Abstract
Cotransplantation of CD34+ hematopoietic stem and progenitor cells (HSPCs) with mesenchymal stromal cells (MSCs) enhances HSPC engraftment. For these applications, MSCs are mostly obtained from bone marrow (BM). However, MSCs can also be isolated from the Wharton's jelly (WJ) of the human umbilical cord. This source, regarded to be a waste product, enables a relatively low-cost MSC acquisition without any burden to the donor. In this study, we evaluated the ability of WJ MSCs to enhance HSPC engraftment. First, we compared cultured human WJ MSCs with human BM-derived MSCs (BM MSCs) for in vitro marker expression, immunomodulatory capacity, and differentiation into three mesenchymal lineages. Although we confirmed that WJ MSCs have a more restricted differentiation capacity, both WJ MSCs and BM MSCs expressed similar levels of surface markers and exhibited similar immune inhibitory capacities. Most importantly, cotransplantation of either WJ MSCs or BM MSCs with CB CD34+ cells into NOD SCID mice showed similar enhanced recovery of human platelets and CD45+ cells in the peripheral blood and a 3-fold higher engraftment in the BM, blood, and spleen 6 weeks after transplantation when compared to transplantation of CD34+ cells alone. Upon coincubation, both MSC sources increased the expression of adhesion molecules on CD34+ cells, although stromal cell-derived factor-1 (SDF-1)-induced migration of CD34+ cells remained unaltered. Interestingly, there was an increase in CFU-GEMM when CB CD34+ cells were cultured on monolayers of WJ MSCs in the presence of exogenous thrombopoietin, and an increase in BFU-E when BM MSCs replaced WJ MSCs in such cultures. Our results suggest that WJ MSC is likely to be a practical alternative for BM MSC to enhance CB CD34+ cell engraftment.
Introduction
Cord blood (CB) is used as an alternative source for hematopoietic stem and progenitor cell (HSPC) transplantation [1–3]. However, the successful outcome of CB transplantation is limited by the relatively low number of transplantable HSPC in these grafts, which results in delayed hematopoietic recovery posttransplant [4]. Double CB transplantation in this respect increases the number of transplantable HSPC, but the time to recovery of donor neutrophils and platelets in the peripheral blood (PB) posttransplant is still inferior to transplantation of bone marrow (BM) or mobilized PB grafts [5]. One method to overcome this CB-associated disadvantage is to enhance the engraftment of HSPC by cotransplantation of accessory cells such as mesenchymal stromal cells (MSCs) [6].
MSCs were first identified in BM as multipotent cells and characterized largely by in vitro attributes [7]. These included their ability to differentiate into mesodermal cells, such as adipocytes, chondrocytes, and osteoblasts, their adherence to plastic, and their expression of specific cell surface markers [8]. In addition, MSCs have the capacity to modulate immune responses [9]. Interestingly, in animal models, cotransplantation of human CB-derived CD34+ cells with human MSCs was shown to improve hematopoietic engraftment [10,11]. Both local and systemic mechanisms may play a role in this latter process, for example, by the MSCs promoting homing to the BM or its vasculature or releasing proangiogenic, immunomodulatory, or growth factors that promote engraftment [9,12,13].
Although originally identified in cultures obtained from BM aspirates [14,15], MSCs can also be isolated from other sources such as adipose tissue [16], compact bone [17], amniotic fluid [18], CB [19], the umbilical cord [20,21], or the placenta [22].
MSCs cultured from Wharton's Jelly (WJ MSCs) of the umbilical cord display unique characteristics such as a greater expansion capacity and faster in vitro growth compared to BM MSCs [23,24]. Moreover, WJ MSCs have some logistical advantages over BM MSCs. Notably, the umbilical cord is considered a waste product and WJ MSCs can, therefore, be obtained from this source at relatively low cost and without burden to the donor. The WJ could, therefore, be a promising source for the clinical application of MSCs [25,26].
With this in mind, we set out to compare the effect of cotransplantation of human CB-derived CD34+ cells with either BM or WJ MSCs on hematopoietic engraftment in immune deficient NOD SCID mice. Furthermore, we assessed whether cotransplantation of WJ MSCs that were autologous to the CB CD34+ cells affected this engraftment when compared to cotransplantation with allogeneic WJ MSCs.
Materials and Methods
Umbilical CB and umbilical cord (UC) collection
CB was drawn from the umbilical vein at birth at >36 weeks gestation after written informed consent from the mother at hospitals in the Netherlands according to NetCord–FACT standards and with ethical permission from the Medical Ethics Board of the Leiden University Medical Center (LUMC), Leiden, The Netherlands. Blood was collected by gravity drainage into MacoPharma collection bags containing 21 mL citrate phosphate dextrose adenine-1 (MacoPharma). The blood was stored at 4°C and processed within 48 h of collection. Umbilical cords were collected concomitantly with the CB in a sterile container containing phosphate-buffered saline (PBS) with 1% (v/v) antibiotic/antimycotic mix (Life Technologies).
CD34+ cell purification
Mononuclear cells (MNCs) were isolated from CB using a sterile Ficoll density gradient (1.077 g/cm3; Pharmacy LUMC). The CD34+ cell fraction was isolated from the MNC fraction by double CD34+ cell selection using immunomagnetic beads (Miltenyi Biotec GmbH). The purity of the isolated CD34+ cell fraction was verified by flow cytometry (Beckman Coulter, Woerden, The Netherlands) using CD45-FITC and CD34-PE antibodies (all Beckman Coulter, ISHAGE protocol [27]), and was higher than 90% for all CD34+ cells used throughout the experiments. Cells were cryopreserved in Iscove's modified Dulbecco's medium (IMDM) with 10% (v/v) dimethyl sulfoxide (DMSO) and 4% (w/v) human serum albumin (Pharmacy LUMC) and stored at −150°C until use.
MSC isolation and culture
Umbilical cord-derived MSCs
MSCs were isolated with an explant method as described in De Bruyn et al. [28]. Briefly, the cords were cut into 5 cm segments and then longitudinally and the vein and arteries tissue removed. The segments were placed on 10-cm culture dishes (Greiner) with the inside of the cord, that is, the WJ, facing the bottom of the plate. MSC medium [Dulbecco's modified Eagle's medium (DMEM) supplemented with 15% (v/v) fetal bovine serum (FBS) and 1% (v/v) antibiotic–antimycotic solution, all Life Technologies] was added to the plate until the segments were submerged in the medium. The culture plates were placed in a humidified incubator at 37°C and 5% CO2. Medium was refreshed every 3 days. After 10 days, the segments were removed and the MSCs adhering to the plate were grown to confluence and passaged into culture flasks.
Since we did not separate the (sub)amnion and the WJ, we cannot exclude that a small part of the MSCs was derived from the (sub)amnion. However, in line with the original description of this method by De Bruyn et al. [28] we decided to use the cells WJ MSCs throughout the article.
BM-derived MSCs
BM was collected from patients undergoing knee or hip replacement surgery at the LUMC with informed consent of the donor and with ethical permission from the Medical Ethics Board of the LUMC. MNCs were isolated from the BM suspensions by gradient centrifugation with Ficoll (1.077 g/mL, pharmacy LUMC) and loaded into culture flasks containing DMEM with 10% (v/v) FBS and 1% (v/v) penicillin and streptomycin (all Life Technologies). After overnight culture in a humidified incubator at 37°C and 5% CO2, nonadhering cells were washed from the flask with PBS. Adherent cells were grown to confluence and passaged. After three passages, cells were cryopreserved in FBS with 10% (v/v) DMSO (pharmacy LUMC). The MSCs that were used throughout this study were between passage 3 and 6.
