Abstract
Kidney transplantation is often the most effective therapy for end-stage renal disease, but there are not enough donor organs to meet the rising demand. Tissue engineering of kidneys is a potential solution to this organ shortage. Achieving microvascular perfusion has been a major barrier to engineering tissues beyond thin muscularized sheets such as the bladder wall. Our laboratory has previously reported that human umbilical vein endothelial cells (ECs) transduced with the antiapoptotic protein Bcl-2 will spontaneously organize into perfused microvessels within type I collagen gels when implanted in immunodeficient mice. To test if this system can be used to perfuse more complex structures, we combined Bcl-2-transduced ECs (Bcl-2-ECs) with renal glomeruli, the specialized vascular filtration units of the kidney. Microdissected green fluorescent protein-expressing rat glomeruli suspended in type I collagen gels were implanted within immunodeficient mice with or without the inclusion of Bcl-2-ECs. Survival of rat glomeruli was enhanced by coimplantation with Bcl-2-ECs. Intravital rhodamine dextran injections demonstrated that surviving glomeruli were perfused through Bcl-2-EC-derived microvessels. Perfused glomeruli maintained podocin staining, but transmission electron microscopy revealed endothelial swelling and podocyte foot process effacement. Anastomosis of microvessels derived from Bcl-2-ECs with glomerular capillaries provides proof of concept that self-assembled microvessels can perfuse specialized organ structures such as glomeruli, but that perfusion alone may be insufficient to maintain normal structure.
Introduction
In 2011, ∼600,000 patients in the United States had end-stage renal disease (ESRD) with Medicare costs totaling more than 30 billion dollars.1 Despite the availability of dialysis, morbidity and mortality in ESRD patients remains high. The best option currently for many ESRD patients is renal transplantation. However, at the present time there are more than 101,000 patients on the waiting list for a kidney transplant in the United States while only 17,106 transplants were done in 2014 (based upon Organ Procurement and Transplantation Network data as of June 2015). While the number of patients needing a kidney transplant is rising, the number of available donor organs remains relatively fixed.2 There is a clear shortage of available donor organs and an urgent need for additional strategies to tackle this problem. Tissue engineering of functional kidneys could potentially bridge this gap.
Successful tissue engineering of organs thicker than 400 μm must overcome the challenge of vascularization to supply oxygen and nutrients as well as to remove waste products.3 A promising approach for delivering nutrients to small organoids is the engineering of microvasculature networks from differentiated endothelial cells (ECs),4,5 which then anastomose with the host microvasculature. When implanted subcutaneously into an immunodeficient mouse, human umbilical vein endothelial cells (HUVECs) transduced with the antiapoptotic protein Bcl-2 led to enhanced EC survival in collagen/fibronectin gels and self-assembled into perfused microvascular networks that enhanced survival of cotransplanted fetal hepatocytes.6 This indicated that establishing perfusion near cotransplanted cells is enhanced by a self-assembled microvasculature.
The kidney is a complex, highly vascular organ that filters >100 L of blood daily to detoxify and tightly regulate fluid, electrolyte, and acid–base balance. The basic functional unit of the kidney is the nephron, and is composed of microvasculature connected to glomeruli that filter into the urinary space created by Bowman's capsule and then into epithelial-lined tubules that process the filtrate before urinary excretion. All three elements of the nephron (the microvasculature, the glomeruli, and the tubules) may be viewed as independent challenges for tissue engineers, but all three must be properly connected for the nephron to function appropriately.
Glomeruli are specialized capillary beds comprised of three cell types: fenestrated glomerular ECs, pericyte-like mesangial cells, and specialized epithelial podocytes. The specific localization and crosstalk between these cells is critical in maintaining the normal filtration function of the kidney.7 A recent report described anastomoses of implanted isolated glomeruli with the microvasculature in the anterior chamber of the mouse eye.8 This prompted us to determine if Bcl-2-transduced EC (Bcl-2-EC) vascular networks could enhance glomerular survival and anastomose with and perfuse specialized capillary beds such as those within renal glomeruli.
