Abstract
Background: Platelet-rich plasma (PRP) contains multiple growth factors and has been shown to enhance fat graft survival after lipotransfer. However, the molecular mechanisms mediating this effect remain unknown. Adipose-derived stem cells (ASCs) play an important role in fat graft survival and are a likely target for PRP-mediated effects. This study seeks to investigate the impact of PRP on ASC proliferation and adipogenic differentiation.
Methods: Human ASCs were isolated using our laboratory protocol. The experiments were divided into four arms: (1) ASCs cultured in general culture medium alone; (2) ASCs in general culture medium + 5%, 10%, 15%, or 20% PRP; (3) ASCs cultured in adipogenic differentiation medium alone; (4) ASCs cultured in adipogenic medium + 5%, 10%, 15%, or 20% PRP. Cell proliferation was analyzed and comparative m-RNA expression of adipogenic genes was assessed by quantitative PCR. Protein expression was determined by western blot.
Results: PRP significantly enhanced proliferation of ASCs, even in the presence of antiproliferative, proadipogenic media. In contrast, PRP inhibited adipogenic differentiation in adipogenic media, evidenced by decreased intracellular lipid accumulation and reduced adipogenic gene expression (PPAR-γ and FABP4). Inhibition appears to occur through downregulation of bone morphogenetic protein receptor IA (BMPRIA) and fibroblast growth factor receptor 1 (FGFR1). Interestingly, PRP elicited these effects across the entire range of doses studied.
Conclusions: PRP appears to modulate ASC function primarily by enhancing cell proliferation. The consequences of its impact on adipogenesis are less clear. Enhanced proliferation initially might set the stage for more robust regeneration and adipogenesis at later time points, providing an important target for ongoing research.
Introduction
Fat grafting allows the restoration of form and function for a wide range of soft tissue defects, such as those derived from traumatic injury, tumor extirpation, and congenital deficiency. Although numerous fillers and implants have been developed, autologous fat grafting remains a gold standard for small to medium-sized defects, averting the problems with immunogenicity seen in many allo- or xeno-derived biologic materials, while avoiding the chronic inflammatory and infectious challenges of foreign materials. Moreover, fat grafting provides a texture and aesthetic closer to that of normal soft tissue. As a material, which can be obtained with minimal morbidity, aspirated fat is an ideal candidate to provide volume for contouring. However, currently, long-term volume retention varies widely (from 30% to 70%), requiring surgeons to subject patients to multiple operations and staged procedures.1,2 Other disadvantages include fat necrosis, calcifications, and oil cyst formation, which further limit the consistency of long-term results. Fortunately, these challenges can be largely overcome by optimizing fat graft survival.
Cell-assisted lipotransfer (CAL), popularized by Yoshimura et al., was one of the first steps attempting to address this problem.3,4 CAL involves isolating cells from the adipose-derived stem cell (ASC)-rich stromal vascular fraction (SVF), a fraction rich in ASCs, and adding them back to lipoaspirate. However, objective data were limited until a relatively recent trial by Kolle et al.5 demonstrated enhanced retention when cultured ASCs, rather than isolated SVF cells, were used to augment grafted fat. In this context, ASCs appear to enhance fat graft survival both through differentiation and through secretion of angiogenic growth factors. Yet, current techniques are far from optimization, and obtaining FDA approval is a major hurdle for stem cell-based therapies.
A promising alternative, platelet-rich plasma (PRP), contains multiple angiogenic growth factors, such as platelet-derived growth factor (PDGF), transforming growth factor beta (TGF-β), epidermal growth factor (EGF), vascular endothelial growth factor (VEGF), and fibroblast growth factor (FGF), and has been demonstrated to enhance proliferation of ASCs in vitro and in vivo6–9 while improving the overall graft survival.8–16 Interestingly, inhibition of VEGF impairs both fat graft retention and adipogenic differentiation, whereas inhibition of adipogenesis impairs angiogenesis.17,18 Although PRP concentration has been found to impact regenerative ability in a variety of other tissue types,19 it has not been well studied in the context of fat grafting. To better understand the role PRP plays in fat graft survival, we examined the impact of PRP concentration on ASC proliferation and adipogenesis in vitro and attempted to probe the underlying molecular mechanisms involved. We hypothesized that concentrations of PRP up to 20% in media would enhance human ASC proliferation and differentiation in short-term cell culture in a dose-dependent fashion.
Materials and Methods
Preparation of PRP lysate
PRP grade concentrated platelets (greater than 1.0 × 106 platelets/μL) were purchased from HemaCare (Van Nuys, CA). A freeze–thaw–freeze cycle was used to collect growth factors within PRP as has been described in the literature.20,21 PRP samples were stored at −80°C for 24 h, rewarmed for 1 h in a 37°C water bath, and then returned to −80°C for another 24 h. The refrozen PRP was then thawed and centrifuged at 2000 g for 10 min. Sterile growth factor-containing lysate was obtained by filtering the supernatant through a 0.22 μm sterile filter. This solution was split into 5 mL aliquots and stored at −80°C.
Growth factor analysis
The PDGF-AB and TGF-β1 concentration in PRP lysate were quantitatively analyzed with a Quantikine Enzyme-Linked Immunosorbent Assay (ELISA) Kit as per the manufacturer's protocol. Standard and samples were added to 96-well microliter plates coated with PDGF-AB and TGF-β1 receptor. Polyclonal antibodies conjugated to horseradish peroxidase and targeted to PDGF-AB or TGF-β1 were used to visualize binding through a chromogen reaction. The results are expressed as mean ± 1 standard deviation (SD).
