Abstract
Here, we tested the hypothesis that a promiscuous bacterial cyclase synthesizes purine and pyrimidine cyclic nucleotides in the pulmonary endothelium. To test this hypothesis, pulmonary endothelial cells were infected with a strain of the Gram-negative bacterium Pseudomonas aeruginosa that introduces only exoenzyme Y (PA103 ΔexoUexoT::Tc pUCPexoY; ExoY+) via a type III secretion system. Purine and pyrimidine cyclic nucleotides were simultaneously detected using mass spectrometry. Pulmonary artery (PAECs) and pulmonary microvascular (PMVECs) endothelial cells both possess basal levels of four different cyclic nucleotides in the following rank order: cAMP > cUMP ≈ cGMP ≈ cCMP. Endothelial gap formation was induced in a time-dependent manner following ExoY+ intoxication. In PAECs, intercellular gaps formed within 2 h and progressively increased in size up to 6 h, when the experiment was terminated. cGMP concentrations increased within 1 h postinfection, whereas cAMP and cUMP concentrations increased within 3 h, and cCMP concentrations increased within 4 h postinfection. In PMVECs, intercellular gaps did not form until 4 h postinfection. Only cGMP and cUMP concentrations increased at 3 and 6 h postinfection, respectively. PAECs generated higher cyclic nucleotide levels than PMVECs, and the cyclic nucleotide levels increased earlier in response to ExoY+ intoxication. Heterogeneity of the cyclic nucleotide signature in response to P. aeruginosa infection exists between PAECs and PMVECs, suggesting the intracellular milieu in PAECs is more conducive to cNMP generation.
Keywords: microtubule, pneumonia, second messenger, compartmentation, permeability
purine cyclic nucleotides (cAMP and cGMP) have been studied extensively and are widely recognized as canonical second messengers. cAMP has two important yet distinct roles in the control of endothelial barrier integrity. When produced near the plasma membrane by transmembrane cyclases, in response to a stimulus such as epinephrine, cAMP activates downstream targets, including exchange protein activated by cAMP (EPAC) and protein kinase A (PKA), which in turn signal effector proteins to stabilize adherens junctions leading to enhanced barrier protection (1, 21, 50). Conversely, activation of exogenous soluble adenylyl cyclases leads to increased cytosolic cAMP. This increase in cytosolic cAMP leads to hyperphosphorylation of the microtubule-associated protein tau and causes its dissociation from microtubules resulting in microtubule breakdown (5, 43, 44, 46, 51, 52), an effect that is sufficient to disrupt the endothelial cell barrier. Activation of soluble adenylyl cyclases causes endothelial cell rounding, loss of cellular adhesions, generation of interendothelial cell gaps, and tissue edema (51, 52, 56).
The Gram-negative bacterium Pseudomonas aeruginosa injects a type III secretion system (T3SS) effector protein ExoY directly in host cells, which acts as a promiscuous soluble cyclase once inside the host cell (4, 14). ExoY enzymatic activity increases cytosolic cAMP resulting in microtubule destabilization and endothelial barrier disruption (44, 52). In addition to cAMP, emerging evidence suggests ExoY generates other intracellular cyclic nucleotides, including cGMP and cUMP (4, 8), in B103 neuroblastoma and A549 lung carcinoma cells and whole lung tissue, although the contribution of specific cell types within the lung to the cyclic nucleotide signature remains unclear. Previously, our lab determined that ExoY+ intoxication results in an increase in both cAMP and cGMP in pulmonary microvascular endothelial cells (PMVECs) (44, 52), although it remains uncertain whether lung endothelium synthesizes pyrimidine cyclic nucleotides.
In the studies described here, the promiscuous P. aeruginosa cyclase ExoY was used as a stimulus for cyclic nucleotide production in the pulmonary endothelium. We tested the hypothesis that the pulmonary endothelium possesses multiple cyclic nucleotides whose levels can be raised by ExoY. Using a mass spectrometry analysis approach (9), we simultaneously measured cAMP, cGMP, cCMP, and cUMP in the same sample. Before ExoY+ infection, we determined whether the pulmonary endothelium possesses baseline levels of the pyrimidine cyclic nucleotides cCMP and cUMP. Our findings support this idea and suggest that the P. aeruginosa cyclase ExoY can selectively elevate these cyclic nucleotides in pulmonary artery and microvascular endothelium.
MATERIALS AND METHODS
Cell culture.
Pulmonary microvascular and pulmonary artery endothelial cells (internal identifications: PMVECR1 and PAECR16B) were obtained from the cell culture core at the University of South Alabama Center for Lung Biology. The isolation and characterization of these cells have been previously described in detail (28, 45). Cells were cultured as described previously (14). Briefly, cells were grown in Dulbecco's modified Eagle medium (DMEM) with 10% heat-inactivated fetal bovine serum (catalog no. 10082; Invitrogen, Carlsbad, CA) and 1% penicillin/streptomycin (catalog no. 15140; Invitrogen) at 37°C, in 21% O2 and 5% CO2.
Bacterial strains.
P. aeruginosa strains have been described in detail elsewhere (52, 60). One strain of P. aeruginosa, with an active ExoY toxin, was used (PA103 ΔexoUexoT::Tc pUCPexoY; ExoY+). Bacteria were taken from frozen stocks, grown overnight on solid agar/carbenicillin (400 μg/ml), and resuspended in PBS to an optical density of 0.25. This was previously determined to equal 2 × 108 bacteria/ml (52). Bacteria were subsequently diluted in Hanks' balanced salt solution (HBSS) to achieve the desired multiplicity of infection (MOI).
For bacterial infection, endothelial cells were trypsinized and counted using a Countess Automated Cell Counter (catalog no. C10227; Invitrogen) according to the manufacturer's instructions. Endothelial cells were grown 12–24 h postconfluence and then infected with P. aeruginosa ExoY+ at a MOI of 20:1 in HBSS and incubated for up to 6 h at 37°C, in 21% oxygen and 5% carbon dioxide.
Measurement of cAMP using enzyme immunoassays.
To assess the potential for cCMP, cUMP, cIMP, and cTMP to contaminate cAMP enzyme immunoassays (EIAs; Cayman Chemical), we assessed the ability of 15, 150, 1,500, and 15,000 pmol of cNMP to contaminate 4 or 150 pmol cAMP samples assayed in six-well plates. Four- and 150-pmol cAMP levels were chosen because they represent typical basal and stimulated (50 μM forskolin and 10 μM rolipram for 20 min) cAMP levels in PMVECs. Samples were treated as described previously (59). Briefly, reactions were terminated by addition of 1 N HCl (0.1 N HCl final), and plates were incubated on ice for 15 min. Apparent cAMP levels were measured using EIAs, and experiments were performed in triplicate. Data are presented as means ± SE.
Cell treatments.
Cells were grown 12–24 h postconfluence in a six-well plate in DMEM supplemented with 10% FBS (vol/vol) and 1% penicillin/streptomycin (vol/vol). Culture medium was removed, and cells were rinsed with 2 ml HBSS for 15 min at 37°C. After 15 min, HBSS was removed and replaced with either 1 ml of HBSS, 1 ml of HBSS with 500 μM isobutyl methylxanthine (IBMX) and 10 μM rolipram, or 1 ml of HBSS with sufficient P. aeruginosa ExoY+ for an infection at an MOI of 20:1. At termination of the experiment, cells were lysed via extraction protocol.