Flow cytometry
Flow cytometry analysis for cell surface marker expression was performed with a Beckman Coulter FC500 or a BD FACSCalibur running CXP or CellQuest Pro software, respectively. Isolated CD34+ cells were analyzed for the expression of CD34 and CD45 (both from Beckman Coulter) and MSCs were analyzed for the expression of CD105, CD90, CD80, CD73, CD45, CD34, CD31, HLA-ABC, and HLA-DR (all from BD Biosciences).
Differentiation of UC and BM MSCs into mesodermal lineages
The WJ MSCs and BM MSCs were analyzed for their ability to differentiate into adipocytes, chondrocytes, and osteoblasts as described previously [29,30]. In brief, MSCs were cultured in specific adipogenic, chondrogenic, and osteogenic differentiation media. After 21 days, the osteogenic cultures were analyzed for the presence of osteoblasts by staining of calcium deposits with Alizarin red and alkaline phosphatase with Fast Blue. In the adipogenic cultures, lipid droplets were visualized with Oil Red O staining and, in the chondrogenic cultures, cells were stained with Toluidine blue. We used an arbitrary scoring system that assesses the degree of differentiation in the cultures. Cultures showing no differentiated cells were scored as 0, a few differentiated cells as 1, moderate differentiation as 2, and full differentiation as 3 (Supplementary Fig. S1; Supplementary Materials and Methods are available online at www.liebertpub.com/scd).
Immune inhibition of adult PBMC and CB MNC by WJ MSCs and BM MSCs
To analyze the effect of MSCs on the proliferation of MNCs obtained from adult peripheral blood (PBMC), 1 × 105 PBMC were cultured for 5 days in 24-well plates with αCD3αCD28 beads (Life Technologies) alone or in combination with different concentrations of BM- or WJ-derived MSCs in a fully humidified incubator at 37°C and 5% CO2. Cell proliferation was measured by 3H-thymidine incorporation.
Coculture of MSCs and CB CD34+ cells with thrombopoietin (TPO)
MSCs obtained from BM and WJ were thawed and plated into a 24-well plate at 1.25 × 105 cells/well and grown overnight in MSC medium [DMEM with 10% (v/v) fetal calf serum (FCS)]. After 24 h, the MSCs were irradiated (10 Gy) and the cells were washed twice with PBS. CB CD34+ cells were added to the wells at 105 cells/well and cultured in expansion medium with Nplate (50 ng/mL, TPO analog; Amgen) as described previously [31]. After 10 days of culture, the hematopoietic cells were harvested by collecting all nonadherent cells by aspirating the supernatant, washing the plates with PBS, and spinning down the collected cell suspension.
The cells were counted and analyzed for the expression of CD34-PE, CD61-PE-Cy7, and CD45-FITC (all Beckman Coulter) by flow cytometry. The HSPCs that were cultured were subsequently analyzed for their capacity to generate myeloid colonies in MethoCult (STEMCELL Technologies) as described previously [32].
Transwell migration experiments
Four different cell suspensions were prepared. These were identical to the in vivo experiment described below. After 30 min of incubation of CB CD34+ cells with MSCs, part of the cell suspension was analyzed for the expression of CD34, CD11a, CD11b, CD184, CD49e, and CD49d (all antibodies from Beckman Coulter) using flow cytometry. The remaining cells were used for migration experiments in transwell plates [Costar (VWR)], 6.5 mm diameter with 5 μm pore filters. The lower compartment of the well and the filter were coated with 2 ng/mL fibronectin (Sigma) for 15 min at 37°C. The lower compartments of the plates were loaded with IMDM and 100 ng/mL SDF-1α (R&D Systems).
All cells were placed in the upper compartment of the plate and incubated for 5 h at 37°C and 5% CO2 in a humidified incubator. After incubation, the number of CD34+ cells that were harvested from the lower compartment was counted to determine the proportion of cells that migrated.
Transplantation in NOD SCID mice
Female 5–6-week-old NOD SCID mice (Charles River) were kept in microisolator cages in laminar flow racks in the LUMC animal facilities. The animal ethics committee of the LUMC approved all animal experiments. NOD SCID mice received 3.5 Gy total body irradiation 24 h before transplantation. Mice were transplanted with 1 × 105 CB-derived CD34+ cells alone or in combination with 1 × 106 MSCs through tail vein injections. PB was collected from the tail vein at weekly intervals starting 3 weeks after transplantation. Blood collection and human platelet measurements were performed as described previously [32]. Briefly, human platelets were stained with a noncross reactive mouse anti-human CD41-PE and mouse anti-human CD45-PC7 (both Beckman Coulter). Erythrocytes were lysed with IOTest 3 lysing solution (Beckman Coulter) for 10 min at room temperature. Flow-Count™ fluorospheres (Beckman Coulter) were added to the cells to enable analysis of the absolute number of circulating human platelets.
Analysis was performed by flow cytometry (FC500, Beckman Coulter) using CXP software. Six weeks after transplantation, mice were sacrificed and BM cells were obtained by flushing femurs with IMDM. Next, human cell engraftment and multilineage chimerism were analyzed by flow cytometry using goat anti-mouse-CD45-PE (LCA, Ly-5, 30-F11; Pharmingen), mouse anti-human CD45-FITC, CD33-FITC, CD34-PE, CD19-PE (all from Beckman Coulter) and the appropriate isotype controls. Erythrocytes were lysed with IOTest 3 Lysing solution (Beckman Coulter). Analysis was performed by flow cytometry (FC500; Beckman Coulter) using CXP software.
Statistics
All statistics were done using IBM SPSS Statistics (version 20, www.ibm.com/SPSS_Statistics). Results are presented as mean ± SEM. To test for statistical significance, a Mann–Whitney test or one-way ANOVA was used. Results were considered to be significant if the P value was ≤0.05.
Results
WJ-derived MSCs have similar marker expression, but limited differentiation potential compared to BM-derived MSCs
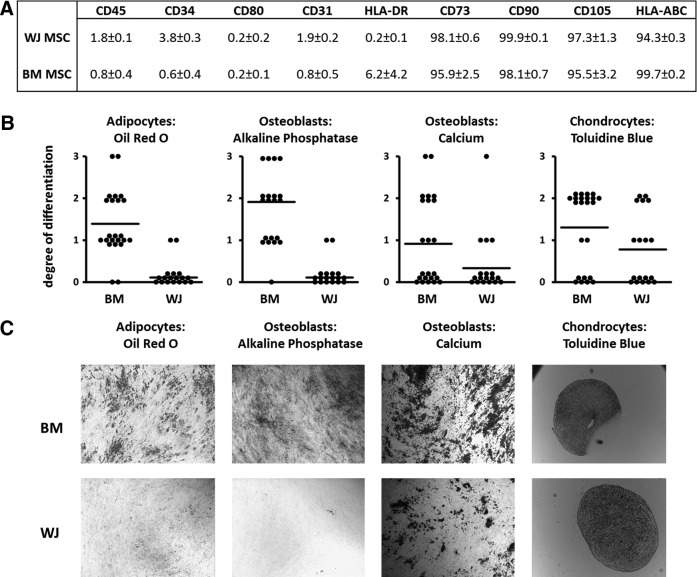
Following isolation and subsequent expansion, the phenotype of the WJ MSCs was determined and compared to BM MSCs (Fig. 1A). Similar to BM MSCs, WJ MSCs expressed HLA-ABC, CD73, CD90, and CD105 and lacked expression of the pan-hematopoietic marker CD45 and the endothelial/hematopoietic marker CD31. A small population of BM MSCs expressed HLA-DR (BM MSCs 6.2% ± 4.2% vs. WJ MSCs 0.2% ± 0.1%); whereas a small subset of WJ MSCs expressed CD34 (WJ MSCs 3.8% ± 0.3% vs. BM MSCs 0.6% ± 0.4%).