Materials and Methods
Cells and rat glomeruli
HUVECs were isolated from deidentified discarded tissues, and cultured as described previously9,10 under protocols approved by the Yale Human Investigations Committee. To generate Bcl-2-ECs, HUVECs were retrovirally transduced with Bcl-2 as previously described.11 Glomeruli were microdissected from euthanized SD-Tg(CAG-EGFP)12 rats by first harvesting the kidneys after perfusion with phosphate buffered saline through the left ventricle. After removal of the medulla, the cortices of the kidneys were minced with a scalpel and then glomeruli were enriched by 40 μm sieving. Glomeruli were then collected manually with a pipette tip coated with 1% bovine serum albumin.
Cell viability assay
To test whether glomerular cells were viable in vitro and in vivo, gels were incubated in HUVEC culture medium M199 with 20% fetal bovine serum, 100 μg/mL penicillin, 100 μg/mL streptomycin, 2 mM l-glutamine containing CellTracker™ red CMTPX dye (Invitrogen, Grand Island, NY) at 5 μg/mL for 1 h 37°C in a tissue culture incubator with 5% CO2–humidified air. CellTracker red dye is freely permeable through cell membranes, and fluorescence is enzymatically activated and retained by living cells, but not by cells that have lost viability. Gels were then washed three times in a medium without dye before visualization.
In vivo implantation of green fluorescent protein-glomeruli and visualization of microvessels and glomeruli
All experimental protocols were approved by the Yale's Institutional Animal Care and Use Committees (IACUC #2012-07863) and conform to the Guide for the Care and Use of Laboratory Animals. To suspend glomeruli and Bcl-2-ECs within collagen gels, rat-tail type 1 collagen (BD Biosciences, San Diego, CA) was added to a final concentration of 1.5 mg/mL in ice-cold M199 and buffered with 25 mM HEPES. The pH was adjusted to 7.4 with 1 M NaOH and the gel solution was kept on ice before polymerization. The collagen solution containing the indicated number of green fluorescent protein (GFP) glomeruli ±1 million Bcl-2-ECs in 400 μL of collagen solution was then added to 48-well dishes and polymerized by incubation for 20 min at 37°C in a tissue culture incubator with 5% CO2–humidified air. Gels were implanted subcutaneously in the abdominal walls of 6–8-week-old female C.B-17 SCID/Bg mice as described previously.13
To visualize through the entirety of gel to assess glomerular survival, extracted gels were incubated in a medium containing CellTracker and fixed in 10% formalin for 10 h and then placed in SCALEVIEW-A2 optical clearing solution (Olympus, Center Valley, PA) for 11 days before analysis. The CellTracker fluorescent signal was retained through the period of fixation and optical clearing. Gels were imaged with a Zeiss Axiovert 200M Fluorescent Microscope with a Hamamatsu ORCA-AG high-resolution camera and Volocity imaging software (PerkinElmer, Waltham, MA).
For multiphoton fluorescence microscopy, host animals were injected with 100 μL of either rhodamine Ulex Europaeus Agglutinin I (UEA-1; Vector Laboratories, Burlingame, CA) at 50 μg/mL or 10 mg/mL 2,000,000 MW rhodamine dextran (Invitrogen) through the tail vein and allowed to perfuse for 10–15 min before extraction of the gel. To generate labeled UEA-1, 10 mg/mL was conjugated to NHS- AlexaFluor 633 (Life Technologies, Norwalk, CT) at ∼four times molar excess of dye to lectin in 100 mM sodium bicarbonate pH 8.3 for 1 h at room temperature. Following conjugation, UEA-1 was purified from unconjugated dye using a 7kD MW cut-off Zebra spin filter column (Thermo Fisher Scientific, Waltham, MA) pre-equilibrated in phosphate buffered saline with pH 7.4. Concentration of conjugated UEA-1 for intravital injection was 2.0 mg/mL. Multiphoton fluorescence microscopy was carried out with a LaVision BioTec TriM Scope.
Histology and electron microscopy
For histological examination, extracted gels were fixed in 10% formalin for 10 h and then paraffin embedded and stained with hematoxylin and eosin, human CD31 (prediluted; BioGenex, Fremont, CA) with hematoxylin counterstain, or podocin (1:1000 Catalog P0372; Sigma, St. Louis, MO) with hematoxylin counterstain.
