Abstract
This study aimed to explore the pattern of accumulation of some of main heavy metals in blood and various organs of rats after exposed to the atmospheric fine particulate matter (PM2.5). Rats were randomly divided into control and three treatment groups (tracheal perfusion with 10 mg/kg, 20 mg/kg and 40 mg/kg of PM2.5 suspension liquid, respectively). Whole blood and the lung, liver, kidney, and cerebral cortex were harvested after rats were treated and sacrificed. The used heavy metals were detected using inductively coupled plasma-mass spectrometry (ICP-MS) instrument. As results, Lead was increased in the liver, lung and cerebral cortex and the level of manganese was significantly elevated in the liver and cerebral cortex in PM2.5 treated rats. Besides, arsenic was prominently enriched both in cerebral cortex and in blood, and so did the aluminum in the cerebral cortex and the copper in the liver. However, cadmium, chromium and nickel have shown no difference between the control group and the three PM2.5 treated groups. Following the exposure of PM2.5, different heavy metals are preferentially accumulated in different body tissues.
Global air pollution became more serious in the recent years and posed public health and safety concerns. Atmospheric particulate matter (PM) is a kind of solid or liquid complex compounds suspended in the atmosphere and a main source of atmospheric pollution. PM, especially fine particulate matter (PM2.5), which has a diameter of no more than 2.5 μm, causes serious harm to human health because of its complicated composition, strong adsorption and rising levels in tandem with rapid industrial development1. It was recognized as the most representative of the atmospheric pollutants. Its monitoring attracts more and more attention worldwide as it aggravates many health problems on prolonged exposure2,3,4.
Because PM2.5 has a long residence time of several days to several weeks in atmosphere, it can travel hundreds to thousands of kilometers. The fine particles in ambient air have been reported to be associated with many health problems including respiratory symptoms, asthma exacerbations, and decrements in lung function5,6. Except for certain insoluble inorganic substances and hydrophobic substances, PM2.5 with water soluble and hygroscopic characteristics could be bio-available7,8. For its large surface area and strong adsorption capacity, PM2.5 can adsorb, combine and transport polycyclic aromatic hydrocarbon (PAH), polychlorinated biphenyls (PCB), heavy metals, bacteria, viruses and other toxic substances and potential carcinogens9,10,11. For insoluble components of PM2.5, once these particulates had been inhaled into the low respiratory tract, they could not only cause inflammatory damage to lung tissues and change the state of relaxation and contraction of blood vessels, but also could diffuse through the alveolar wall into the blood circulation and cause a widespread harm to the body12,13,14,15.
Studies confirmed that PM2.5 with mutagenicity could increase mortality, damage the immune system, as well as cause abnormalities of the nervous system and other serious harm16,17. PM2.5 contains high concentrations of toxic trace metals, such as chromium (Cr), cadmium (Cd), titanium (Ti), manganese (Mn), nickel (Ni), lead (Pb), arsenic (As), zinc (Zn), etc.18,19. These toxic heavy metals incorporated with atmospheric PM2.5 may enter the body through inhalation and have been suggested as causative agents associated with adverse respiratory health effects. Additionally, they can gather in different parts of the body. Heavy metal is not easily biodegradable, and prone to accumulate to hundreds of thousands of times through the food chain under the action of biological amplification enrichment. Synergism or antagonism would occur between all kinds of heavy metal elements in different organisms. A heavy metal element can affect the absorption of another or change its distribution in the body. Studies have shown that Pb, Cd, Cr and Ni in low concentrations from PM2.5 in vivo or in vitro can exhibit genetic toxicity through producing primary DNA or chromosomal damage20. However, researches about intracorporal metabolic distribution of PM2.5 in the major organs are still insufficient.
This study aims at analyzing and comparing the main heavy metals contents of PM2.5 including Pb, aluminum (Al), Mn, copper (Cu), As, Cd, Cr and Ni elements in the blood, lung, liver, kidney, and cerebral cortex of rats after establishment of a rat model which is chronically infected with PM2.5. Eventually, these experimental data can provide scientific evaluation for studying the mechanisms of toxicity induced by atmospheric PM2.5.
Materials and Methods
Reagents and instruments
Normal saline (NS) was obtained from Shandong kangning pharmaceutical Co., Ltd (Shandong, China); Absolute ethyl alcohol was gained from Samtec Tianjin Chemical Reagent Co., Ltd. (Tianjin, China); Diethyl ether and Nitrate (with an excellent level of purity) were purchased from Beijing Chemical Works (Beijing, China); Perchloric acid was purchased from Tianjin zhengcheng chemical products Co., Ltd (Tianjin, China).
