Abstract
Recently, the “glymphatic system” of the brain has been discovered in rodents, which is a paravascular, transparenchymal route for clearance of excess brain metabolites and distribution of compounds in the cerebrospinal fluid. It has already been demonstrated that intrathecally administered gadolinium (Gd) contrast medium distributes along this route in rats, but so far not in humans. A 27-year-old woman underwent magnetic resonance imaging (MRI) with intrathecal administration of gadobutrol, which distributed throughout her entire brain after 1 and 4.5 h. MRI with intrathecal Gd may become a tool to study glymphatic function in the human brain.
Keywords: Magnetic resonance imaging (MRI), intrathecal, contrast medium, brain, paravascular transport, glymphatic circulation
Introduction
Recently, the glympahtic (glia-lymphatic) system was discovered for the first time in mice, which is a brain-wide paravascular pathway for exchange of solutes and clearance of waste metabolites from the brain, including amyloid-β (1). Glymphatic function has also been shown to decrease dramatically with aging (2) and in the injured brain (3).
Depending on the molecular size of tracers injected to the cisterna magna of mice, distribution of small-sized molecular tracers with molecular weight (MW) <800 Daltons (Da) was through the entire brain. Gadolinium (Gd) contrast medium may function as such small tracers, as it has been demonstrated in rats that administration of Gd-GTPA (MW 938 Da) (Magnevist; Bayer HealthCare Pharmaceuticals, New York, NY, USA) into the cisterna magna distributed throughout the entire rat brain (4). It has so far not been established how these observations in rodents relate to human brain physiology, pharmacokinetics, or disease, and such studies are now awaited (5).
In humans, magnetic resonance imaging (MRI) with intrathecal Gd administration is already in use to diagnose the site of cerebrospinal fluid (CSF) leakage in patients with intracranial hypotension and suspected spontaneous CSF leakage (6,7). One of our patients underwent routine magnetic resonance imaging (MRI) following intrathecal gadobutrol (MW 550 Da) administration (Gadovist; Bayer Healthcare, Leverkusen, Germany) due to CSF leakage, and repeated three-dimensional (3D) T1-weighted (T1W) imaging was performed before and at two time-points after intrathecal gadobutrol. The imaging findings support the existence of a glymphatic system also in humans, and add to data previously published in animals.
Case report
Patient history
Written and informed consent was obtained from the patient for case report presentation of data and MRI scans.
The current patient was a 27-year-old previously healthy woman, who was referred to the Department of Neurosurgery, Oslo University Hospital – Rikshospitalet, for suspected intracranial hypotension due to spontaneous CSF leakage. She had a history of headache for more than 2 years, followed by spontaneous CSF leakage to the middle ear, mastoid cells, and throat. An attempt to close the leakage via a trans-mastoid approach was not successful. Later on, she developed bacterial meningitis and need for continued antibiotic treatment. She was referred to an MRI examination with intrathecal gadobutrol to define site of leakage, and subsequent surgical management. Following MRI, we were able to localize the site of leakage, visualized as a pocket of extradural contrast medium accumulation at the apex of the petrosal bone. During the same hospitalization, a subtemporal craniotomy was done, the petrosal bone was dissected free extradurally, the site of CSF leakage was identified and closed using dura substitute and fibrin sealant (Tisseel glue, Baxter AG, Vienna, Austria). Since surgery and during the follow-up time of 6 months, the patient had no evidence of further CSF leakage.
Contrast medium-enhanced MRI
We obtained 3D T1space MR images with fat saturation (repetition time/echo time, 500/15 ms), using the same scanner (1.5 T Siemens Avanto®, Erlangen, Germany), before and 1 and 4.5 h after intrathecal contrast medium. The images were reformatted in a standardized axial plane and to the smallest possible slice thickness (1 mm) to minimize partial averaging effects.
Injection of gadobutrol 0.7 mL 1 mg/mL (Gadovist®) to the intrathecal space was performed with lumbar puncture at L4/5 level preceded by injection of 1 mL iomeprol 300 mg I/mL (Iomeron 300®, Bracco Imaging, Milan, Italy) to verify correct placement of the needle. She had no apparent side effects from the procedure.