Extraction protocol.
At given experimental time points, cell culture plates were placed on ice, supernatant was removed, 300 μl of Extraction Buffer (2 parts acetonitrile, 2 parts methanol, 1 part H2O) were added, and cells were scraped using a cell lifter. Each well and scraper were rinsed two times each with 400 μl Extraction Buffer and dispensed equally in two Eppendorf tubes. The tubes were placed on ice until all time points were completed. Samples were heated at 98°C for 20 min. Samples were cooled on ice and centrifuged at 20,800 g for 10 min at 4°C. Supernatant (1 ml) was removed from each tube, put in individually labeled tubes, and placed in speed vacuum overnight until samples were completely dried. Cell pellets were frozen immediately and kept at −80°C for protein analysis.
Protein analysis.
BCA Protein Assay Kit was used according to the manufacturer's instructions for standard microplate protocol (catalog no. 23225; Pierce, Rockford, IL). Briefly, cell pellets were resuspended in 100 mM NaOH (150–600 μl depending on size of cell pellet). Each standard or sample replicate (25 μl) was dispensed in a microplate well. Kit working reagent (200 μl) was added to each well, and the plate was mixed thoroughly on a plate shaker for 30 s. The plate was covered and incubated at 37°C for 30 min. The microplate was cooled to room temperature, and absorbance was measured at or near 562 nm.
Mass spectrometry analysis.
A Nexera UFLC (Shimadzu) system coupled to the QTrap 5500 was applied for cNMP quantitation (9), except that separation was performed on an Agilent 1100 series (Waldbronn, Germany), and the QTrap 5500 triple quadrupole mass spectrometer (ABSCIEX, Foster City, CA) was used for detection. Ion source settings and collision gas pressure were manually optimized regarding ion source voltage, ion source temperature, nebulizer gas, and curtain gas [ion source voltage of 5,500 V, ion source temperature of 600°C, curtain gas of 30 pounds per square inch (psi), collisionally activated dissociation gas of 9 psi]. Nitrogen was used as the collision gas. Chromatographic data were collected and analyzed with Analyst 1.5.1 software (ABSCIEX).
Image acquisition and processing.
Phase-contrast microscope images were acquired using a Nikon Eclipse TS100 microscope. Images were captured using a Nikon Digital Sight DS-5M camera (no. 121099) at 20–25°C. Objective information is as follows: ×4 objective, Nikon 4x/0.10 WD 30 ∞/−; ×10 objective, Nikon 10x/0.25 Ph1 ADL WD 6.2 ∞/1.2; ×20 objective, Nikon LWD 20x/0.40 Ph1 ADL WD 3.0 ∞/1.2. Software was Nikon Digital Sight DS-L1. After image acquisition, brightness, contrast, setting to grayscale, and crop processing were done using Microsoft Powerpoint Mac 2008. No alterations were made to gamma settings at any point.
Cellular gap and rounded cell quantitation.
After multiple images (x20) were acquired at random using the approach detailed above for each cell type and each time point, number of gaps and rounded cells per field of view were manually counted using ImageJ software (version 1.47). This process was repeated in each of three independent experiments to determine each cell type's sensitivity to the effects of ExoY.
Statistical analyses.
GraphPad Prism 5.0 software package was used to conduct statistical analyses. Student's t-test or one-way ANOVA paired with Tukey's post hoc test was used to determine statistical significance with P < 0.05 considered significant. Specific analyses are detailed in the appropriate legends for Figs. 1–4.
Fig. 1.
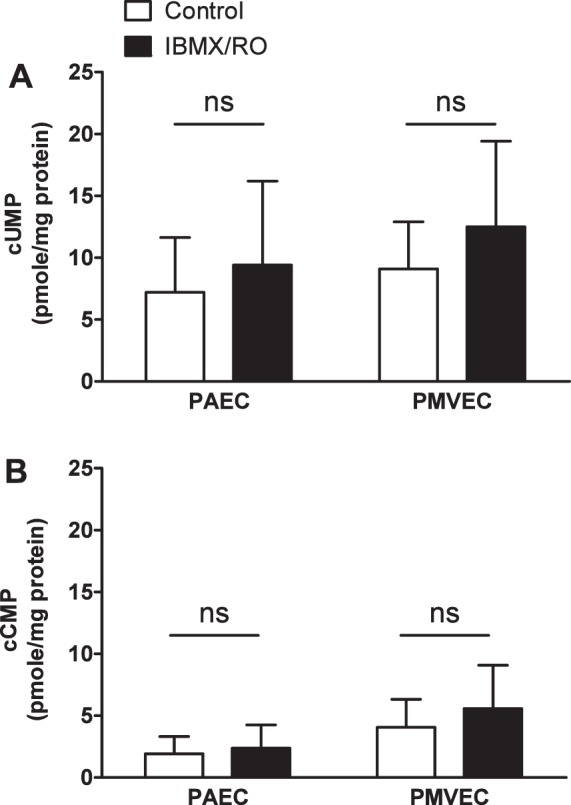
Pulmonary endothelium possesses baseline levels of nonclassical cyclic nucleotides cUMP and cCMP. Pulmonary artery endothelial cells (PAECs) and pulmonary microvascular endothelial cells (PMVECs) at 12–24 h postconfluence were treated with either Hanks' balanced salt solution (HBSS) buffer (control) or 500 μM isobutyl methylxanthine (IBMX) and 10 μM rolipram in HBSS (IBMX/RO). A: baseline cUMP levels range from ∼6 to 12 pmol/mg protein in both PAECs and PMVECs with no significant increase after IBMX/RO treatment. B: baseline cCMP levels range from ∼2 to 7 pmol/mg protein in both PAECs and PMVECs with no significant increase after IBMX/RO treatment. Cells were lysed in extraction buffer (acetonitrile, methanol, H2O) and pelleted by centrifugation. Supernatant was collected and dried and then subjected to mass spectrometry analysis to determine cyclic nucleotide levels. Cyclic nucleotide levels (pmol) were normalized to total protein content (mg protein). Values are averages from 3 independent experiments with error bars representing SD. Student's t-test was used to determine statistical significance between control and IBMX/RO for each cell type. NS, no statistical difference between the two groups analyzed.
Fig. 4.
