Abstract
Background:
We are aware of no systematic review or meta-analysis of published findings about the association between Vitamin D status and risk of gastric cancer (GC). We systematically reviewed the current evidence on the association between Vitamin D intake as well as serum 25-hydroxy Vitamin D (25(OH)D) levels and risk of GC.
Materials and Methods:
Published evidence in this area was searched to August 2014 through the use of ISI Web of Science, Scopus, PubMed/Medline, Ovid Database, EMBASE, and Google Scholar for relevant articles by cross-referencing. Seven articles had reported odds ratios (ORs) or relative risks (RR) as their effect size; four papers had reported the ORs between Vitamin D intake and GC; and three papers had reported the association between serum 25(OH)D and risk of GC.
Results:
Pooled effect size for comparison of highest versus lowest intakes of Vitamin D was 1.09 (95% confidence interval [CI]: 0.94, 1.25; P = 0.26) indicating no significant association between Vitamin D intake and risk of GC. We failed to find a significant association between serum Vitamin D levels and risk of GC (OR: 0.92; 95% CI: 0.74-1.14; P = 0.429). Among men, the pooled effect size or highest versus lowest category of serum Vitamin D levels was 0.92 (95% CI: 0.71, 1.18, P = 0.49). The corresponding figures in women were 1.04 and 95% CI: 0.74-1.47 (P = 0.80).
Conclusion:
We found no evidence for the significant association between Vitamin D status and risk of GC. However, due to limited data in this field, further studies are required to reach a definite conclusion.
Keywords: Cancer, serum 25-hydroxy Vitamin D, stomach, Vitamin D intake
INTRODUCTION
Gastric cancer (GC) remains as a chronic disease with a high rate of morbidity and mortality around the world. GC is the fourth most common cancer diagnosed worldwide.[1] In Iran, the overall incidence rate of GC increased from 2.8 in 2000 to 9.1 per 100,000 persons per year in 2005.[2]
Although the main cause of GC has been reported to be the infection by Helicobacter pylori, several lifestyle factors including diet has also been indicated to contribute to this cancer.[3,4,5,6,7] Among dietary factors, Vitamin D status has received great attention in recent years.[3,4,5,6,7] Several studies have hypothesized that the nutritional status of Vitamin D and serum levels of Vitamin D might effect on the risk of GC.[8,9,10,11,12,13,14] However, the findings are conflicting. While some studies have reported a nonsignificant inverse relationship between Vitamin D status and risk of GC,[8,9] others have shown that high Vitamin D intake and high Vitamin D exposure index was associated with an increased risk of GC. Furthermore, findings from case-control and cohort studies have demonstrated a positive association between dietary intake of Vitamin D or serum 25-hydroxy Vitamin D (25(OH)D) concentrations and risk of stomach cancer; such that those with the cancer or those developed the cancer had higher intakes or higher serum levels of Vitamin D.[10,11,12,13,14] A case-control study investigated the association of selected micronutrient intakes and risk of GC. A significant positive association was seen between Vitamin D intake and risk of GC.[11] In the nested case-control study, no significant association was found between serum 25(OH)D concentrations and risk of cardia (relative risk [RR]: 1.11, 95% confidence interval [CI]: 0.80, 1.55; P = 0.49) or noncardia GC (RR: 1.10, 95% CI: 0.60, 2.01; P = 0.39).[13] Despite these documents, we are aware of no systematic review or meta-analysis of published findings in this regard. Therefore, we performed the present study to systematically review the current evidence on the association between nutritional status of Vitamin D and risk of GC and if possible, to conduct a meta-analysis of published data in this area.
MATERIALS AND METHODS
We performed a systematic review and meta-analysis of studies that examined the association between Vitamin D intake, serum Vitamin D levels, and risk of GC in the adult population.
Literature search strategy
Published evidence in this area was searched through the use of ISI Web of Knowledge, PubMed/Medline, Ovid Database, and Google Scholar for relevant articles. Two authors (SK and PS) independently searched papers published until August 2014 using the following key words: “Vitamin D,” “25-OH-D,” “25-hyroxy Vitamin D,” “1, 25-dihyroxy Vitamin D,” “cholecalciferol,” “calcidiol,” “calcitriol,” “hydroxycholecalciferols,” “25-hydroxy Vitamin D₃” in combination with “stomach,” “gastric,” “cancer,” “tumor,” “tumor,” “carcinoma,” “adenocarcinoma,” or “neoplasm.” Finally, the reference lists of included publications were checked to verify the completeness of the literature search. No restriction about the time of publication or language was made.
