Abstract
Promoting both endogenous and exogenous neural stem cells' (NSCs) survival in the hostile host environments is essential to cell replacement therapy for central nervous system (CNS) disorders. Type 4 metabotropic glutamate receptor (mGluR4), one of the members of mGluRs, has been shown to protect neurons from acute and chronic excitotoxic insults in various brain damages. The present study investigated the preventive effects of mGluR4 on NSC injury induced by oxidative stress. Under challenge with H2O2, loss of cell viability was observed in cultured rat NSCs, and treatment with selective mGluR4 agonist VU0155041 conferred protective effects against the loss of cellular viability in a concentration-dependent manner, as shown by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay. Pretreatment of VU0155041 (30 μM) also inhibited the excessive NSC death induced by H2O2, and group III mGluRs antagonist (RS)-a-methylserine-O-phosphate (MSOP) or gene-targeted knockdown abolished the protective action of mGluR4, indicated by propidium iodide–Hoechst and terminal deoxynucleotidyl transferase-mediated UTP nick end labeling (TUNEL) staining. Western blot assay demonstrated that mGluR4 activation reversed the decreased procaspase-8/9/3and the destructed Bcl-2/Bax expressing balance, and likewise, MSOP and mGluR4 knockdown abrogated the action of mGluR4 activity. Furthermore, inhibition of JNK and p38 mitogen-activated protein kinases (MAPKs) were observed after mGluR4 activation, and as paralleling control, JNK-specific inhibitor SP600125 and p38-specific inhibitor SB203580 significantly rescued the H2O2-mediated NSC apoptosis and cleavage of procaspase-3. We suggest that activation of mGluR4 prevents oxidative stress-induced NSC death and apoptotic-associated protein activities with involvement of inhibiting the JNK and p38 pathways in cell culture. Our findings may help to develop strategies for enhancing the resided and transplanted NSC survival after oxidative stress insult of CNS.
Introduction
Neural stem cell (NSC) replacement promises a novel therapeutic strategy for stroke and other brain diseases. However, this approach is impeded possibly due to the hostile brain environments, which result in massive cell death. A high oxidative stress status in vivo caused by the damaged brain, one of the essential factors accounting for the harsh brain microenvironments, makes a crucial challenge for survival of the resided as well as transplanted NSCs [1–3]. Oxidative stress occurs as a consequence of excessive production of reactive oxygen species (ROS) in the impaired central nervous system (CNS). It has been suggested that oxidative stress could induce NSC apoptosis and/or necrosis due to the activation of cell signaling cascades related to the release of proapoptotic factors and the disturbance of mitochondrial function [4,5]. Hence, conferring antioxidative properties of NSCs may contribute to the potential strategies in favor of combating existing oxidative stimulus.
Type 4 metabotropic glutamate receptor (mGluR4) is a member of group III mGluRs, which belong to family C G-protein-coupled receptors in inhibiting adenylate cyclase activity in heterologous expression systems [6]. Similar to its counterparts in group III mGluRs (mGluR4, 6, 7, and 8), mGluR4 is preferentially localized in presynaptic terminals and is thought to mediate the presynaptic depression of glutamatergic synaptic transmission, most likely through inhibition of glutamate release [7]. In addition, selective activation of mGluR4 also results in neuroprotection against excitotoxic insults in Parkinson's disease, ischemic stroke, and other CNS disorders [8–12]. Moreover, functional mGluR4 has been found to be expressed in embryonic stem cell-derived neural stem/progenitor cells and cerebellar granule cell neuroprecursors, and may play roles in inhibition, cell proliferation, and promotion of neuronal differentiation, although the underlying mechanisms have not been clarified [13–15].
Although an experiment suggested that an mGluR4 ligand, phosphoserine (P-Ser), may improve the survival of rat embryonic cortex-derived neural progenitors in normal condition, it is unknown if mGluR4 plays a role in the protection of NSCs from oxidative injury [13]. These previous findings prompt the potential of mGluR4 as a drug target in NSC replacement therapy, which could enhance neurogenic fate, commitment of NSCs, and/or protects these cells against the insults of harsh CNS microenvironments.
In this study, we attempt to disclose the contribution of mGluR4 to the neuroprotection against oxidative stress-induced NSC death and related cell signaling pathways. We used a selective mGluR4 agonist cis-2-cyclohexanecarboxylic acid (VU1055041) [16], to observe the protective effects of the mGluR4 activity on cell death of NSCs induced by H2O2. Our data showed that mGluR4 activation prevented cultured rat NSCs from H2O2-mediated insults with inhibiting JNK and p38 mitogen-activated protein kinases (MAPKs), which downregulate the expression of procaspase-8/9/3, as well as reversed the disturbance of Bcl-2/Bax expressing balance.
Materials and Methods
Rat cortical NSC culture
Rat cortical NSCs were prepared from E15.5 Sprague-Dawley rat embryos as previously described and with minor modification [17]. Briefly, the cortex was carefully dissected in chilled sterile phosphate-buffered saline (PBS) and incubated with 0.05% trypsin and 200 μM EDTA in PBS at 37°C for 10 min. Then the tissue was mechanically dissociated using a fire-polished Pasteur pipette and filtered using a 40 μm cell strainer (BD Falcon). After centrifugation at 1,000 g for 5 min, cells were suspended and Trypan blue-excluding cells were counted. Then the cells were seeded at a density of 500,000 cells/mL in nonadherent T75 flasks and incubated in a humidified atmosphere of 5% CO2 and 95% air at 37°C. The medium consisted of DMEM/F12 (1:1), 1% of N2, 2% of B27, 20 ng/mL of epidermal growth factor, and 10 ng/mL of basic fibroblast growth factor. After 5–7 days, cells propagated in primary neurospheres (P0 cells) with the diameter of ∼80–200 μm, were dissociated into single cells. These cells were cultured in suspension at a density of 100,000 cells/mL and allowed to form the secondary neurospheres (P1 cells). To obtain a uniform population of NSCs, P1 cells were further dissociated and the single cells were plated onto poly-d-lysine (PDL)-coated dishes, plates, or glass coverslips and grown as a monolayer on the adherent surface. Nestin expressed in NSCs was identified and mGluR4 expression was presented by immunocytochemical staining. All experimental data in this study were obtained from these attached grown NSCs.
Pregnant 15.5-day Sprague-Dawley rats were purchased from the Experimental Animal Center of Xi'an Jiaotong University Health Science Center (Certificate No. 22-9601018). All experimental protocols were approved by the Animal Care and Use Regulation of Xi'an Jiaotong University Health Science Center. All efforts were made to minimize animals' suffering and to keep the numbers of animals used to a minimum.
Experimental treatments
Oxidative stress on NSCs was experimentally induced by adding H2O2. A newly opened bottle of H2O2 solution (30%) was diluted with deionized water and immediately added into the culture medium to achieve the desired working concentration. To optimize the oxidative injury condition for conducting the experiment, NSCs were treated with different concentrations of H2O2 (0, 10, 50, 100, and 200 μM) for 3, 6, 12, or 24 h, then subjected to cell viability [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT)] assay. The dose of 100 μM for 12 h was adopted in the following experiments.
To examine the effects of mGluR4 activation on H2O2-induced NSC oxidative injury, serial concentrations (0, 1, 3, 10, 30, 50 μM) of VU0155041 sodium (Tocris Bioscience) was added to the NSC cultures 1 h before the challenge of H2O2, and incubated for further 12 h. Then, NSCs were subjected to MTT assay, propidium iodide (PI)–Hoechst and terminal deoxynucleotidyl transferase-mediated UTP nick end labeling (TUNEL) staining to observe cell viability and death. Levels of apoptotic proteins procaspase-8/9/3, Bcl-2, and Bax were also examined by western blot analysis. The effects of VU0155041 on NSCs were further confirmed by blockade of mGluR4 using mGluRs antagonist (RS)-a-methylserine-O-phosphate (MSOP, 1 mM; Tocris Bioscience), and knockdown of mGluR4 with RNA interference method.
To observe the involvement of JNK and p38 MAPK signaling in the protective effects of mGluR4 activation on NSC oxidative injury induced by H2O2, NSCs were pretreated by VU0155041 (30 μM) and MSOP (1 mM) solely, or MSOP (1 h) followed VU0155041 for 1 h, then exposed to H2O2 for further 12 h, and phosphorylation levels of JNK and p38 were checked by western blot analysis. The p38 inhibitor SB203580 (10 μM; Sigma) and JNK inhibitor SP600125 (10 μM; Sigma) were used as paralleling control to confirm the action of VU0155041.
All treatments were administered by the direct dilution into the culture medium, and equivalent volume of vehicles was added to the control cultures. All experiments were performed in triplicates and repeated at least three times.
Immunocytochemistry staining
To identify the expression of mGluR4 in NSCs, NSCs plated on PDL-coated coverslips were fixed in 4% paraformaldehyde for 20 min at room temperature and washed in PBS. The cells were permeabilized with 0.1% Triton X-100 for 10 min, followed by blockage for 1 h using 5% bovine serum albumin (BSA). Primary antibodies mouse anti-nestin (1:200; Millipore) and rabbit anti-mGluR4 (1:200; Abcam) were added to the coverslips and incubated overnight at 4°C. All antibodies were diluted in PBS containing 0.05% Triton X-100 and 2% BSA. After washing three times with PBS, NSCs were incubated by secondary antibodies AlexaFluor 488 donkey anti-mouse IgG (1:500; Invitrogen) and AlexaFluor 594 goat anti-rabbit IgG (1:800; Invitrogen) for 2 h at room temperature and washed. Then nuclei were counterstained with 4′,6-diamidino-2-phenylindole (DAPI, 1 μg/mL) for 5 min The coverslips were mounted on glass slides using SlowFade (Molecular probes). Images were observed and acquired under an Olympus BX51 fluorescence microscope equipped with a DP71 digital camera connected to a computer (Olympus).
Cell viability assay
Cell viability was evaluated by MTT assay. NPCs were grown in 96-well plates at 5,000 cells/well for 24 h before experiments. At the end of each treatment, the culture media in each well were added MTT (0.5 mg/mL final concentration, Sigma) and incubated for 2 h at 37°C, while the tetrazolium ring in the MTT was cleaved by mitochondrial dehydrogenases of viable cells, yielding insoluble purple formazan crystals in aqueous media. Then the media were aspirated and the resulting crystals were dissolved by adding 150 μL dimethyl sulfoxide (Sigma). The absorbance was measured at wavelength of 490 nm using a multimicroplate spectrophotometer (Epoch; BioTek). Triplicate paralleled wells were set in all the experiments, and data were collected as the average of at least three independent experiments. The results were presented as percentage of absorbance in the control cells. At least three independent experiments were carried out for each assay.