For transmission electron microscopy, collagen gels were fixed in 2.5% glutaraldehyde in 0.1 M sodium cacodylate buffer (pH 7.4) for 1 h at room temperature, and then rinsed in cacodylate buffer. Samples were further processed by Yale CCMI Electron Microscopy Core and postfixed in 1% osmium tetroxide, en bloc stained in 2% aqueous uranyl acetate for another hour, then rinsed and dehydrated in an ethanol series followed by EMbed 812 EMS resin infiltration, and baked overnight at 60°C. Blocks were cut using a Leica UltraCut UCT, and 60 nm sections were collected on formvar/carbon-coated grids and stained using 2% uranyl acetate and lead citrate. Samples were viewed on FEI Tecnai Biotwin TEM at 80 kV. Images were captured using a Morada CCD and iTEM software (Olympus).
Statistical analysis
Comparisons of groups were carried out by unpaired two-tailed t-tests using Prism Graphpad software (La Jolla, CA).
Results
In this study, we wished to determine if microvessels that have self-assembled from Bcl-2-transduced HUVEC within collagen gels can be used to sustain the viability of isolated glomeruli and result in their perfusion. To readily visualize viable implanted glomeruli, we microdissected glomeruli from transgenic rats expressing enhanced green fluorescent protein (EGFP) driven by an ubiquitous CAG promoter.12 GFP-positive glomeruli were either suspended by themselves or combined with freely suspended Bcl-2-ECs within a collagen gel.
Because gels contract substantially over time after implantation, we selected a gel size, based upon our previous experience4–6,9,13,14 that allows for reproducible surgical manipulation and that results in a final thickness of the gel substantially exceeding the limit by which diffusion from the wound bed alone could sustain most of the glomeruli (∼200 μm). The polymerized gels were placed subcutaneously in the abdominal walls of immunodeficient (C.B-17 SCID/Bg) mice and were harvested 2 weeks later. The average maximal thickness of gels at the time of harvest was 1700 ± 700 μm SD (N = 10). We assessed the numbers of viable glomeruli with and without coimplantation of Bcl-2-ECs, tittering the number of input glomeruli under both conditions.
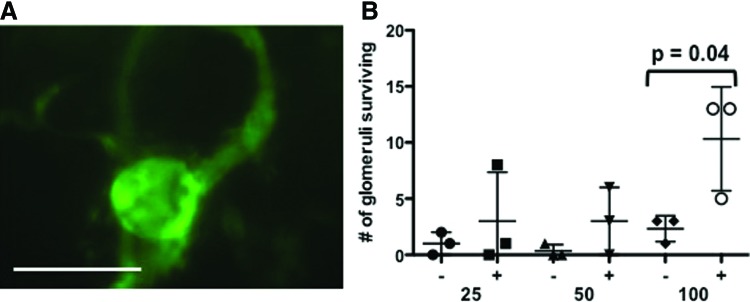
To visualize through the entirety of the explanted gels for glomerular quantification, we performed optical clearing using Scale-A2, a urea-based optical clearing solution that reduces tissue light scattering, yet preserves fluorescence to allow deeper imaging of biological samples.15 Without optical clearing, we were unable to reliably visualize the GFP-positive glomeruli. After clearing for 11 days, we were able to clearly visualize surviving GFP-positive glomeruli (Fig. 1A and Supplementary Fig. S1; Supplementary Data are available online at www.liebertpub.com/tea) through the entire thickness of the gel.
FIG. 1.
