Abstract
Influenza viruses are responsible for the influenza outbreaks that lead to significant burden and cause significant morbidity and mortality worldwide. Based on the core proteins, influenza viruses are classified into three types, A, B, and C, of which only A and B cause significant human disease and so the vaccine is directed against these two subtypes only. The effectiveness of the vaccine depends on boosting the immune system against the serotypes included within it. As influenza viruses undergo periodic changes in their antigen, the vaccine is modified annually to ensure susceptibility. In contrast to other countries, Saudi Arabia faces a unique and challenging situation due to Hajj and Umrah seasons, when millions of people gather at the holy places in Mecca and Madinah, during which influenza outbreaks are commonly found. Such challenges making the adoption of strict vaccination strategy in Saudi Arabia is of great importance. All efforts were made to develop this guideline in an easy-to-read form, making it very handy and easy to use by health care workers. The guideline was designed to provide recommendations for problems frequently encountered in real life, with special consideration for special situations such as Hajj and Umrah seasons and pregnancy.
Keywords: Influenza, vaccination, Hajj, Umrah, Saudi Arabia
Influenza virus causes significant morbidity and mortality worldwide.[1] Persons infected by influenza virus may be asymptomatic or present with self-limited acute febrile respiratory symptoms.[2] However, those presenting with severe illness may have significant morbidity and mortality. Such a presentation has been found to be associated with high-risk patients, e.g., elderly persons, young children, and patients of chronic medical conditions.[3] Though prophylaxis with antiviral agents may be used to prevent influenza transmission, vaccination is considered the best method for this purpose.
As part of the commitment of the Saudi Thoracic Society (STS) toward a long-term enhancement plan for promoting best practices in the field of respiratory diseases,[4,5,6,7,8] a need for development of influenza vaccination guidelines was identified. This is also justified by the fact that Saudi Arabia hosts one of the major global mass gatherings by receiving millions of pilgrims from all over the world for the purpose of the Hajj and Umrah. Therefore, the Scientific Committee for Influenza and Pneumococcal Vaccination (SCIPV) guidelines was created by STS to establish local guidelines based on international recommendations on influenza vaccination, best practices, local literature, and the current settings in Saudi Arabia. The STS guidelines for influenza vaccination aims to standardize the approach among health care professionals (HCPs) in Saudi Arabia in an attempt to support the effort of different governmental agencies in this field, with special attention for specific situations like the Hajj and Umrah. It also aims to disseminate knowledge about vaccination against common respiratory pathogens among HCPs through up-to-date guidelines that are simple to understand and use. Of note, the recommendations related to influenza vaccination apply only to seasonal influenza and do not extend to other pandemics, like the avian flu (H5N1).
Methods
The influenza vaccination guidelines are based on international guidelines and best practices.[2,3,9,10] International guidelines on influenza vaccination were customized based on reviewing the available local literature, whenever available, and the current settings in Saudi Arabia, including statements released from governmental agencies. The SCIPV is a group of Saudi experts with well-respected academic backgrounds and experience in the field of respiratory and infectious diseases. The consensus among the SCIPV was followed whenever there was a lack of appropriate evidence.[11] The following criteria were used to grade the evidence as:
Evidence category A: Randomized controlled trials with rich body of data.
Evidence category B: Randomized controlled trials with limited body of data.
Evidence category C: Nonrandomized trials and observational studies.
Evidence category D: Consensus judgment by SCIPV members. This category is only used in cases where the provision of some guidance was deemed valuable, but the clinical literature addressing the subject was insufficient to justify placement in one of the other categories.
Each section was prepared by a member of the panel and then internally reviewed by other members. The panel conducted round-table discussions frequently and jointly. An international experts reviewed the guidelines, and his recommendations were thoughtfully considered.
Epidemiology
Influenza is a common acute respiratory illness that occurs in outbreaks and epidemics worldwide, mainly during the winter season. Attack rates of seasonal influenza in the general population typically range from 7% to 18%,[3,4,5] whereas attack rates for pandemic influenza are estimated to range from 20% to 50%.[12,13,14,15,16] The peak of influenza attacks occurs between October and May in the Northern Hemisphere and between April and September in the Southern Hemisphere. The World Health Organization (WHO) estimated that influenza virus causes severe illness in 3-5 million people annually.[17] The case fatality of persons with two or more risk factors was estimated to be 377/100,000, compared with 9/100,000 for those without associated risk conditions.[18,19,20]
Influenza vaccination was found to have a positive impact on health care, societal, and individual costs by reducing productivity losses and absenteeism associated with influenza-related illnesses.[21,22,23] Though HCPs are among the high-risk group, there are reports showing their low compliance to annual influenza vaccination.[24,25,26] Saudi Arabia is not an exception as a survey conducted in the eastern province showed lower awareness of the benefit of vaccination among HCPs.[27] In another survey conducted in six major hospitals in Saudi Arabia, the vaccination rate among HCPs was as low as 38%. Nonavailability of vaccine was considered by 43% of HCPs to be the highest barrier for not providing vaccine for patients. Furthermore, lack of awareness of influenza vaccination was reported by almost 75%.[28] In a campaign conducted in a tertiary care hospital in Saudi Arabia from 2003 to 2007, the coverage rate for HCPs was found to be 21-29%.[29] However, changing the methodology by engaging the Nursing Department as a partner in a vaccination campaign increased the coverage among nurses to 80%, physicians to 74%, and paramedical personnel to 67%.
A study during the H1N1 epidemic assessed knowledge and attitudes of the Saudi public reported that 38.3% of participants were not convinced about the truth of published reports about this illness.[30] Of those participants, 16.1% reported receiving information from health providers about influenza-related guidelines. Another study during the same epidemic confirmed the need for prevention strategies, including immunization and improved personal hygiene in the Saudi population.[31]
Virology
Influenza viruses have a single-stranded RNA genome and belong to the family Orthomyxoviridae. Influenza virus is classified into three types based on their core proteins, type A, B, and C. Type A virus is further subdivided based on their outside envelope hemagglutinin activity or neuraminidase activity.[32,33] Influenza B and C viruses mainly affect humans, whereas influenza A virus infects a range of mammalian and avian species and causes all influenza pandemics. While influenza C virus causes a mild form of the disease, types A and B can cause significant human disease.[1] Influenza A virus undergoes high mutation rates. Major changes in the influenza A virus hemagglutinin and the neuraminidase are called antigenic shifts. This results in strains capable of causing epidemics or global pandemics. Minor changes so-called antigenic drifts occur almost annually and usually cause more localized outbreaks.[34]
Clinical Manifestations
Influenza infection should be considered in immunocompetent or immunosuppressed person presents with fever and acute onset of respiratory symptoms and signs. The clinical illness of influenza can present as:
Uncomplicated influenza
In the majority of people, influenza is a self-limited infection that usually lasting 2-5 days following an incubation period of 1-4 days. However, the illness may occasionally last for 1 week or more especially in the elderlies, persons with chronic illnesses, and immunocompromised persons. Influenza infection presents with abrupt onset of fever, headache, myalgia, and malaise. It is also associated with respiratory tract manifestation that includes a sore throat, nonproductive cough, and nasal discharge. Fever usually ranges from 37.8°C to 40.0°C. Influenza may also present without fever especially in the older age group. Gastrointestinal manifestations can occur in 10-20% of influenza infections, especially in children. Some patients may have persistent symptoms of weakness or easy fatigability for several weeks, which is referred to as postinfluenza asthenia.[2]
Complicated influenza
Influenza infection can progress to more serious complications, especially in high-risk groups [Table 1], resulting in increased morbidity and mortality.[35] Pneumonia is the most common complication of influenza, which could be attributed to primary viral pneumonia that presents with severe symptoms, high fever, and dyspnea. It could progress quickly to respiratory failure in 2-5 days.[36,37] Secondary, bacterial pneumonia is also an important cause of morbidity and mortality, especially among older persons. Patients typically relapse with higher fevers and productive cough after initial improvement in the symptoms of acute influenza.[38] The most common bacterial pathogens are Streptococcus pneumoniae followed by Staphylococcus aureus, and occasionally community-associated methicillin-resistant S. aureus. Haemophilus influenzae pneumonia may also complicate influenza.[39,40] Mixed pneumonia can occur with features of both viral and bacterial pneumonia.
