Abstract
Lymph node (LN) status after surgery for rectal cancer is affected by preoperative radiotherapy. The purpose of this study was to perform a population-based evaluation of the impact of pathologic LN status after neoadjuvant radiotherapy on survival. A total of 1,650 patients receiving neoadjuvant chemotherapy in Surveillance, Epidemiology, and End Results Program (SEER)-registered ypIII stage rectal cancer was analyzed. We identified the optimal cutoff for retrieved LNs as 10 (χ2 = 14.006, P < 0.001), which was validated as an independent prognosis factors in a Cox regression model. Further analysis showed that the LN count was only a prognosis factor with the number from 8 to 16(except for 13).After the number 16, the 5-year survival rate decreased gradually. Collectively, our results confirmed that the number of LNs in yp III stage rectal patients was a prognosis factor only with the numbers from 8 to 16(except for 13). Using the total mesorectal excision technique with an adequate pathologic examination, a large number of LNs retrieved (≥17) might indicate worse tumor response grade and poorer survival.
Preoperative radiotherapy (preop-RT) has become part of standard practice offered to improve treatment outcomes in patients with rectal cancer1,2. According to the guidelines for colorectal cancer from the National Comprehensive Cancer Network (NCCN), a minimum of twelve lymph nodes (LNs) must be retrieved and examined for accurate staging and the number of metastatic LNs was validated as an independent prognostic factors3,4,5. Many factors can influence the number of LNs harvested after rectal cancer resection and it has been shown that preop-RT might reduce the number of LNs retrieved6,7. With the decreased LNs retrieval, the prognostic value of the LN count might also diminish. Increasingly, there is doubt regarding the recommended minimum retrieval of 12 LNs for ypTNM staging8. There have been some studies of the optimal LNs retrieval cutoff in ypN0 patients, but few studies have focused on ypN(+) patients. One possible method of identifying a reasonable cutoff value that allows the reliable staging of rectal cancer with LNs metastases treated with preop-RT is to analyze patient survival in a large series of patients diagnosed as ypN(+).
The purpose of this study was to assess the impact of the number of LNs examined on survival of rectal cancer patients treated with preop-RT at ypN(+) stage and to determine the optimal number of LNs that should be examined.
Results
Patient Characteristics in SEER database
In our 8-year study period, a total of 1,650 with rectal cancer met our selection criteria, including 1,042 male and 608 female. The median patient age was 57 years (IQR 48-66). There were 1,125 patients with ypN1 stage and 525 with ypN2 stage. Patient demographics and pathological features are summarized in Table 1. Patients with ypN2 stage had a lower rate of high/moderate grade tumors, a higher proportion of mucinous/signet ring adenocarcinoma, and higher ratios of ypT3/4 tumor stage, which reached the level of significance (p < 0.001).
Table 1. Characteristics of patients included in the study and make comparison between the ypN1 and ypN2 groups.
| Characteristic | Total | ypN1 | ypN2 | P value |
|---|---|---|---|---|
| (n = 1650) (%) | (n = 1125) (%) | (n = 525) | ||
| Media foullow up(mo) | 64(2–155) | 69 (2–155) | 54(2–153) | <0.001 |
| Sex | 0.412 | |||
| male | 1042 | 718(63.8) | 324(61.7) | |
| female | 608 | 407(36.2.) | 201 (38.3) | |
| Age | 57(20–80) | 58(20-80) | 56(21–80) | 0.059 |
| Race | 0.725 | |||
| White | 1345 | 921(81.9) | 424(80.9) | |
| Black | 128 | 88(7.8) | 40(7.6) | |
| Other | 177 | 116(10.3) | 61(11.6) | |