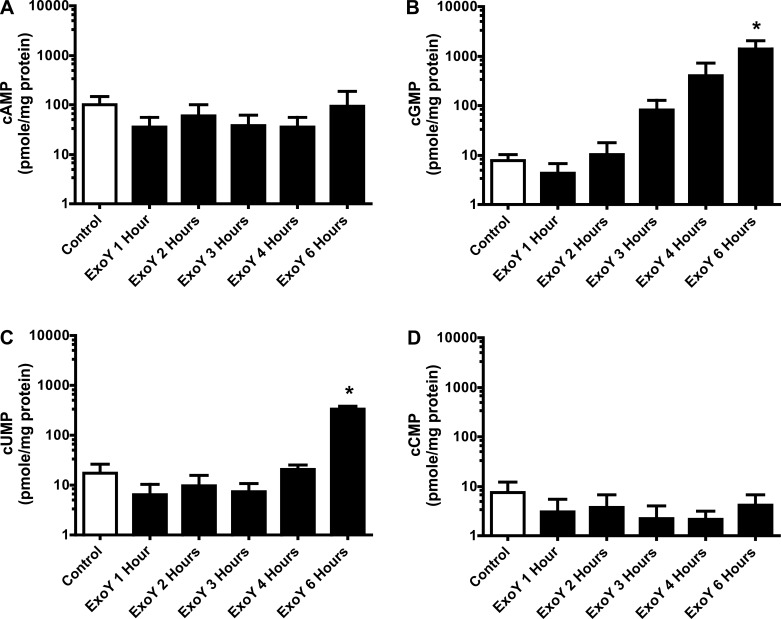
ExoY+ infection increases cGMP and cUMP but not cAMP or cCMP production in PMVECs in a time-dependent manner. PMVECs were inoculated with ExoY+ bacterial strain in HBSS for 6 h at an MOI of 20:1. A: inoculation with ExoY+ does not increase cAMP beyond baseline levels during the 6-h time course. B: beginning as early as 3 h after inoculation with ExoY+, cGMP levels increased in a time-dependent manner from ∼8 to 1,700 pmol/mg protein. C: beginning at 6 h after inoculation with ExoY+, cUMP levels increased from ∼17 to 380 pmol/mg protein. D: inoculation with ExoY+ does not increase cCMP beyond baseline levels during the 6-h time course. Values are averages (y-axes are on a log scale) from 3 independent experiments with error bars representing SD. One-way ANOVA paired with Tukey's post hoc test was used to determine statistical significance at each time point compared with control. *P < 0.05. Absence of * indicates there was no statistical significance between groups analyzed.
RESULTS
Pulmonary endothelium possesses basal levels of pyrimidine and purine cyclic nucleotides.
cAMP and cGMP are both constitutively formed by transmembrane and soluble adenylyl or guanylyl cyclases in many cell types (36, 48, 49), including pulmonary endothelium (29, 43), and hydrolyzed by specific phosphodiesterases (PDEs) (7, 19). The presence or absence of these PDEs plays a large role in the relative abundance of the cNMPs at a given time. The development and use of PDE inhibitors have aided in determining the physiological and pathophysiological roles of these two cNMPs (see Refs. 20, 38, and 39 and references therein). Although there have been sporadic studies focused on other cNMPs, such as cUMP and cCMP, the roles of these pyrimidine cNMPs remain elusive, especially in the endothelium. As such, we sought to determine the baseline levels of cUMP and cCMP in pulmonary artery endothelial cells (PAECs) and PMVECs.
Using a mass spectrometry analysis approach, we were able to simultaneously measure cAMP, cGMP, cUMP, and cCMP. Under baseline conditions, PAECs possessed 55 ± 33 pmol/mg protein cAMP, and PMVECs possessed 44 ± 21 pmol/mg protein cAMP. Addition of IBMX and rolipram increased cAMP approximately two- to threefold in both cell types. PAECs possessed 3.57 ± 2.69 pmol/mg protein cGMP under baseline conditions, and PMVECs possessed 4.83 ± 3.00 pmol/mg protein cGMP under baseline conditions. Treatment with IBMX and rolipram did not increase cGMP in either cell type. By mass spectrometry analysis, PAECs and PMVECs maintain low baseline levels of cGMP compared with cAMP.
Next, we sought to determine baseline levels of the pyrimidine cyclic nucleotides cUMP and cCMP. Under baseline conditions, both PAECs and PMVECs possess ∼5–10 pmol/mg protein of cUMP (Fig. 1A). We found these levels to be unchanged with the addition of IBMX and rolipram over a 2-h time course. Similarly, we found cCMP levels to reside between ∼2 and 4 pmol/mg protein (Fig. 1B). Again, IBMX and rolipram had no significant effect on cCMP levels in the pulmonary endothelium, suggesting that cUMP and cCMP are not targeted for degradation by IBMX and/or rolipram-sensitive PDEs in endothelium.
Pseudomonas aeruginosa ExoY disrupts PAEC and PMVEC barrier integrity.
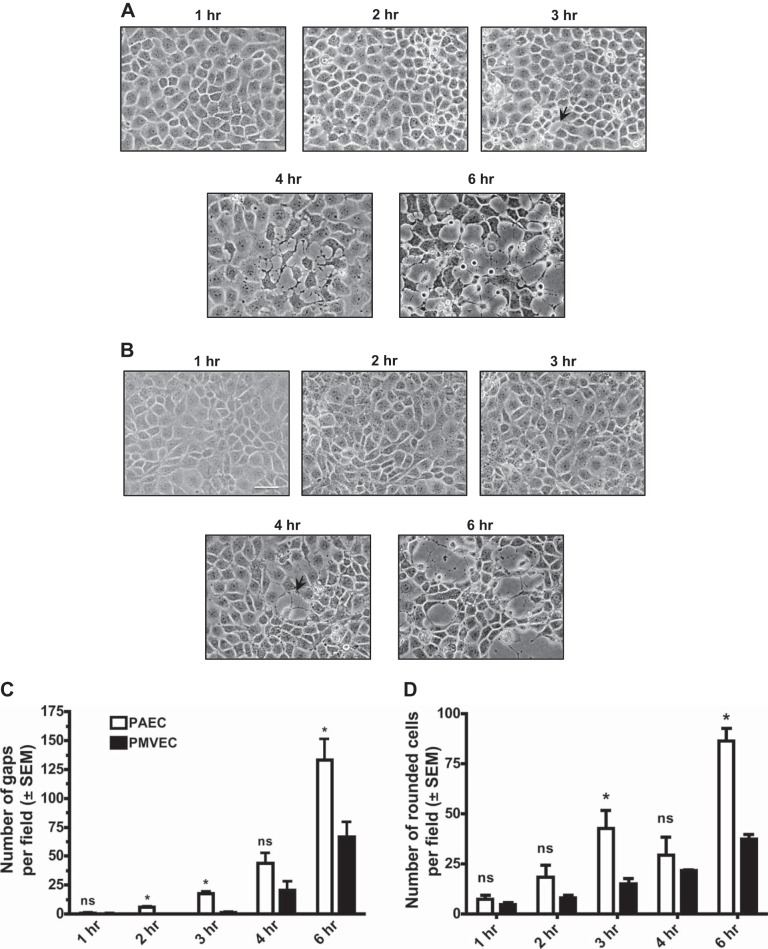
Previous studies have shown that ExoY is a promiscuous cyclase (4, 8), capable of elevating cAMP and cGMP in pulmonary endothelial cells (44). Because PAECs and PMVECs display heterogeneity in a variety of cellular responses, including migration and proliferative capacity (28), we sought to determine whether ExoY differentially increases permeability. PAECs and PMVECs were infected with P. aeruginosa ExoY+ at an MOI of 20:1. By capturing images over a 6-h time course, we determined that PAECs are sensitive to ExoY at earlier time points compared with PMVECs, as evidenced by noticeable interendothelial cell gaps forming by 3 h postinfection (Fig. 2A, 3rd image). In contrast, no gaps in the PMVEC monolayer were observed until 4 h postinfection (Fig. 2B). Thus, PAECs are more sensitive to ExoY-induced barrier disruption than PMVECs. With the use of gap formation as a quantitative measure, the differences in sensitivity to ExoY-induced barrier disruption are described in Fig. 2C. Because cellular gaps may arise from one or more cells, rounded cells were also assessed to determine the sensitivity of each cell type to ExoY. These differences are described in Fig. 2D.