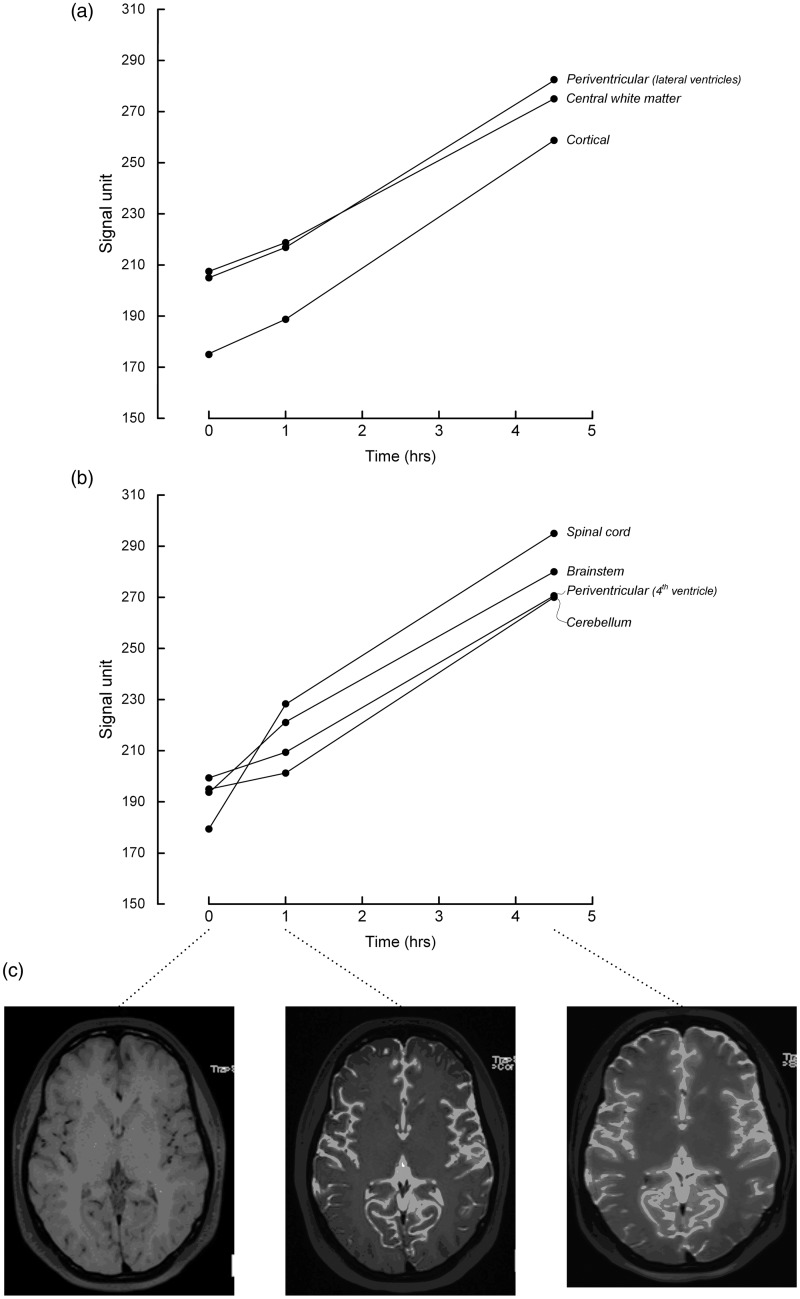
Contrast medium distribution was quantified by measuring signal units (SU) from standardized areas of the brain parenchyma defined by regions of interests (ROIs) applied on the T1 images directly in the picture archiving and communication system (PACS) (Sectra, Linköping, Sweden). The area and placement of ROIs were identical within the same brain segment at different time points, and care was taken not to include CSF spaces at the surface of the brain to avoid partial averaging effects. The numbers of ROIs are presented in Table 1. The supratentorial ROIs were placed subcortically, periventricularly, and in central white matter, all in level with the lateral ventricles. Infratentorial ROIs were placed in the cerebellar hemispheres, around the fourth ventricle, and in the medulla oblongata. The size of a ROI was typically 18 mm2.