Table 1.
Groups at high-risk for influenza complications*
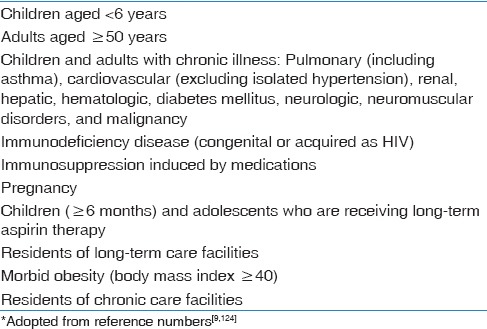
Other complications include myositis, rhabdomyolysis, myocarditis, pericarditis, and toxic shock syndrome. Central nervous system involvement includes encephalopathy, encephalitis, transverse myelitis, aseptic meningitis, and Guillain-Barre syndrome (GBS).[41]
Transmission
Since the virus is present in the respiratory secretions of infected persons, large particle droplets (>5 μm) can easily be transmitted primarily through sneezing and coughing.[42,43] As large particles travel for short distances of <1.5 m, transmission of infection requires close contact with an infected person.[44] The virus can also be transmitted by contact with surfaces that have been contaminated with respiratory droplets, including shaking hands.[45] Infectivity occurs with the shedding of the virus that starts 1–2 days prior to symptoms and until 5-7 days after the appearance of symptoms; 95% of the infectivity occurs in the first 3 days.[46] The period of infectivity may last for 10 days or more in the elderlies, persons with chronic illnesses, and immunocompromised persons.[47]
Influenza Vaccines
Influenza vaccination is the primary tool to prevent influenza infection rather than antiviral chemoprophylaxis.[48] The protection of influenza vaccine depends on inducing humoral immunity, namely neutralizing antibodies against viral capsular antigens, which boost the immune system against the serotypes included in the vaccine. As mentioned, influenza viruses undergo periodic changes in their antigenic envelope glycoproteins, the hemagglutinin (H) and the neuraminidase (N). This explains the spread of infection each year due to the susceptibility of the population to viruses with new antigens.[49] Hence, vaccines are produced annually to match circulating viruses. Three major subtypes of H (H1, H2, and H3) and two subtypes of N (N1 and N2) have been described. Influenza B viruses have less tendency for antigenic changes. Although there are many subtypes of influenza A virus, 1-2 subtypes usually circulate among the human population at any given time. Therefore, most seasonal influenza vaccines include two subtypes of influenza A virus and one subtype of influenza B; hence, it is called a trivalent influenza vaccine (TIV). Recently, quadrivalent vaccines containing two influenza A antigens and two influenza B antigens were introduced. Occasionally, the monovalent vaccine is produced against a candidate pandemic strain, such as the H1N1 vaccine during the 2009/2010 epidemic.
Vaccine production takes about 6 months from the selection of strains to final production and distribution. Two types of vaccines are produced annually; one for the Northern Hemisphere and the other for the Southern Hemisphere. This requires biannual productions of the vaccine to ensure its efficacy against related strains for both Hemispheres. Through the WHO global influenza surveillance and response system, the composition of the vaccine is adjusted based on the characteristics of circulating influenza viruses from the previous season.[50,51] In the Northern Hemisphere, vaccine strains are selected in February and made available in August of each year; in the Southern Hemisphere, vaccine strains are selected in September and made available in April of the following year. Influenza vaccines are manufactured in two forms: Inactivated influenza vaccines (IIVs) and live-attenuated virus vaccines (LAIVs). As the LAIV is not yet available in Saudi Arabia, influenza vaccination guidelines will focus on IIV (mainly TIV).
Inactivated influenza vaccines
The influenza vaccine currently available in Saudi Arabia for persons ≥6 months of age is TIV.[52] As the vaccine includes inactivated or killed virus, this vaccine is not considered infectious. It is administrated through intramuscular injection into the deltoid muscle of the arm [Table 2]. Alternatively, it can be given subcutaneously in those persons with bleeding tendency, albeit with reduced efficacy.[53] The protection of the vaccine depends on several factors, including the age and health status of the person being vaccinated and the match between the virus strains used in the vaccine and those circulating in the community. The overall efficacy of inactivated vaccines in preventing laboratory-confirmed influenza is >60%.[54,55,56,57] However, despite lower efficacy in preventing influenza virus infection in elderly people,[58,59] there is evidence that influenza vaccine lowers hospital admission rates for pneumonia and influenza, and is associated as well with reduction in mortality in elderly vaccines as compared to unvaccinated elderly persons.[60]
Table 2.
Recommended doses in children and adults
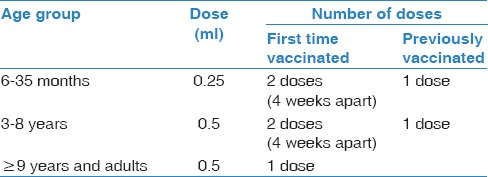
Live-attenuated virus vaccine
LAIV is administrated by the intra-nasal administration. Patients can shed vaccine virus strains from their upper respiratory tract for up to 7 days after receiving the LAIV and can test positive for influenza.[61,62] Hence, the LAIV may cause a mild form of influenza illness. The LAIV is licensed for use among nonpregnant persons aged 2-49 years.[52] LAIV is not yet available in Saudi Arabia.
Indications
The SCIPV recommends the administration of the vaccine annually due to the modification of its composition every year to match the annual circulating influenza virus strains. In Saudi Arabia, the vaccine is recommended during the influenza season that commences annually in September and ends in March of the following year.[63] The vaccination campaign should start soon after the availability of the vaccine. The SCIPV recommends the following for influenza vaccination administration [doses are available in Table 2]:
All persons aged ≥6 months of age, including pregnant, and breastfeeding women (evidence A).[48,52,55,64,65]
-
For children aged 6 months through 8 years, vaccination is recommended based on previous vaccination history:
For children <6 months of age, vaccination is not recommended. However, it is rather recommended to ensure that people around these children are vaccinated:
-
When vaccine supply is limited, vaccination efforts should be prioritized by targeting certain categories:[72,73]
Contraindications
Influenza vaccination is recommended to be postponed if there is an acute illness, especially when associated with fever. However, whenever the acute illness is cured prior to discharge, administration of vaccination is recommended. The IIV is contraindicated in the following situations:
Severe egg allergy. However, a person with mild allergy (e.g., hives) can receive IIV and must wait 30 min under observation in a clinical setting that can handle allergic reactions.[9] To deal with anaphylactic or hypersensitivity reactions, immediate treatment, including epinephrine 1:1000, should be easily accessible during the administration of the vaccine.[84] Formulations that are not produced in eggs are not yet available in Saudi Arabia. Whenever, there are significant concerns, it is recommended to consult a physician specialized in allergic diseases.
