Abstract
Hydroxychloroquine (HCQ) retinopathy can result in permanent vision loss. In early stages of HCQ retinopathy, patients are usually asymptomatic with preservation of visual acuity. We aspire that our review, in conjunction with the American Academy of Ophthalmology screening guidelines, shall shed light on effective screening measures utilizing multimodal imaging techniques to detect early signs of HCQ retinopathy before advanced changes manifest clinically.
Keywords: Hydroxychloroquine, retinal diagnostics, vitreoretinal surgery
Hydroxychloroquine (HCQ) is an anti-malarial medication that has in recent times been utilized as treatment for a variety of autoimmune diseases, such as rheumatoid arthritis and systemic lupus erythematosus, and other inflammatory and dermatologic conditions.[1] Retinal toxicity from HCQ, and its analog, chloroquine, has been recognized for many years.[2,3] By some estimates, in the United States, there are more than 150,000 patients currently on chronic HCQ therapy.[4] The incidence of HCQ retinopathy is very low. In fact, HCQ is estimated at having a 0.5% incidence of retinal toxicity after 5 years of therapy.[5,6] Retinal toxicity secondary to HCQ is irreversible and can continue to progress following cessation of therapy.[7] Prompt screening and serial monitoring, with the utilization of imaging modalities, is paramount importance to early detection. This may not prevent HCQ retinopathy but would prevent vision loss or significant visual impairment. We aspire that our review shall shed light on effective screening measures utilizing multimodal imaging techniques to detect early signs of HCQ retinopathy before advanced changes manifest clinically.
Clinical Presentation
In early stages of HCQ retinopathy, patients are usually asymptomatic with preservation of visual acuity. In fact, objective changes typically precede a patient's complaint of vision loss. Rarely, when investigated, a patient may be found to have subtle changes in night vision, diminished color vision, or a paracentral scotoma.[8] When allowed to advance, HCQ retinopathy leads of significant deterioration of these visual functions (visual acuity, peripheral vision, and night vision). The paracentral scotoma experienced by the patient can worsen, often with a subjective complaint of difficulty reading, before any changes are appreciated on dilated fundus examination. The classic “Bull's eye maculopathy” of HCQ retinopathy, characterized by a perifoveal ring of retinal pigment epithelium (RPE) atrophy that spares the fovea, is a late finding suggestive of advanced and usually irreversible damage. Therefore, the screening of asymptomatic patients with clinical and fundus examination alone is not sufficient, this must be done in conjunction with multimodal imaging to detect early evidence of HCQ retinopathy.[9]
Screening Guidelines
In 2011, revised guidelines for HCQ retinopathy screening were published by the American Academy of Ophthalmology (AAO), with an emphasis on more sensitive diagnostic imaging techniques.[8] The AAO recommends that all patient receive a baseline evaluation before initiating HCQ therapy. This baseline evaluation entails a dilated fundus examination, 10-2 white pattern visual field test (subjective), and at least one of the following objective tests: Spectral-domain optical coherence tomography (SD-OCT), fundus autofluorescence (FAF), or multifocal electroretinogram (mfERG). The frequency of future monitoring is based upon the patient's classification of “low” or “high” risk. The AAO guidelines defined high-risk patients by any one of the following criteria: HCQ treatment >5 years in duration, HCQ dose >6.5 mg/kg/day, significant renal or hepatic disease, preexisting maculopathy due to other etiology, age >60 years, obesity, or cumulative HCQ consumption of 1000 g. Patients who fulfill these high-risk criteria are recommended to have annual follow-up examinations. In contrast, low-risk patients are recommended to have follow-up examinations beginning 5 years after the baseline evaluation. Also, immediate cessation of HCQ therapy should be recommended, in conjunction with the patient's rheumatologist, whenever a patient is found to have probable or definite HCQ retinopathy.