| Pathological grading | <0.001 | |||
| High/Moderate | 1133 | 808(71.8) | 325(61.9) | |
| Poor/Anaplastic | 376 | 216(19.2) | 160(30.5) | |
| Unknown | 141 | 101(9.0) | 40(7.6) | |
| Histotype | <0.001 | |||
| Adenocarcinoma | 1333 | 955(85.0) | 378(72.3) | |
| Mucinous /Signet ring cell | 313 | 168(15.0) | 145(27.7) | |
| T stage | <0.001 | |||
| Tis | 5 | 4(0.4) | 1(0.2) | |
| T1/T2 | 228 | 182(16.2) | 46(8.8) | |
| T3/4 | 1393 | 920(81.9) | 473(90.1) | |
| TX | 23 | 18(1.6) | 5(1.0) | |
| No. of LNs dissected | 12(1–69) | 10(1–58) | 15(4–69) | <0.001 |
Identification of Cutoff points for the minimum number of LNs retrieved in ypN(+) patients in the SEER database
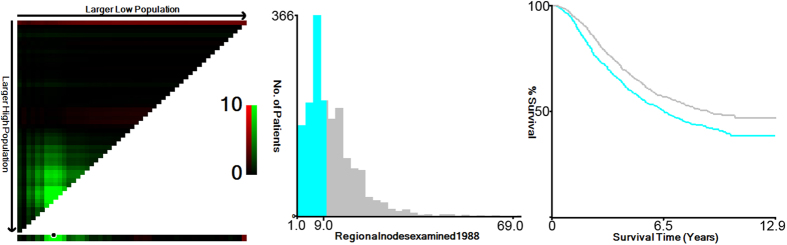
X-tile plots were constructed and the maximum χ2 log-rank value of 14.006 (P < 0.001) was produced, applying 10 as the optimal cutoff value to divide the cohort into high and low risk subsets in terms of rectal cancer cause-specific survival (RCSS) (Fig. 1). To assess the influence of different LN retrieval on RCSS, we analyzed the individual result using different LN number values ranging from 5 to 19. The number below 5 and over 19 weren’t analyzed to guarantee sufficient statistical power. The 5-year RCSSs were calculated for patients with N (LNs number) or more nodes and less than N nodes (Table 2). The number of LNs retrieved was a prognosis factor only for number ranging from 8 to 16 (except for 13, P value = 0.062).The 5-year RCSS of patients with N or more nodes increased gradually when N ranged from 5 to 9 and the result was roughly the same from 10 to 15 peaking to 65.7% at cutoff 11; but after the number 16, the 5-year RCSS gradually decreased.
Figure 1. X-tile analysis of survival data from the SEER registry.
X-tile analysis was performed using patient data, which were equally divided into training and validation sets, from the SEER registry. X-tile plots of the training sets are shown in the left panels, with plots of matched validation sets shown in the smaller inset. The optimal cut-point highlighted by the black circle in the left panels is shown on a histogram of the entire cohort (middle panels), and a Kaplan-Meier plot (right panels). P values were determined using the cutoff point defined in the training set and applying it to the validation set. Figure 1 shows the optimal cutoff point for the ypN (+) patients (10, χ2 = 14.006, P < 0.001).
Table 2. Univariate analysis of the influence of different total lymph node count on RCSS in ypIII stage rectal cancer patients.
| Total lymph node count | No. | 5-year CCS | Log-rank χ2 | P value* |
|---|---|---|---|---|
| <5 | 250 | 58.3% | 0.810 | 0.368 |
| ≥5 | 1400 | 61.4% | ||
| <6 | 359 | 56.1% | 3.871 | 0.049 |
| ≥6 | 1291 | 62.3% | ||
| <7 | 457 | 57.8% | 2.008 | 0.157 |
| ≥7 | 1193 | 62.1% | ||
| <8 | 593 | 55.9% | 9.609 | 0.002 |
| ≥8 | 1057 | 63.7% | ||
| <9 | 703 | 56.6% | 11.164 | 0.001 |
| ≥9 | 947 | 64.1% | ||
| <10 | 788 | 56.6% | 14.006 | <0.001 |
| ≥10 | 862 | 64.8% | ||
| <11 | 902 | 57.0% | 13.492 | 0.001 |
| ≥11 | 748 | 65.7% | ||
| <12 | 991 | 58.0% | 8.515 | 0.004 |
| ≥12 | 659 | 65.4% | ||
| <13 | 1071 | 59.3% | 3.474 | 0.062 |
| ≥13 | 579 | 64.0% | ||
| <14 | 1140 | 59.4% | 4.127 | 0.042 |
| ≥14 | 510 | 64.3% | ||
| <15 | 1192 | 59.2% | 4.771 | 0.029 |
| ≥15 | 458 | 65.3% | ||
| <16 | 1251 | 59.8% | 3.581 | 0.058 |
| ≥16 | 399 | 64.4% | ||
| <17 | 1304 | 60.4% | 2.165 | 0.141 |
| ≥17 | 346 | 62.3% | ||
| <18 | 1355 | 60.6% | 1.058 | 0.304 |
| ≥18 | 295 | 62.6% | ||
| <19 | 1401 | 60.6% | 1.057 | 0.304 |
| ≥19 | 249 | 62.7% |
*P values refer to the log-rank test of the differences between the two survival curves generated using Kaplan-Meier analysis.