Previous history of severe allergy to any of the components of influenza vaccine.
History of GBS within 6 weeks from receiving influenza vaccination.[52]
Children <6 months of age, as no vaccine is yet approved for this category.
If there is an epidemic of influenza in the community, antiviral prophylactic therapy might be administrated during the 2 weeks after receiving the vaccine to protect against the virus till the development of an adequate immune response.[85,86] It can also be administered during the 6 weeks for children not previously vaccinated and who require two doses given at least 4 weeks apart.
Adverse Effects
The IIV is generally safe and well-tolerated in children and adults (evidence A).[87,88,89] However, self-limiting minor side effects have been frequently reported, especially for those who received the influenza vaccination for the first time.[89,90,91,92] The adverse effects include mild redness or swelling at the injection site, low-grade fever, minor body aches, and sore throat. The rate of occurrence of fever has been reported to be 12% in children aged 1-5 years, 5% in children aged 6-15 years and similar to placebo for adults.[52,90] For those who are immunocompromised or have chronic diseases, available evidence does not show that IIV causes clinically important adverse effects.[93,94]
Mild and self-limited oculorespiratory syndrome has been reported to occur within 24 h after receiving IIV.[95,96] However, a Canadian study found low recurrence rates of this complication after revaccination with IIV.[97] The presenting symptoms include red eyes, cough, wheezing, and chest tightness. However, it is still controversial whether this syndrome is a coincidental finding, or it is rather related to an immediate hypersensitivity reaction to any of the IIV components.
Serious adverse events have been rarely reported. The risk of anaphylaxis is very low (0.7 case/million doses) and considered a causal relation with influenza vaccine. Anaphylaxis is presumed to be allergic in nature and may present as hives, angioedema, wheeze and anaphylaxis.[98,99] A slightly increased risk of GBS (1-2 cases/million) has also been reported in association with the influenza vaccine.[41]
Influenza Vaccination for the Hajj and Umrah
The Hajj and Umrah are considered recurrent mass gatherings when millions of Muslims from all over the world come to the holy places in Mecca and Madinah in Saudi Arabia. Infections with influenza viruses are commonly found during these gatherings.[100,101,102,103] In a study that compared the incidence of respiratory tract infections among pilgrims coming from Saudi Arabia and the United Kingdom (UK), the incidence of influenza was 10% in pilgrims from Saudi Arabia and 7% and 14% among vaccinated and nonvaccinated pilgrims from the UK, respectively.[101]
As influenza vaccination is generally considered effective in reducing influenza-related infections, the SCIPV recommends that pilgrims get vaccinated at least 2 weeks before performing the Hajj or Umrah (evidence A).[101,104] It is recommended to pay special attention to those suffering from chronic diseases (cardiac diseases, renal diseases, hepatic disease, respiratory diseases, nervous system disorders, and diabetes mellitus), immune deficient patients (congenital and acquired), metabolic diseases, obese persons, pregnant women, and children aged <5 years. Similar recommendation has also been adopted by the Saudi Ministry of Health that pilgrims are to be vaccinated against seasonal influenza before their arrival into Saudi Arabia.[105]
The Hajj seasons for the next few years will fall during the months of June to September. This raises special concern for the Hajj pilgrims arriving from tropical and subtropical areas, e.g., South and Southeastern Asia, where influenza positivity rates are higher during June to November compared with December to May.[106,107] By collecting a weekly influenza surveillance data from 2006 to 2011 in these countries, Saha et al. reported the positivity rates during June to November to be 86% in Bangladesh, 80% in the Philippines, 70% in India, 43% in Indonesia, and 41% in Malaysia.[107] Therefore, the SCIPV recommends the administration of the Southern Hemisphere influenza vaccine prior to the Hajj and Umrah for pilgrims arriving from the Southern Hemisphere (evidence C).[106,107] Furthermore, as the Northern Hemisphere influenza vaccine is not expected to be available prior to the Hajj in the next seasons for pilgrims from the Northern Hemisphere, the SCIPV also recommends the administration of the Southern Hemisphere influenza vaccine to those pilgrims prior to the Hajj session (evidence D). This should receive special attention as the composition of the current Southern Hemisphere vaccine is based on different characteristics of circulating influenza viruses utilized for the development of the Northern Hemisphere vaccine for the previous season.[108]
Influenza Vaccination for Pregnant and Breastfeeding Women
When compared with nonpregnant women, pregnant women infected with influenza virus are prone to severe illnesses with higher morbidity and mortality.[109,110,111] They also have a greater risk for serious problems for their infants and during delivery.[112,113,114] Hence, seasonal influenza vaccine is commonly used during pregnancy. To assess the safety of influenza vaccine for pregnant women, the vaccine adverse event reporting system (VAERS) project collected reported adverse events in pregnant women receiving the vaccines from 1990 to 2009.[115] The VAERS project did not report unusual patterns of pregnancy complications or adverse fetal outcomes over these two decades. Along the same line, data from six health care organizations in the vaccine safety data link project showed no statistically significant increase in the risk of pregnancy loss 4 weeks after IIV administration.[116] Furthermore, the passive transfer of antibodies from vaccinated women to their infants was found to reduce respiratory illness during the first 6 months of life; hence IIV is not recommended for infants during that period.[117,118] In a randomized controlled study, pregnant women who received seasonal influenza vaccine had a reduction in febrile respiratory illnesses by 36%. The vaccine effectiveness for infants was proven by a 63% reduction of laboratory-confirmed influenza and 29% reduction in febrile respiratory illnesses.[118] Therefore, SCIPV recommends IIV influenza vaccination for pregnant women at any stage of pregnancy (evidence A).[118,119,120,121,122,123] This recommendation is also extended to postpartum and breastfeeding women.
Financial support and sponsorship
The scientific committee for Influenza and Pneumococcal Vaccinations has received full support from the Saudi Thoracic Society.
Conflicts of interest:
There are no conflicts of interest.
Acknowledgment
The Saudi Thoracic Society would like to thank the reviewer of the guidelines, Prof. H. Grant Stiver, Emeritus Professor from the Division of Infectious Diseases, Department of Medicine, Faculty of Medicine, University of British Columbia, Vancouver, BC, Canada.
