Abstract
Severe bacterial infection can lead to inflammation, host tissue damage, and ultimately disseminated septic shock. The mammalian innate immune system responds to microbial infection through the detection of invariant pathogen-associated molecular patterns (PAMPs) by a range of pattern recognition receptors (PRRs) expressed by the host cell. A successful immune response involves tightly coordinated signaling from these receptors, leading to a robust transcriptional response producing cytokines and antimicrobial effectors. While the PRR-expressing phagocytes of the host innate immune system function to contain and degrade internalized bacteria through pathways such as selective autophagy, pathogenic bacteria may subvert this process to replicate in the host cell. We describe the development of imaging assays to investigate these host–pathogen interactions through gene perturbation screens, which could lead to the identification of novel effectors of the host response to bacterial infection. We identify markers of coordinated initial signaling in macrophages challenged with ligands to PRRs of the toll-like receptor (TLR) family and compare this response to that induced by intact bacteria of the Burkholderia cenocepacia complex (Bcc), an opportunistic pathogen that causes life-threatening infections in patients with cystic fibrosis and chronic granulomatous disease. Bcc has been shown to escape the endocytic pathway, activate selective autophagy, and replicate within human macrophages. We demonstrate robust image-based quantification of multiple stages of Bcc infection of macrophages: ubiquitin tagging of cytosolic bacteria, recruitment of selective autophagy effector proteins, and intracellular bacterial replication, and we show perturbation of bacterial replication using drug treatment or siRNA-based gene knockdown. The described panel of imaging assays can be extended to other bacterial infections and pathogenic ligand combinations where high-content siRNA screening could provide significant new insight into regulation of the innate immune response to infection.
Introduction
During bacterial infection, innate immune cells are presented with multiple pathogen-associated molecular patterns (PAMPs), which they recognize through families of pattern recognition receptors (PRRs), such as the toll-like receptor (TLR) family.1 Macrophages reside in tissues and are professional initiators, effectors, and integrators of innate immune signaling through multiple PRR pathways. Engagement of these pathways leads to the activation of components in one or more of the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), interferon regulatory factor (IRF), and mitogen-activated protein kinase (MAPK)-dependent transcription factor families, with the subsequent expression of numerous inflammatory cytokines and immune mediators.2,3 Despite remarkable progress in characterizing the individual PRR signaling responses, relatively few studies have addressed how innate immune cells integrate the cocktail of PAMPs presented by a typical microorganism. It would thus be of great value to identify the molecular mechanisms that underlie the cross talk between different PRR signaling pathways based on the nature of the PAMP combinations presented to the macrophage.
Autophagy is a conserved eukaryotic process by which cytoplasmic material is sequestered by a multi-membraned autophagosome that fuses with a lysosome to degrade intramembranous material.4 Selective autophagy is a means to remove protein aggregates and damaged organelles and is often triggered by ubiquitin tagging of the targeted material.5 It has been established that in addition to the PRR pathways described above, the host selective autophagy pathway can be used as a defense mechanism against intracellular bacteria that enter the host cytosol. Cellular markers for selective autophagy include ubiquitin tagging and colocalization of autophagy effector proteins such as LC3B.6 While there is evidence that PRR ligands can induce colocalization of autophagy effectors with PRR pathway components, the interplay between the autophagy and PRR host defense pathways is poorly understood.
The gram-negative bacterium, Burkholderia cenocepacia, is a member of the Burkholderia cenocepacia complex (Bcc), a group of 17 phenotypically similar species that are found in nature and are opportunistic human pathogens.7,8 B. cenocepacia can be acquired from the environment and in immunocompromised patients such as those with cystic fibrosis or chronic granulomatous disease; an infection with B. cenocepacia can be fatal.7,9 Like other gram-negative bacteria, B. cenocepacia possess numerous PAMPs, including lipopolysaccharide, which activates TLR4.10 Following the initial sensing of the bacterium, human macrophages initiate PRR-activated signaling cascades to activate a host response. Recent studies have shown that B. cenocepacia can infect and replicate inside human macrophages through active subversion of the host autophagy response.11 This bacterium therefore represents a relevant experimental model to investigate multiple aspects of the innate immune response to bacterial infection.
The development of high-throughput screening approaches to microscopy analysis has established a powerful high-content screening (HCS) technology to interrogate host–pathogen interactions. To study the host genes involved in the response to microbial stimuli, it is essential to develop assays conducive to RNAi screening that can monitor host innate pathway activation and quantify intracellular bacteria. Some methods, including transmission electron microscopy and confocal microscopy, do not lend themselves to high-content studies as these methods are exceedingly time-consuming. By contrast, HCS analyses using automated microscopes and sophisticated image analysis software permit the implementation of large-scale screening approaches, such as in testing of antibiotic efficiency,12 measurement of morphological changes in bacteria following drug treatment, and detection of bacteria with amphiphilic carbon dots.13 However, few studies to date have analyzed bacteria in situ within a cellular host using quantitative whole-bacterial imaging assays and combined such assays with RNAi-based gene knockdown to establish a platform for host factor perturbation screening.
We describe the development of assays to quantify host macrophage signaling responses to bacterial stimuli, colocalization of bacteria with host proteins at multiple stages of the host selective autophagy response, and the measurement of intracellular bacterial replication using large-scale in vitro screening platforms. Assay readouts include the detection of post-translational modifications of signaling proteins to interrogate the modulatory mechanisms of TLR4 and TLR2 pathways as a prototype for multiligand signaling in macrophages, with a comparison of cellular responses to synthetic ligands versus intact bacteria. We also monitor bacterial localization with intracellular proteins of the autophagy pathway, using an approach that allows the quantification of intracellular bacteria and the manual determination of parameters that highlight colocalization events, excluding extracellular bacteria and selecting viable cells. Using the CellInsight NXT colocalization protocols with select parameter modifications, we have developed assays amenable to both 96-well and 384-well formats, which, combined with recently developed robust siRNA transfection protocols in macrophages,14 provide a versatile experimental platform for the interrogation of the macrophage response to bacterial infection.
Materials and Methods
Cell Culture
Immortalized murine macrophages (IMMs) derived from C57BL/6 mice were cultured in DMEM, 10% fetal bovine serum (FBS), 20 mM HEPES, and 2 mM glutamine. The IMM cell suspension was passed through a 40 μm cell strainer to prevent clumping of cells and to allow an evenly distributed seeding in the wells. This is especially important if we intend to leave cells in the same wells for more than 48 h. Two hundred microliters of IMM cells at a concentration of 0.5 × 105 cells/mL were seeded in 96-well plates (BD Falcon Catalog No. 353219) and incubated overnight before assay. The human monocytic macrophage cell line THP1 stably expressing GFP-LC3B (generously supplied by Dr. John Kehrl) was cultured in RPMI1640 with 2 mM glutamine and 10% heat-inactivated FBS. One hundred microliters of THP1 cells at a concentration of 5 × 105 cells/mL were seeded in 96-well plates (BD Falcon Catalog No. 353219) in media containing 50 ng/mL phorbol 12-myristate 13-acetate (PMA; Sigma) 48 h before assay to induce differentiation of cells into a macrophage-like state. Both mouse IMM and human THP1 cell lines were passaged at least twice from frozen stocks before use in assays and were not used beyond passage 15. IMM cells were passaged thrice per week to maintain adherent cell confluence at less than 90%. THP1 cells were diluted to a cell density of 5 × 104 cells/mL at passage, and cells were passaged twice per week to maintain suspension cell densities below 3 × 105 cells/mL.
siRNA Transfection
THP1 cells were reverse transfected with siRNA using HiPerFect (Qiagen) following a previously described protocol.14 Nontargeting negative control siRNAs (NTC2 and 5) were from Dharmacon, and siRNA targeting human p62/SQSTM1 were from Dharmacon (D-010230-02) and Ambion (s16961).
TLR Ligand Treatment
IMM culture media was replaced 2 h before TLR ligand treatment. TLR ligands were prepared to their final concentration in IMM media warmed to 37°C, and at the desired time points, the media from the appropriate wells was aspirated and replaced with media containing the ligands. TLR ligand sources: lipid A was from Avanti Polar Lipids, 699500P; Pam3CSK4 (P3C) was from InvivoGen, Cat. No. tlrl-pms.
