Abstract
Background:
Obesity induces endothelial dysfunction even in the pediatric age group. The possible protective effects of fruits and herbal products on the endothelial dysfunction of obese children remain to be determined. This study aims to investigate the effects of lemon and sour orange peels on endothelial function of adolescents with excess weight.
Materials and Methods:
This triple-masked, randomized placebo-controlled trial was conducted for 1-month among 90 overweight and obese participants, aged 6-18 years. They were randomly assigned into three groups of equal number receiving daily oral capsules containing lemon or sour orange powder or placebo. Flow-mediated dilatation (FMD) was compared between three groups by using analysis of covariance.
Results:
Overall, 30 participants in the lemon group, 27 in the sour orange group and 29 in the control group completed the trial. After the trial, mean FMD was significantly (P < 0.001) higher in the lemon group (11.99 ± 4.05) and in the sour orange group (12.79 ± 5.47) than in the placebo group (6.45 ± 2.79). FMD percent change was 145.02 ± 24.34 in the lemon group, 142.04 ± 16.11 in the sour orange group, and 46.73 ± 5.16 in controls (P < 0.001).
Conclusion:
This trial showed that consumption of extracts of lemon and sour orange peels, which contain plenty amounts of antioxidants, flavonoids, pectin, and vitamin C, might have significant benefits on endothelial function in children and adolescents with excess weight. Trial registry code: IRCT201311201434N10.
Keywords: Childhood obesity, endothelial function, Citrus fruits, prevention
INTRODUCTION
Obesity and overweight, especially among children, are considered as major health concerns. A growing body of evidence suggests that the complications of obesity in adulthood begin in early childhood. In recent years, the worldwide prevalence of overweight has increased significantly among children. In addition to the Western countries,[1] childhood obesity is increasing in developing countries; it has become an emerging public health, which in turn would result in socioeconomic and public health burden in these countries in the near future.[2]
In Iran, the prevalence of overweight and obesity in children aged 6-18 years is reported as 10.1% and 4.79%, respectively.[3] A meta-regression analysis showed that the escalating trend of excess weight among Iranian young children is alarming and should be considered by health policy makers.[4]
Childhood obesity plays an important role as a predisposing factor for most noncommunicable diseases. Pediatric obesity has been shown to be associated with increased risk of many chronic diseases;[5,6] it has also been shown to induce endothelial dysfunction even in childhood.[7]
Endothelium has an important role in regulating vascular tone, platelet activity, and thrombosis; it is mainly involved in the development of atherosclerosis. Endothelial dysfunction has been determined in patients with established coronary artery disease or coronary risk factors, both in the coronary and peripheral vessels.[8] Dietary intervention could play an important role in the control of obesity, dyslipidemia, and the regulation of endothelial function.[9] Implementation of treatment and prevention programs in childhood will be more effective than interventions in adulthood.[10]
An increasing body of evidence suggests that increase in oxidative factors accounts for a significant proportion of endothelial dysfunction. In consequence, antioxidants administration are shown to be associated with improvement of coronary and peripheral endothelial function.[11] Epidemiological studies revealed an association between increased intakes of dietary antioxidants and reduced risk of coronary events.[12] Furthermore, it is documented that endothelial dysfunction could be reversed by the use of superoxide scavenging agents, including vitamin C and flavonoids.[13]
Many studies confirmed the effects of flavonoid-rich foods on reducing the markers of cardiovascular disease.[14,15,16,17]
Flavonoids, according to their structure, are reducing agents and can serve as efficient chelators of transition metals involved in cellular oxidation reactions The association of flavonoids, including Citrus flavonoids, with metabolic disorders and atherosclerosis has been linked to their antioxidant properties and reducing oxidative stress.[18,19]
An inverse relationship is documented between flavonoid consumption and heart disease risk factors as reducing blood pressure, improving flow-mediated dilatation (FMD), improving weight management, and dyslipidemia.[15]
Limited experience exists on the role of dietary intervention with fruits rich in Vitamin C and flavonoid on endothelial dysfunction in the pediatric age group. Citrus species fruits are considered to contain flavonoid, pectin, and vitamin C.[20] Lemon and sour orange are two Citrus species Persian fruits, which are popularly used among Iranians as natural additives to several foods and salads.