PI–Hoechst staining
To evaluate the apoptotic nuclear morphology and plasma membrane integrity, NSCs were plated on PDL-coated coverslips and subjected to Hoechst 33258 and PI staining. After treatments, Hoechst dye (5 μg/mL; Sigma) was added to the culture medium and the cells were placed at 37°C for 20 min. Then the NSCs were subsequently stained by PI (10 μg/mL; Sigma) at 37°C for 30 min. The coverslips were rinsed with PBS and mounted. NSC apoptosis was visualized and captured using a Lieca DMI3000B inverted fluorescence inverted microscope (Leica). For quantification, images were imported into Image-Pro Plus software (Version 5.0; Media Cybernetics). Apoptotic cells were characterized by PI staining cells and Hoechst staining nuclei, and Hoechst-positive–PI-negative cells were counted as viable cells. Cells from 10 randomly selected fields on each coverslip (total 300–600 cells/coverslip) were counted using a 20× objective. The percentage of apoptotic cells was determined by the number of apoptotic cells and the total cells. The experiments were performed in triplicates and repeated independently at least three times.
TUNEL staining
To demonstrate cell death of NSCs, TUNEL assay was carried out following the manufacturer's instruction (Roche Diagnostics). Briefly, NSCs were plated on PDL-coated glass coverslips. At the end of each treatment, the cells were fixed in 4% paraformaldehyde in PBS for 20 min at room temperature. After being permeabilized using 0.1% Triton X-100 in 0.1% sodium citrate for 2 min on ice, the cells were incubated with 50 μL of TUNEL reaction mixture for 1 h at 37°C. Cells were further counterstained with DAPI (1 μg/mL) before mounting. Microscopy and imaging were performed in an Olympus BX51 fluorescence microscope. Images were processed using Image-Pro Plus 5.0 software. Ten random fields on each sample were counted using a 20× objective. Data were presented as the percentage of TUNEL-positive cells in the total number of cells (DAPI-stained cells).
siRNA knockdown of mGluR4
The target sequences for rat mGluR4 siRNA were as follows: simGluR4-1, 5′-GCAUGUCACCAUAAUUUGCTT-3′; simGluR4-2, 5′-GGUCAUCGGCUCAUGGACATT-3′. A nonspecific siRNA (sequence: siNC, 5′-CGTACGCGGAATACTTCGATT-3′) was used as a control in all siRNA transfection experiments. All the siRNA duplexes were synthesized by GenePharma. siRNA knockdown was performed as previously described [13]. NSCs were grown on PDL-coated 24-well plates. Before transfection, the culture medium was washed three times by Opti-MEM (Invitrogen). Cells were transfected with 100 nM siRNA duplexes for 6 h in the presence of Lipofectamine 2000 according to the manufacturer's instructions (Invitrogen). Transfection efficiency was examined using a fluorescence inverted microscope. Knockdown of mGluR4 expression was further evaluated using reverse transcriptase PCR and western blot. Cells were cultured for 24 more hours before further treatments.
Western blot analysis
NSCs were cultured on PDL-coated six-well plates. After treatment, the cells were rinsed three times in PBS and incubated in a lysis buffer (50 mM Tris-HCl, pH 7.6, 150 mM NaCl, 1% Triton X-100, 1 mM EDTA, 1 mM EGTA, 0.5% sodium deoxycholate, 0.1% SDS, 1 mM sodium orthovanadate, 50 mM sodium fluoride, 20 mM Na4P2O7, and 10% glycerol; Pierce) supplemented with protease inhibitor cocktail (Roche) for 10 min on ice, followed by sonication (VCX500; Sonics). Cell lysates were cleared by centrifugation for 15 min at 4°C. Protein concentrations of samples were estimated by the BCA assay (Pierce). Samples were mixed with a loading buffer and boiled for 5 min. Proteins (20–40 μg for each sample) were resolved by 10%–12% SDS-polyacrylamide gel electrophoresis and transferred to polyvinylidene fluoride membranes (BioRad). Membranes were blocked with 5% nonfat milk for 1 h at room temperature and subsequently probed with specific primary antibodies overnight at 4°C. The following primary antibodies were used: rabbit anti-mGluR4 polyclonal (1:1,000; Abcam), rabbit anti-caspase-3 (1:1,000; Cell Signaling Technology), rabbit anti-caspase-8 (1:1,000; Cell Signaling Technology), rabbit anti-caspase-9 (1:1,000; Cell Signaling Technology), mouse anti-Bcl-2 (1:1,000; Millipore), mouse anti-Bax (1:1,000; Millipore), rabbit anti-phospho-JNK2 (1:2,000; Cell Signaling Technology), rabbit anti-JNK2 (1:2,000; Cell Signaling Technology), rabbit anti-phospho-p38 (1:2,000; Cell Signaling Technology), rabbit anti-p38 (1:2,000; Cell Signaling Technology), and mouse anti-β-Actin (1:50,000; Sigma-Aldrich). The membranes were rinsed and further incubated with horseradish peroxidase-conjugated anti-rabbit or anti-mouse IgG (both 1:100,000; Sigma-Aldrich) for 1 h at room temperature. Following the secondary antibodies incubation, the membranes were rinsed and immunoreactive bands were visualized by enhanced chemiluminescent substrate according to the manufacturer's protocol (Pierce) and exposed to X-ray film (Fuji). The results were collected using a G:Box gel imaging system (Syngene) and quantified using an NIH ImageJ 3.5 software. The relative levels of target proteins were calculated and normalized by β-Actin, which was used as an internal control. All western blot data were presented in samples from at least three independent experiments.
Statistical analysis
All data are reported as mean ± SD from at least three independent in vitro experiments. Statistical comparisons of cell viability, apoptosis, and western blot data between different groups were made by Tukey's test after one-way ANOVA using SPSS statistical software (version 12.0). P < 0.05 was considered statistically significant.
Results
Activation of mGluR4 increased NSCs viability upon H2O2 injury
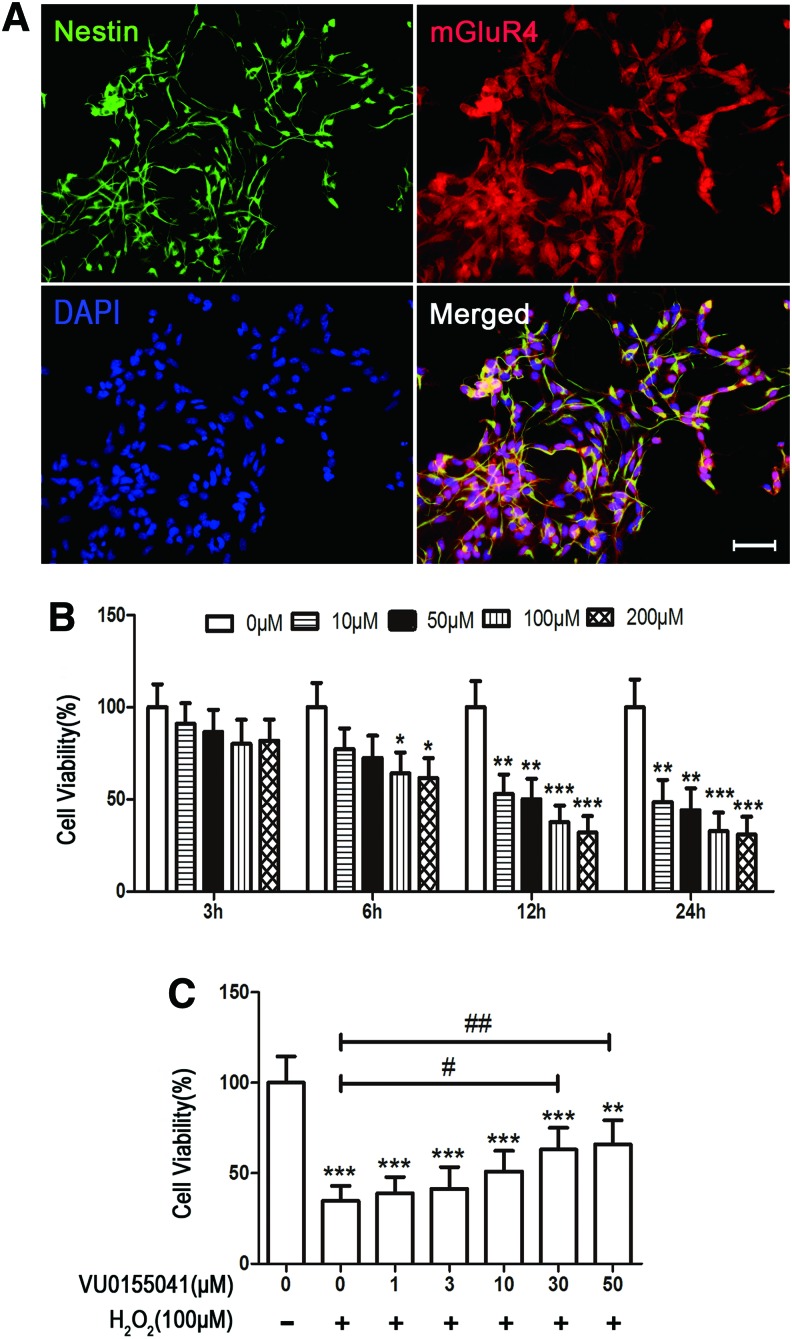
The purity of NSCs and mGluR4 expression were determined by immunocytochemical double labeling for their specific marker nestin and mGluR4. The results showed that this culture procedure yielded 93.2% ± 3.3% nestin-positive cells, of which 91.6% ± 2.8% of them expressed mGluR4 (Fig. 1A). The culture system was used to conduct the following experiments. To identify H2O2-induced NSC injury, MTT assay was carried out as previously described [17]. As expected, H2O2 significantly damaged cell viability in cultured NSCs in time- and concentration-dependent manners (Fig. 1B). Treatment of H2O2 at 100 μM for 12 h resulted in 62.4% ± 8.9% loss of NSC viability (P < 0.001) and was used in the following experiments.
FIG. 1.