Imaging Modalities
Visual fields
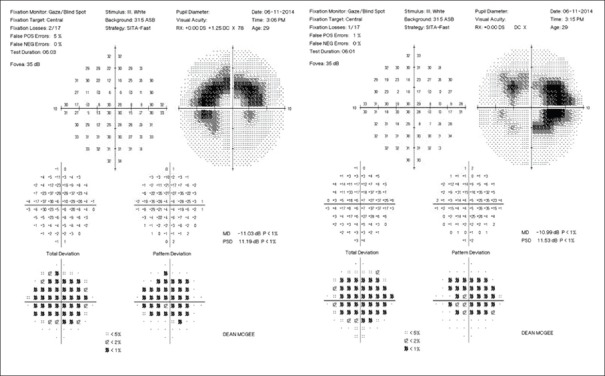
The subjective component to HCQ retinopathy screening is an automated visual field testing the central 10° of vision. The earliest signs of HCQ retinopathy on a central visual field may present as a cluster of paracentral points with decreased sensitivity. These points may be only mildly depressed. Further, HCQ retinopathy leads to a partial bull's eye scotoma that may resemble an arcuate defect although it is more central than that commonly seen from glaucomatous damage [Fig. 1]. Late toxicity may present as complete bull's eye scotoma, with a complete ring defect, relatively sparing the fovea.[9]
Figure 1.
10-2 visual fields: Bilateral paracentral scotoma
Prior recommendations were that Amsler grid screening is sufficient for screening for HCQ retinopathy. However, Amsler grid is not as reliable for detecting subtle decreases in retinal sensitivity in the earliest signs of HCQ retinopathy. Marmor et al. emphasized the importance of not ignoring even subtle defects on a screening visual field because HCQ retinopathy begins with such defects.[8] Unfortunately, patients frequently have nonspecific visual field defects from dry eyes or attention problems, thereby confounding the clinical presentation. It is necessary, therefore, to repeat the visual fields whenever there are even mild defects, in order to determine reproducibility. If the defects are reproducible, further evaluation for HCQ retinopathy is indicated.
Fundus photography
Visible HCQ retinopathy is often a late clinical finding [Fig. 2]. Early signs of HCQ retinopathy may present subtle changes in RPE but may also be subclinical [Fig. 3]. Therefore, fundus photography is not recommended by the AAO guidelines and has an equivalent bearing on HCQ retinopathy screening as dilated fundus examination.[8] However, some clinicians obtain fundus photography to document their findings.
Figure 2.
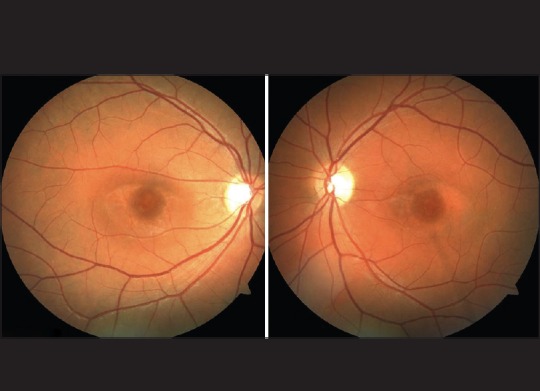
Color fundus photography: Bilateral parafoveal retinal pigment atrophy (“Bull's eye maculopathy”)
Figure 3.
