Abstract
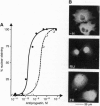
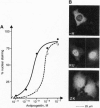
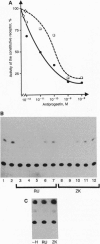
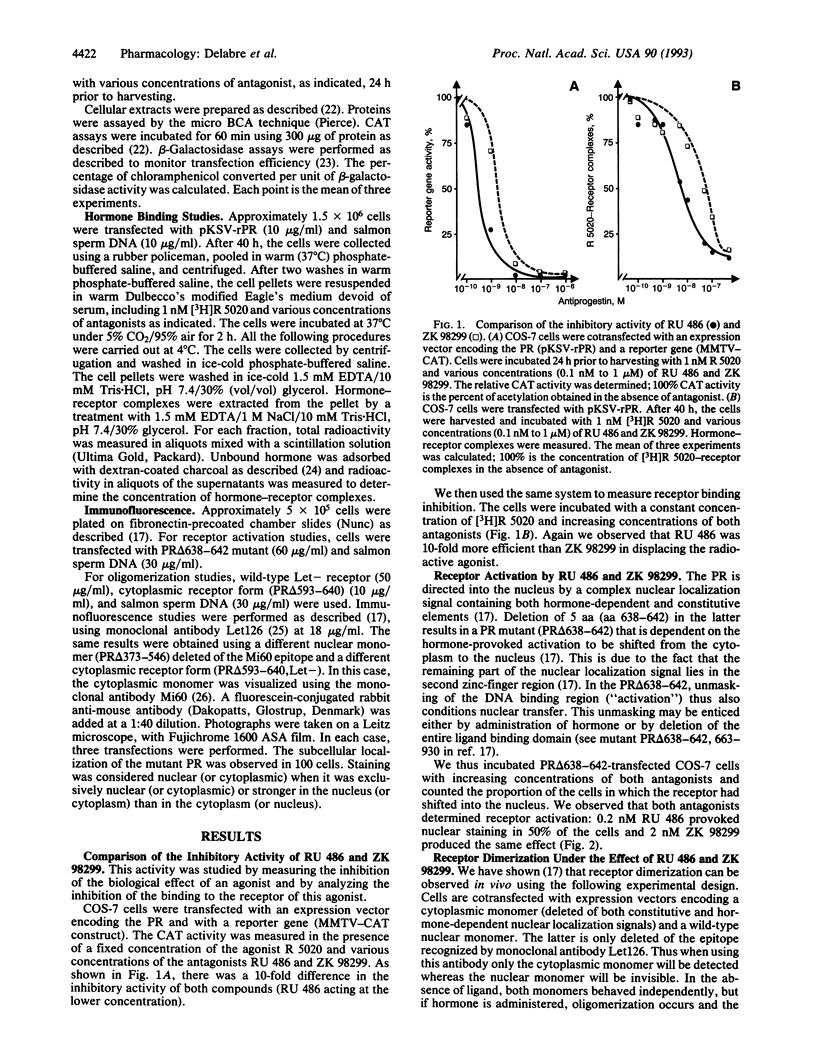
The binding of a steroid hormone to its receptor elicits a sequence of events: activation of the receptor (probably through dissociation from a complex of heat shock proteins), dimerization, binding to hormone responsive elements, and finally modulation of gene transcription. RU 486, the first antiprogestin studied, has been shown to act at the last step of this sequence: provoking an inefficient binding of the receptor to hormone responsive elements. Recently, based on in vitro studies, it has been proposed that ZK 98299 was the prototype of a second class of antiprogestins that were supposed to act through disruption of the binding to DNA. We have devised methods allowing us to study the various steps of agonist or antagonist action in vivo. We show here that RU 486 and ZK 98299 have the same effects on receptor activation, dimerization, and binding to hormone responsive elements; differences in their action are explained by the 10-fold difference in their affinity for the receptor (ZK 98299 having the lower affinity).
