Abstract
BACKGROUND:
Current protocols for detection of circulating fibrocytes (CFs) in peripheral blood described in various pulmonary and nonpulmonary disorders involve complex and time consuming, non standardized techniques.
OBJECTIVE:
Testing a method to rapidly detect and quantify CFs using whole blood lysis flow cytometry-based assay in patients with idiopathic pulmonary fibrosis (IPF) and healthy controls.
METHODS:
One milliliter of venous blood sample in ethylenediaminetetraacetic acid (EDTA) from 33 IPF patients and 35 healthy control subjects was collected. Using whole blood lysis method peripheral blood leukocytes were labeled with monoclonal antibodies for cell surface (CD34 and CD45) and intracellular markers (collagen-1) for flow cytometric analysis. CFs were defined as CD45+ cells coexpressing collagen-I and CD34 molecules.
RESULTS:
In 29 (87.8%) IPF patients and 10 (28.5%) control subjects, a well-defined highly granular CD45+ cell population was detected in dot plots generated by side scatter properties of CD45+ cells. These CD45+ cells were identified as CFs on the basis of coexpression of collagen-I and CD34; none of the other cell types in the peripheral blood were labeled with these monoclonal antibodies. In IPF patients the percentage of CFs was significantly higher compared to healthy controls (median (range): 1.37% (0.52-5.65) and 1.04% (0.1-1.84), respectively; P = 0.03).
CONCLUSIONS:
Whole blood lysis method combined with fluorescence-activated cell sorting (FACS) allows detecting a well-defined homogeneous population of CFs. This method is simple, reproducible, and provides an accurate and rapid estimation of CFs.
Keywords: Bonemarrow-derived cells, circulating fibrocytes, fibrosis, flow cytometry, idiopathic pulmonary fibrosis
The pathogenesis of idiopathic pulmonary fibrosis (IPF) is complex and remains poorly understood. After injury, tissue repair induces a complex process of inflammation, proliferation, angiogenesis, and extracellular matrix production.[1] Defects in this process can result in excessive matrix deposition, leading to scar formation, and dysfunction of the involved organ. Myofibroblasts are considered to be the key cell type in the pathogenesis of pulmonary fibrosis, but the exact origin of these cells has not yet been clearly established. Several studies have suggested that circulating bone marrow-derived cells capable of adopting a mesenchymal phenotype termed as “fibrocytes” may be differentiating into fibroblasts and myofibroblasts in the tissue in response to certain stimuli.[2,3,4,5,6,7,8,9] Since their original description by Bucala and colleagues,[2] fibrocytes have received significant attention in the context of various pulmonary[4,6,7,8,10,11,12,13,14,15,16] and nonpulmonary[17,18,19,20] diseases. The exact origin of circulating fibrocytes (CFs) is yet to be determined. However, there is substantial evidence that these cells are leukocytes as they express the CD45 molecule, carry mesenchymal markers including collagen-I, and are derived from hematopoietic stem cells as they express the CD34 molecule on the cell surface.[2]
A whole spectrum of cell separation techniques are used to isolate and quantify CFs from the circulating blood.[2,6,8,12,13,15,16] All of the methods described to date involve various cell separation techniques, such as aspiration of buffy coats, density-gradient cell separation, and monoclonal antibody-based cell depletion. The different steps involved in ex vivo sample handling may interfere with the accurate quantification of fibrocytes present in the peripheral circulation. Here, we describe a new, relatively simple whole blood lysis flow cytometry-based method for detection and quantification of CFs in peripheral blood samples from patients with IPF and healthy controls.
Methods
Study population
A total of 33 patients with IPF (16 females and 17 males) and 35 healthy volunteers (18 females and 17 males) were included in this study. The study was approved by the Institutional Review Board/Ethics Committee of the College of Medicine, King Saud University (Riyadh, Saudi Arabia) and by the Integrated Ethics Review Board of McMaster University (Hamilton, ON, Canada). Written informed consent was obtained from all study participants. IPF was diagnosed according to established guidelines.[21,22] All IPF cases diagnosed prior to the year 2011 were revaluated to ensure that the diagnosis adhered to the current international guidelines on the diagnosis and management of IPF.[22] All subjects were evaluated as outpatients.
