Abstract
Background:
Creatine monohydrate (CrM) has been shown to be beneficial to health due to its antioxidant potential. Strenuous exercise is associated with oxidative stress, which could lead to apoptosis. We investigated the ability of CrM in amelioration of apoptosis induced by incremental aerobic exercise (AE) to exhaustion in young athletes.
Materials and Methods:
In a placebo-controlled, double-blind, randomized, parallel study, 31 young athletes (age 19.52 ± 2.75 years, body mass 79.24 ± 16.13 kg, height 1.73 ± 6.49 m, body fat 16.37% ± 5.92%) were randomly assigned to CrM (4 × 5 g/day, n = 15) or placebo (PL: 4 × 5 g/day of maltodextrine powder; n = 16) to investigate the effect of 7 days CrM on serum p53 and insulin-like growth factor-1 (IGF-1) concentration after acute incremental AE test to exhaustion. Subjects performed AE before (test 1) and after 7 days of supplementation (test 2).
Results:
Before supplementation, AE to exhaustion induced a significant increase in serum p53 and IGF-1 concentrations at both CrM and PL groups (P < 0.05). After supplementation, serum p53 concentrations were significantly lower in CrM than PL at post-AE (P < 0.05). There were no differences in IGF-1 concentrations between CrM and PL groups at post-AE (P > 0.05).
Conclusion:
Our results suggest that supplementation with CrM prevents apoptosis, as measured by decreases in p53 concentration, induced by AE to exhaustion in young athletes. However, CrM had no effect on IGF-1 concentration after AE to exhaustion in young athletes.
Keywords: Anabolic hormone, creatine monohydrate, p53
INTRODUCTION
Exercise-induced free radicals and reactive oxygen spices (ROS) generation are well documented, which may result in oxidative stress defined as a situation in which an increased level of ROS generation overwhelms the physiological capacity of the antioxidant's system resulting in oxidative damage to lipids, proteins, and DNA.[1,2,3]
Oxidative stress is known to play a role in apoptosis through the products of several cell cycle genes such as p53.[4] P53 is composed of 393 amino acids that is activated in response to a wide variety of stresses including DNA damage, oncogene activation, hypoxia, nutrient deprivation, nucleotide imbalance, and ROS level.[5] Under normal cellular conditions, p53 exists at low concentrations and in an inactive state which controlled by mouse double minute 2 (MDM2) and E2 ubiquitin ligase.[6] When a cell is stressed, p53 degradation diminishes, resulting in rapid accumulation of p53 protein which can result in cell cycle regulation, DNA replication and repair, cell stress response, cell proliferation, and apoptosis.[5,7]
Studies have shown that p53 regulates apoptosis through transcription-dependent (by regulating the expression of apoptotic peptidase activating factor-1 which aids in caspse-9 activation and the expression of the Bcl-2 family) and -independent mechanisms (through translocation to mitochondria that altered mitochondrial membrane potential and lead to release of cytochrome C and by interaction with anti-apoptotic portions Bcl-2 and Bcl-xL in the outer mitochondrial membrane).[8,9,10]
Previous studies have found a relationship between the insulin-like growth factor-1 (IGF-1), cell growth, and p53.[11,12,13] IGF-1 is composed of 70 amino acids which are secreted mainly by the liver.[13] Previous evidences showed that IGF-1 have cellular growth and survival ability,[12] the anti-apoptotic property by its ability to depress p53 transcriptional activity[13] and increases p53 degradation via MDM2 ubiquitination,[14,15,16] ability to promotes DNA repair in damaged cells through p38 mitogen-activated protein kinase (p38 MAPK).[15]
There is limited information regarding the effects of exercise on apoptosis.[17,18,19] In addition, the acute effect of resistance exercise (RE) on circulating p53 remains inconclusive. Previous studies have shown that its circulating levels are either unchanged or exhibit increments following RE.[18,20] Growing evidence indicates that exercise-induced oxidative stress may be associated with muscle fatigue, muscle damage, and a decrease in physical performance.[21] Accordingly, there is a great deal of interest in antioxidant potential against exercise-induced damage.