This study aims to investigate the effects of the peels of lemon (Citrus aurantifolia) and sour orange (Citrus aurantium) on endothelial function in adolescents with excess body weight.
MATERIALS AND METHODS
In this triple-masked, randomized controlled trial, 90 overweight adolescents were enrolled in a 1-month study period. They were recruited from January 2011 to September 2012 from clinics of the Child Growth and Development Research Center, affiliated to Isfahan University of Medical Sciences, Isfahan, Iran.
Written informed consent was obtained from one of the parents or legal guardian. The Research and Ethics Committee of Isfahan University of Medical Sciences approved the study. It was approved and registered in the Iranian Registry of Clinical Trials, which is a Primary Registry in the World Health Organization Registry Network, and received the approval code of: IRCT201311201434N10.
The sample size was calculated by considering an α error of 0.05 and a β error of 20%, and also by considering the effect of fruit juices on FMD in a previous trial among obese children.[7]
Participants and study design
Trained physicians and nurses measured the height and weight of participants; body mass index (BMI) was calculated by dividing weight by the height squared (kg/m2). For determining excess weight, we considered the revised growth charts of the Centers for Disease Control and Prevention, that is, the age- and gender-specific 85-95th BMI percentile was considered as overweight, and >95th percentile as obese.[21] To assess lifestyle habits including diet and physical activity, a validated questionnaire was used. It consisted of questions regarding demographic factors, consumption frequency of various foods, and the time spent on physical activity and sedentary habits.[6]
In this study, we recruited subjects with 10-18 years of age, who had a BMI more than 85th percentile. Those with endocrine disorders, and those who were active or passive smoker were not included to the trial.
For all participants who met the inclusion criteria, a cardiologist determined the FMD. Eligible participants were then randomly assigned into three groups of equal number receiving daily oral capsules containing lemon or sour orange powder or placebo.
Simple randomization was performed by using a random number table. The table was based on the numbers of participants’ records. Participants, physicians, nurses, and the investigators were kept masked about the grouping.
The study medications were prepared in capsule form in Pharmacognosy Department of Isfahan University of Medical Sciences. Well-dried peels and external membranes of lemon (C. aurantifolia) and sour orange (C. aurantium) were used to prepare powder. They were bought from Isfahan and Sari markets (these cities are located in the center and North of Iran, respectively) and identified in the Isfahan Pharmacognosy Department. For placebo receiving group cornstarch powder was prepared.[22] The powders were filled in capsules that were same in color and shape and size. Secret codes were defined for each group and then the medications were packed and delivered to the research center with similar labels to prevent study physician, nurses, and patients from knowing which medication was received.
The patients in first two groups received capsules containing 500 mg powdered peels of lemon or sour orange, twice a day for a period of 1 month. The third group received placebo (500 mg cornstarch) for a similar period of time. The FMD of all participants was assessed again after the trial.
Flow-mediated dilatation
A single cardiologist conducted this study for all patients. He was also kept masked about the grouping of participants. Subjects were requested to fast for at least 8-12 h before the study. All vasoactive medications that might affect FMD such as caffeine, high-fat foods, and vitamin C were withheld for at least for at least 4-6 h before the assessment. Study medications for all three groups were also paused to use during this period of time. In addition, they were asked not exercise before the study. Participants were studied in a quiet, temperature controlled room. They were requested to lie on a flat surface for 10 min to stabilize their blood pressure and heart rate. Afterward, the baseline brachial artery diameter was determined by high-resolution B-mode sonography, using ALOKA 5000 system, 7.5 MHz transducer. The probe was placed 4-5 cm to the cubital fossa of the nondominating hand. Consequently, the pneumatic tourniquet of sphygmomanometer was inflated around the upper part of the forearm to reach 300 Hg millimeter for 5 min. The cuff was then deflated and the brachial diameter was again measured after 90 s of cuff deflation. The distance between tow external borders of the artery wall after the diastolic phase is defined as artery diameter. The difference of artery diameter between before and after inflation is considered as FMD.[23] FMD percent change was defined as: FMDafter treatment — FMDbaseline/FMDbaseline.