Prognostic value of number of LNs retrieved
The number of LNs and other clinicopathological factors, including elderly patients (P < 0.001), black patients (P = 0.043), poor and undifferentiated tumor grades (P < 0.001), mucinous and signet-ring cancer (P = 0.001), advanced TN stages (P < 0.001), higher negative to positive LNs ratio (P < 0.001), and number of LNs (P < 0.001), were significant risk factors for poor survival according to univariate analysis (Table 3). In univariate analysis, age, race, tumor grade, T stage, N stage, higher negative to positive LNs ratio, and LN counts (with an optimal cutoff of 10) were independently and significantly associated with RCSS (Table 3), and a higher number of LNs demonstrated a positive effect on survival (hazard ratio [HR] 0.744; 95% confidence interval [CI] 0.631-0.877, P < 0.001) (Table 3).
Table 3. Univariate and multivariate survival analyses evaluating the number of retrieved LNs influencing RCSS in ypN(+) rectal cancer patients.
| Variable | Univariate analysis | Multivariate analysis |
|||
|---|---|---|---|---|---|
| 5-year RCCS | Log rank χ2test | P* | HR(95%CI) | P | |
| Sex | 0.070 | 0.791 | NI | ||
| Male | 61.1% | ||||
| Female | 60.7% | ||||
| Age | 24.963 | <0.001 | <0.001 | ||
| <60 | 64.6% | Reference | |||
| ≥60 | 55.8% | 1.439(1.247–1.659) | |||
| Race | 6.311 | 0.043 | 0.016 | ||
| Caucasian | 62.1% | Reference | |||
| Black | 51.4% | 1.404(1.095–1.799) | |||
| others | 59.1% | 1.170 (0.931–1.471) | |||
| Grade | 57.252 | <0.001 | <0.001 | ||
| High/ Moderate | 65.3% | Reference | |||
| Poor/ Anaplastic | 44.7% | 1.657(1.412–1.944) | |||
| Unknown | 68.5% | 0.892(0.672–1.184) | |||
| Histotype | 10.876 | 0.001 | 0.462 | ||
| Adenocarcinoma | 62.6% | Reference | |||
| Mucinous/signet ring cell | 54.5% | 1.069(0.895–1.276) | |||
| ypT Stage | 22.284 | <0.001 | 0.003 | ||
| Tis | 60.0% | 1.173(0.287–4.792) | |||
| T1-2 | 76..2% | Reference | |||
| T3-4 | 58.4% | 1.565(1.238–1.979) | |||
| Tx | 60.3% | 1.397(0.722–2.704) | |||
| ypN Stage | 81.663 | <0.001 | <0.001 | ||
| ypN1 | 67.5% | Reference | |||
| ypN2 | 46.7% | 1.638(1.358–1.975) | |||
| N/P LNs ratio | 90.460 | <0.001 | 0.001 | ||
| ≦2.33 | 49.9% | Reference | |||
| >2.33 | 70.6% | 0.735(0.631–0.877) | |||
| No. of LNs | 14.006 | <0.001 | <0.001 | ||
| <10 | 56.6% | Reference | |||
| ≥10 | 64.8% | 0.744(0.631–0.877) | |||
NI: not included in multivariate survival analysis.
N/P LNs ratio: The ratio of the number of negative lymph nodes to the number of metastatic lymph nodes.
*P values refer to the log-rank test of the differences between the two survival curves generated using Kaplan-Meier analysis.