Inclusion criteria
Duplicate publications were deleted. Each title and abstract was reviewed to clarify whether the article was relevant or not. The full-text was reviewed if the abstract indicated that the article reported the association between Vitamin D intake or serum 25(OH)D levels and risk of GC. English and non-English papers that fulfilled the following criteria were included in the current study:
Case-control or cohort studies or pooling projects,
Those that considered Vitamin D intake or serum 25(OH)D concentrations as the exposure variable,
Those that regarded GC as the main outcome variable or as one of the outcomes,
Those that reported odds ratios (ORs), RRs, rate or risk or hazard ratios as their effect size.
Out of 132 retrieved papers in our search, seven articles met the inclusion criteria. The major reasons for exclusion of studies were (a) duplicates and (b) no usable data reported.
Data extraction
Two independent reviewers (SK and PS) extracted the data regarding first author's last name, publication date, sample size, participants’ age, gender, methods used for assessing Vitamin D intake or serum 25(OH)D levels, and reported ORs or RRs as well as covariates. Mean, standard deviation, and range of Vitamin D intake or serum 25(OH)D levels was also extracted. In case of having sex-specific effect sizes reported in the paper, we used these statistics besides the one reported for the whole group, in order to use these effect sizes in subgroup analysis and find gender-specific association between Vitamin D status and risk of GC. Data extraction was conducted independently by two investigators. Any disagreements were resolved through discussion between the authors.
Statistical methods
The association between Vitamin D intake and serum levels of Vitamin D and GC was separately assessed. Main outcome variables were measures of RRs for the association between Vitamin D intake, serum 25(OH)D levels, and GC. Point estimates of RRs or OR, and 95% CI were estimated for each study. The method of Zhang and Yu was used to correct the tendency of ORs for common events to overestimate the RR.[15] Within- and between-study heterogeneities were assessed using Cochran's Q-statistics,[15] and the heterogeneity test was used to assess the null hypothesis that all studies evaluated the same effect. The effect of heterogeneity was quantified using I2,[16] that provides a measure of the degree of inconsistency between studies and determines whether the percentage total variation across studies is due to heterogeneity rather than chance. I2 values range between 0% and 100%, and I2 values of 25%, 50%, and 75% are referred to as low, moderate, and high estimates, respectively.[15] The random effects method[17] was conducted to calculate pooled ORs and 95% CIs, because we found no evidence of heterogeneity. The funnel plot, Begg, and Mazumdar rank correlation test, and Egger's test were employed to assess the publication bias.[18] Although, statistical testing for interaction did not confirm a significant difference between the male and female subgroups, we conducted subgroup analysis to find a gender-specific association between Vitamin D status and risk of GC. Sensitivity analysis was conducted to explore the extent to which inferences might depend on a particular study or number of publications. Statistical analyses were conducted using Stata version 11.2 (Stata Corp, College Station, TX, USA). P < 0.05 was considered statistically significant.
RESULTS
Literature search
An initial search of the PubMed/Medline, ISI Web of Science, Scopus, Ovid, and Google Scholar electronic databases led to 132 papers; 112 papers were eliminated on examination of abstracts as they were not prospective cohort or case-control studies or did not assess the relationship between Vitamin D and risk of GC. Finally, after reviewing the titles and abstracts, 20 papers were initially classified as potentially relevant and the full papers retrieved. After reading full-text of 20 papers, finally 7 articles (4 papers for Vitamin D intake and GC and 3 papers for serum Vitamin D concentrations and GC) were included in the meta-analysis.[8,9,10,11,12,13,14] A flow diagram that detailed the process is presented in Figure 1. Although, the reference list of each paper was checked, but we did not identify further relevant studies. No additional articles were identified by searches of other databases.
Figure 1.