Creatine, or methyl guanidine-acetic acid, is a popular dietary supplement that is used by athletes owing to increase anabolic hormonal response, muscle mass, strength, and sport performance.[22,23,24] Research on the antioxidant action of creatine monohydrate (CrM) suggests free radical scavenging ability,[25,26] ability to reduce lipid peroxidation[27] and DNA susceptibility to oxidative stress[28,29] and ability to boost the anti-inflammatory[30] and neuroprotective activities.[31] Our previous studies also have demonstrated that CrM has anti-oxidant effects,[28,29] but the effects of CrM on exercise-induced apoptosis have not been investigated so far. Therefore, the purpose of this study was to explore the protective effects of CrM against exhaustive RE-induced apoptosis in athletes.
MATERIALS AND METHODS
Subjects
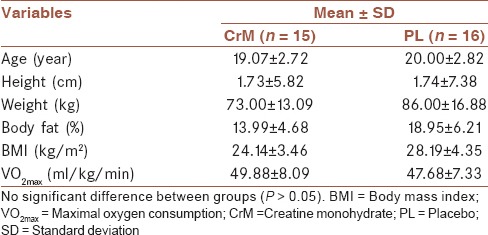
one male freestyle wrestlers (17-23 years) were recruited from the Guilan Province's to participate in this study. Subjects were informed about the nature, purpose, and potential risks of the study and signed an informed consent form approved by the University of Guilan (80DRT, April 9, 2011). The subjects were Freestyle wrestlers, nonsmokers and did not take any routine medications or supplements during the study period. Subjects were recruited from Freestyle wrestling clubs in the Guilan Province of Iran. They all had at least 6 years training experience and were among the top 10 in national championships. Subjects came to the Exercise Physiology Laboratory at Department of Exercise Physiology, Faculty of Physical Education and Sport Science, University of Guilan, 2012 for baseline measurement including subject's body mass, height, percent body fat, and body mass index one week before testing session [Table 1]. This study was approved by the Ethical Committee of Guilan University.
Table 1.
Subjects physical characteristics
This study used a placebo-controlled, double-blind, randomized, and parallel study with a CrM group (n = 15) and a PL group (n = 16) to investigate the effect of CrM on serum p53, a biomarker of apoptosis, and IGF-1, an anabolic hormone, after acute incremental cycle ergometer test to exhaustion. Subjects performed incremental aerobic exercise (AE) test to exhaustion before (test 1) and after 7 days of supplementation (test 2). Venous blood samples for measurement serum p53 and IGF-1 were collected before and after acute cycle ergometer test. Fifteen subjects in supplement group (CrM: 4 × 5 g/day) and 16 subjects in the placebo group (maltodextrine: 4 × 5 g/day) were located with simple randomization method. All subjects completed two AEs testing until exhaustion on cycle ergometer before and after supplementation period [Figure 1]. The exclusion criteria were included consuming supplementation such as CrM, and the amino acids arginine, glycine, and methionine; anabolic steroids or any other anabolic agents known to increase performance and infection or inflammation in the subject's body.
Figure 1.
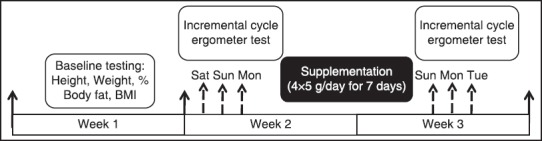
Experimental design
Aerobic exercise test
The subjects completed incremental cycle test to exhaustion on a calibrated electronic cycle ergometer (Tunturi E433) before and after 7 days of supplementation period. Before each incremental cycle test, the seat height of the cycle ergometer was adjusted for near full extension of the subjects’ legs while pedaling. Subjects were instructed to avoid strenuous exercise in the 74 h prior to each testing session. To avoid circadian rhythm effects, testing sessions began on the same day of the week and at the same time of the day. Incremental AE test to exhaustion was commenced by 5 min of warm-up at 50 watts (W) of 60 rpm, then the workload was increased by 30 W every 3 minute until exhaustion at a constant pedaling rate of 60 rpm. Verbal encouragement was used to help motivate the subjects to keep constant pedaling rate of 60 rpm. The power output at exhaustion was recorded to measure endurance capacity by Storer et al.[32] formula:
