Abstract
Severe dengue fever is usually associated with secondary infection by a dengue virus (DENV) serotype (1 to 4) that is different to the serotype of the primary infection. Dengue outbreaks only occur following importations of DENV in Cairns, Australia. However, the majority of imported cases do not result in autochthonous transmission in Cairns. Although DENV transmission is strongly associated with the El Niño-Southern Oscillation (ENSO) climate cycle and local weather conditions, the frequency and potential risk factors of infections with the different DENV serotypes, including whether or not they differ, is unknown. This study used a classification tree model to identify the hierarchical interactions between Southern Oscillation Index (SOI), local weather factors, the presence of imported serotypes and the occurrence of the four autochthonous DENV serotypes from January 2000–December 2009 in Cairns. We found that the 12-week moving average of SOI and the 2-week moving average of maximum temperature were the most important factors influencing the variation in the weekly occurrence of the four DENV serotypes, the likelihoods of the occurrence of the four DENV serotypes may be unequal under the same environmental conditions, and occurrence may be influenced by changes in global and local environmental conditions in Cairns.
Dengue, a disease caused by the dengue virus (DENV), is the most important arbovirus in tropical and subtropical regions1. The number of dengue cases globally has increased 30-fold in the past five decades2. Infection with a specific dengue serotype causes life-long homologous immunity on first serotype-specific infection and short-lived cross-immunity1,3,4,5. People subsequently infected by heterologous serotypes are at higher risk of dengue haemorrhagic fever and dengue shock syndrome, in which the presence of antibodies of heterologous serotypes might increase replication of DENV6. Historically, DENV has been believed to include four serotypes (DENV-1 to 4) with a fifth serotype recently discovered, potentially further complicating dengue transmission control and disease prevention, particularly with regards to the development of a dengue vaccine7. Hence, there is an urgent need to fully understand the transmission dynamics of DENV serotypes.
Previous studies have demonstrated that DENV transmission is strongly associated with the El Niño-Southern Oscillation (ENSO) climate cycle and local weather conditions8,9,10,11,12, and that the risk of occurrence of dengue epidemics has varied according to the strength of ENSO13,14. Moreover, the effects of environmental factors on dengue transmission at different spatial scales have differed, and display complex nonlinear dynamics11,13,14. Some studies also demonstrated that imported dengue cases could initiate local dengue outbreaks only under suitable local weather conditions9,15. Nevertheless, previous studies have aggregated data on all four DENV serotypes. Few studies have paid attention to the effects of weather factors on DENV16. It is necessary to investigate the association between DENV displacement and meteorology to assess the risk of severity of local dengue epidemics15,16. In addition, Co-circulation of the four DENV serotypes has been reported widely and has increased in many regions in the world17. A study in Singapore showed that importation of different DENV serotypes displayed at various transmission levels and a serotype-specific outbreak often has been replaced subsequently by other serotypes18. Although varying predominant DENV serotypes by year from 1973 to 1999 in Thailand was reported19 and transmission dynamics of individual DENV serotypes have been widely described and related to climate, human behaviours and herd immunity20,21,22, the impact of these factors on transmission of different DENV serotypes is still unclear21. The dynamics of DENV are complex, involving a vector component, herd immunity, human behaviours and environmental factors23. The majority of imported DENV cases do not result in autochthonous transmission in non-endemic regions24,25 and little is known about whether there is a differential risk based on specific imported serotype, particularly in non-endemic areas. Uncertainty remains regarding the effects of global and local environmental factors on comparative occurrence of different DENV serotypes26.
Currently, dengue transmission in Australia only occurs in the north-east of Queensland, triggered by viraemic international travellers. In that region, Cairns experiences the highest number of locally acquired dengue fever cases27. The frequency of dengue outbreaks and the number of imported dengue cases have increased in the past two decades in north Queensland. The hyperendemic Asia-Pacific region, where all four DENV serotypes have circulated28,29,30, is the main source of imported dengue throughout the year in Cairns31.