Bacterial Growth
B. cenocepacia J2315 strain bacteria were grown on blood agar plates at 37°C for 3 days. Single colonies removed from the plates were incubated in 25 mL of Luria–Bertani (LB) broth at 37°C with shaking for 16–18 h to grow bacteria to the mid-log phase.
Imaging Assays for Macrophage Signaling Responses to TLR Ligands and Bacterial Infection
IMMs seeded and treated as described in Table 1 were fixed in 4% paraformaldehyde (PFA) at 37°C for 20 min. The fixed cells were blocked in PBST-BSA (5% [w/v] bovine serum albumin [BSA] in 0.1% [v/v] Tween 20 in 1× phosphate-buffered saline [PBS]). The fixed cells were treated with primary antibody against phosphorylated activating transcription factor 2 (pATF2) (Rabbit, 1:200; Cell Signaling Technology Catalog No. 5112P) or acetylated-p65 (Ac-p65, Rabbit, 1:200; Cell Signaling Technology Catalog No. 3045P) at 4°C overnight. Following overnight incubation, the cells were washed three to five times in PBST-BSA. These cells were then incubated for at least 1 h at room temperature with secondary antibody (Goat anti-Rabbit IgG, Alexa Fluor® 488 conjugate, 1:1,000; Life Technologies Catalog No. A-11008). This was followed by PBST-BSA wash (three to five times) and nuclear staining by Hoechst 33342 (NucBlue® Live ReadyProbes® Reagent; Life Technology Catalog No. R37605). The plates were sealed with a clear film (Axygen Catalog No. PCR-SP) and imaged on a CellInsight NXT using the general intensity measurement bioapplication from Thermo Scientific™ HCS Studio™ cell analysis software as per parameters described in Table 2.
Table 1.
Screening of Multi-TLR Signaling in PAMP-Treated or Bacteria-Infected IMMs
| Step | Parameter | Value | Description |
|---|---|---|---|
| Cell culture and ligand treatment (1–5) | |||
| 1 | Plate IMMs | 200 μL | 0.5 × 105 cells/mL; 96-well plates |
| 2 | Incubate cells | 24 h | 37°C, 5% CO2, 95% humidity |
| 3 | Aspirate and incubate with fresh medium | 2 h | cDMEM at 37°C |
| 4 | Treat cells with PAMPs | 200 μL, 0–24 h | Aspirate medium from appropriate wells and add fresh medium containing PAMPs at desired concentration. Incubate at 37°C, 5% CO2, 95% humidity for the desired length of time |
| 5 | Aspirate and wash (2×) with PBS | 100 μL | Prewarmed PBS at 37°C without Ca2+/Mg2+ (100 μL/well, 2×) |
| Fixation and staining (6–14) | |||
| 6 | Fixation | 100 μL, 20 min | Aspirate and add 4% PFA in PBS without Ca2+/Mg2+, 37°C |
| 7 | Blocking and permeabilization | 100 μL | Aspirate and add 5% (w/v) BSA in 0.1% (v/v) Tween 20 in 1× PBS. At room temperature for at least 1 h or overnight at 4°C |
| 8 | Primary antibody staining | 100 μL | Use appropriate dilutions of primary antibodies prepared in blocking solution described above. Incubate on rocker overnight at 4°C |
| 9 | Wash and aspirate (3–5×) | 100 μL | Wash with blocking solution for at least 5 min per wash on rocker at room temperature. Aspirate at end of each wash cycle |
| 10 | Secondary antibody staining | 100 μL | At room temperature for at least 1 h on a rocker. The plate needs to be protected from light from this point onward |
| 11 | Wash and aspirate (3–5×) | 100 μL | Repeat as described in step 9 |
| 12 | Nuclear staining | 100 μL, 20 min | Add four drops of Hoechst 33342 in 20 mL of blocking solution and use this solution for nuclear staining. Incubate on rocker for ∼20 min |
| 13 | Wash and aspirate (1–2×) | 100 μL | Repeat as described in step 11. At the end of the wash cycle, add 200 μL of blocking solution per well |
| 14 | Seal plates | Use clear film to seal the plates | |
| Image acquisition and data analysis (15–17) | |||
| 15 | Acquire images | 20×, 0.45 NA objective | Autofocus was applied using the DAPI channel (Ch1). Up to three channels—green, red, and far red—are imaged in sequence. Imaging was performed using a Cell Insight NXT |
| 16 | Assay readout | The general intensity measurement bioapplication was used to count Ch2 (green stained) objects located within circle (nucleus) and ring (cytoplasm) masks defined by Ch1 objects (Hoechst stained) | |
| 17 | Analysis | Ratios of nuclear (circle) to cytoplasmic (ring) Ch2 intensities are calculated for all objects defined by Ch1. The basal mean +2SD values are calculated for each treatment. Mean intensities are then calculated for all cells above basal mean +2SD. These mean values are then normalized to maximum values in each treatment | |
Step Notes
1. These cells are good for setting up ligand treatment for imaging up to 3 days after which point they become overconfluent.
7. Blocking solution is made in bulk. It is filter sterilized and stored at 4°C. Filtration reduces particles that can cause nonspecific background staining and the sterility helps keep the plates good for imaging for over 4 months when stored in the dark at 4°C.
10–14. Plates should be protected from light.
16. General intensity measurement bioapplication from Thermo Scientific™ HCS Studio™ Cell Analysis Software.
BSA, bovine serum albumin; HCS, high-content screening; IMM, immortalized murine macrophage; PAMP, pathogen-associated molecular pattern; PBS, phosphate-buffered saline; PFA, paraformaldehyde.
Table 2.
| Channel 1: Nuclei | Value | |
|---|---|---|
| Dye/channel | Hoechst 33342 | Excite at 386 |
| Thresholding | Fixed intensity | 100 |
| Segmentation | Shape | 1 |
| Object.Area.Ch1 | Filters | Range within 2SD of mean for basal cells |
| Object.AvgIntensity.Ch1 | Filters | Range within 2SD of mean for basal cells |
| Channel 2: pATF2 or Ac-p65 | ||
| Dye/channel | Green | Excite at 485 |
| Thresholding | None | — |
| Selection | Circle around Ch1 | 10 |
| Region of interest ring Ch2 | Cytoplasm mask | Width 10, distance 0 |
| Region of interest circ Ch2 | Nuclear mask | 0 |
Imaging Assay for Bacterial Replication in Macrophages
THP1 cells were infected with bacteria at a multiplicity of infection (MOI) of 1 as described in Table 3. B. cenocepacia J2315 was used as a wild-type (WT) strain and formalin-killed (FK) bacteria were used as a control. To formalin-kill bacteria, 1 mL of bacteria was centrifuged at 6,000 g for 6 min, then resuspended in 4% formaldehyde in PBS. Following a 30-min incubation, bacteria were centrifuged again at 6,000 g for 6 min and resuspended in RPMI media. Cells were infected at an MOI of 1 as described previously,11 and this time was considered time zero. Following a 1 h infection, the cells were incubated with an antibiotic combination of gentamicin (250 μg/mL) and ceftazidime (500 μg/mL). Following 2 h of antibiotic treatment, media were replaced with antibiotic-free media. Infected cells were subsequently incubated in 5% CO2 at 37°C for variable time intervals as indicated, then cells were washed thrice with PBS to remove extracellular bacteria. Cells were fixed using 4% PFA for 10 min. Following one wash with PBS, cells were permeabilized with 0.1% Triton X-100 diluted in PBS for 10 min. Cells were then washed thrice in PBS and blocked in 5% BSA diluted in PBS for a minimum of 45 min at room temperature. Cells were then incubated with primary antibodies against B. cenocepacia (rabbit 1:1,000) and ubiquitin (mouse 1:300; EnzoLifeSciences BML PW8810) diluted in 5% BSA in PBS overnight at 4°C. The following day, cells were washed thrice in PBS, then incubated in secondary antibodies (anti-rabbit Alexa Fluor 647 [Life Technologies] and anti-mouse IgG Fab2 Alexa Fluor 555 [Cell Signaling Technology], both 1:4,000) diluted in 5% BSA in PBS for 1 h at room temperature. Cells were washed twice in PBS, then incubated for 20 min in NucBlue, diluted one drop/mL in PBS at room temperature. Plates were washed twice more in PBS, then stored in 5% BSA in PBS at 4°C, shielded from light.
