Abstract
Background:
Multiple factors are involved in the development and progression of type 2 diabetes mellitus (DMII) to DMII with metabolic syndrome (MetS) and cardiovascular complications. To identify some of these factors, we aim to investigate the changes in erythrocyte membrane Na+/K+-ATPase activity, serum glucose, insulin, lipid profile, hemoglobin A1C (HbA1c), high-sensitivity C-reactive protein (hs-CRP), anthropometric measurements, and blood pressure in DMII with and without MetS.
Materials and Methods:
This cross-sectional study comprised 155 male subjects distributed into three groups as healthy controls (50 non-DMII volunteers), Group I (50 DMII without MetS), and Group II (55 DMII with MetS). Fasting blood samples were taken for the measurement of glucose, insulin, HbA1c, hs-CRP and lipid profile. Na+/K+-ATPase activity was determined in erythrocyte ghost.
Results:
Na+/K+-ATPase activity was significantly decreased in DMII groups compared with controls. No significant difference was shown in Na+/K+-ATPase activity between DMII groups. Total ATPase activity, total cholesterol and low-density lipoprotein-cholesterol levels were similar in the three groups. Levels of insulin, hs-CRP, triacylglycerols, systolic blood pressure, weight, waist and hip circumference, waist/hip ratio, and body mass index were significantly elevated and high-density lipoprotein-cholesterol significantly decreased only in Group II. Significant differences in serum glucose and hip circumference were seen between the groups. No significant differences in HbA1c levels were observed between DMII groups.
Conclusion:
Changes in many of the measured risk factors that occurred only in Group II compared with controls and Group I may provide an explanation of how DMII progresses to DMII with MetS and future cardiovascular complications.
Keywords: Lipid profile, metabolic syndrome, Na+/K+-ATPase activity, type 2 diabetes
INTRODUCTION
Type 2 diabetes mellitus (DMII) is the most common form of diabetes which accounts for about 90-95% of those with diabetes. It is associated with abnormalities of carbohydrate, lipid, and protein metabolism. Chronic exposure to hyperglycemia can result in dysfunction and failure of various organs especially the eyes, kidneys, nerves, and heart and blood vessels. The long-term micro- and macro-vascular complications in DMII are including retinopathy, nephropathy, neuropathy, myocardial infarction, and stroke.[1,2] According to the American Diabetes Association, cardiovascular disease (CVD) accounts for as many as 75-80% of mortality in DMII patients.[3] The basis of abnormalities in DMII is a deficient action of insulin on target tissues due to impairment of insulin secretion, defects in insulin action, or both. Insulin resistance, which represents a reduced physiological response of the peripheral tissues to the action of the normal levels of insulin, is a major finding in several metabolic disorders, including DMII and metabolic syndrome (MetS).[4,5,6] Initially, insulin resistance is compensated by enhanced insulin secretion but later insulin secretion is impaired. In progression from normal to impaired glucose tolerance and diabetes, insulin secretion deteriorates faster than insulin sensitivity.[7] Insulin resistance is involved in the pathophysiology of diabetic dyslipidemia and commonly occurs as part of MetS.[8] According to the National Cholesterol Education Program's Adult Treatment Panel III (NCEP ATPIII) criteria, the MetS is a combination of modifiable risk factors including hyperglycemia, insulin resistance, hypertension, hypertriglyceridemia, decreased high-density lipoprotein-cholesterol (HDL-C), and abdominal obesity.[9] The consequences associated with the MetS may be responsible for cardiovascular complication and mortality observed in DMII population.[10]
Several studies have reported that Na+/K+-ATPase enzyme may be affected structurally and functionally in DMII. A number of mechanisms have been suggested including glycosylation and impairment of Na+/K+-ATPase enzyme, down regulation of Na+/K+-ATPase due to low insulin secretion or defects in insulin action that results in reduced number of Na+/K+-ATPase enzyme in the cell membrane, low level of ATP in cells, an abnormal ionic distribution between extra and intracellular environment and abnormal sodium metabolism that has critical role in etiology of CVD.[11] Therefore, knowledge of possible relationships between decreased Na+/K+-ATPase activity and CVD risk factors in DMII may contribute to understand better the pathophysiology of complications in diabetes.[12]
Also, recent studies have reported an association between high-sensitivity C-reactive protein (hs-CRP) and increased incident of DMII, MetS, and CVDs. Measurement of hs-CRP, therefore, adds clinically important prognostic information on the MetS.[13,14,15] It is important to understand the potential interrelationships between diabetes, MetS, and CVD risk factors to identify the pathophysiology of cardiovascular outcomes in DMII patients. This study aims to investigate the magnitude and association between the changes in blood and erythrocyte membrane in DMII with MetS compared with DMII without MetS, to identify some of the factors that influence the development and progression of DMII to DMII with MetS and cardiovascular complications. This can be exploited to improve the clinically management and prognosis of the consequences of the disease.