Human subjects
Adipose tissue was harvested from the abdomen of three human female adult patients undergoing elective liposuction and cosmetic contouring of the trunk. Mean ± SD age of the patients was 31.4 ± 11.8 years and mean ± SD body mass index was 30.3 ± 8.6. All patients were healthy and nondiabetic. Adipose tissue collection was approved by the University of Pittsburgh Institutional Review Board. All patients provided written informed consent before the study.
ASC isolation
Primary human ASCs were isolated from adipose tissue as described previously.22,23 Briefly, adipose tissue was digested in collagenase solution (type II; Worthington Biochemical Corp., Lakewood, NJ) with gentle shaking in a 37°C water bath for ∼30 min. The digested tissue was centrifuged at 180 g for 10 min and then filtered to remove large debris. Next, the cellular pellet (SVF) was resuspended in erythrocyte lysis buffer and centrifuged at 180 g for 10 min. The SVF was plated and cultured on Falcon 175-cm2 tissue culture-treated flasks (#353112; Corning Life Sciences, Oneonta, NY) in general ASC culture medium (Dulbecco's modified Eagle's medium/nutrient mixture F12 [DMEM], 10% fetal bovine serum [FBS], 1% penicillin/streptomycin, 1% fungizone [Bristol-Myers Squibb, New York, NY]). Following overnight incubation, nonadherent cells were removed and the cell medium was replaced with fresh plating medium. Afterward, ASCs were expanded to near confluence (passage 0), harvested, frozen, and then stored at −80°C until use. Passage 0 or passage 1 (P0 or P1) cells were used to test the effect of PRP lysate on ASC proliferation and adipogenesis. Five hundred cells per well were seeded on 96-well plates for the cell proliferation assay, and 1.0 × 106 cells per well (1.05 × 105 per cm2) were seeded on 6-well plates for the adipogenesis, quantitative PCR (qPCR), and western blot assays. For analysis of dose–effect of PRP lysate on ASCs under general culture medium and adipogenic medium, the experiments were grouped as follows: Group IA: general culture medium containing DMEM +10% FBS (C); Group IB: adipogenic differentiation medium (Zen-Bio, Inc., Research Triangle Park, NC) (A), Group IIA: general culture medium +5% PRP lysate (C5P); Group IIB: adipogenic differentiation medium +5% PRP lysate (A5P), Group IIIA: general culture medium +10% PRP lysate (C10P); Group IIIB: adipogenic differentiation medium + 10% PRP lysate (A10P), Group IVA: general culture medium + 15% PRP lysate (C15P); Group IVB: adipogenic differentiation medium + 15% PRP lysate (A15P), Group VA: general culture medium + 20% PRP lysate (C20P); and Group VB: adipogenic differentiation medium + 20% PRP lysate (A20P). Media were replaced every other day for 7 days in all groups.
Proliferation assay
Cells (P0-P1) were seeded in triplicate in 96-well plates at a density of 500 cells per well. Cell proliferation was measured using the CyQUANT Cell Proliferation Assay Kit (Invitrogen, Grand Island, NY) according to the manufacturer's instructions. Relative fluorescence was measured using a Tecan SpectraFluor Plus microplate reader (A/E 508/527 nm) (Tecan Group, Ltd., Mannedorf, Switzerland). Morphology was assessed by microscopic examination at 40×.
Adipogenesis assay
Cells (P0-P1) were seeded on six-well plates as described above and cultured overnight at 37°C. Media were replaced every other day for 7 days, and ASC differentiation was quantified by AdipoRed (Lonza Walkersville, Inc., Walkersville, MD) fluorescent staining of intracellular lipid accumulation. Fluorescence was observed using a Nikon T100 fluorescent microscope, and ImageJ software was used to measure relative fluorescent intensity with 10 repeats per group.
Quantitative real-time PCR
Total RNA was isolated using an RNeasy Mini Kit (Qiagen USA, Valencia, CA) according to the manufacturer's protocol. cDNA was prepared using the SuperScript® III Reverse Transcriptase Kit (Life Technologies, Carlsbad, CA). Primer sequences for PPAR-γ, FABP4, FGF receptor 1 (FGFR1), and bone morphogenetic protein receptor IA (BMPRIA) were designed using Oligo 6.0 (Molecular Biology Insights, Inc., Cascade, CO). qPCR was performed using the SYBR Green I Supermix in an iCycler iQ5 real-time detection system (Bio-Rad Laboratories, Inc., Hercules, CA). PCR specificity was assessed by melting curve analysis. β-actin served as the internal control, and the expression of each gene was evaluated in duplicate with three experimental repeats.
Western blot protein assays
Cells were rinsed with ice cold phosphate-buffered saline, and total protein extracts were obtained using M-PER Mammalian Protein Extraction Reagent (Thermo Fisher Scientific, Inc., Waltham, MA). Protein concentrations were measured using a BCA Protein Assay Kit (Pierce, Rockford, IL). Ten micrograms of protein extracts were subjected to SDS-PAGE using the NuPAGE electrophoresis system (Invitrogen, Carlsbad, CA). Proteins were transferred to polyvinylidene difluoride membranes using the iBlot Dry Blotting System (Invitrogen, Carlsbad, CA) as per the manufacturer's protocol. Membrane blocking and immunodetection of proteins were performed using a WesternBreeze Chemiluminescent Detection Kit, anti-rabbit with a secondary solution of alkaline phosphatase-conjugated anti-FABP4 antibody (Invitrogen, Carlsbad, CA). β-actin served as internal control. The resulting protein bands were quantified by volume summation of pixels using ImageJ.
Statistics
All graphed data are reported as mean ± 1 SD. One-way ANOVA with Tukey's post hoc testing was conducted to assess differences between groups. p < 0.05 was considered statistically significant.