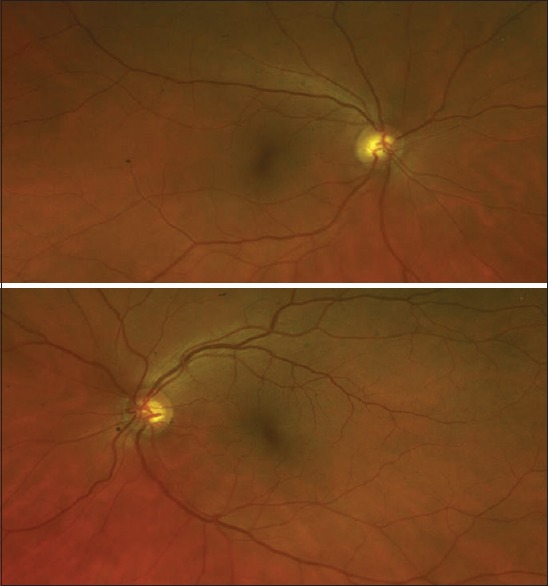
Color Fundus photography: No clinically evident retinopathy, despite corresponding changes in spectral-domain optical coherence tomography [Figure 5]
Spectral-domain optical coherence tomography
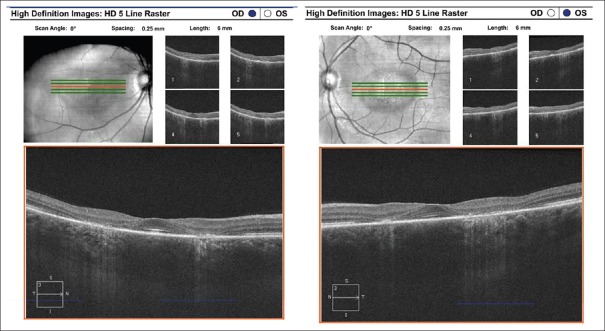
High-resolution cross-sectional images of the retina using SD-OCT may detect distinctive changes before the development of clinically visible HCQ retinopathy.[10] On SD-OCT, HCQ retinopathy manifests as disruption, or complete loss, of the outer nuclear layer, external limiting membrane, inner/outer segment junction, and RPE in the parafoveal region [Fig. 4].[9] Usually, there is a preservation of the subfoveal retinal tissue, leading some to believe in the concept of a relative “foveal resistance,” thereby providing many patients with relatively adequate central visual acuity, in spite of advanced perifoveal HCQ retinopathy.[9] This foveal-sparing accounts for the “flying saucer sign” of HCQ retinopathy [Fig. 4]. Chen et al. first described the “flying saucer sign” as an ovoid appearance secondary to the intact central subfoveal architecture and the loss of adjacent perifoveal outer retinal tissue.[11]
Figure 4.
Spectral-domain optical coherence tomography: Bilateral parafoveal outer retinal and retinal pigment epithelium atrophy with central sparing (“flying saucer sign”)
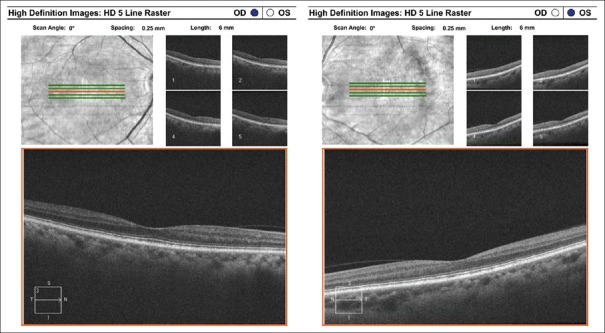
Recently, there has been some focus on the effects of HCQ on the inner retinal layers. In a study by Pasadhika et al., SD-OCT was applied to measure the thickness of certain segments of the retina in three groups of patients: Those with clinical evidence of HCQ retinopathy on fundus examination, those with chronic HCQ use (at least 6 years) but no clinical abnormalities, and a control group of patients with no HCQ exposure. In patients with chronic HCQ exposure, they found a selective thinning of the ganglion cell layer (GCL) and inner plexiform layer (IPL) without any structural changes to the outer retinal layers and RPE.[12] The authors also mentioned that there was no significant thinning of the retinal nerve fiber layer (RNFL), despite GCL thinning. This is attributable to the fact that significant GCL thinning must occur before RNFL thinning can be detected by SD-OCT. Another study by the same group evaluated both inner and outer retinal changes using SD-OCT in chronically treated HCQ-patients with and without clinically evident HCQ retinopathy.[13] It was observed that patients with clinical evidence of HCQ retinopathy had both inner, and outer retinal thinning, whereas those with normal fundus examinations had only selective thinning of the inner retina [Fig. 5]. In short, these studies suggest that developing screening methods that measure the inner layers of the retina may achieve earlier detection of HCQ retinopathy than those that measure the outer retinal layers. However, the temporal relationship between GCL and IPL thinning and decreased visual function remains to be established.
Figure 5.
