Abstract
The peripheral retina is the site of pathology in many ocular diseases and ultra-widefield (UWF) imaging is one of the new technologies available to ophthalmologists to manage some of these diseases. Currently, there are several imaging systems used in practice for the purpose of diagnostic, monitoring disease progression or response to therapy, and telemedicine. These include modalities for both adults and pediatric patients. The current systems are capable of producing wide- and UWF color fundus photographs, fluorescein and indocyanine green angiograms, and autofluorescence images. Using this technology, important clinical observations have been made in diseases such as diabetic retinopathy, uveitides, retinal vascular occlusions and tumors, intraocular tumors, retinopathy of prematurity, and age-related macular degeneration. Widefield imaging offers excellent postoperative documentation of retinal detachment surgery. New applications will soon be available to integrate this technology into large volume routine clinical practice.
Keywords: Retina, ultra-widefield retinal imaging, widefield retinal imaging
The peripheral retina is the site of pathology in many ocular diseases such as diabetic retinopathy (DR), retinal vein occlusions, uveitis, vasculitis, choroidal and retinal masses, retinal tears, and detachments. While careful clinical examination of patient's peripheral retina with scleral indentation is of utmost importance in clinical decision making, there is a need for additional testing and an objective and reliable documentation of findings. Recent advances in the development of diagnostic imaging techniques have played an increasing role in better assessment of retinal periphery. Ultra-widefield (UWF) imaging is one of the new technologies available to retinal specialists. Current UWF imaging modalities can provide several options for posterior segment documentation and evaluation, including options for color images, red-free images, fluorescein and indocyanine green angiography, and fundus autofluorescence.[1,2] Data from these modern imaging devices has truly changed the way ophthalmologists evaluate a patient picture and has led to more understanding of the role of the peripheral pathology in retinal diseases.
Evolution of Ultra-Widefield Imaging Technology
The first reliable fundus camera, which allowed documentation of ocular fundus structures, was introduced by Carl Zeiss and J.W. Nordensen in 1926. This camera provided a 20° fundus image. Years later, Carl Zeiss Company expanded the field of view to 30° which became the standard for the traditional fundus camera.[3] This field of view obtained with traditional fundus cameras was adequate for imaging of the optic nerve and the posterior pole but provided a limited view of the retinal periphery. A protocol consisting of 7-standard 30° images was developed by the DR study for acquiring images of the retinal periphery in a systematic manner. The width of these composite images is approximately 75°. Photographs anterior to the equator may be obtained with this protocol, but they will not image structures in the far periphery.[4] This approach was extended to 9-standard fields protocol for the longitudinal studies of ocular complications of AIDS protocol to capture peripheral cytomegalovirus retinitis. Per specifications of the Fundus Photography Reading Center at the University of Wisconsin, retinal cameras approved for this procedure had 50° or 60° magnification settings. Such photography, however, may be limited by patient alignment problems, focusing irregularities, marginal corneal astigmatism, poor fixation, and light reflex artifacts [5] [Fig. 1].
Figure 1.
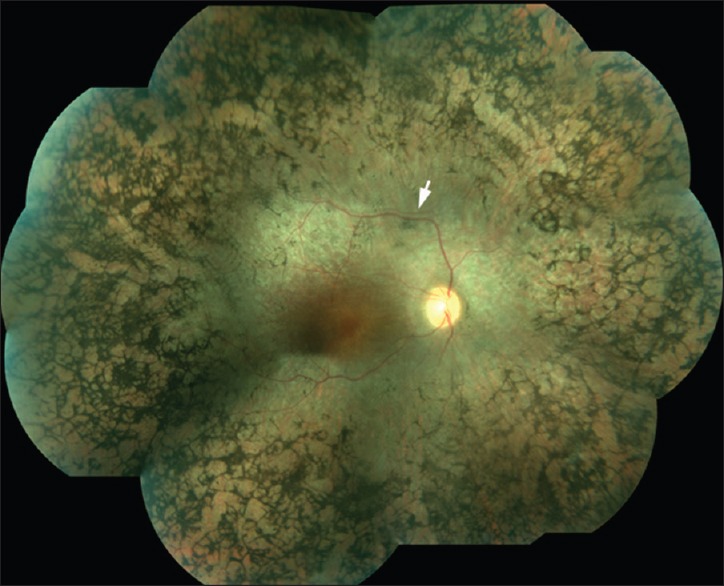
A mosaic fundus photo of the right eye of a 42-year-old female with advanced retinitis pigmentosa showing waxy pallor of the optic nerve head, attenuated retinal blood vessels, numerous bone spicule-like formations approaching the vascular arcades. White arrow points to a blood vessel shadow, an artifact during image alignment
Since that time, several advances have been made to posterior segment imaging that permit visualization of the retinal periphery in a practical manner. Equator-plus camera is a specialized contact lens-based camera that was developed in 1975 by Pomerantzeff. A fiber optic illumination was separated from the camera to minimize lens reflections and obtain a 148° capture from the retina anterior to the equator.[6] The Retcam (Clarity Medical Systems, Pleasanton, California, USA) is a portable wide-angle camera system that was made commercially available in 1997. It is a contact-based, coaxial illumination system which obtains 130° field of view.[7] The system is particularly well-suited for imaging pediatric patients because it is portable and can be placed directly on patients unable to position themselves, such as neonates and infants [Fig. 2a and b]. Specifically, this imaging modality has been well-studied in patients with retinopathy of prematurity. A major limitation in this technology, however, was its inability to image through lens opacities.[7]
Figure 2.