Our previous study assessed the predictors of dengue fever incidence in the Cairns region, finding that temperature and rainfall made a contribution to local dengue fever disease transmission9,10. However, it remains unclear whether environmental factors affect the occurrence of autochthonous cases caused by specific DENV serotypes26. Understanding the drivers of occurrence of different DENV serotypes is important to developing future vaccination programmes, current control strategies and ongoing dengue risk assessments relating to sequential infections with different serotypes.
Results
Associations between autochthonous, reported imported DENV serotypes and SOI
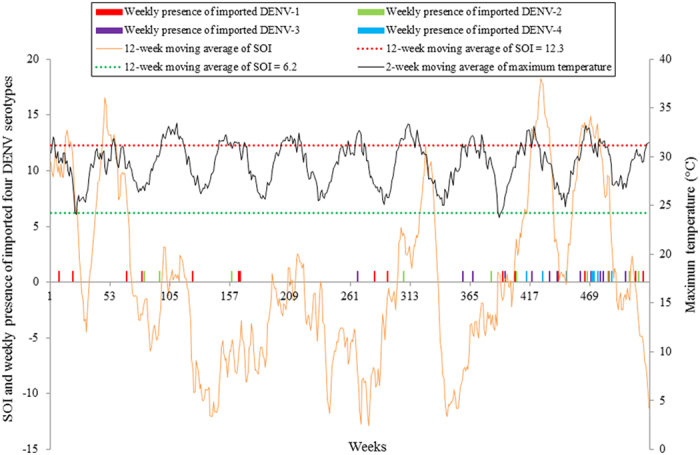
Figure 1 shows the weekly numbers of the reported autochthonous and imported cases for the four DENV serotypes between 1st January 2000 and 31st December 2009. The numbers of autochthonous cases caused by DENV-2 and -3 were much larger than DENV-1 and -4. Large outbreaks of DENV-2 were observed during 2003 and 2004 and a large outbreak of DENV-3 occurred in 2008 and 2009. Figure 1 also showed that many importations did not successfully result in an expectation outbreak. The weekly occurrence of autochthonous cases caused by DENV-2 and -3 were the most frequently observed autochthonous serotypes in the study (Fig. 2). There were only three weekly occurrences of autochthonous DENV-1 during the study period. The occurrence of autochthonous cases caused by the four DENV serotypes appeared to fluctuate randomly over the study period. Sixty-seven percent of the weekly occurrence of autochthonous DENV-1 and 57% of the weekly occurrence of autochthonous DENV-2 were observed when the values of the 12-week moving average of SOI were negative. All the weekly occurrence of autochthonous DENV-3 and 75% of the weekly occurrence of autochthonous DENV-4 were found when the values of the 12-week moving average of SOI were positive. Figure 3 showed that 53% of the weekly presence of reported imported DENV-1 and 60% of the weekly presence of reported imported DENV-2 were observed when the values of the 12-week moving average of SOI were negative. Sixty-seven percent of the weekly presence of reported imported DENV-3 and 88% of the weekly presence of reported imported DENV-4 were detected when the values of the 12-week moving average of SOI were positive. Figure 3 also showed that the diversity and number of imported DENV serotypes increased during the study period. DENV-1 was the most frequently imported DENV serotype in Cairns.
Figure 1. The temporal patterns of the weekly numbers of autochthonous and imported dengue cases caused by the four DENV serotypes during 1st January 2000 and 31st December 2009 in Cairns, Queensland, Australia.

Figure 2. The temporal patterns of the weekly occurrence of the four autochthonous DENV serotypes, 12-week moving average of SOI and 2-week moving average of maximum temperature during 1st January 2000 and 31st December 2009 in Cairns, Queensland, Australia.