Sample collection
Two blood samples (1 and 3ml in EDTA) were collected from both patients and controls; the 1-ml sample was used for flow cytometry, while the 3-ml sample was used for total and differential white blood cell (WBC) counts. Samples with cell concentrations ranging between 3.5 × 103 and 9.8 × 103 WBC/μl were used for flow cytometry, in accordance with the recommendations of the protocol (BD Simul test™ IMK Plus, Cat. No. 349217; Becton Dickinson, San Jose, CA, USA).
Flow cytometric analysis
As previously reported,[13] fibrocytes were phenotypically defined as CD45+ CD34+ Col-1+ expressing cells. One milliliter of venous blood was collected in a vacutainer containing EDTA. For the detection of cell surface markers by flow cytometry, whole blood lysis method was performed using a commercially available kit (Becton Dickinson, Simultest™ IMK Plus; Cat. No. 349217; Becton Dickinson, San Jose, CA, USA) in accordance with the manufacturer'sinstructions. The monoclonal mouseanti-human (immunoglobulin G (IgG) 1) antibodiesused for detection of cell surface markers included anti-CD45 PerCP (Cat. No. 557513; Becton Dickinson Biosciences, San Jose, CA, USA), anti-CD34 PE (Cat. No. 550761; Becton Dickinson Biosciences, San Jose, CA, USA), anti-CD14 PE (Cat. No. 555398; Becton DickinsonBiosciences, San Jose, CA, USA), and the isotype controls, IgG1-FITC (Cat. No. 553443; Becton Dickinson Biosciences, San Jose, CA, USA), and IgG1-PE (Cat. No. 551436; Becton Dickinson Biosciences, San Jose, CA, USA). For intracellular labeling, mouse (IgG) antihuman collagen-1 (Col-1) antibody was used (Cat. No. MA1-83847; ThermoScientific, Rockford, IL, USA).
For the detection of cell surface markers, relevant monoclonal antibodies and isotype controls were dispensed into an aliquot of 100μl of whole blood in 5-ml Falcon polystyrene round-bottom 12 × 75 mm tubes (Becton Dickinson Biosciences, Bedford, MA, USA). Except for CD45 maker where 10 μl of antibody was used, all other monoclonal antibodies were dispensed in a volume of 20 μl. The contents of each tube were vortexed thoroughly at low speed for 3 s, and then incubated for 15 min at room temperature. As a negative control, a separate tube containing 100 μl of blood and 20 μl of phosphate buffered saline (PBS) was included in each experiment. Following this, 2 ml of FACS lysing solution (10% ammonium chloride) was added to each tube and incubated for 10 min in dark for lysis of the red blood cells. The cells were then washed twice with PBS containing 1% bovine serum albumin (DiaMed, GmbH, FR, Switzerland), and fixed with 1% paraformaldehyde solution (PAA Laboratories Inc., Gmbh, Ontario, Canada). Intracellular staining for collagen was performed by indirect staining with a mouse anti-Col-1 antibody. The cells were permeabilized with Perm Buffer II (Becton Dickinson Biosciences, SanJose, CA, USA) for 10 min, washed with PBS containing 0.5% bovine serum albumin, and incubated for 15 min in the dark with 20 μl of 1:100 diluted mouse antihuman Col-1 antibody. The cells were then washed, incubated for 15 min with 10 μl of fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse IgG specific F (ab) 2 (Cat. No. SAB4600042; Sigma-Aldrich Co., St Louis, MO, USA), and washed again with PBS containing 0.5% bovine serum albumin. Finally, the cells were fixed with 2% paraformaldehyde solution. A minimum of 30,000 cells were acquired and flow cytometry was performed on a FACSCalibur (Becton Dickinson Biosciences, San Jose, CA, USA). Data were analyzed using Cell Quest software (Becton Dickinson Biosciences, San Jose, CA, USA). The negative thresholds were set using isotype-control-labeled cells from both patients and normal controls. The data for fibrocytes were recorded as the percentage of the total CD45+ leukocytes.
In order to ensure the reproducibility of the results using the same protocol, a pilot study comprising of five patients was conducted at McMaster University, Hamilton, Ontario, Canada. Results of this pilot study were similar to those obtained in the primary study center.