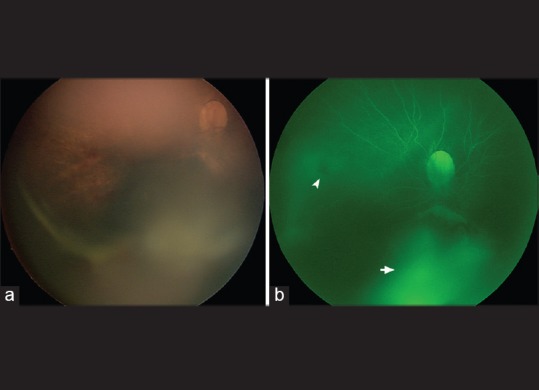
(a) A widefield fundus image of the right eye of a 3-month-old infant with dysplastic optic nerve head, intraretinal, and vitreous hemorrhages (RetCam, Pleasanton, CA, USA), (b) fluorescein angiography of the same eye as in a taken with the same imaging system showing retinal blood vessels filling, blockage from intraretinal hemorrhage (white arrowhead) and staining (white arrow)
A major cause of artifact with any fundus imaging arises from the reflection of light from interfaces in the ocular media. Elimination of these reflections is achieved using confocal scanning laser ophthalmoscopy (SLO), which separates the illuminating and imaging beam within the eye.[5]
Staurenghi et al. developed a combined contact and noncontact handheld lens system coupled with a SLO. The Staurenghi lens system. lens system obtained high-resolution images with a 150° field. However, this technique was cumbersome for the photographer [Fig. 3].[8]
Figure 3.
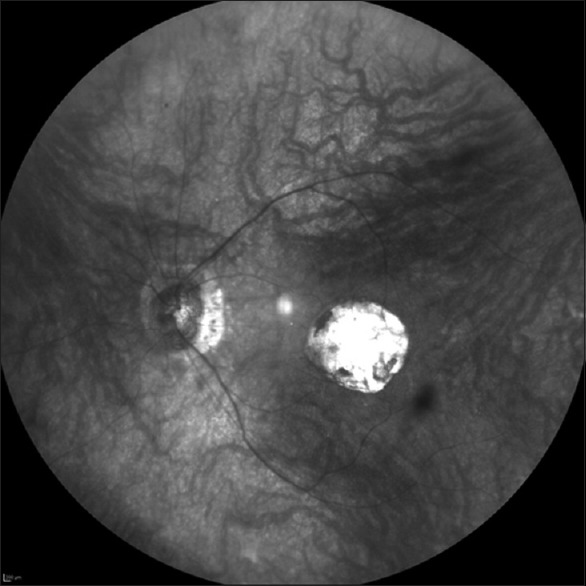
A widefield infrared image of the left eye of a 46-year-old man with macular coloboma of unknown origin. An image taken with noncontact lens (Heidelberg Engineering, Heidelberg, Germany) showing peripapillary atrophy, thin retinal blood vessels, and a well-defined choroidal vasculature
The Optos camera (Optos 200Tx, Dunfermline, UK) is a UWF imaging system, which produces a 200° view of the retina (about 82% of the surface area). The Optos technology utilizes a combined SLO with an ellipsoidal mirror to obtain images of the retinal periphery with one capture without the need for bright illumination lighting or a contact lens, and in some patients, pupillary dilation. The system provides the ability to capture red and green reflectance imaging, as well as fundus autofluorescence, and fluorescein/indocyanine green angiography [Fig. 4a and b].[9] Widefield imaging of the vitreous is now possible with the introduction of a biomicroscopic wide-angle retinal and vitreous observation system utilizing a 3 CCD video camera and a personal computer for image display by Lee and Chang [Figs. 5 and 6].[10] Finally, optical coherence tomography (OCT) technology is making progress toward examining wider areas of the retina. The systems have now expanded from 30° to 55° (Heidelberg Spectralis system, Heidelberg Engineering, Germany). With moving of the scan toward the periphery, some areas can be visualized that were previously unreachable for OCT imaging.[11] Following is a review of wide- and UWF imaging application in some retinochoroidal diseases.
Figure 4.
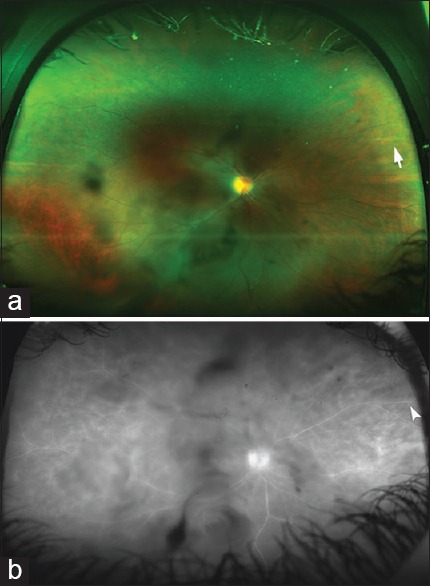
(a) An ultra-widefield color fundus photo of the right eye of a 36-year-old patient with a history of pars planitis in both eyes. The image was taken with an ultra-wide imaging system (Optos 200Tx, Dunfermline, UK). Dark shadows in the central field represent vitreous opacities. There is a sclerotic retinal vessel in the nasal periphery (white arrow) in the area of prior inflammation, (b) an ultra-widefield fluorescein angiogram of the same eye as in a showing central vitreous opacities and staining of the peripheral retinal vessel (white arrowhead)
Figure 5.