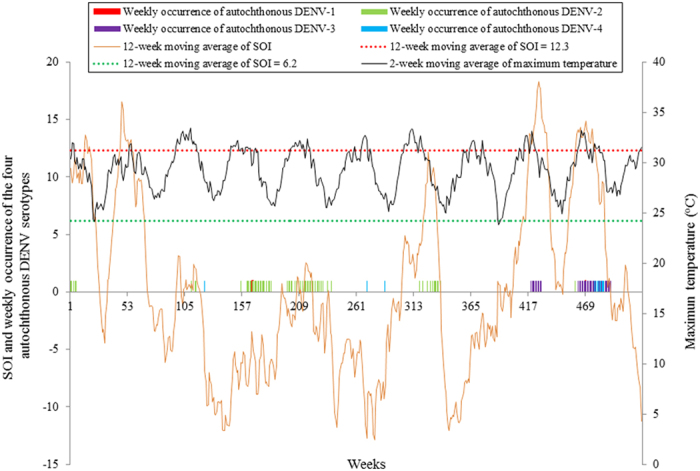
Figure 3. The temporal patterns of the weekly presence of reported imported four DENV serotypes, 2-week moving average of maximum temperature and 12-week moving average of SOI during 1st January 2000 and 31st December 2009 in Cairns, Queensland, Australia.
Classification tree analysis
The misclassification rate of the classification tree model was 19.8% in the study. After accounting for the presence of reported imports of all four DENV serotypes, the first classifying factor in the classification tree model was SOI (Fig. 4). This indicates that the 12-week moving average of SOI was the most important factor influencing the variation in the weekly occurrence of autochthonous cases caused by specific DENV serotypes. There was 90% chance of the weekly occurrence of autochthonous cases caused by DENV-3 when the 12-week moving average of SOI was ≥12.3 (Fig. 4: Terminal node 1); and there was an 85% chance of the occurrence of autochthonous cases caused by DENV-2 in a week when the 12-week moving average of SOI was <6.2 (Fig. 4: Terminal node 2). Moreover, when the 12-week moving average of SOI was ≥6.2 and <12.3, DENV-3 had the highest chance (43%) of occurrence when the 2-week moving average of maximum temperature was ≥30 °C (Fig. 4: Terminal node 3); only DENV-2 occurred when the 2-week moving average of maximum temperature was <30 °C (Fig. 4: Terminal node 4). Rainfall, minimum temperature and imports of the four DENV serotypes were not associated with the weekly occurrence of autochthonous cases caused by the four different DENV serotypes in the model. Finally, although the proportions of the weekly occurrences of autochthonous DENV-1 and DENV-4 were not the largest in the classification tree, 67% of the total weekly occurrences of autochthonous DENV-1 and DENV-4 fell into the terminal node 2 and the terminal node 3, respectively.
Figure 4. The classification tree for the weekly occurrence of autochthonous dengue cases caused by the four DENV serotypes and global/local environmental factors between 1st January 2000 and 31st December 2009 in Cairns, Queensland, Australia.
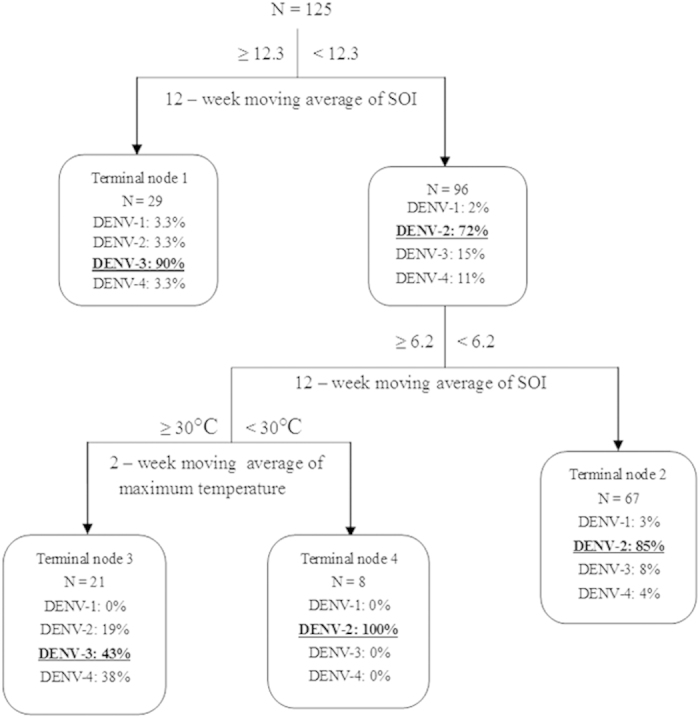
N is the total number of the weekly occurrence of the four autochthonous DENV serotypes in each node.