Results
Characterization of PRP and concentration of growth factors
PRP samples (n = 3) had a mean platelet concentration > million/μL. The concentrations of PDGF-AB and TGF-β1 were 257.8 ± 27.3 ng/mL and 89.8 ± 8.8 ng/mL, respectively.
Cell proliferation and morphology
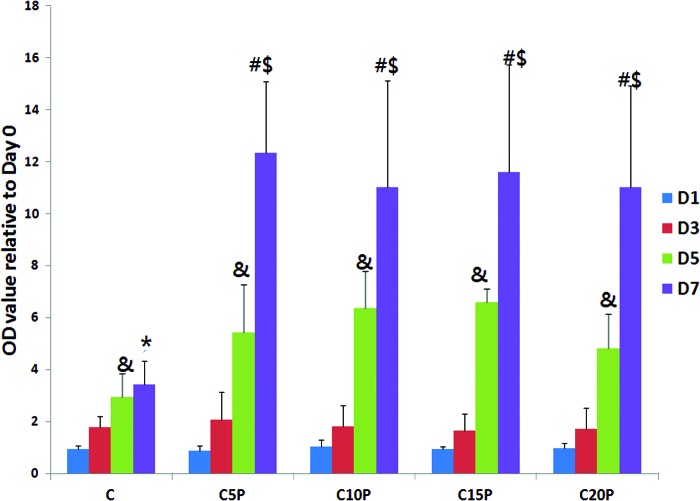
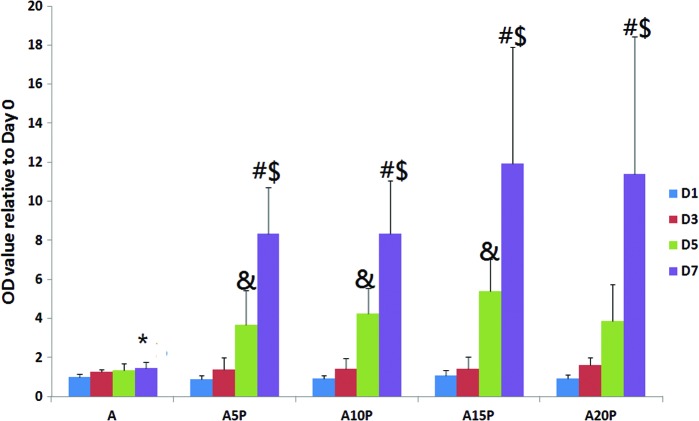
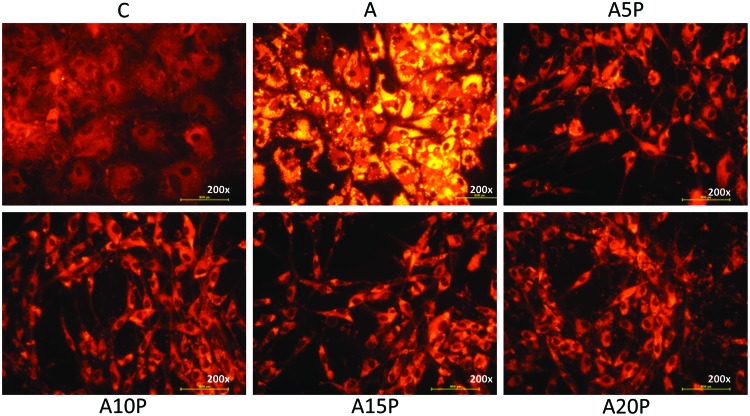
Growth-promoting effects on ASCs were seen in the PRP plus culture media group when compared to media alone with greatest effects seen after 7 days, yet the concentration of PRP in culture media did not significantly impact the growth-promoting activity (Fig. 1). Although the temporal increase from day 1 to 7 was blunted in adipogenic media, the addition of PRP restored the pattern documented with general media+PRP, although the magnitude was reduced somewhat (Fig. 2). Additionally, microscopic examination revealed a smaller cell size and more elongated morphology in all groups receiving PRP. The morphology of cells in the adipogenic medium alone became broader and rounder with accumulation of intracellular lipid droplets (Fig. 3).
FIG. 1.
The effect of platelet-rich plasma (PRP) on adipose-derived stem cell (ASC) proliferation in general culture medium (C), C5P (C+5% PRP), C10P (C+10% PRP), C15P (C+15% PRP), and C20P (C+20% PRP) at days 1, 3, 5, and 7. # indicates D7 > D1, D3, and D5 at each group (p < 0.05); $ indicates D7 at each group > D7 at C group (p < 0.05); & indicates D5 > D1 and D3 at each group (p < 0.05); * indicates D7 > D1 and D3 at C group (p < 0.05). Color images available online at www.liebertpub.com/tea
FIG. 2.
The effect of PRP on ASC proliferation in adipogenic medium (A), A5P (A+5% PRP), A10P (A+10% PRP), A15P (A+15% PRP), and A20P (A+20% PRP) at day 1, 3, 5, and 7. # indicates D7 > D1, D3, and D5 at each group (p < 0.05); $ indicates D7 at each group > D7 at A group (p < 0.05); & indicates D5 > D1 and D3 at each group (p < 0.05); *indicates D7 > D1 at A group (p < 0.05). Color images available online at www.liebertpub.com/tea
FIG. 3.