Subgroup analysis of the effect of LNs retrieved during ypIII stage
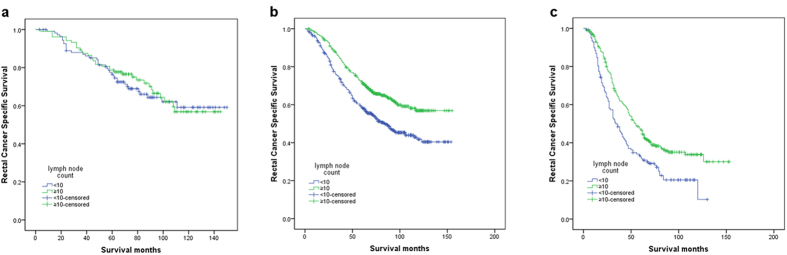
According to the AJCC-7 rectal cancer staging system, patients with LNs metastases were all classified as ypIII stage, which included three subgroups (i.e., ypIIIA, ypIIIB, and ypIIIC). We then further analyzed these three subgroups. Patients with ypTis (n = 5) and ypTx (n = 23) were excluded from this analysis. The results showed that the retrieved LNs (with a cutoff of 10) were significantly associated with 5-year RCSS, according to the univariate analysis in the ypIIIB (χ2 = 22.817, P < 0.001) and ypIIIC subgroups (χ2 = 13.225, P < 0.001), but not in the ypIIIA subgroup (χ2 = 0.121, P = 0.728) (Fig. 2, Table 4 and 5, ). The number of LNs retrieved was also validated as an independent survival factor by multivariate Cox regression in the ypIIIB (LNs≥10, HR 0.698, 95% CI 0.552–0.882, P = 0.003) and ypIIIC subgroups (LNs≥10, HR 0.718, 95%CI 0.553–0.933,P = 0.013) (Table 4 and 5, )
Figure 2. Log-rank tests of rectal cancer cause-specific survival comparing patients with ≥10 lymph nodes with patients who had <10 nodes for (a) stage ypIIIA: χ2 = 0.121, P = 0.728; (b) stage ypIIIB: χ2 = 22.817, P < 0.001; and (c) stage ypIIIC: χ2 = 13.225, P < 0.001.
Table 4. Univariate and multivariate survival analyses evaluating the number of retrieved LNs influencing RCSS in ypIIIB rectal cancer patients.
| Variable | 5-year RCCS | Univariate analysis | P* | Multivariate analysis |
|
|---|---|---|---|---|---|
| Log rank χ2test | HR(95% CI) | P | |||
| Sex | 0.576 | 0.448 | NI | ||
| Male | 65.8% | ||||
| Female | 63.8% | ||||
| Age | 19.534 | <0.001 | <0.001 | ||
| <60 | 69.3% | Reference | |||
| ≥60 | 59.5% | 1.500(1.237–1.820) | |||
| Race | 6.580 | 0.037 | 0.025 | ||
| Caucasian | 66.1% | Reference | |||
| Black | 51.8% | 1.556(1.131–2.141) | |||
| others | 67.9% | 1.025(0.731–1.437) | |||
| Grade | 34.685 | <0.001 | <0.001 | ||
| High/ Moderate | 69.1% | Reference | |||
| Poor/ Anaplastic | 47.8% | 1.728(1.388-2.151) | |||
| Unknown | 74.2% | 0.803(0.536–1.203) | |||
| Histotype | 5.186 | 0.023 | 0.325 | ||
| Adenocarcinoma | 66.5% | Reference | |||
| Mucinous/signet ring cell | 58.9% | 1.134(0.883–1.455) | |||
| ypT Stage | 6.475 | 0.039 | 0.009 | ||
| T1-2 | 53.8% | Reference | |||
| T3 | 67.6% | 0.973(0.460-2.059) | |||
| T4 | 60.5% | 1.364(0.625–2.975) | |||
| ypN Stage | 13.788 | <0.001 | 0.002 | ||
| ypN1 | 67.1% | Reference | |||
| ypN2 | 53.6% | 1.616(1.195–2.185) | |||
| N/P LNs ratio | 27.218 | <0.001 | 0.070 | ||
| ≦2.33 | 56.1% | Reference | |||
| >2.33 | 70.4% | 0.801(0.629–1.019) | |||
| No. of LNs | 22.817 | <0.001 | 0.003 | ||
| <10 | 58.8% | Reference | |||
| ≥10 | 72.0% | 0.698(0.552–0.882) | |||
NI: not included in multivariate survival analysis.
N/P LNs ratio: The ratio of the number of negative lymph nodes to the number of metastatic lymph nodes.
*P values refer to the log-rank test of the differences between the two survival curves generated using Kaplan-Meier analysis.
Table 5. Univariate and Multivariate survival analyses for evaluating the number of LNs retrieved influencing RCSS in ypIIIC rectal cancer patients.