References
- 1.Luckhaupt SE, Sweeney MH, Funk R, Calvert GM, Nowell M, D’Mello T, et al. Influenza-associated hospitalizations by industry, 2009-10 influenza season, United States. Emerg Infect Dis. 2012;18:556–62. doi: 10.3201/eid1804.110337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harper SA, Bradley JS, Englund JA, File TM, Gravenstein S, Hayden FG, et al. Seasonal influenza in adults and children - diagnosis, treatment, chemoprophylaxis, and institutional outbreak management: Clinical practice guidelines of the Infectious Diseases Society of America. Clin Infect Dis. 2009;48:1003–32. doi: 10.1086/604670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.American Academy of Pediatrics Committee on Infectious Diseases. Antiviral therapy and prophylaxis for influenza in children. Pediatrics. 2007;119:852–60. doi: 10.1542/peds.2007-0224. [DOI] [PubMed] [Google Scholar]
- 4.Idrees MM, Saleemi S, Azem MA, Aldammas S, Alhazmi M, Khan J, et al. Saudi guidelines on the diagnosis and treatment of pulmonary hypertension: 2014 updates. Ann Thorac Med. 2014;9(Suppl 1):S1–15. doi: 10.4103/1817-1737.134006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khan JH, Lababidi HM, Al-Moamary MS, Zeitouni MO, Al-Jahdali HH, Al-Amoudi OS, et al. The Saudi Guidelines for the Diagnosis and Management of COPD. Ann Thorac Med. 2014;9:55–76. doi: 10.4103/1817-1737.128843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Al-Hameed F, Al-Dorzi HM, Shamy A, Qadi A, Bakhsh E, Aboelnazar E, et al. The Saudi clinical practice guideline for the diagnosis of the first deep venous thrombosis of the lower extremity. Ann Thorac Med. 2015;10:3–15. doi: 10.4103/1817-1737.146849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Al-Moamary MS, Alhaider SA, Al-Hajjaj MS, Al-Ghobain MO, Idrees MM, Zeitouni MO, et al. The Saudi initiative for asthma - 2012 update: Guidelines for the diagnosis and management of asthma in adults and children. Ann Thorac Med. 2012;7:175–204. doi: 10.4103/1817-1737.102166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Al-Moamary MS, Al-Hajjaj MS, Idrees MM, Zeitouni MO, Alanezi MO, Al-Jahdali HH, et al. The Saudi Initiative for Asthma. Ann Thorac Med. 2009;4:216–33. doi: 10.4103/1817-1737.56001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grohskopf LA, Olsen SJ, Sokolow LZ, Bresee JS, Cox NJ, Broder KR, et al. Prevention and control of seasonal influenza with vaccines: Recommendations of the Advisory Committee on Immunization Practices (ACIP) - United States, 2014-15 influenza season. MMWR Morb Mortal Wkly Rep. 2014;63:691–7. [PMC free article] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention (CDC). Prevention and control of seasonal influenza with vaccines.Recommendations of the Advisory Committee on Immunization Practices – United States, 2013-2014. MMWR Recomm Rep. 2013;62:1–43. [PubMed] [Google Scholar]
- 11.Jadad AR, Moher M, Browman GP, Booker L, Sigouin C, Fuentes M, et al. Systematic reviews and meta-analyses on treatment of asthma: Critical evaluation. BMJ. 2000;320:537–40. doi: 10.1136/bmj.320.7234.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Glezen WP, Couch RB. Interpandemic influenza in the Houston area, 1974-76. N Engl J Med. 1978;298:587–92. doi: 10.1056/NEJM197803162981103. [DOI] [PubMed] [Google Scholar]
- 13.Sullivan KM, Monto AS, Longini IM., Jr Estimates of the US health impact of influenza. Am J Public Health. 1993;83:1712–6. doi: 10.2105/ajph.83.12.1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Neuzil KM, Zhu Y, Griffin MR, Edwards KM, Thompson JM, Tollefson SJ, et al. Burden of interpandemic influenza in children younger than 5 years: A 25-year prospective study. J Infect Dis. 2002;185:147–52. doi: 10.1086/338363. [DOI] [PubMed] [Google Scholar]
- 15.Sharrar RG. National influenza experience in the USA, 1968-69. Bull World Health Organ. 1969;41:361–6. [PMC free article] [PubMed] [Google Scholar]
- 16.Frost W. Epidemiology of influenza. JAMA. 1919;73:313–8. [Google Scholar]
- 17.World Health Organization. Influenza (Seasonal), Fact sheet N 211. April, 2009. Disponibile al link. [Last accessed on 2015 Apr 23]. Avialble from: http://www.who.int/mediacentre/factsheets/fs211/en/
- 18.Barker WH, Mullooly JP. Pneumonia and influenza deaths during epidemics: Implications for prevention. Arch Intern Med. 1982;142:85–9. [PubMed] [Google Scholar]
- 19.Zimmerman RK, Ahwesh ER. Adult vaccination, part 2: Vaccines for persons at high risk.Teaching Immunization for Medical Education (TIME) Project. J Fam Pract. 2000;49(9 Suppl):S51–63. [PubMed] [Google Scholar]
- 20.Zimmerman RK. Adult vaccination, part 1: Vaccines indicated by age.Teaching Immunization for Medical Education (TIME) Project. J Fam Pract. 2000;49(9 Suppl):S41–50. [PubMed] [Google Scholar]
- 21.Molinari NA, Ortega-Sanchez IR, Messonnier ML, Thompson WW, Wortley PM, Weintraub E, et al. The annual impact of seasonal influenza in the US: Measuring disease burden and costs. Vaccine. 2007;25:5086–96. doi: 10.1016/j.vaccine.2007.03.046. [DOI] [PubMed] [Google Scholar]
- 22.Nichol KL, Mallon KP, Mendelman PM. Cost benefit of influenza vaccination in healthy, working adults: An economic analysis based on the results of a clinical trial of trivalent live attenuated influenza virus vaccine. Vaccine. 2003;21:2207–17. doi: 10.1016/s0264-410x(03)00029-x. [DOI] [PubMed] [Google Scholar]
- 23.Nichol KL. Cost-benefit analysis of a strategy to vaccinate healthy working adults against influenza. Arch Intern Med. 2001;161:749–59. doi: 10.1001/archinte.161.5.749. [DOI] [PubMed] [Google Scholar]
- 24.Ballestas T, McEvoy SP, Doyle J. SMAHS Healthcare Worker Influenza Vaccination Working Party. Co-ordinated approach to healthcare worker influenza vaccination in an area health service. J Hosp Infect. 2009;73:203–9. doi: 10.1016/j.jhin.2009.07.028. [DOI] [PubMed] [Google Scholar]
- 25.Maltezou HC, Maragos A, Raftopoulos V, Karageorgou K, Halharapi T, Remoudaki H, et al. Strategies to increase influenza vaccine uptake among health care workers in Greece. Scand J Infect Dis. 2008;40:266–8. doi: 10.1080/00365540701642658. [DOI] [PubMed] [Google Scholar]