AdipoRed staining at 200× magnification showing the intracellular lipid accumulation of ASCs, which were cultured under general culture medium (C), adipogenic medium (A), A5P (A+5% PRP), A10P (A+10% PRP), A15P (A+15% PRP), and A20P (A+20% PRP). Color images available online at www.liebertpub.com/tea
Adipogenic assay
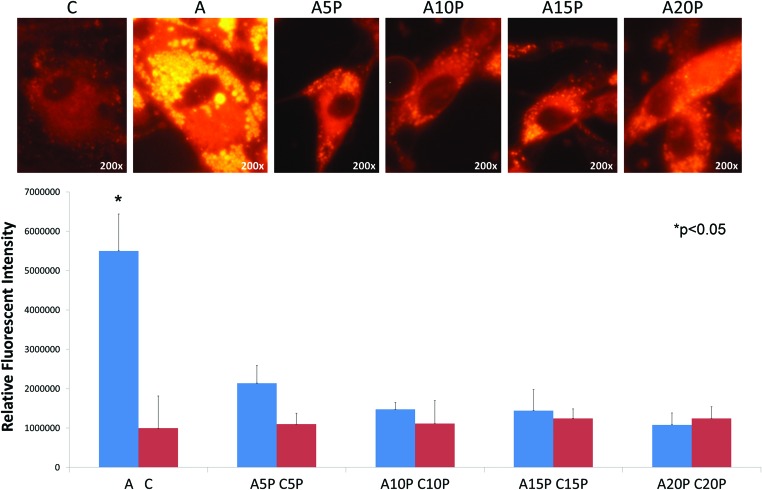
To determine if PRP could enhance ASC adipogenic potential, cells were cultured under adipogenic medium plus different concentrations of PRP (0%, 5%, 10%, 15%, and 20%). Intracellular accumulation of multiple lipid droplets was seen in the adipogenic medium group (Fig. 3). However, no intracellular lipid droplets were seen in the PRP plus adipogenic medium group or the general culture medium control (Fig. 3). This was true across all concentrations of PRP studied. Similarly, relative fluorescent intensity was significantly higher in the adipogenic medium only group than in the PRP or general medium only groups (Fig. 4).
FIG. 4.
Quantitative relative fluorescent intensity measuring AdipoRed-stained intracellular lipid. Images at 200× magnification. Color images available online at www.liebertpub.com/tea
Quantitative PCR
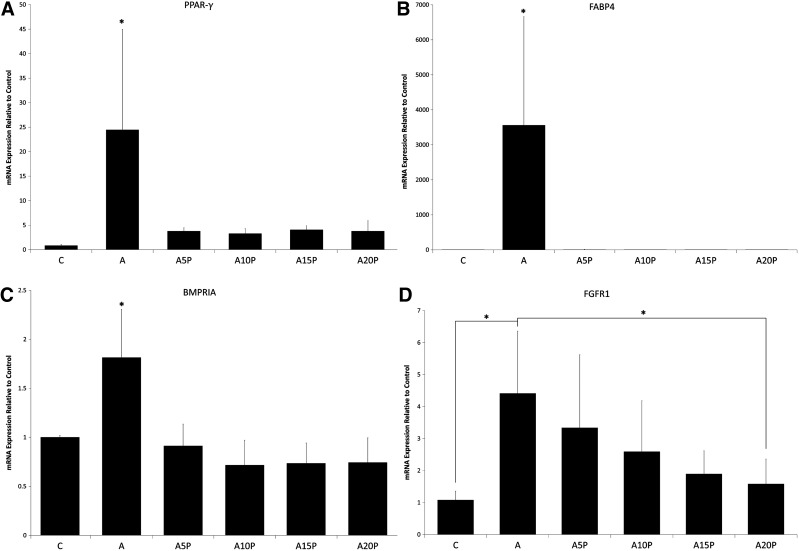
To further confirm the result of the differentiation assay, adipogenic-specific mRNA expression was analyzed. PPAR-γ and FABP4 were expressed at significantly higher levels in adipogenic medium when compared to groups with PRP or general culture media alone (Fig. 5A, B). To further investigate the inhibitory effect of PRP on ASC adipogenesis, we evaluated the major signaling pathways mediating adipogenic differentiation (FGFR and BMPR) by qPCR. FGFR1 and BMPRIA mRNA expression was significantly lower in general culture medium and PRP groups (Fig. 5C, D).
FIG. 5.
(A) The relative mRNA expression of the PPAR-γ gene for A, A5P, A10P, A15P, and A20P as compared to C (general culture medium). (B) The relative mRNA expression of the FABP4 gene of A, A5P, A10P, A15P, and A20P as compared to C (general culture medium). (C) The relative mRNA expression of the bone morphogenetic protein receptor IA (BMPRIA) gene of A, A5P, A10P, A15P, and A20P as compared to C (general culture medium). (D) The relative mRNA expression of the fibroblast growth factor receptor 1 (FGFR1) gene of A, A5P, A10P, A15P, and A20P as compared to C (general culture medium). *indicates p < 0.05.
Western blot
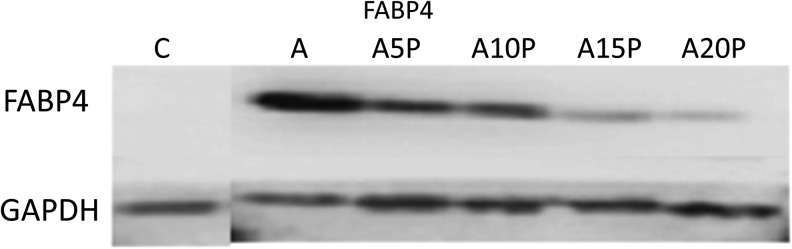
Western blot demonstrated upregulation of FABP4 in adipogenic medium alone, but not in the PRP or general culture medium groups (Fig. 6).
FIG. 6.
Protein expression of FABP4 demonstrated by Western blot at C, A, A5P, A10P, A15P, and A20P.