Fig. 2.
PAECs are more sensitive to ExoY+ infection than PMVECs. PAECs and PMVECS were inoculated with the ExoY+ bacterial strain in HBSS for 6 h at a multiplicity of infection (MOI) of 20:1. A: beginning 1 h after inoculation with ExoY+, small cracks between PAECs progressed to large intercellular gap formation in a time-dependent manner. B: beginning 3 h after inoculation with ExoY+, small cracks between PMVECs progressed to large intercellular gap formation in a time-dependent manner. C: beginning 2 h after inoculation with ExoY+, there is a significant difference (indicated by *) in gap number between PAECs and PMVECs. Images in A and B are each representative of at least 3 separate experiments. Images in A and B were captured at ×20 magnification with the scale bar equal to 10 μm. Arrows indicate interendothelial cell gaps. In C, gaps were manually quantified using ImageJ software as an average no. of gaps across multiple fields of view chosen at random. Values are averages from 3 independent experiments with error bars representing SE. In D, rounded cells were manually quantified using ImageJ software as an average number of rounded cells across multiple fields of view chosen at random. Values are averages from 3 independent experiments with error bars representing SE. Student's t-test was used to determine statistical significance at each time point when comparing PAECs with PMVECs. *P < 0.05. ns, No statistical significance between groups analyzed.
ExoY is a promiscuous cyclase that uses purine and pyrimidine substrates.
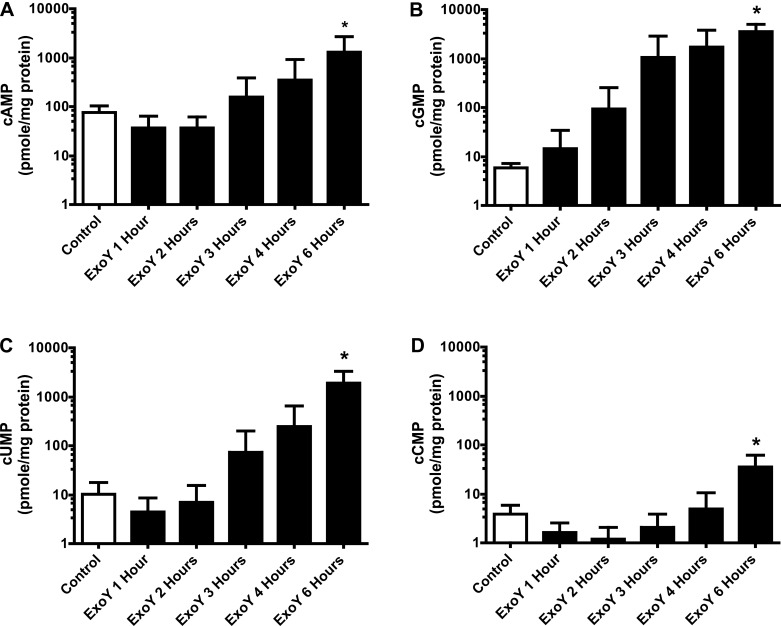
Because PAECs are more sensitive to ExoY-induced barrier disruption, we sought to determine whether ExoY also produced more cAMP in these cells. Employing a similar approach as in Fig. 2, we infected PAECs and PMVECs with ExoY+ at an MOI of 20:1 over a 6-h time course, collected cellular lysate, and subjected the lysate to mass spectrometry analysis to determine cNMP levels. We found that cAMP levels in PAECs began to increase 4 h postinfection, reaching a significant (∼50-fold) increase 6 h postinfection (Fig. 3A) compared with control. The cGMP concentration increased 1 h postinfection and by 6 h postinfection reached an ∼600- to 800-fold increase over control levels (Fig. 3B). cUMP concentrations increased in the same time frame as cAMP, with the initial increase observed 3 h postinfection, and increased ∼200-fold over control (Fig. 3C) by 6 h. Although ExoY induced large increases in cAMP, cGMP, and cUMP, the cCMP levels underwent a more modest increase (∼8-fold) 6 h postinfection, as is seen in Fig. 3D. Therefore, ExoY increased cNMPs in the following rank order in PAECS: cGMP > cUMP >> cAMP >>> cCMP.
Fig. 3.
ExoY+ infection increases PAEC cyclic nucleotide production in a time-dependent manner. PAECS were inoculated with ExoY+ bacterial strain in HBSS for 6 h at an MOI of 20:1. A: beginning at 3 h after inoculation with ExoY+, cAMP levels increased in a time-dependent manner from ∼20 to 1,500 pmol/mg protein. B: beginning as early as 1 h after inoculation with ExoY+, cGMP levels increased in a time-dependent manner from ∼8 to 3,500 pmol/mg protein. C: beginning at 3 h after inoculation with ExoY+, cUMP levels increased in a time-dependent manner from ∼10 to 2,000 pmol/mg protein. D: beginning at 4 h after inoculation with ExoY+, cCMP levels increased in a time-dependent manner from ∼4 to 40 pmol/mg protein. Values are averages (y-axes are on a log scale) from 4 independent experiments with error bars representing SD. One-way ANOVA paired with Tukey's post hoc test was used to determine statistical significance of each time point compared with control. *P < 0.05. Absence of * indicates there was no statistical significance between groups analyzed.
In contrast to the cNMP signature in PAECs, ExoY did not increase cAMP in PMVECs (Fig. 4A) using this mass spectrometry approach. However, ExoY increased cGMP 4–6 h postinfection, where the levels increased 100-fold (Fig. 4B) compared with control. There was no noticeable increase in cUMP until 6 h postinfection (Fig. 4C). Similar to cAMP, there was no observable increase in cCMP levels in PMVECs over the time course in response to ExoY+ infection (Fig. 4D).
Cyclic nucleotide response to ExoY+ infection is greater in PAECs compared with PMVECs.
Although cNMP concentrations were not different between cell types under basal conditions, ExoY generated a greater cNMP response in PAECs than PMVECs. This was especially the case at the 6-h time point, when the cNMP response was at its greatest. Here, ExoY increased cNMPs to 6,769 ± 1,529 pmol/mg protein in PAECs and 1,822 ± 439 pmol/mg protein in PMVECs (P < 0.05).
DISCUSSION
Purine cyclic nucleotides, cAMP and cGMP, are widely recognized canonical second messengers that activate protein effectors necessary to maintain cellular homeostasis. Both transmembrane and soluble purine nucleotidyl cyclases responsible for synthesis of cAMP and cGMP are expressed in the endothelium (17, 26). The physiological role of cAMP in endothelium has been widely studied, whereas the function of cGMP in this cell type is less evident. Agonists such as epinephrine activate transmembrane adenylyl cyclases and increase membrane-associated cAMP (33). The resulting cAMP elevation activates EPAC and PKA, which insert and stabilize adherens junction proteins in the plasma membrane, respectively, thereby reducing tissue edema (1, 21, 55). In contrast to this membrane-delimited cAMP signal, bicarbonate activation of a soluble adenylyl cyclase generates a cAMP signal that induces endothelial tau hyperphosphorylation leading to microtubule breakdown (46, 61), interendothelial cell gap formation, and increased paracellular permeability (5, 44, 56). Thus, the physiological response to cAMP is not only encoded by the purine cyclic nucleotide itself but, most importantly, by its enzymatic source and intracellular location.