TH-150D II PM Sampler was purchased from Wuhan Tianhong Instruments Co., Ltd. (Wuhan, China); Agilent 7500a inductively coupled plasma-mass spectrometry (ICP-MS) was produced from Thermo Scientific Co., Ltd. (Agilent, Santa Clara, USA); Aquaplore ultra-pure water system AWL-2002-Μ was gained from Shanghai bettersize Co., Ltd. (Shanghai, China); ETHOSA Microwave Digestion System (MILESTONE Co., Ltd, USA).
The preparation of mixed PM2.5 suspension
The atmospheric PM2.5 sample was provided by the environmental monitoring center of Tangshan city and the sampling location was at the roof of that center. The sample was collected from December 15, 2013 to February 15, 2014 during the winter season of the city. About 100 m3 sample of air was collected over 24 hours per day each time.
The membrane filter carrying PM2.5 was put into the ultra-pure water and the particles were eluted by ultrasonic oscillator. After 30 min of oscillation, the supernatant fluid was filtered by 5-layer sterile gauze. The obtained liquids were dried to get PM2.5 particles. Control membrane filter was procedurally treated with ultrasonic oscillation in NS as above mentioned and the liquid was utilized in control animals.
PM2.5 particles were weighted and dissolved in NS to make a 4 mg/ml stock solution and the liquid was preserved at 4 °C. Before using, the suspensions were preceded by 30 min ultrasonic oscillation to scatter the particles and then sterilized by autoclaving.
Animal treatment with PM2.5
The 48 adult specific-pathogen-free (SPF) Sprague-Dawley male rats weighting 200–220 g were purchased from the Institute of Hygiene and Environmental Medicine, Academy of Military Medicine (the license number was SCXK- (Army) 2009-003 and the certificate of conformity number was 0001596). The rats were randomly divided into four groups, namely the control group and three treatment groups. They were free feeding and drinking for one week. After ether drugged, each rat in three exposed groups was administrated with PM2.5 working solution (10 ml/kg·body weight) by tracheal perfusion. The exposed dosages used in this study for three groups were 10 mg/kg, 20 mg/kg and 40 mg/kg, respectively. Each working solution was freshly prepared by diluting stock solution with NS. For control group, each rat was treated by the same method with NS (10 ml/kg·body weight) which was processed by the oscillation of the control membrane filter. These experimental rats were treated once a week for up to 12 times. All the experimental protocols were approved by ethics committee of North China University of Science and Technology, Tangshan, Hebei province, China. The methods were carried out in accordance with the approved guidelines.
After finishing the last adminstration, all rats were sacrificed 5 days later. The whole bloods were gathered and the lung, liver, kidney as well as cerebral cortex were removed. All biological samples were immediately stored at −20 °C. 0.1 g of the specimens was respectively put into a small beaker and then digested with 4 ml of mixed concentrated acid (perchloric acid: nitric acid as 1: 4) for 12 h. After that, the beakers were placed on one electric hot plate until white crystal appeared at the bottom of the containers. The capacity was fixed to 5 ml by adding dilute nitric acid (1%) after cooling. Eventually, the contents of heavy metal elements in these samples were determined by using ICP-MS instrument.
The detection of the heavy metal elements
Agilent 7500a ICP-MS was employed to measure the contents of eight kinds of heavy metal elements in these samples. The working conditions and the instrument parameters were listed in table 1. Agilent Calibration Verification Standard solutions were diluted with 1% HNO3 to obtain the standard liquids (STD1). For each heavy metal element, STD1 was diluted into 6 different concentrations by multiple. For STD1, the minimum concentration was 0 ug/L for all these heavy metal elements and the maximum concentrations were 200 ug/L for Al, Pb, Cu, Mn, As, Cr and 20 ug/L for Cd and Ni, respectively. The internal standard elements solution (ISTD, 1 ug/ml) was made by dilution of 10 μg/ml Li6, Sc, Ge, Y, In, Tb as well as Bi and 1% HNO3 was used as the blank (STD0). The ICP-MS was equipped with an autosampler and an Integrated Sample Introduction System with Discrete Sampler (ISIS-DS). A Micromist glass concentric nebulizer (Glass Expansion, MA, USA), quartz torch with a 2.5 mm diameter injector and Shield Torch Technology (Agilent Technologies, CA, USA) were used in the detection.