VO2max (ml/min) = (10.51[W, max]) + (6.35[wt, kg]) − (10.49[age, year]) + 519.3
Supplementation protocol and dietary analyses
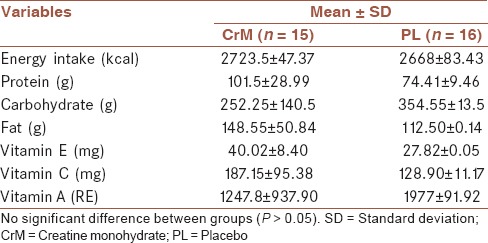
The study was carried out using a randomized double-blind independent groups design with a CrM group and a PL group. CrM group was supplemented for 7 days with 20 g/day of CrM (Mass Global Nutration/ 5460 Yonge St., Suite 1505, Toronto, ON., M2N 6K7, Canada) at 4 × 5 g doses. PL group was supplemented for 7 days with maltodextrine (Mass Global Nutration/ 5460 Yonge St., Suite 1505, Toronto, ON., M2N 6K7, Canada) in a same dosage as CrM group. The powders were identical in taste and shape and were dissolved in 200 mL of water and ingested four times per day for 7 consecutive days. Subjects were instructed to avoid caffeine consumption for 72 h prior to testing. The subjects completed a 6-day diet records, beginning on the 3-day before the first testing session and ending 3 days before the last testing session, to quantify daily kilocalories, macronutrients, and selected vitamins using the Nutritionist IV computer program [Table 2].
Table 2.
Dietary intake assessed during the 3 days prior to each testing session
Blood sampling and biochemical analysis
Blood samples were collected before (pre) and after (post) each incremental cycle test to exhaustion session in both groups. Pretest blood collections were performed after the subjects had rested in a supine position for 15 min, after which 5 ml of blood was drawn from the antecubital vein. After sampling at rest, subjects performed the incremental cycle test to exhaustion and immediately after (post) exercise had another 5 ml of blood drawn using a similar technique. Blood samples were collected into serum vacutainer tubes and approximately after 30 min tubes centrifuged at 6,000 revolutions per minute for 10 min at room temperature to obtain serum for p53 and IGF-1 analysis. Serum samples were stored at 80°C until analysis of the dependent variables. A p53 enzyme-linked immunosorbent assay (ELISA) kit (Human p53 ELISA, Catalog No: BMS256; Bender MedSystems GmbH, Austria, Europe) was used to measure p53 protein concentration in serum samples according to the manufacturer's protocol. Serum IGF-1 was measured using ELISA kit (Immunodiagnostic Systems Ltd, Boldon, Tyne and Wear, United Kingdom) according to the manufacturer's protocol. To eliminate inter-assay variance, all samples for a particular assay were thawed once and analyzed in the same assay run. All samples were run in duplicate with a mean inter- and intra-assay coefficients of variances of 8.9 and 5.5% for serum p53 and 4.3 and 4.6% for serum IGF-1. The detection limits of the p53 and IGF-1 assays were 0.33 U/mL and 3.1 ng/mL, respectively.
Statistical analyses
Statistical evaluation was performed using SPSS software for windows, version 16.0 (SPSS, Chicago, IL, USA). Presupplementation and postsupplementation values for serum p53 and IGF-1 concentrations were analyzed using 2 × 2 (treatment × time) repeated measures analysis of variance (ANOVA). Box's test and Mauchly's test were used to check for equality of variance and sphericity levels, respectively. The Greenhouse-Geisser adjustment was used to correct for any violation of sphericity assumption. Effect sizes (ES) were computed to compare of changes in the CrM and PL groups. Pearson–product moment correlations were used to measure the relationship between p53 and IGF-1 concentrations. The probability level for all the tests was set at 0.05 to indicate significance.
RESULTS
The physical characteristics of subjects in CrM and PL groups were shown in Table 1. There was no statistically significant difference in age, weight, height, % body fat, BMI, and VO2max values between the groups (P > 0.05, Table 1).