Coimplantation of Bcl-2-ECs enhances glomerular survival. (A) Representative epifluorescent image of surviving GFP+ glomeruli after optical clearing of implant containing Bcl-2-ECs and glomeruli. Scale bar is 100 μm. (B) Scatterplot of the number of glomeruli surviving at 2 weeks as determined by GFP expression and CellTracker™ staining without (−) and with (+) Bcl-2-ECs. A titration of 25, 50, and 100 glomeruli were added to each gel before implantation. Means and standard deviations indicated by horizontal bars. ECs, endothelial cells; Bcl-2-ECs, Bcl-2-transduced ECs; GFP, green fluorescent protein. Color images available online at www.liebertpub.com/tea
Retention of GFP fluorescence was associated with cell viability; we also confirmed glomerular viability using the CellTracker red dye. To illustrate the association of GFP with viable cells, we suspended glomeruli within collagen gels and maintained them in the presence or absence of serum for 1 week in vitro. We observed that glomeruli maintained in the presence of serum activated the red fluorescence, while serum-starved glomeruli did not (indicating lack of viability) (Supplementary Fig. S2). Both GFP expression and the CellTracker red staining demonstrated that glomerular cell viability was significantly enhanced by the addition of Bcl-2-ECs in vivo (Fig. 1B), and that the overall survival of glomeruli in the presence of Bcl-2-ECs was 10% at 2 weeks whereas virtually no glomerular cells survived in the absence of Bcl-2-ECs. These data establish that addition of Bcl-2-ECs increased glomerular survival within the implanted gels.
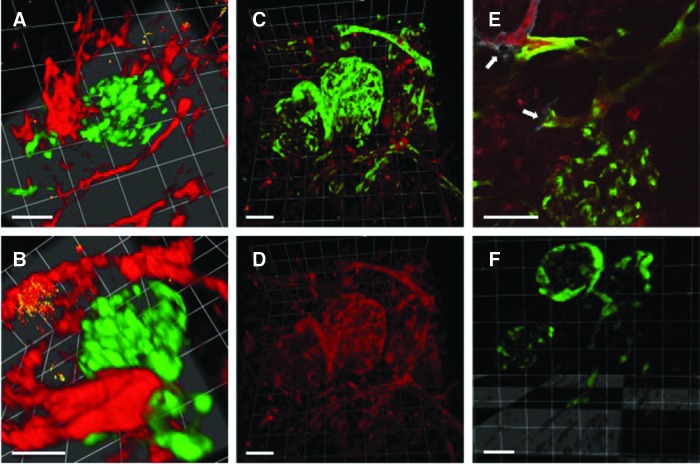
To more closely examine the interactions between the Bcl-2-ECs and the glomeruli, we utilized multiphoton fluorescence microscopy on explanted gels. Fluorescently labeled UEA-1 was intravitally injected to distinguish human ECs from rat or mouse ECs (UEA-1 is specific for human ECs). We observed that the GFP+ rat glomeruli were able to anastomose with the human Bcl-2-EC-derived microvessels. To demonstrate that glomeruli were perfused, high-molecular weight (2,000,000) rhodamine dextran that is restricted to the intravascular space was injected through the tail vein before explant and imaging (Fig. 2A–E). Without addition of Bcl-2-ECs, glomeruli typically were not perfused and no human microvessels were detected (Fig. 2F).
FIG. 2.
GFP glomeruli anastomose with and are perfused by human Bcl-2-EC-derived microvessels. Glomeruli from rats expressing GFP driven by ubiquitous CAG promoter were microdissected and embedded with Bcl-2-ECs in a collagen gel that was implanted in an immunodeficient mouse for 10 days. (A) Multiphoton microscopy of explanted gel with 3D reconstruction of Z-stack of human microvessel (red) and GFP rat glomeruli. (B) Rotation highlighting anastomosis of human microvessel with GFP glomerulus. (A, B) Animals injected with rhodamine-UEA-1, which is specific for human ECs, but not mouse or rat. (C, D) Two million molecular weight rhodamine dextran was injected through the tail vein. Gels were then explanted and visualized with multiphoton microscopy. (C) depicts 3D reconstruction of Z-stack with merged green and red signals, and (D) demonstrates perfusion of the rhodamine dextran within the glomerulus and surrounding vessels in red channel only. (E) Single Z-section of implant after mouse was injected with rhodamine dextran (red) and AlexaFluor 633 (far-red)-conjugated UEA-1 (white). Arrows highlight anastomoses between human and rat vessels. (F) Z-stack of gel without Bcl-2-EC showing glomeruli without detectable dextran perfusion. Scale bars are 100 μm. UEA-1, Ulex Europaeus Agglutinin I.