MATERIALS AND METHODS
Study population
For this cross-sectional study, 155 men between 40 and 60 years of age were recruited from Isfahan Cardiovascular Research Center. Subjects were distributed into three groups, namely control group: Including 50 normoglycemic healthy subjects; Group I: 50 DMII without MetS and apparent sings of complication; and Group II: 55 DMII with MetS. Diagnosis of diabetes was established based on the criteria proposed by the American Diabetes Association (fasting glucose ≥126 mg/dl or 2 h postprandial glucose ≥200 mg/dl,[1] and if they were taking oral anti-diabetic medication but not insulin. Patients were excluded if they were taking medications known to influence the Na+/K+-ATPase enzyme activity, such as thyroxin, glucocorticoids, calcium blockers, mineralocorticoids, digitalis, lipid-lowering agents such as gemfibrozil, clofibrate and smoking cigarettes or other tobacco products.[16] Written informed consent was obtained from each participant, and the study was approved by the Ethics Committee of Isfahan University of Medical Sciences.
Definition of the metabolic syndrome
The MetS was defined according to the NCEP ATPIII. According to ATPIII criteria, a patient is classified as having the MetS when three or more of the following risk determinants are present: Hyperglycemia (fasting plasma glucose ≥100 mg/dl), hypertriglyceridemia (triglyceride ≥150 mg/dl), low HDL cholesterol (HDL-C <40 mg/dl for men and <50 mg/dl for women), blood pressure elevation (≥130/85 mmHg), and increased waist circumference (waist circumference ≥102 cm for men and ≥88 cm for women).[9]
Anthropometric measurements and blood pressure
Weight and height of participants were determined in light clothing and without shoes by portable calibrated electronic weighing scale and portable measuring inflexible bars, respectively. Waist (at umbilicus) and hip (at widest point) circumferences (WC and HC) measured on subjects according to standard conditions using a measuring tape, then waist/hip circumference ratio (WHR) was calculated. Body mass index (BMI) was calculated as weight of individuals divided by the square of their height (kg/m2). Blood pressure was measured after a rest for 15 min using a sphygmomanometer, the mean of the three measurements of systolic and diastolic blood pressure (SBP and DBP) at intervals of 2-5 min was considered as the blood pressure. All measurements were taken by the same person to avoid subjective error.
Blood collection and processing
Venous blood samples were obtained after at least 10 h overnight fasting from the patient and control groups by venipuncture and collected in heparinized, ethylenediaminetetraacetic acid (EDTA), and plain test tubes. The blood samples were centrifuged at 1500 g at 25°C for 10 min, and then serum and plasma were separated into plain test tubes. Serums were processed for the measurement of fasting blood glucose (FBG), triacylglycerols (TGs), total cholesterol (TC), low-density lipoprotein-cholesterol (LDL-C), high density lipoprotein-cholesterol (HDL-C), fasting serum insulin (FINS), and high sensitivity C-reactive protein (hs-CRP). 0.5 ml aliquots of plasma were stored at −20°C for further analyses. The remaining erythrocytes were resuspended and washed twice with 20 mM Tris-buffered saline (pH 7.5) containing 145 mM NaCl. The washed and packed erythrocytes were used for erythrocyte membrane preparation.
Biochemical assays
FBG was measured by enzymatic colorimetric method using glucose oxidase test. Serum TC, TGs, and HDL-C were determined by enzymatic methods using a Hitachi 902 automated analyzer. Serum LDL-C was calculated using Friedewald's formula.[17] When serum TGs concentration was >400 mg/dl, LDL-C was determined directly by enzymatic method using the commercial kit. FINS was measured using an enzyme-linked immunosorbent assay kit. Hemoglobin A1c (HbA1c) was analyzed by latex immunoturbidimetric method and hs-CRP was measured with a latex-enhanced immunoturbidimetric assay using an automated analyzer. The serum hs-CRP level classification as <1, 1-3, and ≥3 mg/l have been defined as lower, moderate, and higher cardiovascular risk, respectively.[18]
Erythrocyte ghost preparation
Erythrocyte ghost was obtained by osmotic lysis as described previously.[19] Briefly, the erythrocyte packed cells were resuspended and lysed by 10 volumes of ice-cold 5 mM Tris/0.1 mM Na2 EDTA, pH 7.6. The hemolysate was then centrifuged at 20,000 g for 20 min at 4°C. Supernatant was discarded, and the pellet was washed three times in 0.017 M NaCl/5 mM Tris-HCl, pH 7.6 and three times with 10 mM Tris-HCl (pH 7.5). Erythrocyte ghost was resuspended in 10 mM Tris-HCl buffer (pH 7.5) and aliquots of 0.5 ml were frozen immediately in liquid nitrogen and then stored at −80°C until analysis of membrane protein concentration and erythrocyte Na+/K+-ATPase activity measurement. Erythrocyte membrane protein was estimated according to the modified method of Markwell et al.[20] using bovine serum albumin as standard.