Serum p53 and IGF-1 concentrations were determined pre- and post-exercise at baseline and after supplementation period. Comparisons between pre- and post-exercise revealed that serum p53 levels increased by 52.82% (P = 0.002) in CrM and 39.46% (P = 0.011) in PL group at before supplementation and by 2.79% (P = 0.788) in CrM and 26.79% (P = 0.048) in PL at after supplementation. After supplementation, serum p53 concentrations were significantly lower in CrM than PL at post-AE (P < 0.05). There were no differences in IGF-1 concentrations between CrM and PL groups at post-AE (P > 0.05). The CrM group demonstrated a significant decrease in the magnitude of p53 response during incremental cycle ergometer tests at postsupplmentation (∆p53 = 0.03 ± 0.39 U/mL) compared with presupplementaion (∆p53 = 0.52 ± 0.47 U/mL), P = 0.024 and ES = 0.35. In contrast, the PL group demonstrated no difference in p53 response during incremental cycle ergometer tests at postsupplmentation (∆p53 = 0.27 ± 0.46 U/mL) compared with presupplementaion (∆p53 = 0.44 ± 0.53 U/mL), P = 0.171 and ES = 0.15. Comparisons between pre- and post-exercise revealed that serum IGF-1 levels increased by 23.16% (P = 0.008) in CrM and 18.23% (P = 0.016) in PL group at before supplementation and by 21.58% (P = 0.001) in CrM and 13.81% (P = 0.046) in PL at after supplementation. No significant changes in the magnitude of IGF-1 response during incremental cycle ergometer tests at postsupplmentation compared with presupplementaion was observed (P > 0.05). There were no significant correlation between serum p53 and IGF-1 concentrations at baseline and after supplementation period (P > 0.05) [Figures 2 and 3].
Figure 2.
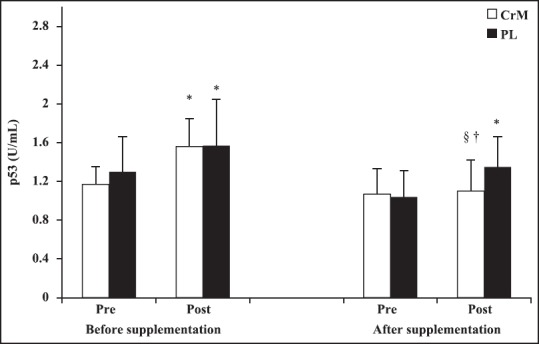
Changes in serum p53 at before- and after supplementation following an acute bout of incremental aerobic exercise test to exhaustion in creatine monohydrate and PL group. *Difference compared to pre exercise (P < 0.05). §Difference compared to post exercise at before supplementation (P < 0.05). †Difference compared to placebo group (P < 0.05)
Figure 3.
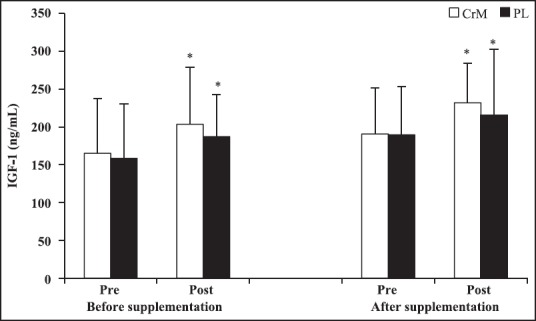
Changes in serum insulin-like growth factor -1 at before- and after supplementation following an acute bout of incremental aerobic exercise test to exhaustion in creatine monohydrate and PL group. *Difference compared to pre exercise (P < 0.05)
DISCUSSION
This study was designed to examine the effects of CrM supplementation on apoptosis following incremental exercise to exhaustion in young athletes. It is well known that exercise to exhaustion result in oxidative stress.[17,18,19] Oxidative stress can activate several important signaling pathways leading to apoptosis through the products of several cell cycle genes such as p53.[4] The tumor suppressor, p53, plays an important role in oxidative stress induced apoptosis through directly regulating the expressions of pro-oxidant and antioxidant genes or through modulating the cellular metabolic pathways.[7]
Few studies investigated the acute effects of exercise on biomarkers of apoptosis,[17,18,19] however, there was no study on the CrM supplementation effects on p53 following acute exercise to exhaustion which is essential for the cell properly adapt its gene expression profile in response to stress. The findings of this study indicate that incremental AE to exhaustion induces a significant increase in serum p53 level. The augmented p53 response is consistent with other studies using high intensity RE to failure in untrained subjects Sharafi and Rahimi,[29] and similar responses have also been observed with acute bout of maximally activated eccentric contractions in skeletal muscle of rats.[33] Furthermore, regarding to p53 response in pathological and in vitro studies, it has been reported that p53 level in lymph node cancer of the prostate tumor cells was higher in the exercise serum-stimulated cells compared to control,[34] similar results have also been reported in patients with colorectal cancer compared with healthy control.[35] Based on the findings of the current and aforementioned studies could be speculated that the increased oxidants after exercise and various oxidative related disease such as cancers lead to activation of p53 which is potentially an important mechanisms by which triggers effectors gene expression programs in order to preserve genomic intensity and cellular homeostasis including DNA repair, apoptosis, cell cycle arrest, and ROS defense.[5]