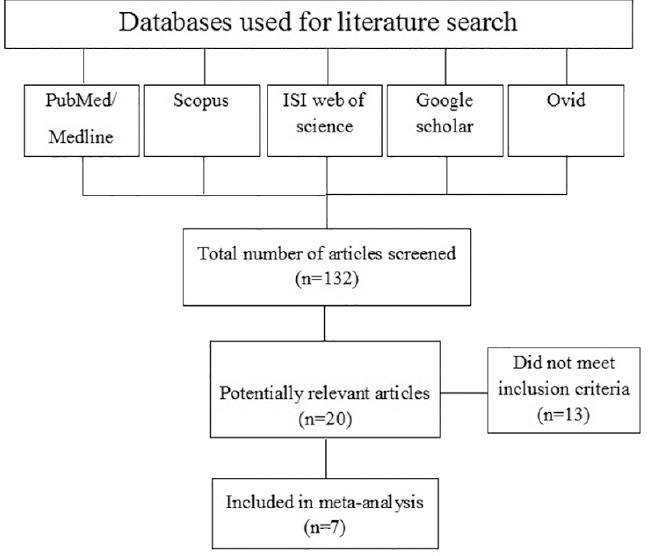
The flow diagram of study selection process
Summary of studies that assessed Vitamin D intake
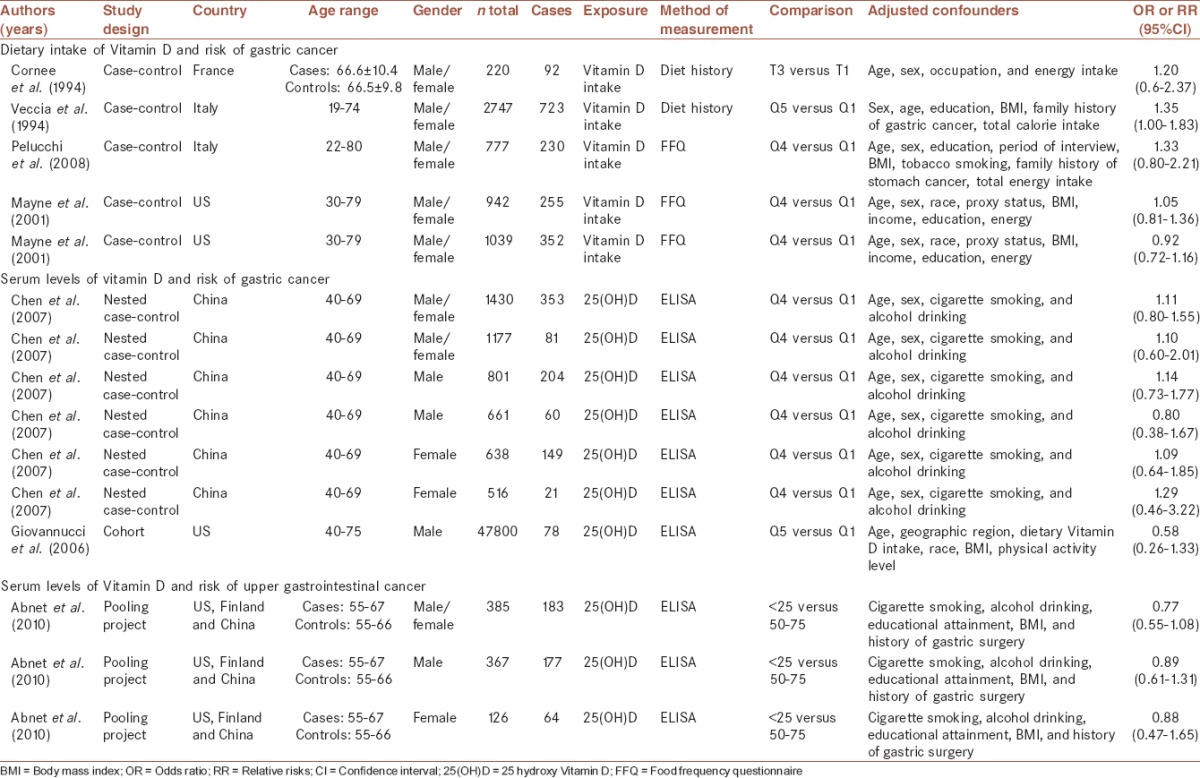
The studies included in this systematic review are summarized and alphabetically listed in Table 1. We found four articles that had reported the association between Vitamin D intake and risk of GC.[10,11,12,14] The publication date for these papers ranged from 1994[10,11] to 2008.[12] All these studies were of case-control design. Two studies were conducted in Italy,[11,12] one in France,[10] and another one in US.[14] One study had reported the association between Vitamin D intake and GC separately for cardia GC and noncardia GC.[14] This study was considered as two separate studies. Others have not clarified if their outcome variable was cardia or noncardia GC.[10,11,12] The sample size for all studies combined was 1652 cases (ranging from 92 to 723) and 4067 controls (ranging from 128 to 2024 individuals). All studies had recruited both genders as their subjects. The age range studied in these papers was 19-80 year. Two studies had assessed dietary Vitamin D intake by the use of a diet history questionnaire[10,11] and two studies had used food frequency questionnaire.[12,14] One study had reported ORs across tertiles,[10] another one across quintiles[11] and two other studies across quartiles of Vitamin D intake.[12,14]
Table 1.