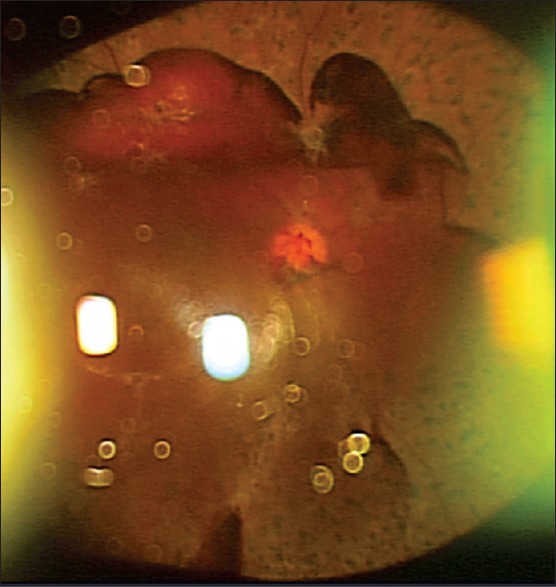
An ultra-widefield color fundus photo of an eye with proliferative diabetic retinopathy taken with slit-lamp and three-dimensional CCD camera. Inverted image shows preretinal hemorrhage inferiorly and scattered laser photocoagulation burns
Figure 6.
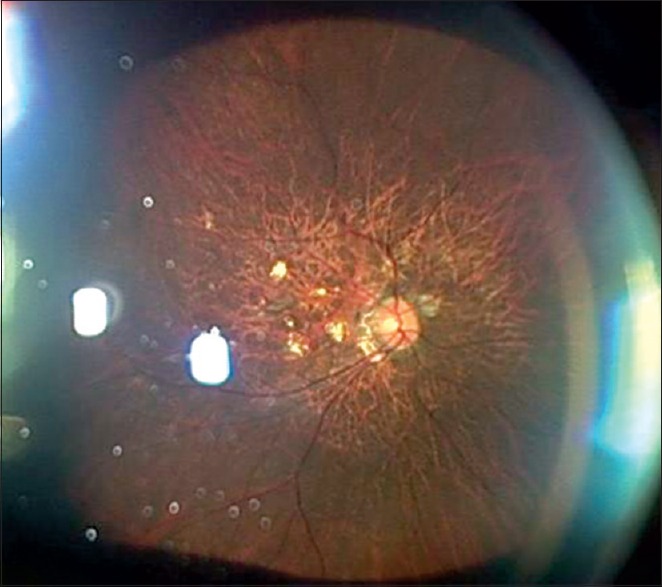
An ultra-widefield color fundus photo of the left eye with high myopia taken with slit-lamp and three-dimensional CCD camera. Inverted image shows rarefaction of the retinal pigment epithelium and patches of chorioretinal atrophy in the posterior pole
Diabetic Retinopathy
DR is a common complication of diabetes mellitus. It is one of the leading causes of blindness among adults and is estimated to be responsible for up to 17% of total blindness.[12,13] Diabetic macular edema (DME) is a major cause of vision loss among diabetic patients.[14,15] Ischemic changes and microvascular abnormalities in patients with DR have long been hypothesized to play a role in the development of DME. Ischemia stimulates the production of vascular endothelial growth factor (VEGF), which can lead to the breakdown of blood-retinal barriers, and development of macular edema.[16,17] Accordingly, ischemic changes in the peripheral retina may induce macular edema. Several studies have demonstrated the association between peripheral retinal nonperfusion and the occurrence of neovascularization (NV) and DME.[18,19] Conventional fluorescein angiography (FA) employs retinal photography that is able to view approximately 30° of the retina at 1 time, and hence missing the peripheral retina. With the advent of ultra-widefield fluorescein angiography (UWFA), it is now possible to view up to 200° of the retina in a single photograph measured from the ocular center. The ability to image the peripheral retina using UWFA provides a more comprehensive assessment of the extent of a retinal disease process, and may lead to detection of abnormalities that may alter treatment plans and give new insights into the pathogenesis of DME [Fig. 7].[1] New treatment modalities, like targeted retinal photocoagulation (TRP) may be effective for treatment of DME. It has been suggested that TRP may replace pan-retinal photocoagulation by directing therapy specifically at ischemic parts of the retina to precisely eliminate the source of VEGF, thus minimizing the sequelae of pan-retinal photocoagulation.[20] In addition, the combination of macular laser and anti-VEGF therapy, and TRP could prove to be an important treatment modality for DME. Despite the significant correlation of retinal ischemia with DME in patients with DR, Wessel et al. failed to detect an association between the amount of retinal ischemia and the degree of macular thickening.[21] In addition to the implication of UWFA in the management of DME, identification of specific areas of retinal nonperfusion with UWFA may allow targeted rather than pan photocoagulation in the treatment of NV.[22] If more laser is required it can at least be applied in a step-wise logical manner. This approach can minimize laser-induced side effects such as field loss and macular edema.[5] A recent study evaluated the efficacy of UWFA in the detection of diabetic pathology found that it was able to demonstrate retinal nonperfusion and NV in 10% of eyes that would have been missed by standard FA.[21]
Figure 7.
