L278, 2007. [DOI] [PubMed] [Google Scholar]
- 15.Chen B, Kan H. Air pollution and population health: a global challenge. Environ Health Prev Med 13: 94–101, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ciencewicki J, Trivedi S, Kleeberger SR. Oxidants and the pathogenesis of lung diseases. J Allergy Clin Immunol 122: 456–468; quiz 469–470, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Connor AJ, Laskin JD, Laskin DL. Ozone-induced lung injury and sterile inflammation. Role of Toll-like receptor 4. Exp Mol Pathol 92: 229–235, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Torres JP, Cote CG, Lopez MV, Casanova C, Diaz O, Marin JM, Pinto-Plata V, de Oca MM, Nekach H, Dordelly LJ, Aguirre-Jaime A, Celli BR. Sex differences in mortality in patients with COPD. Eur Respir J 33: 528–535, 2009. [DOI] [PubMed] [Google Scholar]
- 19.Delfino RJ, Coate BD, Zeiger RS, Seltzer JM, Street DH, Koutrakis P. Daily asthma severity in relation to personal ozone exposure and outdoor fungal spores. Am J Respir Crit Care Med 154: 633–641, 1996. [DOI] [PubMed] [Google Scholar]
- 20.Durrani F, Phelps DS, Weisz J, Silveyra P, Hu S, Mikerov AN, Floros J. Gonadal hormones and oxidative stress interaction differentially affects survival of male and female mice after lung Klebsiella Pneumoniae infection. Exp Lung Res 38: 165–172, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Falagas ME, Mourtzoukou EG, Vardakas KZ. Sex differences in the incidence and severity of respiratory tract infections. Respir Med 101: 1845–1863, 2007. [DOI] [PubMed] [Google Scholar]
- 22.Fielding CA, McLoughlin RM, McLeod L, Colmont CS, Najdovska M, Grail D, Ernst M, Jones SA, Topley N, Jenkins BJ. IL-6 regulates neutrophil trafficking during acute inflammation via STAT3. J Immunol 181: 2189–2195, 2008. [DOI] [PubMed] [Google Scholar]
- 23.Gao H, Guo RF, Speyer CL, Reuben J, Neff TA, Hoesel LM, Riedemann NC, McClintock SD, Sarma JV, Van Rooijen N, Zetoune FS, Ward PA. Stat3 activation in acute lung injury. J Immunol 172: 7703–7712, 2004. [DOI] [PubMed] [Google Scholar]
- 24.Goss CH, Newsom SA, Schildcrout JS, Sheppard L, Kaufman JD. Effect of ambient air pollution on pulmonary exacerbations and lung function in cystic fibrosis. Am J Respir Crit Care Med 169: 816–821, 2004. [DOI] [PubMed] [Google Scholar]
- 25.Haque R, Umstead TM, Ponnuru P, Guo X, Hawgood S, Phelps DS, Floros J. Role of surfactant protein-A (SP-A) in lung injury in response to acute ozone exposure of SP-A deficient mice. Toxicol Appl Pharmacol 220: 72–82, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hatch GE, Koren H, Aissa M. A method for comparison of animal and human alveolar dose and toxic effect of inhaled ozone. Health Phys 57, Suppl 1: 37–40, 1989. [DOI] [PubMed] [Google Scholar]
- 27.Hatch GE, Slade R, Harris LP, McDonnell WF, Devlin RB, Koren HS, Costa DL, McKee J. Ozone dose and effect in humans and rats. A comparison using oxygen-18 labeling and bronchoalveolar lavage. Am J Respir Crit Care Med 150: 676–683, 1994. [DOI] [PubMed] [Google Scholar]