Characteristics of selected studies investigating the association between Vitamin D status and risk of gastric cancer
Summary of studies that assessed serum Vitamin D levels
Three articles assessed the association between serum Vitamin D levels and risk of GC.[8,9,13] The publication date for these papers ranged between 2006[9] and 2010.[8] One study was of a nested case-control nature,[13] another one was a prospective cohort study[9] and the other article was a pooling project of eight cohort studies.[8] The follow-up time for the nested case-control study was 5 years[13] and for the cohort study was 14 years.[9] The median follow-up time from blood collection to cancer diagnosis in the pooling project of eight cohort studies was between 1.7 and 11.8 years.[8] In the pooling project paper, no RRs have been given for GC and all estimates had been provided for upper gastrointestinal cancers. The nested case-control and cohort studies had been done in China (n = 1) and US (n = 1), respectively. The pooling project (n = 1) was a collaborative study between Finland, US, China. One study had reported the RRs separately for cardia and noncardia GC, but had not reported the association for these two types of cancer combined.[13] Therefore, this study was also used as two separate studies. For all studies combined, 79,515 subjects had been examined; among them there were 512 individuals with GC and 1065 individuals with upper gastrointestinal cancer were identified. All studies, except the one in men,[9] had enrolled both genders in the study. The age range in these papers was 40-75 years. All studies had considered serum 25(OH)D levels, quantified by ELISA, as the surrogate biomarker of Vitamin D status. The RRs for GC had been presented by quartiles[13] or quintiles.[9] The pooling project had reported RRs for upper gastrointestinal cancers across different levels (<25, 25-<37.5, 37.5-<50, 50-<75, 75-<100 and ≥100 nmol/L) of serum 25(OH)D and had considered serum 25(OH)D level of 50-<75 nmol/L as a reference category.[8]
Findings from meta-analysis
Vitamin D intake and risk of gastric cancer
There were four papers that examined the association between dietary Vitamin D intake and risk of GC; one study that had reported ORs separately for cardia and noncardia GC was included as two separate studies. Therefore, we had five ORs in the meta-analysis on Vitamin D intake and risk of GC. The results of the meta-analysis revealed no significant association between Vitamin D intake and risk of GC. Based on the pooled effect size obtained from random effects model, the OR (95% CI) for comparison of highest versus lowest intakes of Vitamin D was 1.09 (0.94, 1.25; P = 0.26) [Figure 2]. We found no evidence of between-study heterogeneity (I2 = 13.2%, P = 0.33) as well as publication bias using funnel plot (Egger's test P = 0.35).
Figure 2.