Table 3.
Burkholderia cenocepacia and Ubiquitin Staining for High-Content Imaging
| Step | Parameter | Value | Description |
|---|---|---|---|
| 1 | Differentiation | 48 h | Human THP1 monocytes were differentiated to macrophages by treating with 50 ng/mL PMA and grown in RPMI media with 10% heat-inactivated FBS |
| 2 | Rapamycin treatment | 50 μg/mL | If required for experimental protocol, treat cells with rapamycin for 2 h before infection |
| 3 | Infect cells | 1 h | Burkholderia cenocepacia J2315 was added to cells in fresh media (no rapamycin) at an MOI of 1 (5 × 105 bacteria/mL) |
| 4 | Combination antibiotic treatment | 2 h | Infected media was aspirated and replaced with media treated with 250 μg/mL gentamicin and 500 μg/mL ceftazidime |
| 5 | Media replacement | 3–21 h | Antibiotic media was replaced with nonantibiotic RPMI for the remainder of the time course |
| 6 | Fixation | 3×, 10 min | Cells were washed thrice in PBS and fixed with 4% PFA |
| 7 | Permeabilize cells | 10 min | Cells were washed once in PBS and permeabilized with Triton X-100 0.1% |
| 8 | Block cells | 1 h | Cells were washed twice in PBS and blocked in 5% BSA-PBS |
| 9 | Primary and secondary antibody staining; nuclear staining | As described in Table 1, steps 8–14 |
FBS, fetal bovine serum; PMA, phorbol 12-myristate 13-acetate; MOI, multiplicity of infection.
Bacteria were enumerated using Thermo Scientific HCS Studio Cell Analysis Software according to parameters described in Table 4. First, nuclear dye was excited at 385 nm, and nuclei were gated based on their intensity (>700), clumped nuclei were separated based on intensity differences (>300 was a separate nucleus), and only nuclei that fell within Object.Area.Ch1 of 245–2,400 were considered to be viable nuclei. Bacteria were excited at 650 nm and identified based primarily on their intensity (>5,000). Bacteria were segmented based on their intensities (>1) and had to have a consistently high intensity of ObjectCh2.VarIntensity.Ch2 between 675 and 4,000. The extent of the bacterial infection was measured based on the percentage of cells with at least one bacterium as determined by the %HIGH_ObjectCountCh2, and the number of infected cells was calculated by multiplying %HIGH_ObjectCountCh2 by ValidObjectCount. The total number of bacteria was calculated by multiplying MEAN_ObjectCountCh2 by ValidObjectCount, and the number of bacteria per infected cell was calculated by dividing the total bacteria by the total number of infected cells. In preliminary experiments to establish optimal conditions for imaging of bacterial infection in the microplate format, bacterial replication was quantified by measuring mean fluorescence intensity in the cytoplasm, following a protocol for cytosolic segmentation described previously.14
Table 4.
CellInsight NXT Settings for Figures 3–5
| Channel 1: Nuclei | Value | |
|---|---|---|
| Dye/channel | Hoechst 33342 | Excite at 386 |
| Thresholding | Fixed intensity | 700 |
| Segmentation | Intensity | 300 |
| Object.Area.Ch1 | 245–2,400 | |
| Channel 2: Bacteria | ||
| Dye/channel | Alexa Fluor 647 | Excite at 650 |
| Thresholding | Fixed intensity | 5,000 |
| Segmentation | Intensity | 1 |
| ObjectCh2.VarIntensity.Ch2 | 675–4,000 | |
| Channel 3: Ubiquitina | ||
| Dye/channel | Alexa Fluor 555 | Excite at 549 |
| Thresholding | Fixed intensity | 4,200 |
| Segmentation | Intensity | 1 |
| ObjectCh3.VarIntensity.Ch3 | 180–2,700 | |
| Channel 3: LC3a | ||
| Dye/channel | GFP (stably expressed) | Excite at 488 |
| Thresholding | Fixed intensity | 1,000 |
| Segmentation | Intensity | 1 |
| ObjectCh3.VarIntensity.Ch3 | 180–2,700 | |
| ROI A Mask Creation | Channel 2 | |
| Target I | Channel 3 | |
Ubiquitin and GFP-LC3 are imaged in channel 3 in separate protocols.
Imaging Assays for Macrophage Selective Autophagy Response to Cytosolic Bacteria
Autophagy was induced where indicated using rapamycin 50 μg/mL (Sigma) 2 h before bacterial infection. Bacterial infection and staining protocols were identical to the above stated section, although infections were halted at shorter time points (4–8 h). For 2 h infections, bacteria were allowed to infect for 1 h, then they were challenged with antibiotics for 1 h before cells were washed and fixed. For 30-min infections, bacteria were allowed to infect for 30 min, then cells were washed and fixed.
Colocalization events were determined using Thermo Scientific HCS Studio Cell Analysis Software according to parameters described in Table 5. Nuclear and bacterial identification methods are identical to the above. Ubiquitin puncta were excited at 549 nm, identified based on intensity (>4,200), segmented based on intensity (>1), and had to fall within the ObjectCh3.VarIntensity.Ch3 range of 180–2,700. LC3-GFP was excited at 488 nm, identified based on intensity (>1,000), segmented based on intensity (>1), and likewise had to fall within the ObjectCh3.VarIntensity.Ch3 range of 180–2,700. Bacteria were considered to be the object of interest, where ubiquitin and LC3B were the targets of interest. The MEAN_ROI_A_Target_I_ObjectCount would thus identify the number of times there was a colocalization event between the bacteria and target, averaged over the total number of cells.
Table 5.
CellInsight NXT Colocalization Exported Parameters
| Parameter | Channel | Expected Range |
|---|---|---|
| ValidObjectCount | Ch1 | 300–500 |
| MEAN_ROI_A_Target_I_ObjectCount | Ch3 | 0–0.1 |
| MEAN_ObjectCountCh2 | Ch2 | 0–10 |
| % HIGH_ObjectCountCh2 | Ch2 | 0–20 |
| MEAN_ObjectCountCh3 | Ch3 | 0–7 |
Confocal Microscopy
This method was performed as previously described.11
Results
Quantitative Imaging of MAPK and NF-κB Pathway Activation by TLR Ligands and Bacterial Infection in Mouse Macrophages
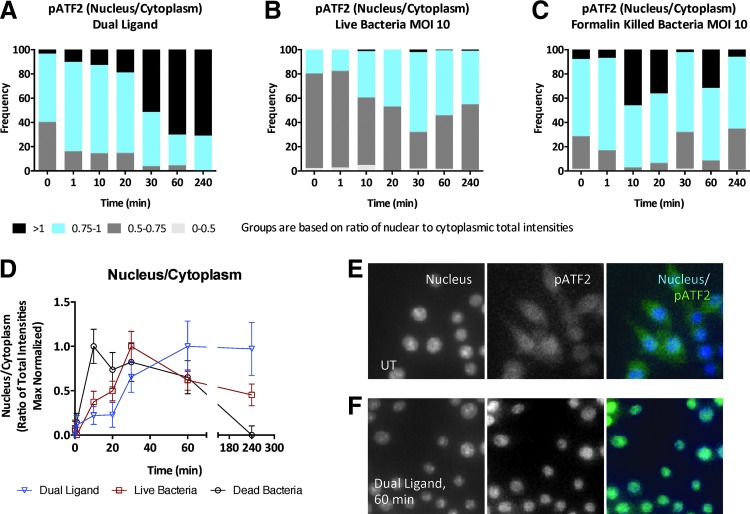
To develop an HCS protocol that would reflect the PRR signaling pathway activation in macrophages, we developed a methodology using phosphoprotein antibodies specific to targets in the two major pathways activated downstream of TLRs, the MAPK/AP1 pathway and the NF-κB pathway (see protocol in Table 1). We first challenged IMMs with a TLR ligand combination of 10 nM lipid A (TLR4) and 10 nM P3C (TLR2/TLR1) and measured the nuclear translocation kinetics of phosphorylated (Thr71) ATF2 (pATF2). ATF2 is a member of the ATF/CREB family of leucine zipper proteins and is a component of activator protein 1 (AP1) transcription factor dimers targeted by the JNK and p38 MAPK signaling pathways. Cells were imaged on a CellInsight NXT high-content imager using the general intensities bioapplication. Dual TLR ligand stimulation induced a robust increase in nuclear translocation of pATF2 that peaked at 60 min and was sustained until 4 h (Fig. 1A). Figure 1E and F show representative images at 20× that show nuclear translocation of pATF2 upon dual TLR stimulation. We then compared pATF2 translocation in IMMs infected with live (Fig. 1B) or FK (Fig. 1C) B. cenocepacia J2315 strain at an MOI of 10. Cells treated with either live or killed B. cenocepacia show an earlier peak in nuclear translocation of pATF2 between 10 and 30 min (Fig. 1B, C). To compare the dynamics across the three stimulations, we calculated the median basal intensity of pATF2 in each stimulation condition. The medians of nucleus-to-cytoplasmic intensity ratios of cells with ratios within the 5th and 95th percentile are plotted normalized to maximum in Figure 1D, demonstrating that the responses to live and killed bacteria peak earlier than the response to dual TLR ligands. However, the overall magnitude of the pATF2 response is greater with dual ligand and killed bacteria and appears attenuated with live bacteria (Fig. 1A–C). A total of ∼2,000 cells were imaged for each experiment.