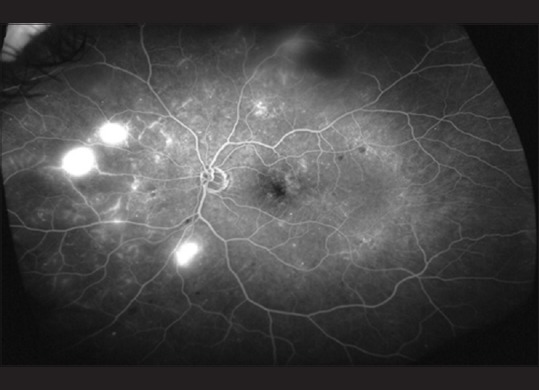
An ultra-widefield fluorescein angiogram of the left eye of a 54-year-old diabetic patient. The images show leakage in the macular area, peripheral areas of nonperfusion, and neovascularization nasally
Uveitis
The diagnosis of posterior uveitis is challenging. Accurate diagnosis often requires careful clinical examination of the retinal periphery as posterior uveitis is associated with significant changes in this region. Management plans depend on the clinical appearance and angiographic pattern of the retinal lesions. Significant retinal findings are likely to be missed by conventional FAs but can be visualized using UWFA.[23] Kaines et al. noted that the high-resolution images obtained with UWF technology allowed clear identification of peripheral retinal lesions and greatly enhanced objective documentation of disease activity and progression. Similar to cases of DR, UWF imaging allowed clear visualization of the areas of NV and peripheral nonperfusion, leading to limited sector pan-retinal photocoagulation, and hence minimizing complications.[5] The added information provided by the UWF images may alter management decisions compared with the standard examination and conventional imaging. Such difference most likely is attributed to peripheral retinal imaging and angiographic findings are not easily identified without widefield imaging.[24] UWF fundus autofluorescence imaging was helpful in monitoring areas of old or new retinal inflammatory activity in patients with uveitis. It revealed areas of focal loss of autofluorescence that were in high concordance with visual field testing results, which showed deep scotomas.[25]
Similarly, UWF imaging may allow earlier detection of disease activity in patients with noninfectious vasculitis, which may lead to earlier treatment and perhaps better patient outcomes [Fig. 8].[26] Leakage from retinal vessels may be seen before there are obvious ophthalmoscopic signs of vasculitis. In some cases of retinal vasculitis, the vessels anterior to the equator may be involved and cause peripheral leakage, ischemia, and NV, which are difficult to detect clinically. Accordingly, visualization of the peripheral retina could be essential to the diagnosis, monitoring, and treatment of retinal vasculitis.[26] Mesquida et al. assessed the role of UWF retinal imaging in the diagnosis and management of retinal vasculitis associated with Behçet disease.[27] They found that UWF retinal imaging had allowed clear documentation of peripheral retinal lesions and greatly simplified longitudinal comparisons for disease activity and progression. Peripheral vein sheathing and retinal infiltrates that denote disease activity were clearly detected with UWF pseudocolor imaging in their study. They also found that UWFA seems to evaluate precisely the early and subclinical retinal involvement. UWFA was a very helpful tool in their patients for determining whether the vasculitis had an occlusive nature and for quantifying the true extent of the capillary nonperfusion. Areas of retinal ischemia and NV were easily identified in their series, aiding targeted laser photocoagulation. The findings of the study also suggested that active retinal vasculitis in patients with Behçet disease may induce retinal epithelium alterations in the retinal periphery. These abnormalities were visible with UWF fundus autofluorescence as multiple hyper fluorescent spots in the retinal periphery.[27]
Figure 8.
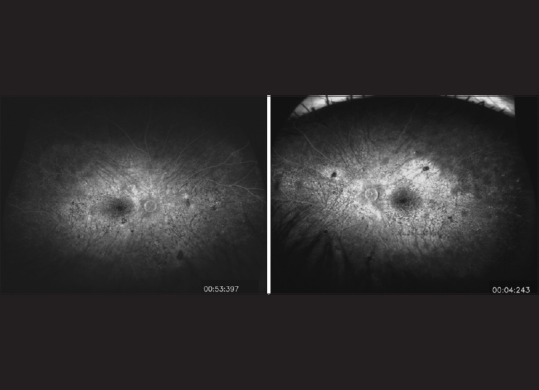
An ultra-widefield fluorescein angiogram of the eyes of a 34-year-old patient with a history of chronic Vogt–Koyanagi–Harada syndrome. The images of right eye (left panel) and left eye (right panel) show disturbance and hyperfluorescence of the retinal pigment epithelium
Retinal Vein Occlusion
Retinovascular occlusive disease is a very common indication for wide and UWF imaging [Fig. 9]. Prasad et al. evaluated the use of UWFA to study the peripheral angiographic features of branch retinal vein occlusions (BRVO) and hemicentral retinal vein occlusions (HRVO). They found that UWFA may provide visualization of peripheral retinal pathology in BRVO and HRVO patients, which may be useful in their evaluation and treatment. They suggested that areas of untreated retinal nonperfusion may be the source of production of biochemical mediators that promote NV and macular edema. Accordingly, they concluded that UWFA may be a powerful tool to identify therapeutic target areas for photocoagulation, allowing for efficient treatment of ischemic retina, and potentially minimizing collateral destruction of adjacent viable perfused retina.[28] A study by Tsui et al.[29] has identified an ischemic index in retinovascular occlusions which describe a ratio of nonperfused retina over the whole retinal area measured manually from ultra-wide angle FAs.
Figure 9.