| Variable | 5-year CCS | Univariate analysis | P* | Multivariate analysis |
|
|---|---|---|---|---|---|
| Log rank χ2test | HR(95% CI) | P | |||
| Sex | 0.716 | 0.397 | NI | ||
| Male | 40.5% | ||||
| Female | 45.2% | ||||
| Age | 7.615 | 0.006 | 0.010 | ||
| <60 | 45.0% | Reference | |||
| ≥60 | 36.6% | 1.383(1.082–1.769) | |||
| Race | 1.001 | 0.606 | NI | ||
| Caucasian | 42.4% | ||||
| Black | 43.0% | ||||
| others | 37.6% | ||||
| Grade | 9.790 | 0.007 | 0.018 | ||
| High/ Moderate | 45.2% | Reference | |||
| Poor/Anaplastic | 31.4% | 1.470(1.126–1.921) | |||
| Unknown | 50.4% | 1.083(0.709–1.654) | |||
| Histotype | 0.149 | 0.699 | NI | ||
| Adenocarcinoma | 41.5% | ||||
| Mucinous/signet ring cell | 43.2% | ||||
| ypT Stage | 4.371 | 0.037 | 0.033 | ||
| T3 | 50.8% | Reference | |||
| T4 | 39.2% | 1.428(1.030–1.979) | |||
| ypN Stage | 0.648 | 0.421 | NI | ||
| ypN1 | 42.4% | ||||
| ypN2 | 41.7% | ||||
| N/P LNs ratio | 13.621 | <0.001 | <0.001 | ||
| ≦2.33 | 37.3% | Reference | |||
| >2.33 | 56.1% | 0.548(0.394–0.762) | |||
| No. of LNs | 13.225 | <0.001 | 0.013 | ||
| <10 | 32.4% | Reference | |||
| ≥10 | 46.6% | 0.718(0.553–0.933) | |||
NI: not included in multivariate survival analysis.
N/P LNs ratio: The ratio of the number of negative lymph nodes to the number of metastatic lymph nodes.
*P values refer to the log-rank test of the differences between the two survival curves generated using Kaplan-Meier analysis.
Discussion
The presence of LN metastases in colorectal cancer is well recognized as one of the most important prognostic factors for long-term outcome9,10. The total number of LNs retrieved is fundamental in most pathological staging systems for colorectal cancer, including the American Joint Committee on Cancer (AJCC), modified Dukes and Astler and Coller. Inadequate LN evaluation is associated with worse outcomes in terms of tumor recurrence and patient survival, particularly in patients with stage II colorectal cancer11,12,13,14. The World Congress of Gastroenterology proposed examining a minimum of 12 LNs to classify stage II colorectal tumors15. In the USA, the American Joint Committee on Cancer (AJCC), the American Society of Clinical Oncology (ASCO) and National Comprehensive Cancer Network (NCCN) have also recommended examining at least 12 LNs to assign Stage II rectal disease16. However, debate exists regarding the importance of increased LN harvests in stage III colorectal cancer. Le Voyer et al. showed that for colon cancer patients with N1 stage, there was an absolute 23% improvement in the 5-year overall survival if >40 LNs were analyzed compared with ≤10 LNs (P < 0.001); and in patients with N2 stage, the 5-year overall survival rates following analysis of >35 and <35 LNs were 71% and 51%, respectively (P = 0.002)11. Vather et al. reported that the mean numbers of LNs examined in stage III patients who died or were alive within 5 years was 13.1 vs 14.8, respectively, and this difference was statistically significant (P < 0.001)17. Chen et al. showed that the median survival times for colon cancer patients with 1–7, 8–14 and ≥15 LN retrieval were 46, 52 and 67 months, respectively (P < 0.001)18. However, several studies failed to demonstrate a similar association between survival and LNs harvest in stage III disease13,14,19,20. Note that our previous study showed that negative LN count, which does not take positive LN into consideration, was an independent prognostic factor for ypIIIB and ypIIIC rectal cancer patients21. However, the relationship between LN count and RCSS in ypIII rectal cancer has not been fully investigated. In all present guidelines for rectal cancer clinical practice, total LN count is the main concern. Hence, in this study, we mainly focused on the prognostic significance of the total LNs count in patients with rectal cancer treated with preoperative radiotherapy. We first used X-tile to identify 10 as the optimal cutoff value, and then it was confirmed as one of the optimal cutoff numbers in an additional one-by-one cutoff value analysis from 5 to 19. The 5-year RCSS rates of patients with N (cutoff number) or more nodes gradually increased when N ranged from 5 to 9, which suggested that inadequate LN retrieval in LN positive rectal cancer patients may also reflect the failure to remove the involved LNs, particularly in IIIB and IIIC stage patients in our study, thus increasing the risk of local recurrence and distant metastasis. In addition, it may be a marker of poor quality surgical or pathologic care, both of which may cause poor survival. However, a 10 LN cutoff was not an independent prognostic factor in stage IIIA patients. This finding could be explained by the fact that there was a low percentage(3.3%) of T1-T2 patients with more advanced stage than stage IIIA22, therefore, the probability of understaging is significantly low.
