Abstract
Purpose:
Optical coherence tomography (OCT) is an important imaging tool assessing retinal architecture. In this article, we report a single centers experience of using handheld spectral domain (SD)-OCT in a pediatric population using the Envisu 2300 (Bioptigen Inc., Research Triangle Park, NC, USA).
Methods:
We studied SD-OCT images from 975 patients imaged from January 2011 to December 2014. The variety of cases that underwent an SD-OCT was analyzed. Cases examples from different case scenarios were selected to showcase unique examples of many diseases.
Results:
Three hundred and sixty-eight infants (37.7%) were imaged for retinopathy of prematurity, 362 children (37.1%) underwent the test for evaluation of suboptimal vision or an unexplained vision loss, 126 children (12.9%) for evaluation of nystagmus or night blindness, 54 children (5.5%) for an intraocular tumor or a mass lesion such as retinoblastoma, and 65 children (6.7%) for other diseases of the pediatric retina. The unique findings in the retinal morphology seen with some of these diseases are discussed.
Conclusion:
The handheld SD-OCT is useful in the evaluation of the pediatric retinal diseases. The test is useful in the assessment of vision development in premature children, evaluation of unexplained vision loss and amblyopia, nystagmus and night blindness, and intraocular tumors (including retinoblastoma).
Keywords: Optical coherence tomography, pediatric retina, retinal dystrophy, retinoblastoma, unexplained vision loss
Optical coherence tomography (OCT) has become an established noncontact and noninvasive imaging tool for the assessment of retinal architecture, making it one of the most frequently ordered outpatient imaging investigation in modern ophthalmology.[1,2] Spectral domain (SD)-OCT has replaced the conventional time domain OCT as it provides images of higher axial resolution and reduced motion artifacts.[3,4,5] The popularity of SD-OCT in the evaluation and management of pediatric retinal disease management has been less than its use in adults. Some of the reasons for this lack of acceptance have been the limitations of the devices available, which are predominantly tabletop devices that precluded their easy use on the infant or the uncooperative child. Most units require a compliant patient who can sit upright. Although the table top device could be dismantled to image an infant in the office sans anesthesia, this method is rather cumbersome and produces images that are laterally inverted causing challenges in image interpretation, and localization.[6]
In order to overcome the limitations of the standard OCT machines in pediatric practice, the technology has evolved to more portable units, which also help image more peripheral lesions. One such device is a portable SD-OCT unit (Envisu 2300, Bioptigen Inc., Research Triangle Park, NC, USA) with a handheld probe. Imaging in the supine position is possible with this device and hence gives the clinician the option of using it during the evaluation of pediatric patients.[7,8] It is comparable with the conventional chin-rest SD-OCT and allows rapid data acquisition. It provides previously unseen details of morphologic features of retinal lesions in these infant eyes thereby influencing prognosis and management and can be considered a useful adjunct to digital wide-field fundus photography with the RetCam (Clarity Medical Systems, Inc. Pleasanton, CA, USA).
In pediatric ophthalmology, OCT imaging has been used in disorders of the optic nerve and vitreous and retina besides the assessment of amblyopia, the anterior chamber angle, and cornea.[9,10] It has been used to quantify the resolution of cystoid macular edema in Coats’ disease after an intravitreal injection of anti-VEGF (pegaptanib sodium).[11] Pediatric choroidal neovascular membranes secondary to a variety of etiologies and the quantitative response to anti-VEGF agents has been assessed using OCT imaging.[12] OCT features of macular toxoplasmosis include retinal thinning, retinal pigment epithelial hyper-reflectivity, excavation, intraretinal cysts, and fibrosis.[13] In Best's disease, OCT can help define the characteristics of the “egg yolk” appearance and visualize CME that is associated with inherited retinal dystrophies.[10] Shaken baby syndrome sequelae have been well studied using the SD-OCT, which has aided in the diagnosis of the chronic full-thickness macular hole with full-thickness retinal scarring, epiretinal membrane, perimacular folds and traumatic retinoschisis.[6]
In this article, we report a single centers experience with the handheld SD-OCT (Envisu 2300, Bioptigen Inc., Research Triangle Park, NC, USA) in the evaluation and management of pediatric eye diseases.
Methods
We reviewed the image database of all consecutive pediatric patients referred to the Pediatric Retina Department of our institute, and who underwent imaging with a handheld SD-OCT device. The Envisu 2300 has a maximum camera line rate of 36 kHz with a typical imaging line rate of 32 kHz and an axial resolution of <4 μm in tissue. The maximum imaging depth in tissue is 2.5 mm with a digital resolution of 2.4 μm/pixel.[14]
During each imaging session, the scans were obtained in rectangular volume scans and/or radial volume scans. For each imaging session, the reference arm length was adjusted according to the axial length of the patient's eye and the focus was adjusted (–10 to + 11 diopter (D); retinal lens; 70° field) on the handheld probe according to the spherical equivalent of the patient's refractive error.
During the study period (January 2011 to December 2014), 975 unique patients with good quality OCT images, adequate for analysis and with complete corroborative clinical diagnoses documented were reviewed for this manuscript. Of these, most of the children (37.7%; 368/975) were examined for evaluation of premature infants with and without retinopathy of prematurity (ROP). The clinical diagnoses that required other children to undergo an OCT are summarized in Table 1 for the remaining 607 children and are the focus of the current manuscript.
Table 1.
Clinical diagnosis of pediatric patients who underwent handheld spectral domain OCT in our institute (n=975 children)
Results
Based on the utility of the handheld OCT in our series, we have categorized the indications that warrant the investigation and include:
Evaluation of premature children
Suboptimal vision and unexplained vision loss
Nystagmus and night blindness
Intraocular tumors and mass lesions
Miscellaneous indications.
When the 975 cases were classified according to the clinical utility of the SD-OCT in our practice, we found that those that 75% (731) were clinically indicated, 20.3% (198) were performed for understanding the pathology further and 4.7% (46) did not add more information to case management than what was known through clinical examination [Table 1].