Fig. 1.
Imaging dual toll-like receptor (TLR) and bacterial infection-induced mitogen-activated protein kinase (MAPK) response in mouse macrophages. Wild-type (WT) immortalized murine macrophages (IMMs) were either (A) stimulated with dual TLR ligands—10 nM each of P3C and lipid A—or (B, C) infected with live or formalin-killed (FK) Burkholderia cenocepacia J2315 at multiplicity of infection (MOI) 10 for up to 4 h and the ratio of nuclear to cytoplasmic intensity of the MAPK-activated transcription factor, phosphorylated activating transcription factor 2 (pATF2), was calculated. (D) The median nuclear-to-cytoplasmic ratios of ATF2 normalized to maximum. (E, F) Images at 20× magnification captured by the CellInsight NXT are shown for untreated and dual ligand treatment for 60 min. Data shown are representative of two independent experiments (D; median+median absolute deviation), and number of cells imaged were 1,964 cells for dual TLR ligand treatment, 2,106 cells for live bacterial infection, and 2,009 cells for killed bacterial infection.
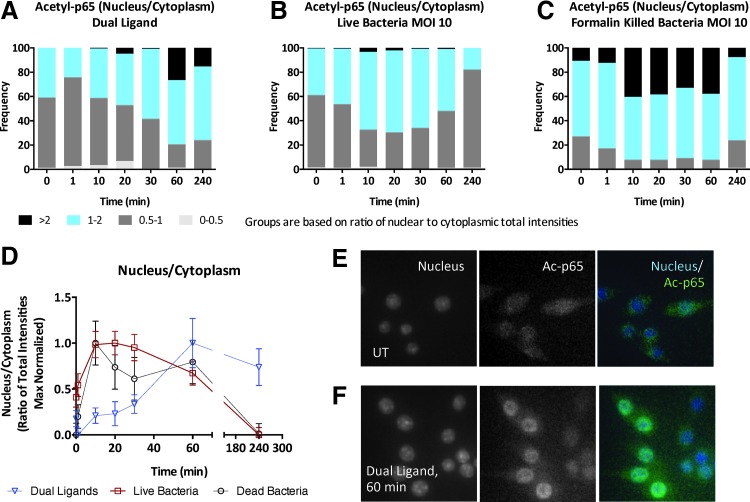
To further compare the signaling response of mouse macrophages to synthetic TLR ligand combinations versus intact bacterial infection, we measured the nuclear translocation kinetics of acetylated (Lys310) p65/RelA. p65 is a component of the canonical NF-κB pathway activated in macrophages in response to bacterial stimuli, and DNA binding and transcriptional activities of p65 are regulated by acetylation of lysines 210, 221, and 310. Dual TLR ligand stimulation induced an increase in nuclear translocation of Ac-p65 that peaked at 60 min and was sustained until 4 h (Fig. 2A). We then compared Ac-p65 translocation in IMMs infected with B. cenocepacia J2315 strain at an MOI of 10. Cells treated with either live (Fig. 2B) or killed (Fig. 2C) B. cenocepacia show an earlier peak in nuclear translocation of Ac-p65 between 10 and 20 min. Similar to the pATF2 response (Fig. 1A–C), the magnitude of the Ac-p65 response was highest in the killed bacteria and dual ligand conditions and lower with live bacteria (Fig. 2A–C). Figure 2D compares the mean of the nuclear-to-cytoplasmic ratios of all three stimulations. Only cells within the 5th and 95th percentile are included and the data are normalized to maximum to allow plotting of different stimulations on the same scale. Comparing Figures 1D and 2D shows that Ac-p65 peaks earlier than pATF2, suggesting that the NF-κB pathway is activated faster than the MAPK pathways on live bacterial challenge. In addition, the slope of pATF2 translocation is sharper than Ac-p65 nuclear translocation and this could be a reflection of the ultrasensitivity inherent in MAPK pathways.15 The dual ligand stimulation appears to induce peak nuclear translocation at 60 min for both pATF2 and Ac-p65, but finer sampling between 30 and 60 min would be required to conclude if pATF2 and Ac-p65 nuclear translocations peak at the same time.
Fig. 2.
Imaging dual TLR and bacterial infection-induced nuclear factor kappa-light-chain-enhancer of activated B cell (NF-κB) response in mouse macrophages. WT IMMs were either (A) stimulated with dual TLR ligands—10 nM each of P3C and lipid A—or (B, C) infected with live or formalin-killed B. cenocepacia J2315 at MOI 10 for up to 4 h and the ratio of nuclear to cytoplasmic intensity of acetylated-p65 (Ac-p65) was calculated. (D) The median nuclear-to-cytoplasmic ratios of Ac-p65 normalized to maximum. (E, F) Images at 20× magnification captured by the CellInsight NXT are shown for untreated and dual ligand treatment for 60 min. Data shown are representative of two independent experiments (D; median+median absolute deviation), and number of cells imaged were 1,930 cells for dual TLR ligand treatment, 2,228 cells for live bacterial infection, and 2,167 cells for killed bacterial infection.
The differences in translocation kinetics (Figs. 1D and 2D) of activated transcription factors in bacterial versus synthetic ligand-treated cells could be because the effective dose of TLR ligands in bacterial infection is different than in the dual ligand system. The differences in the kinetics could also be because the nature of cross talk would be different when dealing with defined synthetic mixtures versus undefined complex mixture of PAMPs presented by the bacteria. Comparison of the response magnitude between live and FK bacteria and dual TLR ligands from the frequency distributions (Figs. 1A–C and 2A–C) also shows that live bacteria induce an attenuated signaling response—this could be due to active evasion mechanisms by the live bacteria, which could alter the host–pathogen interaction during the course of infection. Future studies probing downstream components of TLR signaling in a systematic manner to compare results of dual TLR activation with bacterial infection will address these questions.