Since that increased oxidative stress is responsible for p53 up-regulation and previous study showed that antioxidant can inhibit induction of apoptosis.[36] With this purpose in mind, CrM supplementation as antioxidant was used in order to decrease ROS generation and oxidative stress which addressed in the literature.[25] Results showed that p53 response to acute incremental AE to exhaustion significantly reduced after 7 days of CrM supplementation. In contrast, there was no significant change in p53 response in PL group. The decrement in p53 response due to short-term CrM supplementation may be, in part, attributed to the antioxidant properties of CrM which lead to decrease ROS levels[25] along with reduced oxidative damage to DNA as measured by 8-hydroxy-2’-deoxyguanosine.[29]
The p53 decreasing mechanism following exercise to exhaustion after CrM supplementation compared to pre supplementation is unknown. As previously mentioned, IGF-1 has anti-apoptotic properties and several studies reported that IGF-1 increases p53 degradation via MDM2 ubiquitination.[14,15] The findings of this study demonstrated that acute incremental AE to exhaustion increased circulating IGF-1 level which is consistent with several previous studies that demonstrated the exercise-induced IGF-1 increase occurred following very short high intensity exercise (i.e., 90 second),[37] occurred 10 min following the beginning of endurance exercise[38,39] and occurred in exercise both bellow and above the lactate threshold,[38] as well as Elias et al.[40] reported a transient increase in circulating IGF-1 immediately after exercise to exhaustion. Moreover, after supplementation, IGF-1 significantly increased by 21.85% in CrM group following exercise to exhaustion. Corroborating to our finding Sharafi and Rahimi,[18] reported that plasma IGF-1 level was significantly higher in young resistance trained compared to untrained at immediately after RE while the higher level of p53 in the untrained group was observed in comparison to resistance trained group. With regards to IGF-1 role in suppression of the normal function of p53, it may be the reason for the lower level of p53 in CrM group in comparison to PL group. Based on the previous study, IGF-1 affect both induction of DNA repair and modulation of the MDM2/p19 alternate reading frame/p53 network through p38 MAPK.[15]
In summary, to our knowledge, this is the first study to investigate the influence of short-term CrM supplementation on apoptosis biomarkers following incremental AE to exhaustion in young athletes. This study demonstrated that p53 level was significantly decreased after short-term CrM supplementation following incremental AE to exhaustion. As decreased p53 levels indicate a decrease in apoptosis and oxidative damage to DNA, these results suggest that CrM supplementation is likely to decrease the risk of oxidative damage to DNA and concomitant apoptosis. The possible mechanisms that CrM exert anti-apoptotic properties are unknown. Therefore, it is suggested that future studies evaluate the effect of short-term CrM supplementation on p53 protein level, activity, and expression in vitro and in vivo studies following exercise-induced oxidative stress. Based on previous and current study it could be speculated that the possible mechanism by which CrM induced decrease in p53 level may be, in part, due to anti-oxidative properties of CrM[25] and may be due to increase in circulating IGF-1 level.[13,14,15] Further studies are necessary to determine the role of IGF-1 in regulating p53 following CrM supplementation after intense exercise.
CONCLUSIONS
Our findings revealed that strenuous AE lead to increasing p53 level which is the main inducing factor for mitochondrial-mediated apoptosis and short-term CrM supplementation led to decline in p53 protein following incremental AE to exhaustion. CrM supplementation, which attenuated p53 protein, may lead to inhibition of p53 signaling event and subsequently promoted cell survival and protected the cell from oxidative stress. In this study, the decrease in p53 level and increase in IGF-1 level suggested that short-term CrM supplementation induced a protective effect on apoptosis, thereby; coaches and athletic trainers could utilize CrM supplementation to attenuate this process. However, additional researches regarding CrM effects’ on molecular control points of apoptosis following strenuous exercise are important.