Assay of total and Na+/K+-ATPase activity
The erythrocyte membrane total ATPase activity was determined as described previously.[21] A 50 μl volume of the erythrocyte membrane suspension was incubated with 5 mmol/l ATP, 25 mmol/l KCl, 75 mmol/l NaCl, 5 mmol/l MgCl2, 0.1 mmol/l EGTA, and 25 mmol/l Tris-HCl (pH 7.5) in a total volume of 500 μl for 90 min at 37°C. The reaction was stopped by addition of trichloroacetic acid to a final concentration of 5% (w/v). After centrifugation for 20 min at 1500 g, an aliquot of the supernatant was used to measure the amount of total inorganic phosphorus liberated by the reaction of Fiske and Subbarow.[22] This assay was repeated in the presence of 1 mmol/l ouabain, an inhibitor of Na+/K+-ATPase activity. Total ATPase activity was expressed as μmol of inorganic phosphorus liberated per milligram of membrane protein per hour. The activity of Na+/K+-ATPase was subsequently determined by subtracting the ATPase activity in the presence of ouabain from the total Na+/K+-ATPase activity in the absence of ouabain. Blanks for substrate and membrane were included to compensate for nonenzymatic, hydrolytic breakdown of ATP and endogenous phosphate.[16]
Statistical analysis
Statistical analyzes of data were performed using the SPSS software package version 20 (IBM SPSS Statistics Desktop 20.0 Linux Multilingual eAssembly, CRG2MML). The results were expressed as mean ± standard deviation. The normal distribution of the variable was checked by Kolmogorov–Smirnov test. The equality of variances was calculated with the Levene's test. Differences between groups were assessed by independent samples t-test for continuous variables and Mann–Whitney U-test for nonparametric variables such as DBP. We investigated the association between variables using bivariate correlation analysis. The correlations between quantitative variables were studied with the Pearson's correlation for parametrical variables and Spearman test for nonparametric variables. Levels of P < 0.05 were expressed as statistically significant.
RESULTS
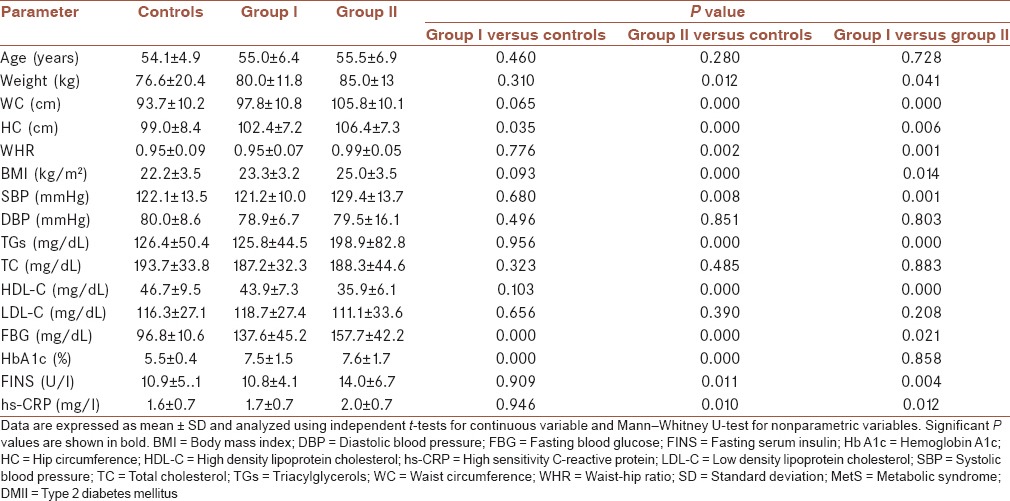
Characteristics of control and DMII groups are presented in Table 1. No significant (P > 0.05) differences were observed between the DMII patients and controls with respect to their age, DBP, serum concentration of TC, and LDL-C. Significant differences in serum levels of FBG between controls and Group I (P = 0.000), controls and Group II (P = 0.000), and Group I and Group II (P = 0.021) were seen. HC was significantly lower in controls as compared with Group I (P = 0.035) and Group II (P = 0.000). In comparison of two DMII groups, HC was significantly lower in Group I (P = 0.006). In Group II, compared with controls and Group I, significant differences in reduced serum levels of HDL-C (P = 0.000), elevated serum levels of FINS (P = 0.011 and P = 0.004 respectively), SBP (P = 0.008 and P = 0.001 respectively) and TGs (P = 0.000), hs-CRP (P = 0.010 and P = 0.012, respectively), weight (P = 0.012 and P = 0.041 respectively), WC (P = 0.000), WHR (P = 0.002 and P = 0.001 respectively), and BMI (P = 0.000 and P = 0.014, respectively) were seen. No significance differences were observed between controls and Group I with regards to the serum levels of FINS (P = 0.909), TGs (P = 0.956), HDL-C (P = 0.103), hs-CRP (P = 0.946), SBP (P = 0.680), weight (P = 0.310), WC (P = 0.065), WHR (P = 0.776), and BMI (P = 0.093). The significant differences were seen in HbA1c results between controls and Group I (P = 0.000), controls and Group II (P = 0.000), but no significant difference was seen between DMII groups (P = 0.857).