Statistical analysis
Data were analyzed using paired t-test, independent t-test, and Chi-square tests, as well as repeated measures analysis of covariance after adjustment for potential covariates as age, gender, dietary, and physical activity habits. Ptime, Pgroup and Ptime × group were calculated for all variables. SPSS for Windows software (SPSS Inc., Chicago, IL, USA) version 20.0 was used for statistical analysis. The statistical significance level was defined as P < 0.05.
RESULTS
The study flowchart is presented in Figure 1. Of total 90 enrolled subjects, four were lost to follow-up: One refused to continue the trial, (1) due to moving of family to another city, and (2) did not return for follow-up. There was no statistically significant difference in the number of withdrawals between the three groups (P = 0.09).
Figure 1.
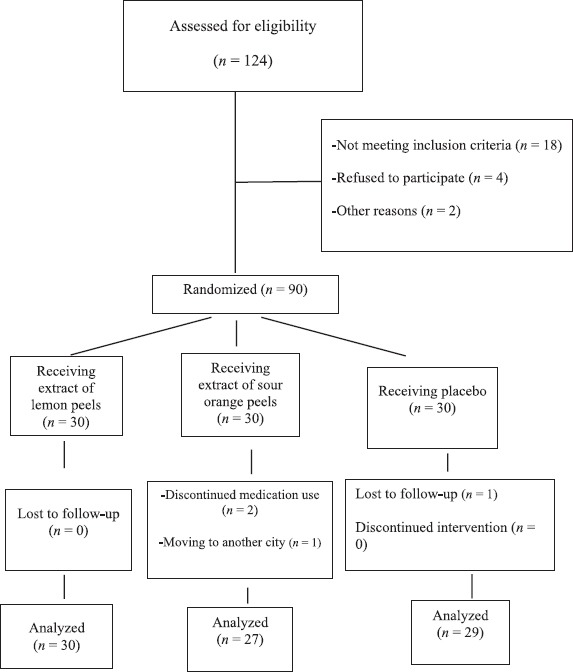
Diagram of the study flowchart
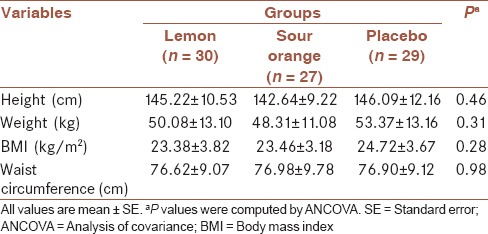
At the end of the trial, 30 participants (mean ± standard deviation [SD] of age = 13.7 ± 7.0 years; 13 boys and 17 girls) in the lemon group, 27 individuals (mean ± SD of age = 12.2 ± 9.2 years; 8 boys and 19 girls) in the Sour Orange group, and 29 subjects (mean ± SD of age = 13.2 ± 9.2 years; 10 boys and 19 girls) in the control group completed the study period. Comparison of age and gender showed no statistically significant difference between three groups (P = 0.45 for age and P = 0.65 for gender). In addition, there was no significant difference between anthropometric measures in three groups. Details of comparisons are shown in Table 1.
Table 1.