- 28.Heinrich PC, Behrmann I, Müller-Newen G, Schaper F, Graeve L. Interleukin-6-type cytokine signalling through the gp130/Jak/STAT pathway. Biochem J 334: 297–314, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Herold S, Mayer K, Lohmeyer J. Acute lung injury: how macrophages orchestrate resolution of inflammation and tissue repair. Front Immunol 2: 65, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hollingsworth JW, Kleeberger SR, Foster WM. Ozone and pulmonary innate immunity. Proc Am Thorac Soc 4: 240–246, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hu HM, Tian Q, Baer M, Spooner CJ, Williams SC, Johnson PF, Schwartz RC. The C/EBP bZIP domain can mediate lipopolysaccharide induction of the proinflammatory cytokines interleukin-6 and monocyte chemoattractant protein-1. J Biol Chem 275: 16373–16381, 2000. [DOI] [PubMed] [Google Scholar]
- 32.Jerrett M, Burnett RT, Pope CA 3rd, Ito K, Thurston G, Krewski D, Shi Y, Calle E, Thun M. Long-term ozone exposure and mortality. N Engl J Med 360: 1085–1095, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Johnston CJ, Oberdörster G, Finkelstein JN. Recovery from oxidant-mediated lung injury: response of metallothionein, MIP-2, and MCP-1 to nitrogen dioxide, oxygen, and ozone exposures. Inhal Toxicol 13: 689–702, 2001. [DOI] [PubMed] [Google Scholar]
- 34.Johnston RA, Mizgerd JP, Flynt L, Quinton LJ, Williams ES, Shore SA. Type I interleukin-1 receptor is required for pulmonary responses to subacute ozone exposure in mice. Am J Respir Cell Mol Biol 37: 477–484, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jones SA. Directing transition from innate to acquired immunity: defining a role for IL-6. J Immunol 175: 3463–3468, 2005. [DOI] [PubMed] [Google Scholar]
- 36.Jones SA, Horiuchi S, Topley N, Yamamoto N, Fuller GM. The soluble interleukin 6 receptor: mechanisms of production and implications in disease. FASEB J 15: 43–58, 2001. [DOI] [PubMed] [Google Scholar]
- 37.Kampa M, Castanas E. Human health effects of air pollution. Environ Pollut 151: 362–367, 2008. [DOI] [PubMed] [Google Scholar]
- 38.Kehrl HR, Vincent LM, Kowalsky RJ, Horstman DH, O'Neil JJ, McCartney WH, Bromberg PA. Ozone exposure increases respiratory epithelial permeability in humans. Am Rev Respir Dis 135: 1124–1128, 1987. [DOI] [PubMed] [Google Scholar]
- 39.Kierstein S, Krytska K, Sharma S, Amrani Y, Salmon M, Panettieri RA, Zangrilli J, Haczku A. Ozone inhalation induces exacerbation of eosinophilic airway inflammation and hyperresponsiveness in allergen-sensitized mice. Allergy 63: 438–446, 2008. [DOI] [PubMed] [Google Scholar]
- 40.Kishimoto T, Akira S, Narazaki M, Taga T. Interleukin-6 family of cytokines and gp130. Blood 86: 1243–1254, 1995. [PubMed] [Google Scholar]
- 41.Kleeberger SR, Levitt RC, Zhang LY. Susceptibility to ozone-induced inflammation. II. Separate loci control responses to acute and subacute exposures. Am J Physiol Lung Cell Mol Physiol 264: L21–L26, 1993. [DOI] [PubMed] [Google Scholar]
- 42.Kleeberger SR, Levitt RC, Zhang LY, Longphre M, Harkema J, Jedlicka A, Eleff SM, DiSilvestre D, Holroyd KJ. Linkage analysis of susceptibility to ozone-induced lung inflammation in inbred mice. Nat Genet 17: 475–478, 1997. [DOI] [PubMed] [Google Scholar]