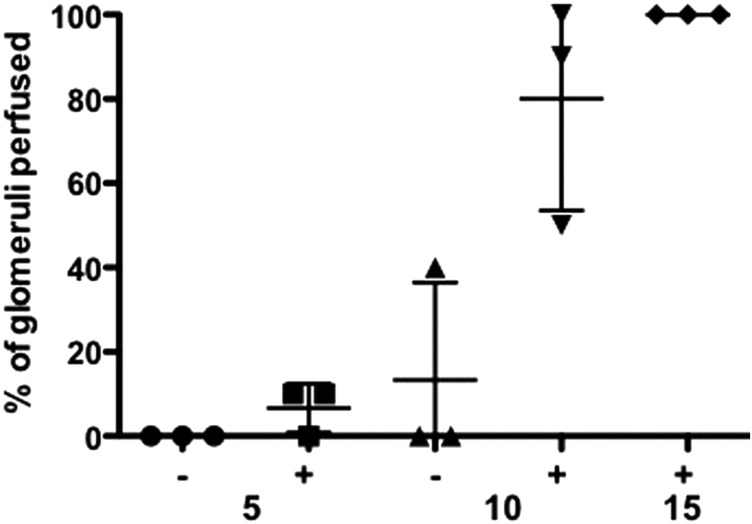
To better characterize the kinetics of Bcl-2-EC-mediated perfusion of glomeruli, collagen gels containing GFP glomeruli were implanted with and without Bcl-2-ECs and examined at 5, 10, and 15 days after perfusion with high-molecular weight rhodamine dextran (Fig. 3). We observed that in the groups with ECs added, the majority of visualized glomeruli were perfused at 10 days (80%), and all surviving glomeruli were perfused at 15 days. Interestingly, in one animal at the 10-day time point, a few glomeruli implanted without Bcl-2-ECs were observed to directly anastomose with the host vasculature and were perfused. Consistent with the limited cell survival in constructs without ECs, we were unable to visualize a sufficient number of glomeruli at the 15-day time point to carry out the perfusion analysis.
FIG. 3.
Scatterplot of glomerular perfusion over time. Percentage of glomeruli perfused at day 5, 10, and 15 without and with Bcl-2-ECs after intravital perfusion of high-molecular weight rhodamine dextran and multiphoton imaging. Ten glomeruli examined for each animal. Note, glomerular perfusion in the EC group at 15 days could not be determined due lack of visible glomeruli after examination of five animals. Means and standard deviations indicated by horizontal bars.
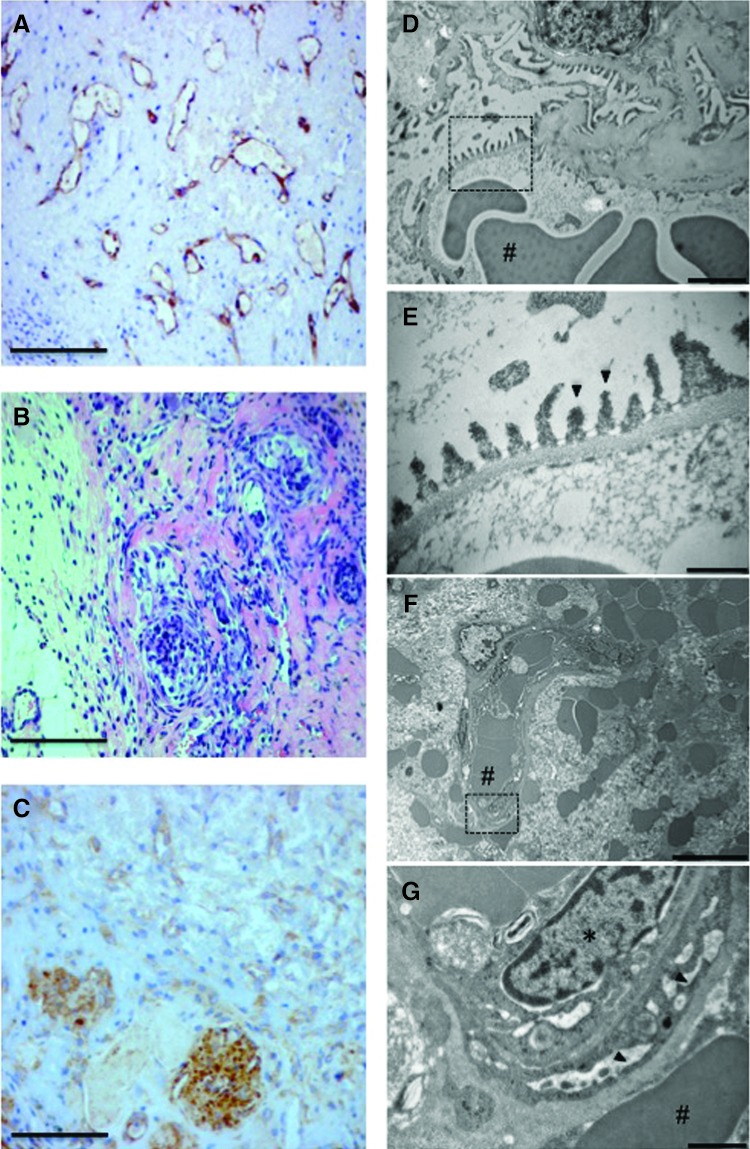
Finally, we examined the morphology of the cells within the gels at the level of light and electron micrcroscopy. In gels containing Bcl-2-ECs, human endothelial CD31 staining was detected (Fig. 4A), and hematoxylin and eosin staining showed glomeruli with segments of open capillary loops that lacked Bowman's (urinary) space (Fig. 4B). In some instances, GFP-positive ECs extended from the glomeruli, consistent with sprouting from either glomerular capillaries or retained segments of the afferent or efferent arterioles. Surviving glomeruli with and without Bcl-2-ECs retained staining for podocin, a protein specific to podocytes (Fig. 4C).
FIG. 4.