Financial support and sponsorship
This project is funded by the Department of Exercise Physiology, University of Guilan (80DRT, April 9, 2011). We thank the participants for participating in this study.
Conflicts of interest
There are no conflicts of interest.
AUTHOR'S CONTRIBUTION
RR contributed in the conception of the work, conducting the study, revising the draft, approval of the final version of the manuscript, and agreed for all aspects of the work. BM contributed in the conception of the work, revising the draft, approval of the final version of the manuscript, and agreed for all aspects of the work. FR contributed in the design of the work, revising the draft, approval of the final version of the manuscript, and agreed for all aspects of the work. ZS contributed in the conception of the work, drafting and revising the draft, approval of the final version of the manuscript, and agreed for all aspects of the work.
Acknowledgments
This project is funded by the Department of Exercise Physiology, University of Guilan (80DRT, April 9, 2011). We thank the participants for participating in this study. The authors thank Mr. Mehdi Ghahramani, PhD student in Exercise Physiology for helpful laboratory assistants with data collection.
REFERENCES
- 1.Bloomer RJ, Goldfarb AH. Anaerobic exercise and oxidative stress: A review. Can J Appl Physiol. 2004;29:245–63. doi: 10.1139/h04-017. [DOI] [PubMed] [Google Scholar]
- 2.Davies KJ, Quintanilha AT, Brooks GA, Packer L. Free radicals tissue damage produced by exercise. Biochem Biophys Res Commun. 1982;107:1198–205. doi: 10.1016/s0006-291x(82)80124-1. [DOI] [PubMed] [Google Scholar]
- 3.Sen CK, Packer L, Hanninen O. Amsterdam: Elsevier; 2000. Handbook of Oxidants Antioxidants in Exercise. [Google Scholar]
- 4.Bonini P, Cicconi S, Cardinale A, Vitale C, Serafino AL, Ciotti MT, et al. Oxidative stress induces p53-mediated apoptosis in glia: p53 transcription-independent way to die. J Neurosci Res. 2004;75:83–95. doi: 10.1002/jnr.10822. [DOI] [PubMed] [Google Scholar]
- 5.Millau JF, Bastien N, Drouin R. P53 transcriptional activities: A general overview and some thoughts. Mutat Res. 2009;681:118–33. doi: 10.1016/j.mrrev.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 6.Momand J, Wu HH, Dasgupta G. MDM2-master regulator of the p53 tumor suppressor protein. Gene. 2000;242:15–29. doi: 10.1016/s0378-1119(99)00487-4. [DOI] [PubMed] [Google Scholar]
- 7.Holley AK, Dhar SK, St Clair DK. Manganese superoxide dismutase vs.p53: Regulation of mitochondrial ROS. Mitochondrion. 2010;10:649–61. doi: 10.1016/j.mito.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 8.Haldar S, Negrini M, Monne M, Sabbioni S, Croce CM. Down-regulation of bcl-2 by p53 in breast cancer cells. Cancer Res. 1994;54:2095–7. [PubMed] [Google Scholar]
- 9.Moll UM, Zaika A. Nuclear and mitochondrial apoptotic pathways of p53. FEBS Lett. 2001;493:65–9. doi: 10.1016/s0014-5793(01)02284-0. [DOI] [PubMed] [Google Scholar]
- 10.Robles AI, Bemmels NA, Foraker AB, Harris CC. APAF-1 is a transcriptional target of p53 in DNA damage-induced apoptosis. Cancer Res. 2001;61:6660–4. [PubMed] [Google Scholar]
- 11.Juul A. Serum levels of insulin-like growth factor I and its binding proteins in health and disease. Growth Horm IGF Res. 2003;13:113–70. doi: 10.1016/s1096-6374(03)00038-8. [DOI] [PubMed] [Google Scholar]
- 12.Kraemer WJ, Rogol AD. Malden, Massachusetts, USA: Blackwell Publishing; 2005. The Endocrine System in Sports and Exercise. [Google Scholar]