Activation of type 4 metabotropic glutamate receptor (mGluR4)-protected rat neural stem cells (NSCs) viability against H2O2-induced injury. The identification of NSC purity and mGluR4 expression were displayed by double staining of nestin and mGluR4, and nuclei were counterstained with 4′,6-diamidino-2-phenylindole (DAPI). Scale bar 50 μm (A). Cytotoxicity of H2O2 to NSCs was evaluated by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay. The viability of NSCs was assessed after exposure to H2O2 (0, 10, 50, 100, and 200 μM) for 3, 6, 12, and 24 h (B). To estimate the protective effects of VU1055041 (VU) on the loss of cell viability induced by H2O2, NSCs were pretreated with selective mGluR4 agonist VU0155041 (0, 1, 3, 10, 30, and 50 μM) for 1 h, followed by treatment of 100 μM of H2O2 for further 12 h (C). Cell viability was presented as a percentage of control in (B, C), and each value represents the mean ± SD of three independent experiments (n = 3). *P < 0.05, **P < 0.01, ***P < 0.001 versus control; #P < 0.05, ##P < 0.01 versus H2O2 control. Color images available online at www.liebertpub.com/scd
To determine whether mGluR4 activation plays a role in protecting NSCs from oxidative stress-induced injury, plated NSCs were pretreated with serial concentration of VU0155041 (a specific mGluR4 agonist, 0, 1, 3, 10, 30, and 50 μM) for 1 h, followed by 100 μM of H2O2 stimulation in the same medium for further 12 h. Cytotoxicity evoked by H2O2 was attenuated by pretreatment with VU0155041 in a concentration-dependent manner, as showed by MTT assay (Fig. 1C). The neuroprotective effect of VU0155041 reached the peak at the concentration of 50 μM (Fig. 1C). VU0155041 concentration ranging from 1 to 50 μM had no significant toxicity to normal cultured NSCs (data not shown). The VU0155041 concentration of 30 μM was used to conduct the following experiments.
Activation of mGluR4-protected NSCs against H2O2-induced apoptosis
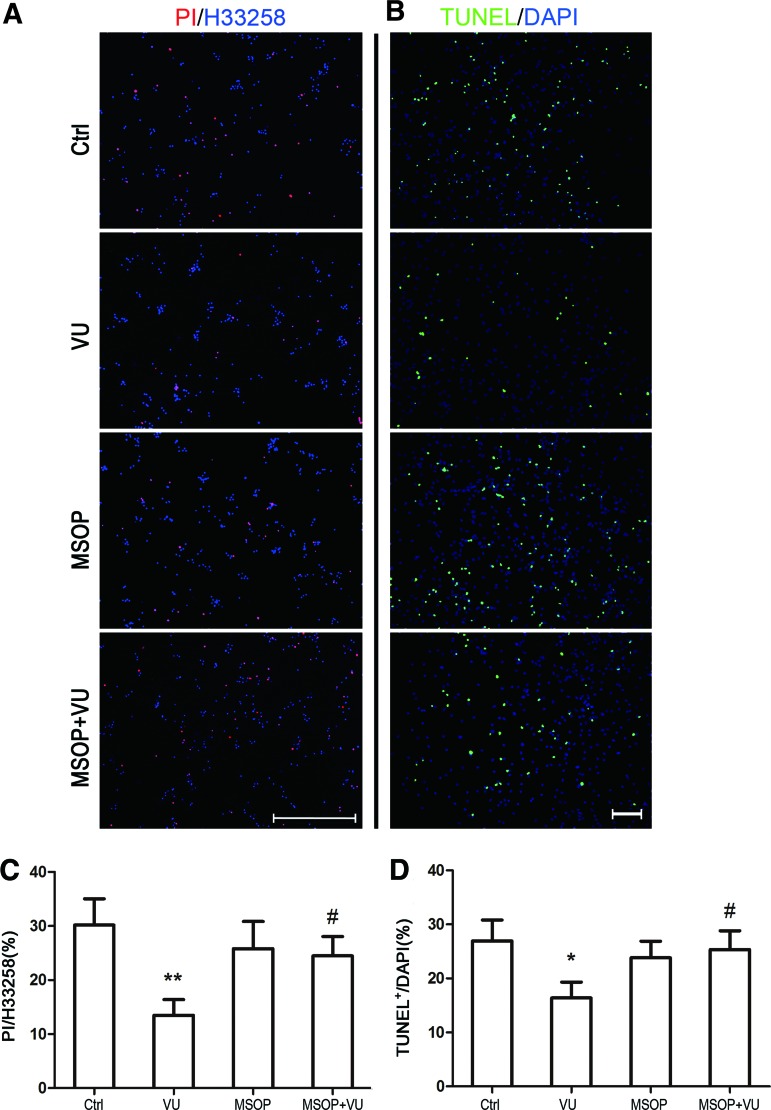
To further determine the neuroprotective effect of mGluR4 activation on H2O2-induced NSC injury, apoptotic cells were distinguished from viable cells by PI–Hoechst and TUNEL staining. As expected, the percentage of apoptotic cells increased markedly after exposure to 100 μM H2O2 for 12 h, which was indicated by 30.2% ± 4.9% PI-positive cells and 28.4% ± 5.7% TUNEL-positive cells (Supplementary Fig. S1 and Fig. 2; Supplementary data are available online at www.liebertpub.com/scd), which significantly increased in comparison with unexposed NSCs (Supplementary Fig. S1, P < 0.001). Treatment of normal cultured NSCs with mGluR4 agonist VU0155041 (30 μM, P > 0.05) or antagonist MSOP (1 mM, P > 0.05) for 12 h had no significant influence on NSC apoptosis, compared with control (Supplementary Fig. S1). Pretreatment with 30 μM VU0155041 significantly decreased H2O2-induced apoptotic NSCs to 13.5% ± 2.9% (P < 0.05) in PI–Hoechst technique and 15.4% ± 2.6% (P < 0.05) in TUNEL staining. An antagonist, MSOP (1 mM, P < 0.05) for mGluR4, markedly blocked the protective effect of VU0155041, while MSOP alone (1 mM, P > 0.05) had no significant influence on H2O2-mediated NSC apoptosis (Fig. 2).
FIG. 2.
Protection of mGluR4 activation on H2O2-induced NSC apoptosis. For estimating cell apoptosis, terminal deoxynucleotidyl transferase-mediated UTP nick end labeling (TUNEL) and propidium iodide–Hoechst (PI–Hoechst) staining were carried out after each treatment. NSC cultures were treated by the vehicle (Ctrl), 30 μM of VU0155041 (VU), 1 mM of mGluRs antagonist (RS)-a-methylserine-O-phosphate (MSOP), and MSOP plus VU0155041 (MSOP + VU) for 1 h, and then exposed to 100 μM of H2O2 for further 12 h. Representative images (A) and quantification (C) were shown after PI–Hoechst staining. Data from three independent experiments (n = 3) were presented as the percentage of PI-positive cells in the total Hoechst-positive cells. **P < 0.01 versus control, #P < 0.05 versus VU0155041 group. Scale bar 200 μm. Representative images and quantitative analysis for TUNEL staining were shown in (B) and (D), respectively. Data from three independent experiments (n = 3) were presented as the percentage of TUNEL-positive cells in total DAPI-stained cells. Scale bar 100 μm. *P < 0.05 versus control, #P < 0.05 versus VU0155041 group. Color images available online at www.liebertpub.com/scd
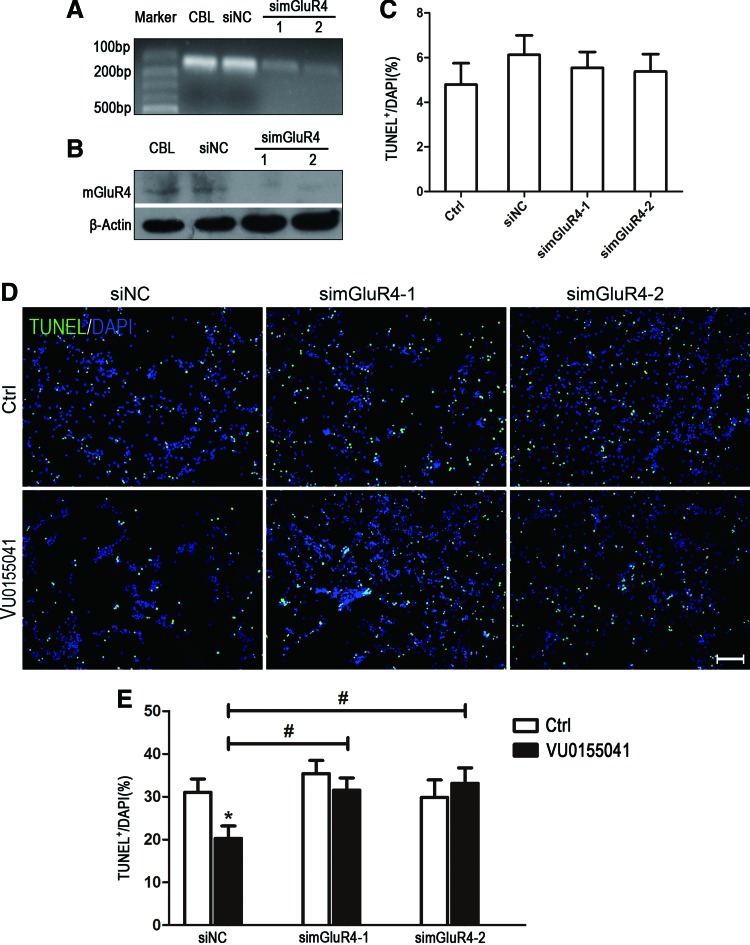
Since MSOP is nonspecific for blocking mGluR4, we further utilized the RNA interfering technique to investigate the neuroprotective effect of mGluR4 activation on H2O2-induced NSC injury. Expression of mGluR4 mRNA and protein in NSCs was significantly downregulated by the transfection of two mGluR4-targeted siRNAs (simGluR4-1 and −2), compared with the nonspecific siRNA (siNC) transfection (Fig. 3A, B). It showed no significant increase of TUNEL-positive NSCs at 24 h after transfection, compared with nontransfected cells (P > 0.05, Fig. 3C). These transfected cells were used to perform the following experiments. TUNEL staining revealed that pretreatment of siNC (P < 0.05)-transfected NSCs with 30 μM VU0155041 significantly decreased the percentage of H2O2-induced apoptotic cells to 20.3% ± 2.8%. However, the protective effect of VU0155041 on NSCs was markedly blocked by transfecting mGluR4-targeted siRNAs (Fig. 3D, E, P > 0.05 for both simGluR-1 and simmGluR-2 transfected). These findings suggested that activation of mGluR4 inhibited the H2O2-induced NSC apoptosis.
FIG. 3.