Histology and ultrastructure of implanted gels with Bcl-2-ECs and glomeruli. (A) Human endothelial CD31 staining (brown) and hematoxylin counterstain (blue). (B) Hematoxylin and eosin staining of cluster of glomeruli. (C) Staining for podocytes with rat podocin antibody (brown) and hematoxylin counterstain (blue). Scale bars for (A–C) are 100 μm. (D–G) Transmission electron microscopy of normal rat and glomeruli implanted with Bcl-2-ECs at 2 weeks. (D) Capillary loops and basement membrane of normal rat glomerulus. Scale bar is 2 μm. (E) Higher magnification of dashed area (D) demonstrating podocyte foot processes and basement membrane of normal glomerulus. Scale bar is 0.5 μm. (F) Microvessels containing red blood cells formed within the collagen gels containing Bcl-2-ECs. Scale bar is 10 μm. (G) Higher magnification of dashed area (F) demonstrating implanted glomerulus with red blood cells, endothelial swelling, and podocyte effacement. Scale bar is 1 μm. * Indicate nuclei and arrows indicate podocyte foot processes. # Indicate red blood cells in the glomerulus.
Transmission electron microscopy of normal glomeruli before microdissection and implantation (Fig. 4D, E) demonstrated normal capillary loops and podocyte foot processes. Intact glomeruli were not detected in the gels without Bcl-2-EC, and those rare glomerular fragments that were seen appeared necrotic (data not shown). Despite anastomoses to local microvessels (Fig. 4F), implanted glomeruli in gels with Bcl-2-ECs demonstrated podocyte foot process effacement and glomerular endothelial swelling (Fig. 4G).
Discussion
Data presented in this study demonstrate that a self-assembled tissue-engineered microvasculature can be used to enhance the survival and reperfusion of isolated renal glomeruli in vivo. This represents a critical first step in tissue engineering of functional renal tissue and highlights the utility and flexibility of EC microvascular self-assembly in combination with specialized organ vasculature.
While glomerular survival was only 10% in the presence of the Bcl-2-ECs at 2 weeks, we anticipate that the efficiency of survival may be enhanced by the addition of proangiogenic factors to the gel constructs before implantation—as we have demonstrated in other related studies16,17—or alternatively by creating vectorial delivery of chemoattractants that function in renal development18 such as semaphorin 3C or neutralizing chemorepulsive factors such as semaphorin 3A.