Spectral-domain optical coherence tomography: Selective inner retinal layer thinning with sparing of the outer retinal or retinal pigment epithelium layers
Fundus autofluorescence
FAF imaging is a novel in-vivo imaging method assessing the distribution of lipofuscin in the outer retina, subretinal space, and RPE.[14] An increased FAF signal intensity indicates the excessive accumulation of lipofuscin granules, in particular the A2E fluorophore, in the lysosomal compartment of the RPE, thereby indicative of abnormal metabolism of outer segment photoreceptors or inadequate phagocytosis of these materials.[14] Conversely, a diminished or complete loss of FAF signal intensity is indicative of RPE cell death [Fig. 6].[15] Preceding the onset of a “Bull's eye maculopathy” suggestive of the perifoveal outer retina and RPE damage, FAF may show subtlety increased signal intensity in this distribution secondary to early photoreceptor damage.[16] These changes have been correlated with abnormalities on coexisting SD-OCT for the same patient.[9] Due to these FAF changes being quite subtle, clinician dependent, and inadequate in detecting subclinical HCQ retinopathy, FAF is not considered to be reliable as a primary screening modality.[17] Instead, it is recommended by the AAO guidelines that FAF be applied in conjunction with other imaging tools, such as 10-2 visual fields and SD-OCT to screen for HCQ retinopathy.[8]
Figure 6.
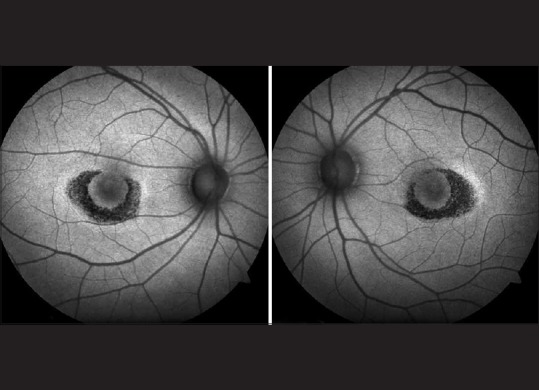
Fundus autofluorescence: Bilateral parafoveal hypoautofluorescence
Multifocal electroretinography
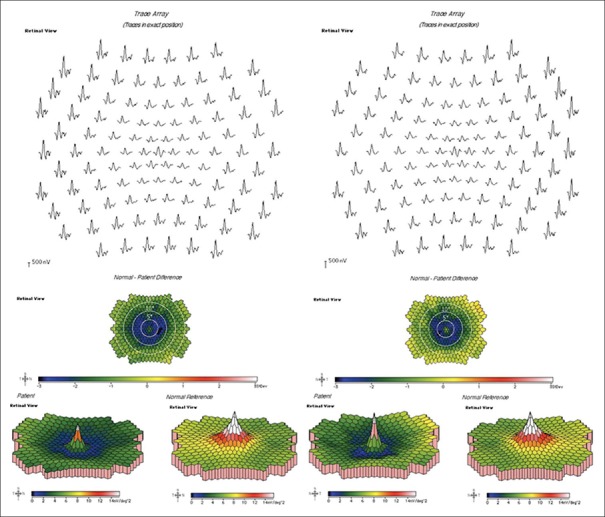
mfERG may be the most sensitive test for early HCQ retinopathy,[18] and numerous studies have evaluated the use of mfERG in the screening of HCQ retinopathy.[19,20] Unlike full-field ERG, mfERG can localize deficiencies to the central macula, thereby detecting the subtle changes characteristic of early HCQ retinopathy.[21,22] Specifically, paracentral reductions in amplitude, indicative of depressed retinal function, are the most specific waveform pattern for HCQ retinopathy [Fig. 7]. Also, when associated with increased implicit times, these paracentral amplitude reductions are more specific for HCQ retinopathy.[23] These responses from mfERG have been shown to have good correlation to the mean deviations seen in 10-2 visual field testing.[24] This provides objective measurements of retinal function to supplement the information gathered from subjective visual field testing. Despite mfERG showing promise as an objective measure of retinal function in screening for early HCQ retinopathy, its utilization is limited by access to equipment, need for trained technician staff to perform and interpret testing, patient cooperation, and cost.
Figure 7.