Table 1.
Change in signal units on T1 MRI after Gadovist® depending on side.
| Before contrast |
Time after contrast (h) |
||
|---|---|---|---|
| 1 | 4 | ||
| SU | SU | SU | |
| Supratentorial | |||
| Subcortical (n = 19) | |||
| Left | 175 ± 9 | 190 ± 12 | 264 ± 30 |
| Right | 176 ± 9 | 185 ± 15 | 251 ± 33 |
| Periventricular (n = 10) | |||
| Left | 205 ± 6 | 216 ± 10 | 286 ± 17 |
| Right | 204 ± 6 | 217 ± 11 | 280 ± 9 |
| Deep white matter (n = 4) | |||
| Left | 207 ± 6 | 216 ± 13 | 273 ± 9 |
| Right | 210 ± 4 | 221 ± 5 | 277 ± 11 |
| Infratentorial | |||
| Brainstem (n = 2) | 194 ± 1 | 223 ± 5 | 280 ± 18 |
| Periventricular (n = 2) | |||
| Left | 200 | 211 | 270 |
| Right | 197 | 207 | 273 |
| Cerebellum (n = 2) | |||
| Left | 192 | 198 | 273 |
| Right | 197 | 207 | 266 |
| Spinal cord (n = 1) | 179 | 228 | 294 |
| Reference | |||
| Air (n = 2) | 1 ± 1 | 2 ± 1 | 1 ± 1 |
n = number of ROIs; SU, signal unit.
Following intrathecal gadobutrol, SU increased in all regions and with similar magnitude on the left and right side (Table 1). Supratentorial SU increased more in periventricular and central white matter than subcortically (Fig. 1). Subcortically, the change in SU was higher in the parietal than frontal and occipital regions after 4.5 h (P < 0.05, Friedman test). Infratentorially, we found higher SU in the spinal cord and brainstem than in the cerebellum and periventricular matter (fourth ventricle).
Fig. 1.
Signal units (SU) measured from 3D T1W MRI before, and then 1 and 4.5 h after intrathecal administration of gadobutrol (Gadovist®), showing SU change in (a) supratentorial regions of subcortical, central white, and periventricular matter, and in (b) infratentorial regions of cerebellar and periventricular (fourth ventricle) matter, and in the brainstem and (upper cervical) spinal cord. In (c) the T1 images obtained after 1 and 4.5 h show that the SU increases in the brain can be directly acknowledged even from pure visual inspection of the images.
Discussion
The present observations of brain-wide distribution of intrathecal gadobutrol suggest free transport of the contrast medium along with CSF and CSF-interstitial fluid (ISF) exchange throughout the entire brain, and shed new light on the pharmacokinetic distribution of intrathecal administered MRI contrast medium.
In this patient, the indication for contrast-enhanced MRI was diagnostic, namely to reveal site of CSF leakage. The diagnostic role of intrathecal contrast medium-enhanced MRI in CSF leakage in spontaneous intracranial hypotension is well documented (6,7), although presently not approved for intrathecal use. With dose levels as used in this present patient, the risk is low (6,7).
In this patient, comparisons of MRI scans were valid as we used the same scanner, unaltered SU in air were measured, being indicative of SU baseline being unaltered, and sequences were done with identical and minimal slice thickness (1 mm) to avoid partial volume effects. Further, identical ROIs were used from all scans.
Gadobutrol has a small molecular size with a MW of 550 Da (8). In comparison, radioactive Alexa Fluor 594 hydrazide (A594; MW 759 Da) administered to the cisterna magna of mice, distributed quickly throughout the brain interstitium, with small amounts within the paravascular spaces (1). Moreover, the contrast medium Gd-DTPA (Magnevist, MW 938 Da) also distributed throughout the rat brain (4). Thus, small-sized molecules seem to pass freely from the subarachnoid space into the brain interstitium, either through transpial and/or transependymal routes. For large-sized molecules, their passage have been shown to be restricted to the paravascular space, but not entering the ISF (1). The MRI method applied does not differentiate between contrast medium within paravascular spaces and the interstitial fluid, as the molecular size of gadobutrol is expectedly small enough to freely pass between those two compartments. Freely moving water is considered to be a requirement for properly functioning paravascular transport of larger molecules, e.g. soluble amyloid-β, with a molecular size about 4 kDa (9).