Table 1. Operating parameters for 7500a ICP-MS instrument.
| Parameters | Setting |
|---|---|
| Flow rate of carrier gas (L/min) | 1.14 |
| Sampling depth (mm) | 5.2 |
| Radio-frequency power (W) | 1480 |
| Spray chamber temp (°C) | 2 |
| Sample cone | Nickel Skimmer |
| Sampling pattern | Quantitative |
| Scanning mode | Jump peak |
| Times of repetition | 3 |
Statistic analysis
All data were analyzed using One-way univariate analysis of variance (ANOVA) followed by Tukey (equal variances assumed or homogeneity of variance after the variable transformation) or Dunnett’s T3 (equal variances not assumed after the variable transformation justification) for Post Hoc test between groups using Statistical Package for Social Sciences software (SPSS version 16.0, Chicago, IL, USA). The results were represented as mean ± SD. All tests were two sided, P < 0.05 was considered statistically significant.
Results
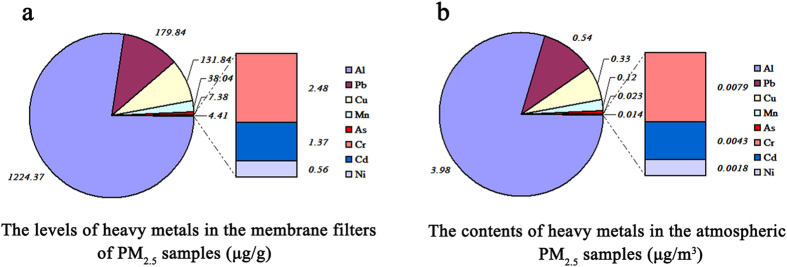
The contents of heavy metal elements in PM2.5 particles
After collection, the membrane filters carrying PM2.5 particles were processed and their heavy metal elements were detected by ICP-MS. The results are shown in Fig. 1(a). The contents of eight kinds of heavy metal elements in the atmospheric PM2.5 samples were ranked from the highest to the lowest level as follows: Al, Pb, Cu, Mn, As, Cr, Cd and Ni. The contents of the first four kinds of metals were higher than 38.0 μg/g. The component analysis showed that airborne concentrations of Al and Pb were the highest (3.98 μg/m3 and 0.54 μg/m3, respectively) among the eight metals of this city’s atmospheric particulate matter in winter, and the contents of rest were followed by Cu (0.33 μg/m3), Mn (0.12 μg/m3), As (0.023 μg/m3), Cr (0.0079 μg/m3), Cd (0.0043 μg/m3) and Ni (0.0018 μg/m3). The results are shown in Fig. 1(b).
Figure 1. The measurements of heavy metal elements in atmospheric PM2.5 particles by ICP-MS during winter in tangshan city.
The levels of heavy metals in the membrane filters of PM2.5 samples are shown in (a). The contents of heavy metals in the atmospheric PM2.5 samples are shown in (b). The eight kinds of heavy metal elements were displayed in order as Al > Pb > Cu > Mn > As > Cr > Cd > Ni.
Heavy metal contents in blood and visceral organs of rats
The eight kinds of heavy metal elements including Al, Pb, Cu, Mn, As, Cr, Cd and Ni in the whole bloods and the liver, lung, cerebral cortex and kidney of rats were detected by ICP-MS.
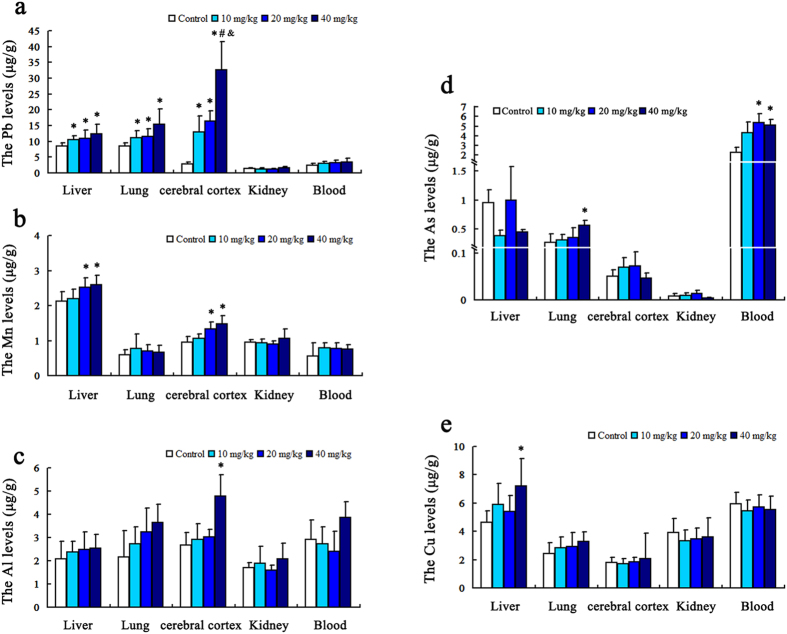
As shown in Fig. 2(a), the visceral lead contents in rats treated with PM2.5 (10 mg/kg, 20 mg/kg and 40 mg/kg) were significantly higher than the control group; the differences were statistically significant (P < 0.05) (F = 3.54, P = 0.033; F = 7.09, P = 0.002 and F = 5.10, P = 0.011, respectively for 40 mg/kg groups, n = 12). Furthermore, lead concentration in the cerebral cortex is creeping upward with the increasing dose and it indicated a remarkable dose-effect relationship.