Target gene knockdown attenuated the protective effects of mGluR4 activation on H2O2-induced NSC apoptosis. NSCs were transfected for 6 h by nonspecific siRNA (siNC) and two mGluR4-targeted siRNAs (simGluR4-1 and simGluR4-2) using Lipofectamine 2000. Representative reverse transcriptase PCR (A) and western blot (WB) (B) images illustrated the downregulation of mGluR4 in mRNA and protein levels. Samples isolated from adult rat cerebellar cortex (CBL) were used as a method control. Quantitative analysis for TUNEL staining (n = 3) was presented for evaluating transfection-induced cell death at 24 h (C). The transfected NSC cultures were added to 30 μM VU0155041 (VU) for 1 h, followed by 100 μM H2O2 for further 12 h, then subjected to TUNEL staining. Representative images (D) were presented. Scale bar 100 μm. Quantitative data from three independent experiments (n = 3) were shown as the percentage of TUNEL-positive cells in total DAPI-stained cells (E). *P < 0.05 versus control, #P < 0.05 versus siNC plus VU0155041 group. Color images available online at www.liebertpub.com/scd
Activation of mGluR4 inhibited H2O2-mediated apoptotic signaling features
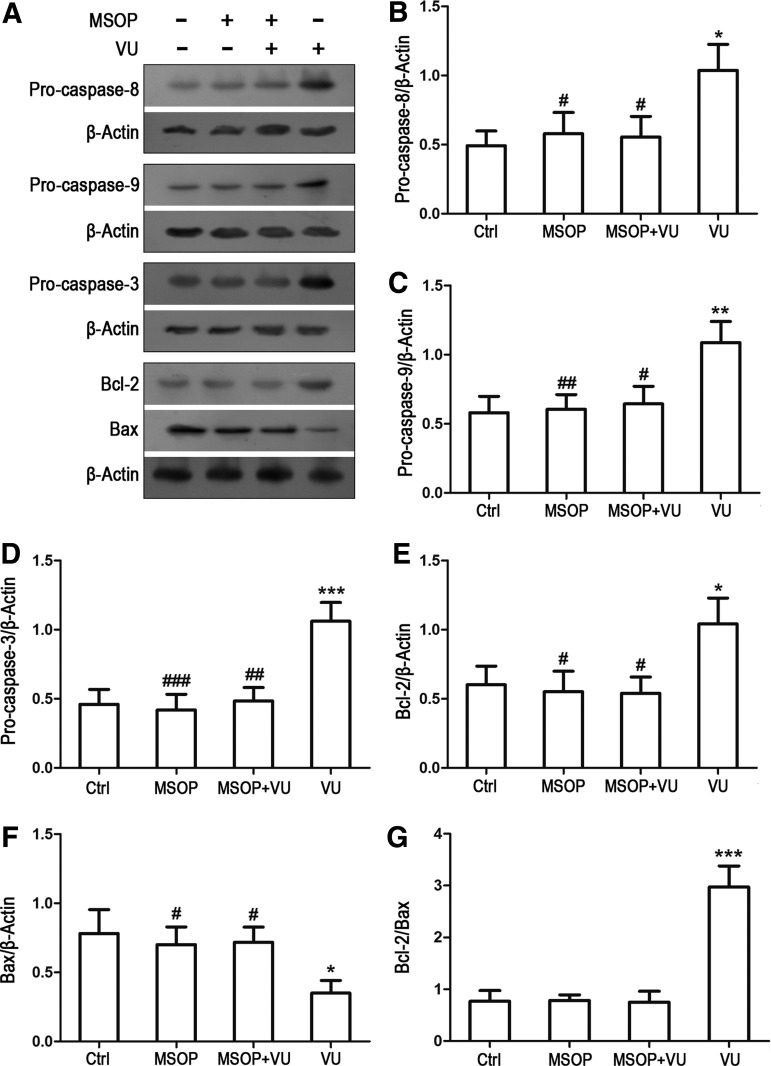
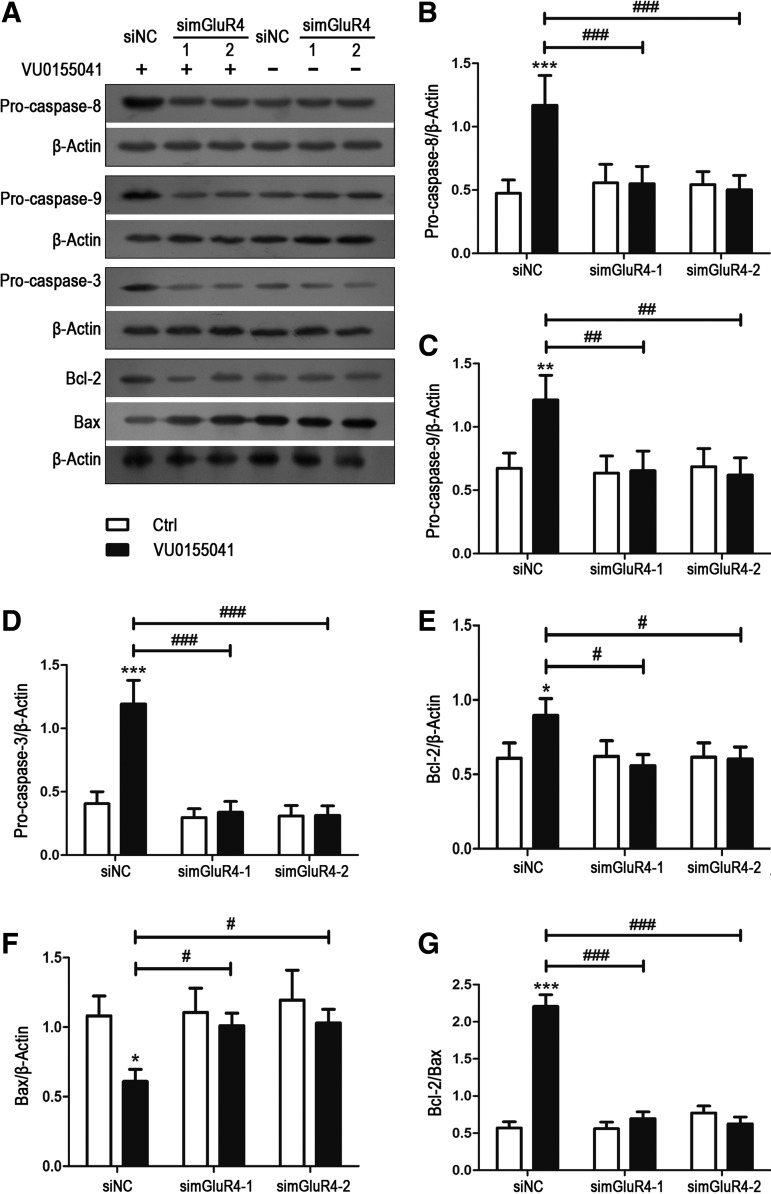
Various apoptotic proteins mediate the oxidative stress-induced cell death, such as antiapoptotic/apoptotic proteins Bcl-2/Bax and the proapoptotic precursor procaspase-8/9/3. Involvement of these potential intracellular mediators was previously assessed in H2O2-induced cell death by determining their intracellular levels [4,18,19]. To investigate the involvement of these apoptotic molecules in the mGluR4-mediated inhibition of H2O2-mediated NSC apoptosis, expression levels of antiapoptotic/apoptotic proteins Bcl-2/Bax and the proapoptotic precursor procaspase-8/9/3were estimated by western blot analysis. Consistent with the previous reports [4,20,21], H2O2 stimulation decreased procaspase-8/9/3 and Bcl-2 levels, and increased the Bax expression level (Supplementary Fig. S2). VU0155041 pretreatment reversed the H2O2-induced reduction of procaspase-8/9/3and Bcl-2, attenuated the Bax level, and resulted in an increase of Bcl-2/Bax ratio, which may contribute to the increased cell viability (Fig. 4A–G). Moreover, antagonism of mGluR4 with MSOP blocked the effects of VU0155041 on the expression levels of these apoptotic proteins (Fig. 4A–G). In mGluR4-targeted siRNAs-transfected NSCs, effect of VU0155041 was also attenuated by mGluR4 knockdown, while nonspecific siRNA transfection did not reduce the effective of VU0155041 on the H2O2-induced apoptotic protein activation (Fig. 5A–G). These data demonstrated that antiapoptotic activity of mGluR4 was associated with inhibition of the cleavage of procaspase-8/9/3, the degradation of Bcl-2, and accumulation of Bax.
FIG. 4.
Activation of mGluR4 inhibited the expression of H2O2-mediated apoptotic signal proteins in NSCs. NSCs cultures were treated by the vehicle (Ctrl), 30 μM of VU0155041 (VU), 1 mM of MSOP, and MSOP plus VU0155041 (MSOP + VU) for 1 h, and then exposed to 100 μM of H2O2 for further 12 h. The differential expression of the apoptotic signal proteins was determined by WB analysis. (A) Representative WB images were illustrating the protein expression of procaspase-8/9/3, Bcl-2, and Bax, and β-Actin was used as a reference protein. WB band quantification for the ratio of procaspase-8 (B), -9 (C), -3 (D), Bcl-2 (E), Bax (F) to β-actin, and the ratio of Bcl-2 to Bax (G) was presented, and each value represents the mean ± SD of three independent experiments (n = 3). *P < 0.05, **P < 0.01, ***P < 0.001 versus control; #P < 0.05, ##P < 0.01, ###P < 0.001 versus VU0155041 group.
FIG. 5.
Knockdown of mGluR4 attenuated the effects of VU0155041 on the H2O2-mediated expression of the apoptotic signal proteins in NSCs. NSCs transfected by nonspecific siRNA (siNC) and two mGluR4-specific siRNA (simGluR4-1 and simGluR4-2) were treated by the vehicle (Ctrl) and 30 μM of VU0155041 (VU) for 1 h, followed by exposure to 100 μM of H2O2 for further 12 h. The differential expression of the apoptotic signal proteins was determined by WB analysis. (A) Representative WB images were illustrating the protein expression of procaspase-8/9/3, Bcl-2, and Bax, and β-Actin was used as a reference protein. WB band quantification for the ratio of procaspase-8 (B), -9 (C), -3 (D), Bcl-2 (E), Bax (F) to β-Actin, and the ratio of Bcl-2 to Bax (G) was presented, and each value represents the mean ± SD of three independent experiments (n = 3). *P < 0.05, **P < 0.01, ***P < 0.001 versus control; #P < 0.05, ##P < 0.01, ###P < 0.001 versus siNC transfection plus VU0155041 group.