Discussion
The fate of nonvascularized fat grafting is still controversial. Long-term retention of fat graft occurs both due to adipocyte survival within transplanted fat and by ASC adipogenesis.24,25 Eto et al.25 found that transplanted fat grafts can be divided into three zones. The outer 300 μm forms the peripheral zone and survives through plasma imbibition. The central zone represents the innermost zone of the graft where all adipocytes and progenitor cells die. In the intermediate zone, adipocytes still die, but ASCs are able to survive and regenerate, contributing to long-term tissue remodeling and fat retention. As such, ASCs are better able to endure ischemic insult when compared to hematopoietic and endothelial cells, meaning that they play a major role in fat graft survival.
Other studies have demonstrated that adding additional ASCs to fat grafts can enhance fat survival.3,4 This likely arises through some combination of secreted proangiogenic growth factors, which trigger early revascularization and direct differentiation into new adipocytes and endothelial cells. Yet, no studies with a high level of evidence had been able to support this hypothesis until Kolle et al.5 reported nearly fivefold greater volume retention, more mature adipose tissue, more newly formed connective tissue, and fewer necrotic zones in ASC-enriched fat grafts when compared to controls. However, CAL techniques have yet to gain FDA approval, making wide-spread clinical implementation unlikely in the near future. A possible alternative might be to stimulate proliferation of native ASCs within the target tissue before grafting.
PRP is a highly promising candidate for promoting ASC proliferation. α-granules within platelets contain multiple growth factors, including PDGF, TGF-β, VEGF, and EGF. A wide range of research has implicated PRP in improved wound healing, bone regeneration, angiogenesis, ligament regeneration, and the treatment of osteoarthritis.26–31 Research has also demonstrated positive effects on fat graft survival through enhanced angiogenesis and reduced inflammation, but this data have been limited to histology. Gentile et al. found similar volume retention rates in fat grafts enhanced with PRP (63%) as those enhanced with SVF cells (69%), whereas the control group retained only 39%.32 The exact molecular mechanism has yet to be established. Meanwhile, several studies in the orthopedic literature have demonstrated that treatment with PRP enhances proliferation33 and reduces osteogenic, chondrogenic, and adipogenic differentiation of synovial and bone marrow-derived stem cells.34–36 We hypothesized that PRP similarly enhances fat graft survival by accelerating ASC proliferation in a dose-dependent fashion.
Kakudo et al.7 found that 5% PRP optimally enhanced ASC proliferation with the effect dissipating at concentrations of 20%. Liu et al.6 demonstrated a similar growth curve for ASCs in concentrations ranging from 5% to 15% over the course of 7 days. 12.5% PRP had the highest growth of any group. Cervelli et al.8 found that PRP enhanced ASC proliferation in a dose-dependent fashion from 0% to 50%. Amable et al.37 tested the dose-dependent effect of PRP (1–30%) on ASC proliferation. They found that 1% PRP was similar to 10% FBS, and ASC proliferation was optimized at 10% PRP. Similarly, our results suggest that 5% PRP is sufficient to significantly enhance proliferation and inhibit adipogenesis, even in adipogenic media. Taken together, the data support the notion that PRP enhances fat graft retention largely through ASC proliferation, even when cells are placed in an adipogenic environment. The relatively high concentration of the mitogens PDGF and TGF-β in PRP could explain this effect. Our ELISA results support this, demonstrating a high concentration of PDGF and TGF-β in PRP lysate comparable to the reported values in the literature.29,38–40
Although PRP can enhance ASC proliferation, the question remains as to whether or not it enhances adipogenesis. When fat grafts undergo ischemic stress and cells become necrotic, ASCs are activated and begin to migrate, proliferate, and differentiate into adipocytes, initially expressing markers such as PPAR-γ, then commencing intracellular lipid accumulation. However, because the process of differentiation consumes ASCs, proliferation is reduced by default. As such, ASCs appear to be unable to pursue self-renewal and adipogenic differentiation simultaneously. Our results support this notion as adipogenesis was reduced dramatically when PRP was present in the medium. The addition of PRP to adipogenic medium dramatically reduced intracellular lipid accumulation as measured by AdipoRed staining, even at PRP concentrations as low as 5%. Similarly, the early differentiation gene PPAR-γ and the late gene FABP4 exhibited dramatically reduced expression in the PRP group. Although the Cervelli et al.8 reported that PRP increased adipogenesis in the adipogenic medium, they did not investigate concentrations greater than 5% PRP. Moreover, the difference from adipogenic media alone was less than twofold at 6 days and could potentially be explained by a dose-related effect at lower concentrations of PRP. In our study, however, PPAR-γ was suppressed by 30-fold any time PRP was added to the adipogenic medium. PRP suppressed the late gene FABP4 even more potently, a finding we corroborated by western blot. Similarly, Amable et al.37 found that adipogenesis of ASCs was inhibited by PRP concentrations as low as 1%. In summary, gene expression, protein expression, and phenotype data all suggest that PRP inhibits ASC differentiation and adipogenesis in adipogenic environments, instead of shifting cellular resources toward proliferation.
Based on these findings, we decided to investigate the mechanism of PRP inhibition on ASC adipogenesis in more detail. The major components of growth factors within PRP are PDGF, TGF-β1, EGF, and VEGF. TGF-β1 has been demonstrated to inhibit adipogenesis in bone marrow mesenchymal progenitor cells through the target gene connective tissue growth factor.41,42 EGF and PDGF are also reported to inhibit adipocyte conversion due to reduced PPAR-γ transcription through action on the mitogen-activated protein kinase (MAPK) cascade. Moreover, VEGF primarily promotes angiogenesis, not adipogenesis. As such, the cocktail of growth factors within PRP seem to promote cell proliferation while inhibiting ASC differentiation by downregulation of BMPRIA and FGFR1, the key receptors mediating adipogenesis. Whitehead and coworkers43,44 found that pharmacological inhibition or total knockdown of FGFR1 inhibited PPAR-γ expression and adipogenic differentiation in preadipocytes. Huang et al.45 showed that BMP can commit preadipocytes to adipocyte lineages through BMPRIA receptor using either the Smad or p38 MAPK pathway. Therefore, we analyzed the correlation between FGFR1/BMPRIA expression and adipogenesis in PRP-treated groups and found that PRP suppresses ASC expression of both genes in a dose-dependent manner when added to adipogenic medium. In summary, our data suggest that PRP inhibits adipogenesis, at least in part, by downregulating expression of FGFR1 and BMPRIA receptors.