Figure 2. The levels of heavy metal elements in the whole blood, liver, lung, cerebral cortex and kidney of rats.
Five heavy metals, including Al, Pb, Cu, Mn and As, showed significantly higher levels in groups treated with PM2.5 than control group. The results were shown in (a–e), representing Pb, Mn, Al, As and Cu respectively. *P < 0.05 = significant as compared to the control; #P < 0.05 = significant as compared to the 10 mg/kg group; &P < 0.05 = significant as compared to the 20 mg/kg group (n = 12).
The results of manganese are shown in Fig. 2(b). Compared with control group, manganese contents of rat’s liver and cerebral cortex in the middle and high dose groups were obviously increased (P < 0.05) (F = 3.82, P = 0.026 and F = 7.30, P = 0.003 for liver; F = 4.78, P = 0.013 and F = 9.22, P = 0.002 for cerebral cortex, respectively, n = 12).
As for aluminum, its content was significantly higher than those of the control group only in rats’ cerebral cortex of the high dose group (P < 0.05) (F = 3.616, P = 0.04), (n = 12), as displayed in Fig. 2(c).
The comparison of arsenic contents in different groups is shown in Fig. 2(d). Compared with control group, the contents in the lung of the high dose group (F = 4.14, P = 0.021) and in the total blood of both the middle and the high dose groups (F = 6.589, P = 0.003; F = 10.649, P = 0. 001) were significantly greater (P < 0.05), (n = 12). The copper content in the liver of 40 mg/kg group was higher than the control group and the difference was significant (F = 3.475, P = 0.035). The results were shown in Fig. 2(e).
The results of cadmium, chromium and nickel elements were shown in Fig. 3(a–c). There were no difference between the control groups and the PM2.5 treated groups regarding the concentrations of these elements in rat’s blood and viscera.
Figure 3. The levels of heavy metal elements in the whole blood, liver, lung, cerebral cortex and kidney of rats.
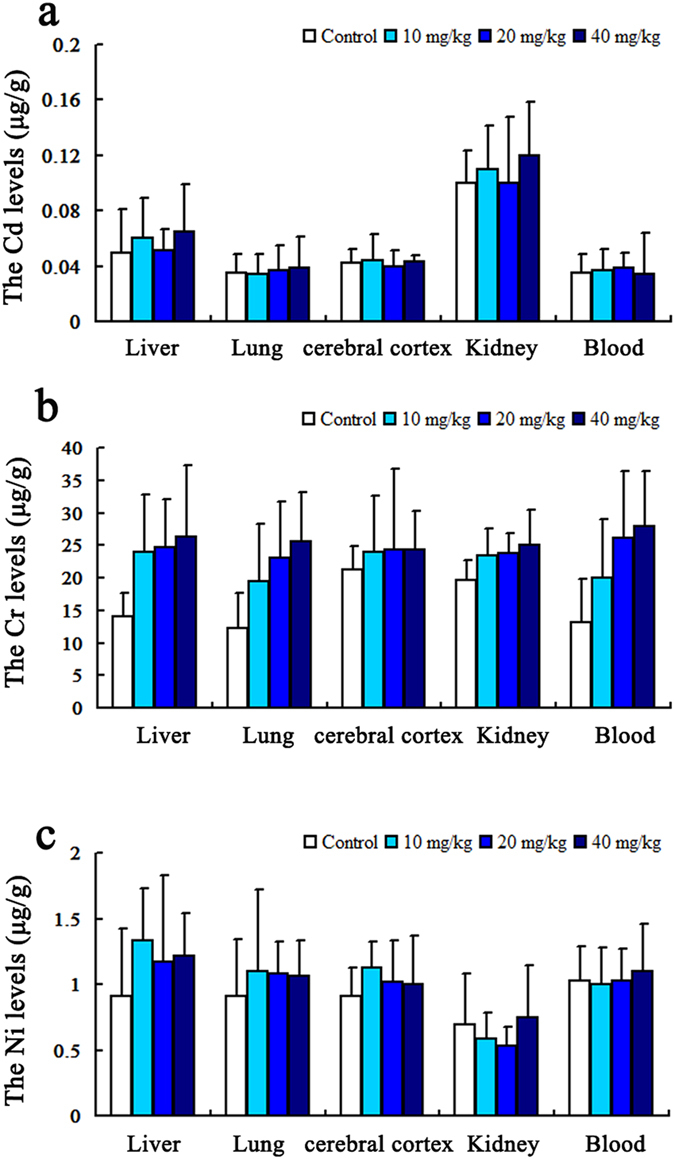
Three heavy metals, including Cd, Cr, and Ni, showed no significant difference between the control group and the PM2.5 treated groups. The results were shown in (a–c). (P > 0.05, respectively) (n = 12).