As indicated by the extraglomerular GFP-positive microvessels that appear to originate from the glomeruli themselves, it seems that the glomerular capillaries may be capable of endothelial sprouting to facilitate reperfusion (although at a much lower efficiency than perfusion by Bcl-2-ECs). Alternatively, these vessels may represent ECs derived from the remnants of the afferent or efferent arterioles. In either case, the presence of rat ECs outside of the glomeruli proper likely explains how rare glomeruli without ECs added can still be reperfused.
Pathologic features of implanted glomeruli such as endothelial swelling and podocyte effacement suggest that perfusion alone is not sufficient to maintain these structures and support the concept that glomerular maintenance is finely balanced. We suspect that these changes are attributable to the absence of normal tubular outflow. Once glomeruli connect to the microvessels, the filtration pressure likely quickly matches the interstitial pressures around the glomeruli thus eliminating pressure gradients for filtration. Alternatively, these changes may be driven by the direct abutment of the podocytes to interstitial collagen, due to the lack of Bowman's space.
Atubular glomeruli, analogous to the perfused glomeruli described in this study, have been described in a number of renal diseases such as obstructive uropathy and cystinosis.19 In a rat model of chronic lithium-induced nephropathy, atubular glomeruli were described to be smaller, to have a reduced urinary space, and an abnormal foot process width distribution20 that did not, however, resemble the more dramatic foot process changes observed here.
The next step in this approach will, therefore, be to determine whether epithelial encapsulation and drainage by epithelial-lined tubules of these perfused glomeruli can preserve glomerular histology and function, which would be an important advance in tissue engineering of a functional kidney.
In conclusion, we describe here a new approach toward renal tissue engineering that incorporates a type 1 collagen-based scaffold, an engineered microvasculature, and isolated intact glomeruli. While such isolated glomeruli clearly maintain the capacity to be reperfused, an important finding for modular tissue engineering,21 the process is inefficient and will need to be further enhanced. In addition, it seems likely that tubular outflow is necessary to maintain the structural integrity of reperfused glomeruli.
Supplementary Material
Acknowledgments
This work was supported by the National Institutes of Health Grants (R01-HL085416 to J.S.P. and W.M.S.) and a Clinical and Translational Science Award (KL2- RR024138 to W.G.C. from the National Center for Advancing Translational Science. A.F. is supported by the NIH/NIDDK (R01-DK090316, DK104753, and 5U24DX076169), and by the National Center for Advancing Translational Sciences (1UL1TR000460). Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NIH. The authors thank Morven Graham (Yale University, New Haven, CT) for assistance with electron microscopy and David Gonzalez (Yale University) for assistance with multiphoton microscopy.
Disclosure Statement
A.F. is an inventor on pending or issued patents aimed to diagnose or treat proteinuric renal diseases. A.F. is vice president of L&F Health LLC and has consulting agreements with Roche, Genentech, Boehringer Ingelheim, Mesoblast, and Bristol-Myers Squibb that are unrelated to the current study. L.E.N. has a financial interest in Humacyte, Inc., a regenerative medicine company. Humacyte did not fund these studies, and Humacyte did not affect the design, interpretation, or reporting of any of the experiments herein.