Statistical analysis
Data are presented as proportions, means, and standard deviations, or as medians with observed ranges. The Mann–Whitney U test was used for nonparametric data. A two-sided P - value of less than 0.05 was considered statistically significant. All analyses were performed using the Statistical Package for Social Sciences (SPSS, version 16.0; SPSS Inc, Chicago, IL, USA).
Results
Detection of CFs
The mean age of participants was 62.9 ± 11.9 years for IPF patients and 27.5 ± 9.9 years for healthy volunteers. Figure 1 shows dot plots for the detection of CFs. Majority of the cells were CD45+ (94.6% ± 2.1) in patients and controls. Figure 1a shows a representative dot plot from 25 (71.5%) normal healthy individuals lacking a unique highly granular cell population which was present in 29 (87.8%) of IPF patients and 28.5% of the controls: The R1 gated cells in Figure 1b. Quadrant analysis of this cell population was performed by setting the vertical and horizontal cursors on cells labeled with isotype control antibody [Figure 1c]. The R1 gated cell population was the only leukocyte subset that was labeled with both the anti-Col-1 and anti-CD34 antibodies [Figure 1d]; none of the other cell types were found binding these antibodies [Figure 1e]. Gating of all CD45+ cell populations clearly identified CFs based on their simultaneous binding of the anti-Col-1 and anti-CD34 antibodies [Figure 1f]; these cells were not labeled with anti-CD14 antibody (data not shown). In patients with IPF, CFs comprised 1.37% (0.52-5.65) (median (range)) of the CD45+ cells, which was higher than the normal controls (1.04% (0.1-1.84); P = 0.03).
Figure 1.
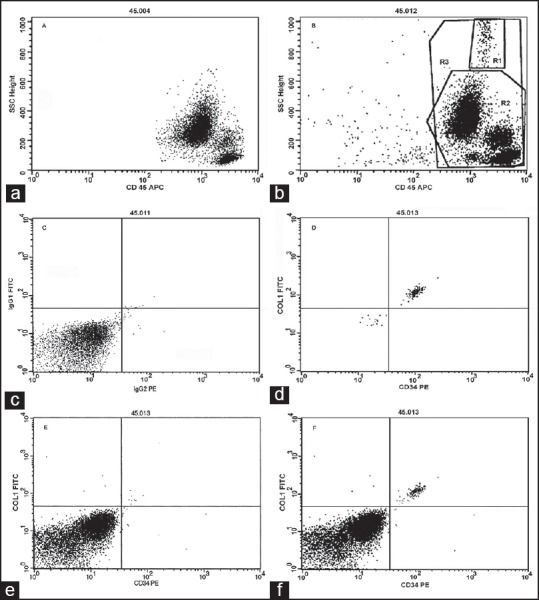
Dot plots from majority of controls lacking circulating fibrocytes (a). Majority of idiopathic pulmonary fibrosis patients and some controls harboring circulating fibrocytes, with cells staining positively with anti-CD45 antibody (b). The horizontal and vertical cursors were set on the isotype control antibodies for quadrant analysis (c). The R1gated cell population identified as fibrocytes based on the coexpression of CD34 and collagen-1 (d). No staining for CD34/collagen-1 antibodies was detected (e). All CD45+ cells clearly revealed a well-defined fibrocyte population (f)
Comparison of whole blood lysis and buffy coat methods
Figure 2 shows data comparing cells isolated by the whole blood lysis method with cells obtained by buffy coat aspiration. Whereas a cluster of a large, complex cells population was observed in the whole blood lysis method [Figure 2a], a rather large population of low to medium complexity with relatively smaller cells was detected in buffy coat isolated peripheral blood cells [Figure 2b]. Distinct clusters of cells were clearly observed with whole blood lysis method [Figure 3a] compared to those observed in cells obtained by buffy coat [Figure 3b], particularly for double positive staining for CD45/Col-1 antibodies. Although the percentages of double positive CD45/Col-1cells detected by buffy coat were higher than those detected by whole blood lysis method [Figure 4a], there was however no difference between the two methods after adjusting for the total cell counts [Figure 4b].
