Abstract
In this study, we reported the clinical results of switching from ranibizumab to aflibercept for the treatment of an insufficient responder with choroidal neovascularization (CNV) secondary to angioid streaks (AS). A 39-year-old female patient with CNV secondary to AS had bilateral persistent intraretinal and subretinal fluid on the optical coherence tomography despite prior intravitreal 0.5 mg ranibizumab injections. The therapy was switched to intravitreal injection of aflibercept. The patient received a loading dose of three intravitreal 2 mg aflibercept injections at 4-week intervals for both eyes. Morphological and functional effects were observed as early as 1-week after the first injection. After the third aflibercept injection, her visual acuity improved, intraretinal and subretinal fluid resolved, and central macular thickness reduced in both eyes. This is an early, but encouraging and promising result indicating that aflibercept might be a good alternative management for CNV secondary to AS that is insufficiently responding to prior ranibizumab injections.
Keywords: Aflibercept, angioid streaks, choroidal neovascularization
Angioid streaks (AS) are linear breaks that are thought to occur due to the cracks in the abnormally calcified and fragile Bruch's membrane. Choroidal neovascularization (CNV) developed in between these cracks is the most challenging and serious complication of the disease.
Natural course of CNV secondary to AS is poor and sight threatening especially when it disturbs the macular anatomy and function. Various managements have been proposed to improve or at least stabilize the visual acuity and restore macular anatomic architecture. Among these, intravitreal injection of anti-vascular endothelial growth factors (VEGF) bevacizumab and ranibizumab were reported to have the most favorable outcomes in long-term studies.[1,2,3,4,5] In the majority of cases, it was demonstrated that activity disease reduced, but repeated injections were often needed to maintain the results.
Aflibercept is a new molecule that binds all of the VEGF forms with a high affinity. Its’ efficacy in the neovascular age-related macular degeneration (AMD) is reported in several studies.[6] It is also indicated that a significant proportion of exudative AMD cases with persistent fluid despite ranibizumab and/or bevacizumab treatment, respond to aflibercept.[7] In this study, we reported the clinical results of switching from ranibizumab to another anti-VEGF, aflibercept, for the treatment of CNV secondary to AS.
Case Report
A 39-year-old female patient with subfoveal CNV secondary to AS had received 7 intravitreal 0.5 mg ranibizumab injections in her right, and 5 intravitreal 0.5 mg ranibizumab injections in her left eye. One month after the last ranibizumab injections, her best-corrected visual acuity (BCVA) was 20/200 in her right eye and counting fingers (CF) at 1 m in the left eye. Fundus examination revealed exudates radially located around the macula and subretinal fluid at the fovea in the right eye [Fig. 1a]. In the left eye, there was a disciform scar with retinal pigment epithelium hypertrophy covering the macula and subretinal hemorrhages at the posterior pole [Fig. 1b]. Fluorescein angiography revealed a subfoveal classic CNV with an active leakage and hyperfluorescence in the right eye and a staining of disciform scarring in the macula of the left eye. On the optical coherence tomography, she had persistent macular edema and subretinal fluid in the right eye with a central foveal thickness (CFT) of 386 μm [Fig. 1c]; intraretinal fluid in the left eye with a CFT of 212 μm [Fig. 1d], despite prior ranibizumab injections. She was accepted as an insufficient responder to ranibizumab, and therapy was switched to aflibercept.
Figure 1.