Evaluation of premature children
The foveal architecture and other retinal features in children with and without ROP were studied in 368 children and have previously reported the utility of SD-OCT imaging in premature infants.[7],8,15,[16,17,18,19,20,21]
Broadly these cases can be described as those with normal looking foveae (clinically) and those with abnormal foveae. We advise SD-OCT for those children with a “normal looking” fovea, but with subnormal visual acuity who are referred from the amblyopia clinic, those not improving visually despite compliant patching therapy, refractive errors which cannot explain the full extent of visual deficit, premature infants who are undergoing longitudinal and serial imaging for correlation of visual milestones, infants who are undergoing vision rehabilitation including those with a diagnosis of delayed visual maturation and cortical vision impairment and other causes of unexplained visual deficit or suboptimal vision that cannot be explained despite a “normal” fovea.
Clinically normal looking foveae can demonstrate abnormal features on the SD-OCT. There have been reports of OCT changes resembling macular edema of adults in Stage 2 ROP [17] in Asian Indian infants and subsequently in other ethnicities and in other stages of ROP as well.[18] Although these changes spontaneously resolved as early as 52 weeks postmenstrual age, we found that the group with macular edema demonstrated early visual and refractive changes compared to age-matched positive and negative controls at the first year follow-up (unpublished data). The importance of documenting even subclinical OCT findings is therefore established.
Premature infants demonstrate OCT features that are different from that of an adult.[7,16,22] The retinal microstructure on OCT has been correlated with visual acuity both retrospectively (unpublished data) and prospectively.[23] Our current understanding of foveal development in these infants suggests that the delay or absence of the inner retinal layer maturation, the growth of the photoreceptor complex and the foveal tent can influence visual acuity in these infants.[8]
The handheld OCT has been used to evaluate macular involvement in cases of advanced ROP. Macular detachments may be shallow and missed clinically. Confirmation of macular detachment alters the diagnosis from Stage 4A to 4B [24] and can be used to prognosticate before surgery and also monitor postsurgical improvement.[25]
Evaluation of children with poor vision
Extending our observations of premature infants and structure-function correlation using the SD-OCT, we have used the same principle to study older toddlers, preschool and preverbal children from our amblyopia clinic who's vision “does not improve” despite seemingly compliant patching therapy and refractive correction, as well as in cases where the low vision cannot be explained based on the other clinical findings alone. The clinical utility in this series is described with the following case examples.
Case 1
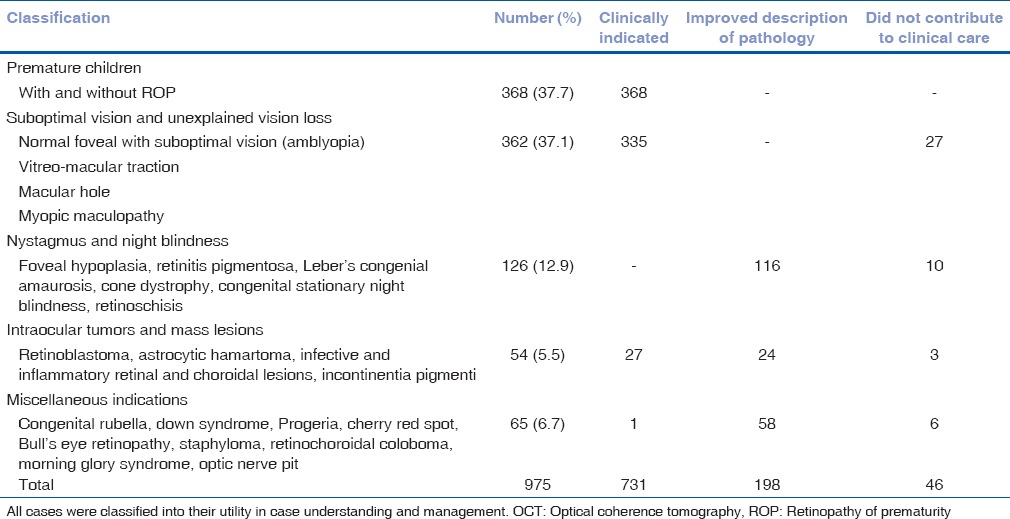
A 5-year-old girl with a refractive error of −1.25 cylinder at 10° and 170° in the right and left eye, respectively with a visual acuity of 20/50 and 20/60 despite spectacle correction for 3 years. Fundus examination and color vision was within normal limits; SD-OCT images [Fig. 1] showed a normal inner retina with a good foveal dip and fusion of the inner retinal layers. The outer retina showed a disruption of the inner segment outer segment (IS-OS) junction at the foveal center and the loss of the foveal tent (in comparison to the normal pediatric retina). The photoreceptors in the cone dense area of the fovea were clearly disrupted. This is similar to what is observed in children with cone dysfunctions.[26,27,28] However, in this case, her color vision was normal.
Figure 1.