Discussion
Cairns was selected as the study site because it is in a non-endemic region, where dengue outbreaks only occur following importations of DENV27. Outbreaks are triggered by incursions of imported DENV throughout the year. The dynamics of dengue outbreaks may not be strongly affected by the immune status of the human population due to limited exposure.
Imported DENV serotypes were introduced into Cairns throughout the year and local transmission of autochthonous DENV serotypes showed that new imports seldom triggered new outbreaks. DENV-1 has been the most frequently imported DENV serotype in Cairns31,32 and this was shown in this study (Fig. 3). However, the probability and the frequency of its subsequent detectable autochthonous transmission were very low. This may be because DENV-1 cases are more likely to be asymptomatic in comparison with cases caused by DENV-2 and -333. Our study confirms that imported DENV serotypes do not necessarily lead to epidemic spread even in susceptible regions24,25,26. This may be explained by the substantial variation in virulence, human behaviour, chance events etc. and transmissions of different DENV genotypes under the same environmental conditions34.
The classification tree model identified that the 12-week moving average of SOI with a threshold value of 12.3 was a key determinant of occurrence of autochthonous cases caused by all four DENV serotypes. Cairns generally experiences La Niña episodes and more rainfall when SOI is >835. The transmission of autochthonous DENV-3 was associated with a wetter environment as 90% of weekly occurrence occurred during La Niña episodes. Conversely, autochthonous DENV-2 presented the highest transmission risk (85%) when the 12-week moving average of SOI was <6.2, which indicated that the transmission of autochthonous DENV-2 was more associated with a hotter and dryer weather condition. It is well known that ENSO is a strong contributor to global inter-annual weather variability, ecosystem dynamics as well as human behaviour36. It is therefore not surprising that the strength of SOI is responsible for local ecological, temperature and rainfall variability. A DENV serotype study in Thailand has demonstrated that the four DENV serotypes have unique epidemiological characteristics and dominant DENV serotypes varied by year19. Temperature variation also influenced the vector efficiency of Aedes. aegypti for DENV-2 and significantly dominates the yearly cyclic pattern of dengue hemorrhagic fever37. Moreover, previous research has suggested that DENV transmission and DENV evolution involves many complex interactions, including cellular, host, environment, vector and population level34. The association between DENV transmission and weather condition might be changed due to virus and vector to adapt to climate variations16. The occurrence of different DENV serotypes might be driven by global environmental factors captured by variables such as SOI, which are likely to be good surrogates for a combination of local environmental variability, socioeconomic and human and mosquito behaviour. We suggest that global SOI appears to mirror a key threshold effect of integrated environmental conditions in determining the occurrence of specific DENV serotypes due to their different characteristics and the efficiency of virus transmission by mosquitoes in Cairns.
This study also found that the 2-week moving average of local maximum temperature played a distinct role in the transmissions of four DENV serotypes when the 12-week moving average of SOI was between 6.2 and 12.3. Typically, increasing temperature can shorten the time interval for the extrinsic incubation period, which is a product of the viral replication speed and Ae. aegypti survival38. This study indicated that transmission of the four DENV serotypes was different for the same global and local environmental conditions. Previous study indicated that the transmission rate of DENV-2 started to upswing during the latter half of hot-dry season and high temperature was suitable for the DENV-2 transmission by Ae. Aegypti35. Our study also evidenced that there was a higher probability of the occurrence of DENV-2 during El Niño episodes. We hypothesise that this may explain why studies at different spatial scales based on aggregated data for the four DENV serotypes have identified different effects of environment on dengue incidence. We also suggest that a new imported DENV serotype could trigger a specific outbreak only under appropriately integrated environmental conditions due to the complex process of transmission of the four DENV serotypes examined here. More research is required to investigate the association between climate factors and viral traits such as virulence indices and vector competence in both humans and mosquitoes based on different serotypes.
