Abstract
Current clinically approved methods for cartilage repair are generally based on either endogenous cell recruitment (e.g., microfracture) or chondrocyte delivery (e.g., autologous chondrocyte implantation). However, both methods culminate in repair tissue with inferior mechanical properties and the addition of biomaterials to these clinical interventions may improve their efficacy. To this end, the objective of this study was to investigate the ability of multipolymer acellular fibrous scaffolds to improve cartilage repair when combined with microfracture in a large animal (i.e., minipig) model. Composite scaffolds were formulated from a combination of hyaluronic acid (HA) fibers and poly(ɛ-caprolactone) (PCL) fibers, either with or without transforming growth factor-β3 (TGFβ3). After 12 weeks in vivo, material choice and TGFβ3 delivery had a significant impact on outcomes; specifically, PCL scaffolds without TGFβ3 had inferior gross appearance and reduced mechanical properties, whereas HA scaffolds that released TGFβ3 resulted in improved histological scores and increased type 2 collagen content. Importantly, analysis of the overall dataset revealed that histology, but not gross appearance, was a better predictor of mechanical properties. This study highlights the importance of scaffold properties on in vivo cartilage repair as well as the need for numerous quantitative outcome measures to fully evaluate treatment methods.
Introduction
Damage to articular cartilage, either through traumatic injuries or chronic osteoarthritis, is one of the leading causes of pain and disability in adults.1,2 Focal defects and degenerated regions tend to heal poorly, as cartilage is avascular and has low cellularity in adults. Current clinical treatments to repair cartilage damage are not without limitation—most approaches rely on damaging the patient's own cartilage to obtain tissue or cells for transplantation into the defect.3–6 For example, autologous chondrocyte implantation involves isolation of cells from cartilage biopsies, expansion ex vivo, and then injection back into the defect site.3,4,7 Alternatively, microfracture, which involves the introduction of holes into the underlying subchondral bone, releases marrow elements (including endogenous stem cells) into the defect that clot in place and provide a template upon which some regeneration can occur.3,4,8 While these methods can provide some level of repair, both approaches result in suboptimal repair tissue with inferior mechanical and biochemical properties and limited durability.
More recently, biomaterial scaffolds (e.g., collagen membranes) have been combined with existing repair strategies to improve clinical outcomes.9 For example, in autologous matrix-induced chondrogenesis (AMIC), an acellular collagen scaffold is implanted into the cartilage defect following microfracture.10 In a retrospective clinical study, AMIC improved various patient-reported outcome measures (i.e., IKDC, Lysholm, Tegner, and VAS pain scores) over time.10–12 While combining microfracture with a biomaterial support may prove beneficial, decellularized collagen membranes are used mainly due to their clinical availability and have not been optimized to promote the most efficacious repair. Other materials have been investigated in a similar technique (i.e., in cartilage defects with microfracture) and have improved outcomes in vivo. For instance, the application of a photocrosslinked poly(ethylene glycol) (PEG) hydrogel with hyaluronic acid (HA) after microfracture showed improvements in human patients based on magnetic resonance imaging and patient self-evaluated clinical outcomes.13 Recently, a 12-week study in rabbits using a nanofibrous self-assembling material based on peptide amphiphiles showed improved type 2 collagen deposition and higher histological scoring with the inclusion of a transforming growth factor β (TGFβ)-binding domain.14 Other materials investigated in cartilage defects with microfracture include HA,15 alginate,16 and poly(lactide-co-glycolide) (PLGA) sponges embedded with a fibrin gel.17 Similarly, electrospun poly(vinyl alcohol) (PVA) and chondroitin sulfate (CS) fibers have been investigated in rat osteochondral defects, with CS fibers increasing glycosaminoglycan (GAG) and type 2 collagen deposition.18 Even with these advances, research toward engineering novel biomaterials to improve repair outcomes following microfracture is limited, and the influence of scaffold properties on in vivo cartilage repair is still not well understood.
For this reason, the objective of this study was to investigate two basic material systems, as well as the delivery of a growth factor, to define the role that scaffold biophysical and biochemical properties play on in vivo cartilage repair. We chose to investigate fibrillar scaffolds, as the nanofibrous architecture of these materials closely mimics the native extracellular matrix, and these scaffolds possess high porosity that can promote cellular infiltration, initial cell–cell contacts, and interconnectivity of deposited matrix.19–22 HA was chosen as the main component due to its presence in cartilage tissue and natural ability to enhance mesenchymal stem cell (MSC) chondrogenesis in vitro.23–25 Poly(ɛ-caprolactone) (PCL) was included as an alternative fiber population due to its history of biomedical use26 and the expected differences in material properties (e.g., degradation and hydrophobicity) between HA and PCL. Both systems were fabricated using a dual jet electrospinning system to produce multipolymer scaffolds, where hydrolytically degradable HA fiber components encapsulated and released TGFβ3 for several weeks to further stimulate cartilaginous matrix production. These materials were implanted for 12 weeks in cartilage defects treated with microfracture using our established large animal (Yucatan minipig) model of cartilage repair,27 and a comprehensive set of repair outcomes were analyzed, including gross appearance, defect fill, regenerate tissue mechanics, subchondral bone remodeling, and quantitative histological scoring, to determine the material combinations that best supported cartilage repair. This study provides a thorough investigation of the influence of various scaffold properties and growth factor release in an in vivo setting toward improved engineered scaffolds for cartilage repair applications.
Materials and Methods
Scaffold fabrication
HA (64 kDa; Lifecore) was modified with either methacrylates (MeHA) or hydroxyethyl methacrylates (HeMA-HA, HH), as described by Tous et al.28 and Burdick et al.,29 and the extent of modification was assessed using 1H NMR. The HH modification includes multiple ester bonds within the crosslinking moiety, allowing for more rapid degradation compared to MeHA. The electrospinning solutions for both MeHA and HH were composed of 4% w/v HA macromer and 2% w/v poly(ethylene oxide) (PEO, 900 kDa; Sigma) with 0.05% w/v Irgacure 2959 (Sigma) in ddH2O. The dissolved HA/PEO solution was electrospun at a voltage of +22.5 kV, flow rate of 1.4 mL/h, and a needle tip to grounded collector distance of 16 cm. For PCL fibers, a 14.3% w/v solution of PCL (80 kDa; Sigma) was dissolved in 1:1 tetrahydrofuran and N,N-dimethylformamide and then electrospun at a voltage of +13 kV, flow rate of 2.0 mL/h, and a needle tip to grounded collector distance of 15 cm.