Discussion
Nowadays, the particulate matter is a main factor affecting global air quality and a primary pollutant for most of the industrial cities. In this study, PM2.5 was collected from Tangshan city which is a heavy industrial port in the north of China with many coal-fired power station, coke-oven plants and iron and steel plants being there. Besides Tangshan, Beijing and Tianjin are also the main areas of atmospheric particulate matter pollution in northern China21.
The analysis of heavy metal components showed that the fine particles PM2.5 in this city primarily consist of aluminum, lead, copper and manganese. In the field of environmental pollution, heavy metal mainly refers to those metal or metalloid elements with obvious biotoxicity such as mercury, cadmium, lead, chromium, copper, cobalt, nickel, tin, arsenic, aluminum, etc. Such pollutants are not easily be degraded by microorganism and may even undergo bioamplification18. Although some other heavy metals are essential elements and a small amount of them show the health benefits such as chromium and manganese, yet their excessive intake can cause damage22. Some heavy metals such as lead and arsenic are well known to be toxic to human body. PM2.5 is an important carrier of heavy metals, and as an atmospheric pollutant, it has potentially serious health hazard to the residents of the contaminated areas16.
Lead as a heavy metal element can pass the blood-brain barrier (BBB), accumulate in brain and eventually cause damage to the central nervous system23. The present study showed that lead was more prominent in the liver, brain and lung of rats when exposed to PM2.5 than the control group. This may be because PM2.5 in the systemic circulation has access to each organ system of the body and they are selectively accumulated in some organs. The liver is the main detoxification organ and accordingly it may have high content of the accumulated lead. Brain may be another important target organ for lead accumulation due to slow excretion24. Brain tissues are relatively sensitive to microenvironment changes. Therefore, even trace amounts of lead can also accumulate in brain tissue and induce neurotoxicity. In this study, with the increase of infected dose, the elevated lead levels in rats’ cortex are obviously detected, presenting significant dose-effect relationship. Kidney is the main excretory organ and it has faster metabolic rate than other organs25,26, so there were no obvious difference in lead levels between the experimental and control groups.
Manganese is one of essential trace elements in different metabolic processes, and as a co-factor of oxidative phosphorylation, it is needed in the enzyme system for catalysing this sequence of oxidative reactions27. Manganese can enter the systemic circulation before being uptaken by mitochondria rich cells in liver, brain, and hair28. High levels of manganese can induce toxic effect on multiple organs so it adversely affects the functions of the liver, cardiovascular, reproductive, immune system and central nervous system29. A study has shown that manganese can pass through the BBB of newborn rat and induce damaging effect on hippocampal development, which finally results in neurobehavioral changes of newborn30.
Due to slow excretion in the brain, excessive accumulation of manganese is the reason that brain is the most affected organ of manganese toxicity31. According to our results, both middle and high dose exposure can lead to selective accumulation of the excessive manganese in the brain and liver, which may induce target organs damage.
The main toxic effect of aluminum is exerted on the nervous system. Aluminum can combine with the phospholipids by complexation and affect the function of nerve cell membrane. Aluminum can also bind the phosphate group in the nuclear chromatin of neurons and disturb DNA transcription and replication to result in abnormal metabolism and protein synthesis32. In addition, it can interfere with cellular energy status and bring about changes in cholinergic neurotransmitter and destruction of BBB function to cause dementia or other degenerative diseases33,34. Related studies have shown that long-term exposure of aluminum increases the susceptibility to Alzheimer’s disease35. In this study, high dose exposure to PM2.5 significantly increased the content of aluminum in cerebral cortex, which confirmed that aluminum can pass the BBB and tend to accumulate in the brain.