- 43.Laskin DL, Sunil VR, Fakhrzadeh L, Groves A, Gow AJ, Laskin JD. Macrophages, reactive nitrogen species, and lung injury. Ann NY Acad Sci 1203: 60–65, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lim SS, Vos T, Flaxman AD, Danaei G, Shibuya K, Adair-Rohani H, Amann M, Anderson HR, Andrews KG, Aryee M, Atkinson C, Bacchus LJ, Bahalim AN, Balakrishnan K, Balmes J, Barker-Collo S, Baxter A, Bell ML, Blore JD, Blyth F, Bonner C, Borges G, Bourne R, Boussinesq M, Brauer M, Brooks P, Bruce NG, Brunekreef B, Bryan-Hancock C, Bucello C, Buchbinder R, Bull F, Burnett RT, Byers TE, Calabria B, Carapetis J, Carnahan E, Chafe Z, Charlson F, Chen H, Chen JS, Cheng AT, Child JC, Cohen A, Colson KE, Cowie BC, Darby S, Darling S, Davis A, Degenhardt L, Dentener F, Des Jarlais DC, Devries K, Dherani M, Ding EL, Dorsey ER, Driscoll T, Edmond K, Ali SE, Engell RE, Erwin PJ, Fahimi S, Falder G, Farzadfar F, Ferrari A, Finucane MM, Flaxman S, Fowkes FG, Freedman G, Freeman MK, Gakidou E, Ghosh S, Giovannucci E, Gmel G, Graham K, Grainger R, Grant B, Gunnell D, Gutierrez HR, Hall W, Hoek HW, Hogan A, Hosgood HD, Hoy D, Hu H, Hubbell BJ, Hutchings SJ, Ibeanusi SE, Jacklyn GL, Jasrasaria R, Jonas JB, Kan H, Kanis JA, Kassebaum N, Kawakami N, Khang YH, Khatibzadeh S, Khoo JP, Kok C, Laden F, Lalloo R, Lan Q, Lathlean T, Leasher JL, Leigh J, Li Y, Lin JK, Lipshultz SE, London S, Lozano R, Lu Y, Mak J, Malekzadeh R, Mallinger L, Marcenes W, March L, Marks R, Martin R, McGale P, McGrath J, Mehta S, Mensah GA, Merriman TR, Micha R, Michaud C, Mishra V, Mohd Hanafiah K, Mokdad AA, Morawska L, Mozaffarian D, Murphy T, Naghavi M, Neal B, Nelson PK, Nolla JM, Norman R, Olives C, Omer SB, Orchard J, Osborne R, Ostro B, Page A, Pandey KD, Parry CDH, Passmore E, Patra J, Pearce N, Pelizzari PM, Petzold M, Phillips MR, Pope D, Pope CA III, Powles J, Rao M, Razavi H, Rehfuess EA, Rehm JT, Ritz B, Rivara FP, Roberts T, Robinson C, Rodriguez-Portales JA, Romieu I, Room R, Rosenfeld LC, Roy A, Rushton L, Salomon JA, Sampson U, Sanchez-Riera L, Sanman E, Sapkota A, Seedat S, Shi P, Shield K, Shivakoti R, Singh GM, Sleet DA, Smith E, Smith KR, Stapelberg NJC, Steenland K, Stöckl H, Stovner LJ, Straif K, Straney L, Thurston GD, Tran JH, Van Dingenen R, van Donkelaar A, Veerman JL, Vijayakumar L, Weintraub R, Weissman MM, White RA, Whiteford H, Wiersma ST, Wilkinson JD, Williams HC, Williams W, Wilson N, Woolf AD, Yip P, Zielinski JM, Lopez AD, Murray CJL, Ezzati M. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 380: 2224–2260, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25: 402–408, 2001. [DOI] [PubMed] [Google Scholar]
- 46.Mandhane PJ, Hanna SE, Inman MD, Duncan JM, Greene JM, Wang HY, Sears MR. Changes in exhaled nitric oxide related to estrogen and progesterone during the menstrual cycle. Chest 136: 1301–1307, 2009. [DOI] [PubMed] [Google Scholar]
- 47.Martinez FJ, Curtis JL, Sciurba F, Mumford J, Giardino ND, Weinmann G, Kazerooni E, Murray S, Criner GJ, Sin DD, Hogg J, Ries AL, Han M, Fishman AP, Make B, Hoffman EA, Mohsenifar Z, Wise R; National Emphysema Treatment Trial Research Group. Sex differences in severe pulmonary emphysema. Am J Respir Crit Care Med 176: 243–252, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Matute-Bello G, Downey G, Moore BB, Groshong SD, Matthay MA, Slutsky AS, Kuebler WM; Acute Lung Injury in Animals Study. An official American Thoracic Society workshop report: features and measurements of experimental acute lung injury in animals. Am J Respir Cell Mol Biol 44: 725–738, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McBride DE, Koenig JQ, Luchtel DL, Williams PV, Henderson WR Jr. Inflammatory effects of ozone in the upper airways of subjects with asthma. Am J Respir Crit Care Med 149: 1192–1197, 1994. [DOI] [PubMed] [Google Scholar]