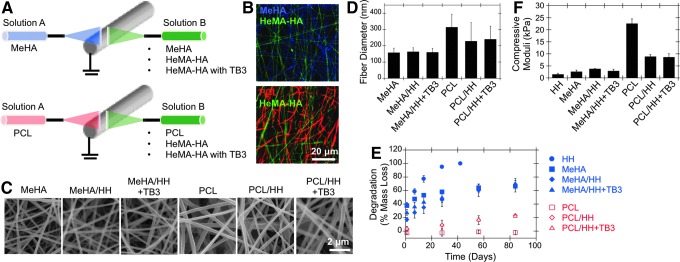
Multipolymer fibrous scaffolds were electrospun into various composite formulations with distinct MeHA, HH, and PCL fiber populations and with or without TGFβ3 (TB3) encapsulated in the HH fiber fraction (Fig. 1A, B). Specifically, these variables resulted in six separate fibrous scaffolds (i.e., MeHA, MeHA/HH, MeHA/HH+TB3, PCL, PCL/HH, and PCL/HH+TB3) that were fabricated using dual jet electrospinning,30 in which two electrospinning jets were directly opposed on either side of a rotating grounded collecting mandrel. All scaffold conditions were electrospun to an overall thickness of ∼1 mm. After electrospinning, HA-containing fibrous scaffolds were purged with nitrogen and crosslinked with UV light (OmniCure S1000 UV Spot Cure Systems). Before surgery, 4-mm samples were cut using biopsy punches (Millipore), and samples were sterilized using germicidal UV. Samples from TGFβ3 groups included a total of ∼35 ng of TGFβ3 per scaffold.
FIG. 1.
Engineered fibrillar scaffold processing and characterization. (A) Multipolymer fibrillar scaffold design and imaging of fibrous scaffolds through (B) confocal microscopy images of swollen multipolymer scaffolds and (C) SEM images of dry multipolymer scaffolds. Swollen scaffolds had multiple fluorescent dyes encapsulated before electrospinning to enable visualization. Scaffolds were also characterized through quantification of (D) dry fiber diameter (n > 70), (E) degradation (i.e., % mass loss) (n = 4), and (F) bulk compressive moduli (n = 5). HA, hyaluronic acid; HeMA-HA (HH), hydroxyethyl methacrylated HA (degradable fiber component); MeHA, methacrylated HA; PCL, poly(ɛ-caprolactone); SEM, scanning electron microscopy; TB3, transforming growth factor-β3. Color images available online at www.liebertpub.com/tea
Scaffold characterization
After fabrication, scaffolds were imaged using scanning electron microscopy and confocal microscopy (with fluorescent dyes included into the solutions before electrospinning) for dry and swollen fiber morphology, respectively. To visualize swollen scaffolds, fluorescent dyes were designed and synthesized to covalently bind to HA fibers, and these dyes were incorporated before electrospinning. Briefly, cysteine-containing peptides were coupled to multiple dyes (i.e., FITC and coumarin) using solid-phase peptide synthesis. After dye synthesis, Rhodamine B (Sigma), a cysteine-conjugated FITC, and a cysteine-conjugated coumarin dye were added to PCL, HeMA-HA, and MeHA electrospinning solutions, respectively, before electrospinning. The solutions were then electrospun and crosslinked, as previously described in previous section, onto methacrylated coverslips, and swollen scaffolds were imaged using confocal microscopy (Zeiss Axio Observer Inverted microscope; Penn CDB Microscopy Core).
Mechanical properties were measured by unconfined compressive testing after swelling overnight with a dynamic mechanical analyzer (Q800 TA Instruments) at a strain rate of 10%/min, and moduli were calculated at a strain rate from 10% to 20% (n = 5). Degradation profiles were quantified from scaffolds incubated in phosphate-buffered saline (PBS) at 37°C through overall mass loss and uronic acid release for HA-containing scaffolds (n = 4). For all HA-containing groups, samples were incubated in PBS at 37°C, and the incubation solution was collected at the desired time points. Total HA within the samples was quantified by degrading scaffolds with hyaluronidase (Sigma) at the final time point. The solutions were then analyzed for HA content using the uronic acid assay, as defined by Tous et al.28 For all PCL-containing conditions, samples were weighed dry and then incubated in PBS at 37°C. At each time point, separate samples were then snap-frozen, freeze-dried, and weighed. Mass loss was quantified by comparing those weights to the initial weight of the samples (i.e., before swelling).
Assessment of TGFβ3 activity in vitro through MSC pellet chondrogenesis
To characterize the release of encapsulated TGFβ3, samples were incubated in PBS with 1% w/v bovine serum albumin (BSA) (R&D Systems), and TGFβ3 was quantified using a DuoSet® ELISA kit (R&D Systems) (n = 4). To measure the activity of released TGFβ3, MSC pellets were cultured within the same wells as fibrous scaffolds. MSC pellets were formed through centrifugation of 250,000 MSCs (Lonza) in 1 mL of incomplete chondrogenic media (ICM; high glucose Dulbecco's modified Eagle's medium with 1× penicillin/streptomycin, 0.1 mM dexamethasone, 50 μg/mL ascorbate 2-phosphate, 40 μg/mL l-proline, 1 mM sodium pyruvate, 6.25 μg/mL insulin, 6.25 μg/mL transferrin, 6.25 ng/mL selenous acid, 1.25 mg/mL BSA, and 5.35 μg/mL linoleic acid) in 15-mL conical tubes, which were loosely capped to allow for gas exchange. Pellets were allowed to form for 72 h before transferring to 24-well plates. Scaffolds were sterilized with germicidal UV for 45 min on either side and then added to the appropriate wells along with 1 mL of ICM. As a positive control, 10 ng/mL recombinant human TGFβ3 (R&D Systems) was added to the ICM to create complete chondrogenic media (CM+), and ICM without any supplement was used as a negative control. Media were refreshed once per week. After 4 weeks of in vitro culture, pellets were digested with papain and GAG content quantified using the 1,9-dimethylmethylene blue dye-binding assay, DNA using Picogreen (Invitrogen), and collagen using the hydroxyproline assay (n = 4).31,32 Pellets were also processed for histology and stained with alcian blue, picrosirius red, hematoxylin and eosin (H&E), and immunohistochemistry (IHC) for type 2 collagen (n = 4).