Although our data provide a detailed examination of several mechanisms by which PRP enhances the regenerative capacity of ASCs, this study is not without limitations. Assessments were performed in vitro, and corroboration of these findings in in vivo models is the target of ongoing research. Moreover, because we did not test PRP doses lower than 5%, comparison to prior articles using 1% and 2.5% is limited. Finally, we cannot say for certain that PDGF and TGF-β are the primary factors responsible for the proliferative effects seen without testing for effect reversal using anti-PDGF or anti-TGF-β antibodies.
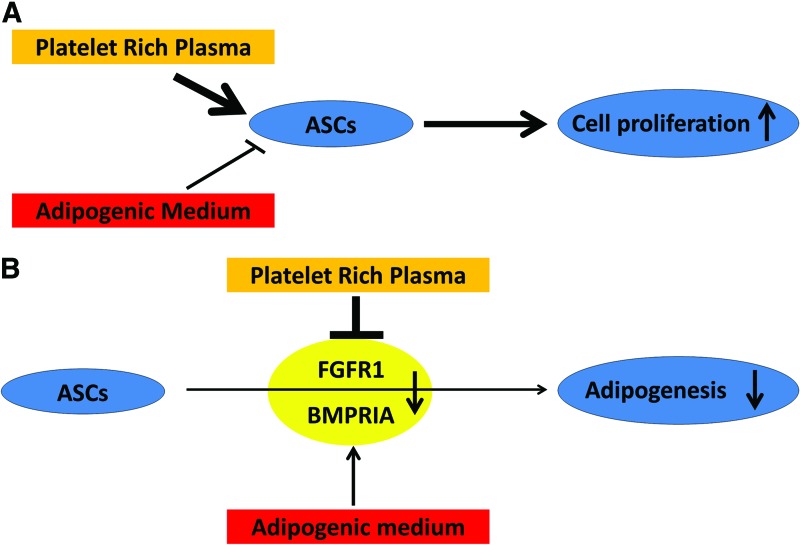
Nevertheless, our data suggest that PRP exhibits its primary effects on ASCs through enhanced proliferation, not adipogenesis, even when cells are placed in an adipogenic environment (Fig. 7A). This effect was present across the entire dose range tested and is most likely mediated through suppression of FGFR1 and BMPRIA (Fig. 7B). Our data also suggest a possible mechanism by which PRP enhances fat graft survival. Initially, when PRP is mixed with fat graft and activated through thrombin or calcium chloride, multiple growth factors are released and abruptly stimulate proliferation of ASCs within the graft. The larger population of ASCs postproliferation can then participate in further hormone production and differentiation into adipocytes or endothelial cells after the PRP and associated hormones are resorbed (usually within a week). As such, supplementation with PRP would be particularly valuable when adequate fat is difficult to obtain or when fewer ASCs are available to enhance grafting than would be desired. Understanding the means by which PRP enhances ASC-mediated regeneration will provide insight into enhancing fat graft survival and could have important applications in future clinical practice.
FIG. 7.
(A) Suggested impact of PRP on ASC proliferation. (B) PRP-induced inhibition of ASC adipogenesis through the FGFR1 and BMPRIA receptors. Color images available online at www.liebertpub.com/tea
Acknowledgments
This research was supported by the National Institutes of Health, RO1-CA114246 (to J.P.R.), USA and the NSC 103-2314-B-182A-036, NSC 97-2314-B-182A-074 from the Ministry of Science and Technology, Taiwan. The authors appreciate the technical support from Sudheer K. Ravuri.
Disclosure Statement
None of the authors have relevant financial interests in the work described.