Activation of mGluR4 inhibited H2O2-induced phosphorylation of JNK and p38 in NSCs
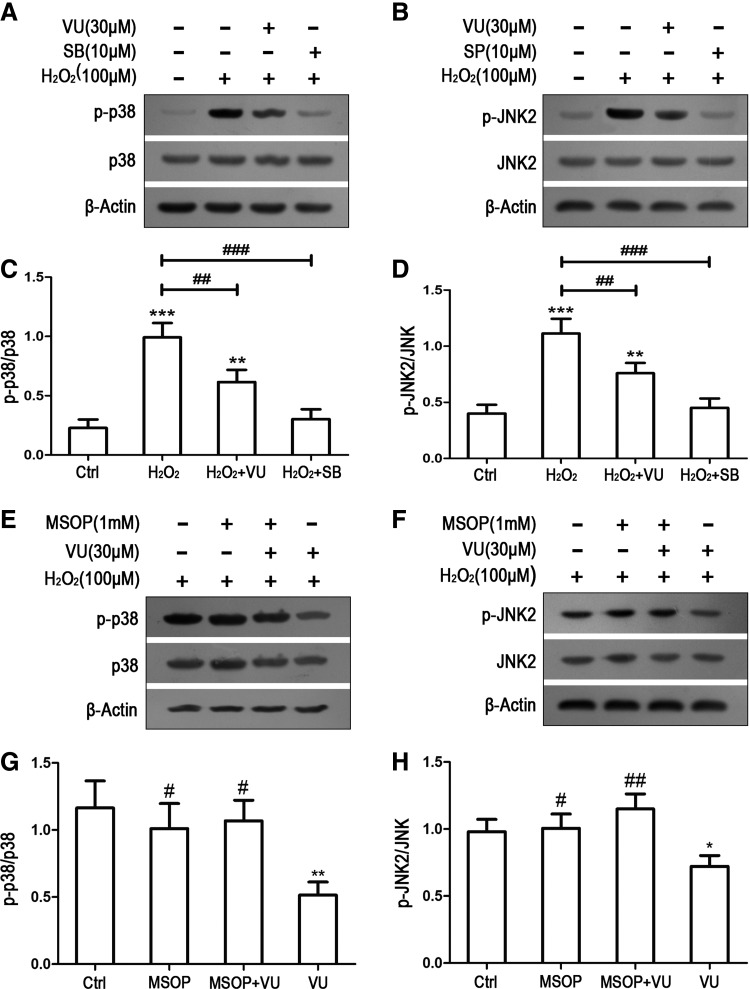
To explore the intracellular pathways responsible for mGluR4-mediated neuroprotection on oxidative stress-induced NSC injury, we investigated the effects of mGluR4 activation on JNK and p38 MAPKs by western blot analysis. Before 12 h of H2O2 stimulation, NSCs were pretreated with 30 μM VU0155041, p38 inhibitor SB203580 (SB, 10 μM), and JNK inhibitor SP600125 (SP, 10 μM), and mGluR4 activation by VU0155041 were preblocked by MSOP (1 mM). The cell lysates were analyzed for the presence of phosphorylated JNK2 and p38 by antibodies to the specific phosphorylation sites of p38 (Thr180/Tyr182) and JNK2 (Thr183/Tyr185), as shown in Fig. 6. As expected, H2O2 stimulation led to sustained JNK2 and p38 phosphorylation, while pretreatment with VU0155041 significantly inhibited the H2O2-induced phosphorylation of JNK2 and p38. As paralleled control for VU0155041, SB203580 or SP600125 also markedly suppressed the H2O2-mediated activation of JNK2 and p38, respectively (Fig. 6A–D). For blocking mGluR4 activation, MSOP treatment competed with the inhibiting effect of VU0155041 on H2O2-induced JNK2 and p38 activation (Fig. 6E–H). As JNK and p38 are poorly activated in normal proliferating NSC cultures (Fig. 6A–D), we were able to examine whether mGluR4 activation affects JNK2 and p38 activity.
FIG. 6.
Activation of mGluR4 inhibited H2O2-induced JNK and p38 phosphorylation in NSCs. NSCs were pretreated by VU0155041 (VU), p38 inhibitor SB203580 (SB), and JNK inhibitor SP600125 (SP) for 1 h, followed by 100 μM of H2O2 for further 12 h. Representative WB images were illustrating the differential expression of p-p38/p38 (A) and p-JNK2/JNK2 (B) after treatments. WB band quantification for the ratio of p-p38/p38 (C) and P-JNK2/JNK2 (D) was presented. **P < 0.01, ***P < 0.001 versus control; ##P < 0.01, ###P < 0.001 versus H2O2 group. To further confirm the inhibiting effects of mGluR4 on JNK and p38, 1 mM MSOP was used to preblock the action of VU0155041 on JNK and p38. Representative WB images and corresponding quantifications demonstrated the expressing levels of p-p38/p38 (E, G) and p-JNK2/JNK2 (F, H) after treatments. Each value represents the mean ± SD of three independent experiments (n = 3). *P < 0.05, **P < 0.01, versus control; #P < 0.05, ##P < 0.01, versus VU0155041 group.
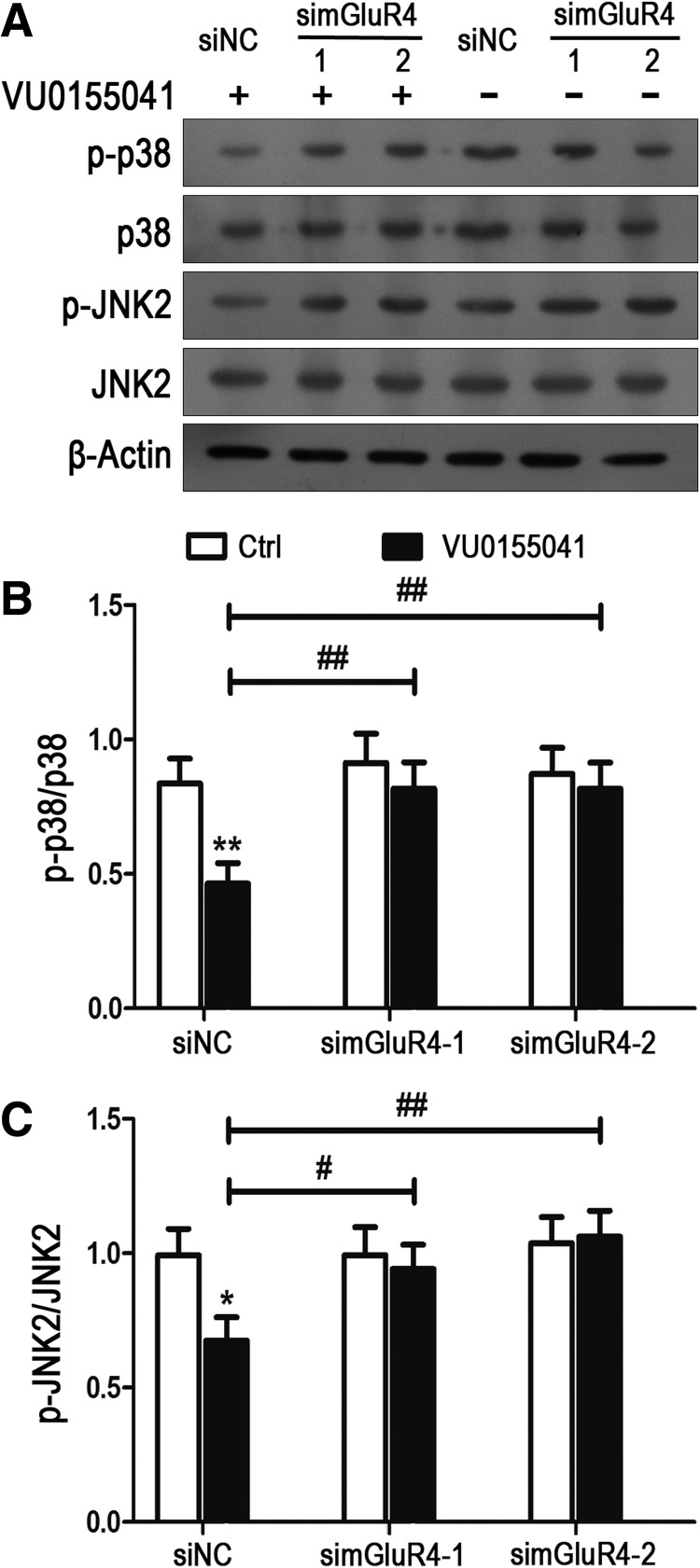
To confirm the inhibition of VU0155041 on H2O2-induced p38 and JNK activation, phosphorylation of p38 and JNK2 was further observed in NSCs with mGluR4 knockdown (Fig. 7). Cultured NSCs were transfected using nonspecific siRNA (siNC) and two mGluR4-specific siRNA (simGluR4-1 and simGluR4-2), then pretreated by the vehicle (Ctrl) and 30 μM of VU0155041 for 1 h and exposed to 100 μM of H2O2 for further 12 h. Similar to the counterpart of nontransfected NSCs, VU0155041 significantly decreased the levels of p-p38 and p-JNK induced by H2O2 in the siNC-transfected NSCs. Whereas inhibition of VU0155041 on p-p38 and p-JNK expression was abolished by mGluR4-specific siRNA transfection (Fig. 7A–C). Combining these data, it was suggested that activation of mGluR4 in NSC culture attenuates p38 and JNK phosphorylation induced by H2O2.
FIG. 7.
Knockdown of mGluR4 depleted the inhibition of VU0155041 on the H2O2-mediated JNK and p38 phosphorylation in NSCs. NSCs transfected by nonspecific siRNA (siNC) and two mGluR4-specific siRNA (simGluR4-1 and simGluR4-2) were pretreated by the vehicle (Ctrl) and 30 μM of VU0155041 for 1 h, followed by exposure to 100 μM of H2O2 for further 12 h. Representative WB bands were shown (A) to illustrate the differential expression of p-p38/p38 and p-JNK2/JNK2 among groups, and β-actin was used as an internal control. Quantitative analysis on ratio of p-p38/p38 (B) and p-JNK2/JNK2 (C) was presented. Each value represents the mean ± SD of three independent experiments (n = 3). *P < 0.05, **P < 0.01 versus control; #P < 0.05, ##P < 0.01 versus siNC transfection plus VU0155041 group.