Surgical procedure
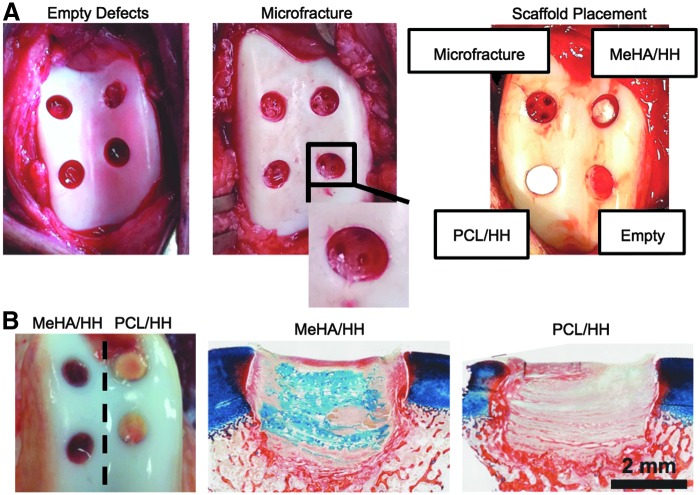
All animal procedures were performed under protocols approved by the Institutional Animal Care and Use Committee at the Philadelphia VA Medical Center and the University of Pennsylvania and in accordance with policies set forth by the National Institutes of Health. In total, 16 juvenile (i.e., 6 months old), castrated male Yucatan minipigs (Sinclair Bioresources) were utilized. Surgical protocols were as defined in Fisher et al.27 and involved bilateral surgeries, in which four defects were created in the trochlear groove of both hind limbs. Chondral defects were created using a 4-mm biopsy punch to outline the defect, followed by a curved blunt probe to remove cartilage from the defect area. The cartilage was removed down to the level, but without penetration, of the subchondral bone plate. Microfracture was performed using an awl and consisted of three equally spaced holes in a triangular configuration within each defect. These microfracture holes were ∼0.3–0.5 mm in diameter (as determined by the awl) and just deep enough to allow for bleeding from the bone marrow cavity. For defects treated with fibrous scaffolds, the material was placed into the defect after microfracture and allowed to swell in place for 3–5 min before closure. Scaffolds were randomized and preassigned between the different defect locations to minimize any possible confounding effects of defect location on results. After surgery, animals were allowed normal cage activity as tolerated for the duration of the study.
In terms of specific groups included for each study, two pilot studies (i.e., 2 and 6 weeks) and one longer term study (i.e., 12 weeks) were conducted. The objective of the 2-week pilot study was to assess scaffold stability in the defects with and without sutures, and this short pilot study included only MeHA/HH and PCL/HH scaffolds, both with and without sutures (n = 4 for all four groups). Regarding the 6-week pilot study, the objective was to measure any potential short-term effects of released TGFβ3 and differences in scaffold degradation or porosity (i.e., differences between scaffolds with and without a HeMA-HA fiber population). Thus, the 6-week study included the following groups: microfracture, MeHA, MeHA/HH, MeHA/HH+TB3 (n = 5 for all groups, please note that only one hind limb was used for this pilot study). Finally, the 12-week study included all scaffolds (i.e., six scaffold groups) along with empty, microfracture, and normal tissue controls (n = 7–10). For the 12-week study, scaffolds with TGFβ3 were implanted in separate joints from groups without TGFβ3 to avoid leakage of TGFβ3 into the surrounding defects. However, all groups were implanted in each joint for the 6-week study to distribute samples between animals, as the sample size was limited for this smaller preliminary study.
Processing and analysis of in vivo cartilage defect samples
Animals were sacrificed 2, 6, and 12 weeks after surgery. Images were taken of the entire trochlear groove before sample harvest, and these gross images were later scored by four independent blinded observers using the system developed by Goebel et al.33 Samples were immediately immersed in PBS with protease inhibitors (Sigma) and frozen at −80°C until processing. At 2 and 6 weeks, samples were processed only for histology (n = 4 at 2 weeks, n = 5 at 6 weeks). For the 12-week study, empty defects, microfracture (with no scaffold) defects, and normal osteochondral tissue samples were also investigated, along with the six fibrous scaffolds discussed previously. The 12-week samples were thawed, separated into individual osteochondral samples, and potted in PMMA with ∼8 mm of the subchondral bone and cartilage surface projecting from the PMMA. Mechanical testing was performed on PBS-immersed samples, using an Instron 5948 electromechanical test system affixed with a 2-mm-diameter spherical indentation probe. Indentation was applied at 0.1%/s strain ramp for 50 s. Resulting data were then fit using a Hertz model to obtain the Young's modulus (n = 7–10).34,35 After mechanical testing, samples were fixed in 4% paraformaldehyde and imaged by microcomputed tomography (microCT; Scanco Viva CT75), with and without an iodine-based contrast solution (Lugol solution; Sigma), as in Fisher et al.27 Finally, samples were decalcified, processed for histology, and stained using Safranin-O/Fast Green, H&E, and immunohistochemistry for type 2 collagen (n = 7–10). Four blinded independent observers scored the resulting histology, using a modified version of the ICRS-II scoring system,36 and images were quantified for percent stained area and staining intensity, as described in the following section.
Quantification of histological staining
Quantification of percent positive stained area and staining intensity was performed using ImageJ (NIH) and CellProfiler (Broad Institute). For in vivo data, the defect area (i.e., the initial defect area, as defined by the level of the surrounding cartilage and thus excluding the remodeled subchondral bone) was outlined and a mask was created in ImageJ. CellProfiler was then used to quantify both the percent positive stained area and staining intensity of the masked defect area using a custom script. For in vitro data, no mask was created, and the entire image was quantified. Importantly, each image was treated equally with the same threshold used for quantification of percent positive stained area for all samples.
Statistical analysis
For comparison of two groups, a Student's t-test with a p-value of 0.05 was used to quantify statistical significance. For analyses with more than one comparison, a one-way ANOVA was performed followed by a Tukey post hoc test. Principal component analysis (PCA) was performed on the entire dataset using Jmp (SAS).
Results
Multipolymer fibrillar scaffolds with varied mechanics and degradation
As described previously, a dual jet electrospinning system with directly opposing jets was utilized to achieve a relatively equal mix of both fiber populations for the following six multipolymer fibrous scaffolds: MeHA, MeHA/HH, MeHA/HH+TB3, PCL, PCL/HH, and PCL/HH+TB3. All scaffolds had consistently smooth fibers (Fig. 1C) and average dry fiber diameters of ∼150 and 300 nm for HA and PCL fibers, respectively (Fig. 1D). The increased hydrophobicity of PCL-containing scaffolds was apparent when samples were immersed in PBS, as HA-only scaffolds became transparent and swelled significantly, whereas PCL-containing scaffolds retained their opaque or white appearance. These differences were further reflected in the mass swelling ratios (Supplementary Fig. S1A, B; Supplementary data are available at www.liebertpub.com/tea). Degradation profiles were similar for most HA-only scaffolds (except for the HH group, which degraded much more rapidly), with an initial burst release of HA during the initial overnight swelling period and a steady degradation profile that plateaued at later times (Fig. 1E). This initial burst release was most likely due to the release of unreacted HA macromers from the scaffold. Thus, the in vitro scaffold characterization validated our hypothesis that the incorporation of PCL would significantly decrease degradation rates and mass swelling ratios (Fig. 1E and Supplementary Fig. S1C) and increase mechanical properties (Fig. 1F).