Anthropometric measures of the study participants assigned to the lemon, sour orange, and placebo groups
Before administration of medication, FMD was compared between three groups and no significant difference was detected (P = 0.07). After the trial, the anthropometric measures had no significant change, but the mean FMD was significantly (P < 0.001) higher in the lemon group (11.99 ± 4.05) and in the sour orange group (12.79 ± 5.47) than in those who were on placebo (6.45 ± 2.79).
Treatment effects were noted by increase in mean FMD within the first two groups, which were 5.50 ± 2.12 versus 5.55 ± 2.17 at baseline (P = 0.08), 11.99 ± 4.05 versus 12.79 ± 5.47 at the end of treatment period in the lemon and sour orange groups, respectively. FMD had no significant change within the third group who received placebo which were 6.50 ± 2.43 at the baseline and 6.45 ± 2.79 at the end (P = 0.91).
FMD percent change was 145.02 ± 24.34 in the lemon group, 142.04 ± 16.11 in the sour orange group, and 46.73 ± 5.16 in controls (P < 0.001).
DISCUSSION
Our study showed that consumption of lemon and sour orange extracts, which contain plenty amounts of antioxidants, flavonoids, pectin, and vitamin C might have significant benefits on endothelial function in children and adolescents with excess weight.
Obesity is associated with molecular and cellular alterations in adipose tissue, as well as with various inflammatory processes. As the body fat increases, several pro-inflammatory factors are produced in adipose tissue. Additionally, clinical and experimental data have demonstrated a link between systemic inflammation and endothelial dysfunction. Recent evidence show that disturbed endothelial function may be an early marker of an ongoing atherosclerotic process.[24] Serum markers of inflammation and oxidative stress are linked to the early inflammatory processes of atherosclerosis.[25] FMD of the brachial artery, used as a noninvasive endothelial function testing, shows early stages of endothelial dysfunction and is associated with the early inflammatory state in obese children.[26]
In this study, FMD was well improved in children who were treated with extracts of lemon and sour orange peels. These effects are suggested to be because of the antioxidative effects of their ingredients, including flavonoids, and vitamin C on endothelium function. Vitamin C inhibits peroxidation of membrane phospholipids and might have a scavenger role for free radicals; moreover it is necessary for the synthesis of several hormones and neurotransmitters. Vitamin C may improve the function of immune system and may be useful for controlling inflammation; such effects include activities of antimicrobial and natural killer cells, lymphocyte proliferation, chemotaxis, and delayed-type hypersensitivity.[27] In previous studies, it has been documented that antioxidant therapy by supplementation of vitamins can improve the endothelial function in the pediatric age group.[28]
Citrus flavonoid, which is another main component in Citrus species, has marked potential activities in lowering serum lipid and lipoproteins, as well as in reducing the progression of atherosclerosis and endothelial dysfunction.[29] Flavonoids are suggested to act directly in decreasing the inflammatory response within macrophages of the arterial intima, and therefore in slowing the progress of endothelial dysfunction and atherosclerosis.[30] Some effects might be mediated by increased hepatic glutathione peroxidase and superoxide dismutase activities, as well as decreased hepatic and plasma thiobarbituric acid reactive substances.[31] Moreover, experimental studies it might significantly increase enzymes with antioxidant activity in pancreas and plasma, and might decrease serum activities of liver enzymes.[32]
Consequently, consumption of fruits rich in vitamin C and flavonoids could result in favorable changes in FMD, and might have protective effects against the process of atherosclerosis. Pectin as a soluble fiber of Citrus fruits has also mild hypocholestrolemic effects.[33]
The findings of this study are also in line with the results of some other studies, which have investigated the use of antioxidant and other vitamin-rich fruits on endothelial function in adults. It is demonstrated that daily consumption of pomegranate juice for 3 months has beneficial effects on improvement in FMD or endothelial function. Pomegranate is considered as an antioxidant-rich fruit; it is suggested that its certain natural antioxidants or flavonoids are responsible for these effects on endothelial function.[34]
Another study confirmed that flavonoids or some natural antioxidants might have strong effects on endothelial function; for instance, it is shown that daily ingestion of moderate amounts of grape juice might improve endothelial function in adult patients with atherosclerotic vascular diseases.[35] Similarly, in a study in the pediatric age group, daily consumption of natural grape and/or pomegranate juice had acute and long-term benefits on FMD of adolescents with metabolic syndrome.[36] It is suggested that parts of the beneficial effects of Citrus peels might be because of their antioxidant activity.[37] Many recent review articles underscored the protective effects of Citrus fruits against atherosclerotic diseases and have suggested different mechanisms.[38,39,40,41,42] The current findings serve as confirmatory evidence for such beneficial effects from childhood.