The symptoms of arsenicism may appear very soon or may appear after more than ten years or even decades36. It primarily depends on the nature of exposure including the amount and duration of intake of arsenic compounds and the general individual health condition. Unbound arsenic exerts its toxicity by generating reactive oxygen intermediates during their redox cycling and metabolic activation processes that may cause lipid peroxidation and DNA damage. Moreover, it can bind thiol or sulfhydryl groups in tissue proteins of the liver, lung, kidney, spleen, gastrointestinal mucosa, and keratin-rich tissues (skin, hair, and nails)37. Chronic arsenic exposure may be associated with the higher probability of lung cancer occurrence38. This study has found that after exposure to PM2.5, the accumulation of arsenic in blood and lung were obviously increased in a dose dependent manner. Thus it can be understood that hematopoietic dysfunction and an increase in the risk of lung cancer are related to the effect of chronic arsenic exposure.
Just like other essential trace elements, excessive intake of copper can also cause toxic reaction. Copper is mainly concentrated in the liver and once the amount exceeds the ability of liver detoxification, it would be released into the blood39,40. Chronic copper poisoning can cause hepatomegaly and abnormal liver function41. In addition, chronic copper poisoning can lead to lung fibrosis and nervous system disorders including poor memory and attention, instability, multiple neuritis42,43. Selective hepatic lodging of copper was proved by this study so it can be inferred that long-term exposure to PM2.5 would probably lead to liver damage in the first instance.
Although chromium is one of the essential elements, long-term exposure to chromium compounds can lead to lung cancer and hepatocellular carcinoma44. Respiratory tract is the main entry port of cadmium resulting inhalation toxicity, and likewise, it may induce acute liver and kidney damage as well as chronic damage of many organs and systems45. Nickel represents a good example of a metal whose use is increasing in modern technologies. Among the known health related effects of nickel are skin allergies, lung fibrosis, various degrees of kidney injury, cardiovascular system deterioration and stimulation of neoplastic transformation. Nevertheless, the mechanisms of these effects remain not well known46. The results of our study showed that there was no difference of chromium, cadmium and cickel between control group and those treated with different concentrations of PM2.5 in the whole blood and mainly organs of rats. This may be correlated to the low levels of these elements in PM2.5 samples.
Collectively, PM2.5 is a complex mixture of a variety of constituents. After sedimentation in the lung, the heavy metals in PM2.5 particles can easily get into the circulatory system and then accumulate in the target organs such as liver, brain and kidney to cause their dysfunction. They have not only teratogenic, carcinogenic and mutagenic effects but also a huge potential damage to their target organs.
The present studies about in-vivo metabonomics research of important PM2.5 constituents are very limited and there is a need to strengthen the basic research and the epidemiological investigations. This study showed that heavy metals such as lead, manganese, aluminum, and copper carried by PM2.5 can enter the circulation after respiratory exposure and selectively accumulate in the target organs including blood, brain, liver and lungs. This study of heavy metals distribution and metabolism in rat body highlights the potential harm of the atmospheric PM2.5 to various organs of rat providing scientific basis for further studies on the health hazards of the atmospheric PM2.5.
Additional Information
How to cite this article: Li, Q. et al. The preferential accumulation of heavy metals in different tissues following frequent respiratory exposure to PM2.5 in rats. Sci. Rep. 5, 16936; doi: 10.1038/srep16936 (2015).
Footnotes
Author Contributions C.Y.J., Q.Z.L. and H.B.L conceived, designed and carried out the experiments, analyzed the experimental data and wrote the paper; S.F.J., Q.Z.L. and H.B.L guided the experiments and prepared all figures and table; A.M. co-performed writing the paper; J.H. and Y.J.M. co-performed animal model experiments and the detection of ICP-MS; C.Y.J. supervised and directed the project. All authors discussed the results and commented on the manuscript and reviewed the manuscript.