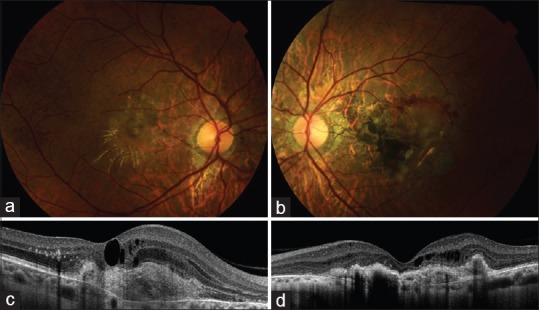
Fundus photograph and optic coherence tomography 1-month after the last ranibizumab injections. (a) Retinal exudates radially located around the macula in the right eye. (b) Disciform scar with subretinal hemorrhages at the posterior pole in the left eye. (c) Choroidal neovascularization with persistent macular edema and subretinal fluid on the optic coherence tomography of the right eye. (d) Choroidal neovascularization and intraretinal fluid on the optic coherence tomography of the left eye
Aflibercept 2.0 mg was administered as three consecutive monthly injections. Both morphological and functional effects were observed in the right and left eyes as early as 1-week after the first aflibercept injections. One month after the third aflibercept injections, her BCVA was 20/40, CFT was 274 μm in the right eye; BCVA was CF at 5 m, and CFT was 188 μm in the left eye. Subretinal and intraretinal fluid reduced considerably in the right eye [Fig. 2a and c], subretinal hemorrhages and intraretinal fluid disappeared in the left eye [Fig. 2b and d].
Figure 2.
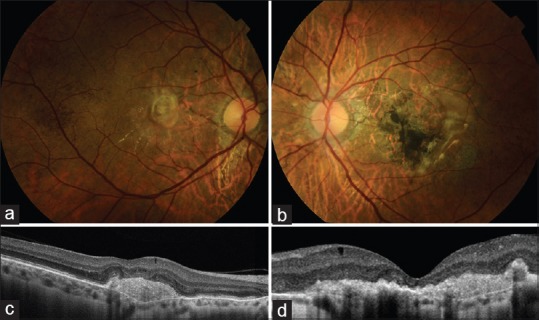
Fundus photograph and optic coherence tomography 1-month after three intravitreal injections of aflibercept. (a) Retinal exudates reduced considerably in the right eye. (b) Subetinal hemorrhages disappeared in the left eye. (c) Optic coherence tomography of the right eye showing the resolution of macular edema. (d) Intraretinal fluid disappeared on the optic coherence tomography of the left eye
Discussion
In AS various managements, such as laser photocoagulation, transpupillary thermotherapy and photodynamic therapy (PDT), have been proposed for the treatment of CNV, but they are no longer used in clinical practice because of rather discouraging results.[1] After the drugs that inhibit VEGF had been introduced in the treatment of CNV secondary to AMD, it became a therapeutic option for other neovascular disorders like AS.
After Teixeira et al.[8] described theirfirst case of intravitreal bevacizumab injection in CNV secondary to AS, several studies were conducted to assess the efficacy of anti-VEGF therapy in these patients. In all these studies, either bevacizumab or ranibizumab was used, both functional and anatomical recovery was achieved in most of the cases.
However, because of being a rare disorder, usually a small number of patients participate in the studies that makes difficult to decide about a standardized treatment protocol. By a majority, injections were administered pro re nata (PRN) with monthly follow-up visits.[2,3,9] In some studies with ranibizumab, injections were applied fixed monthly or with a loading dose, followed by PRN injections.[4,5] The present case was treated with three initial monthly ranibizumab injections for both eyes, followed by four PRN injections for the right, two PRN injections for the left eye. Because the visual acuity did not improve, the change in CFT was not significant, persistent retinal fluid, and disease activation signs like retinal hemorrhages were observed, we accepted patient as an insufficient responder.
Combination therapies such as PDT and intravitreal triamsinolon injection, PDT and bevacizumab, PDT and ranibizumab have been attempted, but none of them was found superior to the anti-VEGF monotheraphy.[1] So we decided to switch the therapy to another anti-VEGF drug.
Knowing the fact that bevacizumab is an off-label drug for ophthalmic applications and associated with a higher risk of systemic side effects, we preferred to administer aflibercept. We applied 2 mg aflibercept with a loading dose of three intravitreal injections at 4 weeks intervals as recommended for AMD patients. Early results of our patient with refractory CNV secondary to AS were satisfactory and promising. The reason of the favorable results with aflibercept might be related to binding not only all isomers of the VEGF-A family but also VEGF-B and placental growth factor.[10] But it is not enough to say that it is better than the others because of the absence of studies comparing anti-VEGF drugs in treatment naïve patients with CNV secondary to AS.