Development of a Human Macrophage Model for Image-Based Analysis of Bacterial Infection
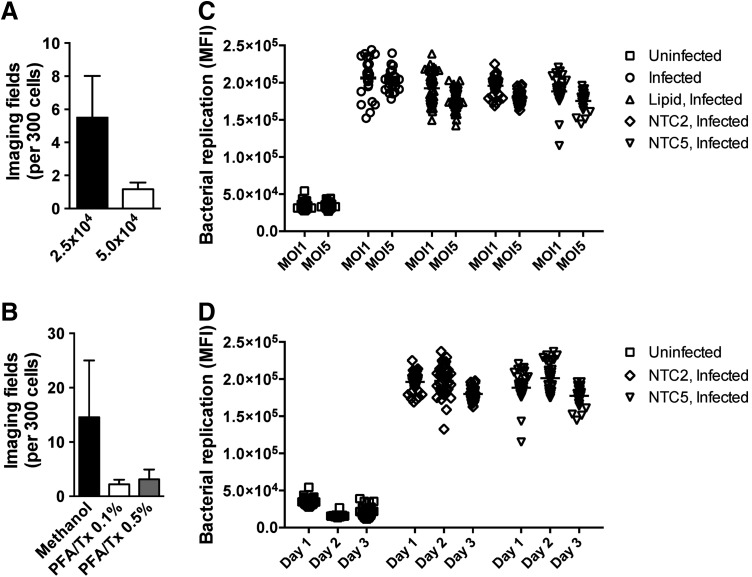
The human monocytic THP1 cell line provides a viable human macrophage-like model system after differentiation with PMA.16 The parental THP1 cell line grows in suspension; however, the cells become adherent during differentiation over 2–3 days. We have described the development of robust protocols for siRNA-based gene perturbation in these cells where the differentiation period provides a convenient time window in which cells can be reverse transfected with siRNA.14 Since the bacterial infection assays we describe were designed for siRNA screening applications, the cell seeding density was maintained in the range (2.5 × 104–5 × 104 cells/well of a 96-well plate or 5 × 103–1 × 104 cells/well of a 384-well plate) that we have previously found to give optimal gene target knockdown in THP1 cells.14 We tested different cell seeding densities in this range for bacterial infection assays in THP1 cells and found that higher cell densities led to better maintenance of cell number, as evidenced by the lower number of imaging fields required to capture data from at least 300 cells (Fig. 3A). Since our bacterial imaging assays require cell permeabilization for antibody-based readouts, we also compared cell fixation and permeabilization methods to identify optimal conditions for maintaining high cell numbers per imaging field (Fig. 3B).
Fig. 3.
Identification of optimal experimental parameters for image-based quantification of bacterial replication. (A) The average number of imaging fields required to identify at least 300 THP1 cells plated at different cell densities per well of a 96-well plate and infected with B. cenocepacia for 24 h at MOI 1. (B) The average number of imaging fields required to identify at least 300 THP1 cells plated at 5 × 104 cells per well of a 96-well plate, infected with B. cenocepacia for 24 h at MOI 1, and subjected to different fixation and permeabilization conditions. (C) THP1 cells were infected with B. cenocepacia at MOI 1 or MOI 5 for 24 h, and bacterial replication was quantified by imaging as described in the Materials and Methods section. Before infection, cells were treated with either HiPerFect transfection lipid or transfected with nontargeting control (NTC) siRNA where indicated. (D) THP1 cells were infected with B. cenocepacia at MOI 1 for 24 h, and bacterial replication was quantified by imaging as described in the Materials and Methods section. Data are shown from infections conducted on 3 separate days. Before infection, cells were transfected with NTC siRNA where indicated. Data shown are representative of two independent experiments for (A) and (B) (mean + SD) and (C) Individual data points from 24 to 48 wells of a 96-well plate. (D) Individual data points from 32 separate wells for each condition from three replicate experiments.
Robust Image-Based Quantification of Bacterial Infection in Human Macrophages
Having identified optimal conditions for cell handling, we sought to establish a reproducible HCS assay for measurement of bacterial replication in macrophages using a previously developed specific antibody raised against the J2315 strain of B. cenocepacia.11 Considering our intent to develop the assay for siRNA screening applications, we compared bacterial infection levels in human THP1 cells either treated with siRNA transfection lipid or transfected with different nontargeting control (NTC) siRNA. Cells were infected with B. cenocepacia J2315 at an MOI of 1 or 5, and after 1 h of incubation to permit bacterial uptake, cells were treated with antibiotics for 2 h to remove any residual extracellular bacteria. Media was then changed to antibiotic-free media, and the bacteria were allowed to replicate intracellularly for a further 21 h for a total 24 h infection time. We show that for all the above conditions, we detect reproducible bacterial infection levels at MOIs of both 1 and 5 (Fig. 3C), suggesting that an MOI of 1 is sufficient for consistent detection of bacterial replication. Moreover, we observe no adverse affect on bacterial replication in cells treated with siRNA transfection lipid or nontargeting siRNA (Fig. 3C), and the reproducibility of the MOI 1 infection across wells in the plate (24–48 wells per condition) shows the following robust % coefficient of variation (CV) for each condition: infected 12.84%, lipid 10.84%, NTC2 6.91%, and NTC5 10.89%. To further assess the reproducibility of the assay across multiple infection experiments carried out on different days, we compared bacterial infection levels in cells treated with two different NTC siRNAs, NTC2 and NTC5, with at least 32 wells per condition. Across three independent experiments, we observe highly reproducible levels of bacterial replication (Fig. 3D), with %CVs of 5.1% (NTC2) and 6.3% (NTC5).
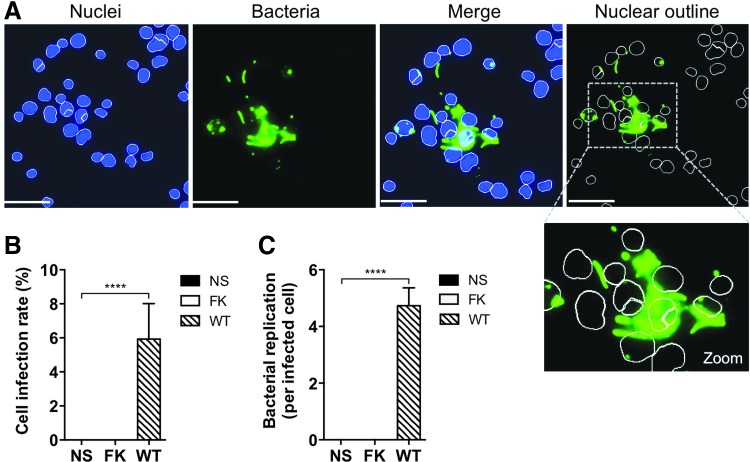
High-content imaging parameters were established to delineate infected cells based on nuclear staining and visualization of cytosolic bacteria (see the Materials and Methods section, Tables 3 and 4). To establish these parameters, images of an uninfected negative control and an infected WT positive control were displayed side by side. Bacteria were identified based on high intensity and shape recognition software and these parameters were set to identify the infected positive control cells (Fig. 4A). Objects with low intensity and low variable intensity were excluded from enumeration. Bacteria were also identified based on object shape, that is, only objects shaped as B. cenocepacia rods or spots (bacteria in a different orientation) were counted. When multiple bacteria overlapped, the software was often able to identify individual B. cenocepacia based on intensity differences at the perimeter of each bacterium. Excessively large areas of low-intensity staining were not counted as bacteria. The program segmented bacteria as strictly as possible, creating a segment when the intensity difference between pixels was as low as possible (setting of 1 in the segmentation parameter). This ensured the bacteria would be counted as separate if there was at least one pixel of low intensity between two high-intensity pixels. These parameters identified no bacteria in the uninfected control, nor any bacteria in the FK, nonreplicative infection control (Fig. 4B). We set the program to count at least three hundred viable nuclei per well before moving to another well, and on average, our image analysis parameters identified at least one hundred distinct bacteria per well. At an MOI of 1, ∼6% of cells were infected with WT B. cenocepacia following a 24 h infection (Fig. 4B), and the bacterial count per infected cell showed a mean value of around 5 (Fig. 4C).
Fig. 4.
Quantification of bacterial replication using high-content imaging. (A) THP1 cells infected with B. cenocepacia at MOI 1 for 24 h. Nuclei (blue) of viable cells were identified using parameters described in the Materials and Methods section. Bacteria (green) were enumerated within 10 μm of the center of viable nuclei to restrict counting to intracellular bacteria. (B) Infection rate of THP1 cells infected with B. cenocepacia at MOI 1 for 24 h. (C) Average bacterial replication rate (per infected cell) in THP1 cells infected with B. cenocepacia at MOI 1 for 24 h. Scale bars = 50 μm. NS, nonstimulated; FK, formalin-killed bacteria; WT, wild-type bacteria. Data shown are representative images (A) or combined measurements (B and C; mean + SEM) from four independent experiments. ****P < 0.0001 (two-tailed t-test).