Multifocal electroretinogram: Paracentral and generalized signal attenuation
Discussion
Despite the widespread application of these imaging modalities, there exists neither consensus on which test has the best sensitivity for screening nor the best specificity for confirming the presence of early HCQ retinopathy. We advise that a comprehensive approach should be taken in evaluation for HCQ retinopathy, and not to solely rely on a single imaging modality or clinical finding.
Conclusion
The integrity of the foveal outer segment structures is paramount importance in maintaining visual acuity and should be assessed periodically using multimodal imaging. Once HCQ retinopathy is documented, discontinuation of medication should be recommended, in cooperation with the patient's internist or rheumatologist. Patients should be advised that HCQ retinopathy may progress despite discontinuation of medication, and the etiology of this progression is poorly understood. We aspire that larger clinical studies will be performed, focusing on various SD-OCT indices, in order to better evaluate earlier detection methods for HCQ retinopathy.
Financial support and sponsorship
National Eye Institute, National institute of Health (EY-022071, EY-025256); The Foundation Fighting Blindness Inc. USA; and The Research to Prevent Blindness, Inc. USA.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Ben-Zvi I, Kivity S, Langevitz P, Shoenfeld Y. Hydroxychloroquine: From malaria to autoimmunity. Clin Rev Allergy Immunol. 2012;42:145–53. doi: 10.1007/s12016-010-8243-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Easterbrook M. Long-term course of antimalarial maculopathy after cessation of treatment. Can J Ophthalmol. 1992;27:237–9. [PubMed] [Google Scholar]
- 3.Elman A, Gullberg R, Nilsson E, Rendahl I, Wachtmeister L. Chloroquine retinopathy in patients with rheumatoid arthritis. Scand J Rheumatol. 1976;5:161–6. doi: 10.3109/03009747609165456. [DOI] [PubMed] [Google Scholar]
- 4.Semmer AE, Lee MS, Harrison AR, Olsen TW. Hydroxychloroquine retinopathy screening. Br J Ophthalmol. 2008;92:1653–5. doi: 10.1136/bjo.2008.144402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mavrikakis M, Papazoglou S, Sfikakis PP, Vaiopoulos G, Rougas K. Retinal toxicity in long term hydroxychloroquine treatment. Ann Rheum Dis. 1996;55:187–9. doi: 10.1136/ard.55.3.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wolfe F, Marmor MF. Rates and predictors of hydroxychloroquine retinal toxicity in patients with rheumatoid arthritis and systemic lupus erythematosus. Arthritis Care Res (Hoboken) 2010;62:775–84. doi: 10.1002/acr.20133. [DOI] [PubMed] [Google Scholar]
- 7.Mavrikakis I, Sfikakis PP, Mavrikakis E, Rougas K, Nikolaou A, Kostopoulos C, et al. The incidence of irreversible retinal toxicity in patients treated with hydroxychloroquine: A reappraisal. Ophthalmology. 2003;110:1321–6. doi: 10.1016/S0161-6420(03)00409-3. [DOI] [PubMed] [Google Scholar]
- 8.Marmor MF, Kellner U, Lai TY, Lyons JS, Mieler WF. American Academy of Ophthalmology. Revised recommendations on screening for chloroquine and hydroxychloroquine retinopathy. Ophthalmology. 2011;118:415–22. doi: 10.1016/j.ophtha.2010.11.017. [DOI] [PubMed] [Google Scholar]
- 9.Marmor MF. Comparison of screening procedures in hydroxychloroquine toxicity. Arch Ophthalmol. 2012;130:461–9. doi: 10.1001/archophthalmol.2011.371. [DOI] [PubMed] [Google Scholar]