Study limitations and strengths
One of the study limitations is the relatively small sample size, and this could be regarded as the main reason significant between-group differences of assessed parameters are suggested to be studied in future investigations. Moreover, the treatment period in the current trial was limited to 1-month. This period may not have been sufficient for detection of all significant or nonsignificant differences between groups. Finally, it would be ideal to check proper cell mediators and other complementary serologic and biochemical factors in order to determine the treatment effects of lemon and sour orange, which was not possible in this study.
This study has strengths in a number of ways, that is, it was novel in the pediatric age group, moreover it was conducted as a triple masked and placebo-controlled trial, which causes elimination of selection and observational biases; moreover it was a randomized trial and the analyses were adjusted for potential confounders.
CONCLUSION
The findings of this trial suggest that consumption of extracts of lemon and sour orange peels might have significant benefits on endothelial function in children and adolescents with excess weight. Longitudinal studies are necessary to assess the long-term clinical effects of these herbal extracts.
Financial support and sponsorship
This study was conducted as a thesis funded by Isfahan University of Medical Sciences. The project number was: 392115.
Conflicts of interest
There are no conflicts of interest.
AUTHOR'S CONTRIBUTION
All authors contributed to the study concept and design; in conducting the trial; in drafting and editing the paper. All authors have read and approved the final draft for submission, and accept the responsibility for the paper content.
REFERENCES
- 1.Hedley AA, Ogden CL, Johnson CL, Carroll MD, Curtin LR, Flegal KM. Prevalence of overweight and obesity among US children, adolescents, and adults, 1999-2002. JAMA. 2004;291:2847–50. doi: 10.1001/jama.291.23.2847. [DOI] [PubMed] [Google Scholar]
- 2.Kelishadi R. Childhood overweight, obesity, and the metabolic syndrome in developing countries. Epidemiol Rev. 2007;29:62–76. doi: 10.1093/epirev/mxm003. [DOI] [PubMed] [Google Scholar]
- 3.Kelishadi R, Ardalan G, Gheiratmand R, Majdzadeh R, Hosseini M, Gouya MM, et al. Thinness, overweight and obesity in a national sample of Iranian children and adolescents: CASPIAN Study. Child Care Health Dev. 2008;34:44–54. doi: 10.1111/j.1365-2214.2007.00744.x. [DOI] [PubMed] [Google Scholar]
- 4.Kelishadi R, Haghdoost AA, Sadeghirad B, Khajehkazemi R. Trend in the prevalence of obesity and overweight among Iranian children and adolescents: A systematic review and meta-analysis. Nutrition. 2014;30:393–400. doi: 10.1016/j.nut.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 5.Van Gaal LF, Mertens IL, De Block CE. Mechanisms linking obesity with cardiovascular disease. Nature. 2006;444:875–80. doi: 10.1038/nature05487. [DOI] [PubMed] [Google Scholar]