Figure 2.
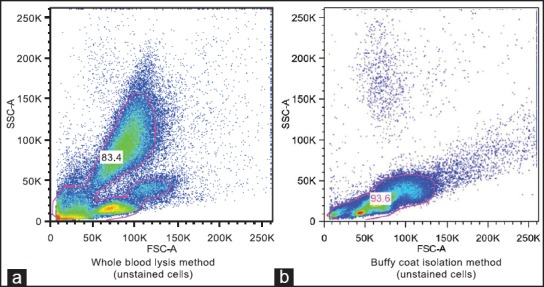
Comparison of dot plots of unstained cell clusters of peripheral blood leukocytes isolated by whole blood lysis method (a) and buffy coat method (b)
Figure 3.
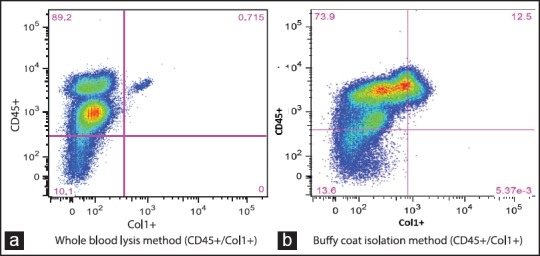
Comparison of dot plots of double positive (CD45/Col-1) peripheral blood leukocytes isolated by whole blood lysis method showing a discrete cell population (a) and a rather ill-defined double positive population of peripheral blood leukocytes isolated by buffy coat method (b)
Figure 4.
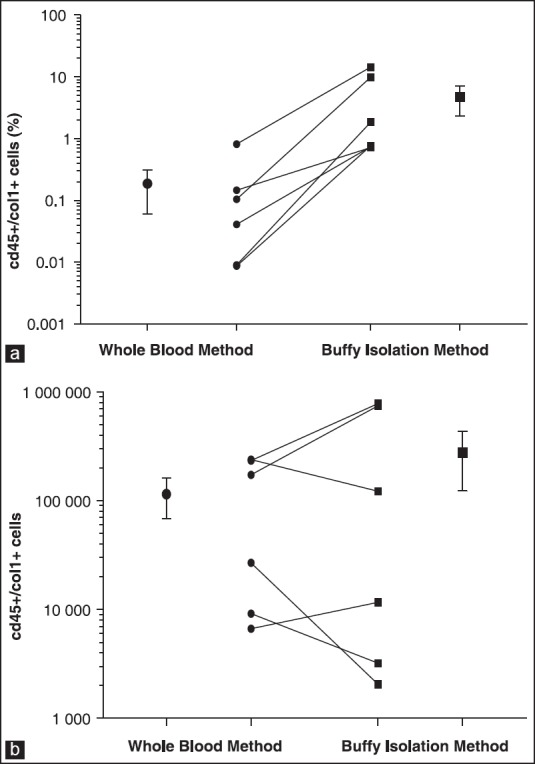
Comparison of percentages of double positive (CD45/Col-1) cells detected by whole blood lysis method and buffy coat method before (a) and after adjustment for total cell counts (b)
Discussion
The present study describes a new whole blood lysis method to detect CFs with flow cytometry. It demonstrates that this method is capable of detecting a well-defined homogeneous population of CFs defined as CD34+, CD45+, and Col-1+ cells in a majority of patients with IPF, and in a relatively small number of healthy controls.
The role of CFs in the pathogenesis of pulmonary fibrosis is supported by studies in animal models.[4,7,10] However, the role of CFs in human fibrotic lung disease appears to be limited. Mehrad and colleagues[6] reported in a small number of patients with fibrotic interstitial lung diseases that fibrocytes comprised 6-10% of the buffy coat leukocyte populations. Furthermore, they found an increased expression of chemokine receptor CXCL12 in the lung tissues which is considered to be a homing receptor for fibrocytes in areas of tissue injury.[6] In another study, Andersson-Sjoland and colleagues[11] found a significant positive correlation between the number of fibroblastic foci and the amount of fibrocytes in the lung tissues of patients with IPF. In another study, Moeller and colleagues[15] reported a significant increase in the percentage of CFs in stable IPF patients compared with healthy control subjects. They also noted a marked increase in the fibrocyte levels of IPF patients with acute exacerbations, and reported that these increased levels were predictive of shortened survival.[15] Our study confirms the findings of Moeller et al.,[15] and Mehrad et al.,[6] showing that CFs are present and increased in the blood of patients with IPF and are also found in normal individuals, albeit at much lower numbers.