Table 1.
Characteristics of control group and DMII with and without MetS
The results of erythrocyte membrane protein content and Na+/K+-ATPase activities are shown in Table 2. Significantly lowered values of erythrocyte membranes Na+/K+-ATPase activity were seen in Group I and Group II (P = 0.035 and P = 0.002, respectively), compared with controls.
Table 2.
Erythrocyte membrane protein content, total and Na+/K+-ATPase activity
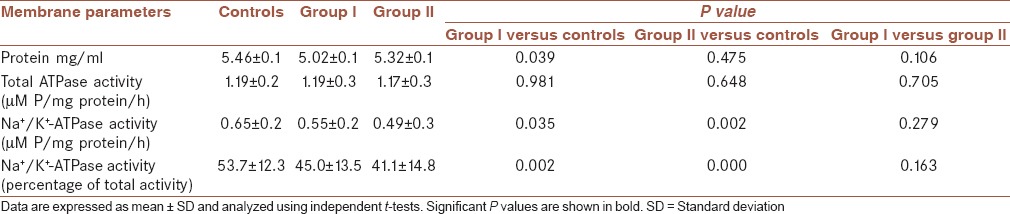
There was no significant (P = 0.279) difference in Na+/K+-ATPase activity between Group I and Group II. The erythrocyte total ATPase activity was similar in both DMII and control groups. A decrease in membrane protein content was observed in Group I in comparison to controls (P = 0.039). There was no significant (P = 0.106) difference in membrane protein content between Group I and Group II.
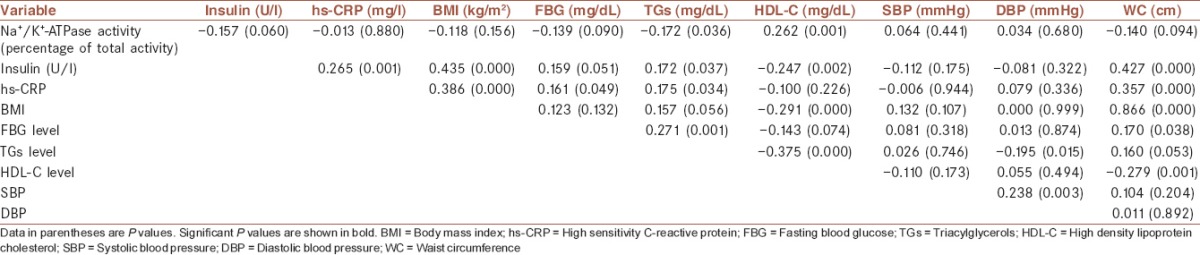
Table 3 shows the Pearson correlation coefficients between Na+/K+-ATPase activity, insulin, hs-CRP, BMI, and components of MetS in all the subjects. Erythrocyte membranes Na+/K+-ATPase activity was negatively associated with TGs (r = −0.172, P = 0.036) and positively correlated with HDL-C (r = 0.262, P = 0.001). No significant correlation was observed between the enzyme activity, hs-CRP (r = −0.013, P = 0.880), BMI (r = −0.118, P = 0.156), SBP (r = 0.064, P = 0.441), and DBP (r = 0.034, P = 0.680). However, there was negative correlations between the enzyme activity and insulin (r = −0.157, P = 0.060), FBG (r = −0.139, P = 0.090), and WC (r = −0.140, P = 0.094). Significant positive correlations were found between insulin and hs-CRP (r = 0.265, P = 0.001), BMI (r = 0.435, P = 0.000), WC (r = 0.427, P = 0.000), TGs (r = 0.172, P = 0.037) and FBG (r = 0.159, P = 0.051) and negative correlation with HDL-C (r = −0.247, P = 0.002). hs-CRP was significantly associated with BMI (r = 0.386, P = 0.000), WC (r = 0.357, P = 0.000), FBG (r = 0.161, P = 0.049), and TGs (r = 0.175, P = 0.034). There was a significant positive correlation between BMI and WC (r = 0.866, P = 0.000) and significant negative correlation between BMI and HDL-C (r = −0.291, P = 0.000). FBG was significantly correlated with TGs (r = 0.271, P = 0.001) and WC (r = 0.170, P = 0.038). Positive correlations were found between TGs and WC (r = 0.160, P = 0.053) and inverse significant relationship between TGs and DBP (r = −0.195, P = 0.015), and HDL-C (r = −0.375, P = 0.000) was seen. HDL-C was significantly negative associated with WC (r = −0.279, P = 0.001) and negatively associated with FBG (r = −0.143, P = 0.074). Significant positive correlation between SBP and DBP (r = 0.238, P = 0.003) was seen.
Table 3.
Pearson correlation coefficient between Na+/K+-ATPase activity, insulin, hs-CRP, BMI and components of metabolic syndrome in all subjects
DISCUSSION