- 13.Leri A, Liu Y, Claudio PP, Kajstura J, Wang X, Wang S, et al. Up-Regulation of AT1 and AT2 receptors in postinfarcted hypertrophied myocytes and stretch-mediated apoptotic cell death. Am J Pathol. 1999;154:567–80. doi: 10.1016/S0002-9440(10)65037-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heron-Milhavet L, LeRoith D. Insulin-like growth factor I induces MDM2-dependen degradation of p53 via the p38 MAPK pathway in response to DNA damage. J Biol Chem. 2002;277:15600–6. doi: 10.1074/jbc.M111142200. [DOI] [PubMed] [Google Scholar]
- 15.Héron-Milhavet L, Karas M, Goldsmith CM, Baum BJ, LeRoith D. Insulin-like growth factor-I (IGF-I) receptor activation rescues UV-damaged cells through a p38 signaling pathway. Potential role of the IGF-I receptor in DNA repair. J Biol Chem. 2001;276:18185–92. doi: 10.1074/jbc.M011490200. [DOI] [PubMed] [Google Scholar]
- 16.Maki CG, Huibregtse JM, Howley PM. In vivo ubiquitination and proteasome-mediated degradation of p53. Cancer Res. 1996;56:2649–54. [PubMed] [Google Scholar]
- 17.Ma H, Lin H, Feng H, Putheti R. Effects of anagelica polysaccharide on hepatocytes apoptosis induced by exhaustive exercise. Afr J Microbiol Res. 2009;3:774–7. [Google Scholar]
- 18.Sharafi H, Rahimi R. The effect of resistance exercise on p53, caspase-9, and caspase-3 in trained and untrained men. J Strength Cond Res. 2012;26:1142–8. doi: 10.1519/JSC.0b013e31822e58e5. [DOI] [PubMed] [Google Scholar]
- 19.Koçtürk S, Kayatekin BM, Resmi H, Açikgöz O, Kaynak C, Ozer E. The apoptotic response to strenuous exercise of the gastrocnemius and solues muscle fibers in rats. Eur J Appl Physiol. 2008;102:515–24. doi: 10.1007/s00421-007-0612-7. [DOI] [PubMed] [Google Scholar]
- 20.Boroujerdi SS, Rahimi R. The apoptotic response to resistance exercise with different intensities in athletes. Med Sport. 2011;64:31–44. [Google Scholar]
- 21.Powers SK, Jackson MJ. Exercise-induced oxidative stress: Cellular mechanisms and impact on muscle force production. Physiol Rev. 2008;88:1243–76. doi: 10.1152/physrev.00031.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eckerson JM, Stout JR, Moore GA, Stone NJ, Nishimura K, Tamura K. Effect of two and five days of creatine loading on anaerobic working capacity in women. J Strength Cond Res. 2004;18:168–73. doi: 10.1519/1533-4287(2004)018<0168:eotafd>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 23.Rahimi R, Faraji H, Sheikholeslami-Vatani D, Qaderi M. Creatine supplementation alters the hormonal response to resistance exercise. Kinesiology. 2010;42:136–43. [Google Scholar]
- 24.Rawson ES, Persky AM. Mechanisms of muscular adaptations to creatine supplementation. Int Sport Med J. 2007;8:43–53. [Google Scholar]
- 25.Lawler JM, Barnes WS, Wu G, Song W, Demaree S. Direct antioxidant properties of creatine. Biochem Biophys Res Commun. 2002;290:47–52. doi: 10.1006/bbrc.2001.6164. [DOI] [PubMed] [Google Scholar]
- 26.Young JF, Larsen LB, Malmendal A, Nielsen NC, Straadt IK, Oksbjerg N, et al. Creatine-induced activation of antioxidative defence in myotube cultures revealed by explorative NMR-based metabonomics and proteomics. J Int Soc Sports Nutr. 2010;7:9. doi: 10.1186/1550-2783-7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Basta P, Skarpan´ska-Stejnborn A, Pilaczyn´ska-Szczes´niak L. Creatine supplementation and parameters of exercise-induced oxidative stress after a standard rowing test. Stud Phys Cult Tour. 2006;13:17–23. [Google Scholar]