Fibrocytes constitute approximately 0.5% of circulating leukocytes in normal healthy individuals.[2] CFs in peripheral blood of otherwise normal healthy individuals ranging from 0.1 to 1% have also been reported in several studies indicating that it is not unusual for these cells to be present in circulation in healthy state.[6,10,23,24] In agreement with these observations, a relatively small number of healthy controls in the present study had CFs in the peripheral blood and the percentage was no different from what has been reported previously.
Previous studies of CFs have used different methods to quantify fibrocytes in peripheral blood; these include detecting CFs by manual aspiration of the peripheral blood leukocytes that form buffy coats at the interface after centrifugation of non-clotted blood samples,[6,12,15,16] detection of CFs in the nonadherent non-T cell population obtained by Ficoll-Hypaque density gradient centrifugation of peripheral blood mononuclear cells followed by manual aspiration of cells from the interface, depletion of T cells by E-rosetting and treatment with monoclonal anti-CD3 antibodies,[13] and by in vitro differentiation of peripheral blood mononuclear cells after 2 weeks in culture.[2] All of these methods, however, involve extensive sample handling in laboratory prior to estimation. For example, there is a risk of in adequate manual aspiration of the cells at the interface during cell separation, and cell depletion methods applied for recovery of CFs may be subject to significant cell loss. Similarly, experiments involving prolonged cell culture include the risk of in vitro cell proliferation, which may adversely affect the accurate assessment of CFs. Moreover, the density gradient cell separation technique that is frequently used for cell separation in experiments designed for detection of CFs has been associated with a selective loss of certain cell populations. This has been demonstrated in the analysis of blood samples from patients with hematological malignancies containing relatively small numbers of neoplastic cells.[25] In addition, Ficoll-Hypaque separated peripheral blood mononuclear cell population when compared with whole blood lysis samples from patients with hematological malignancies has clearly shown a selective loss of CD34+ cells from a significant number of samples.[26] It is possible that the increased granularity of CFs may have prevented these cells from settling at the interphase, particularly in procedures involving Ficoll-Hypaque density gradient cell separation.
Apart from sample preparation, the placement of gates is an important aspect of quality assurance in flow cytometric analysis. One of the published flow cytometric methods for assessing CFs involves excluding CD45-positive leukocytes and enumerating the CD45/Col-I positive cells that shift into the gated area over a broad spread of laser side scatter.[15] One limitation and criticism of this method is that it may be less accurate and reproducible, because the broad spread of side scatter could suggest that CFs are not a distinct population, but rather is a mixture of different leukocytes. From a clinical perspective, this relative inaccuracy may be an acceptable limitation as long as the CF counts determined by this approach are useful for monitoring disease activity.[27] Further, as the gate lines often appear to bifurcate, dense cell populations and a minor inconsistency in the application of gates may alter the accuracy of the estimation. In contrast, the whole blood lysis method in the present study allowed detecting a more precisely defined discrete population of CFs. Because of its relative simplicity, this method may be very useful for serial measurements for monitoring the alterations in fibrocyte counts in response to disease activity or therapeutic interventions. The whole blood lysis method for flow cytometric analysis is less labor intensive, cost effective, and requires significantly less amount of blood. It offers a reproducible and accurate estimation of CFs, and avoids extensive sample handling in the laboratory.
In summary, we used a commercially available protocol for whole blood lysis followed by flow cytometry to detect CFs in the peripheral circulation. These cells, which expressed CD45+ CD34+ Col-I+ markers, were found in the majority of patients with IPF and a relatively small number of normal healthy individuals. The validity and usefulness of this easier and less expensive method of fibrocyte detection should be evaluated in a larger multicenter study.
Acknowledgments
The authors thank Actelion Pharmaceuticals Ltd., Riyadh, Saudi Arabia for providing the monoclonal antibodies used in the present study.