- 26.Maltezou HC, Maragos A, Halharapi T, Karagiannis I, Karageorgou K, Remoudaki H, et al. Factors influencing influenza vaccination rates among healthcare workers in Greek hospitals. J Hosp Infect. 2007;66:156–9. doi: 10.1016/j.jhin.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 27.Al-Tawfiq JA, Antony A, Abed MS. Attitudes towards influenza vaccination of multi-nationality health-care workers in Saudi Arabia. Vaccine. 2009;27:5538–41. doi: 10.1016/j.vaccine.2009.06.108. [DOI] [PubMed] [Google Scholar]
- 28.Alshammari TM, AlFehaid LS, AlFraih JK, Aljadhey HS. Health care professionals’ awareness of, knowledge about and attitude to influenza vaccination. Vaccine. 2014;32:5957–61. doi: 10.1016/j.vaccine.2014.08.061. [DOI] [PubMed] [Google Scholar]
- 29.Al-Otaibi BM, El-Saed A, Balkhy HH. Influenza vaccination among healthcare workers at a tertiary care hospital in Saudi Arabia: Facing challenges. Ann Thorac Med. 2010;5:120–1. doi: 10.4103/1817-1737.62480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Balkhy HH, Abolfotouh MA, Al-Hathlool RH, Al-Jumah MA. Awareness, attitudes, and practices related to the swine influenza pandemic among the Saudi public. BMC Infect Dis. 2010;10:42. doi: 10.1186/1471-2334-10-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.BinSaeed AA. Characteristics of pandemic influenza A (H1N1) infection in patients presenting to a university hospital in Riyadh, Saudi Arabia. Ann Saudi Med. 2010;30:59. doi: 10.4103/0256-4947.59377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Francki RI, Fauquet C, Knudson D, Brown F. Classification and Nomenclature of Viruses: Fifth Report of the International Committee on Taxonomy of Viruses. Virology Division of the International Union of Microbiological Societies: Springer Science & Business Media. 2012 [Google Scholar]
- 33.Petrosillo N, Di Bella S, Drapeau CM, Grilli E. The novel influenza A (H1N1) virus pandemic: An update. Ann Thorac Med. 2009;4:163–72. doi: 10.4103/1817-1737.56008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dolin R. Influenza. In: Braunwald E, Fauci AS, Kasper DL, et al., editors. Harrison's Principles of Internal Medicine. New York: McGraw-Hill; 2008. p. 1127. [Google Scholar]
- 35.Memoli MJ, Athota R, Reed S, Czajkowski L, Bristol T, Proudfoot K, et al. The natural history of influenza infection in the severely immunocompromised vs nonimmunocompromised hosts. Clin Infect Dis. 2014;58:214–24. doi: 10.1093/cid/cit725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Martin CM, Kunin CM, Gottlieb LS, Barnes MW, Liu C, Finland M. Asian influenza A in Boston, 1957-1958. I. Observations in thirty-two influenza-associated fatal cases. AMA Arch Intern Med. 1959;103:515–31. doi: 10.1001/archinte.1959.00270040001001. [DOI] [PubMed] [Google Scholar]
- 37.Rello J, Pop-Vicas A. Clinical review: Primary influenza viral pneumonia. Crit Care. 2009;13:235. doi: 10.1186/cc8183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chertow DS, Memoli MJ. Bacterial coinfection in influenza: A grand rounds review. JAMA. 2013;309:275–82. doi: 10.1001/jama.2012.194139. [DOI] [PubMed] [Google Scholar]
- 39.Schwarzmann SW, Adler JL, Sullivan RJ, Jr, Marine WM. Bacterial pneumonia during the Hong Kong influenza epidemic of 1968-1969. Arch Intern Med. 1971;127:1037–41. [PubMed] [Google Scholar]
- 40.Tabarsi P, Moradi A, Marjani M, Baghaei P, Hashemian SM, Nadji SA, et al. Factors associated with death or intensive care unit admission due to pandemic 2009 influenza A (H1N1) infection. Ann Thorac Med. 2011;6:91–5. doi: 10.4103/1817-1737.78429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vellozzi C, Iqbal S, Broder K. Guillain-Barre syndrome, influenza, and influenza vaccination: The epidemiologic evidence. Clin Infect Dis. 2014;58:1149–55. doi: 10.1093/cid/ciu005. [DOI] [PubMed] [Google Scholar]
- 42.Brankston G, Gitterman L, Hirji Z, Lemieux C, Gardam M. Transmission of influenza A in human beings. Lancet Infect Dis. 2007;7:257–65. doi: 10.1016/S1473-3099(07)70029-4. [DOI] [PubMed] [Google Scholar]
- 43.Bischoff WE, Reid T, Russell GB, Peters TR. Transocular entry of seasonal influenza-attenuated virus aerosols and the efficacy of n95 respirators, surgical masks, and eye protection in humans. J Infect Dis. 2011;204:193–9. doi: 10.1093/infdis/jir238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Center for Disease Control. Influenza antiviral medications: Summary for clinicians. [Last retrieved on 2015 Mar 17]. Available from: http://www.cdc.gov/flu/professionals/antivirals/summary-clinicians.htm .
- 45.Larson EL, Liverman CT. Preventing transmission of pandemic influenza and other viral respiratory diseases: Personal protective equipment for healthcare workers. Update 2010. National Academies Press. 2011. [Last accessed on 2015 Apr 25]. Available at: http://www.cdc.gov/niosh/docket/review/docket129A/pdfs/NIOSH-129-A_IOMreport.pdf . [PubMed]
- 46.Cowling BJ, Chan KH, Fang VJ, Lau LL, So HC, Fung RO, et al. Comparative epidemiology of pandemic and seasonal influenza A in households. N Engl J Med. 2010;362:2175–84. doi: 10.1056/NEJMoa0911530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Leekha S, Zitterkopf NL, Espy MJ, Smith TF, Thompson RL, Sampathkumar P. Duration of influenza A virus shedding in hospitalized patients and implications for infection control. Infect Control Hosp Epidemiol. 2007;28:1071–6. doi: 10.1086/520101. [DOI] [PubMed] [Google Scholar]
- 48.Grohskopf LA, Shay DK, Shimabukuro TT, Sokolow LZ, Keitel WA, Bresee JS, et al. Prevention and control of seasonal influenza with vaccines: Recommendations of the Advisory Committee on Immunization Practices - United States, 2013-2014. MMWR Recomm Rep. 2013;62:1–43. [PubMed] [Google Scholar]
- 49.Balkhy H. Avian influenza: The tip of the iceberg. Ann Thorac Med. 2008;3:154–7. doi: 10.4103/1817-1737.43085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Geneva: WHO; 2013. World Health Organization. Global Influenza Surveillance and Response System (GISRS) [Google Scholar]