Some limitations of this study should be acknowledged. Firstly, the data did not capture all dengue cases due to asymptomatic or subclinical dengue cases, and incomplete data on DENV serotypes. This is likely to result in underestimation of the probability of the occurrence of autochthonous cases caused by specific DENV serotype (e.g. the small number of DENV-1 in the study). Secondly, other potential confounding variables such as social factors, vector control, population cross-immunity, mosquito populations, house construction, specific behaviours and occupation, were not available for the current study. Thirdly, failure to detect an effect of imported DENV serotypes on autochthonous DENV serotypes might have arisen from incomplete reporting of imported dengue cases to local health authorities. Finally, temporal autocorrelation of dengue cases may be a potential confounder, leading to biased estimation of effects. However, our study is a starting point to discuss the relationship between DENV serotypes and environmental factors and showed that a relationship may exist. In future research, further epidemiological investigation after considering potential confounders is needed to confirm the findings.
Although many studies have reported that dengue dynamics have fluctuated with ENSO using aggregated data on all four DENV serotypes14,39, our study presents a further investigation of the relationship between global and local environmental factors and dengue dynamics based on each of four autochthonous DENV serotypes. Our data suggest that suitable environmental conditions (i.e., rainfall and temperature) are more important than imported cases because they are key drivers of mosquito population dynamics. Importations happen sufficiently frequently that importation is not the main driver to an outbreak occurring.
Conclusion
Twelve-week moving average of SOI and the 2-week moving average of maximum temperature may be important factors influencing the temporal distribution of the weekly occurrence of autochthonous cases caused by specific DENV serotypes in Cairns. Our findings suggest that the likelihoods of the occurrence of the four serotypes might be unequal under the same environmental conditions and vary with changes in global and local environments. Knowledge of the association between the occurrence of DENV serotypes and global climate and local environmental variability might be important for guiding improvements in allocating resources for disease prevention and control. Such interventions currently rely on vector control methods but may in the future include vaccines. Understanding the predictors of different serotypes might prove helpful in establishing optimal vaccination programmes and quantifying the risk and population health burden of severe dengue infections. Moreover, this study suggests that the role of global climate change in the transmission dynamics of the four DENV serotypes needs further work to inform future dengue control.
Methods
Study site and data collection
Cairns (latitude 16.55°S, longitude 145.46°E) is the second-largest city in north Queensland, and is a popular destination for domestic and international travellers. Cairns experiences a tropical climate with wet and dry seasons throughout the year. The highest and lowest annual mean temperatures are approximately 31°C and 18°C, respectively40. Annual mean total rainfall is 2008 mm41. Variability of climate in Australia is often related to changes in Southern Oscillation Index (SOI). Typically, Cairns experiences a reduction in rainfall during an El Niño episode and wetter weather than usual during a La Niña episode.
Data on the numbers of daily confirmed autochthonous and imported dengue cases between 1st January 2000 and 31st December 2009 were obtained from Queensland Health (the Queensland State government health authority). Approximately 19.8% of dengue cases with an unknown country of origin, or unknown DENV serotype, were excluded from the analysis. Imported dengue cases were defined as confirmed patients with a travel history to dengue endemic regions in the past 12 days. Autochthonous dengue cases had not recently experienced overseas travel27.
Daily minimum and maximum temperature, daily relative humidity and daily rainfall for 1st January 2000 to 31st December 2009 were obtained from the Australian Bureau of Meteorology. In this study, the SOI was used to assess global climatic variability since SOI describes interannual climate variability and the pattern of the ENSO cycle in the Pacific Ocean. SOI is calculated from the daily fluctuations in the Seal Level Pressure difference between Tahiti and Darwin. SOI is typically used to estimate the intensity of El Niño and La Niña events (two extreme events in the ENSO cycle), and to predict trends in climate variability globally. SOI yields constant negative values for a period of months when an El Niño episode is developing. Typically, the global climate experiences an El Niño episode when SOI values are negative and a La Niña episode when the SOI is positive42. Daily SOI was obtained from the Queensland Government, Australia43.