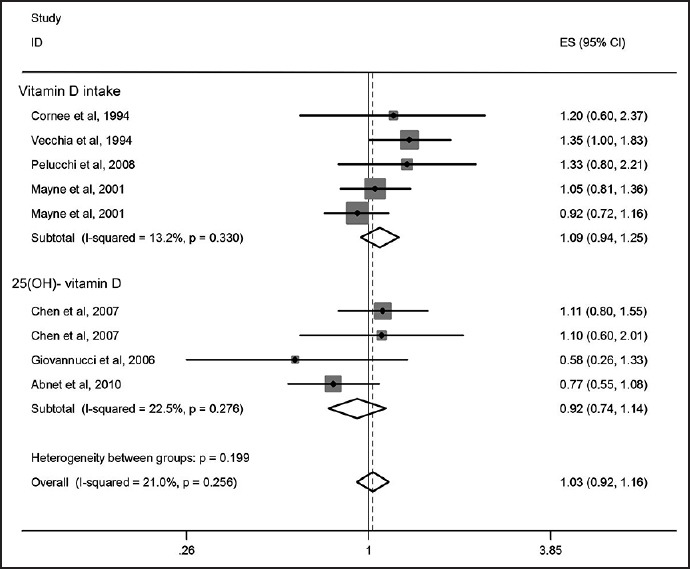
Forest plot of the association between Vitamin D status and risk of gastric cancer for nine included papers; ES: Effect size has been shown as odds ratio
Serum Vitamin D levels and risk of gastric cancer
We found three papers that assessed the relationship between serum levels of Vitamin D and risk of GC. No significant association was observed between serum Vitamin D levels and risk of GC. The pooled effect size, obtained from random effects model, for highest versus lowest serum Vitamin D levels was 0.92 (95% CI: 0.74, 1.14; P = 0.42) [Figure 2]. No evidence of heterogeneity was found (I2: 22.5%, P = 0.27); Also funnel plot did not reveal any evidence of publication bias (Egger's test P = 0.69). As some studies had reported a gender-stratified relationship between serum Vitamin D levels and risk of GC, the analyses were performed separately for men and women. Among men, the pooled effect size in highest versus lowest category of serum Vitamin D levels was 0.92 (95% CI: 0.71, 1.18, P = 0.49) [Figure 3]. For further assurance of the relationship between serum Vitamin D levels and risk of GC in men, we eliminated one study that investigated the association between serum Vitamin D levels and risk of upper gastrointestinal cancers. Despite removing this study from the analysis, no significant association was observed (Pooled OR: 0.94; 95% CI: 0.66, 1.32; P = 0.70) [Figure 4]. No evidence of heterogeneity was found either for all studies in men (I2: 0%, P = 0.51) or when we removed the one assessed upper GI cancer (I2: 11.3%, P = 0.32). We also failed to find any evidence of publication bias from funnel plot (Egger's test; P = 0.32). We failed to find a significant association between serum levels of Vitamin D and risk of GC in women (Pooled OR: 1.04; 95% CI: 0.74, 1.47; P = 0.80). There was no evidence of heterogeneity and publication bias (I2: 0.0%, P = 0.77; Egger's test P = 0.69, respectively) [Figure 5].
Figure 3.
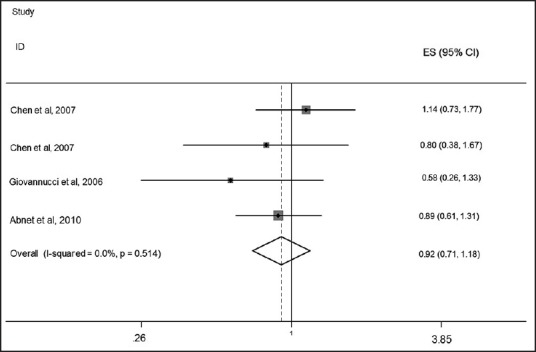
Forest plot of the association between serum 25-hydroxy Vitamin D levels and risk of gastric cancer among men for four included studies
Figure 4.
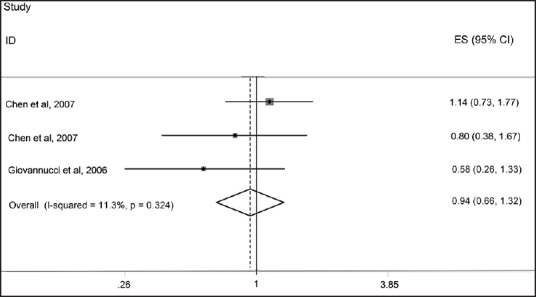
Forest plot of the association between serum 25-hydroxy Vitamin D levels and risk of gastric cancer among men for three included studies
Figure 5.
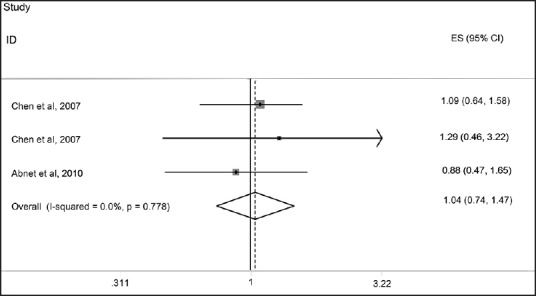
Forest plot of the association between serum 25-hydroxy Vitamin D levels and risk of gastric cancer among women for three included studies
DISCUSSION
In the current meta-analysis, we summarized the results of papers published so far on the association between Vitamin D intake, serum Vitamin D levels, and risk of GC. Overall, no significant association was found between Vitamin D status and risk of GC.