- 51.Fiore AE, Shay DK, Broder K, Iskander JK, Uyeki TM, Mootrey G, et al. Prevention and control of seasonal influenza with vaccines: Recommendations of the Advisory Committee on Immunization Practices (ACIP), 2009. MMWR Recomm Rep. 2009;58:1–52. [PubMed] [Google Scholar]
- 52.Fiore AE, Uyeki TM, Broder K, Finelli L, Euler GL, Singleton JA, et al. Prevention and control of influenza with vaccines: Recommendations of the Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Recomm Rep. 2010;59:1–62. [PubMed] [Google Scholar]
- 53.Cook IF, Barr I, Hartel G, Pond D, Hampson AW. Reactogenicity and immunogenicity of an inactivated influenza vaccine administered by intramuscular or subcutaneous injection in elderly adults. Vaccine. 2006;24:2395–402. doi: 10.1016/j.vaccine.2005.11.057. [DOI] [PubMed] [Google Scholar]
- 54.Jefferson TO, Rivetti D, Di Pietrantonj C, Rivetti A, Demicheli V. Vaccines for preventing influenza in healthy adults. Cochrane Database Syst Rev. 2007;Rev 2:CD001269. doi: 10.1002/14651858.CD001269.pub3. [DOI] [PubMed] [Google Scholar]
- 55.Loeb M, Russell ML, Moss L, Fonseca K, Fox J, Earn DJ, et al. Effect of influenza vaccination of children on infection rates in Hutterite communities: A randomized trial. JAMA. 2010;303:943–50. doi: 10.1001/jama.2010.250. [DOI] [PubMed] [Google Scholar]
- 56.Castilla J, Godoy P, Domínguez A, Martínez-Baz I, Astray J, Martín V, et al. Influenza vaccine effectiveness in preventing outpatient, inpatient, and severe cases of laboratory-confirmed influenza. Clin Infect Dis. 2013;57:167–75. doi: 10.1093/cid/cit194. [DOI] [PubMed] [Google Scholar]
- 57.Martínez-Baz I, Navascués A, Pozo F, Chamorro J, Albeniz E, Casado I, et al. Influenza vaccine effectiveness in preventing inpatient and outpatient cases in a season dominated by vaccine-matched influenza B virus. Hum Vaccin Immunother. 2015;11:1626–33. doi: 10.1080/21645515.2015.1038002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bellei NC, Carraro E, Castelo A, Granato CF. Risk factors for poor immune response to influenza vaccination in elderly people. Braz J Infect Dis. 2006;10:269–73. doi: 10.1590/s1413-86702006000400011. [DOI] [PubMed] [Google Scholar]
- 59.Thomas RE, Jefferson TO, Demicheli V, Rivetti D. Influenza vaccination for health-care workers who work with elderly people in institutions: A systematic review. Lancet Infect Dis. 2006;6:273–9. doi: 10.1016/S1473-3099(06)70462-5. [DOI] [PubMed] [Google Scholar]
- 60.Nichol KL, Nordin JD, Nelson DB, Mullooly JP, Hak E. Effectiveness of influenza vaccine in the community-dwelling elderly. N Engl J Med. 2007;357:1373–81. doi: 10.1056/NEJMoa070844. [DOI] [PubMed] [Google Scholar]
- 61.Ali T, Scott N, Kallas W, Halliwell ME, Savino C, Rosenberg E, et al. Detection of influenza antigen with rapid antibody-based tests after intranasal influenza vaccination (FluMist) Clin Infect Dis. 2004;38:760–2. doi: 10.1086/382887. [DOI] [PubMed] [Google Scholar]
- 62.Vesikari T, Karvonen A, Korhonen T, Edelman K, Vainionpää R, Salmi A, et al. A randomized, double-blind study of the safety, transmissibility and phenotypic and genotypic stability of cold-adapted influenza virus vaccine. Pediatr Infect Dis J. 2006;25:590–5. doi: 10.1097/01.inf.0000220229.51531.47. [DOI] [PubMed] [Google Scholar]
- 63.al-Hajjar S, Akhter J, al Jumaah S, Hussain Qadri SM. Respiratory viruses in children attending a major referral centre in Saudi Arabia. Ann Trop Paediatr. 1998;18:87–92. doi: 10.1080/02724936.1998.11747933. [DOI] [PubMed] [Google Scholar]
- 64.Govaert TM, Thijs CT, Masurel N, Sprenger MJ, Dinant GJ, Knottnerus JA. The efficacy of influenza vaccination in elderly individuals.A randomized double-blind placebo-controlled trial. JAMA. 1994;272:1661–5. [PubMed] [Google Scholar]
- 65.Osterholm MT, Kelley NS, Sommer A, Belongia EA. Efficacy and effectiveness of influenza vaccines: A systematic review and meta-analysis. Lancet Infect Dis. 2012;12:36–44. doi: 10.1016/S1473-3099(11)70295-X. [DOI] [PubMed] [Google Scholar]
- 66.Englund JA, Walter EB, Gbadebo A, Monto AS, Zhu Y, Neuzil KM. Immunization with trivalent inactivated influenza vaccine in partially immunized toddlers. Pediatrics. 2006;118:e579–85. doi: 10.1542/peds.2006-0201. [DOI] [PubMed] [Google Scholar]
- 67.Manzoli L, Schioppa F, Boccia A, Villari P. The efficacy of influenza vaccine for healthy children: A meta-analysis evaluating potential sources of variation in efficacy estimates including study quality. Pediatr Infect Dis J. 2007;26:97–106. doi: 10.1097/01.inf.0000253053.01151.bd. [DOI] [PubMed] [Google Scholar]
- 68.Negri E, Colombo C, Giordano L, Groth N, Apolone G, La Vecchia C. Influenza vaccine in healthy children: A meta-analysis. Vaccine. 2005;23:2851–61. doi: 10.1016/j.vaccine.2004.11.053. [DOI] [PubMed] [Google Scholar]
- 69.Neuzil KM, Jackson LA, Nelson J, Klimov A, Cox N, Bridges CB, et al. Immunogenicity and reactogenicity of 1 versus 2 doses of trivalent inactivated influenza vaccine in vaccine-naive 5-8-year-old children. J Infect Dis. 2006;194:1032–9. doi: 10.1086/507309. [DOI] [PubMed] [Google Scholar]
- 70.Rhorer J, Ambrose CS, Dickinson S, Hamilton H, Oleka NA, Malinoski FJ, et al. Efficacy of live attenuated influenza vaccine in children: A meta-analysis of nine randomized clinical trials. Vaccine. 2009;27:1101–10. doi: 10.1016/j.vaccine.2008.11.093. [DOI] [PubMed] [Google Scholar]
- 71.Jefferson T, Smith S, Demicheli V, Harnden A, Rivetti A, Di Pietrantonj C. Assessment of the efficacy and effectiveness of influenza vaccines in healthy children: Systematic review. Lancet. 2005;365:773–80. doi: 10.1016/S0140-6736(05)17984-7. [DOI] [PubMed] [Google Scholar]
- 72.Ndiaye SM, Hopkins DP, Shefer AM, Hinman AR, Briss PA, Rodewald L, et al. Interventions to improve influenza, pneumococcal polysaccharide, and hepatitis B vaccination coverage among high-risk adults: A systematic review. Am J Prev Med. 2005;28(5 Suppl):248–79. doi: 10.1016/j.amepre.2005.02.016. [DOI] [PubMed] [Google Scholar]
- 73.Annoynymous. Seasonal Influenza Vaccination – FAQ 2015. [Last accessed on 2015 Jul 17]. Available from: http://www.moh.gov.sa/en/Flu/Pages/QA.aspx .