References
- Zhang R. J., Ho K. F. & Shen Z. X. The role of aerosol in climate change, the environment, and human health. Atmos Ocea Sci Lett 5, 156–161 (2011). [Google Scholar]
- Stajner I. et al. US national air quality forecast capability: expanding coverage to include particulate matter in Air Pollution Modeling and its Application XXI (eds Douw G. et al. ) 379–384 (Springer, Netherlands, 2012). [Google Scholar]
- Hu X. et al. Bioaccessibility and health risk of arsenic, mercury and other metals in urban street dusts from a mega-city, Nanjing, China. Environ pollut 159, 1215–1221 (2011). [DOI] [PubMed] [Google Scholar]
- Brown D. M. et al. Occupational Exposure to PM2.5 and Incidence of Ischemic Heart Disease: Longitudinal Targeted Minimum Loss-based Estimation. Epidemiology , 10.1097/EDE.0000000000000329 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzivian L. Outdoor air pollution and asthma in children. J Asthma 48, 470–481 (2011). [DOI] [PubMed] [Google Scholar]
- Rice M. B. et al. Long-term exposure to traffic emissions and fine particulate matter and lung function decline in the Framingham heart study. Am J Respir Crit Care Med 191, 656–664 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y. et al. Characterization and source apportionment of water-soluble organic matter in atmospheric fine particles (PM2.5) with high-resolution aerosol mass spectrometry and GC-MS. Environ Sci Technol 45, 4854–4861 (2011). [DOI] [PubMed] [Google Scholar]
- Chen J. et al. Impact of Relative Humidity and Water Soluble Constituents of PM 2.5 on Visibility Impairment in Beijing, China. Aerosol Air Qual Res 14, 260–268 (2014). [Google Scholar]
- Tucker W. An overview of PM 2.5 sources and control strategies. Fuel Process Technol 65, 379–392 (2000). [Google Scholar]
- Alexis N. E. et al. Biological material on inhaled coarse fraction particulate matter activates airway phagocytes in vivo in healthy volunteers. J Allergy Clin Immunol 117, 1396–1403 (2006). [DOI] [PubMed] [Google Scholar]
- Dergham M. et al. Prooxidant and proinflammatory potency of air pollution particulate matter (PM(2).(5)(-)(0).(3)) produced in rural, urban, or industrial surroundings in human bronchial epithelial cells (BEAS-2B). Chem Res Toxicol 25, 904–919 (2012). [DOI] [PubMed] [Google Scholar]
- Roberts S. & Martin M. A. Methods for bias reduction in time-series studies of particulate matter air pollution and mortality. J Toxicol Environ Health A 70, 665–675 (2007).17365620 [Google Scholar]
- Park S. K. et al. Particulate air pollution, metabolic syndrome, and heart rate variability: the multi-ethnic study of atherosclerosis (MESA). Environ Health Persp 118, 1406–1411 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terzano C., Di Stefano F., Conti V., Graziani E. & Petroianni A. Air pollution ultrafine particles: toxicity beyond the lung. Eur Rev Med Pharmacol Sci 14, 809–821 (2010). [PubMed] [Google Scholar]
- Grahame T. J. Distinguishing health effects among different PM 2.5 components in Urban Airborne Particulate Matter (eds Fathi Z. et al. ) 575–597 (Springer Berlin, Heidelberg, 2011). [Google Scholar]
- Kampa M. & Castanas E. Human health effects of air pollution. Environ Pollut 151, 362–367 (2008). [DOI] [PubMed] [Google Scholar]
- Ying Z. et al. Long-term exposure to concentrated ambient PM2.5 increases mouse blood pressure through abnormal activation of the sympathetic nervous system: a role for hypothalamic inflammation. Environ Health Persp 122, 79–86 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srimuruganandam B. & Shiva Nagendra S. M. Source characterization of PM10 and PM2.5 mass using a chemical mass balance model at urban roadside. Sci Total Environ 433, 8–19 (2012). [DOI] [PubMed] [Google Scholar]
- Saldarriaga-Norena H. et al. Characterization of trace metals of risk to human health in airborne particulate matter (PM2.5) at two sites in Guadalajara, Mexico. J Environ Monitor 11, 887–894 (2009). [DOI] [PubMed] [Google Scholar]
- de Kok T. M. et al. Genotoxicity and physicochemical characteristics of traffic-related ambient particulate matter. Environ Mol Mutagen 46, 71–80 (2005). [DOI] [PubMed] [Google Scholar]
- Yu M., Carmichael G. R., Zhu T. & Cheng Y. F. Sensitivity of predicted pollutant levels to urbanization in China. Atmos Environ 60, 544–554 (2012). [Google Scholar]
- Pilarczyk R. et al. Concentrations of toxic heavy metals and trace elements in raw milk of Simmental and Holstein-Friesian cows from organic farm. Environ Monit Assess 185, 8383–8392 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlet L. et al. Neurodegenerative diseases and exposure to the environmental metals Mn, Pb, and Hg. Coordin Chem Rev 256, 2147–2163 (2012). [Google Scholar]