Footnotes
Source of Support: Nil
Conflicts of interest: None declared.
References
- 1.Martin P. Wound healing — aiming for perfect skin regeneration. Science. 1997;276:75–81. doi: 10.1126/science.276.5309.75. [DOI] [PubMed] [Google Scholar]
- 2.Bucala R, Spiegel LA, Chesney J, Hogan M, Cerami A. Circulating fibrocytes define a new leukocyte subpopulation that mediates tissue repair. Mol Med. 1994;1:71–81. [PMC free article] [PubMed] [Google Scholar]
- 3.Bellini A, Mattoli S. The role of the fibrocyte, a bone marrow-derived mesenchymal progenitor, in reactive and reparative fibroses. Lab Invest. 2007;87:858–70. doi: 10.1038/labinvest.3700654. [DOI] [PubMed] [Google Scholar]
- 4.Hashimoto N, Jin H, Liu T, Chensue SW, Phan SH. Bone marrow-derived progenitor cells in pulmonary fibrosis. J Clin Invest. 2004;113:243–52. doi: 10.1172/JCI18847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hong KM, Belperio JA, Keane MP, Burdick MD, Strieter RM. Differentiation of human circulating fibrocytes as mediated by transforming growth factor-beta and peroxisome proliferator-activated receptor gamma. J Biol Chem. 2007;282:22910–20. doi: 10.1074/jbc.M703597200. [DOI] [PubMed] [Google Scholar]
- 6.Mehrad B, Burdick MD, Zisman DA, Keane MP, Belperio JA, Strieter RM. Circulating peripheral blood fibrocytes in human fibrotic interstitial lung disease. Biochem Biophys Res Commun. 2007;353:104–8. doi: 10.1016/j.bbrc.2006.11.149. [DOI] [PubMed] [Google Scholar]
- 7.Moore BB, Murray L, Das A, Wilke CA, Herrygers AB, Toews GB. The role of CCL12 in the recruitment of fibrocytes and lung fibrosis. Am J Respir Cell Mol Biol. 2006;35:175–81. doi: 10.1165/rcmb.2005-0239OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schmidt M, Sun G, Stacey MA, Mori L, Mattoli S. Identification of circulating fibrocytes as precursors of bronchial myofibroblasts in asthma. J Immunol. 2003;171:380–9. doi: 10.4049/jimmunol.171.1.380. [DOI] [PubMed] [Google Scholar]
- 9.Mori L, Bellini A, Stacey MA, Schmidt M, Mattoli S. Fibrocytes contribute to the myofibroblast population in wounded skin and originate from the bone marrow. Exp Cell Res. 2005;304:81–90. doi: 10.1016/j.yexcr.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 10.Phillips RJ, Burdick MD, Hong K, Lutz MA, Murray LA, Xue YY, et al. Circulating fibrocytes traffic to the lungs in response to CXCL12 and mediate fibrosis. J Clin Invest. 2004;114:438–46. doi: 10.1172/JCI20997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Andersson-Sjoland A, de Alba CG, Nihlberg K, Becerril C, Ramirez R, Pardo A, et al. Fibrocytes are a potential source of lung fibroblasts in idiopathic pulmonary fibrosis. Int J Biochem Cell Biol. 2008;40:2129–40. doi: 10.1016/j.biocel.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 12.LaPar DJ, Burdick MD, Emaminia A, Harris DA, Strieter BA, Liu L, et al. Circulating fibrocytes correlate with bronchiolitis obliterans syndrome development after lung transplantation: A novel clinical biomarker. Ann Thorac Surg. 2011;92:470–7. doi: 10.1016/j.athoracsur.2011.04.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang CH, Huang CD, Lin HC, Lee KY, Lin SM, Liu CY, et al. Increased circulating fibrocytes in asthma with chronic airflow obstruction. Am J Respir Crit Care Med. 2008;178:583–91. doi: 10.1164/rccm.200710-1557OC. [DOI] [PubMed] [Google Scholar]
- 14.Saunders R, Siddiqui S, Kaur D, Doe C, Sutcliffe A, Hollins F, et al. Fibrocyte localization to the airway smooth muscle is a feature of asthma. J Allergy Clin Immunol. 2009;123:376–84. doi: 10.1016/j.jaci.2008.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moeller A, Gilpin SE, Ask K, Cox G, Cook D, Gauldie J, et al. Circulating fibrocytes are an indicator of poor prognosis in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2009;179:588–94. doi: 10.1164/rccm.200810-1534OC. [DOI] [PubMed] [Google Scholar]