Whereas cAMP, and to a lesser extent cGMP, have garnered considerable attention in endothelial cell biology, the mere existence of pyrimidine cyclic nucleotides in any cell type has been questioned. Pyrimidine cyclic nucleotides were first identified in the 1960s to 1980s (10, 11, 18, 23, 41, 42), with evidence for expression of enzymes that synthesize and degrade cUMP and/or cCMP (12, 23, 30, 31). However, studies were confounded by limitations in the available research tools, including antibodies that were not sufficiently selective to discriminate between contaminating molecular species. Because of controversy in the pyrimidine cyclic nucleotide field, and because of the emerging importance of purine cyclic nucleotides, cUMP and cCMP were not studied for several decades. Technological advances in mass spectrometry have enabled molecular detection of pyrimidine cyclic nucleotides with relatively high sensitivity and specificity (9). Recently, a variety of cells and tissues have been shown to constitutively possess cUMP and cCMP (4, 24). Mammalian soluble adenylyl and guanylyl cyclases are capable of synthesizing these cyclic nucleotides under some experimental conditions (3, 25), and phosphodiesterase 3A and 3B degrade cUMP (47) while phosphodiesterase 7A1 inactivates cCMP (40). Multidrug resistance proteins export cUMP and cCMP out of the cell (34), raising the possibility that cellular production of pyrimidine cyclic nucleotides may result in their extracellular accumulation. Although these data reveal the existence of cUMP and cCMP and mechanisms of their synthesis and degradation, the role(s) of these cyclic nucleotides in control of physiological processes remains uncertain. Here, we demonstrate that both PAECs and PMVECs possess cUMP and cCMP, at constitutive concentrations that resemble cGMP. However, a combination of IBMX and rolipram, which is sufficient to inhibit the activity of all of the cAMP-sensitive endothelial phosphodiesterases (including phosphodiesterase 3 and 7) (37), does not increase cUMP and cCMP concentrations. Future studies will be required to determine how pyrimidine cyclic nucleotides are synthesized and degraded in endothelium.
Although endogenous pyrimidine nucleotidyl cyclases have yet to be discovered, certain bacterial enzymes are capable of synthesizing cUMP and cCMP in mammalian cells. The Bacillus anthracis edema factor and the Bordetella pertussis toxin CyaA were first shown to be adenylyl cyclase toxins (27, 35), and later shown to also synthesize pyrimidine cyclic nucleotides (22). The P. aeruginosa type III secretion effector ExoY was initially described as an adenylyl cyclase (60), later recognized to be a promiscuous cyclase with both guanylyl and adenylyl cyclase activity (44), and most recently shown to be both a(n) uridylyl and cytidylyl cyclase (4, 54). In this latter example, studies were performed in B103 neuroblastoma and A549 lung carcinoma cells. While these studies laid the foundation for the ability of ExoY to synthesize pyrimidine cyclic nucleotides, we provide direct evidence that ExoY produces purine and pyrimidine cyclic nucleotides in lung endothelium, a cell type of high physiological relevance to the pulmonary vascular community. We now recognize ExoY as a promiscuous cyclase, meaning that it is capable of simultaneously synthesizing more than one cyclic nucleotide. This finding was a surprise. Endogenously expressed mammalian cyclic nucleotidyl cyclases are highly selective in their substrate selection, where adenylyl cyclases synthesize cAMP but not cGMP, and guanylyl cyclases synthesize cGMP but not cAMP. Point mutations in these cyclases interconvert substrate specificity, yet even in this case, the mammalian enzymes do not synthesize more than one cyclic nucleotide species. In this regard, the bacterial nucleotidyl cyclase enzymes represent an exception to the rule. Interestingly, ExoY's nucleotidyl cyclase activity is attributed to residues 1–212 (of 384 amino acids), where the lysine at residue 81 is required for substrate recognition (60). Substituting lysine 81 for methionine abolishes all of ExoY's nucleotidyl cyclase activity, inclusive of purine and pyrimidine cyclic nucleotides (4, 15, 52, 60). In our system, infection with ExoYK81M coincides with a distinct lack of pulmonary endothelial barrier disruption (data not shown). In a recent study using A549 and B103 cells as the target of P. aeruginosa infection, cNMP levels were unchanged in response to ExoYK81M (8). In a pathophysiologically relevant model of lung injury, Bahre et al. infected mice with P. aeruginosa ExoY+ and found that cGMP and cUMP levels increase, whereas ExoYK81M did not alter cyclic nucleotide levels (3). These findings suggest that ExoY, and not other bacterial factors, is responsible for the increase in purine and pyrimidine cyclic nucleotides in multiple systems.
The idea that ExoY is a promiscuous cyclase was borne out in our present studies, where ExoY+ intoxication of PAECs induced a time-dependent increase in cGMP, followed by cUMP, cAMP, and cCMP. The cGMP elevation not only occurred first, but it was the cyclic nucleotide that increased to the greatest degree. Mechanisms responsible for the promiscuous ExoY enzymatic activity remain unknown, especially as it relates to the temporal nature and relative magnitude of the respective cyclic nucleotide signatures. It may be that the intracellular ExoY location, the localized substrate abundance, or ExoY posttranslational modification(s) determine which cyclic nucleotide is synthesized. ExoY requires a mammalian cofactor for activity, and preliminary work suggests that actin stimulates cyclase activity (53). There are a number of prokaryotic enzymes requiring eukaryotic cofactors (2). The enzymes tend to be highly flexible molecules perhaps facilitating their transfer in eukaryotic cells and allowing wider substrate recognition. Cofactors tend to be molecules in high cellular concentrations, which provide conditions for bacterial toxins or enzymes to evolve to find these binding partners. The binding of cofactors in the correct eukaryotic environment regulates activity and ensures that the toxin is not prematurely activated within the producing bacterium, which due to the promiscuous nature of substrate recognition could be toxic. Overall, further studies will be required to more fully understand the molecular basis of ExoY's enzymatic activity.
ExoY generated purine and pyrimidine cyclic nucleotides in both PAECs and PMVECs. However, we found that the cyclic nucleotides accumulated earlier and in greater abundance in PAECs than they did in PMVECs. The T3SS introduces ExoY in both cell types (data not shown), yet ExoY appears to have much greater catalytic activity in PAECs than in PMVECs. The reason for such differential catalytic activity among cell types is unknown, but may provide insight into the enzyme's cofactor. Alternatively, the microvascular cells may extrude or degrade cyclic nucleotides more rapidly, possibilities that have not been fully ruled out.