- 50.McKittrick T, Adams WC. Pulmonary function response to equivalent doses of ozone consequent to intermittent and continuous exercise. Arch Environ Health 50: 153–158, 1995. [DOI] [PubMed] [Google Scholar]
- 51.Mikerov A, Umstead T, Gan X, Huang W, Guo X, Wang G, Phelps D, Floros J. Impact of ozone exposure on the phagocytic activity of human surfactant protein A (SP-A) and SP-A variants. Am J Physiol Lung Cell Mol Physiol 294: L121–L130, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mikerov AN, Cooper TK, Wang G, Hu S, Umstead TM, Phelps DS, Floros J. Histopathologic evaluation of lung and extrapulmonary tissues show sex differences in Klebsiella pneumoniae — infected mice under different exposure conditions. Int J Physiol Pathophysiol Pharmacol 3: 176–190, 2011. [PMC free article] [PubMed] [Google Scholar]
- 53.Mikerov AN, Gan X, Umstead TM, Miller L, Chinchilli VM, Phelps DS, Floros J. Sex differences in the impact of ozone on survival and alveolar macrophage function of mice after Klebsiella pneumoniae infection. Respir Res 9: 24, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mikerov AN, Haque R, Gan X, Guo X, Phelps DS, Floros J. Ablation of SP-A has a negative impact on the susceptibility of mice to Klebsiella pneumoniae infection after ozone exposure: sex differences. Respir Res 9: 77, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mudway IS, Kelly FJ. An investigation of inhaled ozone dose and the magnitude of airway inflammation in healthy adults. Am J Respir Crit Care Med 169: 1089–1095, 2004. [DOI] [PubMed] [Google Scholar]
- 56.Myers JR, Sherman CB. Should supplemental estrogens be used as steroid-sparing agents in asthmatic women? Chest 106: 318–319, 1994. [DOI] [PubMed] [Google Scholar]
- 57.Peng RD, Samoli E, Pham L, Dominici F, Touloumi G, Ramsay T, Burnett RT, Krewski D, Le Tertre A, Cohen A, Atkinson RW, Anderson HR, Katsouyanni K, Samet JM. Acute effects of ambient ozone on mortality in Europe and North America: results from the APHENA study. Air Qual Atmos Health 6: 445–453, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Poli V. The role of C/EBP isoforms in the control of inflammatory and native immunity functions. J Biol Chem 273: 29279–29282, 1998. [DOI] [PubMed] [Google Scholar]
- 59.Pope CA, Muhlestein JB, May HT, Renlund DG, Anderson JL, Horne BD. Ischemic heart disease events triggered by short-term exposure to fine particulate air pollution. Circulation 114: 2443–2448, 2006. [DOI] [PubMed] [Google Scholar]
- 60.Rao CK, Moore CG, Bleecker E, Busse WW, Calhoun W, Castro M, Chung KF, Erzurum SC, Israel E, Curran-Everett D, Wenzel SE. Characteristics of perimenstrual asthma and its relation to asthma severity and control: data from the Severe Asthma Research Program. Chest 143: 984–992, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Reibman J, Hsu Y, Chen LC, Bleck B, Gordon T. Airway epithelial cells release MIP-3alpha/CCL20 in response to cytokines and ambient particulate matter. Am J Respir Cell Mol Biol 28: 648–654, 2003. [DOI] [PubMed] [Google Scholar]