- Steuerwald A. J., Blaisdell F. S., Geraghty C. M. & Parsons P. J. Regional distribution and accumulation of lead in caprine brain tissues following a long-term oral dosing regimen. J Toxicol Env Heathl A 77, 663–678 (2014). [DOI] [PubMed] [Google Scholar]
- Pollinger K. et al. Biodistribution of quantum dots in the kidney after intravenous injection. J Nanosci Nanotechno 14, 3313–3319 (2014). [DOI] [PubMed] [Google Scholar]
- Kokot F. Dosage of drugs in patients with kidney diseases. Wiad Lek 49, 87–91 (1996). [PubMed] [Google Scholar]
- Oubrahim H., Chock P. B. & Stadtman E. R. Manganese(II) induces apoptotic cell death in NIH3T3 cells via a caspase-12-dependent pathway. J Biol Chem 277, 20135–20138 (2002). [DOI] [PubMed] [Google Scholar]
- Richardson C., Roberts E., Nelms S. & Roberts N. B. Optimisation of whole blood and plasma manganese assay by ICP-MS without use of a collision cell. Clin Chem Lab Med 50, 317–323 (2012). [DOI] [PubMed] [Google Scholar]
- Lu X., Zhu Y., Bai R., Li S. & Teng X. The effect of manganese-induced toxicity on the cytokine mRNA expression of chicken spleen lymphocytes in vitro. Res Vet Sci 101, 165–167 (2015). [DOI] [PubMed] [Google Scholar]
- Garcia S. J., Gellein K., Syversen T. & Aschner M. Iron deficient and manganese supplemented diets alter metals and transporters in the developing rat brain. Toxicol Sci 95, 205–214 (2007). [DOI] [PubMed] [Google Scholar]
- Fernsebner K., Zorn J., Kanawati B., Walker A. & Michalke B. Manganese leads to an increase in markers of oxidative stress as well as to a shift in the ratio of Fe(II)/(III) in rat brain tissue. Metallomics 6, 921–931 (2014). [DOI] [PubMed] [Google Scholar]
- Crapper McLachlan D. R., Lukiw W. J. & Kruck T. P. Aluminum, altered transcription, and the pathogenesis of Alzheimer’s disease. Environ Geochem Health 12, 103–114 (1990). [DOI] [PubMed] [Google Scholar]
- Zatta P., Ibn-Lkhayat-Idrissi M., Zambenedetti P., Kilyen M. & Kiss T. In vivo and in vitro effects of aluminum on the activity of mouse brain acetylcholinesterase. Brain Res Bull 59, 41–45 (2002). [DOI] [PubMed] [Google Scholar]
- Shaw C. A. & Tomljenovic L. Aluminum in the central nervous system (CNS): toxicity in humans and animals, vaccine adjuvants, and autoimmunity. Immunol Res 56, 304–316 (2013). [DOI] [PubMed] [Google Scholar]
- Tomljenovic L. Aluminum and Alzheimer’s disease: after a century of controversy, is there a plausible link? J Alzheimers Dis 23, 567–598 (2011). [DOI] [PubMed] [Google Scholar]
- Kapaj S., Peterson H., Liber K. & Bhattacharya P. Human health effects from chronic arsenic poisoning-a review. J Environ Sci Health A Tox Hazard Subst Environ Eng 41, 2399–2428 (2006). [DOI] [PubMed] [Google Scholar]
- Ratnaike R. N. Acute and chronic arsenic toxicity. Postgrad Med J 79, 391–396 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argos M. et al. Arsenic and lung disease mortality in Bangladeshi adults. Epidemiology 25, 536–543 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer J. F. The molecular basis of copper-transport diseases. Trends Mol Med 7, 64–69 (2001). [DOI] [PubMed] [Google Scholar]
- López-Alonso M. et al. Assessment of some blood parameters as potential markers of hepatic copper accumulation in cattle. J Vet Diagn Invest 18, 71–75 (2006). [DOI] [PubMed] [Google Scholar]
- Zietz B. P. et al. Epidemiological investigation on chronic copper toxicity to children exposed via the public drinking water supply. Sci Total Environ 302, 127–144 (2003). [DOI] [PubMed] [Google Scholar]
- Cadet J. L. Free radical mechanisms in the central nervous system: an overview. Int J Neurosci 40, 13–18 (1988). [DOI] [PubMed] [Google Scholar]
- Magaye R., Zhao J., Bowman L. & Ding M. Genotoxicity and carcinogenicity of cobalt-, nickel- and copper-based nanoparticles. Exp Ther Med 4, 551–561 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arita A. & Costa M. Epigenetics in metal carcinogenesis: nickel, arsenic, chromium and cadmium. Metallomics 1, 222–228 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ognjanovic B. I. et al. Effect of chronic cadmium exposure on antioxidant defense system in some tissues of rats: protective effect of selenium. Physiol Res 57, 403–411 (2008). [DOI] [PubMed] [Google Scholar]
- Denkhaus E. & Salnikow K. Nickel essentiality, toxicity, and carcinogenicity. Crit Rev Oncol Hematol 42, 35–56 (2002). [DOI] [PubMed] [Google Scholar]