The intended purpose of preop-RT is tumor down-staging by decreasing the primary tumor bulk and associated LN metastases. Some authors have demonstrated that neoadjuvant therapy may result in radiation-induced lymphocyte destruction and stromal fibrosis resulting in alterations of the morphology of the LNs, making it difficult to detect them23,24. Recent research has shown that a decreased number of LNs is related to good tumor response. de Campos-Lobato et al. have shown that retrieving fewer than 12 LNs in the proctectomy specimen of rectal cancer patients treated with preoperative chemoradiation does not affect their overall survival and may be a marker of greater tumor response and, consequently, decreased LR rate25. Rullier et al. were also unable to identify any relationship between a reduced number of LNs examined and decreased patient survival26. Habr-Gama et al. have demonstrated good disease-free survival rates in patients with a complete absence of LNs in their specimens27. We also found that with a cutoff of less than 7, the number of LNs retrieved was not a prognostic factor. Decreased LN retrieval might reflect improved response to preop-RT rather than inappropriate or suboptimal surgical resection in this setting. Most previously published articles determined LN cutoffs considering only a single value, while they barely used one-by-one analysis. In our study, we found that if the cutoff value was greater than 16, the 5-year RCSS rate in patients with a greater number of LNs (i.e., more than the cutoff) would decrease gradually, losing its prognosis value after the number 17(P > 0.05). Several hypotheses could explain this finding. First, the fact that harvesting a higher total number of LNs may result in higher detection rate of metastatic LNs with resultant upstaging of cancer, - a widely accepted concept in surgery; Second, previous study showed that greater LN retrieval could indicate a worse preop-RT response25. On average, 5 more LNs were retrieved from ypN2 stage patients compared with ypN1 stage patients (15 VS 10), and a poorer tumor regression score results in shorter RCSS rates25,26,27.
This large population-based study has several potential limitations. First, the SEER database does not include information on the administration of chemotherapy. It is possible that additional or concurrent chemotherapy decreases the number of LNs more than preop-RT alone. Second, individual surgeons, pathologists, and other factors may affect LN harvest, but we cannot adjust for these factors. Finally, SEER database also does not report data on preoperative clinical stage, or tumor regression score, so we cannot analyze the relationship between tumor regression score and LNs retrieval in this study.
In conclusion, our analysis of the SEER database revealed that the number of LNs retrieved was a prognostic factor only for numbers ranging from 8 to 16(except for 13) with a maximum χ2 log-rank value of 14.006 at a cutoff of 10. The good tumor response associated with a reduced number of examined LNs may offset the influence of potential under staging with a cutoff of less than 7. Increased LN retrieval (≥17) could indicate worse preop-RT response and poorer RCSS.
Methods
Patient selection in the SEER database
Data from the Surveillance Epidemiology and End Results (SEER) Program of the United States National Cancer Institute, release 2015, were utilized for this study. SEER, a population-based cancer registry, collects cancer incidence and survival data from 18 regional population-based registries covering approximately 26% of the US population. The SEER data contain no identifiers and are publicly available for studies of cancer-based epidemiology and TNM staging of colorectal22,28, gastric29, esophageal cancer30 and other cancers.
SEER registry patients eligible for this cohort included those with adenocarcinoma, NOS (8150/3), adenocarcinoma in adenomatous polyps (8210/3), villous adenoma (8261/3) or tubulovillous adenoma (8263/3), mucinous adenocarcinoma (8480/3), signet ring cell carcinoma (8490/3) of the rectum diagnosed from 1998 through 2005 and treated with surgical resection. The inclusive study dates were chosen to allow a known treatment sequence of “radiation prior to surgery”. The patient age was limited to 18 to 80 years old. Patients diagnosed after 2006 were excluded to ensure adequate follow-up times. Additional exclusion criteria were as follows: no LNs examined pathologically, ypN0 patients, with synchronous distance metastases, and patients who died within 30 postoperative days.
The following information was retrieved from the SEER database: the patient age, sex, race, extension of primary tumor invasion, radiation sequence, total number of LNs examined, number of involved LNs, grade, survival time, and SEER cause-specific death classification. All cases were restaged based on the AJCC-7 staging. The primary endpoint of the study is RCSS which was calculated from the date of diagnosis to the date of cancer specific death. Deaths attributed to the cancer of interest are treated as events and deaths from other causes are treated as censored observation.