Imaging of the Macrophage Autophagy Response to Bacterial Infection
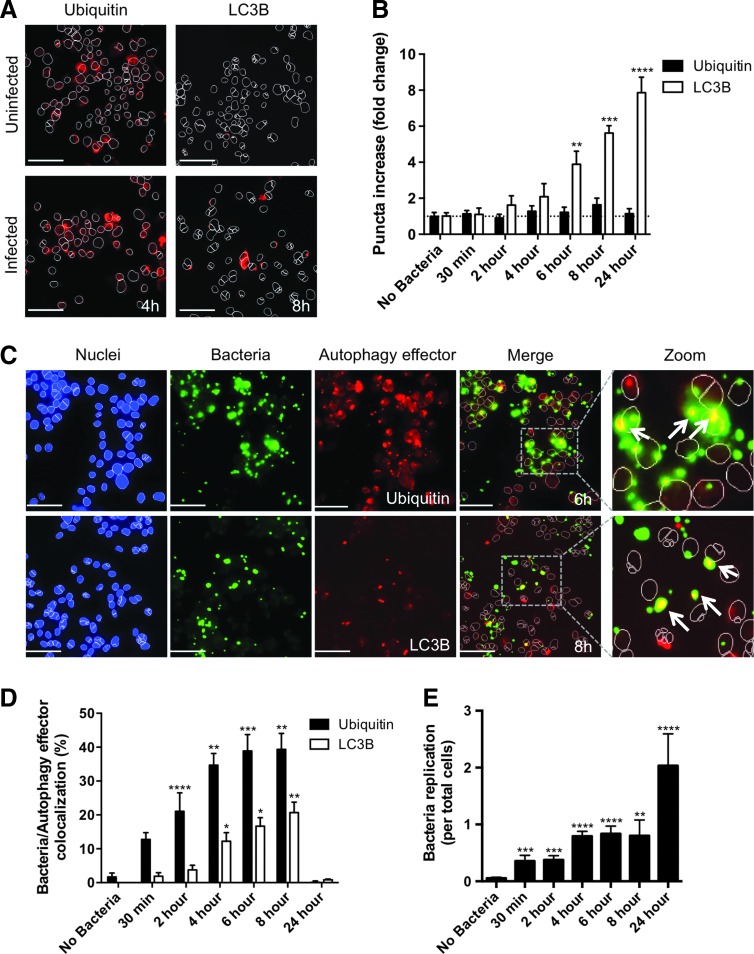
We have previously shown that during B. cenocepacia infection of human macrophages, the bacteria escape the endocytic pathway and enter the host cell cytosol.11 This induces a selective autophagy response characterized by the ubiquitination of bacteria and the recruitment of autophagy effector proteins such as LC3B.17 To develop HCS assays for the selective autophagy response to B. cenocepacia infection, we employed a THP1 cell line stably expressing GFP-LC3 and also stained these cells for ubiquitin using a specific antibody (see the Materials and Methods section, Tables 3–5). We first measured the frequency of ubiquitin and LC3B puncta in response to bacterial infection (Fig. 5A). While the frequency of ubiquitin puncta remained constant, we observed a substantial increase in LC3B puncta in THP1 cells infected with B. cenocepacia (Fig. 5B).
Fig. 5.
Imaging of autophagy induction and bacterial colocalization with autophagy factors. (A) Imaging and (B) quantification of ubiquitin and LC3B puncta in THP1 cells infected with B. cenocepacia at MOI 1 for 4 and 8 h, respectively. (C) Imaging of nuclei (blue), bacteria (green), and autophagy effector (red) colocalization in THP1 cells infected with B. cenocepacia at MOI 1 for 6 h (Ubiquitin) and 8 h (LC3B), respectively. Merge and zoomed panels show only the nuclear outline. Arrows highlight examples of bacteria/autophagy effector colocalization. (D) Quantification of percent bacterial colocalization with autophagy effectors over the duration of a 24 h infection (E) Quantification of bacterial replication (average bacteria per total cells) over the duration of a 24 h infection. Scale bars = 50 μm. Data shown are representative images (A and C) or combined measurements (B, D, and E; mean + SEM) from three independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 (two-tailed t-test).
We then assessed whether the ubiquitin and LC3B puncta showed significant colocalization with intracellular bacteria (Fig. 5C). When determining this colocalization with the CellInsight NXT, MEAN_ROI_A_Target_I_ObjectCount was divided by MEAN_ObjectCountCh2, which equates to the fraction of bacteria/factor colocalization events divided by the total number of bacteria. Multiplying this value by 100 determined our percent colocalization. Using these parameters, ubiquitin colocalization with bacteria was detectable as early as 30 min postinfection, reaching a peak of 40% colocalization by 6 h (Fig. 5D). In contrast, LC3B colocalization with bacteria increased with slower kinetics, showing a substantial increase after 4 h and reaching a peak of 20% colocalization at 8 h postinfection (Fig. 5D).
Comparing these trends to the average number of bacteria per total cells (MEAN_ObjectCountCh2), as the number of bacteria per cell increases, the activation of autophagy proteins increases (Fig. 5D, E). However, by 24 h of infection, we see a dramatic drop in autophagy protein colocalization with bacteria (Fig. 5D), although there is a large increase in bacterial replication at this time point (Fig. 5E). Our HCS imaging assays therefore accurately reflect the progression of B. cenocepacia pathogenesis in human macrophages,11 showing that while ubiquitin and LC3B are recruited to the bacteria during infection to promote bacterial control through selective autophagy, this response fails to induce effective bacterial degradation and B. cenocepacia replication proceeds.
Pharmacological Stimulation of Autophagy Leads to Measurable Increases in Colocalization Events Between Bacteria and Autophagy Effectors
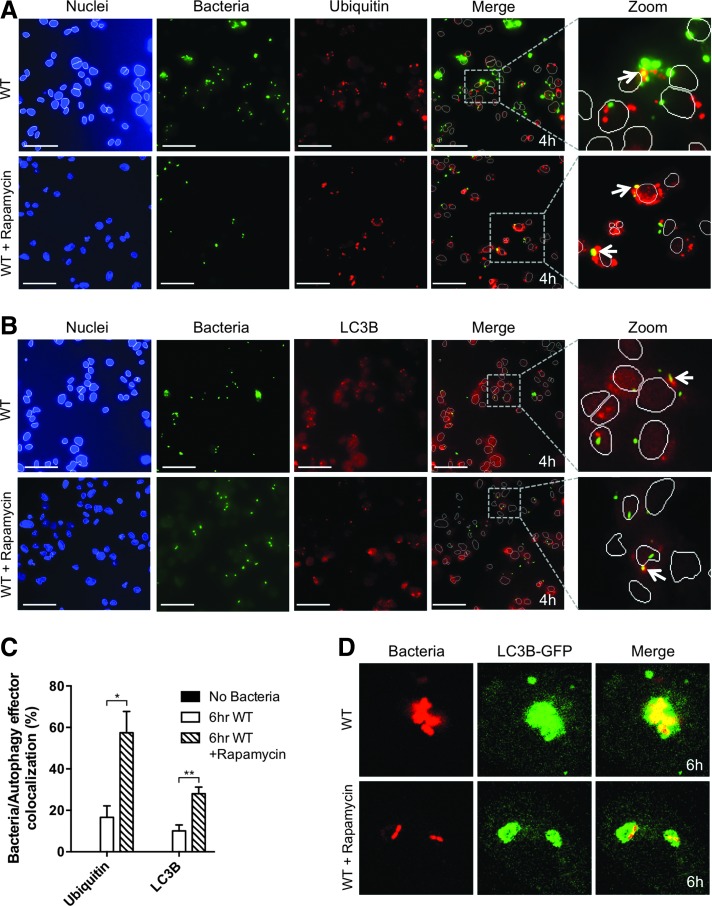
To determine if our HCS assays for selective autophagy targeting of intracellular bacteria were sensitive to manipulation of autophagy flux, we treated cells with rapamycin, a well-established activator of autophagy.18 As described earlier, a 6 h infection led to increased colocalization of both ubiquitin and LC3B with B. cenocepacia (Fig. 5D). We induced autophagy by treating THP1 cells with rapamycin for 2 h before infection and observed increased colocalization of ubiquitin and LC3B with bacteria as early as 4 h postinfection (Fig. 6A, B). Rapamycin treatment led to significant increases in colocalization of both autophagy effectors with bacteria (Fig. 6C), and this increase was most pronounced for LC3B colocalization, consistent with prior studies showing that rapamycin treatment significantly increases active LC3B levels.19 As a confirmation of the substantial increase in LC3B colocalization measured by our HCS assay, confocal microscopy analysis also showed a similar rapamycin-induced increase in the colocalization of LC3B with bacteria at 6 h postinfection, especially the ratio of LC3B:bacterial area (Fig. 6D). Most bacteria are enveloped by a substantial amount of LC3B in the rapamycin-treated cells, while bacteria are associated with a smaller relative amount of LC3B without rapamycin treatment (Fig. 6D).