- 10.Rodriguez-Padilla JA, Hedges TR, 3rd, Monson B, Srinivasan V, Wojtkowski M, Reichel E, et al. High-speed ultra-high-resolution optical coherence tomography findings in hydroxychloroquine retinopathy. Arch Ophthalmol. 2007;125:775–80. doi: 10.1001/archopht.125.6.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen E, Brown DM, Benz MS, Fish RH, Wong TP, Kim RY, et al. Spectral domain optical coherence tomography as an effective screening test for hydroxychloroquine retinopathy (the “flying saucer” sign) Clin Ophthalmol. 2010;4:1151–8. doi: 10.2147/OPTH.S14257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pasadhika S, Fishman GA, Choi D, Shahidi M. Selective thinning of the perifoveal inner retina as an early sign of hydroxychloroquine retinal toxicity. Eye (Lond) 2010;24:756–62. doi: 10.1038/eye.2010.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pasadhika S, Fishman GA. Effects of chronic exposure to hydroxychloroquine or chloroquine on inner retinal structures. Eye (Lond) 2010;24:340–6. doi: 10.1038/eye.2009.65. [DOI] [PubMed] [Google Scholar]
- 14.Schmitz-Valckenberg S, Holz FG, Bird AC, Spaide RF. Fundus autofluorescence imaging: Review and perspectives. Retina. 2008;28:385–409. doi: 10.1097/IAE.0b013e318164a907. [DOI] [PubMed] [Google Scholar]
- 15.Holz FG, Bellman C, Staudt S, Schütt F, Völcker HE. Fundus autofluorescence and development of geographic atrophy in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2001;42:1051–6. [PubMed] [Google Scholar]
- 16.Kellner U, Renner AB, Tillack H. Fundus autofluorescence and mfERG for early detection of retinal alterations in patients using chloroquine/hydroxychloroquine. Invest Ophthalmol Vis Sci. 2006;47:3531–8. doi: 10.1167/iovs.05-1290. [DOI] [PubMed] [Google Scholar]
- 17.Marmor MF. Fundus autofluorescence is not the best early screen for hydroxychloroquine toxicity. JAMA Ophthalmol. 2013;131:1487–8. doi: 10.1001/jamaophthalmol.2013.4835. [DOI] [PubMed] [Google Scholar]
- 18.Penrose PJ, Tzekov RT, Sutter EE, Fu AD, Allen AW, Jr, Fung WE, et al. Multifocal electroretinography evaluation for early detection of retinal dysfunction in patients taking hydroxychloroquine. Retina. 2003;23:503–12. doi: 10.1097/00006982-200308000-00010. [DOI] [PubMed] [Google Scholar]
- 19.Chang WH, Katz BJ, Warner JE, Vitale AT, Creel D, Digre KB. A novel method for screening the multifocal electroretonogram in patients using hydroxychloroquine. Retina. 2008;28:1478–86. doi: 10.1097/IAE.0b013e318181445b. [DOI] [PubMed] [Google Scholar]
- 20.Lyons JS, Severns ML. Using multifocal ERG ring ratios to detect and follow Plaquenil retinal toxicity: A review: Review of mfERG ring ratios in Plaquenil toxicity. Doc Ophthalmol. 2009;118:29–36. doi: 10.1007/s10633-008-9130-0. [DOI] [PubMed] [Google Scholar]
- 21.Lyons JS, Severns ML. Detection of early hydroxychloroquine retinal toxicity enhanced by ring ratio analysis of multifocal electroretinography. Am J Ophthalmol. 2007;143:801–9. doi: 10.1016/j.ajo.2006.12.042. [DOI] [PubMed] [Google Scholar]
- 22.Maturi RK, Folk JC, Nichols B, Oetting TT, Kardon RH. Hydroxychloroquine retinopathy. Arch Ophthalmol. 1999;117:1262–3. doi: 10.1001/archopht.117.9.1262. [DOI] [PubMed] [Google Scholar]
- 23.Maturi RK, Yu M, Weleber RG. Multifocal electroretinographic evaluation of long-term hydroxychloroquine users. Arch Ophthalmol. 2004;122:973–81. doi: 10.1001/archopht.122.7.973. [DOI] [PubMed] [Google Scholar]
- 24.Lai TY, Ngai JW, Chan WM, Lam DS. Visual field and multifocal electroretinography and their correlations in patients on hydroxychloroquine therapy. Doc Ophthalmol. 2009;119:29–36. doi: 10.1007/s10633-006-9006-0. [DOI] [PubMed] [Google Scholar]