- 62.Rice MB, Ljungman PL, Wilker EH, Gold DR, Schwartz JD, Koutrakis P, Washko GR, O'Connor GT, Mittleman MA. Short-term exposure to air pollution and lung function in the Framingham Heart Study. Am J Respir Crit Care Med 188: 1351–1357, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Samet JM. The Clean Air Act and health—a clearer view from 2011. N Engl J Med 365: 198–201, 2011. [DOI] [PubMed] [Google Scholar]
- 64.Samet JM, Dominici F, Curriero FC, Coursac I, Zeger SL. Fine particulate air pollution and mortality in 20 U. S. cities, 1987–1994. N Engl J Med 343: 1742–1749, 2000. [DOI] [PubMed] [Google Scholar]
- 65.Sims EJ, Green MW, Mehta A. Decreased lung function in female but not male subjects with established cystic fibrosis-related diabetes. Diabetes Care 28: 1581–1587, 2005. [DOI] [PubMed] [Google Scholar]
- 66.Sørheim IC, Johannessen A, Gulsvik A, Bakke PS, Silverman EK, DeMeo DL. Gender differences in COPD: are women more susceptible to smoking effects than men? Thorax 65: 480–485, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Thorley AJ, Goldstraw P, Young A, Tetley TD. Primary human alveolar type II epithelial cell CCL20 (macrophage inflammatory protein-3alpha)-induced dendritic cell migration. Am J Respir Cell Mol Biol 32: 262–267, 2005. [DOI] [PubMed] [Google Scholar]
- 68.Umstead T, Phelps D, Floros J. The toll of ozone. Pneumon 24: 20–23, 2011. [Google Scholar]
- 69.Verthelyi D. Sex hormones as immunomodulators in health and disease. Int Immunopharmacol 1: 983–993, 2001. [DOI] [PubMed] [Google Scholar]
- 70.Williams AS, Issa R, Leung SY, Nath P, Ferguson GD, Bennett BL, Adcock IM, Chung KF. Attenuation of ozone-induced airway inflammation and hyper-responsiveness by c-Jun NH2 terminal kinase inhibitor SP600125. J Pharmacol Exp Ther 322: 351–359, 2007. [DOI] [PubMed] [Google Scholar]
- 71.Williams AS, Leung SY, Nath P, Khorasani NM, Bhavsar P, Issa R, Mitchell JA, Adcock IM, Chung KF. Role of TLR2, TLR4, and MyD88 in murine ozone-induced airway hyperresponsiveness and neutrophilia. J Appl Physiol 103: 1189–1195, 2007. [DOI] [PubMed] [Google Scholar]
- 72.Wulfsohn NL, Politzer WM, Henrico JS. Testosterone therapy in bronchial asthma. S Afr Med J 38: 170–172, 1964. [PubMed] [Google Scholar]
- 73.Yamazaki S, Shima M, Yoda Y, Oka K, Kurosaka F, Shimizu S, Takahashi H, Nakatani Y, Nishikawa J, Fujiwara K, Mizumori Y, Mogami A, Yamada T, Yamamoto N. Association of ambient air pollution and meteorological factors with primary care visits at night due to asthma attack. Environ Health Prev Med 18: 401–406, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yan C, Wu M, Cao J, Tang H, Zhu M, Johnson PF, Gao H. Critical role for CCAAT/enhancer-binding protein β in immune complex-induced acute lung injury. J Immunol 189: 1480–1490, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yancey AL, Watson HL, Cartner SC, Simecka JW. Gender is a major factor in determining the severity of mycoplasma respiratory disease in mice. Infect Immun 69: 2865–2871, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]