Statistical analysis and modelling
In this study, the number of autochthonous cases was very skew between the four DENV serotypes during the study period. Hence, we used the weekly occurrence of each of the four serotypes instead of using the weekly number of cases caused by the four serotypes in order to make the data less skewed, particularly to DENV-1 and DENV-4. Data on the weekly occurrence of the four autochthonous DENV serotypes were available for a total of 125 weeks between 1st January 2000 and 31st December 2009. A classification tree model was used to determine threshold effects of hierarchical relationships between environmental factors and the occurrence of the four autochthonous DENV serotypes44. In the model, the dependent variable was a categorical variable with four categories (i.e., DENV-1 to -4). The independent variables were the strength of the ENSO45,46, local meteorological data and importation of specific DENV serotypes, each of which has biologically plausible impact on dengue transmission due, for example, to potential impacts on extrinsic incubation period (e.g. 8–12 days) and intrinsic incubation periods (e.g. 4–10 days) in the mosquito and human47. Specific variables included minimum and maximum temperature, relative humidity and rainfall, at a lag of 1 week, the presence of reported imported cases of each DENV serotype at lags of 1–5 weeks, 2–4 week moving averages of local weather and 8 and 12-week moving averages of SOI.
A classification tree model was used to segment the distribution of the weekly occurrence of the four autochthonous DENV serotypes into subsets that were most likely to be associated with a particular combination of environmental conditions. The classification tree was built by repeating best splits of the environmental factors and the weekly presence of the four imported DENV serotypes in predicting the probability of the weekly occurrence of the four autochthonous DENV serotypes. The principle of impurity reduction (measured using the Gini improvement statistic) was used as the criterion for defining the best split. Cross-validation was used to select the tree size using estimated prediction errors. The ‘best’ tree is defined as having the smallest tree size and an estimated error rate within one standard error of the minimum44.
Additional Information
How to cite this article: Huang, X. et al. El Niño-Southern Oscillation, local weather and occurrences of dengue virus serotypes. Sci. Rep. 5, 16806; doi: 10.1038/srep16806 (2015).
Acknowledgments
We thank National Health and Medical Research Council in Australia for funding (#1002608) this study. We thank the Queensland Department of Health and Australian Bureau of Meteorology for providing the data on notified dengue fever cases and climate, respectively. The study was partly funded by the NHMRC project Grant (App1002608).
Footnotes
Author Contributions W.H. developed the original idea for this research. X.H. analysed the data and drafted this manuscript. W.H., A.C., G.W., G.D. and S.T. contributed to writing of the manuscript.
References
- Halstead S. B. Dengue. The Lancet 370, 1644–1652 (2007). [DOI] [PubMed] [Google Scholar]
- Lam S. K., Burke D., Gubler D., Méndez-Gálvan J. & Thomas L. Call for a World Dengue Day. The Lancet 379, 411–412 (2012). [DOI] [PubMed] [Google Scholar]
- Hay S. I. et al. Etiology of interepidemic periods of mosquito-borne disease. Proc. Natl. Acad. Sci. USA 97, 9335–9339 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirisena P. & Noordeen F. Evolution of dengue in Sri Lanka—changes in the virus, vector, and climate. Int. J. Infect. Dis. 19, 6–12 (2014). [DOI] [PubMed] [Google Scholar]
- Deen J. L. et al. The WHO dengue classification and case definitions: time for a reassessment. The Lancet 368, 170–173 (2006). [DOI] [PubMed] [Google Scholar]
- Kochel T. J. et al. Effect of dengue-1 antibodies on American dengue-2 viral infection and dengue haemorrhagic fever. The Lancet 360, 310–312 (2002). [DOI] [PubMed] [Google Scholar]