Scaffolds are stable within defects without sutures
To determine whether suture fixation of scaffolds was necessary to ensure stable integration of the scaffolds within cartilage defects in vivo, both HA-only and PCL/HA composite scaffolds were investigated in a 2-week, preliminary in vivo study either with or without sutures, resulting in four investigational groups (i.e., MeHA/HH with and without sutures, PCL/HH with and without sutures). Materials were implanted after cartilage defect creation and microfracture. Differences in scaffold swelling were again apparent during surgery, with HA-only scaffolds (i.e., MeHA/HH) immediately swelling with blood and turning red after scaffold placement, whereas the PCL/HH composites remained relatively white and opaque (Fig. 2A). After 2 weeks in vivo, histological findings validated that scaffolds remained present in all four groups, as staining with alcian blue at a pH of 2.5 stained the nonsulfated GAG (i.e., HA) component within the scaffolds (Fig. 2B and Supplementary Fig. S2). Due to the similarities in scaffold retention with and without sutures and also the increased time of surgery and possible damage to the scaffold and surrounding cartilage from suturing, all subsequent in vivo studies did not include sutures to secure scaffolds within the defects.
FIG. 2.
Scaffold implantation and stability in cartilage defects. (A) Images of the surgical procedure for the implantation of fibrillar scaffolds postmicrofracture into chondral defects and representative image of scaffolds and controls immediately after implantation. (B) Gross appearance and histology (staining with alcian blue and picrosirius red) for MeHA/HH and PCL/HH composite scaffolds after 2 weeks in vivo (n = 4). Color images available online at www.liebertpub.com/tea
Released TGFβ3 remains active in vitro and in vivo
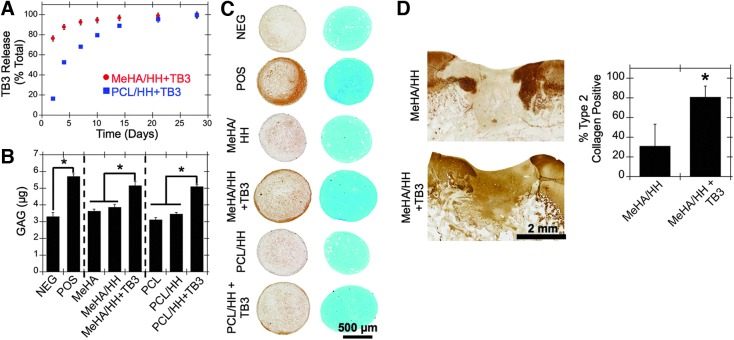
An ELISA was performed to quantify TGFβ3 release in vitro. The incorporation of PCL significantly slowed the release of encapsulated TGFβ3, possibly due to differences in scaffold swelling (Fig. 3A). To assess whether TGFβ3 released from scaffolds remained active in vitro, MSC pellets were cultured in the same wells as scaffolds with or without TGFβ3 release. MSC pellets situated in the same wells as TGFβ3-releasing scaffolds showed significantly increased GAG and type 2 collagen deposition, as observed through biochemical analysis (Fig. 3B), histology (Fig. 3C), and quantification of staining (Supplementary Figs. S3 and S4).
FIG. 3.
Release and activity of encapsulated TGFβ3. (A) TGFβ3 release profiles from HA-only (red) and PCL/HA (blue) composite scaffolds (n = 4). (B) GAG content (as quantified through biochemical analysis) (n = 4) and (C) histology (left: immunohistochemistry for type 2 collagen, right: alcian blue staining) of MSC pellets cultured in the same wells as scaffolds with or without encapsulated TGFβ3 after 28 days in vitro (n = 4). (D) TGFβ3 activity was also assessed in vivo through immunohistochemistry for type 2 collagen within cartilage defects 6 weeks after scaffold implantation (n = 5). *p < 0.05. GAG, glycosaminoglycan; POS, positive (with 10 ng/mL exogenous TB3, no scaffold); MSC, mesenchymal stem cell; NEG, negative (no exogenous TB3, no scaffold). Color images available online at www.liebertpub.com/tea
To further assess TGFβ3 activity in an in vivo setting, scaffolds were implanted as described previously into porcine cartilage defects after microfracture. For this study, HA-only scaffolds with (i.e., MeHA/HH+TB3) and without TGFβ3 (i.e., both MeHA and MeHA/HH) were investigated (n = 5), along with positive (i.e., native tissue) and negative (i.e., microfracture) controls. After 6 weeks in vivo, no differences were observed in GAG content as seen through staining with alcian blue and picrosirius red; however, type 2 collagen content was significantly increased in the TGFβ3-releasing group (i.e., MeHA/HH+TB3) compared to the MeHA/HH group, further validating the activity of the released TGFβ3 (Fig. 3D).