Statistical analysis
The LNs cutoff points were analyzed using the X-tile program (http://www.tissuearray.org/rimmlab/), which identified the cutoff with the minimum P values from log-rank χ2 statistics for the categorical LNs in terms of survival31. The relationship between various clinical and histological variables and survival was evaluated using the Kaplan-Meier method. Differences between survival curves were tested for statistical significance by using log rank test. The Cox proportional hazard regression model was used to identify the variables that could independently influence survival in preop-RT rectal cancer patients. The Mann-Whitney U test was used for continuous variables, and the chi-square test was used for categorical variables. The 5-year RCSS rate was estimated from Kaplan-Meier curves. Deaths attributed to the rectal cancer of interest are treated as events and deaths from other causes are treated as censored observation. Statistical analyses were performed with the statistical software package SPSS (Statistical Package for the Social Sciences) for Windows, version 17 (SPSS Inc, Chicago, IL, USA). Two-sided p values of less than 0.05 were considered to be statistically significant.
Additional Information
How to cite this article: Li, Q. et al. Effect of Lymph Node Count on Pathological Stage III Rectal Cancer with Preoperative Radiotherapy. Sci. Rep. 5, 16990; doi: 10.1038/srep16990 (2015).
Acknowledgments
The authors would like to thank SEER for open access to the database and Huixun Jia, and Jing Zhu from Center for Biomedical Statistical, Fudan University Shanghai Cancer Center for providing support on statistics help. This study was partially supported by grants from the National Natural Science Foundation of China (No. 81372646, 81101586).
Footnotes
Author Contributions Q.G.L., X.X.L and S.J.C. conceived of and designed the study. X.X.L, L.L. and L.G. performed the analyses. G.X.C and X.X.L prepared all figures and tables. Q.G.L and X.X.L wrote the main manuscript. All authors reviewed the manuscript.
References
- Kapiteijn E. et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. N Engl J Med 345, 638–646 (2001). [DOI] [PubMed] [Google Scholar]
- Sauer R. et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med 351, 1731–1740 (2004). [DOI] [PubMed] [Google Scholar]
- Gunderson L. L., Jessup J. M., Sargent D. J., Greene F. L. & Stewart A. K. Revised TN categorization for colon cancer based on national survival outcomes data. J Clin Oncol 28, 264–271 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong K. D., Lee S. I. & Moon H. Y. Lymph node ratio as determined by the 7th edition of the American Joint Committee on Cancer staging system predicts survival in stage III colon cancer. J Surg Oncol 103, 406–410 (2011). [DOI] [PubMed] [Google Scholar]
- Suzuki O. et al. Number of lymph node metastases is better predictor of prognosis than level of lymph node metastasis in patients with node-positive colon cancer. J Am Coll Surg 202, 732–736 (2006). [DOI] [PubMed] [Google Scholar]
- Baxter N. N., Morris A. M., Rothenberger D. A. & Tepper J. E. Impact of preoperative radiation for rectal cancer on subsequent lymph node evaluation: a population-based analysis. Int J Radiat Oncol Biol Phys 61, 426–431 (2005). [DOI] [PubMed] [Google Scholar]
- Li Q. et al. Lymph node count after preoperative radiotherapy is an independently prognostic factor for pathologically lymph node-negative patients with rectal cancer. Medicine 94, e395 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibold T. et al. Prognostic implications of the distribution of lymph node metastases in rectal cancer after neoadjuvant chemoradiotherapy. J Clin Oncol 26, 2106–2111 (2008). [DOI] [PubMed] [Google Scholar]
- Compton C. C. et al. Prognostic factors in colorectal cancer. College of American Pathologists Consensus Statement 1999. Arch Pathol Lab Med 124, 979–994 (2000). [DOI] [PubMed] [Google Scholar]
- Baxter N. N. et al. Lymph node evaluation in colorectal cancer patients: a population-based study. J Natl Cancer Inst 97, 219–225 (2005). [DOI] [PubMed] [Google Scholar]
- Le Voyer T. E. et al. Colon cancer survival is associated with increasing number of lymph nodes analyzed: a secondary survey of intergroup trial INT-0089. J Clin Oncol 21, 2912–2919 (2003). [DOI] [PubMed] [Google Scholar]