FIG. 1.
Characterization of the Wharton's Jelly MSCs (WJ MSCs). (A) Expression of cell surface markers by WJ MSCs and BM MSCs. The percentage of cells ( ± SEM; n = 10) that expresses the respective markers is shown. (B) Ability of WJ MSCs (n = 18 different isolates) and BM MSCs (n = 23 different isolates) to differentiate into adipocytes (Oil Red O), osteoblasts (alkaline phosphatase and calcium deposition) and chondrocytes (Toluidine blue staining). 0, no differentiated cells; 1, <20% differentiated cells d; 2, <60% differentiated cells; 3, >60% differentiated cells (see also Supplementary Fig. S1). The bar represents the mean of all experiments. (C) Representative images of differentiation cultures after incubation with cell differentiation specific stains. BM MSCs, bone marrow-derived MSCs; MSCs, mesenchymal stromal cells.
We next analyzed the ability of BM MSCs and WJ MSCs to differentiate into adipocytes, chondrocytes, and osteoblasts. The majority of BM-derived MSC isolates had the capacity to differentiate into chondrocytes and adipocytes and half of the BM MSC isolates differentiated into osteoblasts. In contrast, more than 25% of the WJ MSC isolates (4 out of 18) showed adipocyte generation and <15% of the WJ MSC isolates (2 out of 18) showed osteoblast differentiation (Fig. 1B, C). Moreover, in these respective four and two cultures adipocyte and osteoblast differentiation was sporadic (grade 1). Chondrogenic differentiation occurred in half of the WJ isolates (9 out of 18). Thus, although BM MSCs and WJ MSCs are phenotypically similar, WJ MSCs are limited with respect to their capacity to differentiate into mature mesodermal cell types as shown by their lower degree of differentiation compared to BM MSCs.
For one MSC donor of each type of MSCs, these functional differences were also investigated by the comparison of differentiation-specific gene expression. As shown before, no osteoblast or adipocyte-specific staining was observed in the WJ MSC culture, whereas positive staining for both cell types could be found in the BM MSC cultures (Supplementary Fig. S2A). The expression of the adipocyte-related genes peroxisome proliferator-activated receptor-γ (PPARG), fatty acid-binding protein 4 (FABP4) and perilipin (PLIN) was clearly upregulated in the BM MSC cultures, whereas WJ MSCs only showed upregulation of PPARG (Supplementary Fig. S2B). For osteoblast-related genes, this method showed to be unreliable since osteogenic differentiated BM MSCs did not show any increase in osteogenic gene expression, despite the presence of osteogenic-specific staining (Supplementary Fig. S2A, C).
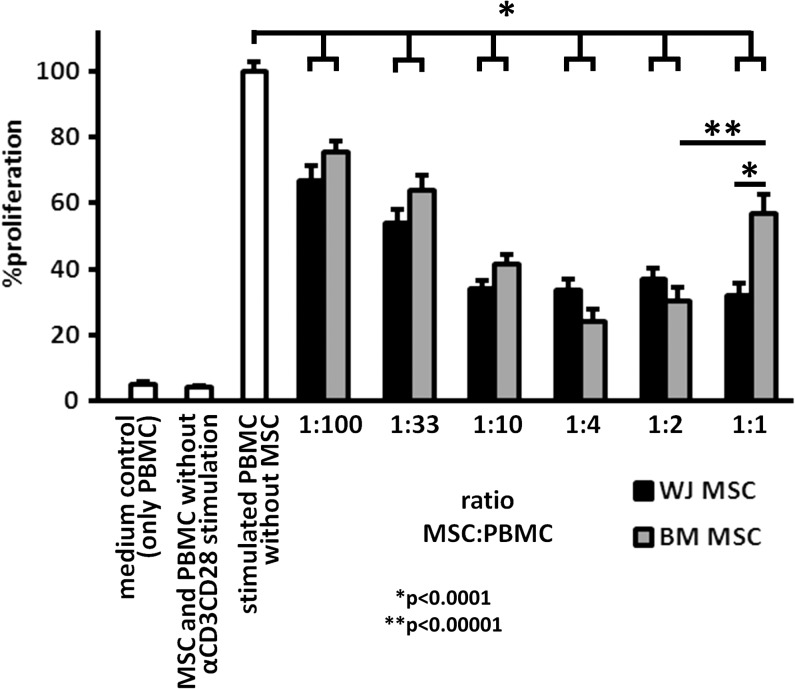
WJ MSCs and BM MSCs inhibit T cell proliferation of PBMC stimulated with αCD3αCD28 beads
Next, we compared in vitro immunomodulatory properties of WJ MSCs and BM MSCs in cocultures with unstimulated and αCD3αCD28-stimulated PBMC. In this setting, MSCs were not immunogenic themselves, since coincubation with MSCs did not lead to proliferation of unstimulated PBMC. Moreover, WJ MSCs and BM MSCs inhibited proliferation of stimulated PBMC and this reduction was MSC dose dependent (Fig. 2, P < 0.0001 for all ratios of MSCs and PBMC compared to stimulated PBMC alone). Interestingly, coculture of BM MSCs and PBMC at a 1:1 ratio resulted in decreased inhibition of proliferation (43% ± 6%) as compared to a 1:2 ratio of BM MSCs (70% ± 4%, P < 0.00001). Additionally, at this 1:1 ratio, WJ MSCs were more inhibitory than BM MSCs (Fig. 2; 68% ± 4% vs. 43% ± 6% inhibition, respectively, P < 0.0001).
FIG. 2.
WJ and BM MSCs inhibit T cell proliferation. BM or WJ MSCs were mixed with 1 × 105 PBMC at different ratios and stimulated with αCD3αCD28 beads for 5 days. Proliferation of the PBMC was measured by (3H) thymidine incorporation on day 5 and compared to a control without MSCs. MSCs obtained from both sources significantly inhibited the proliferation of the PBMC in a dose-dependent manner (n = 8, *P < 0.0001, **P < 0.00001). PBMC, peripheral blood mononuclear cell.