Subchondral bone remodeling is dependent on scaffold chemistry and presence of TGFβ3
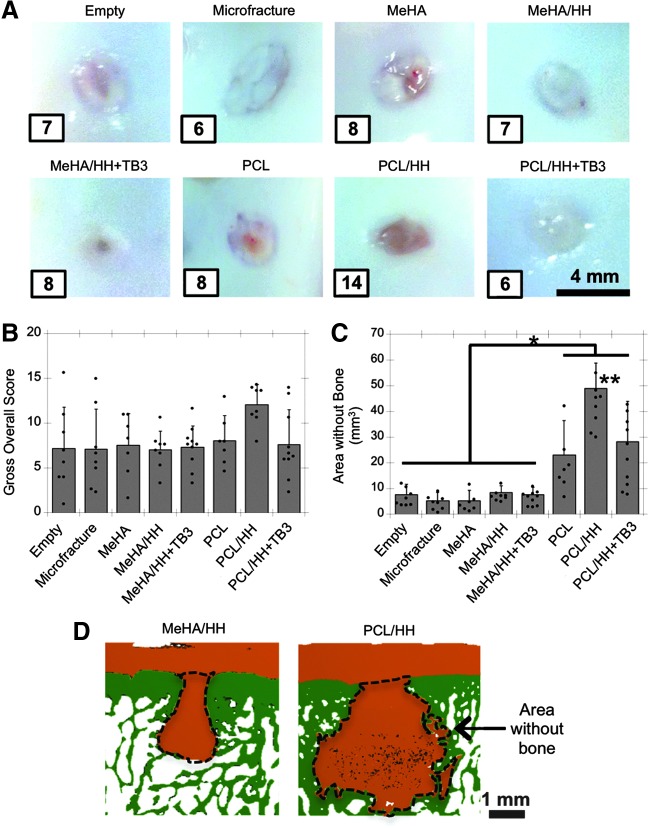
All scaffold groups and controls (i.e., six scaffold groups along with empty, microfracture, and normal tissue controls) were investigated for 12 weeks in vivo using the same porcine cartilage defect model, as previously described (n = 7–10). After 12 weeks, the gross appearance of most cartilage defects showed regenerate tissue that was relatively opaque, white-blue, and with in-level fill with the surrounding cartilage (Fig. 4A and Supplementary Fig. S5). In almost all defects, the center of the defect appeared slightly less opaque and more reddish in comparison to the surrounding areas. The PCL/HH group had inferior gross appearance (both in terms of color and blood vessels); these differences were validated through scoring with the system developed by Goebel et al.33 (Supplementary Fig. S6). Still, the overall score for gross appearance did not show any significant differences between conditions (Fig. 4B). The high level of defect fill for all treatment groups was also validated through quantification of contrast-enhanced (Lugol's solution) microCT reconstructions of the defect (Supplementary Fig. S7A).
FIG. 4.
Gross appearance and subchondral bone remodeling of cartilage defects after 12 weeks in vivo. (A) Representative gross images (i.e., median overall gross scores, which are shown in inset) and (B) overall gross score for all cartilage defects (n = 7–10). Note that normal tissue overall gross score = 0. (C) Subchondral bone remodeling, as quantified through area without bone underneath the cartilage (mm3) (n = 7–10). (D) Representative microCT reconstructions depicting cartilage (orange) and bone (green), with the area without bone underneath the cartilage outlined. *p < 0.05 between specified groups and **p < 0.05 in comparison to all other groups. microCT, microcomputed tomography. Color images available online at www.liebertpub.com/tea
Remodeling of the subchondral bone was quantified through microCT imaging in two ways, through overall bone density (i.e., percent bone volume/total volume of the region directly under the defect) and the area underneath the cartilage without bone (Fig. 4C, D and Supplementary Fig. S7). Although overall bone density levels were within the normal range for HA-only and control groups even directly under the defect (i.e., 0–1 mm depth from the defect surface), there was some level of bony remodeling with all groups, as quantified by the area without bone. Interestingly, it was clear from both metrics that the PCL-containing scaffolds (i.e., PCL, PCL/HH, and PCL/HH+TB3) had significantly more subchondral bone remodeling than other groups and that the PCL/HH group had the most severe remodeling response.
Semiquantitative histological assessment of cartilage repair
Histological analysis is considered an important metric in the evaluation of cartilage repair and is certainly one of the most common outcomes presented in in vivo studies. In this study, we stained with Safranin-O/fast green (for proteoglycans and fibrous tissue), H&E (for cells and tissue structure), and performed IHC for type 2 collagen. Sections were scored using a modified version of the well-validated ICRS-II visual analog scoring system, across 13 parameters related to regenerate and surrounding tissue.36 Consistent with other metrics noted previously, Safranin-O/fast green revealed wide variability in cartilage repair, ranging from a high level of Safranin-O-stained area within the defect to a complete lack of proteoglycan staining or even incomplete defect fill (Fig. 5A and Supplementary Figs. S8 and S9). All defects were well infiltrated with cells, as observed by H&E staining (Supplementary Fig. S10). H&E staining also suggested that the PCL scaffolds, in particular, had sunken into the remodeling subchondral bone.
FIG. 5.
Histological assessment of cartilage repair after 12 weeks in vivo. (A, C) Representative images after staining with Safranin-O/fast green and immunohistochemistry for type 2 collagen, respectively. Images shown represent median values for overall histological score and percent type 2 collagen-positive area, respectively (shown in inset). (B) Scoring of histology using ICRS-II system (overall score shown) and (D) quantification of percent positive type 2 collagen-stained area (n = 7–10). *p < 0.05 between specified groups and **p < 0.05 in comparison to all other groups, unless that group is marked with n.s. Color images available online at www.liebertpub.com/tea
Scoring of these histological observations using the ICRS-II scoring system revealed high levels of integration and defect fill for most groups and also underscored the severe bone remodeling response in the PCL and PCL/HH groups, as was noted earlier by microCT (Supplementary Fig. S11). Both TGFβ3-releasing groups had significantly higher overall scores compared to the PCL/HH group, and these TGFβ3-releasing groups along with the MeHA group also scored significantly higher for the superficial zone compared to the PCL/HH group (Fig. 5B). Moreover, both TGFβ3-releasing groups and the MeHA group showed increased type 2 collagen staining within the defect area, and these three conditions were the only groups that reached levels comparable to native tissue in terms of both type 2 collagen-positive area and staining intensity (Fig. 5C, D and Supplementary Fig. S12).
Mechanical properties and correlative relationships between outcome parameters
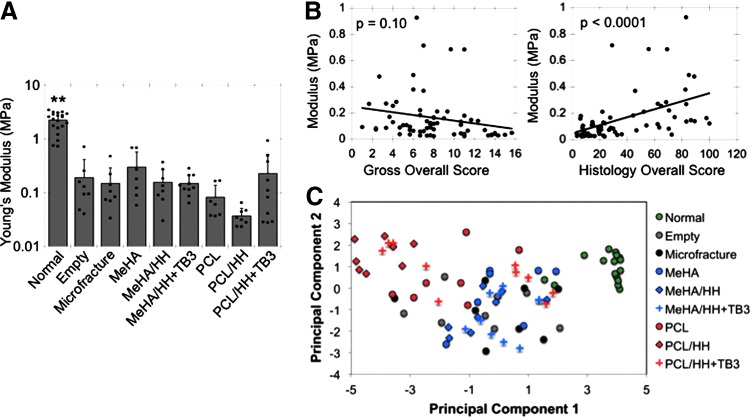
Microindentation testing was performed on explanted tissue, with the testing location focused on the central portion of each defect (Supplementary Fig. S13). As with the histological measures detailed earlier, a high standard deviation was observed in all groups. Indeed, even the Young's modulus for normal tissue ranged from 0.7 to 3.5 MPa (Fig. 6A). Generally, most treatment groups had mechanical properties that were similar to both the empty and microfracture groups. Although there were no significant differences due to the high spread of data, the MeHA group had an average modulus of ∼299 kPa, which was twice that of many other groups, including microfracture (151 kPa) and MeHA/HH (157 kPa). Also, the PCL and PCL/HH groups had markedly lower moduli than most other groups (∼82 and ∼37 kPa, respectively), which could be due to the increased subchondral bone remodeling and inferior matrix composition, as mentioned previously. As seen through histology, it again appeared that the incorporation of TGFβ3 offset the inclusion of PCL, as the PCL/HH+TB3 group had an average modulus that was closer to those of the HA groups.