- 74.Gross PA, Hermogenes AW, Sacks HS, Lau J, Levandowski RA. The efficacy of influenza vaccine in elderly persons.A meta-analysis and review of the literature. Ann Intern Med. 1995;123:518–27. doi: 10.7326/0003-4819-123-7-199510010-00008. [DOI] [PubMed] [Google Scholar]
- 75.Carman WF, Elder AG, Wallace LA, McAulay K, Walker A, Murray GD, et al. Effects of influenza vaccination of health-care workers on mortality of elderly people in long-term care: A randomised controlled trial. Lancet. 2000;355:93–7. doi: 10.1016/S0140-6736(99)05190-9. [DOI] [PubMed] [Google Scholar]
- 76.Potter J, Stott DJ, Roberts MA, Elder AG, O’Donnell B, Knight PV, et al. Influenza vaccination of health care workers in long-term-care hospitals reduces the mortality of elderly patients. J Infect Dis. 1997;175:1–6. doi: 10.1093/infdis/175.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lee PY, Matchar DB, Clements DA, Huber J, Hamilton JD, Peterson ED. Economic analysis of influenza vaccination and antiviral treatment for healthy working adults. Ann Intern Med. 2002;137:225–31. doi: 10.7326/0003-4819-137-4-200208200-00005. [DOI] [PubMed] [Google Scholar]
- 78.Hayward AC, Harling R, Wetten S, Johnson AM, Munro S, Smedley J, et al. Effectiveness of an influenza vaccine programme for care home staff to prevent death, morbidity, and health service use among residents: Cluster randomised controlled trial. BMJ. 2006;333:1241. doi: 10.1136/bmj.39010.581354.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hurwitz ES, Haber M, Chang A, Shope T, Teo S, Ginsberg M, et al. Effectiveness of influenza vaccination of day care children in reducing influenza-related morbidity among household contacts. JAMA. 2000;284:1677–82. doi: 10.1001/jama.284.13.1677. [DOI] [PubMed] [Google Scholar]
- 80.Esposito S, Marchisio P, Cavagna R, Gironi S, Bosis S, Lambertini L, et al. Effectiveness of influenza vaccination of children with recurrent respiratory tract infections in reducing respiratory-related morbidity within the households. Vaccine. 2003;21:3162–8. doi: 10.1016/s0264-410x(03)00253-6. [DOI] [PubMed] [Google Scholar]
- 81.Nordin J, Mullooly J, Poblete S, Strikas R, Petrucci R, Wei F, et al. Influenza vaccine effectiveness in preventing hospitalizations and deaths in persons 65 years or older in Minnesota, New York, and Oregon: Data from 3 health plans. J Infect Dis. 2001;184:665–70. doi: 10.1086/323085. [DOI] [PubMed] [Google Scholar]
- 82.Hak E, Nordin J, Wei F, Mullooly J, Poblete S, Strikas R, et al. Influence of high-risk medical conditions on the effectiveness of influenza vaccination among elderly members of 3 large managed-care organizations. Clin Infect Dis. 2002;35:370–7. doi: 10.1086/341403. [DOI] [PubMed] [Google Scholar]
- 83.Vu T, Farish S, Jenkins M, Kelly H. A meta-analysis of effectiveness of influenza vaccine in persons aged 65 years and over living in the community. Vaccine. 2002;20:1831–6. doi: 10.1016/s0264-410x(02)00041-5. [DOI] [PubMed] [Google Scholar]
- 84.Erlewyn-Lajeunesse M, Brathwaite N, Lucas JS, Warner JO. Recommendations for the administration of influenza vaccine in children allergic to egg. BMJ. 2009;339:b3680. doi: 10.1136/bmj.b3680. [DOI] [PubMed] [Google Scholar]
- 85.Puig-Barberà J, Diez-Domingo J, Pérez Hoyos S, Belenguer Varea A, González Vidal D. Effectiveness of the MF59-adjuvanted influenza vaccine in preventing emergency admissions for pneumonia in the elderly over 64 years of age. Vaccine. 2004;23:283–9. doi: 10.1016/j.vaccine.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 86.Joseph C, Goddard N. Influenza vaccine uptake in the elderly: Results from a rapid assessment of the effectiveness of new government policy in England for the winters 2000/2001 and 2001/2002. Vaccine. 2003;21:1137–48. doi: 10.1016/s0264-410x(02)00505-4. [DOI] [PubMed] [Google Scholar]
- 87.France EK, Glanz JM, Xu S, Davis RL, Black SB, Shinefield HR, et al. Safety of the trivalent inactivated influenza vaccine among children: A population-based study. Arch Pediatr Adolesc Med. 2004;158:1031–6. doi: 10.1001/archpedi.158.11.1031. [DOI] [PubMed] [Google Scholar]
- 88.Hambidge SJ, Glanz JM, France EK, McClure D, Xu S, Yamasaki K, et al. Safety of trivalent inactivated influenza vaccine in children 6 to 23 months old. JAMA. 2006;296:1990–7. doi: 10.1001/jama.296.16.1990. [DOI] [PubMed] [Google Scholar]
- 89.Govaert TM, Dinant GJ, Aretz K, Masurel N, Sprenger MJ, Knottnerus JA. Adverse reactions to influenza vaccine in elderly people: Randomised double blind placebo controlled trial. BMJ. 1993;307:988–90. doi: 10.1136/bmj.307.6910.988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Neuzil KM, Dupont WD, Wright PF, Edwards KM. Efficacy of inactivated and cold-adapted vaccines against influenza A infection, 1985 to 1990: The pediatric experience. Pediatr Infect Dis J. 2001;20:733–40. doi: 10.1097/00006454-200108000-00004. [DOI] [PubMed] [Google Scholar]
- 91.Rosenberg M, Sparks R, McMahon A, Iskander J, Campbell JD, Edwards KM. Serious adverse events rarely reported after trivalent inactivated influenza vaccine (TIV) in children 6-23 months of age. Vaccine. 2009;27:4278–83. doi: 10.1016/j.vaccine.2009.05.023. [DOI] [PubMed] [Google Scholar]
- 92.Berry BB, Ehlert DA, Battiola RJ, Sedmak G. Influenza vaccination is safe and immunogenic when administered to hospitalized patients. Vaccine. 2001;19:3493–8. doi: 10.1016/s0264-410x(01)00068-8. [DOI] [PubMed] [Google Scholar]
- 93.Neuzil KM. The safety of inactivated influenza vaccine adults and children with asthma. J Pediatr. 2002;140:632. [PubMed] [Google Scholar]
- 94.Wright PF, Thompson J, Vaughn WK, Folland DS, Sell SH, Karzon DT. Trials of influenza A/New Jersey/76 virus vaccine in normal children: An overview of age-related antigenicity and reactogenicity. J Infect Dis. 1977;136(Suppl):S731–41. doi: 10.1093/infdis/136.supplement_3.s731. [DOI] [PubMed] [Google Scholar]
- 95.Ohmit SE, Victor JC, Teich ER, Truscon RK, Rotthoff JR, Newton DW, et al. Prevention of symptomatic seasonal influenza in 2005-2006 by inactivated and live attenuated vaccines. J Infect Dis. 2008;198:312–7. doi: 10.1086/589885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ohmit SE, Victor JC, Rotthoff JR, Teich ER, Truscon RK, Baum LL, et al. Prevention of antigenically drifted influenza by inactivated and live attenuated vaccines. N Engl J Med. 2006;355:2513–22. doi: 10.1056/NEJMoa061850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Skowronski DM, Strauss B, Kendall P, Duval B, De Serres G. Low risk of recurrence of oculorespiratory syndrome following influenza revaccination. CMAJ. 2002;167:853–8. [PMC free article] [PubMed] [Google Scholar]
- 98.Nakayama T, Onoda K. Vaccine adverse events reported in post-marketing study of the Kitasato Institute from 1994 to 2004. Vaccine. 2007;25:570–6. doi: 10.1016/j.vaccine.2006.05.130. [DOI] [PubMed] [Google Scholar]
- 99.Vellozzi C, Burwen DR, Dobardzic A, Ball R, Walton K, Haber P. Safety of trivalent inactivated influenza vaccines in adults: Background for pandemic influenza vaccine safety monitoring. Vaccine. 2009;27:2114–20. doi: 10.1016/j.vaccine.2009.01.125. [DOI] [PubMed] [Google Scholar]
- 100.Alzeer AH. Respiratory tract infection during Hajj. Ann Thorac Med. 2009;4:50–3. doi: 10.4103/1817-1737.49412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Rashid H, Shafi S, Haworth E, El Bashir H, Memish ZA, Sudhanva M, et al. Viral respiratory infections at the Hajj: Comparison between UK and Saudi pilgrims. Clin Microbiol Infect. 2008;14:569–74. doi: 10.1111/j.1469-0691.2008.01987.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Balkhy HH, Memish ZA, Bafaqeer S, Almuneef MA. Influenza a common viral infection among Hajj pilgrims: Time for routine surveillance and vaccination. J Travel Med. 2004;11:82–6. doi: 10.2310/7060.2004.17027. [DOI] [PubMed] [Google Scholar]
- 103.Qureshi H, Gessner BD, Leboulleux D, Hasan H, Alam SE, Moulton LH. The incidence of vaccine preventable influenza-like illness and medication use among Pakistani pilgrims to the Haj in Saudi Arabia. Vaccine. 2000;18:2956–62. doi: 10.1016/s0264-410x(00)00116-x. [DOI] [PubMed] [Google Scholar]
- 104.Mustafa AN, Gessner BD, Ismail R, Yusoff AF, Abdullah N, Ishak I, et al. A case-control study of influenza vaccine effectiveness among Malaysian pilgrims attending the Haj in Saudi Arabia. Int J Infect Dis. 2003;7:210–4. doi: 10.1016/s1201-9712(03)90054-3. [DOI] [PubMed] [Google Scholar]
- 105.Annoynymous. MOH Reiterates Adherence to the Health Requirements by Umrah and Hajj Pilgrims 2015. [Last accessed on 2015 Jul 10]. Available from: http://www.moh.gov.sa/en/Ministry/MediaCenter/News/Pages/news-2015-07-06-001.aspx .