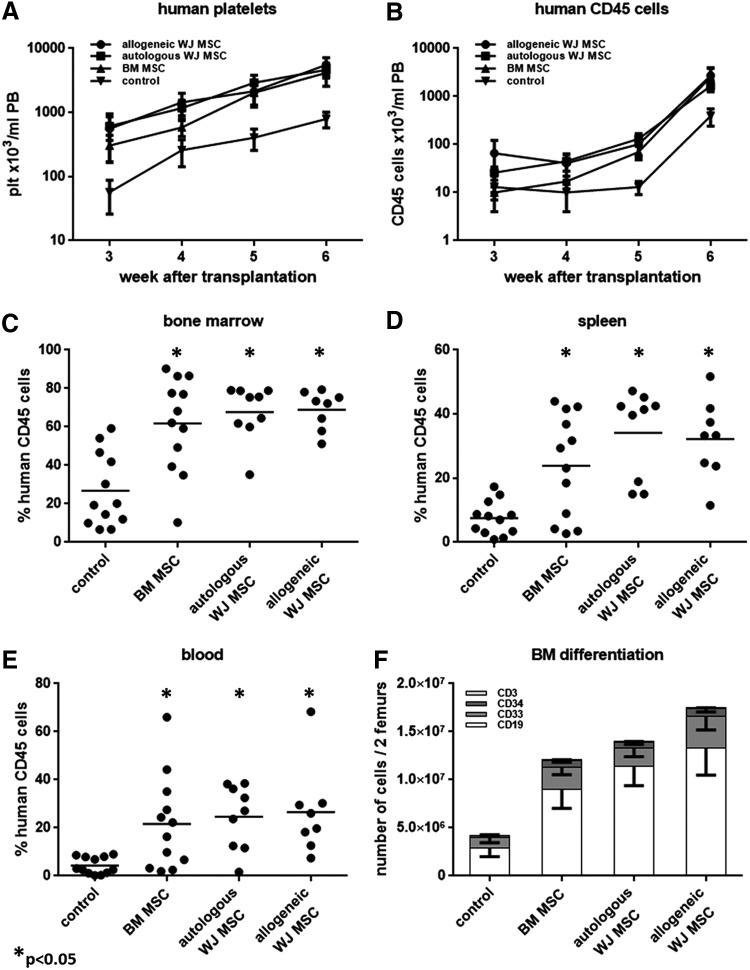
Cotransplantation of MSCs enhances the engraftment of CB-derived CD34+ cells in NOD SCID mice irrespective of the donor source
To evaluate and compare the effects of the different MSCs on HSC engraftment in vivo, we cotransplanted human CB-derived CD34+ cells and human MSCs into sublethally irradiated NOD SCID mice (n = 3 experiments with three different donors). Additionally, autologous WJ MSCs (ie, MSCs generated from the umbilical cord of the CD34+ cell donor), and allogeneic WJ MSCs (MSCs generated from the umbilical cord of another donor) were compared with cotransplantation of allogeneic BM-derived MSCs and transplantation of CD34+ cells alone. Starting from 3 weeks after transplantation until the mice were sacrificed at week 6, we analyzed the PB of the mice for the presence of human platelets (Fig. 3A) and human CD45+ cells at weekly intervals (Fig. 3B). All recipient mice had detectable levels of human platelets in their PB as early as week 3.
FIG. 3.
Cotransplantation of MSCs and CD34+ cells enhances peripheral blood recovery and bone marrow engraftment in NOD SCID mice. (A) Human platelet recovery in the PB of the mice after transplantation. (B) Human CD45+ cell recovery in the PB of the mice after transplantation. (C) Percentage of human CD45+ cells in the BM of the mice 6 weeks after transplantation. (D) Percentage of human CD45+ cells in the spleen of the mice 6 weeks after transplantation. (E) Percentage of human CD45+ cells in the blood of the mice 6 weeks after transplantation. Bars represent the mean of all mice. (F) Lineage differentiation of the human CD45+ cells in the BM 6 weeks after transplantation. The total number of human CD45+ cells in the femurs of all mice was analyzed for the expression of lymphoid markers CD19 and CD3, myeloid marker CD33 and stem/progenitor cell marker CD34 (n = 3 experiments with three different CB donors/MSC isolates). PB, peripheral blood.
Cotransplantation of MSCs from all sources resulted in higher levels of circulating platelets compared to transplantation of CD34+ cells alone (CD34+ cells alone: 57 ± 31 plt/μL PB vs. with BM MSCs: 304 ± 135 plt/μL PB, P = 0.073, with autologous WJ MSCs: 610 ± 244 plt/μL PB, P < 0.05, or with allogeneic MSCs: 556 ± 390 plt/μL PB, P < 0.05). At 6 weeks after transplantation, platelet levels were on an average 5-fold higher in the CD34+ and MSC cotransplanted groups when compared to the platelet levels in recipients of CD34+ cells alone (CD34+ cells alone: 790 ± 216 plt/μL PB vs. BM MSCs and CD34+ cells: 4,146 ± 1,586 plt/μL PB, P < 0.005, autologous WJ MSCs and CD34+ cells: 4,649 ± 1,203 plt/μL PB, P < 0.005, and allogeneic WJ MSCs and CD34+ cells: 5,546 ± 1,654 plt/μL PB, P < 0.05). Similarly, cotransplantation of WJ MSCs significantly increased human CD45+ cells in the PB from week 4 onward as compared to transplantation with CD34+ cells alone (40.6 ± 22.7 × 103 and 45.1 ± 17 × 103 vs. 10.1 ± 5.7 × 103 CD45+ cells/mL for CD34+ cells with autologous and allogeneic WJ MSCs vs. CD34+ cells alone, respectively, P < 0.05). Slower recovery of circulating human leukocytes was observed with cotransplanted BM MSCs as compared to cotransplanted WJ MSCs. Cotransplantation of BM MSCs increased circulating CD45+ cells 5 weeks after transplantation reaching levels similar to cotransplanting WJ MSCs at this time point (69.7 ± 21.9 × 103 vs. 12.6 ± 3.9 × 103 CD45+ cells/mL for CD34+ cells with BM MSCs or for CD34+ transplantation only, P < 0.05).
Six weeks after transplantation, the mice were sacrificed and the BM was analyzed for the presence of human hematopoietic cells. Cotransplantation of MSCs obtained from both WJ and BM increased the frequency of human CD45+ cells in the BM by at least 2-fold as compared to transplantation of CD34+ cells alone (Fig. 3C, CD34+ cells alone: 26.7% ± 5.5% human CD45+ cells vs. CD34+ cells with BM MSCs 61.8% ± 7.1%, P < 0.0005, with autologous WJ MSCs: 67.7% ± 4.8%, P < 0.0001, or with allogeneic WJ MSCs 68.9% ± 3.6%, P < 0.0001). A similar pattern was observed for human CD45+ cell chimerism in spleen and PB.
Cotransplantation of MSCs and CD34+ cells increased chimerism in the spleen by at least 3-fold compared to transplantation of CD34+ cells alone (Fig. 3D, CD34+ cells alone: 7.5% ± 1.5% vs. cotransplantation with BM MSCs: 23.9% ± 4.6%, P < 0.05, with autologous WJ MSCs: 34.1% ± 4.5%, P < 0.005, or with allogeneic WJ MSCs: 32.2% ± 4.3%, P < 0.001) and in the blood at least 5-fold (Fig. 3E, CD34+ cells alone: 4.2% ± 1.0% human CD45+ cells vs. cotransplantation with BM MSCs: 21.7% ± 5.6%, P < 0.05, with autologous WJ MSCs: 24.7% ± 4.5% or with allogeneic WJ MSCs: 26.5% ± 6.6%, P < 0.005). BM cells harvested from the femurs of the mice expressed similar percentages of the common myeloid marker CD33, the lymphoid markers CD19 and CD3 and the stem/progenitor cell marker CD34 irrespective of the cotransplantation of MSCs (Fig. 3F).
In our model, autologous and allogeneic WJ MSCs enhanced total human CD45+ cell reconstitution to a similar extent.
