Abstract
The inhalation of nanosized air pollutant particles is a recognised risk factor for cardiovascular disease; however, the link between occupational exposure to engineered nanoparticles and adverse cardiovascular events remains unclear. In the present study, the authors demonstrated that pulmonary exposure of rats to ultrafine titanium dioxide (UFTiO2) significantly increased heart rate and depressed diastolic function of the heart in response to isoproterenol. Moreover, pulmonary inhalation of UFTiO2 elevated mean and diastolic blood pressure in response to norepinephrine. Pretreatment of the rats ip with the transient receptor potential (TRP) channel blocker ruthenium red inhibited substance P synthesis in nodose ganglia and associated functional and biological changes in the cardiovascular system. In conclusion, the effects of pulmonary inhalation of UFTiO2 on cardiovascular function are most likely triggered by a lung-nodose ganglia-regulated pathway via the activation of TRP channels in the lung.
Keywords: nanoparticles, neural pathways, cardiovascular system, inhalation study
Introduction
Evidence accumulated from epidemiological studies suggests that there is a significant pathogenic correlation between the inhalation of small-sized or nanosized particles from ambient air and cardiovascular events, such as angina, arrhythmia, ischemic heart failure and sudden death (Dockery et al. 1992; Donaldson et al. 2001; Huang & Ghio 2009; Simkhovich et al. 2008). Recently, a number of studies have shown that pulmonary exposure to engineered nanoparticles (with a diameter <100 nm), such as single-walled carbon nanotubes (SWCNTs), ultrafine titanium dioxide (UFTiO2) and silicon dioxide (SiO2), can cause biological and pathophysiological effects on the cardiovascular system. For instance, pulmonary inhalation of SWCNTs or UFTiO2 results in the development of vascular abnormalities, including atherosclerosis, increased vascular tone and impaired endothelial-dependent vascular dilation (Cascio et al. 2007; Li et al. 2007; Nurkiewicz et al. 2008), while pulmonary inhalation of SiO2 causes alterations in both blood vessels and the heart (Chen et al. 2008). All of these observations suggest that engineered nanoparticles exhibit the potential to cause adverse effects on the cardiovascular system. In addition, the most interesting finding is that the adverse effects observed on the cardiovascular system induced by engineered nanoparticles are very similar to those reported in epidemiological studies of air pollution (Brook et al. 2009; Niwa et al. 2008; Simeonova & Erdely 2009). This finding indicates that engineered nanoparticles and nanosized particles from ambient air may share a common pathophysiological pathway(s) in the development of adverse cardiovascular effects following pulmonary exposure. Engineered nanoparticles are more homogeneous in their physical and chemical properties than naturally existing nanoparticles; therefore, they may induce more consistent and reproducible adverse health effects. These characteristics of engineered nanoparticles provide researchers with a rationale for using engineered nanoparticles to elucidate the mechanisms underlying adverse cardiovascular effects induced not only by engineered nanoparticles but also by air pollution.
Recently, studies conducted by other investigators have shown that inhalation of ultrafine particles from ambient air can alter cardiovascular autonomic nerve activity and baro-receptor reflex sensitivity (Bartoli et al. 2009; Timonen et al. 2006), providing evidence to support the hypothetical mechanism of an autonomic sensory neuron-regulated pathway. Interestingly, the same observation has also been made in a study conducted by Legramante et al. (2009), who demonstrated that intratracheal instillation of engineered nanoparticles reduces the baroreflex response, thereby altering cardiac autonomic neuron activity. The authors of this study also demonstrated that pulmonary exposure of rats to UFTiO2 enhanced substance P synthesis in nodose ganglia of the vagus nerve and was associated with biological changes in the heart, which was evidenced by an increased phosphorylation level of cardiac troponin I (cTnI) (Kan et al. 2012). All of these studies provide strong evidence, suggesting that a neuron-regulated pathway could play an important contributing role in cardiovascular dysfunction caused by air pollution or engineered nanoparticles (Kang et al. 2011).