The objective of the present study was to evaluate the magnitude and significance of the relationship between the changes in blood and erythrocyte membrane in DMII to identify some of the factors that effect on the progression of DMII to DMII with MetS and cardiovascular complications.
All of the cardiovascular risk factors presented in Table 1 show a significant increase in DMII with MetS in comparison with DMII without MetS and control subjects.
The results of this study shows the primarily change in Na+/K+-ATPase activity of the erythrocyte membrane in DMII. Then, the poor disease control that is associated with abnormalities in plasma parameters can progress DMII to DMII with MetS and cardiovascular complications, the cause of death in as many as 75-80% of individuals with diabetes.[3]
Pearson correlation analyses presented in Table 3 show that TGs had the most association with the following parameters such as positive correlations with hs-CRP, FINS, FBG, and WC, and inverse relationship with the Na+/K+-ATPase activity, DBP, and HDL-C in all subjects. Many clinical studies have shown that hypertriglyceridemia is associated with further hyperinsulinemia and thus is associated with weight gain and obesity.[23] Weight gain in turn increases insulin resistance and promotes atherogenesis through several mechanisms, including the promotion of hypertension and dyslipidemia. Visceral obesity is also associated with peripheral resistance to insulin actions and hyperinsulinemia, dyslipidemia, type 2 diabetes, hypertension, and increased risk for CVD. Results of many prospective studies have shown that high fasting insulin, as an independent predictor of CAD, has association with the risk of coronary heart disease.[23,24,25] All these risk factors are presented in Group II patients in our study as shown in Table 1.
Results presented in Table 2 show that total ATPase activity is similar in three groups and is consistent with Kumar study,[26] whereas membrane protein content and Na+/K+-ATPase activity in Group I showed significant decrease compared to controls. Calculating the percentage of the enzyme activity indicated that ouabain-sensitive ATPase activity that accounts for 53.67% of total ATPase activity in erythrocytes from controls, only accounts for 45.01% in Group I and 41.07% in Group II patients. As the patients were of the same race and ethnic group and the same gender, the reason for the low enzyme levels seen in patients with type 2 diabetes seems to be due to factors other than individual variability in age and selection bias. Reduced activity of ATPase may be caused by changes in the viscosity and fluidity of the membrane as well as direct protein oxidative damage by free radical species or other lipid peroxidation products.[27] This could lead to changes in the permeability of the membrane to various ions and transport mechanisms that have been proposed as a primary cause of complications of diabetes mellitus and its progression to a number of metabolic disorders.[28] Therefore, the observed decreased in Na+/K+-ATPase activity probably plays an important role for the pathophysiology of diabetes. However, more research is needed to improve our understanding about factors responsible for decreased Na+/K+-ATPase, observed in type 2 diabetes.