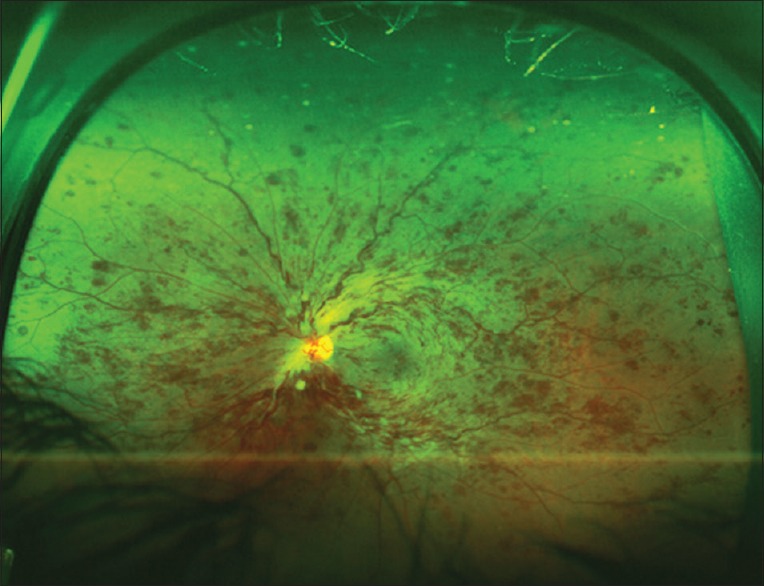
An ultra-widefield color fundus photo of the left eye of a 60-year-old patient with central retinal vein occlusion. The image shows intraretinal hemorrhages in all retinal quadrants
Age-Related Macular Degeneration
Age-related macular degeneration (AMD) is the leading cause of irreversible central vision loss among the elderly population and seriously compromises their quality of life. The vast majority of AMD-related vision loss results from exudative AMD, characterized by invasion of blood vessels into the subretinal space.[30,31] AMD is a multifactorial disease. The pathogenesis of AMD is complex with genetic, degenerative, and environmental predisposing factors. Hypoxia and ischemia are thought to play a role in the progression of AMD to neovascular AMD.[32] It is possible that peripheral retinal ischemia may contribute significantly to an abnormal angiogenic drive, mediated primarily by VEGF.[33] In a recent study by Madhusudhan and Beare, peripheral leakage, as indicated on UWFA, was noted to be associated with active neovascular AMD in a proportion of patients compared to the fellow eyes without active neovascular AMD. However, the authors found that the association between peripheral retinal nonperfusion and neovascular AMD was not significant.[32] Further studies to evaluate the significance of the abnormal findings in the peripheral retina in patients with AMD are required to improve our understanding of the pathogenesis of the disease. In a prospective study that was conducted to characterize peripheral fundus autofluorescence abnormalities in patients with AMD, significant risk factors for peripheral autofluorescence abnormalities were neovascular AMD compared with nonneovascular AMD and normal eyes, older age, and female sex. These findings may contribute to more understanding of the pathogenesis and the prognosis of the disease.[33]
Retinopathy of Prematurity
UWFA images showed clear views of the different stages of ROP features at the posterior pole and peripheral retina. With the help of UWFA images, regression of ROP features was identified, following laser and intravitreal bevacizumab treatment. In addition, it was made possible that the “skip areas” that were missed by initial laser treatment could be identified in the peripheral retina and managed accordingly.[34]
Choroidal Melanoma
Choroidal melanoma is the most common primary malignant ocular neoplasm in adults. It metastasizes into several organs and metastasis may occur before the primary tumor is diagnosed.[35] Therefore, early detection is essential. Clinical differentiation of malignant melanoma from benign choroidal nevus may be difficult. Shields et al. identified five predictive clinical features in order to help clinicians better differentiate small malignant melanoma from a benign choroidal nevus. These included: Tumor thickness > 2 mm, presence of subretinal fluid, clinical symptoms, orange pigment overlying the surface of the tumor, and tumor margins touching or being located within 3 mm of the optic disc.[36]
Reznicek et al. obtained images using UWF technology for more accurate evaluation of the criteria established by Shields et al. such as evaluation of location of the lesion in relation to the optic nerve, subretinal fluid, and maximal horizontal and vertical diameter. In addition they found, that the mean fundus autofluorescence intensity of melanomas was significantly lower than the autofluorescence of choroidal nevi and this combined with clinical criteria, and UWF images may help in the differential diagnosis.[37] In addition, measurements of the size of pigmented choroidal lesions using UWF imaging were found to be reasonably correlated with ultrasound measurements.[38]
Coats’ Disease
Coats’ disease is an idiopathic, retinal vascular abnormality of young males characterized by telangiectatic retinal vessels with aneurysms [Fig. 10]. Abnormal permeability of these vessels leads to exudative retinal detachment and subretinal lipid deposits. Often, subfoveal exudation leading to permanent severe vision loss occurs prior to the presentation. The severe forms of the disease often involve neovascular glaucoma and phthisis bulbi.[39,40] UWF fundus photography and angiography can be used successfully as an outpatient procedure in the pediatric patient population without the necessity of examination under anesthesia and can aid the physician in the documentation and evaluation of Coat's disease.[41]
Figure 10.
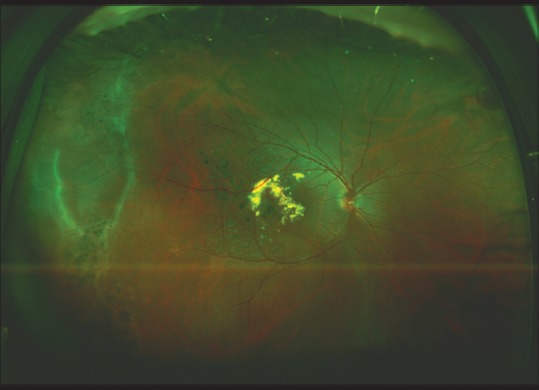
An ultra-widefield color fundus photo of the right eye of a 13-year-old male with Coat's disease showing both peripheral and central retinal involvement. Laser photocoagulation was applied to ablate telangiectatic retinal vessels, which led to reduction in exudation
Von Hippel-Lindau Disease
Von Hippel–Lindau disease (VHL) is an autosomal dominant inherited systemic cancer syndrome that gives rise to cystic and highly vascularized tumors in many organs, including the eye.[42] Patients with VHL are at increased risk of developing central nervous system and retinal hemangioblastomas, clear cell renal carcinoma, pheochromocytomas, neuroendocrine tumors and cysts of the pancreas, endolymphatic sac tumors, papillary cystadenomas of the epididymis, and broad ligament.[43] Retinal hemangioblastomas, present in up to 85% of individuals with VHL, are the most common lesion of VHL disease.[44]
Retinal Detachment
UWF autofluorescence imaging may reveal abnormalities in the rhegmatogenous retinal detachment that allow excellent demarcation of the extent of the retinal detachment and assist in the preoperative characterization of the detachment and may help in postoperative counseling [Fig. 11].[45] It may also be useful in documenting multiple foci of exudative detachment and response to treatment as well as for patient education.