In recent years, numerous studies have reported the associations of circulating 25(OH)D levels with a variety of cancers. Although, inverse associations were rather consistently found for colorectal cancer,[19] findings from studies that assessed the relationship between Vitamin D and breast cancer were more heterogeneous, where strong inverse association has been found in case-control studies, but not in longitudinal studies.[20,21] No associations have been found for other cancers, such as prostate cancer,[22] but risk increase has been suggested at both the lower and the upper levels of the 25(OH)D distributions.[23]
We found a nonsignificant positive association between dietary intake of Vitamin D and risk of GC in adult populations. The four papers on Vitamin D intake we included in the current analysis were as follow:
-
(1)
A case-control study on nutritional factors and GC among 220 people (92 cases and 128 controls) that was performed in 1994. The sample size of this study was very small in comparison with others. Diet history questionnaire had been used for dietary assessment. The questionnaire was particularly detailed and covered extensively the variety of foods typical of the Mediterranean diet. The authors reached a nonsignificant positive relationship between Vitamin D intake and risk of GC (OR: 1.20, 95% CI: 0.60, 2.37; P = 0.61).[10]
-
(2)
Another one was a case-control study that investigated the association of selected micronutrient intakes and risk of GC among 723 cases and 2024 controls. The size of this data set allowed sufficient statistical power to obtain reasonably precise risk estimates for micronutrients. A significant positive association was seen between Vitamin D intake and risk of GC (OR: 1.35, 95% CI: 1.00; 1.83);[11]
-
(3)
In an Italian case-control study, the investigators assessed the relationship between dietary intake of selected micronutrients and risk of GC among 230 patients with GC and 547 matched controls. A major strength of this study was the use of a reproducible and valid FFQ for assessing dietary intakes of macro- and micro-nutrients. A nonsignificant positive association was found between Vitamin D intake and risk of GC (1.33, 95% CI: 0.80, 2.21; P = 0.67);[12]
-
(4)
Another case-control study that was conducted in US assessed the association of nutrient intakes and risk of subtypes of esophageal and GC.
This paper reported the association of Vitamin D intake with risk of gastric cardia-adenocarcinoma and noncardia GC, separately. Totally, 255 patients with gastric cardia-adenocarcinoma, 352 with noncardia GC and 687 controls participated in this study. The strength of this study was considering the use of Vitamin supplements that had not been taken into account in other studies. Again, the authors found no significant association between Vitamin D intake and risk of gastric cardia-adenocarcinoma (OR: 1.05, 95% CI: 0.81, 1.36) or noncardia GC (OR: 0.92, 95% CI: 0.72, 1.16).[14] Taking all studies together, it seems that dietary intake of Vitamin D cannot contribute to the risk of GC; however, the association between high intakes of Vitamin D and risk of GC must be interpreted cautiously. It must be kept in mind that all studies we included in our meta-analysis on Vitamin D intake and risk of GC were of case-control design. A possible limitation inherent in case-control study designs are that cases might have recalled their diet and supplement-taking practices differently after cancer diagnosis. We found no information on H. pylori infection in these studies; while this infection is known as a major determinant of GC. However, case-control studies have limited ability to measure H. pylori because blood samples obtained at stomach cancer diagnosis are of low value.
Our meta-analysis revealed no statistically significant relationship between serum levels of Vitamin D and risk of GC, neither for the whole population nor for gender-stratified analyses. Three studies were included in this meta-analysis:[8,9,13] One prospective study that was performed in men,[9] a nested case-control study[13] and a pooling project.[8] In the cohort study, after following-up the participants for 14 years, 78 GC cases were found. The authors reached a nonstatistically significant but suggestive inverse association between serum 25(OH)D levels and risk of stomach cancer (RR: 0.58, 95% CI: 0.26, 1.33). They found that an increment of 25 nmol/L in 25(OH)D levels was associated with a 43% and 45% reduction in incidence and mortality, respectively, of digestive system cancers.[9] In the nested case-control study, no significant association was found between serum 25(OH)D concentrations and risk of cardia (RR: 1.11, 95% CI: 0.80, 1.55; P = 0.49) or noncardia GC (RR: 1.10, 95% CI: 0.60, 2.01; P = 0.39).[13] One might attribute the lack of associations between serum Vitamin D levels and risk of GC in the above-mentioned studies to the low incidence of this cancer. However, the same findings were obtained in a pooling project of eight prospective studies from China, Finland, and the United States.[8] The authors of this project failed to find any significant association between 25(OH)D concentrations and risk of upper GI cancers (RR: 0.90, 95% CI: 0.65, 1.24) as well as total GC (RR: 0.77, 95% CI: 0.55, 1.08). The strength of this study was the combination of multiple populations from diverse geographic locations that could provide a wide distribution of exposure variable. Furthermore, using eight cohorts supplied a relatively large sample size. As with all the previous studies, the weakness of this study was also the lack of information on H. pylori status. Taken together, it seems that no significant association exists between low serum Vitamin D status and risk of GC. However, due to limited data in this field, further studies are required to reach a definite conclusion.