An optical coherence tomography image from Case 1 (above) with unexplained vision loss shows disruption in the ellipsoid zone at the fovea with an intact external limiting membrane. An example of normal retina is represented below (CC: Choriocapillaries; EZ: Ellipsoid zone, FP: Foveal pit, FT: Foveal tent, GCL: Ganglion cell layer, INL: Inner nuclear layer, IPL: Inner plexiform layer, IS: Inner segment of the photoreceptor, NFL: Nerve fiber layer, OLM: Outer limiting membrane, ONL: Outer nuclear layer, OPL: Outer plexiform layer, OS: Outer segment of the photoreceptor, RPE: Retinal pigment epithelium complex)
Case 2
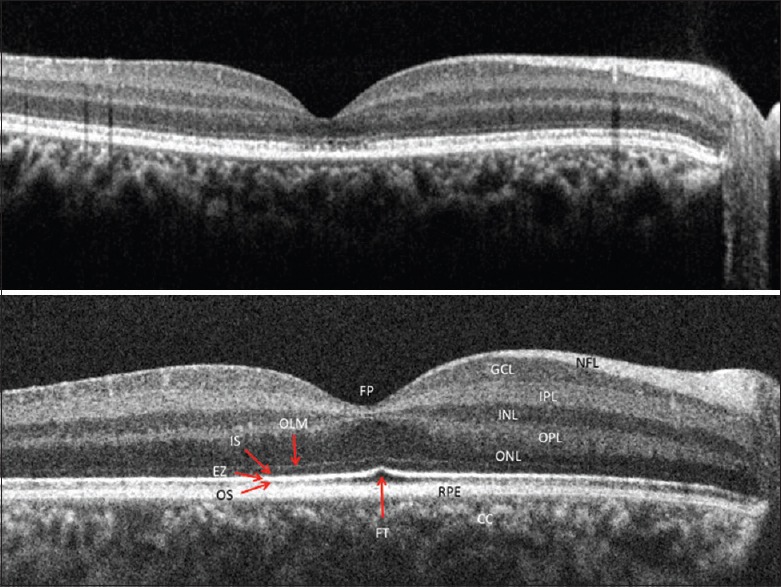
An 8-year-old girl showing no improvement with spectacle correction or patching with a visual acuity constant at 20/50 and 20/60, respectively had a myopic correction of −2.25 × 180° and −2.50 × 180°, respectively. The SD-OCT [Fig. 2] revealed a normal inner retina, but a thickened and irregular IS-OS layer in both eyes more in the foveal center with the loss of the foveal tent. The OS RPE layer was attenuated or near absent in the foveal center. The full-field electroretinogram (ERG) was normal. This child was unable to cooperate for multifocal ERG testing.
Figure 2.
A spectral domain-optical coherence tomography image of Case 2, an 8-year-old girl with unexplained vision loss showing an abnormal ellipsoid zone, and outer segment of photoreceptors layer
Evaluation of children with nystagmus and night blindness
Nystagmus and night blindness are both early signs of retinal dystrophy. In addition to an ERG demonstrating abnormal retinal function, the SD-OCT was able to pick up early abnormalities of the retinal microstructure in an otherwise normal looking fundus. Many authors have noted the importance of SD-OCT in the diagnosis of nystagmus.[22,29] In our experience, SD-OCT imaging was possible in most children with nystagmus in the office without anesthesia. Despite the abnormal eye movement, the procedure could be performed in supine and upright position. SD-OCT images provide micro-structural evidence of the photoreceptor morphology in the foveal center and the retinal mid-periphery that can help diagnose, prognosticate and follow-up these cases. We were also able to investigate children with night blindness to diagnosis a rod-cone dystrophy.
Case 3
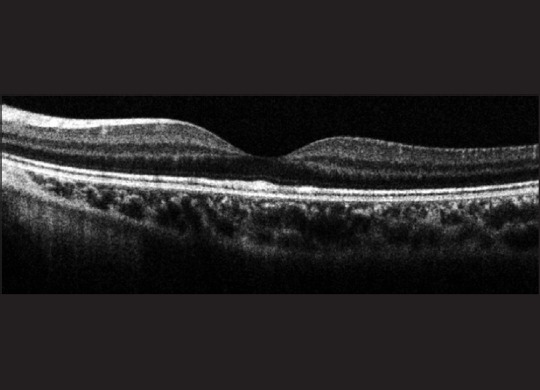
A 4-month-old girl presented when her parents noticed that she was unable to maintain a steady gaze. She was found to have a horizontal pendular nystagmus, with an absent foveal pit and absent foveal reflexes on fundus examination. An ERG performed earlier for the nystagmus had indicated normal photoreceptor responses. The SD-OCT [Fig. 3] demonstrated an absent foveal pit, persistence of the inner retinal layers in the foveal center with normal outer retinal layers. These findings are typical of foveal hypoplasia.[30,31]
Figure 3.
A spectral domain-optical coherence tomography image of Case 3 demonstrating foveal hypoplasia (box inset showing the area of the retina scanned, with “*” to indicate where the foveal pit was anticipated). There is an absence of the central foveal pit and the inner retinal layers are present throughout the scan length
Case 4
A 1-year-old girl presented when her parents noticed that she could not make eye contact and lacked a social smile. The child had a pendular nystagmus in both eyes. The fundus showed a normal retina with minimal peripheral tessellation and a normal foveal contour. A full field ERG showed abnormal scotopic and photopic waveforms, with the latter affected more. The SD-OCT [Fig. 4] showed an absent foveal tent and a uniform hyper-reflectivity of the layer of cone OS tips, with a reflectivity of the other retinal layers seeming normal. The true significance of this potentially early OCT sign of abnormal photoreceptor function is not known and is being reported for the first time in this child with a cone-rod dystrophy.
Figure 4.
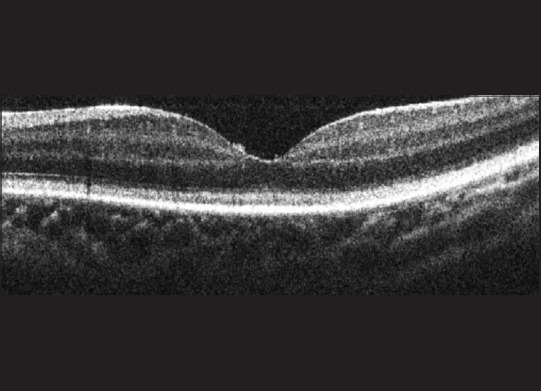
A spectral domain-optical coherence tomography image of Case 4 with a cone-rod dystrophy demonstrating an absent foveal tent and an irregularly thickened and hyper-reflective layer of cone outer segment tips
Case 5
A 5-year-old boy presented to us when his parents noticed he was avoiding brightly lit areas. He was found to have a best-corrected vision of 20/120 vision in each eye, with severe loss of color perception. He demonstrated a pendular nystagmus. His fundus examination was unremarkable. A full field ERG performed showed a normal scotopic response, with an absent photopic response, confirming a diagnosis of complete achromatopsia. The SD-OCT [Fig. 5] performed showed a disrupted photoreceptor OS layer only at the fovea (referred to as the “hypo-reflective zone”), which has been previously reported in complete achromatopsia.[28,31,32]
Figure 5.