Figure 11.
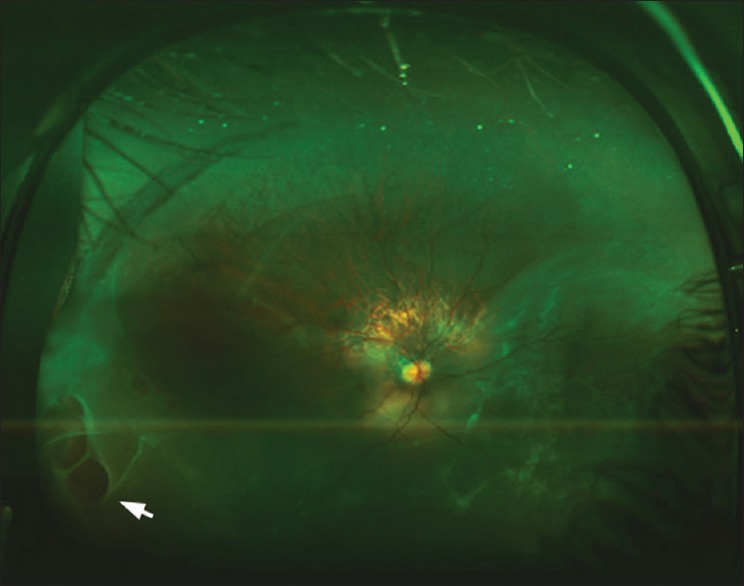
An ultra-widefield color fundus photo of the right eye of a 37-year-old patient with rhegmatogenous retinal detachment. There is inferior retinal detachment with large breaks in the inferotemporal periphery (white arrow)
Future Directions
Imaging of the peripheral retina has significantly improved over the past years. Widefield technology has become important clinically with regards to early diagnosis, effective treatment, and monitoring of most sight-threatening retinal diseases. In the future, widefield imaging may have significant utility as a research tool particularly for evaluating new treatment approaches. It is likely that with additional time, the full utility of widefield imaging will be revealed, and this may enable more rapid progress in understanding retinal pathology. Advancements in telemedicine methods and the development of portable fundus cameras have increased the accessibility of retinal imaging, but most of these approaches rely on separate computers for viewing and transmission of fundus images. Recently, a novel portable handheld smartphone-based retinal camera capable of capturing high-quality, widefield fundus images was developed. The use of the mobile phone platform creates a fully embedded system capable of acquisition, storage, and analysis of fundus images that can be directly transmitted from the phone via wireless telecommunication system for remote evaluation.[46] Telemedicine programs have shown that nonphysician operators can be trained to obtain images for remote expert interpretations.[47] Photographic documentation can also be a valuable asset for medico-legal and educational purposes.[48]
A significant amount of work is going on to validate and expand the utilization of UWF imaging. Not so far in the future, we believe that UWF imaging technology will be indispensable for the routine daily retina practice.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Tan CS, Sadda SR, Hariprasad SM. Ultra-widefield retinal imaging in the management of diabetic eye diseases. Ophthalmic Surg Lasers Imaging Retina. 2014;45:363–6. doi: 10.3928/23258160-20140909-07. [DOI] [PubMed] [Google Scholar]
- 2.Pang CE, Shah VP, Sarraf D, Freund KB. Ultra-widefield imaging with autofluorescence and indocyanine green angiography in central serous chorioretinopathy. Am J Ophthalmol. 2014;158:362–71.e2. doi: 10.1016/j.ajo.2014.04.021. [DOI] [PubMed] [Google Scholar]
- 3.Ciardella A, Brown D. Wide field imaging. In: Agarwal A, editor. Fundus Fluorescein and Indocyanine Green Angiography: A Textbook and Atlas. New York: Slack Inc.; 2007. pp. 79–83. [Google Scholar]
- 4.Diabetic retinopathy study. Report Number 6. Design, methods, and baseline results. Report Number 7. A modification of the Airlie House classification of diabetic retinopathy. Prepared by the Diabetic Retinopathy. Invest Ophthalmol Vis Sci. 1981;21(1 Pt 2):1–226. [PubMed] [Google Scholar]
- 5.Kaines A, Oliver S, Reddy S, Schwartz SD. Ultrawide angle angiography for the detection and management of diabetic retinopathy. Int Ophthalmol Clin. 2009;49:53–9. doi: 10.1097/IIO.0b013e31819fd471. [DOI] [PubMed] [Google Scholar]
- 6.Pomerantzeff O. Equator-plus camera. Invest Ophthalmol. 1975;14:401–6. [PubMed] [Google Scholar]
- 7.Roth DB, Morales D, Feuer WJ, Hess D, Johnson RA, Flynn JT. Screening for retinopathy of prematurity employing the retcam 120: Sensitivity and specificity. Arch Ophthalmol. 2001;119:268–72. [PubMed] [Google Scholar]
- 8.Staurenghi G, Viola F, Mainster MA, Graham RD, Harrington PG. Scanning laser ophthalmoscopy and angiography with a wide-field contact lens system. Arch Ophthalmol. 2005;123:244–52. doi: 10.1001/archopht.123.2.244. [DOI] [PubMed] [Google Scholar]
- 9.Witmer MT, Parlitsis G, Patel S, Kiss S. Comparison of ultra-widefield fluorescein angiography with the Heidelberg Spectralis(®) noncontact ultra-widefield module versus the Optos(®) Optomap(®) Clin Ophthalmol. 2013;7:389–94. doi: 10.2147/OPTH.S41731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee BR, Chang HR. Biomicroscopic vitreous observation using a 3 CCD video camera and a personal computer for image capture and archiving. Korean J Ophthalmol. 2000;14:74–9. doi: 10.3341/kjo.2000.14.2.74. [DOI] [PubMed] [Google Scholar]