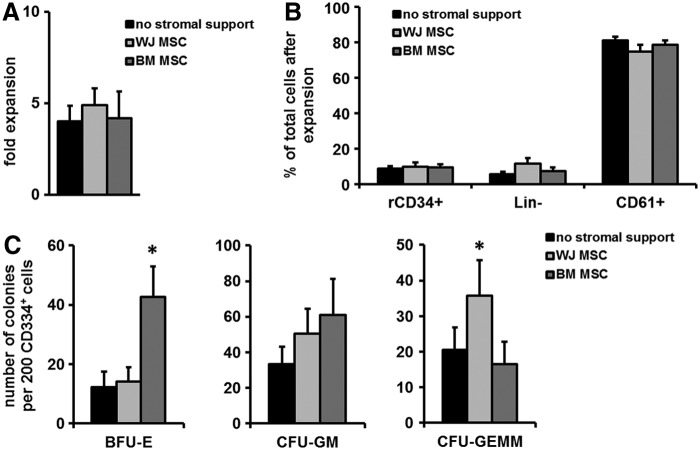
Coculture of WJ MSCs and CB CD34+ cells with TPO enhances CFU-GEMM formation, while BM MSCs enhances BFU-E formation
Several functional characteristics of MSCs might play a role in their observed ability to facilitate engraftment. MSCs have been shown previously to support the growth of human CB CD34+ cells [33–37]. Furthermore, we have shown that transplantation of TPO-expanded CB CD34+ enhanced early platelet repopulation while retaining long-term hematopoietic engraftment capacity in NOD SCID mice [31,32]. Combining these two potential engraftment-enhancing strategies, we compared the capacity of human WJ MSCs and BM MSCs to support differentiation and expansion of human CB-derived CD34+ cells in the presence of exogenous TPO. To this end, CD34+ cells were cultured for 10 days on monolayers of MSCs obtained from different sources in the presence of exogenous TPO and analyzed for expansion of total MNC and CFU formation of the cultured CD34+ cells.
Cultures of CD34+ cells on either BM MSCs or WJ MSCs monolayers did not enhance the TPO-induced expansion of total nucleated cells (Fig. 4A, WJ MSCs 4.8 ± 0.9-fold expansion and BM MSCs 4.1 ± 1.5-fold expansion vs. no stromal support 4.0 ± 0.8-fold expansion) over this time period. Additionally, the ratio between the three main cell subpopulations that are typically formed when CB CD34+ are cultured with TPO, namely residual CD34+ cells (rCD34+), CD34−CD61− cells (Lin−), and CD34−61+ cells (CD61+) [38], were similar between cultures without stromal support and those on MSC monolayers from different sources (Fig. 4B).
FIG. 4.
Coculture of BM or WJ MSCs with CB CD34+ cells supports the expansion of CB CD34+ cells in the presence of TPO. CB CD34+ cells obtained from different donors (n = 5) were cultured with TPO for 10 days in the presence or absence of MSCs obtained from different sources. Next, the composition of the expanded cells was analyzed using flow cytometry and the capacity to form myeloid colonies was analyzed with CFU assays. (A) Fold expansion (depicted as the total number of hematopoietic nucleated cells after culture divided by the number of input cells) in the absence or presence of stromal cells. (B) Percentage of the three major populations of the total hematopoietic cells observed after expansion in the absence or presence of stromal support from MSCs from different sources; rCD34+ = residual CD34 cells, Lin− = CD34−CD61−Lineage− cells, and CD61+ = CD34−CD61+ cells. (C) Colony-forming capacity of TPO-expanded CD34+ cells after culture in the absence or presence of stromal support (*P < 0.05).
To investigate the differentiation potential of the remaining HSPCs in the TPO-induced cultures, we next analyzed their colony-forming capacity in CFU assays. To this end, HSPC were first separated from the MSCs after coculture and subsequently cultured in semisolid cultures in the presence of cytokines. Interestingly, HSPC derived from cultures on BM MSC monolayers exhibited an increased capacity to form BFU-E colonies (Fig. 4C, BM MSCs 42.8 ± 10.2 BFU-E/1,000 rCD34+ cells compared to no MSCs 14.5 ± 6.1 BFU-E/1,000 rCD34+ cells, P < 0.05). HSPC cultured in the presence of WJ MSCs gave rise to higher numbers of CFU-GEMM (35.8 ± 9.9 colonies/1,000 CD34+ cells vs. 20.6 ± 6.3/1,000 CD34+ without stromal support; Fig. 4C, right panel, n = 5 experiments, P < 0.05).
Incubation of CB-derived CD34+ cells with MSCs does not significantly alter their migration toward CXCL12, but increases the expression of adhesion markers
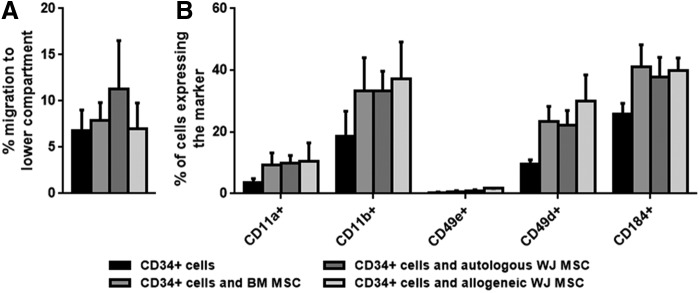
Hematopoietic stem cell homing to the marrow is the primary step for their engraftment and relies on their adhesive and migratory capacities. We investigated whether MSCs change the migratory characteristics of CB CD34+ cells toward CXCL12 in transwell migration studies. To this end, CB CD34+ cells and MSCs were incubated together for 30 min to mimic the time that CD34+ cells and MSCs are in the same tube before infusion. Aliquots of the cell suspensions were placed in transwell plates while others were analyzed in parallel for adhesion marker expression. Additionally, the effect of the presence of either autologous or allogeneic WJ MSCs on the migration of CD34+ cells was tested (Fig. 5A).
FIG. 5.
In vitro homing characteristics of and adhesion molecule expression of CD34+ in the presence of MSCs obtained from different sources. (A) Migration of CD34+ cells toward stromal cell-derived factor-1α in the presence of MSCs obtained from WJ or BM. (B) Expression of adhesion markers and CXCR4 on CB-derived CD34+ cells after incubation with WJ MSCs or BM MSCs.
Autologous WJ MSCs tended to increase CD34+ migration when compared to the migration of CD34+ cells alone; this difference however, was not significant (11.3% ± 5.2% vs. 6.8% ± 2.2% for CD34+ cell migration with or without autologous WJ MSCs).
Next, we investigated the expression of several adhesion markers known to be involved in homing to the BM [39]. In this respect, we analyzed the expression of CD11a, CD11b, CD49d, CD49e, and the CXCL12 receptor CD184 (CXCR4) on CD34+ cells after 30 min incubation with MSCs (Fig. 5B). Regardless of the MSC source, incubation of CB CD34+ cells with MSCs seemed to induce a general increase in the marker expression. However, even between MSCs from the same source, expression levels varied considerably. Therefore, only CD49d and CD49e expression was significantly increased after incubation with BM MSCs and allogeneic WJ MSCs, respectively (P < 0.05).
Discussion
In the present study, we compared the effect of human MSCs obtained from WJ and BM on the engraftment of CB-derived CD34+ cells in an immune-deficient murine transplant model. The recovery of human platelets and CD45+ cells in the PB of these mice was significantly enhanced by cotranspantation of either WJ or BM MSCs from 3 weeks onward. At 6 weeks posttransplantation, the percentage of human CD45+ cells in the BM, spleen, and PB was at least 3-fold higher when MSCs were cotransplanted with CD34+ cells compared to transplantation of CD34+ cells alone. MSCs obtained from BM and WJ were comparable in their capacity to enhance the engraftment of CB-derived CD34+ cells.
Although the mechanism is so far not determined, MSCs induced CD34+ cell engraftment has been suggested to be associated with their immune-modulatory capacity. MSCs from different tissues, including WJ are known to modulate immunological responses [9,40]. At high levels of interferon-γ (IFN-γ) and tumor necrosis factor-α (TNF-α), MSCs have an anti-inflammatory effect for which secreted indoleamine 2,3-dioxygenase is one of the proposed mediators [41,42]. Conversely, in steady-state conditions at low levels of IFN-γ and TNF-α, allogeneic MSCs are able to stimulate an immunological response [9]. The absence of inflammatory signals directly after transplantation could, therefore, not only diminish the immunosuppressive effect of the cotransplanted MSCs, but may also have an immune-activating effect.