- 106.Chadha MS, Potdar VA, Saha S, Koul PA, Broor S, Dar L, et al. Dynamics of influenza seasonality at sub-regional levels in India and implications for vaccination timing. PLoS One. 2015;10:e0124122. doi: 10.1371/journal.pone.0124122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Saha S, Chadha M, Al Mamun A, Rahman M, Sturm-Ramirez K, Chittaganpitch M, et al. Influenza seasonality and vaccination timing in tropical and subtropical areas of southern and South-Eastern Asia. Bull World Health Organ. 2014;92:318–30. doi: 10.2471/BLT.13.124412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Brookes L, Pavia AT, Poland GA. Why Is Influenza So Difficult to Prevent and Treat?, Will We See Improvement Any Time Soon? [Last accessed on 2015 Apr 11]. Available at: http://www.avogel.it/pubblicazioni/Doc/Why-is-Influenza-so-difficult-to-prevent-and-treat.pdf .
- 109.Neuzil KM, Reed GW, Mitchel EF, Simonsen L, Griffin MR. Impact of influenza on acute cardiopulmonary hospitalizations in pregnant women. Am J Epidemiol. 1998;148:1094–102. doi: 10.1093/oxfordjournals.aje.a009587. [DOI] [PubMed] [Google Scholar]
- 110.Dodds L, McNeil SA, Fell DB, Allen VM, Coombs A, Scott J, et al. Impact of influenza exposure on rates of hospital admissions and physician visits because of respiratory illness among pregnant women. CMAJ. 2007;176:463–8. doi: 10.1503/cmaj.061435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Schanzer DL, Langley JM, Tam TW. Influenza-attributed hospitalization rates among pregnant women in Canada 1994-2000. J Obstet Gynaecol Can. 2007;29:622–9. doi: 10.1016/s1701-2163(16)32559-2. [DOI] [PubMed] [Google Scholar]
- 112.McNeil SA, Dodds LA, Fell DB, Allen VM, Halperin BA, Steinhoff MC, et al. Effect of respiratory hospitalization during pregnancy on infant outcomes. Am J Obstet Gynecol. 2011;204(6 Suppl 1):S54–7. doi: 10.1016/j.ajog.2011.04.031. [DOI] [PubMed] [Google Scholar]
- 113.Bloom-Feshbach K, Simonsen L, Viboud C, Mølbak K, Miller MA, Gottfredsson M, et al. Natality decline and miscarriages associated with the 1918 influenza pandemic: The Scandinavian and United States experiences. J Infect Dis. 2011;204:1157–64. doi: 10.1093/infdis/jir510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Håberg SE, Trogstad L, Gunnes N, Wilcox AJ, Gjessing HK, Samuelsen SO, et al. Risk of fetal death after pandemic influenza virus infection or vaccination. N Engl J Med. 2013;368:333–40. doi: 10.1056/NEJMoa1207210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Moro PL, Broder K, Zheteyeva Y, Walton K, Rohan P, Sutherland A, et al. Adverse events in pregnant women following administration of trivalent inactivated influenza vaccine and live attenuated influenza vaccine in the Vaccine Adverse Event Reporting System, 1990-2009. Am J Obstet Gynecol. 2011;204:146.e1–7. doi: 10.1016/j.ajog.2010.08.050. [DOI] [PubMed] [Google Scholar]
- 116.Irving SA, Kieke BA, Donahue JG, Mascola MA, Baggs J, DeStefano F, et al. Trivalent inactivated influenza vaccine and spontaneous abortion. Obstet Gynecol. 2013;121:159–65. doi: 10.1097/aog.0b013e318279f56f. [DOI] [PubMed] [Google Scholar]
- 117.Puck JM, Glezen WP, Frank AL, Six HR. Protection of infants from infection with influenza A virus by transplacentally acquired antibody. J Infect Dis. 1980;142:844–9. doi: 10.1093/infdis/142.6.844. [DOI] [PubMed] [Google Scholar]
- 118.Zaman K, Roy E, Arifeen SE, Rahman M, Raqib R, Wilson E, et al. Effectiveness of maternal influenza immunization in mothers and infants. N Engl J Med. 2008;359:1555–64. doi: 10.1056/NEJMoa0708630. [DOI] [PubMed] [Google Scholar]
- 119.Munoz FM, Greisinger AJ, Wehmanen OA, Mouzoon ME, Hoyle JC, Smith FA, et al. Safety of influenza vaccination during pregnancy. Am J Obstet Gynecol. 2005;192:1098–106. doi: 10.1016/j.ajog.2004.12.019. [DOI] [PubMed] [Google Scholar]
- 120.Heinonen OP, Shapiro S, Monson RR, Hartz SC, Rosenberg L, Slone D. Immunization during pregnancy against poliomyelitis and influenza in relation to childhood malignancy. Int J Epidemiol. 1973;2:229–35. doi: 10.1093/ije/2.3.229. [DOI] [PubMed] [Google Scholar]
- 121.Pool V, Iskander J. Safety of influenza vaccination during pregnancy. Am J Obstet Gynecol. 2006;194:1200. doi: 10.1016/j.ajog.2005.07.091. [DOI] [PubMed] [Google Scholar]
- 122.Benowitz I, Esposito DB, Gracey KD, Shapiro ED, Vázquez M. Influenza vaccine given to pregnant women reduces hospitalization due to influenza in their infants. Clin Infect Dis. 2010;51:1355–61. doi: 10.1086/657309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Vaccines against influenza WHO position paper - November 2012. Wkly Epidemiol Rec. 2012;87:461–76. [PubMed] [Google Scholar]
- 124.Fiore AE, Fry A, Shay D, Gubareva L, Bresee JS, Uyeki TM. Centers for Disease Control and Prevention (CDC). Antiviral agents for the treatment and chemoprophylaxis of influenza - Recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2011;60:1–24. [PubMed] [Google Scholar]