- 11.Gregori NZ, Lam BL, Gregori G, Ranganathan S, Stone EM, Morante A, et al. Wide-field spectral-domain optical coherence tomography in patients and carriers of X-linked retinoschisis. Ophthalmology. 2013;120:169–74. doi: 10.1016/j.ophtha.2012.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.World Health Organization. Global Initiative for the Elimination of Avoidable Blindness: Action Plan 2006e2011. Geneva, Switzerland: World Health Organization; 2007. [Google Scholar]
- 13.Yau JW, Rogers SL, Kawasaki R, Lamoureux EL, Kowalski JW, Bek T, et al. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care. 2012;35:556–64. doi: 10.2337/dc11-1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nussenblatt RB, Kaufman SC, Palestine AG, Davis MD, Ferris FL., 3rd Macular thickening and visual acuity. Measurement in patients with cystoid macular edema. Ophthalmology. 1987;94:1134–9. doi: 10.1016/s0161-6420(87)33314-7. [DOI] [PubMed] [Google Scholar]
- 15.Klein R. Diabetic retinopathy. Annu Rev Public Health. 1996;17:137–58. doi: 10.1146/annurev.pu.17.050196.001033. [DOI] [PubMed] [Google Scholar]
- 16.Aiello LP, Avery RL, Arrigg PG, Keyt BA, Jampel HD, Shah ST, et al. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N Engl J Med. 1994;331:1480–7. doi: 10.1056/NEJM199412013312203. [DOI] [PubMed] [Google Scholar]
- 17.Adamis AP, Miller JW, Bernal MT, D’Amico DJ, Folkman J, Yeo TK, et al. Increased vascular endothelial growth factor levels in the vitreous of eyes with proliferative diabetic retinopathy. Am J Ophthalmol. 1994;118:445–50. doi: 10.1016/s0002-9394(14)75794-0. [DOI] [PubMed] [Google Scholar]
- 18.Patel RD, Messner LV, Teitelbaum B, Michel KA, Hariprasad SM. Characterization of ischemic index using ultra-widefield fluorescein angiography in patients with focal and diffuse recalcitrant diabetic macular edema. Am J Ophthalmol. 2013;155:1083–44.e2. doi: 10.1016/j.ajo.2013.01.007. [DOI] [PubMed] [Google Scholar]
- 19.Wessel MM, Nair N, Aaker GD, Ehrlich JR, D’Amico DJ, Kiss S. Peripheral retinal ischaemia, as evaluated by ultra-widefield fluorescein angiography, is associated with diabetic macular oedema. Br J Ophthalmol. 2012;96:694–8. doi: 10.1136/bjophthalmol-2011-300774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oliver SC, Schwartz SD. Ultra-widefield fluorescein angiography. In: Arevalo JF, editor. Retinal Angiography and Optical Coherence Tomography. New York: Springer Science and Business Media, LLC; 2009. pp. 407–17. [Google Scholar]
- 21.Wessel MM, Aaker GD, Parlitsis G, Cho M, D’Amico DJ, Kiss S. Ultra-wide-field angiography improves the detection and classification of diabetic retinopathy. Retina. 2012;32:785–91. doi: 10.1097/IAE.0b013e3182278b64. [DOI] [PubMed] [Google Scholar]
- 22.Muqit MM, Marcellino GR, Henson DB, Young LB, Patton N, Charles SJ, et al. Optos-guided pattern scan laser (Pascal)-targeted retinal photocoagulation in proliferative diabetic retinopathy. Acta Ophthalmol. 2013;91:251–8. doi: 10.1111/j.1755-3768.2011.02307.x. [DOI] [PubMed] [Google Scholar]
- 23.Gupta V, Al-Dhibi HA, Arevalo JF. Retinal imaging in uveitis. Saudi J Ophthalmol. 2014;28:95–103. doi: 10.1016/j.sjopt.2014.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Campbell JP, Leder HA, Sepah YJ, Gan T, Dunn JP, Hatef E, et al. Wide-field retinal imaging in the management of noninfectious posterior uveitis. Am J Ophthalmol. 2012;154:908–11.e2. doi: 10.1016/j.ajo.2012.05.019. [DOI] [PubMed] [Google Scholar]
- 25.Seidensticker F, Neubauer AS, Wasfy T, Stumpf C, Thurau SR, Kampik A, et al. Wide-field fundus autofluorescence corresponds to visual fields in chorioretinitis patients. Clin Ophthalmol. 2011;5:1667–71. doi: 10.2147/OPTH.S26224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leder HA, Campbell JP, Sepah YJ, Gan T, Dunn JP, Hatef E, et al. Ultra-wide-field retinal imaging in the management of non-infectious retinal vasculitis. J Ophthalmic Inflamm Infect. 2013;3:30. doi: 10.1186/1869-5760-3-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mesquida M, Llorenç V, Fontenla JR, Navarro MJ, Adán A. Use of ultra-wide-field retinal imaging in the management of active Behçet retinal vasculitis. Retina. 2014;34:2121–7. doi: 10.1097/IAE.0000000000000197. [DOI] [PubMed] [Google Scholar]
- 28.Prasad PS, Oliver SC, Coffee RE, Hubschman JP, Schwartz SD. Ultra wide-field angiographic characteristics of branch retinal and hemicentral retinal vein occlusion. Ophthalmology. 2010;117:780–4. doi: 10.1016/j.ophtha.2009.09.019. [DOI] [PubMed] [Google Scholar]
- 29.Tsui I, Kaines A, Havunjian MA, Hubschman S, Heilweil G, Prasad PS, et al. Ischemic index and neovascularization in central retinal vein occlusion. Retina. 2011;31:105–10. doi: 10.1097/IAE.0b013e3181e36c6d. [DOI] [PubMed] [Google Scholar]