- Normile D. Surprising new dengue virus throws a spanner in disease control efforts. Science 342, 415–415 (2013). [DOI] [PubMed] [Google Scholar]
- Hales S., de Wet N., Maindonald J. & Woodward A. Potential effect of population and climate changes on global distribution of dengue fever: an empirical model. The Lancet 360, 830–834 (2002). [DOI] [PubMed] [Google Scholar]
- Huang X., Clements A. C., Williams G., Milinovich G. & Hu W. A threshold analysis of dengue transmission in terms of weather variables and imported dengue cases in Australia. Emerg. Microbes Infect. 2, e87, doi: 10.1038/emi.2013.85 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X., Williams G., Clements A. C. & Hu W. Imported Dengue Cases, Weather Variation and Autochthonous Dengue Incidence in Cairns, Australia. PloS ONE 8, e81887, doi: 10.1371/journal.pone.0081887 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson M. A., Dominici F. & Glass G. E. Local and global effects of climate on dengue transmission in Puerto Rico. PLoS Negl. Trop. Dis. 3, e382, doi: 10.1371/journal.pntd.0000382 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu W., Clements A., Williams G. & Tong S. Dengue fever and El Nino/Southern Oscillation in Queensland, Australia: a time series predictive model. Occup. Environ. Med. 67, 307–311 (2010). [DOI] [PubMed] [Google Scholar]
- Tipayamongkholgul M., Fang C.-T., Klinchan S., Liu C.-M. & King C. C. Effects of the El Niño-Southern Oscillation on dengue epidemics in Thailand, 1996-2005. BMC Public Health 9, 422 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cazelles B., Chavez M., McMichael A. J. & Hales S. Nonstationary influence of El Niño on the synchronous dengue epidemics in Thailand. PLoS Med. 2, e106, doi: 10.1371/journal.pmed.0020106 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang C. S. et al. The role of imported cases and favorable meteorological conditions in the onset of dengue epidemics. PLoS Negl Trop Dis 4, e775, doi: 10.1371/journal.pntd.0000775 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin C. W., Comrie A. C. & Ernst K. Climate and dengue transmission: evidence and implications. Environ. Health Perspect. 121, 1264–1272 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messina J. P. et al. Global spread of dengue virus types: mapping the 70 year history. Trends Microbiol. 22, 138–146 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K.-S. et al. Dengue virus surveillance for early warning, Singapore. Emerg. Infect. Dis. 16, 847 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nisalak A. et al. Serotype-specific dengue virus circulation and dengue disease in Bangkok, Thailand from 1973 to 1999. Am. J. Trop Med. Hyg. 68, 191–202 (2003). [PubMed] [Google Scholar]
- Carrington C. V., Foster J. E., Pybus O. G., Bennett S. N. & Holmes E. C. Invasion and maintenance of dengue virus type 2 and type 4 in the Americas. J. Virol. 79, 14680–14687 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halstead S. B. Dengue virus-mosquito interactions. Annu. Rev. Entomol. 53, 273–291 (2008). [DOI] [PubMed] [Google Scholar]
- Adams B. et al. Cross-protective immunity can account for the alternating epidemic pattern of dengue virus serotypes circulating in Bangkok. Proc. Natl. Acad. Sci. USA 103, 14234–14239 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duong V. et al. Complex dynamic of dengue virus serotypes 2 and 3 in Cambodia following series of climate disasters. Infect. Genet. Evol. 15, 77–86 (2013). [DOI] [PubMed] [Google Scholar]
- De Simone T. et al. Dengue virus surveillance: the co-circulation of DENV-1, DENV-2 and DENV-3 in the State of Rio de Janeiro, Brazil. Trans. R. Soc. Trop. Med. Hyg. 98, 553–562 (2004). [DOI] [PubMed] [Google Scholar]
- Rigau-Pérez J. G. et al. The reappearance of dengue-3 and a subsequent dengue-4 and dengue-1 epidemic in Puerto Rico in 1998. Am. J. Trop. Med. Hyg. 67, 355–362 (2002). [DOI] [PubMed] [Google Scholar]