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baulieu E. E. Contragestion and other clinical applications of RU 486, an antiprogesterone at the receptor. Science. 1989 Sep 22;245(4924):1351–1357. doi: 10.1126/science.2781282. [DOI] [PubMed] [Google Scholar]
- Beato M. Induction of transcription by steroid hormones. Biochim Biophys Acta. 1987 Nov 20;910(2):95–102. doi: 10.1016/0167-4781(87)90060-1. [DOI] [PubMed] [Google Scholar]
- Benhamou B., Garcia T., Lerouge T., Vergezac A., Gofflo D., Bigogne C., Chambon P., Gronemeyer H. A single amino acid that determines the sensitivity of progesterone receptors to RU486. Science. 1992 Jan 10;255(5041):206–209. doi: 10.1126/science.1372753. [DOI] [PubMed] [Google Scholar]
- Cabrol D., Bouvier D'Yvoire M., Mermet E., Cedard L., Sureau C., Baulieu E. E. Induction of labour with mifepristone after intrauterine fetal death. Lancet. 1985 Nov 2;2(8462):1019–1019. doi: 10.1016/s0140-6736(85)90575-6. [DOI] [PubMed] [Google Scholar]
- Chauchereau A., Loosfelt H., Milgrom E. Phosphorylation of transfected wild type and mutated progesterone receptors. J Biol Chem. 1991 Sep 25;266(27):18280–18286. [PubMed] [Google Scholar]
- Couzinet B., Le Strat N., Ulmann A., Baulieu E. E., Schaison G. Termination of early pregnancy by the progesterone antagonist RU 486 (Mifepristone). N Engl J Med. 1986 Dec 18;315(25):1565–1570. doi: 10.1056/NEJM198612183152501. [DOI] [PubMed] [Google Scholar]
- DeMarzo A. M., Beck C. A., Onate S. A., Edwards D. P. Dimerization of mammalian progesterone receptors occurs in the absence of DNA and is related to the release of the 90-kDa heat shock protein. Proc Natl Acad Sci U S A. 1991 Jan 1;88(1):72–76. doi: 10.1073/pnas.88.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluzman Y. SV40-transformed simian cells support the replication of early SV40 mutants. Cell. 1981 Jan;23(1):175–182. doi: 10.1016/0092-8674(81)90282-8. [DOI] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Gravanis A., Schaison G., George M., de Brux J., Satyaswaroop P. G., Baulieu E. E., Robel P. Endometrial and pituitary responses to the steroidal antiprogestin RU 486 in postmenopausal women. J Clin Endocrinol Metab. 1985 Jan;60(1):156–163. doi: 10.1210/jcem-60-1-156. [DOI] [PubMed] [Google Scholar]
- Gronemeyer H., Benhamou B., Berry M., Bocquel M. T., Gofflo D., Garcia T., Lerouge T., Metzger D., Meyer M. E., Tora L. Mechanisms of antihormone action. J Steroid Biochem Mol Biol. 1992 Mar;41(3-8):217–221. doi: 10.1016/0960-0760(92)90347-l. [DOI] [PubMed] [Google Scholar]
- Gronemeyer H. Transcription activation by estrogen and progesterone receptors. Annu Rev Genet. 1991;25:89–123. doi: 10.1146/annurev.ge.25.120191.000513. [DOI] [PubMed] [Google Scholar]
- Groyer A., Schweizer-Groyer G., Cadepond F., Mariller M., Baulieu E. E. Antiglucocorticosteroid effects suggest why steroid hormone is required for receptors to bind DNA in vivo but not in vitro. Nature. 1987 Aug 13;328(6131):624–626. doi: 10.1038/328624a0. [DOI] [PubMed] [Google Scholar]
- Guiochon-Mantel A., Loosfelt H., Lescop P., Sar S., Atger M., Perrot-Applanat M., Milgrom E. Mechanisms of nuclear localization of the progesterone receptor: evidence for interaction between monomers. Cell. 1989 Jun 30;57(7):1147–1154. doi: 10.1016/0092-8674(89)90052-4. [DOI] [PubMed] [Google Scholar]