- 30.Friedman DS, O’Colmain BJ, Muñoz B, Tomany SC, McCarty C, de Jong PT, et al. Prevalence of age-related macular degeneration in the United States. Arch Ophthalmol. 2004;122:564–72. doi: 10.1001/archopht.122.4.564. [DOI] [PubMed] [Google Scholar]
- 31.Gorin MB. Genetic insights into age-related macular degeneration: Controversies addressing risk, causality, and therapeutics. Mol Aspects Med. 2012;33:467–86. doi: 10.1016/j.mam.2012.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Madhusudhan S, Beare N. Wide-field fluorescein angiography in wet age-related macular degeneration. Scientific World Journal. 2014;2014:536161. doi: 10.1155/2014/536161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tan CS, Heussen F, Sadda SR. Peripheral autofluorescence and clinical findings in neovascular and non-neovascular age-related macular degeneration. Ophthalmology. 2013;120:1271–7. doi: 10.1016/j.ophtha.2012.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Patel CK, Fung TH, Muqit MM, Mordant DJ, Brett J, Smith L, et al. Non-contact ultra-widefield imaging of retinopathy of prematurity using the Optos dual wavelength scanning laser ophthalmoscope. Eye (Lond) 2013;27:589–96. doi: 10.1038/eye.2013.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bell DJ, Wilson MW. Choroidal melanoma: Natural history and management options. Cancer Control. 2004;11:296–303. doi: 10.1177/107327480401100503. [DOI] [PubMed] [Google Scholar]
- 36.Shields CL, Shields JA, Kiratli H, De Potter P, Cater JR. Risk factors for growth and metastasis of small choroidal melanocytic lesions. Ophthalmology. 1995;102:1351–61. [PubMed] [Google Scholar]
- 37.Reznicek L, Stumpf C, Seidensticker F, Kampik A, Neubauer AS, Kernt M. Role of wide-field autofluorescence imaging and scanning laser ophthalmoscopy in differentiation of choroidal pigmented lesions. Int J Ophthalmol. 2014;7:697–703. doi: 10.3980/j.issn.2222-3959.2014.04.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kernt M, Schaller UC, Stumpf C, Ulbig MW, Kampik A, Neubauer AS. Choroidal pigmented lesions imaged by ultra-wide-field scanning laser ophthalmoscopy with two laser wavelengths (Optomap) Clin Ophthalmol. 2010;4:829–36. doi: 10.2147/opth.s11864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Do DV, Haller JA. Coats disease. In: Ryan SJ, Schachat AP, editors. Retina. Philadelphia, PA: Elsevier; 2006. pp. 1417–27. [Google Scholar]
- 40.Shields JA, Shields CL, Honavar SG, Demirci H. Clinical variations and complications of Coats disease in 150 cases: The 2000 Sanford Gifford Memorial Lecture. Am J Ophthalmol. 2001;131:561–71. doi: 10.1016/s0002-9394(00)00883-7. [DOI] [PubMed] [Google Scholar]
- 41.Kang KB, Wessel MM, Tong J, D’Amico DJ, Chan RV. Ultra-widefield imaging for the management of pediatric retinal diseases. J Pediatr Ophthalmol Strabismus. 2013;50:282–8. doi: 10.3928/01913913-20130528-04. [DOI] [PubMed] [Google Scholar]
- 42.Haddad NM, Cavallerano JD, Silva PS. Von hippel-lindau disease: A genetic and clinical review. Semin Ophthalmol. 2013;28:377–86. doi: 10.3109/08820538.2013.825281. [DOI] [PubMed] [Google Scholar]
- 43.Maher ER. Von Hippel-Lindau disease. Curr Mol Med. 2004;4:833–42. doi: 10.2174/1566524043359827. [DOI] [PubMed] [Google Scholar]
- 44.Chew EY. Ocular manifestations of von Hippel-Lindau disease: Clinical and genetic investigations. Trans Am Ophthalmol Soc. 2005;103:495–511. [PMC free article] [PubMed] [Google Scholar]
- 45.Witmer MT, Cho M, Favarone G, Chan RV, D’Amico DJ, Kiss S. Ultra-wide-field autofluorescence imaging in non-traumatic rhegmatogenous retinal detachment. Eye (Lond) 2012;26:1209–16. doi: 10.1038/eye.2012.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Maamari RN, Keenan JD, Fletcher DA, Margolis TP. A mobile phone-based retinal camera for portable wide field imaging. Br J Ophthalmol. 2014;98:438–41. doi: 10.1136/bjophthalmol-2013-303797. [DOI] [PubMed] [Google Scholar]
- 47.Fijalkowski N, Zheng LL, Henderson MT, Wang SK, Wallenstein MB, Leng T, et al. Stanford University Network for Diagnosis of Retinopathy of Prematurity (SUNDROP): Five years of screening with telemedicine. Ophthalmic Surg Lasers Imaging Retina. 2014;45:106–13. doi: 10.3928/23258160-20140122-01. [DOI] [PubMed] [Google Scholar]
- 48.Paul Chan RV, Williams SL, Yonekawa Y, Weissgold DJ, Lee TC, Chiang MF. Accuracy of retinopathy of prematurity diagnosis by retinal fellows. Retina. 2010;30:958–65. doi: 10.1097/IAE.0b013e3181c9696a. [DOI] [PMC free article] [PubMed] [Google Scholar]