- 28.Mirzaei B, Rahmani-Nia F, Salehi Z, Rahimi R. Effects of creatine monohydrate supplementation on oxidative DNA damage and lipid peroxidation induced by acute incremental exercise to exhaustion in wrestlers. Kinesiology. 2013;45:30–40. [Google Scholar]
- 29.Rahimi R. Creatine supplementation decreases oxidative DNA damage and lipid peroxidation induced by a single bout of resistance exercise. J Strength Cond Res. 2011;25:3448–55. doi: 10.1519/JSC.0b013e3182162f2b. [DOI] [PubMed] [Google Scholar]
- 30.Santos RV, Bassit RA, Caperuto EC, Costa Rosa LF. The effect of creatine supplementation upon inflammatory and muscle soreness markers after a 30km race. Life Sci. 2004;75:1917–24. doi: 10.1016/j.lfs.2003.11.036. [DOI] [PubMed] [Google Scholar]
- 31.Matthews RT, Yang L, Jenkins BG, Ferrante RJ, Rosen BR, Kaddurah-Daouk R, et al. Neuroprotective effects of creatine and cyclocreatine in animal models of Huntington's disease. J Neurosci. 1998;18:156–63. doi: 10.1523/JNEUROSCI.18-01-00156.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Storer TW, Davis JA, Caiozzo VJ. Accurate prediction of VO2max in cycle ergometry. Med Sci Sports Exerc. 1990;22:704–12. doi: 10.1249/00005768-199010000-00024. [DOI] [PubMed] [Google Scholar]
- 33.Chen YW, Nader GA, Baar KR, Fedele MJ, Hoffman EP, Esser KA. Response of rat muscle to acute resistance exercise defined by transcriptional and translational profiling. J Physiol. 2002;545:27–41. doi: 10.1113/jphysiol.2002.021220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barnard RJ, Leung PS, Aronson WJ, Cohen P, Golding LA. A mechanism to explain how regular exercise might reduce the risk for clinical prostate cancer. Eur J Cancer Prev. 2007;16:415–21. doi: 10.1097/01.cej.0000243851.66985.e4. [DOI] [PubMed] [Google Scholar]
- 35.Abdel-Aziz MM, Lotfy M, El-Kady IM, Abozaid M. Mutant p53 protein in the serum of patients with colorectal cancer. Correlation with the level of carcinoembryonic antigen and serum epidermal growth factor receptor. Cancer Detect Prev. 2009;32:329–35. doi: 10.1016/j.cdp.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 36.Johnson TM, Yu ZX, Ferrans VJ, Lowenstein RA, Finkel T. Reactive oxygen species are downstream mediators of p53-dependent apoptosis. Proc Natl Acad Sci U S A. 1996;93:11848–52. doi: 10.1073/pnas.93.21.11848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kraemer WJ, Harman FS, Vos NH, Gordon SE, Nindl BC, Marx JO, et al. Effects of exercise and alkalosis on serum insulin-like growth factor I and IGF-binding protein-3. Can J Appl Physiol. 2000;25:127–38. doi: 10.1139/h00-009. [DOI] [PubMed] [Google Scholar]
- 38.Bang P, Brandt J, Degerblad M, Enberg G, Kaijser L, Thorén M, et al. Exercise-induced changes in insulin-like growth factors and their low molecular weight binding protein in healthy subjects and patients with growth hormone deficiency. Eur J Clin Invest. 1990;20:285–92. doi: 10.1111/j.1365-2362.1990.tb01857.x. [DOI] [PubMed] [Google Scholar]
- 39.Schwarz AJ, Brasel JA, Hintz RL, Mohan S, Cooper DM. Acute effect of brief low- and high-intensity exercise on circulating insulin-like growth factor (IGF) I, II, and IGF-binding protein-3 and its proteolysis in young healthy men. J Clin Endocrinol Metab. 1996;81:3492–7. doi: 10.1210/jcem.81.10.8855791. [DOI] [PubMed] [Google Scholar]
- 40.Elias AN, Pandian MR, Wang L, Suarez E, James N, Wilson AF. Leptin and IGF-I levels in unconditioned male volunteers after short-term exercise. Psychoneuroendocrinology. 2000;25:453–61. doi: 10.1016/s0306-4530(99)00070-0. [DOI] [PubMed] [Google Scholar]