- 6.Kelishadi R, Ardalan G, Qorbani M, Ataie-Jafari A, Bahreynian M, Taslimi M, et al. Methodology and Early Findings of the Fourth Survey of Childhood and Adolescence Surveillance and Prevention of Adult Non-Communicable Disease in Iran: The CASPIAN-IV Study. Int J Prev Med. 2013;4:1451–60. [PMC free article] [PubMed] [Google Scholar]
- 7.Hashemi M, Kelishadi R, Hashemipour M, Zakerameli A, Khavarian N, Ghatrehsamani S, et al. Acute and long-term effects of grape and pomegranate juice consumption on vascular reactivity in paediatric metabolic syndrome. Cardiol Young. 2010;20:73–7. doi: 10.1017/S1047951109990850. [DOI] [PubMed] [Google Scholar]
- 8.Heitzer T, Schlinzig T, Krohn K, Meinertz T, Münzel T. Endothelial dysfunction, oxidative stress, and risk of cardiovascular events in patients with coronary artery disease. Circulation. 2001;104:2673–8. doi: 10.1161/hc4601.099485. [DOI] [PubMed] [Google Scholar]
- 9.Kelly RB. Diet and exercise in the management of hyperlipidemia. Am Fam Physician. 2010;81:1097–102. [PubMed] [Google Scholar]
- 10.Kelishadi R, Azizi-Soleiman F. Controlling childhood obesity: A systematic review on strategies and challenges. J Res Med Sci. 2014;19:993–1008. [PMC free article] [PubMed] [Google Scholar]
- 11.Heitzer T, Just H, Münzel T. Antioxidant vitamin C improves endothelial dysfunction in chronic smokers. Circulation. 1996;94:6–9. doi: 10.1161/01.cir.94.1.6. [DOI] [PubMed] [Google Scholar]
- 12.Kushi LH, Folsom AR, Prineas RJ, Mink PJ, Wu Y, Bostick RM. Dietary antioxidant vitamins and death from coronary heart disease in postmenopausal women. N Engl J Med. 1996;334:1156–62. doi: 10.1056/NEJM199605023341803. [DOI] [PubMed] [Google Scholar]
- 13.Ting HH, Timimi FK, Haley EA, Roddy MA, Ganz P, Creager MA. Vitamin C improves endothelium-dependent vasodilation in forearm resistance vessels of humans with hypercholesterolemia. Circulation. 1997;95:2617–22. doi: 10.1161/01.cir.95.12.2617. [DOI] [PubMed] [Google Scholar]
- 14.Bazzano LA. The high cost of not consuming fruits and vegetables. J Am Diet Assoc. 2006;106:1364–8. doi: 10.1016/j.jada.2006.06.021. [DOI] [PubMed] [Google Scholar]
- 15.Hooper L, Kroon PA, Rimm EB, Cohn JS, Harvey I, Le Cornu KA, et al. Flavonoids, flavonoid-rich foods, and cardiovascular risk: A meta-analysis of randomized controlled trials. Am J Clin Nutr. 2008;88:38–50. doi: 10.1093/ajcn/88.1.38. [DOI] [PubMed] [Google Scholar]
- 16.Tohill BC, Seymour J, Serdula M, Kettel-Khan L, Rolls BJ. What epidemiologic studies tell us about the relationship between fruit and vegetable consumption and body weight. Nutr Rev. 2004;62:365–74. doi: 10.1111/j.1753-4887.2004.tb00007.x. [DOI] [PubMed] [Google Scholar]
- 17.Kurowska EM, Spence JD, Jordan J, Wetmore S, Freeman DJ, Piché LA, et al. HDL-cholesterol-raising effect of orange juice in subjects with hypercholesterolemia. Am J Clin Nutr. 2000;72:1095–100. doi: 10.1093/ajcn/72.5.1095. [DOI] [PubMed] [Google Scholar]
- 18.Huxley RR, Neil HA. The relation between dietary flavonol intake and coronary heart disease mortality: A meta-analysis of prospective cohort studies. Eur J Clin Nutr. 2003;57:904–8. doi: 10.1038/sj.ejcn.1601624. [DOI] [PubMed] [Google Scholar]