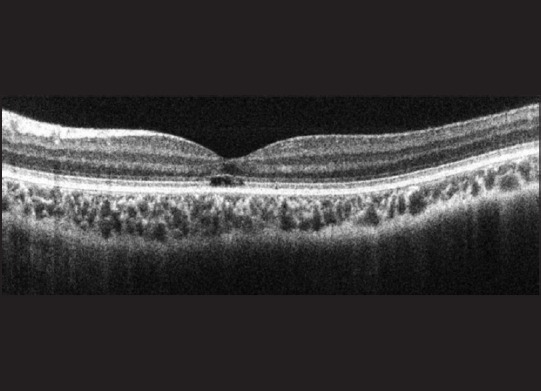
A spectral domain-optical coherence tomography image of Case 5 with complete achromatopsia showing a disrupted outer segment layer at the fovea demonstrating the hypo-reflective zone seen with cone dysfunction
Case 6
A 2-year-old girl presented with a history of poor vision in dark and hearing loss. The child was found to have a vision of 20/2700 in the right eye and 20/960 in the left eye with tellers acuity cards. Cycloplegic refraction showed a hypermetropia of +7.50D in both eyes. A fundus examination showed peripheral retinal pigment epithelium mottling, pale optic discs, narrow arterioles and a grossly normal macula. A full field ERG showed no response from either scotopic or photopic stimuli, as expected in a severe rod-cone dystrophy. The SD-OCT [Fig. 6] showed preserved retinal layers at the fovea, with the loss of the outer layers of the retina with increasing distance from the fovea. The photoreceptor layers were completely lost 2–3 mm away from the fovea and the outer plexiform layer was lost further into the mid-peripheral retina.
Figure 6.
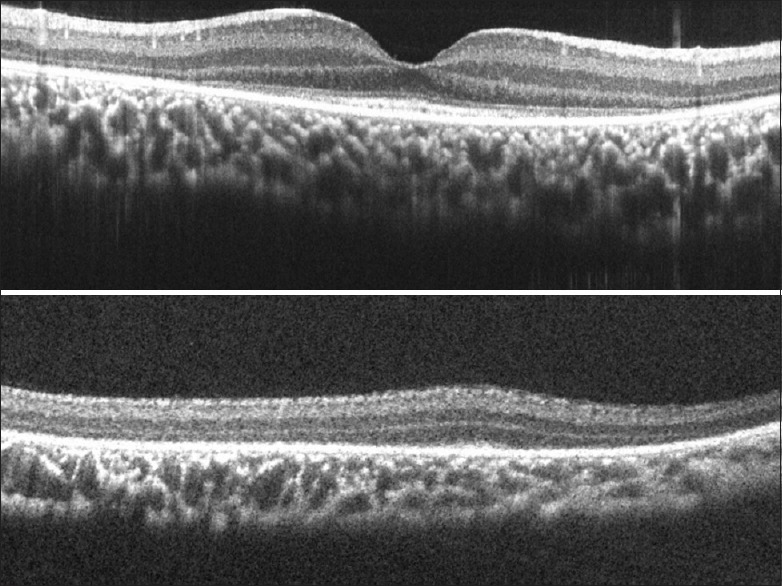
Spectral domain-optical coherence tomography images of Case 6 with a rod-cone dystrophy showing the central retina (above) and peripheral retina (below). Progressive losses of the outer retinal layers are shown in these images as the retina is imaged from the fovea to the periphery
Children with retinal tumors and other retinal lesions
The diagnosis of an intraocular tumor such as retinoblastoma is usually through fundus examination and ultrasonography and rarely requires confirmation using an SD-OCT. Most often in our practice, in an obviously affected eye, a small retinoblastoma lesion is almost always seen in the opposite eye, therefore making the diagnosis clear.
An astrocytic hamartoma in a child with ash-leaf macules of the skin and the presence of other tumors in the myocardium and brain usually confirm a clinical diagnosis of tuberous sclerosis. It is a lesion that rarely gets referred for differentiation from a retinoblastoma, and an SD-OCT can help differentiate the two. While an astrocytic hamartoma arises from the nerve fiber layer [33,34] [Fig. 7], retinoblastomas arise from the inner nuclear layer.[35] We have reported the OCT appearance of a treated retinoblastoma before and after chemotherapy [Fig. 8], with a reduction in tumor size and more homogeneity postchemotherapy. There was the disappearance of the dark streaks caused by posterior shadowing from tumor vasculature (bar-code like appearance) which was seen prior to chemotherapy.[36]
Figure 7.
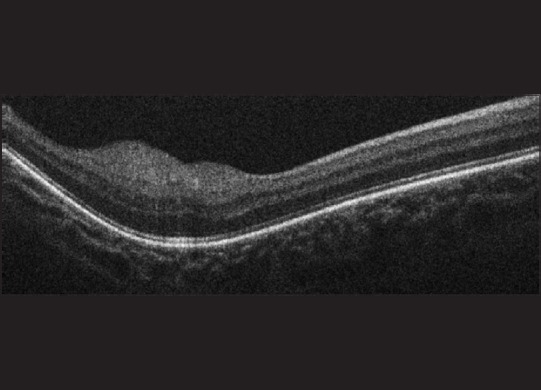
A spectral domain-optical coherence tomography image of an astrocytic hamartoma in a 3 years old demonstrating a smooth, regular, and well defined lesion involving the nerve fiber layer
Figure 8.