Fig. 6.
Pharmacological induction of autophagy increases bacterial colocalization with autophagy factors. (A, B) THP1 cells infected with B. cenocepacia at MOI 1 for 4 h either without (upper panels) or with 50 μg/mL rapamycin pretreatment (lower panels). Imaging of nuclei (blue), bacteria (green), and autophagy effectors (red) is shown for (A) Ubiquitin and (B) LC3B, respectively. Merge and zoomed panels show only the nuclear outline. Arrows highlight examples of bacteria/autophagy effector colocalization. (C) Quantification of percent bacterial colocalization with autophagy effectors after a 6 h infection with and without rapamycin pretreatment. (D) Confocal imaging of bacteria (red) and LC3B-GFP (green) in THP1 cells infected with B. cenocepacia at MOI 1 for 6 h. Scale bars = 50 μm. Data shown are representative images (A, B, and D) or combined measurements (C; mean + SEM) from three independent experiments. *P < 0.05, **P < 0.01 (two-tailed t-test).
Pharmacological Stimulation of Autophagy Leads to an Attenuation of Bacterial Replication That Is Reversed by siRNA-Based Perturbation of Host Autophagy Effectors
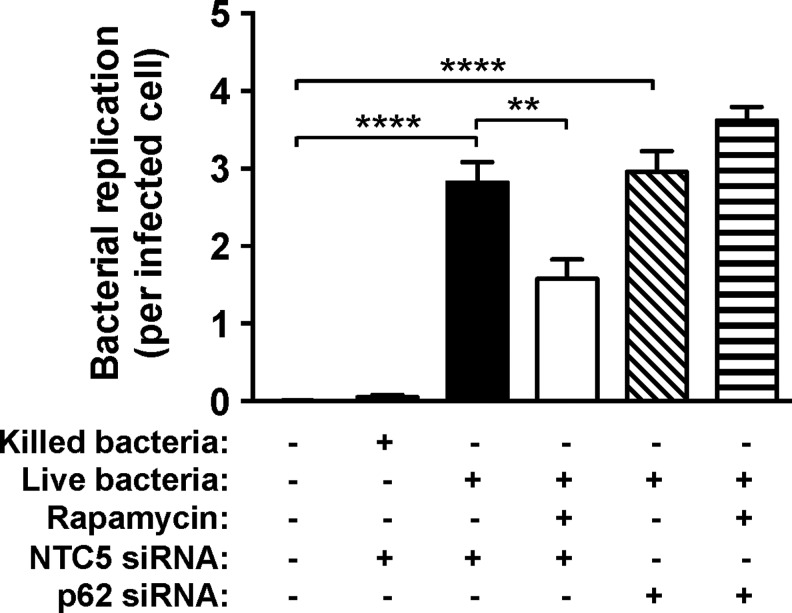
We have shown that increasing host macrophage autophagy flux with rapamycin leads to increased colocalization of host autophagy effector proteins with invading Bcc bacteria. To determine if this increased host autophagy response is reflected in the level of bacterial replication, we quantified intracellular bacteria in THP1 cells pretreated with rapamycin. We find that activation of host autophagy with rapamycin leads to a significant decrease in bacterial replication (Fig. 7). To determine whether previously optimized protocols for siRNA-based gene perturbation in THP1 human macrophages14 could be used to attenuate the rapamycin-induced host autophagy response, we targeted the autophagy effector, p62/SQSTM1, which recognizes ubiquitinated autophagy cargo and recruits LC3B to initiate formation of the autophagosome.20 We find that in the presence of p62 siRNA, addition of rapamycin induces no significant change in bacterial replication (Fig. 7). This confirms that the rapamycin effect is through the autophagy pathway and demonstrates the utility of our described intracellular bacterial imaging assays for siRNA screening applications.
Fig. 7.
Effect of manipulation of autophagy flux and perturbation of host cell autophagy effectors on bacterial replication. THP1 cells were infected with live or killed B. cenocepacia at MOI 1 for 24 h, and bacterial replication was quantified by imaging as described in the Materials and Methods section. Before infection, cells were treated with 50 μg/mL rapamycin and transfected with either nontargeting control (NTC5) or p62 siRNA (50 nM) where indicated. Data shown are combined measurements (median+median absolute deviation) from three independent experiments. **P ≤ 0.01, ****P ≤ 0.0001 (two-tailed t-test).
Discussion
Antibody staining of signaling components downstream of TLRs allowed us to interrogate the mechanisms of responses to multi-TLR ligands or intact bacteria. This staining in a 96-well format allowed the multiplexing of dose responses and time courses through the measurement of multiple fluorescent channels. As with any antibody staining protocol, one needs to optimize conditions of fixation, blocking, and antibody incubation. Appropriate single color; isotype; primary antibody; secondary antibody; and staining controls are needed to ensure that the signal measured is true.21,22 When we screen multiple antibodies or cell types in one 96-well assay, a fixed threshold for background correction is not optimal, instead an isodata threshold-based background correction was implemented. Cell density also affects the quality of the results; if the wells are too sparsely seeded, there is significant delay in autofocusing and in capturing images of a sufficient number of cells. If the cells are too crowded or not in a monolayer, it becomes difficult to avoid cell loss during washing steps, and it is also a challenge to segment cells and demarcate cell-specific channel intensities during image analysis. We find that image sampling of nonadjacent fields in a well rather than imaging only adjoining fields is more practical as it increases the likelihood of identifying at least one field with optimal cell density. Our protocol for imaging macrophage TLR pathway activation identified differential signaling activation kinetics in macrophages challenged with combined synthetic ligands versus killed or live bacteria, indicating that the macrophage can discriminate the context of TLR ligand presentation. This may have important implications for understanding multisignal integration in macrophages and will be further investigated using this platform.
The development of reliable quantitative assays for intracellular bacterial replication can facilitate small-molecule screens for novel antibiotics as well as phenotypic screens using gene silencing or the more recently developed genome editing technologies. In this study, we describe the quantification of intracellular bacteria using an antibody staining method. One advantage of this method is that it can allow the rapid screening of bacterial replication phenotypes in a 384- or 96-well format. However, as described above for signaling assay readouts, this method depends on carefully controlled cell concentrations and appropriate fixation techniques to achieve an optimal number of cells for accurate data collection. It should be noted that we have found 96-well assays to be more consistent in this regard than 384-well assays using THP1 cells; however, this could be, in part, due to the semi-adherent nature of these cells, and this may not be a factor using more adherent cell types.