We also evaluated the relationship between erythrocyte Na+/K+-ATPase activity, fasting serum insulin, hs-CRP and BMI with components of MetS in all subjects as shown in Table 3. Erythrocyte Na+/K+-ATPase activity had a significant negative correlation with TGs and significant positive correlation with HDL-C. Negative correlations were also seen between the enzyme activity and insulin, FBG and WC. These results clearly confirm a relationship between the parameters of low metabolic control and low erythrocyte Na+/K+-ATPase enzyme activity level. Reduced enzyme activity induced by diabetes can be involved in the pathogenesis of diabetes and DMII complications. However, the present study design is not suitable to say that the low Na+/K+-ATPase activity precedes the developments of DMII vascular complications because our study population was DMII patients without any vascular complication. It could be considered as our study's limitations and should be considered in further studies. These data provide evidence to suggest that the decreased Na+/K+-ATPase activity in DMII patients with poor disease control may, therefore, progress DMII to DMII with MetS and further cardiovascular complications.
The Third National Health and Nutrition Examination Survey reports that “people with diabetes without MetS did not have an increase in CHD prevalence and those with both MetS and diabetes had the highest prevalence of CHD compared with those with nei ther” It is noteworthy to say all the criteria proposed for the clinical diagnosis of the MetS are established cardiovascular risk factors.[29]
According to Esteghamati et al., the prevalence of MetS in adult Iranian individuals aging 25-64 years, using the ATPIII definitions, is 33.6%, which is relatively higher than other reports (23-40%, depending on ethnicity and the criteria used).[30]
Consistent with several other studies, our results showed positive and significant correlations between hs-CRP levels and insulin, BMI and three components of the MetS including WC, FBG, and TGs. Results in Group II showed significantly elevated hs-CRP levels compared to controls and Group I [Table 1]. Several prospective cohort studies have indicated that hs-CRP concentration independent of other risk factors is associated with the future risk of CVD events.[31,32,33]
It is conceivable to suggest that poor glycemic control (e.g., HbA1c >9%) is related to decreased erythrocyte Na+/K+-ATPase levels. Although Koc, B et al. reported that HbA1c levels are not related to Na+/K+-ATPase levels in DMII and control groups,[34] however, in our study the significant inverse correlation between HbA1c levels and Na+/K+-ATPase levels (r = −0.185 with a P = 0.039) was seen in all subjects (data not shown). This difference is likely due to correlation analysis since our analysis was performed in all subjects. It has been reported that for every 1% decrease in HbA1c, the incidence of microvascular complications is reduced by 25-35%.[35] Therefore, improved glycemic control prevents or delays microvascular complications.
CONCLUSION
Given the above observations, the decreased Na+/K+-ATPase activity in DMII with poor disease control, may further progress DMII to DMII with MetS and cardiovascular complications. These results underline the need for initial and continued assessment of CVD risk in people with newly diagnosed diabetes. In these patients an ongoing measurement of risk factors such as MetS components and implementing timely interventions, will improve the management of diabetes and prevent or delay the progression of diabetes to cardiovascular complications.
Financial support and sponsorship
This study was supported by grants from Isfahan University of Medical Sciences (grant No. 393087) and the Isfahan Cardiovascular Research Institute.
Conflicts of interest
There are no conflicts of interest.
AUTHOR'S CONTRIBUTION
MP participated in hypothesis generation, study design, data interpretation and manuscript development (revision, editing and finalyzing). FZ conducted experiments, participated in statistical analysis, data interpretation and manuscript development (draft and revision). MS contributed to patient selection and management, data interpretation, and manuscript review.
Acknowledgements
This study was supported by grants from Isfahan University of Medical Sciences (grant No. 393087) and the Isfahan Cardiovascular Research Institute. The authors thank the staff of Isfahan Cardiovascular Research Institute laboratory for their kind cooperation. They also thank all of the patients who participated in this study.