The immunosuppressive properties of MSCs, however, are most likely to mediate a beneficial effect on the outcome of hematopoietic cell transplantation, for example, by exhibiting a prophylactic effect on the occurrence or severity of Graft versus Host Disease or host-mediated graft rejection [43,44]. In agreement, a study in which MNCs obtained from two CB units were transplanted demonstrated that either removal of the immune-competent cells from the graft or MSC cotransplantation alleviated single CB donor dominance and improved the overall engraftment [10].
In our in vitro experiments, MSCs from the BM and WJ were equally potent in inhibiting the proliferation of αCD3αCD28-stimulated PBMC. However, as we use immunodeficient NOD SCID mice for our in vivo transplantation experiments and transplants without any immune-competent cells, these immune-modulating qualities do not seem to be instrumental for the similar engraftment enhancing effect of both MSC sources since. Alloimmunization between the recipient and the transplanted cells or between the CD34+ cell-purified HSC graft and the cotransplanted allogeneic MSCs are, therefore, not likely to occur. In agreement, no difference in the engraftment-enhancing capacity of autologous and allogeneic WJ MSCs was found. Since immune-related components cannot be assessed in our model, the marked increase of engraftment must, therefore, be caused by other MSC-derived factors.
Alternatively, MSCs may play a direct role in regenerating the BM niche, first by homing to the marrow and differentiating into stromal tissues and second by inducing the proliferation and differentiation of HSC. Concerning the first option, our studies show that the differentiation capacity of WJ MSCs is variable and limited and thus unlikely to be the cause for their engraftment-stimulating effect. Corroborated by a study from Bosch et al. [45], this also makes WJ MSCs less interesting candidates for therapeutic bone or cartilage regeneration.
Concerning the second option, WJ MSCs have been shown to support the growth of CB-derived CD34+ cells ex vivo [33–37]. As we previously showed that TPO-expanded CB CD34+ cells contributed to both improved platelet recovery and BM engraftment [31], we combined these two different mechanisms in an in vitro experiment and investigated whether MSCs could further enhance TPO-induced effects on CD34+ cultures. In this regard, additional presence of MSCs did not change TPO-induced expansion and neither was the composition of the formed subpopulations changed. CFU cultures of the expanded cells did, however, show that the MSCs have a different effect on the types of cells that are formed after expansion.
Cultures of HSPC on BM MSC monolayers exhibited an increased capacity to form BFU-E colonies while HSPC cultured in the presence of WJ MSCs gave rise to higher numbers of CFU-GEMM. These observations might be of conceptual importance since CFU-GEMM is correlated with the presence of more primitive stem cells [46]. Hence, WJ MSCs, in this respect, might have a better potential to preserve the more immature CD34+ cells in culture with TPO than BM MSCs.
Other studies have shown that the coculture of MSCs, including WJ MSCs, can enhance the fold expansion of both total nucleated cells and CD34+ cells [35–37]. These studies used a cocktail of cytokines, including SCF, Flt3L, and TPO, in their culture protocol. Adding SCF and Flt3L to the expansion medium can significantly enhance the fold expansion of the cells [47], and the absence of these cytokines could, therefore, explain the lack of TNC and CD34+ cell expansion in our experiments.
However, for both the MSCs induced marrow niche regenerating mechanisms to become relevant, homing of MSCs with the HSPCs to the BM is necessary. This has so far not been convincingly shown. [11,48,49]. Although colocalization of MSCs and CD34+ cells in the pelvis has been reported [49], MSCs are more often detected in various organs, but not in the BM [11] and entrapment of MSCs in the lungs has been described as a possible explanation for the lack of homing of the MSCs [49].
MSC-induced homing of CD34+ cells to the HSC niche might be another explanation for increased engraftment by local (eg, by cell to cell contact in the marrow) or systemic (eg, by paracrine factors production) support. Our in vitro studies, with brief exposure of CD34+ cells to MSCs, could not consistently show enhanced migration of CD34+ cells toward CXCL12. However, in the presence of MSCs, CD34+ cells upregulate surface markers that are associated with their homing to or retention in the BM marrow (eg, CD11a, CD11b, CD184, CD49e, and CD49d) [39,50,51].
In conclusion, our data support the use of human WJ MSCs as an alternative source to enhance the engraftment of human CB-derived CD34+ cells. Although MSC-induced homing of HPSC to the BM seems an interesting explanation from our studies, for a real life estimate of MSCs role in engraftment support, other mechanisms also need to be considered. Engraftment in this regard is likely dependent on (interacting) factors like the immune status of the recipient and hematopoietic immune (activating) cells in the transplants. However, the immune-deficient recipient mice and the CD34+ isolated stem cell transplant that were used in our studies, makes MSC-induced immunomodulation an unlikely explanation for the observed enhanced engraftment in vivo.
Our in vitro experiments, however, do suggest that WJ MSCs may serve as an alternative source to BM MSCs for immunomodulatory applications. A general advantage of WJ MSCs is that the umbilical cord can be regarded as a waste product, and that WJ MSCs can, therefore, be obtained at a relatively low cost without harm or risk for the donor.
Since cotransplantation of allogeneic MSCs did not show particular disadvantages [52–54], and while cotransplantation of autologous MSCs with donor hematopoietic stem cells might even inhibit engraftment [55], our study does not support the paired use and banking of CB CD34+ cells and WJ MSCs of the same umbilical cord. However, concurrent collection of the umbilical cord with CB collection enables sharing the logistics, tissue typing, and virological testing for two products from only one donor with additional saving of costs and effort to obtain MSCs from BM. This new CB cord strategy would, therefore, create a relatively cheap, off-the-shelf MSC product that can be provided by tissue banks to hospitals. The value of such an approach, however, will eventually depend on the therapeutic efficacy of MSCs and more specifically WJ MSCs.