Several points need to be considered in the interpretation of the current findings. Our systematic review and meta-analysis are the most up-to-date investigation of the worldwide evidence on the association between Vitamin D status and GC. This is the only evidence that included both dietary intake of Vitamin D and serum 25(OH)D levels and assessed their association with GC. Although, methods of assessment of Vitamin D intake were partly different, all studies had used the same method for assessing serum 25(OH)D levels. This might reduce of heterogeneity between studies. Furthermore, given the limited information on both Vitamin D intake and serum Vitamin D levels in relation to GC, additional studies are required.
CONCLUSION
We found no evidence for the significant association between Vitamin D status and risk of GC. However, due to limited data in this field, further studies are required to reach a definite conclusion.
Financial support and sponsorship
The study was supported by a grant from Isfahan University of Medical Sciences, Isfahan, Iran.
Conflicts of interest
There are no conflicts of interest.
AUTHOR'S CONTRIBUTION
SK contributed in the conception of the work, conducting the study, revising the draft, approval of the final version of the manuscript, and agreed for all aspects of the work. AF contributed in the conception of the work, conducting the study, revising the draft, approval of the final version of the manuscript, and agreed for all aspects of the work. PS contributed in the conception of the work, conducting the study, revising the draft, approval of the final version of the manuscript, and agreed for all aspects of the work. AE contributed in the conception of the work, conducting the study, revising the draft, approval of the final version of the manuscript, supervised the study, and agreed for all aspects of the work.
Acknowledgments
Financial support for conception, design, data analysis and manuscript drafting comes from the Food Security Research Center, Isfahan University of Medical Sciences, Isfahan, Iran (no. 294017).
REFERENCES
- 1.Parkin DM. International variation. Oncogene. 2004;23:6329–40. doi: 10.1038/sj.onc.1207726. [DOI] [PubMed] [Google Scholar]
- 2.Haidari M, Nikbakht MR, Pasdar Y, Najaf F. Trend analysis of gastric cancer incidence in Iran and its six geographical areas during 2000-2005. Asian Pac J Cancer Prev. 2012;13:3335–41. doi: 10.7314/apjcp.2012.13.7.3335. [DOI] [PubMed] [Google Scholar]
- 3.Mayne SA, Navarro S. Diet, obesity and reflux in the etiology of adenocarcinomas of the esophagus and gastric cardia in humans. J Nutr. 2002;132:3467S–70S. doi: 10.1093/jn/132.11.3467S. [DOI] [PubMed] [Google Scholar]
- 4.Roder DM. The epidemiology of gastric cancer. Gastric Cancer. 2002;5(Suppl 1):5–11. doi: 10.1007/s10120-002-0203-6. [DOI] [PubMed] [Google Scholar]
- 5.Nomura A. Stomach cancer. In: Schottenfeld D, Fraumeni JF, editors. Cancer Epidemiology and Prevention. 2nd ed. New York: Oxford University Press; 1996. pp. 707–24. [Google Scholar]
- 6.Parsonnet J, Friedman GD, Vandersteen DP, Chang Y, Vogelman JH, Orentreich N, et al. Helicobacter pylori infection and the risk of gastric carcinoma. N Engl J Med. 1991;325:1127–31. doi: 10.1056/NEJM199110173251603. [DOI] [PubMed] [Google Scholar]
- 7.Fuchs CS, Mayer RJ. Gastric carcinoma. N Engl J Med. 1995;333:2–41. doi: 10.1056/NEJM199507063330107. [DOI] [PubMed] [Google Scholar]