While the present patient suffered intracranial hypotension due to CSF leakage, she had no brain disease or CSF absorption disorder. Hence, the intrathecal contrast medium-enhanced MRI reflects contrast medium distribution from CSF to a brain otherwise expected to be healthy. In patients with CSF circulation disorders, alterations in the cerebral distribution of contrast medium might be expected. Accordingly, brain clearance of MRI contrast medium visualized by sequential T1W images might be used to diagnose type and quantify severity of CSF circulation disorders. Also, different routes for solute transport and clearance could be explored depending on the molecular size of the contrast medium agent. In a mouse-model of subarachnoid hemorrhage (SAH), contrast medium-enhanced MRI revealed severely impaired glymphatic system after SAH (10).
The present MRI scans revealed an increase in SU at both time points after contrast medium administration while subsequent repeated measures would also reveal the efflux of gadobutrol from the brain and cranial cavity. Accordingly, the present observations provide no information about site for ISF absorption, such as capillary absorption at the venous side, efflux along paravascular transport routes, or transport along the cranial nerve sheaths to the extracranial lymphatics, e.g. cervical lymph nodes. We are in progress to further study these issues in upcoming studies.
In conclusion, intrathecal gadobutrol distributed widely throughout the entire brain and spinal cord, and thereby indicates the presence of a glymphatic system also in the human brain, as recently discovered in rodents. This case illustrates the potential for application of intrathecal Gd contrast medium to study water and solute transport in the human brain.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Iliff JJ, Wang M, Liao Y, et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid beta. Sci Transl Med 2012; 4: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kress BT, Iliff JJ, Xia M, et al. Impairment of paravascular clearance pathways in the aging brain. Ann Neurol 2014; 76: 845–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iliff JJ, Chen MJ, Plog BA, et al. Impairment of glymphatic pathway function promotes tau pathology after traumatic brain injury. J Neurosci 2014; 34: 16180–16193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Iliff JJ, Lee H, Yu M, et al. Brain-wide pathway for waste clearance captured by contrast-enhanced MRI. J Clin Invest 2013; 123: 1299–1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jessen NA, Munk AS, Lundgaard I, et al. The glymphatic system: a beginner’s guide. Neurochem Res 2015. Epub ahead of print. [DOI] [PMC free article] [PubMed]
- 6.Aydin K, Terzibasioglu E, Sencer S, et al. Localization of cerebrospinal fluid leaks by gadolinium-enhanced magnetic resonance cisternography: a 5-year single-center experience. Neurosurgery 2008; 62: 584–589. [DOI] [PubMed] [Google Scholar]
- 7.Akbar JJ, Luetmer PH, Schwartz KM, et al. The role of MR myelography with intrathecal gadolinium in localization of spinal CSF leaks in patients with spontaneous intracranial hypotension. Am J Neuroradiol 2012; 33: 535–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Adam G, Neuerburg J, Spuntrup E, et al. Dynamic contrast-enhanced MR imaging of the upper abdomen: enhancement properties of gadobutrol, gadolinium-DTPA-polylysine, and gadolinium-DTPA-cascade-polymer. Magn Reson Med 1994; 32: 622–628. [DOI] [PubMed] [Google Scholar]
- 9.Shoji M, Golde TE, Ghiso J, et al. Production of the Alzheimer amyloid beta protein by normal proteolytic processing. Science 1992; 258: 126–129. [DOI] [PubMed] [Google Scholar]
- 10.Gaberel T, Gakuba C, Goulay R, et al. Impaired glymphatic perfusion after strokes revealed by contrast-enhanced MRI: a new target for fibrinolysis? Stroke 2014; 45: 3092–3096. [DOI] [PubMed] [Google Scholar]