- Swanson R. S., Compton C. C., Stewart A. K. & Bland K. I. The prognosis of T3N0 colon cancer is dependent on the number of lymph nodes examined. Ann Surg Oncol 10, 65–71 (2003). [DOI] [PubMed] [Google Scholar]
- Prandi M. et al. Prognostic evaluation of stage B colon cancer patients is improved by an adequate lymphadenectomy: results of a secondary analysis of a large scale adjuvant trial. Ann Surg 235, 458–463 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tepper J. E. et al. Impact of number of nodes retrieved on outcome in patients with rectal cancer. J Clin Oncol 19, 157–163 (2001). [DOI] [PubMed] [Google Scholar]
- Fielding L. P. et al. Clinicopathological staging for colorectal cancer: an International Documentation System (IDS) and an International Comprehensive Anatomical Terminology (ICAT). J Gastroenterol Hepatol 6, 325–344 (1991). [DOI] [PubMed] [Google Scholar]
- Klopfleisch R., Weiss A. T. & Gruber A. D. Excavation of a buried treasure–DNA, mRNA, miRNA and protein analysis in formalin fixed, paraffin embedded tissues. Histol Histopathol 26, 797–810 (2011). [DOI] [PubMed] [Google Scholar]
- Vather R. et al. Lymph node examination as a predictor of long-term outcome in Dukes B colon cancer. Int J Colorectal Dis 24, 283–288 (2009). [DOI] [PubMed] [Google Scholar]
- Chen S. L. & Bilchik A. J. More extensive nodal dissection improves survival for stages I to III of colon cancer: a population-based study. Ann Surg 244, 602–610 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caplin S., Cerottini J. P., Bosman F. T., Constanda M. T. & Givel J. C. For patients with Dukes’ B (TNM Stage II) colorectal carcinoma, examination of six or fewer lymph nodes is related to poor prognosis. Cancer 83, 666–672 (1998). [PubMed] [Google Scholar]
- Sarli L. et al. Number of lymph nodes examined and prognosis of TNM stage II colorectal cancer. Eur J Cancer 41, 272–279 (2005). [DOI] [PubMed] [Google Scholar]
- Li Q. et al. Increased number of negative lymph nodes is associated with improved cancer specific survival in pathological IIIB and IIIC rectal cancer treated with preoperative radiotherapy. Oncotarget 5, 12459–12471 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunderson L. L., Jessup J. M., Sargent D. J., Greene F. L. & Stewart A. Revised tumor and node categorization for rectal cancer based on surveillance, epidemiology, and end results and rectal pooled analysis outcomes. J Clin Oncol 28, 256–263 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fajardo L. F. Effects of ionizing radiation on lymph nodes. A review. Front Radiat Ther Oncol 28, 37–45 (1994). [DOI] [PubMed] [Google Scholar]
- Shvero J. et al. Histological changes in the cervical lymph nodes after radiotherapy. Oncol Rep 8, 909–911 (2001). [DOI] [PubMed] [Google Scholar]
- de Campos-Lobato L. F. et al. Less than 12 nodes in the surgical specimen after total mesorectal excision following neoadjuvant chemoradiation: it means more than you think! Ann Surg Oncol 20, 3398–3406 (2013). [DOI] [PubMed] [Google Scholar]
- Rullier A. et al. Lymph nodes after preoperative chemoradiotherapy for rectal carcinoma: number, status, and impact on survival. Am J Surg Pathol 32, 45–50 (2008). [DOI] [PubMed] [Google Scholar]
- Habr-Gama A. et al. Absence of lymph nodes in the resected specimen after radical surgery for distal rectal cancer and neoadjuvant chemoradiation therapy: what does it mean? Dis Colon Rectum 51, 277–283 (2008). [DOI] [PubMed] [Google Scholar]
- Gao P. et al. Is the prediction of prognosis not improved by the seventh edition of the TNM classification for colorectal cancer? Analysis of the surveillance, epidemiology, and end results (SEER) database. BMC Cancer 13, 123 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X. et al. Comparison of three lymph node staging schemes for predicting outcome in patients with gastric cancer. Br J Surg 100, 505–514 (2013). [DOI] [PubMed] [Google Scholar]
- Hu Y., Hu C., Zhang H., Ping Y. & Chen L. Q. How does the number of resected lymph nodes influence TNM staging and prognosis for esophageal carcinoma? Ann Surg Oncol 17, 784–790 (2010). [DOI] [PubMed] [Google Scholar]
- Camp R. L., Dolled-Filhart M. & Rimm D. L. X-tile: a new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin Cancer Res 10, 7252–7259 (2004). [DOI] [PubMed] [Google Scholar]