- 8.Abnet CC, Chen Y, Chow WH, Gao YT, Helzlsouer KJ, Le Marchand L, et al. Circulating 25-hydroxyvitamin D and risk of esophageal and gastric cancer: Cohort Consortium Vitamin D Pooling Project of Rarer Cancers. Am J Epidemiol. 2010;172:94–106. doi: 10.1093/aje/kwq121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Giovannucci E, Liu Y, Rimm EB, Hollis BW, Fuchs CS, Stampfer MJ, et al. Prospective study of predictors of vitamin D status and cancer incidence and mortality in men. J Natl Cancer Inst. 2006;98:451–9. doi: 10.1093/jnci/djj101. [DOI] [PubMed] [Google Scholar]
- 10.Cornée J, Pobel D, Riboli E, Guyader M, Hémon B. A case-control study of gastric cancer and nutritional factors in Marseille, France. Eur J Epidemiol. 1995;11:55–65. doi: 10.1007/BF01719946. [DOI] [PubMed] [Google Scholar]
- 11.La Vecchia C, Ferraroni M, D’Avanzo B, Decarli A, Franceschi S. Selected micronutrient intake and the risk of gastric cancer. Cancer Epidemiol Biomarkers Prev. 1994;3:393–8. [PubMed] [Google Scholar]
- 12.Pelucchi C, Tramacere I, Bertuccio P, Tavani A, Negri E, La Vecchia C. Dietary intake of selected micronutrients and gastric cancer risk: An Italian case-control study. Ann Oncol. 2009;20:160–5. doi: 10.1093/annonc/mdn536. [DOI] [PubMed] [Google Scholar]
- 13.Chen W, Dawsey SM, Qiao YL, Mark SD, Dong ZW, Taylor PR, et al. Prospective study of serum 25(OH)-vitamin D concentration and risk of oesophageal and gastric cancers. Br J Cancer. 2007;97:123–8. doi: 10.1038/sj.bjc.6603834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mayne ST, Risch HA, Dubrow R, Chow WH, Gammon MD, Vaughan TL, et al. Nutrient intake and risk of subtypes of esophageal and gastric cancer. Cancer Epidemiol Biomarkers Prev. 2001;10:1055–62. [PubMed] [Google Scholar]
- 15.Lipsey MW, Wilson DB. Thousand Oaks, CA: Sage; 2001. Practical Meta-Analysis. [Google Scholar]
- 16.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–58. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 17.Harris RJ, Bradburn MJ, Deeks JJ, Harbord RM, Altman DG, Sterne JAC. Metan: Random-and random-effects meta-analysis. Stata J. 2008;8:3–28. [Google Scholar]
- 18.Ioannidis JP, Trikalinos TA. The appropriateness of asymmetry tests for publication bias in meta-analyses: A large survey. CMAJ. 2007;176:1091–6. doi: 10.1503/cmaj.060410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bosetti C, Bravi F, Negri E, La Vecchia C. Oral contraceptives and colorectal cancer risk: A systematic review and meta-analysis. Hum Reprod Update. 2009;15:489–98. doi: 10.1093/humupd/dmp017. [DOI] [PubMed] [Google Scholar]
- 20.Nickson C, Mason KE, English DR, Kavanagh AM. Mammographic screening and breast cancer mortality: A case-control study and meta-analysis. Cancer Epidemiol Biomarkers Prev. 2012;21:1479–88. doi: 10.1158/1055-9965.EPI-12-0468. [DOI] [PubMed] [Google Scholar]
- 21.Qin LQ, Xu JY, Wang PY, Hoshi K. Soyfood intake in the prevention of breast cancer risk in women: A meta-analysis of observational epidemiological studies. J Nutr Sci Vitaminol (Tokyo) 2006;52:428–36. doi: 10.3177/jnsv.52.428. [DOI] [PubMed] [Google Scholar]
- 22.Ip S, Dahabreh IJ, Chung M, Yu WW, Balk EM, Iovin RC, et al. An evidence review of active surveillance in men with localized prostate cancer. Evid Rep Technol Assess (Full Rep) 2011;204:1–341. [PMC free article] [PubMed] [Google Scholar]
- 23.Ma Y, Zhang P, Wang F, Yang J, Liu Z, Qin H. Association between vitamin D and risk of colorectal cancer: A systematic review of prospective studies. J Clin Oncol. 2011;29:3775–82. doi: 10.1200/JCO.2011.35.7566. [DOI] [PubMed] [Google Scholar]