FIG. 6.
Mechanical properties of cartilage defects after 12 weeks in vivo and correlative relationships between various outcome measures. (A) Young's modulus, as obtained through microindentation testing, and Hertz model approximation of cartilage defects (n = 7–10). (B) Correlations between gross or histological overall scores and tissue modulus measured through microindentation. (C) Principal component analysis of entire dataset from 12-week in vivo cartilage repair study. **p < 0.05 in comparison to all other groups.
Due to the high number of outcomes in this study, analyzing the dataset in its entirety allowed for comparisons of how well each outcome predicted mechanical properties. Most metrics were reasonably good predictors of mechanics, with linear regression resulting in significant p-values for all outcomes with mechanics, except for defect fill and overall score of gross appearance (Fig. 6B and Supplementary Fig. S14). PCA was also performed to reduce all assessment variables into two principal components that accounted for most of the variability within the data, allowing for two-dimensional visualization of the entire dataset (Fig. 6C). This analysis suggested that the HA-only scaffolds were more similar to normal tissue compared to PCL-containing scaffolds, as the PCL groups (especially without TGFβ3) were generally furthest from the cluster of normal cartilage samples.
Discussion
Even with recent advances in scaffold development, the number of studies applying acellular biomaterials with microfracture to cartilage defects is limited, and thus, the effect of material parameters on cartilage repair outcomes in vivo is still not well understood. The scaffolds investigated here were specifically chosen to investigate the effects of various material properties (e.g., degradation, mechanics, and growth factor release) on cartilage repair in a porcine cartilage defect model.
In terms of scaffold design, one major finding of this study is the difference between PCL composites and HA-only scaffolds on cartilage repair. Both PCL groups without TGFβ3 trended toward inferior gross appearance and reduced mechanical properties, possibly due to extensive remodeling of the subchondral bone. The underlying reason for this enhanced bony remodeling with PCL scaffolds is not entirely clear, although possible factors include differences in degradation rates or even a heightened inflammatory response to the PCL material.37 Histology suggested that the PCL scaffolds had sunken into the remodeling bone, which may have exacerbated the remodeling or inhibited the reossification of the remodeled bony area. Regardless, these differences in subchondral bone remodeling may have negatively impacted mechanical properties, as a softer underlying surface removes the support structure underneath the cartilage and can also lead to eventual subchondral bone overgrowth and hypertrophy and degeneration of the cartilage region.38–40
The TGFβ superfamily is involved in a number of important cellular processes, including proliferation and stimulation of matrix production, depending on the cell or tissue type and a variety of other factors.41 Aberrant TGFβ signaling has been linked to the onset of inflammation and osteoarthritis;42 however, it is generally viewed as a positive factor for cartilage repair. In this study, the retained bioactivity of electrospun TGFβ3 was validated through both in vitro and in vivo studies, and this finding highlights an important benefit of HA, as HA can be electrospun from water without the need for harsh organic solvents that significantly reduce growth factor activity and stability. Interestingly, delivery of TGFβ3 from PCL-containing scaffolds seemed to offset the high level of remodeling (possibly due to inflammation). Indeed, PCL-containing scaffolds with TGFβ3 had similar levels of cartilage repair compared to the other treatment groups. Both TGFβ3-releasing groups and the MeHA group had improved histological scoring and increased type 2 collagen content, affirming the stimulatory role that both TGFβ3 and the sustained presence of HA can have on cartilaginous matrix production.
Along with these findings on the effect of scaffold properties and growth factor delivery on cartilage repair, our study also highlights important considerations regarding animal model choice, sample size, study duration, and the incorporation of multiple outcome measures when investigating cartilage repair in vivo. Although commonly used and significantly less expensive, small animal models such as rabbits and rodents can be problematic. For instance, cartilage defects tend to heal spontaneously with rabbits, and it is difficult to create purely chondral defects with rodents due to the extremely thin cartilage layer.43,44 Large animal models are more appropriate for translation of cartilage treatment strategies toward use with humans27,43,44; for example, the porcine joint size and gait characteristics more accurately represent humans compared to smaller animals.44 However, the cost, housing needs, and other factors associated with these larger animals (e.g., equine models) can be prohibitive for preliminary studies.
In this study, we chose to utilize a juvenile minipig cartilage defect model, in which chondral defects were created within the trochlear grooves to assess cartilage repair. The choice of juvenile minipigs mitigates some of the aforementioned issues with small animal models while not being significantly cost-prohibitive for feasibility or preliminary studies that investigate a large number of treatment conditions, as with our study. Even with the increased clinical relevancy with larger animals, our findings suggest that a great degree of variability may be an inherent factor with such large animal models, as we observed large standard deviations in all outcomes even for the control groups (i.e., empty, microfracture, and normal tissue). This increased variability should be considered when determining the minimum sample size needed to differentiate between treatment groups and the type and number of outcome measures needed for a full understanding of cartilage repair in in vivo studies, especially for large animal models.
Typical in vivo cartilage-focused repair studies report only a few outcomes for assessment, with histology and gross appearance as the most common. Surprisingly, mechanical testing is still an infrequent consideration for cartilage repair assessment, despite the fact that cartilage predominantly serves as a load-bearing tissue. In this study, we were able to mechanically test samples with low strains that should not cause irreversible damage to the tissue; this allowed for those same samples to be assessed through other metrics, including microCT imaging and histology. Moreover, although samples were frozen and thawed before testing, multiple groups have reported that freeze–thawing does not significantly impact mechanical properties of cartilage and other tissues in most cases,45,46 and the values we calculated from our normal tissue samples were similar to those reported previously in literature.35,47 Although not statistically significant, there were trends between the scaffold groups, with the sustained presence of HA (i.e., MeHA group) and the inclusion of TGFβ3 in the PCL/HA composite scaffold generally resulting in higher moduli. It is important to note that these trends may have reached statistical significance with longer term studies, as 12 weeks is still a relatively short time period for cartilage repair. The significant differences evident through histological analysis provide additional evidence for this, as differences in production or organization of cartilaginous matrix may require more time to culminate in improved mechanical properties. Although longer studies may be more clinically relevant, these shorter time periods are essential to screen an initially large number of treatment groups and to focus on the most promising treatments for future long-term studies (e.g., 6 months to years) and larger animal models (e.g., equine).