References
- 1.Pinski K.S., and Roenigk H.H. Autologous fat transplantation. Long-term follow-up. J Dermatol Surg Oncol 18, 179, 1992 [DOI] [PubMed] [Google Scholar]
- 2.Kaufman M.R., Bradley J.P., Dickinson B., Heller J.B., Wasson K., O'Hara C., et al. Autologous fat transfer national consensus survey: trends in techniques for harvest, preparation, and application, and perception of short- and long-term results. Plast Reconstr Surg 119, 323, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Yoshimura K., Sato K., Aoi N., Kurita M., Hirohi T., and Harii K. Cell-assisted lipotransfer for cosmetic breast augmentation: supportive use of adipose-derived stem/stromal cells. Aesthetic Plast Surg 32, 48, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yoshimura K., Sato K., Aoi N., Kurita M., Inoue K., Suga H., et al. Cell-assisted lipotransfer for facial lipoatrophy: efficacy of clinical use of adipose-derived stem cells. Dermatol Surg 34, 1178, 2008 [DOI] [PubMed] [Google Scholar]
- 5.Kolle S.-F.T., Fischer-Nielsen A., Mathiasen A.B., Elberg J.J., Oliveri R.S., Glovinski P.V., et al. Enrichment of autologous fat grafts with ex-vivo expanded adipose tissue-derived stem cells for graft survival: a randomised placebo-controlled trial. Lancet 382, 1113, 2013 [DOI] [PubMed] [Google Scholar]
- 6.Liu H.-Y., Wu A.T.H., Tsai C.-Y., Chou K.-R., Zeng R., Wang M.-F., et al. The balance between adipogenesis and osteogenesis in bone regeneration by platelet-rich plasma for age-related osteoporosis. Biomaterials 32, 6773, 2011 [DOI] [PubMed] [Google Scholar]
- 7.Kakudo N., Minakata T., Mitsui T., Kushida S., Notodihardjo F.Z., and Kusumoto K. Proliferation-promoting effect of platelet-rich plasma on human adipose-derived stem cells and human dermal fibroblasts. Plast Reconstr Surg 122, 1352, 2008 [DOI] [PubMed] [Google Scholar]
- 8.Cervelli V., Scioli M.G., Gentile P., Doldo E., Bonanno E., Spagnoli L.G., et al. Platelet-rich plasma greatly potentiates insulin-induced adipogenic differentiation of human adipose-derived stem cells through a serine/threonine kinase Akt-dependent mechanism and promotes clinical fat graft maintenance. Stem Cells Transl Med 1, 206, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nakamura S., Ishihara M., Takikawa M., Murakami K., Kishimoto S., Nakamura S., et al. Platelet-rich plasma (PRP) promotes survival of fat-grafts in rats. Ann Plast Surg 65, 101, 2010 [DOI] [PubMed] [Google Scholar]
- 10.Gentile P., Di Pasquali C., Bocchini I., Floris M., Eleonora T., Fiaschetti V., et al. Breast reconstruction with autologous fat graft mixed with platelet-rich plasma. Surg Innov 20, 370, 2013 [DOI] [PubMed] [Google Scholar]
- 11.Oh D.S., Cheon Y.W., Jeon Y.R., and Lew D.H. Activated platelet-rich plasma improves fat graft survival in nude mice: a pilot study. Dermatol Surg 37, 619, 2011 [DOI] [PubMed] [Google Scholar]
- 12.Pires Fraga M.F., Nishio R.T., Ishikawa R.S., Perin L.F., Helene A., and Malheiros C.A. Increased survival of free fat grafts with platelet-rich plasma in rabbits. J Plast Reconstr Aesthet Surg 63, e818, 2010 [DOI] [PubMed] [Google Scholar]
- 13.Rodríguez-Flores J., Palomar-Gallego M.A., Enguita-Valls A.B., Rodríguez-Peralto J.L., and Torres J. Influence of platelet-rich plasma on the histologic characteristics of the autologous fat graft to the upper lip of rabbits. Aesthetic Plast Surg 35, 480, 2011 [DOI] [PubMed] [Google Scholar]
- 14.Ullmann Y., Hyams M., Ramon Y., Beach D., Peled I.J., and Lindenbaum E.S. Enhancing the survival of aspirated human fat injected into nude mice. Plast Reconstr Surg 101, 1940, 1998 [DOI] [PubMed] [Google Scholar]
- 15.Liao H.-T., Marra K.G., and Rubin J.P. Application of platelet-rich plasma and platelet-rich fibrin in fat grafting: basic science and literature review. Tissue Eng Part B Rev 20, 267, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yuksel E., Weinfeld A.B., Cleek R., Wamsley S., Jensen J., Boutros S., et al. Increased free fat-graft survival with the long-term, local delivery of insulin, insulin-like growth factor-I, and basic fibroblast growth factor by PLGA/PEG microspheres. Plast Reconstr Surg 105, 1712, 2000 [DOI] [PubMed] [Google Scholar]
- 17.Yamaguchi M., Matsumoto F., Bujo H., Shibasaki M., Takahashi K., Yoshimoto S., et al. Revascularization determines volume retention and gene expression by fat grafts in mice. Exp Biol Med (Maywood) 230, 742, 2005 [DOI] [PubMed] [Google Scholar]
- 18.Fukumura D., Ushiyama A., Duda D.G., Xu L., Tam J., Krishna V., et al. Paracrine regulation of angiogenesis and adipocyte differentiation during in vivo adipogenesis. Circ Res 93, e88, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anitua E., Sánchez M., Zalduendo M.M., de la Fuente M., Prado R., Orive G., et al. Fibroblastic response to treatment with different preparations rich in growth factors. Cell Prolif 42, 162, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sell S.A., Wolfe P.S., Ericksen J.J., Simpson D.G., and Bowlin G.L. Incorporating platelet-rich plasma into electrospun scaffolds for tissue engineering applications. Tissue Eng Part A 17, 2723, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weibrich G., Kleis W.K.G., Hafner G., and Hitzler W.E. Growth factor levels in platelet-rich plasma and correlations with donor age, sex, and platelet count. J Craniomaxillofac Surg 30, 97, 2002 [DOI] [PubMed] [Google Scholar]