- Guiochon-Mantel A., Loosfelt H., Ragot T., Bailly A., Atger M., Misrahi M., Perricaudet M., Milgrom E. Receptors bound to antiprogestin from abortive complexes with hormone responsive elements. Nature. 1988 Dec 15;336(6200):695–698. doi: 10.1038/336695a0. [DOI] [PubMed] [Google Scholar]
- Herbomel P., Bourachot B., Yaniv M. Two distinct enhancers with different cell specificities coexist in the regulatory region of polyoma. Cell. 1984 Dec;39(3 Pt 2):653–662. doi: 10.1016/0092-8674(84)90472-0. [DOI] [PubMed] [Google Scholar]
- Herrmann W., Wyss R., Riondel A., Philibert D., Teutsch G., Sakiz E., Baulieu E. E. Effet d'un stéroide anti-progestérone chez la femme: interruption du cycle menstruel et de la grossesse au début. C R Seances Acad Sci III. 1982 May 17;294(18):933–938. [PubMed] [Google Scholar]
- Horwitz K. B. The antiprogestin RU38 486: receptor-mediated progestin versus antiprogestin actions screened in estrogen-insensitive T47Dco human breast cancer cells. Endocrinology. 1985 Jun;116(6):2236–2245. doi: 10.1210/endo-116-6-2236. [DOI] [PubMed] [Google Scholar]
- Klein-Hitpass L., Cato A. C., Henderson D., Ryffel G. U. Two types of antiprogestins identified by their differential action in transcriptionally active extracts from T47D cells. Nucleic Acids Res. 1991 Mar 25;19(6):1227–1234. doi: 10.1093/nar/19.6.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klijn J. G., de Jong F. H., Bakker G. H., Lamberts S. W., Rodenburg C. J., Alexieva-Figusch J. Antiprogestins, a new form of endocrine therapy for human breast cancer. Cancer Res. 1989 Jun 1;49(11):2851–2856. [PubMed] [Google Scholar]
- Kumar V., Chambon P. The estrogen receptor binds tightly to its responsive element as a ligand-induced homodimer. Cell. 1988 Oct 7;55(1):145–156. doi: 10.1016/0092-8674(88)90017-7. [DOI] [PubMed] [Google Scholar]
- Logeat F., Le Cunff M., Pamphile R., Milgrom E. The nuclear-bound form of the progesterone receptor is generated through a hormone-dependent phosphorylation. Biochem Biophys Res Commun. 1985 Aug 30;131(1):421–427. doi: 10.1016/0006-291x(85)91819-4. [DOI] [PubMed] [Google Scholar]
- Logeat F., Vu Hai M. T., Fournier A., Legrain P., Buttin G., Milgrom E. Monoclonal antibodies to rabbit progesterone receptor: crossreaction with other mammalian progesterone receptors. Proc Natl Acad Sci U S A. 1983 Nov;80(21):6456–6459. doi: 10.1073/pnas.80.21.6456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzo F., Jolivet A., Loosfelt H., Thu vu Hai M., Brailly S., Perrot-Applanat M., Milgrom E. A rapid method of epitope mapping. Application to the study of immunogenic domains and to the characterization of various forms of rabbit progesterone receptor. Eur J Biochem. 1988 Sep 1;176(1):53–60. doi: 10.1111/j.1432-1033.1988.tb14250.x. [DOI] [PubMed] [Google Scholar]
- Meyer M. E., Pornon A., Ji J. W., Bocquel M. T., Chambon P., Gronemeyer H. Agonistic and antagonistic activities of RU486 on the functions of the human progesterone receptor. EMBO J. 1990 Dec;9(12):3923–3932. doi: 10.1002/j.1460-2075.1990.tb07613.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michna H., Schneider M. R., Nishino Y., el Etreby M. F. Antitumor activity of the antiprogestins ZK 98.299 and RU 38.486 in hormone dependent rat and mouse mammary tumors: mechanistic studies. Breast Cancer Res Treat. 1989 Dec;14(3):275–288. doi: 10.1007/BF01806299. [DOI] [PubMed] [Google Scholar]