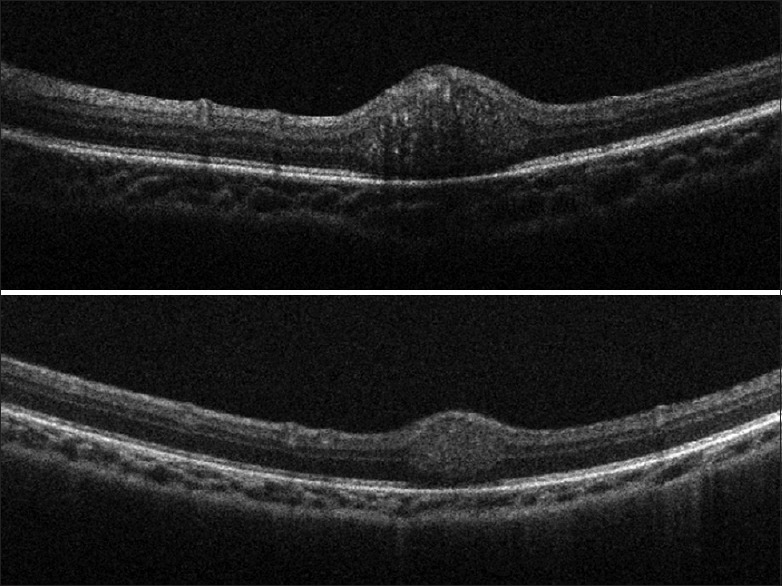
Spectral domain-optical coherence tomography images of a small retinoblastoma tumor in a 3-month-old boy before (above) and after (below) the first cycle of chemotherapy (Image courtesy: Mallipatna A, Suren V. Retinoblastoma. In: Vinekar A, Avadhani K, editors. Spectral Domain Optical Coherence Imaging of the Eye. New Delhi: Elsevier Health Sciences; 2014.)
Other indications of SD-OCT in children with retinoblastoma have included the detection of small tumors in the screening of children who are germline predisposed to having retinoblastoma, detection of the source of vitreous seeding to help target focal laser therapy [Fig. 9], determining tumor recurrences in laser scars [Fig. 10], follow-up of vitreo-macular traction during the course of treatment [Fig. 11] and the presumed involvement of the optic nerve head in children with a tumor adjacent to the optic disc [Fig. 12]. A previous paper has commented on the utility of the SD-OCT in the management of retinoblastoma by assisting in the diagnosis of new lesions, monitoring response to laser therapy and identifying tumor recurrences.[35]
Figure 9.
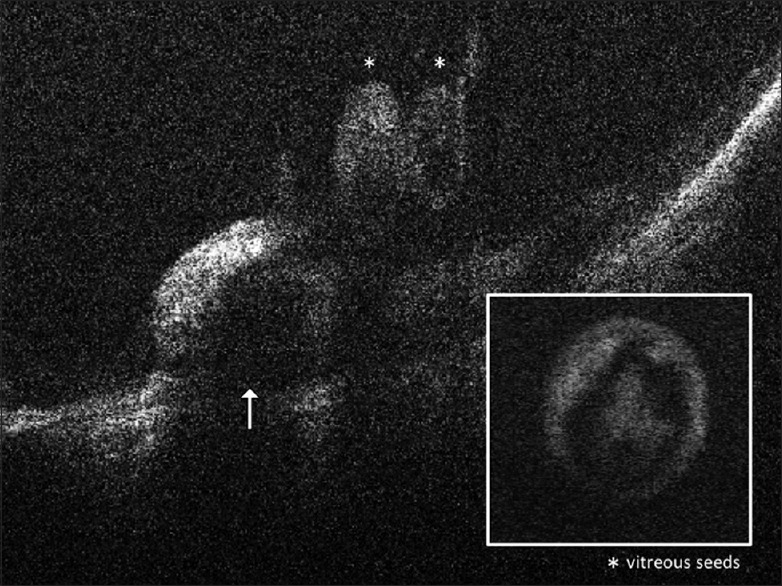
Spectral domain-optical coherence tomography images of a retinoblastoma tumor (indicated by an arrow) dispersing vitreous seeds (indicated by FNx01) into the vitreous. The box (inset) shows a single pearl-like vitreous seed with an outer layer of viable cells surrounding a core of necrotic cell debris
Figure 10.
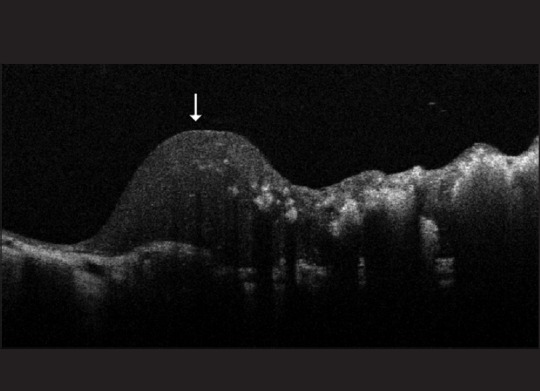
A spectral domain-optical coherence tomography image of a recurring tumor (indicated by an arrow) occurring at the edge of a previously treated tumor (irregularly hyper-reflective lesion to the right of the arrow)
Figure 11.
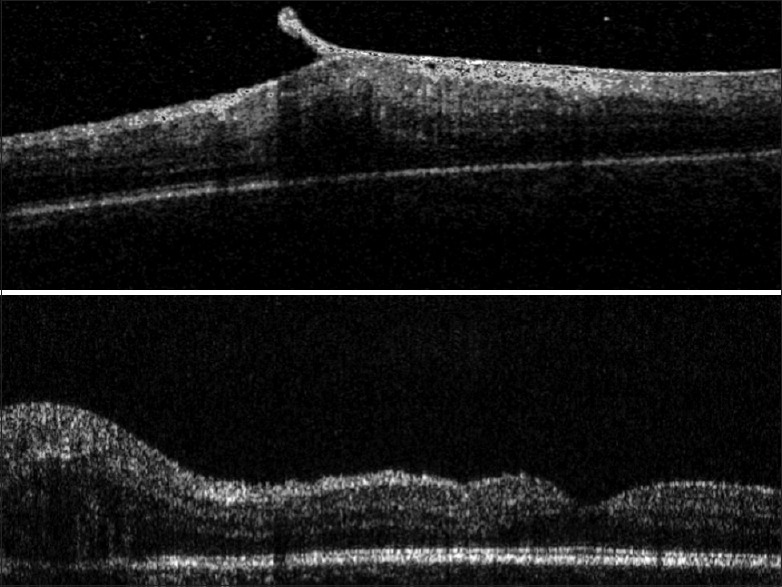
Serial OCT images of the fovea of a 4-year-old boy treated with focal lasers, who developed a vitreo-macular traction after treatment (above image from a tabletop SD-OCT: Spectral OCT-SLO, Ophthalmic Technologies Inc., Toronto, Canada), with the traction band spontaneously detaching from the fovea after four months (below image from the hand-held SD-OCT: Envisue 2300)
Figure 12.