REFERENCES
- 1.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2013;36:S67–74. doi: 10.2337/dc13-S067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Donaghue KC, Wadwa RP, Dimeglio LA, Wong TY, Chiarelli F, Marcovecchio ML, et al. ISPAD Clinical Practice Consensus Guidelines 2014. Microvascular and macrovascular complications in children and adolescents. Pediatr Diabetes. 2014;15(Suppl 20):257–69. doi: 10.1111/pedi.12180. [DOI] [PubMed] [Google Scholar]
- 3.Martín-Timón I, Sevillano-Collantes C, Segura-Galindo A, Del Cañizo-Gómez FJ. Type 2 diabetes and cardiovascular disease: Have all risk factors the same strength? World J Diabetes. 2014;5:444–70. doi: 10.4239/wjd.v5.i4.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adams-Huet B, Devaraj S, Siegel D, Jialal I. Increased adipose tissue insulin resistance in metabolic syndrome: Relationship to circulating adipokines. Metab Syndr Relat Disord. 2014;12:503–7. doi: 10.1089/met.2014.0092. [DOI] [PubMed] [Google Scholar]
- 5.Rutter MK, Sullivan LM, Fox CS, Wilson PW, Nathan DM, Vasan RS, et al. Baseline levels, and changes over time in body mass index and fasting insulin, and their relationship to change in metabolic trait clustering. Metab Syndr Relat Disord. 2014;12:372–80. doi: 10.1089/met.2013.0148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nolan CJ, Ruderman NB, Kahn SE, Pedersen O, Prentki M. Insulin resistance as a physiological defense against metabolic stress: implications for the management of subsets of type 2 diabetes. Diabetes. 2015;64:673–86. doi: 10.2337/db14-0694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cavaghan MK, Ehrmann DA, Polonsky KS. Interactions between insulin resistance and insulin secretion in the development of glucose intolerance. J Clin Invest. 2000;106:329. doi: 10.1172/JCI10761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vergès B. Pathophysiology of diabetic dyslipidaemia: Where are we? Diabetologia. 2015;58:886–99. doi: 10.1007/s00125-015-3525-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive summary of the third report of the national cholesterol education program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) JAMA. 2001;285:2486–97. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 10.Alberti K, Zimmet P, Shaw J. Metabolic syndrome—a new world-wide definition. A consensus statement from the international diabetes federation. Diabet Med. 2006;23:469–80. doi: 10.1111/j.1464-5491.2006.01858.x. [DOI] [PubMed] [Google Scholar]
- 11.Jokhio R, Khan Y, Ali L, Seehar G. Effect of fbs and blood cholesterol on Na+/K+-ATPase activity in type-I diabetes. Pak J Pharmacol. 2008;25:21–30. [Google Scholar]
- 12.Iwalokun BA, Iwalokun SO. Association between erythrocyte Na+ K+-ATPase activity and some blood lipids in type 1 diabetic patients from Lagos, Nigeria. BMC Endocr Disord. 2007;7:7. doi: 10.1186/1472-6823-7-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Esser N, Legrand-Poels S, Piette J, Scheen AJ, Paquot N. Inflammation as a link between obesity, metabolic syndrome and type 2 diabetes. Diabetes Res Clin Pract. 2014;105:141–50. doi: 10.1016/j.diabres.2014.04.006. [DOI] [PubMed] [Google Scholar]
- 14.Abu-Farha M, Behbehani K, Elkum N. Comprehensive analysis of circulating adipokines and hsCRP association with cardiovascular disease risk factors and metabolic syndrome in Arabs. Cardiovasc Diabetol. 2014;13:76. doi: 10.1186/1475-2840-13-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Al-Hamodi Z, Al-Habori M, Al-Meeri A, Saif-Ali R. Association of adipokines, leptin/adiponectin ratio and C-reactive protein with obesity and type 2 diabetes mellitus. Diabetol Metab Syndr. 2014;6:99. doi: 10.1186/1758-5996-6-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shahid SM, Rafique R, Mahboob T. Electrolytes and sodium transport mechanism in diabetes mellitus. Pak J Pharm Sci. 2005;18:6–10. [PubMed] [Google Scholar]
- 17.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 18.Assadpour Piranfar M. The Correlation between High-Sensitivity C-Reactive Protein (hsCRP) Serum Levels and Severity of Coronary Atherosclerosis. Int Cardiovasc Res J. 2014;8:6–8. [PMC free article] [PubMed] [Google Scholar]