Even mechanical testing alone is not sufficient as an assessment of cartilage repair, and ideally, a plethora of outcomes should be evaluated to fully understand the impact of treatment. Another strength of this study is that each sample was tested using multiple outcome measures, including gross appearance (imaging and scoring), histology (imaging, quantification, and scoring), mechanical testing, and microCT analysis for defect fill and subchondral bone remodeling. This large number of metrics allowed for comparisons between groups and also the generation of a big picture of cartilage repair using PCA to combine all samples and outcome measures into one graph. Comparisons of outcome measures suggested that histology was a much better predictor of mechanical properties in comparison to gross appearance, even though both histology and gross appearance are well-accepted and commonly reported measures of cartilage repair. Importantly, PCA further highlighted the differences seen through individual metrics based on material choice and inclusion of TGFβ3, with PCL scaffolds without TGFβ3 generally furthest from the cluster of normal tissue samples. As stated previously, these results demonstrate the effect of scaffold properties on cartilage repair and also highlight important study design considerations for in vivo studies, especially the need to assess treatment groups through multiple measures for a full understanding of the mechanisms behind and true level of cartilage repair.
Conclusions
In summary, we demonstrated tunable control over fibrillar scaffolds and investigated the effect of these material properties and growth factor delivery on cartilage repair in vivo. Without the need for tissue or cell harvest and extensive in vitro culture, implantation of acellular fibrillar scaffolds in combination with microfracture represents a promising technique to improve upon current clinical limitations. Moreover, the work presented here highlights important considerations for in vivo studies, including the choice of animal model, study length, and outcome measures needed to fully assess cartilage repair.
Supplementary Material
Acknowledgments
This work was supported by a graduate research fellowship from the National Science Foundation (I.L.K.) and grants from the National Institutes of Health (R01 EB008722) and the Department of Veterans Affairs (I01 RX000700). The authors also acknowledge Dr. Nicole Belkin and Dr. Andrew Milby for assistance with surgeries and Dr. Ross Marklein for assistance with principal component analysis. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the Department of Veterans Affairs.
Disclosure Statement
No competing financial interests exist.
References
- 1.Knecht S., Vanwanseele B., and Stussi E. A review on the mechanical quality of articular cartilage—implications for the diagnosis of osteoarthritis. Clin Biomech (Bristol, Avon) 21, 999, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Hootman J., Helmick C., and Brady T. A public health approach to addressing arthritis in older adults: the most common cause of disability. Am J Public Health 102, 426, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahmed T.A.E., and Hincke M.T. Strategies for articular cartilage lesion repair and functional restoration. Tissue Eng Part B Rev 16, 305, 2010 [DOI] [PubMed] [Google Scholar]
- 4.Bedi A., Feeley B., and Williams R. Management of articular cartilage defects of the knee. J Bone Joint Surg 92, 994, 2010 [DOI] [PubMed] [Google Scholar]
- 5.Lutzner J., Kasten P., and Gunther K-P., and Kirschner S. Surgical options for patients with osteoarthritis of the knee. Nat Rev Rheumatol 5, 309, 2009 [DOI] [PubMed] [Google Scholar]
- 6.Mollon B., Kandel R., Chahal J., and Theodoropoulos J. The clinical status of cartilage tissue regeneration in humans. Osteoarthritis Cartilage 21, 1824, 2013 [DOI] [PubMed] [Google Scholar]
- 7.Roelofs A., Rocke J., and De Bari C. Cell-based approaches to joint surface repair: a research perspective. Osteoarthritis Cartilage 21, 892, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goyal D., Keyhani S., Lee E., and Hui J. Evidence-based status of microfracture technique: a systematic review of level I and II studies. Arthroscopy 29, 1579, 2013 [DOI] [PubMed] [Google Scholar]
- 9.Burdick J.A., Mauck R.L., Gorman J.H., III, and Gorman R. Acellular biomaterials: an evolving alternative to cell-based therapies. Sci Transl Med 5, 176ps4, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Benthien J., and Behrens P. Autologous matrix-induced chondrogenesis (AMIC). A one-step procedure for retropatellar articular resurfacing. Acta Orthop Belg 76, 260, 2010 [PubMed] [Google Scholar]
- 11.Benthien J., and Behrens P. The treatment of chondral and osteochondral defects of the knee with autologous matrix-induced chondrogenesis (AMIC): method description and recent developments. Knee Surg Sports Traumatol Arthrosc 19, 1316, 2011 [DOI] [PubMed] [Google Scholar]
- 12.Kusano T., Jakob R.P., Gautier E., Magnussen R.A., Hoogewoud H., and Jacobi M. Treatment of isolated chondral and osteochondral defects in the knee by autologous matrix-induced chondrogenesis (AMIC). Knee Surg Sports Traumatol Arthrosc 20, 2109, 2011 [DOI] [PubMed] [Google Scholar]
- 13.Sharma B., Fermanian S., Gibson M., Unterman S., Herzka D.A., Cascio B., et al. Human cartilage repair with a photoreactive adhesive-hydrogel composite. Sci Transl Med 5, 1, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shah R.N., Shah N.A., Del Rosario Lim M.M., Hsieh C., Nuber G., and Stupp S.I. Supramolecular design of self-assembling nanofibers for cartilage regeneration. Proc Natl Acad Sci U S A 107, 3293, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kang S.W., Bada L.P., Kang C.S., Lee J.S., Kim C.H., Park J.H., et al. Articular cartilage regeneration with microfracture and hyaluronic acid. Biotechnol Lett 30, 435, 2008 [DOI] [PubMed] [Google Scholar]
- 16.Sukegawa A., Iwasaki N., Kasahara Y., Onodera T., Igarashi T., and Minami A. Repair of rabbit osteochondral defects by an acellular technique with an ultrapurified alginate gel containing stromal cell-derived factor-1. Tissue Eng Part A 18, 934, 2012 [DOI] [PubMed] [Google Scholar]
- 17.Wang W., Li B., Yang J., Xin L., Li Y., Yin H., et al. The restoration of full-thickness cartilage defects with BMSCs and TGF-beta 1 loaded PLGA/fibrin gel constructs. Biomaterials 31, 8964, 2010 [DOI] [PubMed] [Google Scholar]