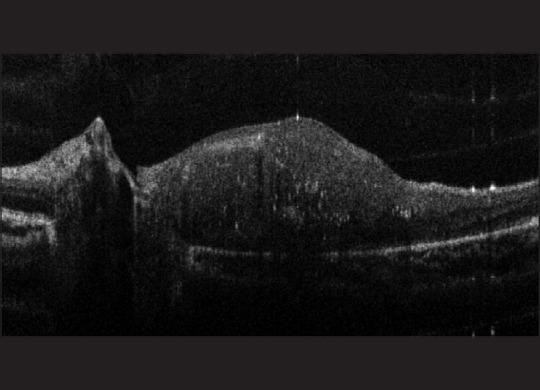
A spectral domain-optical coherence tomography image of a retinoblastoma tumor in a 3-year-old girl seeming to extend into the optic nerve in her only remaining eye
Retinal mid-peripheral and peripheral lesions such as choroidal nevi, retinitis, choroiditis, and flecked retinal syndromes can also be imaged on SD-OCT. We have reported an early variant of a combined hamartoma of the retina and retinal pigment epithelium in a 6-week-old infant undergoing routine ROP screening on the RetCam.[37] The lesion demonstrated a smooth contoured, homogenous lesion arising from the inner retinal layers. The outer retinal layers were imaged using the enhanced depth imaging technique.
Discussion
With the handheld SD-OCT, we now have a useful tool for the evaluation of the pediatric retina. It can assess vision development in premature children, children with unexplained vision loss and amblyopia, children with nystagmus and night blindness and children with intraocular tumors, and retinoblastoma. The list of indications is expanding, especially considering that the test can be performed with reasonable quality in some children without sedation or anesthesia. The requirement for invasive tests that sometimes need to be done under anesthesia, such as a fluorescein angiography, can be minimized with the effective use of this device.
Using the handheld device requires a degree of skill to obtain good quality images and is dependent on the degree of co-operation by the children. Additionally, although the coverage of the peripheral retina is better than with the tabletop devices, image clarity limits how far in the periphery we can capture usable images.
In this manuscript, we report our clinical experience in imaging retinal conditions in infants and children. This cohort may not represent the general prevalence of these entities because of the referral bias in the study subjects. Our institute is a tertiary care referral unit and is likely to receive patients with a higher incidence of unexplained visual loss or less common retinal conditions, which will skew the outcome and make it less generalizable. Hence, we described the indications under different clinical scenarios, to demonstrate the scope of handheld OCT imaging in the pediatric population. We also use OCT images to counsel the parents of these children by demonstrating the structural and morphological changes and correlating it with either vision or therapy at the time of diagnosis or during follow-up.
Though SD-OCT imaging of the pediatric retina has several applications, as Toth CA (2012) has summarized,[38] some concerns include:
The pediatric eye is shorter and optically differs from the adult, and measurements of the lateral extent of lesions reported by OCT systems must be examined and most likely corrected based on the infant eye's unique optical pathway [8]
The infant, and especially the premature infant, retina is notably different from the adult. Inner retinal layers persist and outer retinal layers may be extremely immature. Thus the SD-OCT reflective bands that define adult retinal layers are very different for the premature and term infant eye [39]
SD-OCT findings in infant eyes such as macular edema of prematurity and subfoveal fluid in healthy term infants at birth may represent normal development or unrecognized disease processes [17,40]
Studies correlating SD-OCT findings in infants to visual acuity are few.