The present studies have revealed a discrepancy in the ExoY-induced cAMP signal in PMVECs, especially when compared with earlier work. Here, we did not observe an ExoY-induced increase in cAMP above baseline levels in PMVECs; rather, the response was dominated by increases in cGMP and cUMP. Previously we have observed very prominent ExoY-induced increases in cAMP beginning ∼3 h postinfection (44, 52). A principal difference in our earlier studies and those reported here is the approach used to detect cAMP. Previously we used radioimmunoassays or EIAs (14, 54), which rely on antibody recognition of cAMP for resolving signal sensitivity and specificity. We wondered whether either cGMP or cUMP could cross-react with the cAMP antibody and contaminate the cAMP signal (Table 1). To test this idea, cAMP was clamped at either a low or high concentration in the presence of ascending purine and pyrimidine cyclic nucleotides. At low cAMP concentrations, 3,750-fold excess cGMP and cUMP contaminated the cAMP measurement ∼10-fold; this contamination was not seen at high cAMP concentrations. It is therefore possible that, in the absence of a rise in basal cAMP concentrations, substantial elevations in cGMP and cUMP may be detected by cAMP radioimmunoassays and EIAs, leading to a modest false positive cAMP measurement. This contaminated signal cannot explain the pronounced cAMP elevation previously reported, however. Thus, we cannot explain why a cAMP response was absent in PMVECs in our present experiments, especially since a typical ExoY-induced cAMP response was observed in PAECs using the mass spectrometry approach.
Table 1.
Assessment of the potential for cCMP, cUMP, cIMP, and cTMP to contaminate cAMP enzyme immunoassays
| Normalized cAMP Reading |
||
|---|---|---|
| cNMP Added, pmol | 4 pmol cAMP | 150 pmol cAMP |
| 0 cNMP | 1.00 ± 0.00 | 1.00 ± 0.00 |
| 15 cGMP | 1.37 ± 0.49 | 1.05 ± 0.08 |
| 150 cGMP | 1.48 ± 0.02 | 1.06 ± 0.08 |
| 1,500 cGMP | 4.3 ± 0.1 | 1.23 ± 0.14 |
| 15,000 cGMP | 9.6 ± 0.3 | 1.71 ± 0.26 |
| 15 cCMP | 0.88 ± 0.07 | 1.10 ± 0.10 |
| 150 cCMP | 0.73 ± 0.19 | 1.04 ± 0.12 |
| 1,500 cCMP | 1.70 ± 0.28 | 1.08 ± 0.11 |
| 15,000 cCMP | 5.3 ± 0.4 | 1.36 ± 0.15 |
| 15 cUMP | 0.99 ± 0.14 | 1.39 ± 0.31 |
| 150 cUMP | 1.21 ± 0.30 | 0.95 ± 0.07 |
| 1,500 cUMP | 3.6 ± 2.4 | 0.97 ± 0.04 |
| 15,000 cUMP | 11.7 ± 8.6 | 1.18 ± 0.17 |
Data were normalized to readings for cAMP alone and are presented as means ± SE, performed in triplicate. Indicated amounts of cNMP were added to known amounts of cAMP (4 or 150 pmol cAMP as indicated). Apparent cAMP levels were measured using cAMP enzyme immunoassays.
ExoY acts as an edema factor because of its ability to generate purine and pyrimidine cyclic nucleotides. Both cAMP and cGMP contribute to endothelial cell barrier disruption, although the cAMP signal is most effective at disrupting the endothelial cell barrier (14). cAMP activates PKA, which phosphorylates the endothelial cell tau protein resulting in microtubule disruption, cell rounding, and loss of cell-cell adhesions (14). The cGMP signal can also activate PKA and protein kinas G, resulting in modest endothelial tau phosphorylation. We have questioned whether ExoY consumes GTP from the microtubule cap, impairing microtubule assembly and growth; this possibility has not yet been tested. The contribution(s) of cUMP and cCMP to endothelial barrier disruption remain unknown.
P. aeruginosa gains access to pulmonary endothelium through the distal airway following disruption of alveolar epithelium (15) or, alternatively, through the circulation following systemic infection (16). This bacterium displays a vascular tropism, especially for the lung microcirculation (32, 58). However, there is no evidence for direct interaction between P. aeruginosa and pulmonary artery endothelium, since bacilli associated with the bronchovascular bundle tend to accumulate in the perivascular interstitium (56a, 13, 57). Thus, we would anticipate that PMVECs would represent a pathophysiologically relevant ExoY target cell, whereas PAECs would be less likely to encounter the exoenzyme. Interestingly, PAECs were more sensitive to ExoY-induced barrier disruption than were PMVECs, consistent with their increase in cyclic nucleotide accumulation.
In summary, we report the presence of pyrimidine cyclic nucleotides in PAECs and PMVECs under constitutive conditions. ExoY increases both purine and pyrimidine cyclic nucleotides in the following rank order: cGMP > cUMP >>> cAMP (in PAECs) >> cCMP. This increase in cyclic nucleotides occurs in parallel with endothelial cell barrier disruption. ExoY enzymatic activity is greater in PAECs than it is in PMVECs, which results in earlier and more profound barrier disruption in the macrovascular endothelial cell type. Whereas the production of cGMP and cAMP by soluble nucleotidyl cyclases is sufficient to disrupt the endothelial cell barrier, at present, the role(s) of cUMP and cCMP and their effector proteins on endothelial cell barrier disruption remain undetermined.
GRANTS
This work was supported by American Heart Association postdoctoral fellowship Grant 14POST18080004 (K. A. Morrow) and National Heart, Lung, and Blood Institute Grants HL-60024 and HL-66299 (T. Stevens) and HL-07612 and HL-107122 (C. D. Ochoa).
DISCLOSURES
No conflicts of interest, financial or otherwise are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: K.A.M., R.S., and T.S. conception and design of research; K.A.M., V.K., A.L.B., S.L.S., C.D.O., and E.A.C. performed experiments; K.A.M., V.K., A.L.B., and T.S. analyzed data; K.A.M., T.C.R., and T.S. interpreted results of experiments; K.A.M., T.C.R., and T.S. prepared figures; K.A.M. drafted manuscript; K.A.M., R.S., V.K., S.L.S., C.D.O., D.W.F., T.C.R., and T.S. edited and revised manuscript; K.A.M., R.S., V.K., A.L.B., S.L.S., C.D.O., E.A.C., D.W.F., T.C.R., and T.S. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Linn Ayers, Annette Garbe, Allison Lawrence, and Anna Buford for their contribution to the development of this work.