- 16.Yeager ME, Nguyen CM, Belchenko DD, Colvin KL, Takatsuki S, Ivy DD, et al. Circulating fibrocytes are increased in children and young adults with pulmonary hypertension. Eur Respir J. 2012;39:104–11. doi: 10.1183/09031936.00072311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang L, Scott PG, Dodd C, Medina A, Jiao H, Shankowsky HA, et al. Identification of fibrocytes in postburn hypertrophic scar. Wound Repair Regen. 2005;13:398–404. doi: 10.1111/j.1067-1927.2005.130407.x. [DOI] [PubMed] [Google Scholar]
- 18.Haudek SB, Xia Y, Huebener P, Lee JM, Carlson S, Crawford JR, et al. Bone marrow-derived fibroblast precursors mediate ischemic cardiomyopathy in mice. Proc Natl Acad Sci U S A. 2006;103:18284–9. doi: 10.1073/pnas.0608799103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pilling D, Buckley CD, Salmon M, Gomer RH. Inhibition of fibrocyte differentiation by serum amyloid P. J Immunol. 2003;171:5537–46. doi: 10.4049/jimmunol.171.10.5537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vakil V, Sung JJ, Piecychna M, Crawford JR, Kuo P, Abu-Alfa AK, et al. Gadolinium-containing magnetic resonance image contrast agent promotes fibrocyte differentiation. J Magn Reson Imaging. 2009;30:1284–8. doi: 10.1002/jmri.21800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.American Thoracic Society, European Respiratory Society. American Thoracic Society/European Respiratory Society International Multidisciplinary Consensus Classification of the Idiopathic Interstitial Pneumonias.This joint statement of the American Thoracic Society (ATS), and the European Respiratory Society (ERS) was adopted by the ATS board of directors, June 2001 and by the ERS Executive Committee, June 2001. Am J Respir Crit Care Med. 2002;165:277–304. doi: 10.1164/ajrccm.165.2.ats01. [DOI] [PubMed] [Google Scholar]
- 22.Raghu G, Collard HR, Egan JJ, Martinez FJ, Behr J, Brown KK, et al. ATS/ERS/JRS/ALAT Committee on Idiopathic Pulmonary Fibrosis.An official ATS/ERS/JRS/ALAT statement: Idiopathic pulmonary fibrosis: Evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med. 2011;183:788–824. doi: 10.1164/rccm.2009-040GL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Metz CN. Fibrocytes: A unique cell population implicated in wound healing. Cell Mol Life Sci. 2003;60:1342–50. doi: 10.1007/s00018-003-2328-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Quan TE, Cowper S, Wu SP, Bockenstedt LK, Bucala R. Circulating fibrocytes: Collagen-secreting cells of the peripheral blood. Int J Biochem Cell Biol. 2004;36:598–606. doi: 10.1016/j.biocel.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 25.Villanova, PA: 1993. National Committee for Clinical Laboratory Standards. Clinical applications of flow cytometry: Immunophenotyping of leukemic cells. Proposed guideline. NCCLS document H43-P. ISBN 1-56238-219-5. [Google Scholar]
- 26.Tamul KR, Schmitz JL, Kane K, Folds JD. Comparison of the effects of Ficoll-Hypaque separation and whole blood lysis on results of immunophenotypic analysis of blood and bone marrow samples from patients with hematologic malignancies. Clin Diagn Lab Immunol. 1995;2:337–42. doi: 10.1128/cdli.2.3.337-342.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moore BB, Kolb M. Fibrocytes and progression of fibrotic lung disease.Ready for show time? Am J Respir Crit Care Med. 2014;190:1338–9. doi: 10.1164/rccm.201411-2013ED. [DOI] [PubMed] [Google Scholar]