References
- 1.US Renal Data System, USRDS. 2013 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD, 2013 [Google Scholar]
- 2.Orlando G., Soker S., Stratta R.J., and Atala A. Will regenerative medicine replace transplantation? Cold Spring Harb Perspect Med 3, a015693, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Auger F.A., Gibot L., and Lacroix D. The pivotal role of vascularization in tissue engineering. Annu Rev Biomed Eng 15, 177, 2013 [DOI] [PubMed] [Google Scholar]
- 4.Schechner J., Nath A., Zheng L., Kluger M., Hughes C., Sierra-Honigmann M., Lorber M., Tellides G., Kashgarian M., Bothwell A., and Pober J. In vivo formation of complex microvessels lined by human endothelial cells in an immunodeficient mouse. Proc Natl Acad Sci U S A 97, 9191, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Enis D.R., Shepherd B.R., Wang Y., Qasim A., Shanahan C.M., Weissberg P.L., Kashgarian M., Pober J.S., and Schechner J.S. Induction, differentiation, and remodeling of blood vessels after transplantation of Bcl-2-transduced endothelial cells. Proc Natl Acad Sci U S A 102, 425, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harding M.J., Lepus C.M., Gibson T.F., Shepherd B.R., Gerber S.A., Graham M., Paturzo F.X., Rahner C., Madri J.A., Bothwell A.L., Lindenbach B.D., and Pober J.S. An implantable vascularized protein gel construct that supports human fetal hepatoblast survival and infection by hepatitis C virus in mice. PLoS One 5, e9987, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schlöndorff D., and Banas B. The mesangial cell revisited: no cell is an island. J Am Soc Nephrol 20, 1179, 2009 [DOI] [PubMed] [Google Scholar]
- 8.Kistler A.D., Caicedo A., Abdulreda M.H., Faul C., Kerjaschki D., Berggren P.O., Reiser J., and Fornoni A. In vivo imaging of kidney glomeruli transplanted into the anterior chamber of the mouse eye. Sci Rep 4, 3872, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maier C., Shepherd B., Yi T., and Pober J. Explant outgrowth, propagation and characterization of human pericytes. Microcirculation 17, 367, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gimbrone M.A. Culture of vascular endothelium. Prog Hemost Thromb 3, 1, 1976 [PubMed] [Google Scholar]
- 11.Zheng L., Ben L.H., Pober J.S., and Bothwell A.L. Porcine endothelial cells, unlike human endothelial cells, can be killed by human CTL via Fas ligand and cannot be protected by Bcl-2. J Immunol 169, 6850, 2002 [DOI] [PubMed] [Google Scholar]
- 12.Okabe M., Ikawa M., Kominami K., Nakanishi T., and Nishimune Y. ‘Green mice’ as a source of ubiquitous green cells. FEBS Lett 407, 313, 1997 [DOI] [PubMed] [Google Scholar]
- 13.Shepherd B.R., Jay S.M., Saltzman W.M., Tellides G., and Pober J.S. Human aortic smooth muscle cells promote arteriole formation by coengrafted endothelial cells. Tissue Eng Part A 15, 165, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chang W.G., Andrejecsk J.W., Kluger M.S., Saltzman W.M., and Pober J.S. Pericytes modulate endothelial sprouting. Cardiovasc Res 100, 492, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hama H., Kurokawa H., Kawano H., Ando R., Shimogori T., Noda H., Fukami K., Sakaue-Sawano A., and Miyawaki A. Scale: a chemical approach for fluorescence imaging and reconstruction of transparent mouse brain. Nat Neurosci 14, 1481, 2011 [DOI] [PubMed] [Google Scholar]
- 16.Jay S.M., Shepherd B.R., Bertram J.P., Pober J.S., and Saltzman W.M. Engineering of multifunctional gels integrating highly efficient growth factor delivery with endothelial cell transplantation. FASEB J 22, 2949, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Devalliere J., Chang W.G., Andrejecsk J.W., Abrahimi P., Cheng C.J., Jane-wit D., Saltzman W.M., and Pober J.S. Sustained delivery of proangiogenic microRNA-132 by nanoparticle transfection improves endothelial cell transplantation. FASEB J 28, 908, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reidy K., and Tufro A. Semaphorins in kidney development and disease: modulators of ureteric bud branching, vascular morphogenesis, and podocyte-endothelial crosstalk. Pediatr Nephrol 26, 1407, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chevalier R.L., and Forbes M.S. Generation and evolution of atubular glomeruli in the progression of renal disorders. J Am Soc Nephrol 19, 197, 2008 [DOI] [PubMed] [Google Scholar]
- 20.Marcussen N., Ottosen P.D., and Christensen S. Ultrastructural quantitation of atubular and hypertrophic glomeruli in rats with lithium-induced chronic nephropathy. Virchows Arch A Pathol Anat Histopathol 417, 513, 1990 [DOI] [PubMed] [Google Scholar]
- 21.Nichol J.W., and Khademhosseini A. Modular tissue engineering: engineering biological tissues from the bottom up. Soft Matter 5, 1312, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.