REFERENCES
- 1.Adamson RH, Liu B, Fry GN, Rubin LL, Curry FE. Microvascular permeability and number of tight junctions are modulated by cAMP. Am J Physiol Heart Circ Physiol 274: H1885–H1894, 1998. [DOI] [PubMed] [Google Scholar]
- 2.Anderson DM, Sato H, Dirck AT, Feix JB, Frank DW. Ubiquitin activates patatin-like phospholipases from multiple bacterial species. J Bacteriol 197: 529–541, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bahre H, Danker KY, Stasch JP, Kaever V, Seifert R. Nucleotidyl cyclase activity of soluble guanylyl cyclase in intact cells. Biochem Biophys Res Commun 443: 1195–1199, 2014. [DOI] [PubMed] [Google Scholar]
- 4.Bahre H, Hartwig C, Munder A, Wolter S, Stelzer T, Schirmer B, Beckert U, Frank DW, Tummler B, Kaever V, Seifert R. cCMP and cUMP occur in vivo. Biochem Biophys Res Commun 460: 909–914, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Balczon R, Prasain N, Ochoa C, Prater J, Zhu B, Alexeyev M, Sayner S, Frank DW, Stevens T. Pseudomonas aeruginosa exotoxin Y-mediated tau hyperphosphorylation impairs microtubule assembly in pulmonary microvascular endothelial cells. PloS one 8: e74343, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beavo JA. Cyclic nucleotide phosphodiesterases: functional implications of multiple isoforms. Physiol Rev 75: 725–748, 1995. [DOI] [PubMed] [Google Scholar]
- 8.Beckert U, Wolter S, Hartwig C, Bahre H, Kaever V, Ladant D, Frank DW, Seifert R. ExoY from Pseudomonas aeruginosa is a nucleotidyl cyclase with preference for cGMP and cUMP formation. Biochem Biophys Res Commun 450: 870–874, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beste KY, Burhenne H, Kaever V, Stasch JP, Seifert R. Nucleotidyl cyclase activity of soluble guanylyl cyclase alpha1beta1. Biochemistry 51: 194–204, 2012. [DOI] [PubMed] [Google Scholar]
- 10.Bloch A. Cytidine 3′,5′-monophosphate (cyclic CMP). I. Isolation from extracts of leukemia L-1210 cells. Biochem Biophys Res Commun 58: 652–659, 1974. [DOI] [PubMed] [Google Scholar]
- 11.Bloch A. Uridine 3′,5′-monophosphate (cyclic UMP). I. Isolation from rat liver extracts. Biochem Biophys Res Commun 64: 210–218, 1975. [DOI] [PubMed] [Google Scholar]
- 12.Bloch A, Cheng YC. Modulation of cyclic CMP-specific phosphodiesterase activity by polyamines and by cyclic purine nucleotides. Adv Enzyme Regul 17: 283–287, 1978. [DOI] [PubMed] [Google Scholar]
- 13.Bonifacio SL, Kitterman JA, Ursell PC. Pseudomonas pneumonia in infants: an autopsy study. Hum Pathol 34: 929–938, 2003. [DOI] [PubMed] [Google Scholar]
- 14.Bonilla-Abadia F, Munoz-Buitron E, Ochoa CD, Carrascal E, Canas CA. A rare association of localized scleroderma type morphea, vitiligo, autoimmune hypothyroidism, pneumonitis, autoimmune thrombocytopenic purpura and central nervous system vasculitis. Case report. BMC Res Notes 5: 689, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boyer S, Faure K, Ader F, Husson MO, Kipnis E, Prangere T, Leroy X, Guery BP. Chronic pneumonia with Pseudomonas aeruginosa and impaired alveolar fluid clearance. Respir Res 6: 17, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brigham KL, Woolverton WC, Blake LH, Staub NC. Increased sheep lung vascular permeability caused by pseudomonas bacteremia. J Clin Invest 54: 792–804, 1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Buchan KW, Martin W. Modulation of barrier function of bovine aortic and pulmonary artery endothelial cells: dissociation from cytosolic calcium content. Br J Pharmacol 107: 932–938, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cech SY, Ignarro LJ. Cytidine 3′,5′-monophosphate (cyclic CMP) formation by homogenates of mouse liver. Biochem Biophys Res Commun 80: 119–125, 1978. [DOI] [PubMed] [Google Scholar]
- 19.Drummond GI, Perrott-Yee S. Enzymatic hydrolysis of adenosine 3′,5′-phosphoric acid. J Biol Chem 236: 1126–1129, 1961. [PubMed] [Google Scholar]
- 20.Fan Chung K. Phosphodiesterase inhibitors in airways disease. Eur J Pharmacol 533: 110–117, 2006. [DOI] [PubMed] [Google Scholar]
- 21.Fukuhara S, Sakurai A, Sano H, Yamagishi A, Somekawa S, Takakura N, Saito Y, Kangawa K, Mochizuki N. Cyclic AMP potentiates vascular endothelial cadherin-mediated cell-cell contact to enhance endothelial barrier function through an Epac-Rap1 signaling pathway. Mol Cell Biol 25: 136–146, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gottle M, Dove S, Kees F, Schlossmann J, Geduhn J, Konig B, Shen Y, Tang WJ, Kaever V, Seifert R. Cytidylyl and uridylyl cyclase activity of bacillus anthracis edema factor and Bordetella pertussis CyaA. Biochemistry 49: 5494–5503, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hardman JG, Sutherland EW. A cyclic 3′,5′-nucleotide phosphodiesterase from heart with specificity for uridine 3′,5′-phosphate. J Biol Chem 240: 3704–3705, 1965. [PubMed] [Google Scholar]
- 24.Hartwig C, Bahre H, Wolter S, Beckert U, Kaever V, Seifert R. cAMP, cGMP, cCMP and cUMP concentrations across the tree of life: High cCMP and cUMP levels in astrocytes. Neurosci Lett 579: 183–187, 2014. [DOI] [PubMed] [Google Scholar]
- 25.Hasan A, Danker KY, Wolter S, Bahre H, Kaever V, Seifert R. Soluble adenylyl cyclase accounts for high basal cCMP and cUMP concentrations in HEK293 and B103 cells. Biochem Biophys Res Commun 448: 236–240, 2014. [DOI] [PubMed] [Google Scholar]
- 26.He P, Curry FE. Differential actions of cAMP on endothelial [Ca2+]i and permeability in microvessels exposed to ATP. Am J Physiol Heart Circ Physiol 265: H1019–H1023, 1993. [DOI] [PubMed] [Google Scholar]
- 27.Hewlett EL, Urban MA, Manclark CR, Wolff J. Extracytoplasmic adenylate cyclase of Bordetella pertussis. Proc Natl Acad Sci USA 73: 1926–1930, 1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.King J, Hamil T, Creighton J, Wu S, Bhat P, McDonald F, Stevens T. Structural and functional characteristics of lung macro- and microvascular endothelial cell phenotypes. Microvasc Res 67: 139–151, 2004. [DOI] [PubMed] [Google Scholar]
- 29.Klinger JR. The nitric oxide/cGMP signaling pathway in pulmonary hypertension. Clin Chest Med 28: 143–167, 2007. [DOI] [PubMed] [Google Scholar]
- 30.Klotz U, Stock K. Evidence for a cyclic nucleotide-phosphodiesterase with high specificity for cyclic uridine-3′,5′-monophosphate in rat adipose tissue. Naunyn Schmiedebergs Arch Pharmakol 269: 117–120, 1971. [DOI] [PubMed] [Google Scholar]
- 31.Kuo JF, Brackett NL, Shoji M, Tse J. Cytidine 3′:5′-monophosphate phosphodiesterase in mammalian tissues. Occurrence and biological involvement. J Biol Chem 253: 2518–2521, 1978. [PubMed] [Google Scholar]
- 32.Lange M, Hamahata A, Enkhbaatar P, Esechie A, Connelly R, Nakano Y, Jonkam C, Cox RA, Traber LD, Herndon DN, Traber DL. Assessment of vascular permeability in an ovine model of acute lung injury and pneumonia-induced Pseudomonas aeruginosa sepsis. Crit Care Med 36: 1284–1289, 2008. [DOI] [PubMed] [Google Scholar]