- 19.Aherne SA, O’Brien NM. Mechanism of protection by the flavonoids, quercetin and rutin, against tert-butylhydroperoxide-and menadione-induced DNA single strand breaks in Caco-2 cells. Free Radic Biol Med. 2000;29:507–14. doi: 10.1016/s0891-5849(00)00360-9. [DOI] [PubMed] [Google Scholar]
- 20.Mulvihill EE, Allister EM, Sutherland BG, Telford DE, Sawyez CG, Edwards JY, et al. Naringenin prevents dyslipidemia, apolipoprotein B overproduction, and hyperinsulinemia in LDL receptor-null mice with diet-induced insulin resistance. Diabetes. 2009;8:2198–210. doi: 10.2337/db09-0634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grummer-Strawn LM, Garza C, Johnson CL. Childhood growth charts. Pediatrics. 2002;109:141–2. doi: 10.1542/peds.109.1.141. [DOI] [PubMed] [Google Scholar]
- 22.Sabzghabaee AM, Dianatkhah M, Sarrafzadegan N, Asgary S, Ghannadi A. Clinical evaluation of Nigella sativa seeds for the treatment of hyperlipidemia: A randomized, placebo controlled clinical trial. Med Arch. 2012;66:198–200. doi: 10.5455/medarh.2012.66.198-200. [DOI] [PubMed] [Google Scholar]
- 23.Corretti MC, Anderson TJ, Benjamin EJ, Celermajer D, Charbonneau F, Creager MA, et al. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: A report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol. 2002;39:257–65. doi: 10.1016/s0735-1097(01)01746-6. [DOI] [PubMed] [Google Scholar]
- 24.Wang Z, Nakayama T. Inflammation, a link between obesity and cardiovascular disease. Mediators Inflamm. 2010;2010:535918. doi: 10.1155/2010/535918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kelishadi R, Sharifi M, Khosravi A, Adeli K. Relationship between C-reactive protein and atherosclerotic risk factors and oxidative stress markers among young persons 10-18 years old. Clin Chem. 2007;53:456–64. doi: 10.1373/clinchem.2006.073668. [DOI] [PubMed] [Google Scholar]
- 26.Kelishadi R, Hashemi M, Mohammadifard N, Asgary S, Khavarian N. Association of changes in oxidative and proinflammatory states with changes in vascular function after a lifestyle modification trial among obese children. Clin Chem. 2008;54:147–53. doi: 10.1373/clinchem.2007.089953. [DOI] [PubMed] [Google Scholar]
- 27.Shaik-Dasthagirisaheb YB, Varvara G, Murmura G, Saggini A, Caraffa A, Antinolfi P, et al. Role of vitamins D, E and C in immunity and inflammation. J Biol Regul Homeost Agents. 2013;27:291–5. [PubMed] [Google Scholar]
- 28.Engler MM, Engler MB, Malloy MJ, Chiu EY, Schloetter MC, Paul SM, et al. Antioxidant vitamins C and E improve endothelial function in children with hyperlipidemia: Endothelial Assessment of Risk from Lipids in Youth (EARLY) Trial. Circulation. 2003;108:1059–63. doi: 10.1161/01.CIR.0000086345.09861.A0. [DOI] [PubMed] [Google Scholar]
- 29.Yuting C, Rongliang Z, Zhongjian J, Yong J. Flavonoids as superoxide scavengers and antioxidants. Free Radic Biol Med. 1990;9:19–21. doi: 10.1016/0891-5849(90)90045-k. [DOI] [PubMed] [Google Scholar]
- 30.Yen J, Weng C, Li S. Citrus flavonoid 5-demethylnobiletin suppresses scavenger receptor expression in THP-1 cells and alters lipid homeostasis in HepG2 liver cells. Mol Nutr Food Res. 2011;55:733–48. doi: 10.1002/mnfr.201000226. [DOI] [PubMed] [Google Scholar]