- 19.DeLuise M, Flier JS. Functionally abnormal Na -K pump in erythrocytes of a morbidly obese patient. J Clin Invest. 1982;69:38–44. doi: 10.1172/JCI110439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Markwell MA, Haas SM, Bieber LL, Tolbert NE. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem. 1978;87:206–10. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- 21.Namazi G, Jamshidi Rad S, Attar AM, Sarrafzadegan N, Sadeghi M, Naderi G, et al. Increased membrane lipid peroxidation and decreased Na /K -ATPase activity in erythrocytes of patients with stable coronary artery disease. Coron Artery Dis. 2015;26:239–44. doi: 10.1097/MCA.0000000000000196. [DOI] [PubMed] [Google Scholar]
- 22.Fiske CH, Subbarow Y. The colorimetric determination of phosphorus. J Biol Chem. 1925;66:375–400. [Google Scholar]
- 23.Matsuzawa Y. Obesity and metabolic syndrome: The contribution of visceral fat and adiponectin. Diabetes Manage. 2014;4:391–401. [Google Scholar]
- 24.Steinberger J, Daniels SR American Heart Association Atherosclerosis, Hypertension, and Obesity in the Young Committee (Council on Cardiovascular Disease in the Young); American Heart Association Diabetes Committee (Council on Nutrition, Physical Activity, and Metabolism) Obesity, insulin resistance, diabetes, and cardiovascular risk in children: An American Heart Association scientific statement from the Atherosclerosis, Hypertension, and Obesity in the Young Committee (Council on Cardiovascular Disease in the Young) and the Diabetes Committee (Council on Nutrition, Physical Activity, and Metabolism) Circulation. 2003;107:1448–53. doi: 10.1161/01.cir.0000060923.07573.f2. [DOI] [PubMed] [Google Scholar]
- 25.Welsh P, Preiss D, Lloyd SM, de Craen AJ, Jukema JW, Westendorp RG, et al. Contrasting associations of insulin resistance with diabetes, cardiovascular disease and all-cause mortality in the elderly: PROSPER long-term follow-up. Diabetologia. 2014;57:2513–20. doi: 10.1007/s00125-014-3383-9. [DOI] [PubMed] [Google Scholar]
- 26.Kumar R. Biochemical changes in erythrocyte membrane in type 2 diabetes mellitus. Indian J Med Sci. 2012;66:131–5. [PubMed] [Google Scholar]
- 27.Ziobro A, Duchnowicz P, Mulik A, Koter-Michalak M, Broncel M. Oxidative damages in erythrocytes of patients with metabolic syndrome. Mol Cell Biochem. 2013;378:267–73. doi: 10.1007/s11010-013-1617-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rosic NK, Standaert ML, Pollet RJ. The mechanism of insulin stimulation of (Na+,K+)-ATPase transport activity in muscle. J Biol Chem. 1985;260:6206–12. [PubMed] [Google Scholar]
- 29.Alexander CM, Landsman PB, Teutsch SM, Haffner SM. Third National Health and Nutrition Examination Survey (NHANES III); National Cholesterol Education Program (NCEP). NCEP-defined metabolic syndrome, diabetes, and prevalence of coronary heart disease among NHANES III participants age 50 years and older. Diabetes. 2003;52:1210–4. doi: 10.2337/diabetes.52.5.1210. [DOI] [PubMed] [Google Scholar]
- 30.Esteghamati A, Ashraf H, Khalilzadeh O, Zandieh A, Nakhjavani M, Rashidi A, et al. Optimal cut-off of homeostasis model assessment of insulin resistance (HOMA-IR) for the diagnosis of metabolic syndrome: Third national surveillance of risk factors of non-communicable diseases in Iran (SuRFNCD-2007) Nutr Metab (Lond) 2010;7:26. doi: 10.1186/1743-7075-7-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ridker PM, Silvertown JD. Inflammation, C-reactive protein, and atherothrombosis. J Periodontol. 2008;79(8 Suppl):1544–51. doi: 10.1902/jop.2008.080249. [DOI] [PubMed] [Google Scholar]
- 32.Ridker PM. High-sensitivity C-reactive protein as a predictor of all-cause mortality: Implications for research and patient care. Clin Chem. 2008;54:234–7. doi: 10.1373/clinchem.2007.099465. [DOI] [PubMed] [Google Scholar]
- 33.Rifai N. High-sensitivity C-reactive protein: A useful marker for cardiovascular disease risk prediction and the metabolic syndrome. Clin Chem. 2005;51:504–5. doi: 10.1373/clinchem.2004.044990. [DOI] [PubMed] [Google Scholar]
- 34.Koc B, Erten V, Yilmaz MI, Sonmez A, Kocar IH. The relationship between red blood cell Na/K-ATPase activities and diabetic complications in patients with type 2 diabetes mellitus. Endocrine. 2003;21:273–8. doi: 10.1385/ENDO:21:3:273. [DOI] [PubMed] [Google Scholar]
- 35.DeFronzo RA. Insulin resistance, lipotoxicity, type 2 diabetes and atherosclerosis: The missing links. The Claude Bernard Lecture 2009. Diabetologia. 2010;53:1270–87. doi: 10.1007/s00125-010-1684-1. [DOI] [PMC free article] [PubMed] [Google Scholar]