This case illustrates the efficacy of intravitreal aflibercept therapy for persistent retinal fluid due to subfoveal CNV in AS that is insufficient responder to ranibizumab. It also demonstrates switching anti-VEGF agents may also have favorable outcomes in such refractory cases. This is an early, but also encouraging and promising result of intravitreal aflibercept that may be a good alternative management for CNV secondary to AS. Further prospective studies on larger number of patients with a longer follow-up should help to establish the real therapeutic effect of this agent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Gliem M, Finger RP, Fimmers R, Brinkmann CK, Holz FG, Charbel Issa P. Treatment of choroidal neovascularization due to angioid streaks: A comprehensive review. Retina. 2013;33:1300–14. doi: 10.1097/IAE.0b013e3182914d2b. [DOI] [PubMed] [Google Scholar]
- 2.Teixeira A, Mattos T, Velletri R, Teixeira R, Freire J, Moares N, et al. Clinical course of choroidal neovascularization secondary to angioid streaks treated with intravitreal bevacizumab. Ophthalmic Surg Lasers Imaging. 2010;41:546–9. doi: 10.3928/15428877-20100726-04. [DOI] [PubMed] [Google Scholar]
- 3.Finger RP, Charbel Issa P, Schmitz-Valckenberg S, Holz FG, Scholl HN. Long-term effectiveness of intravitreal bevacizumab for choroidal neovascularization secondary to angioid streaks in pseudoxanthoma elasticum. Retina. 2011;31:1268–78. doi: 10.1097/IAE.0b013e318207d1dc. [DOI] [PubMed] [Google Scholar]
- 4.Ladas ID, Kotsolis AI, Ladas DS, Niskopoulou M, Georgalas I, Papakonstantinou D, et al. Intravitreal ranibizumab treatment of macular choroidal neovascularization secondary to angioid streaks: One-year results of a prospective study. Retina. 2010;30:1185–9. doi: 10.1097/IAE.0b013e3181d2f11d. [DOI] [PubMed] [Google Scholar]
- 5.Finger RP, Charbel Issa P, Hendig D, Scholl HP, Holz FG. Monthly ranibizumab for choroidal neovascularizations secondary to angioid streaks in pseudoxanthoma elasticum: A one-year prospective study. Am J Ophthalmol. 2011;152:695–703. doi: 10.1016/j.ajo.2011.03.022. [DOI] [PubMed] [Google Scholar]
- 6.Heier JS, Brown DM, Chong V, Korobelnik JF, Kaiser PK, Nguyen QD, et al. Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration. Ophthalmology. 2012;119:2537–48. doi: 10.1016/j.ophtha.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 7.Fassnacht-Riederle H, Becker M, Graf N, Michels S. Effect of aflibercept in insufficient responders to prior anti-VEGF therapy in neovascular AMD. Graefes Arch Clin Exp Ophthalmol. 2014;252:1705–9. doi: 10.1007/s00417-014-2589-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Teixeira A, Moraes N, Farah ME, Bonomo PP. Choroidal neovascularization treated with intravitreal injection of bevacizumab (Avastin) in angioid streaks. Acta Ophthalmol Scand. 2006;84:835–6. doi: 10.1111/j.1600-0420.2006.00762.x. [DOI] [PubMed] [Google Scholar]
- 9.Shah M, Amoaku WM. Intravitreal ranibizumab for the treatment of choroidal neovascularisation secondary to angioid streaks. Eye (Lond) 2012;26:1194–8. doi: 10.1038/eye.2012.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Papadopoulos N, Martin J, Ruan Q, Rafique A, Rosconi MP, Shi E, et al. Binding and neutralization of vascular endothelial growth factor (VEGF) and related ligands by VEGF Trap, ranibizumab and bevacizumab. Angiogenesis. 2012;15:171–85. doi: 10.1007/s10456-011-9249-6. [DOI] [PMC free article] [PubMed] [Google Scholar]