- 31.Jeon SM, Kim HK, Kim HJ, Do GM, Jeong TS, Park YB, et al. Hypocholesterolemic and antioxidative effects of naringenin and its two metabolites in high-cholesterol fed rats. Transl Res. 2007;149:15–21. doi: 10.1016/j.trsl.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 32.Annadurai T, Muralidharan AR, Joseph T, Hsu MJ, Thomas PA, Geraldine P. Antihyperglycemic and antioxidant effects of a flavanone, naringenin, in streptozotocin-nicotinamide-induced experimental diabetic rats. J Physiol Biochem. 2012;68:307–18. doi: 10.1007/s13105-011-0142-y. [DOI] [PubMed] [Google Scholar]
- 33.Sabzghabaee AM, Khayam I, Kelishadi R, Ghannadi A, Soltani R, Badri S, et al. Effect of Zizyphus jujuba fruits on dyslipidemia in obese adolescents: A triple-masked randomized controlled clinical trial. Med Arch. 2013;67:156–9. [PubMed] [Google Scholar]
- 34.Sumner MD, Elliott-Eller M, Weidner G, Daubenmier JJ, Chew MH, Marlin R, et al. Effects of pomegranate juice consumption on myocardial perfusion in patients with coronary heart disease. Am J Cardiol. 2005;96:810–4. doi: 10.1016/j.amjcard.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 35.Stein JH, Keevil JG, Wiebe DA, Aeschlimann S, Folts JD. Purple grape juice improves endothelial function and reduces the susceptibility of LDL cholesterol to oxidation in patients with coronary artery disease. Circulation. 1999;100:1050–5. doi: 10.1161/01.cir.100.10.1050. [DOI] [PubMed] [Google Scholar]
- 36.Valle Jiménez M, Estepa RM, Camacho RM, Estrada RC, Luna FG, Guitarte FB. Endothelial dysfunction is related to insulin resistance and inflammatory biomarker levels in obese prepubertal children. Eur J Endocrinol. 2007;156:497–502. doi: 10.1530/EJE-06-0662. [DOI] [PubMed] [Google Scholar]
- 37.Di Majo D, Giammanco M, La Guardia M, Tripoli E, Giammanco S, Finotti E. Flavanones in Citrus fruit — Structure-antioxidant activity relationships. Food Res Int. 2005;38:1161–6. [Google Scholar]
- 38.Grosso G, Galvano F, Mistretta A, Marventano S, Nolfo F, Calabrese G, et al. Red orange: Experimental models and epidemiological evidence of its benefits on human health. Oxid Med Cell Longev. 2013;2013:157240. doi: 10.1155/2013/157240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mulvihill EE, Huff MW. Citrus flavonoids and the prevention of atherosclerosis. Cardiovasc Hematol Disord Drug Targets. 2012;12:84–91. doi: 10.2174/1871529x11202020084. [DOI] [PubMed] [Google Scholar]
- 40.Assini JM, Mulvihill EE, Huff MW. Citrus flavonoids and lipid metabolism. Curr Opin Lipidol. 2013;24:34–40. doi: 10.1097/MOL.0b013e32835c07fd. [DOI] [PubMed] [Google Scholar]
- 41.Landberg R, Naidoo N, van Dam RM. Diet and endothelial function: From individual components to dietary patterns. Curr Opin Lipidol. 2012;23:147–55. doi: 10.1097/MOL.0b013e328351123a. [DOI] [PubMed] [Google Scholar]
- 42.Cappello AR, Dolce V, Iacopetta D, Martello M, Fiorillo M, Curcio R, et al. Bergamot (Citrus bergamia Risso) flavonoids and their potential benefits in human hyperlipidemia and atherosclerosis: An overview. Mini Rev Med Chem. 2015;15 doi: 10.2174/1389557515666150709110222. Epub ahead of print. [DOI] [PubMed] [Google Scholar]