Activation of mGluR4 prevented H2O2-mediated cell death involving the inhibition of JNK and p38 signaling pathways
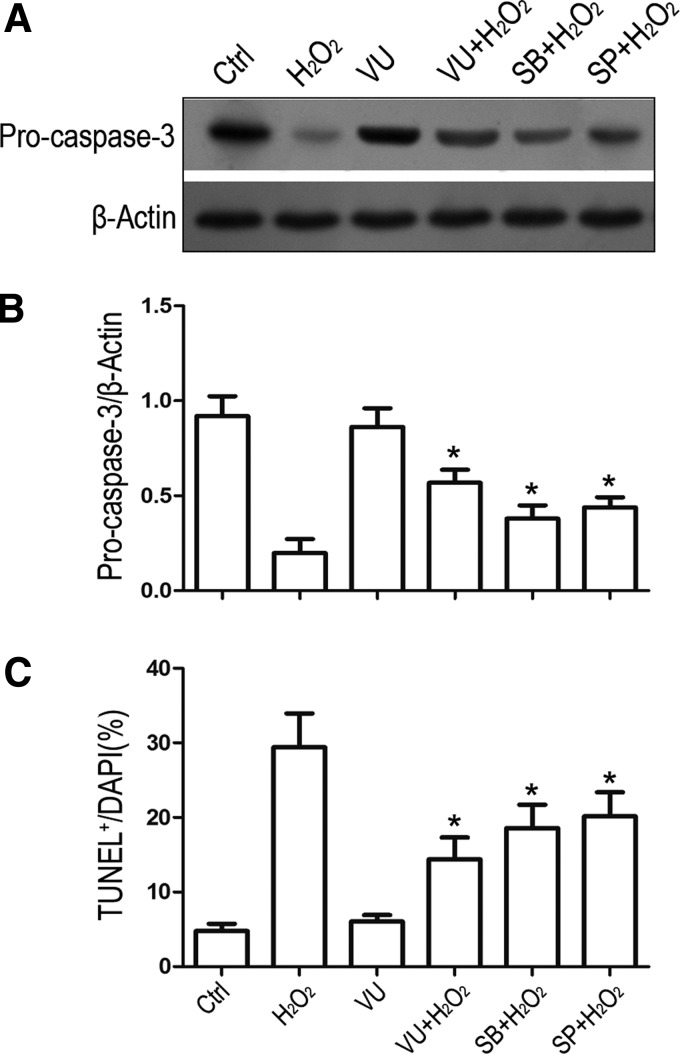
It is understood that JNK and p38 account for NSC death in certain oxidative stress models, which their inhibitors play a role in protecting cells from oxidative injury [22,23]. To examine whether JNK and p38 inactivation contributes to the protective effects of mGluR4 on oxidative NSC apoptosis, we further estimated the H2O2-mediated procaspase-3 cleavage and NSC apoptotic levels in the presence of VU0155041, JNK, or p38 inhibitors. NSCs were pretreated with 30 μM VU0155041, 10 μM SB203580, or 10 μM SP600125, followed by exposure to H2O2 for 12h. Western blot analysis revealed that JNK and p38 inhibitors as well as VU0155041 markedly rescued the procaspase-3 cleavage, compared to H2O2 alone group (Fig. 8A, B). Furthermore, activation of mGluR4 by VU0155041 showed similar tendency compared to JNK and p38 inhibitors in protecting NSC apoptosis against H2O2 injury (Fig. 8C). Hence, inactivation of JNK and p38 is likely to entail neuroprotection of mGluR4 resistant to H2O2-induced oxidative injury of NSCs.
FIG. 8.
Protective effect of VU0155041 on H2O2-induced NSC apoptosis is involved in the inactivation of JNK and p38 MAPK pathways. NSCs were pretreated with 30 μM VU0155041, 10 μM SB203580, or 10 μM SP600125, then exposed or unexposed to 100 μM H2O2 for further 12h. Representative WB image (A) and band quantification (B) for the ratio of procaspase-3to β-Actin was presented. Quantitative analysis for TUNEL staining was presented for demonstrating the cell apoptosis after each treatment (C). Each statistical value represents the mean ± SD of three independent experiments (n = 3). *P < 0.05 versus H2O2 group.
Discussion
In the present study, we reported that pharmacological activation of mGluR4, one of the group III mGluRs, attenuated H2O2-induced rat NSC apoptosis, which was accompanied by reversing the decreased procaspase-8/9/3 levels and balancing Bcl-2/Bax expression. Furthermore, the neuroprotective effects of the mGluR4 agonist might be due to the inhibition of JNK and p38 phosphorylation in cultured rat NSCs. These data may provide in vitro evidence for investigating the strategy of preventing NSCs against oxidative-induced apoptosis.
Studies from mice and rats show that new neurons are continuously generated through adult lives, especially from NSCs located in the subgranular zone of hippocampal dentate gyrus and the subventricular zone of lateral ventricles [24,25]. Moreover, implantable NSCs can be acquired by isolation from fetal or adult brain, or induced from embryonic stem cells as well. These endogenous and exogenous cells go through neurogenesis of proliferation, differentiation, migration, and maturation, and are expected to substitute for lost neurons during CNS disorders [26,27]. Although the potential value of transplanted and endogenous NSCs for the therapy of the CNS diseases has widely been accepted, effective therapy for damaged CNS using NSCs is still far from reality, crucially due to the hostile microenvironment, as well as the possibilities of host immunological rejection and tumorigenic drift of transplanted NSCs. Impaired neurogenesis has been reported in brains suffering from Alzheimer's disease [28], epilepsy [29], and Parkinson's disease [30]. Several CNS injuries, such as ischemic stroke and trauma, have been shown to increase endogenous neurogenesis and promote NSC immigration to the lesion regions and differentiation [31–33]. However, NSC activation is transient and most of these newborn cells are subjected to apoptosis eventually, and the number of survivors is far from adequate to repair the damages [32]. Following these acute or chronic brain injuries, the pathophysiological process, including heightened release of inflammatory cytokines, overproduction of ROS, glutamate metabolism impairment, and other cascade of interactive events as well, contributes to the hostile microenvironment, which not only exacerbates neuron loss, but also impedes repair mechanisms by, for instance, limiting the survival of the host and graft NSCs [34,35]. Oxidative stress, induced by overproduction of ROS, is one of the key factors that inhibit survival signals and activate death signals in NSCs [27,36].
Under normal conditions, the ROS level is balanced between the production and removal mechanisms. Physiological levels of ROS are essential for controlling redox-sensitive proteins involved in various biochemical reactions, such as cell proliferation, differentiation, and survival [37,38]. Under conditions leading to the accumulation of ROS, oxidative stress occurs and causes disturbances in cellular homeostasis, and results in apoptotic cell death, or in extreme cases, necrotic cell death. The major ROS include free radicals, the superoxide anion radical (O2−), the hydroxyl radical (OH−), and the nonradical H2O2 [39]. H2O2 has been employed as a well-established model by many researchers to investigate the mechanisms of oxidative stress-induced cell injuries because of its rapid membrane permeability [40,41]. In the present study, we used H2O2 as a source of ROS to induce NSC injuries, and the results indicated that H2O2 caused not only loss of cell viability, but also apoptosis, similar to the investigations of other researchers [4,42,43]. NSCs are exquisitely sensitive to oxidative stress and related damages, which obstructs the normal cellular functions and even leads to cell apoptosis [2,44]. It is suggested that the enhancement of ROS scavenging capacity or the increase of cellular antioxidant mechanisms might facilitate not only the survival of NSCs in disease conditions, but may also regulate their fate [44–46]. Our experimental results provide data to support mGluR4 activation, which was considered to protect mature neurons against excitotoxic cell death and maintain the homeostasis of extracellular glutamate levels [8,9], enhance the antioxidant capacity of NSCs, which may promote the survival of NSCs in hostile surrounding microenvironment of disease conditions.
H2O2-induced neuronal apoptosis is a mixture response and involved multiple apoptotic pathways, such as activity of caspase cascades and perturbation of Bcl-2/Bax balance [18,47]. In oxidative stress conditions caspase-3, a key effector caspase, is activated as a major target of initiator caspase-8 and caspase-9. Caspase-8 can be cleaved activated, although death receptor-dependent pathway, such as TNF and FasL, and caspase-9 activity, are triggered by the opening of mitochondrial permeability transition pores resulting in the release of cytochrome c from the inner mitochondrial membrane space into the cytosol [23,48–50]. Moreover, destruction of mitochondrial membrane causes the degradation of Bcl-2, an inhibitor for cell death, and also leads to the accumulation and oligomer formation of Bax, which mediate cell apoptosis [41,43]. In our experiments, we detected that treatment of NSCs by H2O2 mediated the cleavage of procaspase-8/9/3, the downregulation of Bcl-2, and upregulation of Bax expression levels, suggesting that the abovementioned apoptotic signals also occurred during oxidative stress-induced NSC death, and these data are consistent with previous investigations [20,49,51]. We further demonstrated that preactivation of mGluR4 in NSCs inhibited the cleavage of procaspase-8/9/3and reversed the H2O2-induced Bcl-2/Bax expression levels and the antagonist or gene knockdown abrogated the effects of mGluR4, corroborating the concept of the protection of mGluR4 against NSC oxidative injuries.
JNK as well as p38 MPAKs are the key intracellular signaling molecules involved in the regulation of cellular responses to various stresses, such as ionizing radiation, inflammatory cytokines, and ischemia/hypoxia [52,53]. Links between JNK/p38 phosphorylation and cell death upon oxidative stress are well characterized in various types of cells, including neural cells [23,54–57]. During experimental oxidative stress-induced cell apoptosis, JNK and p38 were phosphorylated by H2O2 stimulation and caspase cascades in suffered cells were consequently activated, which resulted in cell death [41,58–60], and inhibition of JNK and p38 phosphorylation may play a role in protecting cells against oxidative injuries [22,41,43,54]. We cannot identify if there is crosstalking between the two signal pathways, which may play the synergistic or antistatic roles with each other in oxidative injuries of NSCs. Certain intracellular signal molecules, such as TNF receptor-associated factor 2 [61], mixed lineage kinase-3 [62], and mitogen-activated protein kinase kinase kinase 4 [63] seems to be the nodes that transmit the hurtful signals and activate both JNK and p38. Future studies should include the identification of these possible molecules, which might be used as targets for protecting cells from apoptosis through inhibiting the JNK and p38 signaling pathways. JNK and p38 MAPKs are also referred to various types of mGluRs in regulating cell apoptosis and survival in CNS insults [64–66]. The present study showed that mGluR4 activation attenuated the JNK and p38 phosphorylation induced by H2O2 in cultured NSCs and the action is possibly linked with the inhibition of procaspase-3cleavage and the suppression of NSC apoptosis, suggesting mGluR4-mediated protection of NSCs might be due to the inhibition of JNK/p38 MAPKs. Interestingly, these data also present a new insight about how mGluR4, a typical G protein-coupled receptor, inhibits JNK/p38 phosphorylation during oxidative stress. Previous reports indicated that ROS inhibited the MAPK phosphatases, resulting in sustained JNK and p38 activation [23,67], and mGluR subtypes were coupled to activating certain types of phosphatases [68,69]. Hence, a possible mechanism of mGluR4 regulating JNK/p38 phosphorylation may refer to the rescue of MAPK phosphatases and the underlying links are needed for investigation.
It is well known that mGluR subtypes mediate excitatory (group I mGluRs), or inhibitory (group II and III mGluRs) actions on acute or chronic neuronal injuries. Drugs interacting with mGluRs are expected to contribute to the therapy of neuronal damages without influencing the efficiency of excitatory synaptic transmission typically mediated by ionotropic glutamate receptors. For these reasons, mGluR subtypes may be activated or inhibited without generating severe side effects [70–73]. Therefore, mGluR4 may serve as a possible drug target for preventing oxidative-induced NSC apoptosis, and the findings may help to develop strategies for enhancing graft and host NSC survival after oxidative stress insult of CNS.
Conclusion
In summary, the present study in rat NSCs has demonstrated that mGluR4 activation could decrease cell death induced by H2O2-mediated oxidative stress. Moreover, the protective action of mGluR4 would seem to be involved in the inhibition of JNK/p38 pathways and the subsequent suppression of caspase-8/9/3 activation, as well as Bcl-2/Bax balance destruction, in turn, inhibits NSC apoptosis. The mechanisms of mGluR4 regulating JNK/p38 phosphorylation in protection of oxidative stress-induced NSC death remain open and need to be investigated further.