- 22.Philips B.J., Grahovac T.L., Valentin J.E., Chung C.W., Bliley J.M., Pfeifer M.E., et al. Prevalence of endogenous CD34+ adipose stem cells predicts human fat graft retention in a xenograft model. Plast Reconstr Surg 132, 845, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Coleman S.R. Structural fat grafts: the ideal filler? Clin Plast Surg 28, 111, 2001 [PubMed] [Google Scholar]
- 24.PEER LA. Cell survival theory versus replacement theory. Plast Reconstr Surg (1946). 16, 161, 1955 [DOI] [PubMed] [Google Scholar]
- 25.Eto H., Kato H., Suga H., Aoi N., Doi K., Kuno S., et al. The fate of adipocytes after nonvascularized fat grafting: evidence of early death and replacement of adipocytes. Plast Reconstr Surg 129, 1081, 2012 [DOI] [PubMed] [Google Scholar]
- 26.Foster T.E., Puskas B.L., Mandelbaum B.R., Gerhardt M.B., and Rodeo S.A. Platelet-rich plasma: from basic science to clinical applications. Am J Sports Med 37, 2259, 2009 [DOI] [PubMed] [Google Scholar]
- 27.Marx R.E. Platelet-rich plasma (PRP): what is PRP and what is not PRP? Implant Dent 10, 225, 2001 [DOI] [PubMed] [Google Scholar]
- 28.Sugimori E., Shintani S., Ishikawa K., and Hamakawa H. Effects of apatite foam combined with platelet-rich plasma on regeneration of bone defects. Dent Mater J 25, 591, 2006 [DOI] [PubMed] [Google Scholar]
- 29.Tsay R.C., Vo J., Burke A., Eisig S.B., Lu H.H., and Landesberg R. Differential growth factor retention by platelet-rich plasma composites. J Oral Maxillofac Surg 63, 521, 2005 [DOI] [PubMed] [Google Scholar]
- 30.Pietramaggiori G., Kaipainen A., Czeczuga J.M., Wagner C.T., and Orgill D.P. Freeze-dried platelet-rich plasma shows beneficial healing properties in chronic wounds. Wound Repair Regen 14, 573, 2006 [DOI] [PubMed] [Google Scholar]
- 31.Liao H.-T., Chen C.-T., Chen C.-H., Chen J.-P., and Tsai J.-C. Combination of guided osteogenesis with autologous platelet-rich fibrin glue and mesenchymal stem cell for mandibular reconstruction. J Trauma 70, 228, 2011 [DOI] [PubMed] [Google Scholar]
- 32.Gentile P., De Angelis B., Pasin M., Cervelli G., Curcio C.B., Floris M., et al. Adipose-derived stromal vascular fraction cells and platelet-rich plasma: basic and clinical evaluation for cell-based therapies in patients with scars on the face. J Craniofac Surg 25, 267, 2014 [DOI] [PubMed] [Google Scholar]
- 33.Mojica-Henshaw M.P., Jacobson P., Morris J., Kelley L., Pierce J., Boyer M., et al. Serum-converted platelet lysate can substitute for fetal bovine serum in human mesenchymal stromal cell cultures. Cytotherapy 15, 1458, 2013 [DOI] [PubMed] [Google Scholar]
- 34.Giovanini A.F., Grossi J.R.A., Gonzaga C.C., Zielak J.C., Göhringer I., Vieira J.D.S., et al Leukocyte-platelet-rich plasma (L-PRP) induces an abnormal histophenotype in craniofacial bone repair associated with changes in the immunopositivity of the hematopoietic clusters of differentiation, osteoproteins, and TGF-β1. Clin Implant Dent Relat Res 16, 259, 2014 [DOI] [PubMed] [Google Scholar]
- 35.Chen L., Yang X., Huang G., Song D., Ye X.-S., Xu H., et al. Platelet-rich plasma promotes healing of osteoporotic fractures. Orthopedics 36, e687, 2013 [DOI] [PubMed] [Google Scholar]
- 36.Lee J.K., Lee S., Han S.A., Seong S.C., and Lee M.C. The effect of platelet-rich plasma on the differentiation of synovium-derived mesenchymal stem cells. J Orthop Res 32, 1317, 2014 [DOI] [PubMed] [Google Scholar]
- 37.Amable P.R., Teixeira M.V.T., Carias R.B.V., Granjeiro J.M., and Borojevic R. Mesenchymal stromal cell proliferation, gene expression and protein production in human platelet-rich plasma-supplemented media. PLoS One 9, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weibrich G., Kleis W.K.G., and Hafner G. Growth factor levels in the platelet-rich plasma produced by 2 different methods: curasan-type PRP kit versus PCCS PRP system. Int J Oral Maxillofac Implants 17, 184, 2002 [PubMed] [Google Scholar]
- 39.Huang Q., Wang Y., Wu T., Jiang S., Hu Y., and Pei G. Preliminary separation of the growth factors in platelet-rich plasma: effects on the proliferation of human marrow-derived mesenchymal stem cells. Chin Med J (Engl) 122, 83, 2009 [PubMed] [Google Scholar]
- 40.Weibrich G., Kleis W.K.G., Streckbein P., Moergel M., Hitzler W.E., and Hafner G. Comparison of point-of-care methods for preparation of platelet concentrate (platelet-rich plasma). Int J Oral Maxillofac. Implants 27, 762, 2012 [PubMed] [Google Scholar]
- 41.Kumar A., Ruan M., Clifton K., Syed F., Khosla S., and Oursler M.J. TGF-β mediates suppression of adipogenesis by estradiol through connective tissue growth factor induction. Endocrinology 153, 254, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Camp H.S., and Tafuri S.R. Regulation of peroxisome proliferator-activated receptor gamma activity by mitogen-activated protein kinase. J Biol Chem 272, 10811, 1997 [DOI] [PubMed] [Google Scholar]
- 43.Widberg C.H., Newell F.S., Bachmann A.W., Ramnoruth S.N., Spelta M.C., Whitehead J.P., et al. Fibroblast growth factor receptor 1 is a key regulator of early adipogenic events in human preadipocytes. Am J Physiol Endocrinol Metab 296, E121, 2009 [DOI] [PubMed] [Google Scholar]
- 44.Hutley L., Shurety W., Newell F., McGeary R., Pelton N., Grant J., et al. Fibroblast growth factor 1: a key regulator of human adipogenesis. Diabetes 53, 3097, 2004 [DOI] [PubMed] [Google Scholar]
- 45.Huang H., Song T.-J., Li X., Hu L., He Q., Liu M., et al. BMP signaling pathway is required for commitment of C3H10T1/2 pluripotent stem cells to the adipocyte lineage. Proc Natl Acad Sci U S A 106, 12670, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]