- 18.Coburn J.M., Gibson M., Monagle S., Patterson Z., and Elisseeff J.H. Bioinspired nanofibers support chondrogenesis for articular cartilage repair. Proc Natl Acad Sci U S A 109, 10012, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kai D., Jin G., Prabhakaran M.P., and Ramakrishna S. Electrospun synthetic and natural nanofibers for regenerative medicine and stem cells. Biotechnol J 8, 59, 2013 [DOI] [PubMed] [Google Scholar]
- 20.Lee K., Jeong L., Kang Y., and Lee S. Electrospinning of polysaccharides for regenerative medicine. Adv Drug Deliv Rev 61, 1020, 2009 [DOI] [PubMed] [Google Scholar]
- 21.Liu W., Thomopoulos S., and Xia Y. Electrospun nanofibers for regenerative medicine. Adv Healthc Mater 1, 10, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sill T.J., and von Recum H.A. Electrospinning: applications in drug delivery and tissue engineering. Biomaterials 29, 1989, 2008 [DOI] [PubMed] [Google Scholar]
- 23.Kim I.L., Khetan S., Baker B.M., Chen C.S., and Burdick J.A. Fibrous hyaluronic acid hydrogels that direct MSC chondrogenesis through mechanical and adhesive cues. Biomaterials 34, 5571, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chung C., and Burdick J.A. Influence of three-dimensional hyaluronic acid microenvironments on mesenchymal stem cell chondrogenesis. Tissue Eng Part A 15, 243, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bian L., Guvendiren M., Mauck R.L., and Burdick J.A. Hydrogels that mimic developmentally relevant matrix and N-cadherin interactions enhance MSC chondrogenesis. Proc Natl Acad Sci U S A 110, 10117, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dash T., and Konkimalla V. Poly-ε-caprolactone based formulations for drug delivery and tissue engineering: a review. J Control Release 158, 15, 2012 [DOI] [PubMed] [Google Scholar]
- 27.Fisher M., Belkin N., Milby A., Henning E., Bostrom M., Kim M., et al. Cartilage repair and subchondral bone remodeling in response to focal lesions in a mini-pig model: implications for tissue engineering. Tissue Eng Part A 21, 850, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tous E., Ifkovits J.L., Koomalsingh K.J., Shuto T., Soeda T., Kondo N., et al. Influence of injectable hyaluronic acid hydrogel degradation behavior on infarction-induced ventricular remodeling. Biomacromolecules 12, 4127, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Burdick J.A., Chung C., Jia X.Q., Randolph M.A., and Langer R. Controlled degradation and mechanical behavior of photopolymerized hyaluronic acid networks. Biomacromolecules 6, 386, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baker B.M., Gee A.O., Metter R.B., Nathan A.S., Marklein R.A., Burdick J.A., et al. The potential to improve cell infiltration in composite fiber-aligned electrospun scaffolds by the selective removal of sacrificial fibers. Biomaterials 29, 2348, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Neuman R.E., and Logan M.A. The determination of hydroxyproline. J Biol Chem 184, 299, 1950 [PubMed] [Google Scholar]
- 32.Stegemann H., and Stalder K. Determination of hydroxyproline. Clin Chim Acta 18, 267, 1967 [DOI] [PubMed] [Google Scholar]
- 33.Goebel L., Orth P., Müller A., Zurakowski D., Bücker A., Cucchiarini M., et al. Experimental scoring systems for macroscopic articular cartilage repair correlate with the MOCART score assessed by a high-field MRI at 9.4 T—comparative evaluation of five macroscopic scoring systems in a large animal cartilage defect model. Osteoarthritis Cartilage 20, 1046, 2012 [DOI] [PubMed] [Google Scholar]
- 34.Solon J., Levental I., Sengupta K., Georges P., and Janmey P. Fibroblast adaptation and stiffness matching to soft elastic substrates. Biophys J 93, 4453, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li X., An Y.H., Wu Y.D., Song Y.C., Chao Y.J., and Chien C.H. Microindentation test for assessing the mechanical properties of cartilaginous tissues. J Biomed Mater Res B Appl Biomater 80, 25, 2007 [DOI] [PubMed] [Google Scholar]
- 36.Mainil-Varlet P., Van Damme B., Nesic D., Knutsen G., Kandel R., and Roberts S. A new histology scoring system for the assessment of the quality of human cartilage repair: ICRS II. Am J Sports Med 38, 880, 2010 [DOI] [PubMed] [Google Scholar]
- 37.Vacanti N., Cheng H., Hill P., Guerreiro J., Dang T., Ma M., et al. Localized delivery of dexamethasone from electrospun fibers reduces the foreign body response. Biomacromolecules 13, 3031, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Orth P., Cucchiarini M., Kaul G., Ong M.F., Graeber S., Kohn D.M., et al. Temporal and spatial migration pattern of the subchondral bone plate in a rabbit osteochondral defect model. Osteoarthritis Cartilage 20, 1161, 2012 [DOI] [PubMed] [Google Scholar]
- 39.Dorotka R., Bindreiter U., Macfelda K., Windberger U., and Nehrer S. Marrow stimulation and chondrocyte transplantation using a collagen matrix for cartilage repair. Osteoarthritis Cartilage 13, 655, 2005 [DOI] [PubMed] [Google Scholar]
- 40.Wei X., and Messner K. Maturation-dependent durability of spontaneous cartilage repair in rabbit knee joint. J Biomed Mater Res 46, 539, 1999 [DOI] [PubMed] [Google Scholar]
- 41.Leask A., and Abraham D.J. TGF-beta signaling and the fibrotic response. FASEB J 18, 816, 2004 [DOI] [PubMed] [Google Scholar]
- 42.Zhen G., and Cao X. Targeting TGFβ signaling in subchondral bone and articular cartilage homeostasis. Trends Pharmacol Sci 35, 227, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cook J., Hung C., Kuroki K., Stoker A., Cook C., Pfeiffer F., et al. Animal models of cartilage repair. Bone Joint Res 3, 89, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chu C., Szczodry M., and Bruno S. Animal models for cartilage regeneration and repair. Tissue Eng Part B Rev 16, 105, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lewis P.B., Williams J.M., Hallab N., Virdi A., Yanke A., and Cole B.J. Multiple freeze-thaw cycled meniscal allograft tissue: a biomechanical, biochemical, and histologic analysis. J Orthop Res 26, 49, 2008 [DOI] [PubMed] [Google Scholar]
- 46.Szarko M., Muldrew K., and Bertram J.E. Freeze-thaw treatment effects on the dynamic mechanical properties of articular cartilage. BMC Musculoskelet Disord 11, 231, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Miller G.J., and Morgan E.F. Use of microindentation to characterize the mechanical properties of articular cartilage: comparison of biphasic material properties across length scales. Osteoarthritis Cartilage 18, 1051, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.