Supplementary Material
Acknowledgments
The authors would like to thank the staff of the experimental animal facility of the Leiden University Medical Center for technical assistance. This work was funded by grant no. PPOC 08-009 of the Sanquin Blood Supply Foundation, the Netherlands and by a grant from the Dutch Government to the Netherlands Institute for Regenerative Medicine (NIRM, grant no. FES0908). SMW and MvdG received grant support from the National Institute of Health Research under its program grants scheme (RP-PG-0310-1003) and NHS Blood and Transplant.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Ballen KK, Gluckman E. and Broxmeyer HE. (2013). Umbilical cord blood transplantation: the first 25 years and beyond. Blood 122:491–498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ballen KK, Koreth J, Chen YB, Dey BR. and Spitzer TR. (2012). Selection of optimal alternative graft source: mismatched unrelated donor, umbilical cord blood, or haploidentical transplant. Blood 119:1972–1980 [DOI] [PubMed] [Google Scholar]
- 3.Fuchs E, O'Donnell PV. and Brunstein CG. (2013). Alternative transplant donor sources: is there any consensus?. Curr Opin Oncol 25:173–179 [DOI] [PubMed] [Google Scholar]
- 4.Eapen M, Rocha V, Sanz G, Scaradavou A, Zhang M-J, Arcese W, Sirvent A, Champlin RE, Chao N. and Gee AP. (2010). Effect of graft source on unrelated donor haemopoietic stem-cell transplantation in adults with acute leukaemia: a retrospective analysis. Lancet Oncol 11:653–660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brunstein CG, Gutman JA, Weisdorf DJ, Woolfrey AE, DeFor TE, Gooley TA, Verneris MR, Appelbaum FR, Wagner JE. and Delaney C. (2010). Allogeneic hematopoietic cell transplantation for hematologic malignancy: relative risks and benefits of double umbilical cord blood. Blood 116:4693–4699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Danby R. and Rocha V. (2014). Current strategies to improve engraftment in cord blood transplantation. J Stem Cell Res Ther 4:172 [Google Scholar]
- 7.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S. and Marshak DR. (1999). Multilineage potential of adult human mesenchymal stem cells. Science 284:143–147 [DOI] [PubMed] [Google Scholar]
- 8.Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop D. and Horwitz E. (2006). Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 8:315–317 [DOI] [PubMed] [Google Scholar]
- 9.Bernardo ME. and Fibbe WE. (2013). Mesenchymal stromal cells: sensors and switchers of inflammation. Cell Stem Cell 13:392–402 [DOI] [PubMed] [Google Scholar]
- 10.Kim D-W, Chung Y-J, Kim T-G, Kim Y-L. and Oh I-H. (2004). Cotransplantation of third-party mesenchymal stromal cells can alleviate single-donor predominance and increase engraftment from double cord transplantation. Blood 103:1941–1948 [DOI] [PubMed] [Google Scholar]
- 11.Noort WA, Kruisselbrink AB, Kruger M, van Bezooijen RL, de Paus RA, Heemskerk MH, Löwik CW, Falkenburg J, Willemze R. and Fibbe WE. (2002). Mesenchymal stem cells promote engraftment of human umbilical cord blood–derived CD34+ cells in NOD/SCID mice. Exp Hematol 30:870–878 [DOI] [PubMed] [Google Scholar]
- 12.Watt SM, Gullo F, van der Garde M, Markeson D, Camicia R, Khoo CP. and Zwaginga JJ. (2013). The angiogenic properties of mesenchymal stem/stromal cells and their therapeutic potential. Br Med Bull 108:25–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bonnet D, Bhatia M, Wang J, Kapp U. and Dick J. (1999). Cytokine treatment or accessory cells are required to initiate engraftment of purified primitive human hematopoietic cells transplanted at limiting doses into NOD/SCID mice. Bone Marrow Transplant 23:203–209 [DOI] [PubMed] [Google Scholar]
- 14.Caplan AI. (1991). Mesenchymal stem cells. J Orthop Res 9:641–650 [DOI] [PubMed] [Google Scholar]
- 15.Friedenstein AJ, Gorskaja J. and Kulagina N. (1976). Fibroblast precursors in normal and irradiated mouse hematopoietic organs. Exp Hematol 4:267–274 [PubMed] [Google Scholar]
- 16.Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, Alfonso ZC, Fraser JK, Benhaim P. and Hedrick MH. (2002). Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell 13:4279–4295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guo Z, Li H, Li X, Yu X, Wang H, Tang P. and Mao N. (2006). In vitro characteristics and in vivo immunosuppressive activity of compact bone‐derived murine mesenchymal progenitor cells. Stem Cells 24:992–1000 [DOI] [PubMed] [Google Scholar]
- 18.Scherjon SA, Kleijburg-van der Keur C, Noort WA, Claas FH, Willemze R, Fibbe WE. and Kanhai HH. (2003). Amniotic fluid as a novel source of mesenchymal stem cells for therapeutic transplantation. Blood 102:1548–1549 [DOI] [PubMed] [Google Scholar]
- 19.Erices A, Conget P. and Minguell JJ. (2000). Mesenchymal progenitor cells in human umbilical cord blood. Br J Haematol 109:235–242 [DOI] [PubMed] [Google Scholar]
- 20.Sarugaser R, Lickorish D, Baksh D, Hosseini MM. and Davies JE. (2005). Human umbilical cord perivascular (HUCPV) cells: a source of mesenchymal progenitors. Stem Cells 23:220–229 [DOI] [PubMed] [Google Scholar]
- 21.Seshareddy K, Troyer D. and Weiss ML. (2008). Method to isolate mesenchymal‐like cells from Wharton's Jelly of umbilical cord. Methods Cell Biol 86:101–119 [DOI] [PubMed] [Google Scholar]
- 22.Brooke G, Rossetti T, Pelekanos R, Ilic N, Murray P, Hancock S, Antonenas V, Huang G, Gottlieb D. and Bradstock K. (2009). Manufacturing of human placenta‐derived mesenchymal stem cells for clinical trials. Br J Haematol 144:571–579 [DOI] [PubMed] [Google Scholar]
- 23.Fong C-Y, Richards M, Manasi N, Biswas A. and Bongso A. (2007). Comparative growth behaviour and characterization of stem cells from human Wharton's jelly. Reprod Biomed Online 15:708–718 [DOI] [PubMed] [Google Scholar]
- 24.Troyer DL. and Weiss ML. (2008). Concise review: Wharton's Jelly‐derived cells are a primitive stromal cell population. Stem cells 26:591–599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bongso A. and Fong C-Y. (2013). The therapeutic potential, challenges and future clinical directions of stem cells from the Wharton's jelly of the human umbilical cord. Stem Cell Rev 9:226–240 [DOI] [PubMed] [Google Scholar]
- 26.El Omar R, Beroud J, Stoltz J-F, Menu P, Velot E. and Decot V. (2014). Umbilical cord mesenchymal stem cells: the new gold standard for mesenchymal stem cell-based therapies?. Tissue Eng Part B Rev 20:523–544 [DOI] [PubMed] [Google Scholar]
- 27.Sutherland DR, Anderson L, Keeney M, Nayar R. and Chin-Yee I. (1996). The ISHAGE guidelines for CD34+ cell determination by flow cytometry. J Hematother 5:213–226 [DOI] [PubMed] [Google Scholar]
- 28.De Bruyn C, Najar M, Raicevic G, Meuleman N, Pieters K, Stamatopoulos B, Delforge A, Bron D. and Lagneaux L. (2010). A rapid, simple, and reproducible method for the isolation of mesenchymal stromal cells from Wharton's jelly without enzymatic treatment. Stem Cells Dev 20:547–557 [DOI] [PubMed] [Google Scholar]
- 29.Duijvestein M, Wildenberg ME, Welling MM, Hennink S, Molendijk I, van Zuylen VL, Bosse T, Vos ACW, de Jonge‐Muller ES. and Roelofs H. (2011). Pretreatment with interferon‐γ enhances the therapeutic activity of mesenchymal stromal cells in animal models of colitis. Stem Cells 29:1549–1558 [DOI] [PubMed] [Google Scholar]