- Runge‐Ranzinger S., Horstick O., Marx M. & Kroeger A. What does dengue disease surveillance contribute to predicting and detecting outbreaks and describing trends? Trop. Med. Int. Health 13, 1022–1041 (2008). [DOI] [PubMed] [Google Scholar]
- Queensland Government. Queensland Dengue Management Plan. (Queensland Government, 2011). [Google Scholar]
- Kyle J. L. & Harris E. Global spread and persistence of dengue. Annu. Rev. Microbiol. 62, 71–92 (2008). [DOI] [PubMed] [Google Scholar]
- Messer W. B., Gubler D. J., Harris E., Sivananthan K. & de Silva A. M. Emergence and global spread of a dengue serotype 3, subtype III virus. Emerg. Infect. Dis. 9, 800–809 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubler D. J. The changing epidemiology of yellow fever and dengue, 1900 to 2003: full circle? Comp. Immunol. Microbiol. Infect. Dis. 27, 319–330 (2004). [DOI] [PubMed] [Google Scholar]
- Warrilow D., Northill J. A. & Pyke A. T. Sources of Dengue Viruses Imported into Queensland, Australia, 2002–2010. Emerg. Infect. Dis. 18, 1850–1857 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katrina Knope & Carolein Giele. Increasing notifications of dengue in Australia related to overseas travel, 1991 to 2012. Commun. Dis. Intell. Q. Rep. 37, E55–E59 (2013). [PubMed] [Google Scholar]
- Rodenhuis-Zybert I. A., Wilschut J. & Smit J. M. Dengue virus life cycle: viral and host factors modulating infectivity. Cell. Mol. Life Sci. 67, 2773–2786 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebeca Rico-Hesse. Microevolution and virulence of dengue viruses. Adv. Virus Res. 59, 315–341 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bureau of Meteorology. Climate Glossary Southern Oscillation Index (2014). Available at: http://www.bom.gov.au/climate/glossary/soi.shtml. (Accessed: 19th March 2014).
- Cobb K. M. et al. Highly variable El Niño–Southern Oscillation throughout the Holocene. Science 339, 67–70 (2013). [DOI] [PubMed] [Google Scholar]
- Watts D. M., Burke D. S., Harrison B. A., Whitmire R. E. & Nisalak A. Effect of temperature on the vector efficiency of Aedes aegypti for dengue 2 virus. Am. J. Trop. Med. Hyg. 36, 143–152 (1987). [DOI] [PubMed] [Google Scholar]
- Focks D. A., Brenner R. J., Hayes J. & Daniels E. Transmission thresholds for dengue in terms of Aedes aegypti pupae per person with discussion of their utility in source reduction efforts. Am. J. Trop. Med. Hyg. 62, 11–18 (2000). [PubMed] [Google Scholar]
- Gagnon A. S., Bush Andrew BG & Karen E. Smoyer-Tomic. Dengue epidemics and the El Niño southern oscillation. Clim. Res. 19, 35–43 (2001). [Google Scholar]
- Bureau of Meteorology. Climate of Cairns (2012). Available at: http://www.bom.gov.au/qld/cairns/climate.shtml . (Accessed: 15th January 2013).
- Bureau of Meteorology. Climate statistics for Australian locations-Summary statistics CAIRNS AERO (2015). Available at: http://www.bom.gov.au/climate/averages/tables/cw_031011.shtml. (Accessed: 6th July 2015).
- Australian Government Bureau of Meteorology. El Niño, La Niña and Australia’s climate (2005). Available at: http://www.bom.gov.au/info/leaflets/nino-nina.pdf. (Accessed:15th September 2014).
- Queensland Government. SOI data files (2014). Available at: http://www.longpaddock.qld.gov.au/seasonalclimateoutlook/southernoscillationindex/soidatafiles/index.php. (Accessed: 1st April 2014).
- Breiman L., Friedman J. H., Olshen R. A. & Stone C. G. Classification and Regression Trees. (Wadsworth International Group, California, 1984). [Google Scholar]
- Banu S., Hu W., Hurst C. & Tong S. Dengue transmission in the Asia-Pacific region: impact of climate change and socio-environmental factors. Trop. Med. Int. Health 16, 598–607 (2011). [DOI] [PubMed] [Google Scholar]
- Chan M. & Michael A. Johansson. The incubation periods of dengue viruses. PLoS One 7, e50972, doi: 10.1371/journal.pone.0050972 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organisation. Dengue guidelines for diagnosis, treatment, prevention and control. (WHO, France, 2009). [PubMed] [Google Scholar]