Nevertheless, SD-OCT remains an invaluable tool for the assessment of pediatric retinal diseases. Newer technologies such as the OCT angiography can help take it further by providing more detailed information.[41] Intraoperative OCT could help study the retinal morphological changes during surgery in ways that can help improve the surgical outcome. Analyzing quantified data with image analysis and comparing it to age-specific normative data will help identify the more subtle disease in the future.[42,43]
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Puliafito CA, Hee MR, Lin CP, Reichel E, Schuman JS, Duker JS, et al. Imaging of macular diseases with optical coherence tomography. Ophthalmology. 1995;102:217–29. doi: 10.1016/s0161-6420(95)31032-9. [DOI] [PubMed] [Google Scholar]
- 2.Huang D, Swanson EA, Lin CP, Schuman JS, Stinson WG, Chang W, et al. Optical coherence tomography. Science. 1991;254:1178–81. doi: 10.1126/science.1957169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Drexler W. Ultrahigh-resolution optical coherence tomography. J Biomed Opt. 2004;9:47–74. doi: 10.1117/1.1629679. [DOI] [PubMed] [Google Scholar]
- 4.Gupta V, Gupta P, Singh R, Dogra MR, Gupta A. Spectral-domain Cirrus high-definition optical coherence tomography is better than time-domain Stratus optical coherence tomography for evaluation of macular pathologic features in uveitis. Am J Ophthalmol. 2008;145:1018–22. doi: 10.1016/j.ajo.2008.01.021. [DOI] [PubMed] [Google Scholar]
- 5.Drexler W, Sattmann H, Hermann B, Ko TH, Stur M, Unterhuber A, et al. Enhanced visualization of macular pathology with the use of ultrahigh-resolution optical coherence tomography. Arch Ophthalmol. 2003;121:695–706. doi: 10.1001/archopht.121.5.695. [DOI] [PubMed] [Google Scholar]
- 6.Vinekar A, Sivakumar M, Shetty R, Mahendradas P, Krishnan N, Mallipatna A, et al. A novel technique using spectral-domain optical coherence tomography (Spectralis, SD-OCT HRA) to image supine non-anaesthetized infants: Utility demonstrated in aggressive posterior retinopathy of prematurity. Eye (Lond) 2010;24:379–82. doi: 10.1038/eye.2009.313. [DOI] [PubMed] [Google Scholar]
- 7.Scott AW, Farsiu S, Enyedi LB, Wallace DK, Toth CA. Imaging the infant retina with a hand-held spectral-domain optical coherence tomography device. Am J Ophthalmol. 2009;147:364–73.e2. doi: 10.1016/j.ajo.2008.08.010. [DOI] [PubMed] [Google Scholar]
- 8.Maldonado RS, Izatt JA, Sarin N, Wallace DK, Freedman S, Cotten CM, et al. Optimizing hand-held spectral domain optical coherence tomography imaging for neonates, infants, and children. Invest Ophthalmol Vis Sci. 2010;51:2678–85. doi: 10.1167/iovs.09-4403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Salchow DJ, Hutcheson KA. Optical coherence tomography applications in pediatric ophthalmology. J Pediatr Ophthalmol Strabismus. 2007;44:335–49. doi: 10.3928/01913913-20071101-01. [DOI] [PubMed] [Google Scholar]
- 10.Alasil T, Keane PA, Sim DA, Tufail A, Rauser ME. Optical coherence tomography in pediatric ophthalmology: Current roles and future directions. Ophthalmic Surg Lasers Imaging Retina. 2013;44(6 Suppl):S19–29. doi: 10.3928/23258160-20131101-04. [DOI] [PubMed] [Google Scholar]
- 11.Kaul S, Uparkar M, Mody K, Walinjkar J, Kothari M, Natarajan S. Intravitreal anti-vascular endothelial growth factor agents as an adjunct in the management of Coats’ disease in children. Indian J Ophthalmol. 2010;58:76–8. doi: 10.4103/0301-4738.58480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kohly RP, Muni RH, Kertes PJ, Lam WC. Management of pediatric choroidal neovascular membranes with intravitreal anti-VEGF agents: A retrospective consecutive case series. Can J Ophthalmol. 2011;46:46–50. doi: 10.3129/i10-123. [DOI] [PubMed] [Google Scholar]
- 13.Garg S, Mets MB, Bearelly S, Mets R. Imaging of congenital toxoplasmosis macular scars with optical coherence tomography. Retina. 2009;29:631–7. doi: 10.1097/IAE.0b013e318198d8de. [DOI] [PubMed] [Google Scholar]
- 14.Buckland E. Bioptigen En-visu SD-OCT primer. In: Vinekar A, Avadhani K, editors. Spectral Domain Optical Coherence Imaging of the Eye. New Delhi: Elsevier Health Sciences; 2014. pp. 18–27. [Google Scholar]
- 15.Lee AC, Maldonado RS, Sarin N, O’Connell RV, Wallace DK, Freedman SF, et al. Macular features from spectral-domain optical coherence tomography as an adjunct to indirect ophthalmoscopy in retinopathy of prematurity. Retina. 2011;31:1470–82. doi: 10.1097/IAE.0b013e31821dfa6d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tick S, Rossant F, Ghorbel I, Gaudric A, Sahel JA, Chaumet-Riffaud P, et al. Foveal shape and structure in a normal population. Invest Ophthalmol Vis Sci. 2011;52:5105–10. doi: 10.1167/iovs.10-7005. [DOI] [PubMed] [Google Scholar]
- 17.Vinekar A, Avadhani K, Sivakumar M, Mahendradas P, Kurian M, Braganza S, et al. Understanding clinically undetected macular changes in early retinopathy of prematurity on spectral domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2011;52:5183–8. doi: 10.1167/iovs.10-7155. [DOI] [PubMed] [Google Scholar]
- 18.Dubis AM, Subramaniam CD, Godara P, Carroll J, Costakos DM. Subclinical macular findings in infants screened for retinopathy of prematurity with spectral-domain optical coherence tomography. Ophthalmology. 2013;120:1665–71. doi: 10.1016/j.ophtha.2013.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vinekar A, Maldonado RS, Toth CA. Foveal layers in an infant. In: Vinekar A, Avadhani K, editors. Spectral Domain Optical Coherence Imaging of the Eye. New Delhi: Elsevier Health Sciences; 2014. [Google Scholar]
- 20.Vinekar A, Avadhani K. Retinopathy of prematurity-macular changes. In: Vinekar A, Avadhani K, editors. Spectral Domain Optical Coherence Imaging of the Eye. New Delhi: Elsevier Health Sciences; 2014. [Google Scholar]
- 21.Vinekar A, Avadhani K, Sivakumar M, Mahendradas P, Kurian M, Braganza S, et al. Macular edema in premature infants. Ophthalmology. 2012;119:1288–9.e1. doi: 10.1016/j.ophtha.2012.03.029. [DOI] [PubMed] [Google Scholar]