Supplementary Material
Acknowledgment
This work was supported by grants from the National Natural Science Foundation of China (no. 81371348, 31100774).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Ceccatelli S, Tamm C, Sleeper E. and Orrenius S. (2004). Neural stem cells and cell death. Toxicol Lett 149:59–66 [DOI] [PubMed] [Google Scholar]
- 2.Ceccatelli S, Tamm C, Zhang Q. and Chen M. (2007). Mechanisms and modulation of neural cell damage induced by oxidative stress. Physiol Behav 92:87–92 [DOI] [PubMed] [Google Scholar]
- 3.Silva-Vargas V, Crouch EE. and Doetsch F. (2013). Adult neural stem cells and their niche: a dynamic duo during homeostasis, regeneration, and aging. Curr Opin Neurobiol 23:935–942 [DOI] [PubMed] [Google Scholar]
- 4.Lin HJ, Wang X, Shaffer KM, Sasaki CY. and Ma W. (2004). Characterization of H2O2-induced acute apoptosis in cultured neural stem/progenitor cells. FEBS Lett 570:102–106 [DOI] [PubMed] [Google Scholar]
- 5.Finkel T. (2003). Oxidant signals and oxidative stress. Curr Opin Cell Biol 15:247–254 [DOI] [PubMed] [Google Scholar]
- 6.Pin JP. and Duvoisin R. (1995). The metabotropic glutamate receptors: structure and functions. Neuropharmacology 34:1–26 [DOI] [PubMed] [Google Scholar]
- 7.Corti C, Aldegheri L, Somogyi P. and Ferraguti F. (2002). Distribution and synaptic localisation of the metabotropic glutamate receptor 4 (mGluR4) in the rodent CNS. Neuroscience 110:403–420 [DOI] [PubMed] [Google Scholar]
- 8.Moyanova SG, Mastroiacovo F, Kortenska LV, Mitreva RG, Fardone E, Santolini I, Sobrado M, Battaglia G, Bruno V, Nicoletti F. and Ngomba RT. (2011). Protective role for type 4 metabotropic glutamate receptors against ischemic brain damage. J Cereb Blood Flow Metab 31:1107–1118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bruno V, Battaglia G, Ksiazek I, van der Putten H, Catania MV, Giuffrida R, Lukic S, Leonhardt T, Inderbitzin W, et al. (2000). Selective activation of mGlu4 metabotropic glutamate receptors is protective against excitotoxic neuronal death. J Neurosci 20:6413–6420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maj M, Bruno V, Dragic Z, Yamamoto R, Battaglia G, Inderbitzin W, Stoehr N, Stein T, Gasparini F, et al. (2003). (-)-PHCCC, a positive allosteric modulator of mGluR4: characterization, mechanism of action, and neuroprotection. Neuropharmacology 45:895–906 [DOI] [PubMed] [Google Scholar]
- 11.Wang H, Jiang W, Yang R. and Li Y. (2011). Spinal metabotropic glutamate receptor 4 is involved in neuropathic pain. Neuroreport 22:244–248 [DOI] [PubMed] [Google Scholar]
- 12.Faden AI, Ivanova SA, Yakovlev AG. and Mukhin AG. (1997). Neuroprotective effects of group III mGluR in traumatic neuronal injury. J Neurotrauma 14:885–895 [DOI] [PubMed] [Google Scholar]
- 13.Saxe JP, Wu H, Kelly TK, Phelps ME, Sun YE, Kornblum HI. and Huang J. (2007). A phenotypic small-molecule screen identifies an orphan ligand-receptor pair that regulates neural stem cell differentiation. Chem Biol 14:1019–1030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Canudas AM, Di Giorgi-Gerevini V, Iacovelli L, Nano G, D'Onofrio M, Arcella A, Giangaspero F, Busceti C, Ricci-Vitiani L, et al. (2004). PHCCC, a specific enhancer of type 4 metabotropic glutamate receptors, reduces proliferation and promotes differentiation of cerebellar granule cell neuroprecursors. J Neurosci 24:10343–10352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cappuccio I, Verani R, Spinsanti P, Niccolini C, Gradini R, Costantino S, Nicoletti F. and Melchiorri D. (2006). Context-dependent regulation of embryonic stem cell differentiation by mGlu4 metabotropic glutamate receptors. Neuropharmacology 51:606–611 [DOI] [PubMed] [Google Scholar]
- 16.Wang WY, Wang H, Luo Y, Jia LJ, Zhao JN, Zhang HH, Ma ZW, Xue QS. and Yu BW. (2012). The effects of metabotropic glutamate receptor 7 allosteric agonist N,N′-dibenzhydrylethane-1,2-diamine dihydrochloride on developmental sevoflurane neurotoxicity: role of extracellular signal-regulated kinase 1 and 2 mitogen-activated protein kinase signaling pathway. Neuroscience 205:167–177 [DOI] [PubMed] [Google Scholar]
- 17.Chen X, Tian Y, Yao L, Zhang J. and Liu Y. (2010). Hypoxia stimulates proliferation of rat neural stem cells with influence on the expression of cyclin D1 and c-Jun N-terminal protein kinase signaling pathway in vitro. Neuroscience 165:705–714 [DOI] [PubMed] [Google Scholar]
- 18.Wu Y, Wang D, Wang X, Wang Y, Ren F, Chang D, Chang Z. and Jia B. (2011). Caspase 3 is activated through caspase 8 instead of caspase 9 during H2O2-induced apoptosis in HeLa cells. Cell Physiol Biochem 27:539–546 [DOI] [PubMed] [Google Scholar]
- 19.Yamakawa H, Ito Y, Naganawa T, Banno Y, Nakashima S, Yoshimura S, Sawada M, Nishimura Y, Nozawa Y. and Sakai N. (2000). Activation of caspase-9 and −3 during H2O2-induced apoptosis of PC12 cells independent of ceramide formation. Neurol Res 22:556–564 [DOI] [PubMed] [Google Scholar]
- 20.Choi NY, Choi H, Park HH, Lee EH, Yu HJ, Lee KY, Joo Lee Y. and Koh SH. (2014). Neuroprotective effects of amlodipine besylate and benidipine hydrochloride on oxidative stress-injured neural stem cells. Brain Res 1551:1–12 [DOI] [PubMed] [Google Scholar]
- 21.Wu YM, Jin R, Yang L, Zhang J, Yang Q, Guo YY, Li XB, Liu SB, Luo XX. and Zhao MG. (2013). Phosphatidylinositol 3 kinase/protein kinase B is responsible for the protection of paeoniflorin upon H(2)O(2)-induced neural progenitor cell injury. Neuroscience 240:54–62 [DOI] [PubMed] [Google Scholar]
- 22.Kim JH, Choi W, Lee JH, Jeon SJ, Choi YH, Kim BW, Chang HI. and Nam SW. (2009). Astaxanthin inhibits H2O2-mediated apoptotic cell death in mouse neural progenitor cells via modulation of P38 and MEK signaling pathways. J Microbiol Biotechnol 19:1355–1363 [DOI] [PubMed] [Google Scholar]
- 23.Kamata H, Honda S, Maeda S, Chang L, Hirata H. and Karin M. (2005). Reactive oxygen species promote TNFalpha-induced death and sustained JNK activation by inhibiting MAP kinase phosphatases. Cell 120:649–661 [DOI] [PubMed] [Google Scholar]
- 24.Ming GL. and Song H. (2005). Adult neurogenesis in the mammalian central nervous system. Annu Rev Neurosci 28:223–250 [DOI] [PubMed] [Google Scholar]
- 25.Eriksson PS, Perfilieva E, Bjork-Eriksson T, Alborn AM, Nordborg C, Peterson DA. and Gage FH. (1998). Neurogenesis in the adult human hippocampus. Nat Med 4:1313–1317 [DOI] [PubMed] [Google Scholar]
- 26.Brilli E, Reitano E, Conti L, Conforti P, Gulino R, Consalez GG, Cesana E, Smith A, Rossi F. and Cattaneo E. (2013). Neural stem cells engrafted in the adult brain fuse with endogenous neurons. Stem Cells Dev 22:538–547 [DOI] [PubMed] [Google Scholar]
- 27.Madhavan L, Ourednik V. and Ourednik J. (2005). Grafted neural stem cells shield the host environment from oxidative stress. Ann N Y Acad Sci 1049:185–188 [DOI] [PubMed] [Google Scholar]
- 28.Ziabreva I, Perry E, Perry R, Minger SL, Ekonomou A, Przyborski S. and Ballard C. (2006). Altered neurogenesis in Alzheimer's disease. J Psychosom Res 61:311–316 [DOI] [PubMed] [Google Scholar]
- 29.Hattiangady B. and Shetty AK. (2010). Decreased neuronal differentiation of newly generated cells underlies reduced hippocampal neurogenesis in chronic temporal lobe epilepsy. Hippocampus 20:97–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hoglinger GU, Rizk P, Muriel MP, Duyckaerts C, Oertel WH, Caille I. and Hirsch EC. (2004). Dopamine depletion impairs precursor cell proliferation in Parkinson disease. Nat Neurosci 7:726–735 [DOI] [PubMed] [Google Scholar]
- 31.Felling RJ. and Levison SW. (2003). Enhanced neurogenesis following stroke. J Neurosci Res 73:277–283 [DOI] [PubMed] [Google Scholar]
- 32.Niv F, Keiner S, Krishna , Witte OW, Lie DC. and Redecker C. (2012). Aberrant neurogenesis after stroke: a retroviral cell labeling study. Stroke 43:2468–2475 [DOI] [PubMed] [Google Scholar]
- 33.Braun H, Schafer K. and Hollt V. (2002). BetaIII tubulin-expressing neurons reveal enhanced neurogenesis in hippocampal and cortical structures after a contusion trauma in rats. J Neurotrauma 19:975–983 [DOI] [PubMed] [Google Scholar]
- 34.Adibhatla RM. and Hatcher JF. (2010). Lipid oxidation and peroxidation in CNS health and disease: from molecular mechanisms to therapeutic opportunities. Antioxid Redox Signal 12:125–169 [DOI] [PubMed] [Google Scholar]
- 35.Lucas SM, Rothwell NJ. and Gibson RM. (2006). The role of inflammation in CNS injury and disease. Br J Pharmacol 147 Suppl 1:S232–S240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang TT, Zou Y. and Corniola R. (2012). Oxidative stress and adult neurogenesis—effects of radiation and superoxide dismutase deficiency. Semin Cell Dev Biol 23:738–744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ray PD, Huang BW. and Tsuji Y. (2012). Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell Signal 24:981–990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yeo H, Lyssiotis CA, Zhang Y, Ying H, Asara JM, Cantley LC. and Paik JH. (2013). FoxO3 coordinates metabolic pathways to maintain redox balance in neural stem cells. EMBO J 32:2589–2602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Labinskyy N, Csiszar A, Orosz Z, Smith K, Rivera A, Buffenstein R. and Ungvari Z. (2006). Comparison of endothelial function, O2-* and H2O2 production, and vascular oxidative stress resistance between the longest-living rodent, the naked mole rat, and mice. Am J Physiol Heart Circ Physiol 291:H2698–H2704 [DOI] [PubMed] [Google Scholar]