- Milgrom E., Baulieu E. E. A method for studying binding proteins, based upon differential dissociation of small ligand. Biochim Biophys Acta. 1969 Dec 23;194(2):602–605. doi: 10.1016/0005-2795(69)90124-x. [DOI] [PubMed] [Google Scholar]
- Moudgil V. K., Nath R., Bhakta A., Nakao M. ZK98299, a novel antiprogesterone that does not interact with chicken oviduct progesterone receptor. Biochim Biophys Acta. 1991 Sep 3;1094(2):185–192. doi: 10.1016/0167-4889(91)90007-k. [DOI] [PubMed] [Google Scholar]
- Nath R., Bhakta A., Moudgil V. K. ZK98299--a new antiprogesterone: biochemical characterization of steroid binding parameters in the calf uterine cytosol. Arch Biochem Biophys. 1992 Jan;292(1):303–310. doi: 10.1016/0003-9861(92)90083-9. [DOI] [PubMed] [Google Scholar]
- Neef G., Beier S., Elger W., Henderson D., Wiechert R. New steroids with antiprogestational and antiglucocorticoid activities. Steroids. 1984 Oct;44(4):349–372. doi: 10.1016/s0039-128x(84)80027-6. [DOI] [PubMed] [Google Scholar]
- Sullivan W. P., Madden B. J., McCormick D. J., Toft D. O. Hormone-dependent phosphorylation of the avian progesterone receptor. J Biol Chem. 1988 Oct 15;263(29):14717–14723. [PubMed] [Google Scholar]
- Takimoto G. S., Tasset D. M., Eppert A. C., Horwitz K. B. Hormone-induced progesterone receptor phosphorylation consists of sequential DNA-independent and DNA-dependent stages: analysis with zinc finger mutants and the progesterone antagonist ZK98299. Proc Natl Acad Sci U S A. 1992 Apr 1;89(7):3050–3054. doi: 10.1073/pnas.89.7.3050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teutsch G., Gaillard-Moguilewsky M., Lemoine G., Nique F., Philibert D. Design of ligands for the glucocorticoid and progestin receptors. Biochem Soc Trans. 1991 Nov;19(4):901–908. doi: 10.1042/bst0190901. [DOI] [PubMed] [Google Scholar]
- Tsai S. Y., Carlstedt-Duke J., Weigel N. L., Dahlman K., Gustafsson J. A., Tsai M. J., O'Malley B. W. Molecular interactions of steroid hormone receptor with its enhancer element: evidence for receptor dimer formation. Cell. 1988 Oct 21;55(2):361–369. doi: 10.1016/0092-8674(88)90059-1. [DOI] [PubMed] [Google Scholar]
- Vegeto E., Allan G. F., Schrader W. T., Tsai M. J., McDonnell D. P., O'Malley B. W. The mechanism of RU486 antagonism is dependent on the conformation of the carboxy-terminal tail of the human progesterone receptor. Cell. 1992 May 15;69(4):703–713. doi: 10.1016/0092-8674(92)90234-4. [DOI] [PubMed] [Google Scholar]
- Wei L. L., Sheridan P. L., Krett N. L., Francis M. D., Toft D. O., Edwards D. P., Horwitz K. B. Immunologic analysis of human breast cancer progesterone receptors. 2. Structure, phosphorylation, and processing. Biochemistry. 1987 Sep 22;26(19):6262–6272. doi: 10.1021/bi00393a046. [DOI] [PubMed] [Google Scholar]
- Wiechert R., Neef G. Synthesis of antiprogestational steroids. J Steroid Biochem. 1987;27(4-6):851–858. doi: 10.1016/0022-4731(87)90159-2. [DOI] [PubMed] [Google Scholar]