Our screening parameters establish a protocol for enumerating single intracellular bacteria based on staining intensity and bacterial shape. However, when bacteria appear in replicative clusters on top of one another, it is challenging to distinguish single bacteria based on intensity peaks. This may lead to undercounting of bacteria with the described approach; however, we are still able to enumerate an average of 5 to 6 bacteria per cell during infection, which is consistent with a range of 3–15 bacteria per cell that we have described previously using other methods.11
To complement image-based quantification of intracellular bacterial replication, the measurement of colocalization of host response factors with bacteria provides an orthogonal assay readout that can be particularly advantageous for screening applications. For example, perturbation of host factors that mediate the selective autophagy response would be expected to both decrease the colocalization of autophagy effectors with bacteria and also lead to increased bacterial replication. This type of paired assay approach can lead to the identification of higher confidence hits in primary screens. In the colocalization HCS assays described here, setting standardized colocalization parameters ensures a consistent enumeration of colocalization events that are unbiased across fields and conditions. In a 96-well format, we were able to observe and quantify significant increases in colocalization of both ubiquitin and LC3B with bacteria in THP1 cells infected with B. cenocepacia. Moreover, our assay was able to detect the expected kinetics of the bacterial recognition and autophagy initiation as the initial ubiquitination of bacteria preceded the increase in LC3B colocalization, which is dependent on the recruitment of a ubiquitin-binding adapter such as p62, which in turn can directly recruit LC3B to the nascent autophagosome.17 We also demonstrated that pharmacological induction of autophagy with rapamycin led to measurable increases in colocalization of selective autophagy effectors with internalized bacteria, consistent with prior studies showing that increased autophagy flux can facilitate host defense against B. cenocepacia infection. Accordingly, rapamycin pretreatment of human macrophages also led to a reduction in bacterial replication, which could be reversed by siRNA-based knockdown of the key host autophagy adaptor protein, p62.
In this study, we have described the development of high-throughput imaging assays to measure multiple aspects of the macrophage response to synthetic mimics of bacterial ligands and to infection with both live and killed gram-negative bacteria, and we demonstrate the modulation of the bacterial infection outcome through both pharmacological and RNAi-based perturbations. The application of such assays combined with established gene perturbation screening methodology, to interrogate host innate immune responses to bacterial stimuli, will provide opportunities to gain substantial new insight into host–pathogen interactions.
Abbreviations Used
- Ac-p65
acetylated-p65
- AP1
activator protein 1
- ATF2
activating transcription factor 2
- Bcc
Burkholderia cenocepacia complex
- BSA
bovine serum albumin
- CREB
cyclic adenosine monophosphate response element-binding protein
- CV
coefficient of variation
- DMEM
Dulbecco's modified Eagle's medium
- FBS
fetal bovine serum
- FK
formalin-killed
- HCS
high-content screening
- HEPES
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid
- IMMs
immortalized murine macrophages
- IRF
interferon regulatory factor
- LB
Luria–Bertani
- LC3B
microtubule-associated protein 1A/1B-light chain 3 beta
- MAPK
mitogen-activated protein kinase
- MOI
multiplicity of infection
- NF-κB
nuclear factor kappa-light-chain-enhancer of activated B cells
- NTC
nontargeting control
- P3C
Pam3CSK4
- p62
sequestosome 1
- PAMPs
pathogen-associated molecular patterns
- PBS
phosphate-buffered saline
- PFA
paraformaldehyde
- PMA
phorbol 12-myristate 13-acetate
- PRRs
pattern recognition receptors
- RPMI1640
Roswell Park Memorial Institute 1640 media
- THP1
human acute monocytic leukemia cell line
- TLR
toll-like receptor
- WT
wild-type
Acknowledgment
This work was generously supported by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases (A.H.M., S.J.V., and I.D.C.F).
Disclosure Statement
No competing financial interests exist.
References
- 1.Akira S, Uematsu S, Takeuchi O: Pathogen recognition and innate immunity. Cell 2006;124:783–801 [DOI] [PubMed] [Google Scholar]
- 2.Creagh EM, O'Neill LA: TLRs, NLRs and RLRs: a trinity of pathogen sensors that co-operate in innate immunity. Trends Immunol 2006;27:352–357 [DOI] [PubMed] [Google Scholar]
- 3.Ostuni R, Zanoni I, Granucci F: Deciphering the complexity of toll-like receptor signaling. Cell Mol Life Sci 2010;67:4109–4134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Deretic V, Levine B: Autophagy, immunity, and microbial adaptations. Cell Host Microbe 2009;5:527–549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Youle RJ, Narendra DP: Mechanisms of mitophagy. Nat Rev Mol Cell Biol 2011;12:9–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ding WX, Yin XM: Mitophagy: mechanisms, pathophysiological roles, and analysis. Biol Chem 2012;393:547–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vandamme P, Holmes B, Vancanneyt M, et al. : Occurrence of multiple genomovars of Burkholderia cepacia in cystic fibrosis patients and proposal of Burkholderia multivorans sp. nov. Int J Syst Bacteriol 1997;47:1188–1200 [DOI] [PubMed] [Google Scholar]
- 8.Mahenthiralingam E, Urban TA, Goldberg JB: The multifarious, multireplicon Burkholderia cepacia complex. Nat Rev Microbiol 2005;3:144–156 [DOI] [PubMed] [Google Scholar]
- 9.Bernhardt SA, Spilker T, Coffey T, LiPuma JJ: Burkholderia cepacia complex in cystic fibrosis: frequency of strain replacement during chronic infection. Clin Infect Dis 2003;37:780–785 [DOI] [PubMed] [Google Scholar]
- 10.Bamford S, Ryley H, Jackson SK: Highly purified lipopolysaccharides from Burkholderia cepacia complex clinical isolates induce inflammatory cytokine responses via TLR4-mediated MAPK signalling pathways and activation of NFkappaB. Cell Microbiol 2007;9:532–543 [DOI] [PubMed] [Google Scholar]
- 11.Al-Khodor S, Marshall-Batty K, Nair V, Ding L, Greenberg DE, Fraser ID: Burkholderia cenocepacia J2315 escapes to the cytosol and actively subverts autophagy in human macrophages. Cell Microbiol 2014;16:378–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peach KC, Bray WM, Winslow D, Linington PF, Linington RG: Mechanism of action-based classification of antibiotics using high-content bacterial image analysis. Mol Biosyst 2013;9:1837–1848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nandi S, Ritenberg M, Jelinek R: Bacterial detection with amphiphilic carbon dots. Analyst 2015;140:4232–4237 [DOI] [PubMed] [Google Scholar]
- 14.Li N, Sun J, Benet ZL, et al. : Development of a cell system for siRNA screening of pathogen responses in human and mouse macrophages. Sci Rep 2015;5:9559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang CY, Ferrell JE, Jr.: Ultrasensitivity in the mitogen-activated protein kinase cascade. Proc Natl Acad Sci U S A 1996;93:10078–10083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tsuchiya S, Yamabe M, Yamaguchi Y, Kobayashi Y, Konno T, Tada K: Establishment and characterization of a human acute monocytic leukemia cell line (THP-1). Int J Cancer 1980;26:171–176 [DOI] [PubMed] [Google Scholar]
- 17.Pankiv S, Clausen TH, Lamark T, et al. : p62/SQSTM1 binds directly to Atg8/LC3 to facilitate degradation of ubiquitinated protein aggregates by autophagy. J Biol Chem 2007;282:24131–24145 [DOI] [PubMed] [Google Scholar]
- 18.Blommaart EF, Luiken JJ, Blommaart PJ, van Woerkom GM, Meijer AJ: Phosphorylation of ribosomal protein S6 is inhibitory for autophagy in isolated rat hepatocytes. J Biol Chem 1995;270:2320–2326 [DOI] [PubMed] [Google Scholar]
- 19.Tanemura M, Ohmura Y, Deguchi T, et al. : Rapamycin causes upregulation of autophagy and impairs islets function both in vitro and in vivo. Am J Transplant 2012;12:102–114 [DOI] [PubMed] [Google Scholar]
- 20.Zheng YT, Shahnazari S, Brech A, Lamark T, Johansen T, Brumell JH: The adaptor protein p62/SQSTM1 targets invading bacteria to the autophagy pathway. J Immunol 2009;183:5909–5916 [DOI] [PubMed] [Google Scholar]
- 21.Ivell R, Teerds K, Hoffman GE: Proper application of antibodies for immunohistochemical detection: antibody crimes and how to prevent them. Endocrinology 2014;155:676–687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Burry RW: Controls for immunocytochemistry: an update. J Histochem Cytochem 2011;59:6–12 [DOI] [PMC free article] [PubMed] [Google Scholar]