- 30.Perez-Galarza J, Carlotti F, Rabelink MJ, Cramer S, Hoeben RC, Fibbe WE. and van Pel M. (2014). Optimizing reporter constructs for in vivo bioluminescence imaging of IFN-γ stimulated mesenchymal stromal cells. Exp Hematol 42:793–803 [DOI] [PubMed] [Google Scholar]
- 31.van der Garde M, van Hensbergen Y, Brand A, Slot MC, de Graaf-Dijkstra A, Mulder A, Watt SM. and Zwaginga JJ. (2015). Thrombopoietin treatment of one graft in a double cord blood transplant provides early platelet recovery while contributing to long-term engraftment in NSG mice. Stem Cells Dev 24:67–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van Hensbergen Y, Schipper LF, Brand A, Slot MC, Welling M, Nauta AJ. and Fibbe WE. (2006). Ex vivo culture of human CD34+ cord blood cells with thrombopoietin (TPO) accelerates platelet engraftment in a NOD/SCID mouse model. Exp Hematol 34:943–950 [DOI] [PubMed] [Google Scholar]
- 33.Bakhshi T, Zabriskie RC, Bodie S, Kidd S, Ramin S, Paganessi LA, Gregory SA, Fung HC. and Christopherson KW. (2008). Mesenchymal stem cells from the Wharton's jelly of umbilical cord segments provide stromal support for the maintenance of cord blood hematopoietic stem cells during long‐term ex vivo culture. Transfusion 48:2638–2644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Magin A, Körfer N, Partenheimer H, Lange C, Zander A. and Noll T. (2009). Primary cells as feeder cells for coculture expansion of human hematopoietic stem cells from umbilical cord blood-a comparative study. Stem Cells Dev 18:173–186 [DOI] [PubMed] [Google Scholar]
- 35.Zhang Y, Chai C, Jiang X-S, Teoh S-H. and Leong KW. (2006). Co-culture of umbilical cord blood CD34+ cells with human mesenchymal stem cells. Tissue Eng 12:2161–2170 [DOI] [PubMed] [Google Scholar]
- 36.Fong C, Gauthaman K, Cheyyatraivendran S, Lin H, Biswas A. and Bongso A. (2012). Human umbilical cord Wharton's jelly stem cells and its conditioned medium support hematopoietic stem cell expansion ex vivo. J Cell Biochem 113:658–668 [DOI] [PubMed] [Google Scholar]
- 37.Alakel N, Jing D, Muller K, Bornhauser M, Ehninger G. and Ordemann R. (2009). Direct contact with mesenchymal stromal cells affects migratory behavior and gene expression profile of CD133+ hematopoietic stem cells during ex vivo expansion. Exp Hematol 37:504–513 [DOI] [PubMed] [Google Scholar]
- 38.Schipper LF, Brand A, Reniers N, Melief CJ, Willemze R. and Fibbe WE. (2003). Differential maturation of megakaryocyte progenitor cells from cord blood and mobilized peripheral blood. Exp Hematol 31:324–330 [DOI] [PubMed] [Google Scholar]
- 39.Voermans C, Rood P, Hordijk P, Gerritsen W. and Van Der Schoot C. (2000). Adhesion molecules involved in transendothelial migration of human hematopoietic progenitor cells. Stem Cells 18:435–443 [DOI] [PubMed] [Google Scholar]
- 40.Weiss ML, Anderson C, Medicetty S, Seshareddy KB, Weiss RJ, VanderWerff I, Troyer D. and McIntosh KR. (2008). Immune properties of human umbilical cord Wharton's jelly‐derived cells. Stem Cells 26:2865–2874 [DOI] [PubMed] [Google Scholar]
- 41.Valencic E, Piscianz E, Andolina M, Ventura A. and Tommasini A. (2010). The immunosuppressive effect of Wharton's jelly stromal cells depends on the timing of their licensing and on lymphocyte activation. Cytotherapy 12:154–160 [DOI] [PubMed] [Google Scholar]
- 42.Tipnis S, Viswanathan C. and Majumdar AS. (2010). Immunosuppressive properties of human umbilical cord-derived mesenchymal stem cells: role of B7-H1 and IDO. Immunol Cell Biol 88:795–806 [DOI] [PubMed] [Google Scholar]
- 43.Baron F, Lechanteur C, Willems E, Bruck F, Baudoux E, Seidel L, Vanbellinghen J-F, Hafraoui K, Lejeune M. and Gothot A. (2010). Cotransplantation of mesenchymal stem cells might prevent death from graft-versus-host disease (GVHD) without abrogating graft-versus-tumor effects after HLA-mismatched allogeneic transplantation following nonmyeloablative conditioning. Biol Blood Marrow Transplant 16:838–847 [DOI] [PubMed] [Google Scholar]
- 44.Kuzmina LA, Petinati NA, Parovichnikova EN, Lubimova LS, Gribanova EO, Gaponova TV, Shipounova IN, Zhironkina OA, Bigildeev AE. and Svinareva DA. (2011). Multipotent mesenchymal stromal cells for the prophylaxis of acute graft-versus-host disease—a phase II study. Stem Cells Int 2012:968213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bosch J, Houben AP, Radke TF, Stapelkamp D, Bünemann E, Balan P, Buchheiser A, Liedtke S. and Kögler G. (2011). Distinct differentiation potential of “MSC”. derived from cord blood and umbilical cord: are cord-derived cells true mesenchymal stromal cells?. Stem Cells Dev 21:1977–1988 [DOI] [PubMed] [Google Scholar]
- 46.Broxmeyer HE, Douglas GW, Hangoc G, Cooper S, Bard J, English D, Arny M, Thomas L. and Boyse EA. (1989). Human umbilical cord blood as a potential source of transplantable hematopoietic stem/progenitor cells. Proc Natl Acad Sci 86:3828–3832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gullo F, van der Garde M, Russo G, Pennisi M, Motta S, Pappalardo F. and Watt S. (2015). Computational modeling of the expansion of human cord blood CD133+ hematopoietic stem/progenitor cells with different cytokine combinations. Bioinformatics 31:2514–2522 [DOI] [PubMed] [Google Scholar]
- 48.Hiwase SD, Dyson PG, To LB. and Lewis ID. (2009). Cotransplantation of placental mesenchymal stromal cells enhances single and double cord blood engraftment in nonobese diabetic/severe combined immune deficient mice. Stem Cells 27:2293–2300 [DOI] [PubMed] [Google Scholar]
- 49.Schrepfer S, Deuse T, Reichenspurner H, Fischbein M, Robbins R. and Pelletier M. (2007). Stem cell transplantation: the lung barrier. Transplant Proc 39:573–576 [DOI] [PubMed] [Google Scholar]
- 50.Coombe DR, Watt S. and Parish C. (1994). Mac-1 (CD11b/CD18) and CD45 mediate the adhesion of hematopoietic progenitor cells to stromal cell elements via recognition of stromal heparan sulfate. Blood 84:739–752 [PubMed] [Google Scholar]
- 51.Lai CY, Yamazaki S, Okabe M, Suzuki S, Maeyama Y, Iimura Y, Onodera M, Kakuta S, Iwakura Y. and Nojima M. (2014). Stage‐specific roles for CXCR4 signaling in murine hematopoietic stem/progenitor cells in the process of bone marrow repopulation. Stem Cells 32:1929–1942 [DOI] [PubMed] [Google Scholar]
- 52.Bernardo M, Ball L, Cometa A, Roelofs H, Zecca M, Avanzini M, Bertaina A, Vinti L, Lankester A. and Maccario R. (2011). Co-infusion of ex vivo-expanded, parental MSCs prevents life-threatening acute GVHD, but does not reduce the risk of graft failure in pediatric patients undergoing allogeneic umbilical cord blood transplantation. Bone Marrow Transplant 46:200–207 [DOI] [PubMed] [Google Scholar]
- 53.Le Blanc K, Frassoni F, Ball L, Locatelli F, Roelofs H, Lewis I, Lanino E, Sundberg B, Bernardo ME. and Remberger M. (2008). Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet 371:1579–1586 [DOI] [PubMed] [Google Scholar]
- 54.Macmillan M, Blazar B, DeFor T. and Wagner J. (2008). Transplantation of ex-vivo culture-expanded parental haploidentical mesenchymal stem cells to promote engraftment in pediatric recipients of unrelated donor umbilical cord blood: results of a phase I–II clinical trial. Bone Marrow Transplant 43:447–454 [DOI] [PubMed] [Google Scholar]
- 55.Nauta AJ, Westerhuis G, Kruisselbrink AB, Lurvink EG, Willemze R. and Fibbe WE. (2006). Donor-derived mesenchymal stem cells are immunogenic in an allogeneic host and stimulate donor graft rejection in a nonmyeloablative setting. Blood 108:2114–2120 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.