- 40.Kim J. and Wong PK. (2009). Loss of ATM impairs proliferation of neural stem cells through oxidative stress-mediated p38 MAPK signaling. Stem Cells 27:1987–1998 [DOI] [PubMed] [Google Scholar]
- 41.Naderi J, Hung M. and Pandey S. (2003). Oxidative stress-induced apoptosis in dividing fibroblasts involves activation of p38 MAP kinase and over-expression of Bax: resistance of quiescent cells to oxidative stress. Apoptosis 8:91–100 [DOI] [PubMed] [Google Scholar]
- 42.St-Pierre J, Drori S, Uldry M, Silvaggi JM, Rhee J, Jager S, Handschin C, Zheng K, Lin J, et al. (2006). Suppression of reactive oxygen species and neurodegeneration by the PGC-1 transcriptional coactivators. Cell 127:397–408 [DOI] [PubMed] [Google Scholar]
- 43.Bokara KK, Kwon KH, Nho Y, Lee WT, Park KA. and Lee JE. (2011). Retroviral expression of arginine decarboxylase attenuates oxidative burden in mouse cortical neural stem cells. Stem Cells Dev 20:527–537 [DOI] [PubMed] [Google Scholar]
- 44.Chuikov S, Levi BP, Smith ML. and Morrison SJ. (2010). Prdm16 promotes stem cell maintenance in multiple tissues, partly by regulating oxidative stress. Nat Cell Biol 12:999–1006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Madhavan L, Ourednik V. and Ourednik J. (2006). Increased “vigilance”. of antioxidant mechanisms in neural stem cells potentiates their capability to resist oxidative stress. Stem Cells 24:2110–2119 [DOI] [PubMed] [Google Scholar]
- 46.Lei S, Zhang P, Li W, Gao M, He X, Zheng J, Li X, Wang X, Wang N, et al. (2014). Pre- and posttreatment with edaravone protects CA1 hippocampus and enhances neurogenesis in the subgranular zone of dentate gyrus after transient global cerebral ischemia in rats. ASN Neuro 6:pii: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tamm C, Zhivotovsky B. and Ceccatelli S. (2008). Caspase-2 activation in neural stem cells undergoing oxidative stress-induced apoptosis. Apoptosis 13:354–363 [DOI] [PubMed] [Google Scholar]
- 48.Hunter AM, LaCasse EC. and Korneluk RG. (2007). The inhibitors of apoptosis (IAPs) as cancer targets. Apoptosis 12:1543–1568 [DOI] [PubMed] [Google Scholar]
- 49.Tamm C, Robertson JD, Sleeper E, Enoksson M, Emgard M, Orrenius S. and Ceccatelli S. (2004). Differential regulation of the mitochondrial and death receptor pathways in neural stem cells. Eur J Neurosci 19:2613–2621 [DOI] [PubMed] [Google Scholar]
- 50.Cheng B, Lu H, Bai B. and Chen J. (2013). d-beta-Hydroxybutyrate inhibited the apoptosis of PC12 cells induced by H2O2 via inhibiting oxidative stress. Neurochem Int 62:620–625 [DOI] [PubMed] [Google Scholar]
- 51.Li J, Johnson D, Calkins M, Wright L, Svendsen C. and Johnson J. (2005). Stabilization of Nrf2 by tBHQ confers protection against oxidative stress-induced cell death in human neural stem cells. Toxicol Sci 83:313–328 [DOI] [PubMed] [Google Scholar]
- 52.Matsukawa J, Matsuzawa A, Takeda K. and Ichijo H. (2004). The ASK1-MAP kinase cascades in mammalian stress response. J Biochem 136:261–265 [DOI] [PubMed] [Google Scholar]
- 53.McCubrey JA, Lahair MM. and Franklin RA. (2006). Reactive oxygen species-induced activation of the MAP kinase signaling pathways. Antioxid Redox Signal 8:1775–1789 [DOI] [PubMed] [Google Scholar]
- 54.Fukunaga M, Miyata S, Higo S, Hamada Y, Ueyama S. and Kasuga M. (2005). Methylglyoxal induces apoptosis through oxidative stress-mediated activation of p38 mitogen-activated protein kinase in rat Schwann cells. Ann N Y Acad Sci 1043:151–157 [DOI] [PubMed] [Google Scholar]
- 55.Eisenberg-Lerner A. and Kimchi A. (2007). DAP kinase regulates JNK signaling by binding and activating protein kinase D under oxidative stress. Cell Death Differ 14:1908–1915 [DOI] [PubMed] [Google Scholar]
- 56.Ki YW, Park JH, Lee JE, Shin IC. and Koh HC. (2013). JNK and p38 MAPK regulate oxidative stress and the inflammatory response in chlorpyrifos-induced apoptosis. Toxicol Lett 218:235–245 [DOI] [PubMed] [Google Scholar]
- 57.Feligioni M, Brambilla E, Camassa A, Sclip A, Arnaboldi A, Morelli F, Antoniou X. and Borsello T. (2011). Crosstalk between JNK and SUMO signaling pathways: deSUMOylation is protective against H2O2-induced cell injury. PLoS One 6:e28185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Song X, Xu A, Pan W, Wallin B, Kivlin R, Lu S, Cao C, Bi Z. and Wan Y. (2008). Minocycline protects melanocytes against H2O2-induced cell death via JNK and p38 MAPK pathways. Int J Mol Med 22:9–16 [PubMed] [Google Scholar]
- 59.Murakami T, Takagi H, Suzuma K, Suzuma I, Ohashi H, Watanabe D, Ojima T, Suganami E, Kurimoto M, et al. (2005). Angiopoietin-1 attenuates H2O2-induced SEK1/JNK phosphorylation through the phosphatidylinositol 3-kinase/Akt pathway in vascular endothelial cells. J Biol Chem 280:31841–31849 [DOI] [PubMed] [Google Scholar]
- 60.Junn E. and Mouradian MM. (2001). Apoptotic signaling in dopamine-induced cell death: the role of oxidative stress, p38 mitogen-activated protein kinase, cytochrome c and caspases. J Neurochem 78:374–383 [DOI] [PubMed] [Google Scholar]
- 61.Mauro C, Crescenzi E, De Mattia R, Pacifico F, Mellone S, Salzano S, de Luca C, D'Adamio L, Palumbo G, et al. (2006). Central role of the scaffold protein tumor necrosis factor receptor-associated factor 2 in regulating endoplasmic reticulum stress-induced apoptosis. J Biol Chem 281:2631–2638 [DOI] [PubMed] [Google Scholar]
- 62.Tibbles LA, Ing YL, Kiefer F, Chan J, Iscove N, Woodgett JR. and Lassam NJ. (1996). MLK-3 activates the SAPK/JNK and p38/RK pathways via SEK1 and MKK3/6. EMBO J 15:7026–7035 [PMC free article] [PubMed] [Google Scholar]
- 63.Abell AN, Granger DA. and Johnson GL. (2007). MEKK4 stimulation of p38 and JNK activity is negatively regulated by GSK3beta. J Biol Chem 282:30476–30484 [DOI] [PubMed] [Google Scholar]
- 64.Iacovelli L, Bruno V, Salvatore L, Melchiorri D, Gradini R, Caricasole A, Barletta E, De Blasi A. and Nicoletti F. (2002). Native group-III metabotropic glutamate receptors are coupled to the mitogen-activated protein kinase/phosphatidylinositol-3-kinase pathways. J Neurochem 82:216–223 [DOI] [PubMed] [Google Scholar]
- 65.Ciccarelli R, D'Alimonte I, Ballerini P, D'Auro M, Nargi E, Buccella S, Di Iorio P, Bruno V, Nicoletti F. and Caciagli F. (2007). Molecular signalling mediating the protective effect of A1 adenosine and mGlu3 metabotropic glutamate receptor activation against apoptosis by oxygen/glucose deprivation in cultured astrocytes. Mol Pharmacol 71:1369–1380 [DOI] [PubMed] [Google Scholar]
- 66.Iacovelli L, Felicioni M, Nistico R, Nicoletti F. and De Blasi A. (2014). Selective regulation of recombinantly expressed mGlu7 metabotropic glutamate receptors by G protein-coupled receptor kinases and arrestins. Neuropharmacology 77:303–312 [DOI] [PubMed] [Google Scholar]
- 67.Staples CJ, Owens DM, Maier JV, Cato AC. and Keyse SM. (2010). Cross-talk between the p38alpha and JNK MAPK pathways mediated by MAP kinase phosphatase-1 determines cellular sensitivity to UV radiation. J Biol Chem 285:25928–25940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mao L, Yang L, Arora A, Choe ES, Zhang G, Liu Z, Fibuch EE. and Wang JQ. (2005). Role of protein phosphatase 2A in mGluR5-regulated MEK/ERK phosphorylation in neurons. J Biol Chem 280:12602–12610 [DOI] [PubMed] [Google Scholar]
- 69.Enz R. (2002). The metabotropic glutamate receptor mGluR7b binds to the catalytic gamma-subunit of protein phosphatase 1. J Neurochem 81:1130–1140 [DOI] [PubMed] [Google Scholar]
- 70.Caraci F, Battaglia G, Sortino MA, Spampinato S, Molinaro G, Copani A, Nicoletti F. and Bruno V. (2012). Metabotropic glutamate receptors in neurodegeneration/neuroprotection: still a hot topic?. Neurochem Int 61:559–565 [DOI] [PubMed] [Google Scholar]
- 71.Nicoletti F, Bockaert J, Collingridge GL, Conn PJ, Ferraguti F, Schoepp DD, Wroblewski JT. and Pin JP. (2011). Metabotropic glutamate receptors: from the workbench to the bedside. Neuropharmacology 60:1017–1041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Melchiorri D, Cappuccio I, Ciceroni C, Spinsanti P, Mosillo P, Sarichelou I, Sale P. and Nicoletti F. (2007). Metabotropic glutamate receptors in stem/progenitor cells. Neuropharmacology 53:473–480 [DOI] [PubMed] [Google Scholar]
- 73.Zhu DY, Zhou LM, Zhang YY, Huang JQ, Pan X. and Lou YJ. (2012). Involvement of metabotropic glutamate receptor 5 in cardiomyocyte differentiation from mouse embryonic stem cells. Stem Cells Dev 21:2130–2141 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.