- 22.Cronin TH, Hertle RW, Ishikawa H, Schuman JS. Spectral domain optical coherence tomography for detection of foveal morphology in patients with nystagmus. J AAPOS. 2009;13:563–6. doi: 10.1016/j.jaapos.2009.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vinekar A, Zope A, Mangalesh S, Jayadev C, Sivakumar M, Kemmanu V, et al., editors. Correlating Foveal Spectral Domain Optical Coherence Tomography with Visual Acuity in Premature Infants with and Without Retinopathy of Prematurity. Denver, CO: ARVO; 2015. [Google Scholar]
- 24.Joshi MM, Trese MT, Capone A., Jr Optical coherence tomography findings in stage 4A retinopathy of prematurity: A theory for visual variability. Ophthalmology. 2006;113:657–60. doi: 10.1016/j.ophtha.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 25.Vinekar A, Yadav NK. ROP - Postvirectomy. In: Vinekar A, Avadhani K, editors. Spectral Domain Optical Coherence Imaging of the Eye. New Delhi: Elsevier; 2013. pp. 381–3. [Google Scholar]
- 26.Hood DC, Zhang X, Ramachandran R, Talamini CL, Raza A, Greenberg JP, et al. The inner segment/outer segment border seen on optical coherence tomography is less intense in patients with diminished cone function. Invest Ophthalmol Vis Sci. 2011;52:9703–9. doi: 10.1167/iovs.11-8650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thomas MG, Kumar A, Kohl S, Proudlock FA, Gottlob I. High-resolution in vivo imaging in achromatopsia. Ophthalmology. 2011;118:882–7. doi: 10.1016/j.ophtha.2010.08.053. [DOI] [PubMed] [Google Scholar]
- 28.Leng T, Marmor MF, Kellner U, Thompson DA, Renner AB, Moore W, et al. Foveal cavitation as an optical coherence tomography finding in central cone dysfunction. Retina. 2012;32:1411–9. doi: 10.1097/IAE.0b013e318236e4ea. [DOI] [PubMed] [Google Scholar]
- 29.Lee H, Sheth V, Bibi M, Maconachie G, Patel A, McLean RJ, et al. Potential of handheld optical coherence tomography to determine cause of infantile nystagmus in children by using foveal morphology. Ophthalmology. 2013;120:2714–24. doi: 10.1016/j.ophtha.2013.07.018. [DOI] [PubMed] [Google Scholar]
- 30.Recchia FM, Carvalho-Recchia CA, Trese MT. Optical coherence tomography in the diagnosis of foveal hypoplasia. Arch Ophthalmol. 2002;120:1587–8. [PubMed] [Google Scholar]
- 31.Thomas MG, Kumar A, Mohammad S, Proudlock FA, Engle EC, Andrews C, et al. Structural grading of foveal hypoplasia using spectral-domain optical coherence tomography a predictor of visual acuity? Ophthalmology. 2011;118:1653–60. doi: 10.1016/j.ophtha.2011.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thiadens AA, Somervuo V, van den Born LI, Roosing S, van Schooneveld MJ, Kuijpers RW, et al. Progressive loss of cones in achromatopsia: An imaging study using spectral-domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2010;51:5952–7. doi: 10.1167/iovs.10-5680. [DOI] [PubMed] [Google Scholar]
- 33.Shields CL, Materin MA, Shields JA. Review of optical coherence tomography for intraocular tumors. Curr Opin Ophthalmol. 2005;16:141–54. doi: 10.1097/01.icu.0000158258.01681.40. [DOI] [PubMed] [Google Scholar]
- 34.Medina CA, Plesec T, Singh AD. Optical coherence tomography imaging of ocular and periocular tumours. Br J Ophthalmol. 2014;98(Suppl 2):ii40–6. doi: 10.1136/bjophthalmol-2013-304299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rootman DB, Gonzalez E, Mallipatna A, Vandenhoven C, Hampton L, Dimaras H, et al. Hand-held high-resolution spectral domain optical coherence tomography in retinoblastoma: Clinical and morphologic considerations. Br J Ophthalmol. 2013;97:59–65. doi: 10.1136/bjophthalmol-2012-302133. [DOI] [PubMed] [Google Scholar]
- 36.Mallipatna A, Suren V. Retinoblastoma. In: Vinekar A, Avadhani K, editors. Spectral Domain Optical Coherence Imaging of the Eye. New Delhi: Elsevier Health Sciences; 2014. [Google Scholar]
- 37.Vinekar A, Quiram P, Sund N, Wilson J, Capone A., Jr Plasmin-assisted vitrectomy for bilateral combined hamartoma of the retina and retinal pigment epithelium: Histopathology, immunohistochemistry, and optical coherence tomography. Retin Cases Brief Rep. 2009;3:186–9. doi: 10.1097/ICB.0b013e318185ea69. [DOI] [PubMed] [Google Scholar]
- 38.Toth CA. New devices for posterior segment imaging: Spectral domain OCT in infants. In: Christiansen S, Summers G, editors. Pediatric Ophthalmology 2012 Learning from Our Past, Looking to Our Future: AAO Subspeciality Day. Chicago, Illinois, USA: American Academy of Ophthalmology; 2012. pp. 47–8. [Google Scholar]
- 39.Maldonado RS, O’Connell RV, Sarin N, Freedman SF, Wallace DK, Cotten CM, et al. Dynamics of human foveal development after premature birth. Ophthalmology. 2011;118:2315–25. doi: 10.1016/j.ophtha.2011.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cabrera MT, Maldonado RS, Toth CA, O’Connell RV, Chen BB, Chiu SJ, et al. Subfoveal fluid in healthy full-term newborns observed by handheld spectral-domain optical coherence tomography. Am J Ophthalmol. 2012;153:167–75.e3. doi: 10.1016/j.ajo.2011.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Spaide RF, Klancnik JM, Jr, Cooney MJ. Retinal vascular layers imaged by fluorescein angiography and optical coherence tomography angiography. JAMA Ophthalmol. 2015;133:45–50. doi: 10.1001/jamaophthalmol.2014.3616. [DOI] [PubMed] [Google Scholar]
- 42.Liu X, Shen M, Huang S, Leng L, Zhu D, Lu F. Repeatability and reproducibility of eight macular intra-retinal layer thicknesses determined by an automated segmentation algorithm using two SD-OCT instruments. PLoS One. 2014;9:e87996. doi: 10.1371/journal.pone.0087996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ehnes A, Wenner Y, Friedburg C, Preising MN, Bowl W, Sekundo W, et al. Optical Coherence Tomography (OCT) Device Independent Intraretinal layer segmentation. Transl Vis Sci Technol. 2014;3:1. doi: 10.1167/tvst.3.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]