- 33.Langeler EG, van Hinsbergh VW. Norepinephrine and iloprost improve barrier function of human endothelial cell monolayers: role of cAMP. Am J Physiol Cell Physiol 260: C1052–C1059, 1991. [DOI] [PubMed] [Google Scholar]
- 34.Laue S, Winterhoff M, Kaever V, van den Heuvel JJ, Russel FG, Seifert R. cCMP is a substrate for MRP5. Naunyn Schmiedebergs Arch Pharmacol 387: 893–895, 2014. [DOI] [PubMed] [Google Scholar]
- 35.Leppla SH. Anthrax toxin edema factor: a bacterial adenylate cyclase that increases cyclic AMP concentrations of eukaryotic cells. Proc Natl Acad Sci USA 79: 3162–3166, 1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lucas KA, Pitari GM, Kazerounian S, Ruiz-Stewart I, Park J, Schulz S, Chepenik KP, Waldman SA. Guanylyl cyclases and signaling by cyclic GMP. Pharmacol Rev 52: 375–414, 2000. [PubMed] [Google Scholar]
- 37.Lugnier C, Schoeffter P, Le Bec A, Strouthou E, Stoclet JC. Selective inhibition of cyclic nucleotide phosphodiesterases of human, bovine and rat aorta. Biochem Pharmacol 35: 1743–1751, 1986. [DOI] [PubMed] [Google Scholar]
- 38.Maurice DH. Cardiovascular implications in the use of PDE5 inhibitor therapy. Int J Impot Res 16, Suppl 1: S20–S23, 2004. [DOI] [PubMed] [Google Scholar]
- 39.Mayer M, Stief CG, Truss MC, Uckert S. Phosphodiesterase inhibitors in female sexual dysfunction. World J Urol 23: 393–397, 2005. [DOI] [PubMed] [Google Scholar]
- 40.Monzel M, Kuhn M, Bahre H, Seifert R, Schneider EH. PDE7A1 hydrolyzes cCMP. FEBS Lett 588: 3469–3474, 2014. [DOI] [PubMed] [Google Scholar]
- 41.Newton RP, Hakeem NA, Salvage BJ, Wassenaar G, Kingston EE. Cytidylate cyclase activity: identification of cytidine 3′,5′-cyclic monophosphate and four novel cytidine cyclic phosphates as biosynthetic products from cytidine triphosphate. Rapid Commun Mass Spectrom 2: 118–126, 1988. [DOI] [PubMed] [Google Scholar]
- 42.Newton RP, Kingston EE, Hakeem NA, Salih SG, Beynon JH, Moyse CD. Extraction, purification, identification and metabolism of 3′,5′-cyclic UMP, 3′,5′-cyclic IMP and 3′,5′-cyclic dTMP from rat tissues. Biochem J 236: 431–439, 1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Obiako B, Calchary W, Xu N, Kunstadt R, Richardson B, Nix J, Sayner SL. Bicarbonate disruption of the pulmonary endothelial barrier via activation of endogenous soluble adenylyl cyclase, isoform 10. Am J Physiol Lung Cell Mol Physiol 305: L185–L192, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ochoa CD, Alexeyev M, Pastukh V, Balczon R, Stevens T. Pseudomonas aeruginosa exotoxin Y is a promiscuous cyclase that increases endothelial tau phosphorylation and permeability. J Biol Chem 287: 25407–25418, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ochoa CD, Stevens T, Balczon R. Cold exposure reveals two populations of microtubules in pulmonary endothelia. Am J Physiol Lung Cell Mol Physiol 300: L132–L138, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Prasain N, Alexeyev M, Balczon R, Stevens T. Soluble adenylyl cyclase-dependent microtubule disassembly reveals a novel mechanism of endothelial cell retraction. Am J Physiol Lung Cell Mol Physiol 297: L73–L83, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Reinecke D, Burhenne H, Sandner P, Kaever V, Seifert R. Human cyclic nucleotide phosphodiesterases possess a much broader substrate-specificity than previously appreciated. FEBS Lett 585: 3259–3262, 2011. [DOI] [PubMed] [Google Scholar]
- 48.Rich TC, Fagan KA, Nakata H, Schaack J, Cooper DM, Karpen JW. Cyclic nucleotide-gated channels colocalize with adenylyl cyclase in regions of restricted cAMP diffusion. J Gen Physiol 116: 147–161, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rich TC, Fagan KA, Tse TE, Schaack J, Cooper DM, Karpen JW. A uniform extracellular stimulus triggers distinct cAMP signals in different compartments of a simple cell. Proc Natl Acad Sci USA 98: 13049–13054, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sayner SL. Emerging themes of cAMP regulation of the pulmonary endothelial barrier. Am J Physiol Lung Cell Mol Physiol 300: L667–L678, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sayner SL, Alexeyev M, Dessauer CW, Stevens T. Soluble adenylyl cyclase reveals the significance of cAMP compartmentation on pulmonary microvascular endothelial cell barrier. Circ Res 98: 675–681, 2006. [DOI] [PubMed] [Google Scholar]
- 52.Sayner SL, Frank DW, King J, Chen H, VandeWaa J, Stevens T. Paradoxical cAMP-induced lung endothelial hyperpermeability revealed by Pseudomonas aeruginosa ExoY. Circ Res 95: 196–203, 2004. [DOI] [PubMed] [Google Scholar]
- 53.Schneider EH, Seifert R. Report on the Third Symposium “cCMP and cUMP as New Second Messengers”. Naunyn Schmiedebergs Arch Pharmacol 388: 1–3, 2015. [DOI] [PubMed] [Google Scholar]
- 54.Seifert R. cCMP and cUMP: emerging second messengers. Trends Biochem Sci 40: 8–15, 2015. [DOI] [PubMed] [Google Scholar]
- 55.Siflinger-Birnboim A, Bode DC, Malik AB. Adenosine 3′,5′-cyclic monophosphate attenuates neutrophil-mediated increase in endothelial permeability. Am J Physiol Heart Circ Physiol 264: H370–H375, 1993. [DOI] [PubMed] [Google Scholar]
- 56.Stevens TC, Ochoa CD, Morrow KA, Robson MJ, Prasain N, Zhou C, Alvarez DF, Frank DW, Balczon R, Stevens T. The Pseudomonas aeruginosa exoenzyme Y impairs endothelial cell proliferation and vascular repair following lung injury. Am J Physiol Lung Cell Mol Physiol 306: L915–L924, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56a.Swartz MN, Castleman B. Case records of the Massachusetts General Hospital. Case 15–1966. Extensive burn followed by Pseudomonas septicemia, hypotension, anuria, and death. N Engl J Med 274: 736–744, 1966.4956063 [Google Scholar]
- 57.Teplitz C. Pathogenesis of Pseudomonas vasculitis and septic legions. Arch Pathol 80: 297–307, 1965. [PubMed] [Google Scholar]
- 58.Winn WC, LaSala PR, Leslie KO. Bacterial Infections. In: Pulmonary Pathology, edited by Tomashefski JF, Cagle PT, Farver CF, and Fraire AE. New York, NY: Springer, 2008, p. 228–315. [Google Scholar]
- 59.Xin W, Tran TM, Richter W, Clark RB, Rich TC. Roles of GRK and PDE4 activities in the regulation of beta2 adrenergic signaling. J Gen Physiol 131: 349–364, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yahr TL, Vallis AJ, Hancock MK, Barbieri JT, Frank DW. ExoY, an adenylate cyclase secreted by the Pseudomonas aeruginosa type III system. Proc Natl Acad Sci USA 95: 13899–13904, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhu B, Zhang L, Creighton J, Alexeyev M, Strada SJ, Stevens T. Protein kinase A phosphorylation of tau-serine 214 reorganizes microtubules and disrupts the endothelial cell barrier. Am J Physiol Lung Cell Mol Physiol 299: L493–L501, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]