Nodose and dorsal root ganglia contain pulmonary C-fibre sensory neurons. These sensory neurons detect major irritants in the lung and can be stimulated mechanically, chemically and biologically via the activation of the transient receptor potential (TRP) channels. Increased activity in C-fibre sensory neurons can result in changes to the gene expression of preprotachykinin and neuronal function in nodose and dorsal root ganglia (Curran et al. 2002; Dinh et al. 2004). Moreover, the nerve fibres from the nodose ganglia also project to the brainstem, including the medullar cardiovascular regulatory centre, to regulate autonomic efferent neuron activity (Spyer 1982; Stansfeld & Wallis 1985) or innervate the different chambers of the rat heart and the wall of the aortic arch, where they exhibit a variety of neurochemical phenotypes (Ai et al. 2009; Guic et al. 2010; Kosta et al. 2010). Thus, the sole connection of nodose ganglia could be a critical neuronal pathway that regulates cardiovascular function in response to pulmonary inhalation of nanoparticles. The objective of the present study was to determine cardiovascular reactions following pulmonary exposure to UFTiO2 and elucidate the underlying mechanism(s). The authors conducted cardiovascular haemodynamic measurements, including heart rate, left ventricular function and systemic blood pressure, and evaluated the role of nodose ganglia in the regulation of cardiovascular function by applying the TRP channel blocker in an in vivo animal model.
Methods and materials
Animals
Male Sprague-Dawley rats (Hla:(SD) CVF) from Hilltop Lab Animals (Scottdale, PA, USA), 6–7 weeks of age and free of viral pathogens, parasites, mycoplasmas, Helicobacter and cilia-associated respiratory (CAR) bacillus were used for all experiments. The rats were housed in cages ventilated with HEPA (high-efficiency particulate air)- filtered air under controlled temperature and humidity conditions and a 12-h light/12-h dark cycle. Food (Teklad 7913) and tap water were provided ad libitum. The rats were allowed to acclimatise to the facilities for 1 week before exposure was performed. The animal facilities are specific pathogen-free and accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International. All experimental procedures were approved by the Animal Care and Use Committee of the National Institute for Occupational Safety and Health and conducted in accordance with the guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health.
Pulmonary UFTiO2 exposure and ruthenium red treatment
Rats (7–8 weeks of age) received UFTiO2 (primary particle diameter ~21 nm) by inhalation. For this study, the UFTiO2 aerosol concentration was 6 mg/m3, and the exposure duration was 4 h. The aerosol generation system, exposure chamber and physical characterisation of UFTiO2 aerosol have been described previously (Nurkiewicz et al. 2008). Previous studies have shown that this exposure scheme produced an actual pulmonary deposition of 10 μg UFTiO2 in rats, which is equivalent to workers exposed to 0.1 mg/m3 for 27 workdays in a typical occupational environment, and resulted in biological and functional changes in the systemic and cardiac vascular system (Kan et al. 2012; LeBlanc et al. 2009; Nurkiewicz et al. 2008). This equivalency is calculated based on the deposited mass of UFTiO2 aerosol assuming a slow or no clearance. The rationale for the 27 days was based on the equivalent deposited mass per surface area of alveolar epithelium. Morphometric data for rat and human alveolar surface area are from Stone et al. (1992). Non-selective TRP channel blocker ruthenium red (2.5 mg/kg) (Sigma-Aldrich, St. Louis, MO, USA) was injected intraperitoneally 1 h prior to the pulmonary exposure of rats to UFTiO2 or filtered air.
In vivo haemodynamic measurements
At 24 h post-exposure, rats (7–8 per group) were anaesthetised with inhaled 3% isoflurane mixed with oxygen at a flow rate of 2 l/min. A temperature-regulated heating pad was used to maintain the normal body temperature of the rat. Using aseptic technique, a custom catheter made according to the method described by Khanna et al. (2007) was inserted into the left ventricle through the carotid artery. The correct position of the catheter tip in the left ventricle was confirmed by the waveform of left ventricular pressure visualised on a computer monitor. Heart function was assessed by measuring the maximum rate of increase in left ventricular pressure (dP/dtmax) and minimum rate of decrease in left ventricular pressure (dP/dtmin). To study vascular function in vivo, systemic arterial blood pressure was determined by using a fluid-filled arterial catheter placed in the femoral artery and connected to a pressure transducer coupled to a computerised cardiovascular continuous monitoring system, a PowerLab/4SP analog-to-digital converter (AD Instruments, Colorado Springs, CO, USA). The heart rate was recorded by the same monitoring system at a sampling rate of 1000 Hz. Isoproterenol (ISO) or norepinephrine (NE) (Sigma-Aldrich) was administrated through a catheter (polyurethane, 3 French size) preplaced in the jugular vein. Cardiopulmonary responses and spinal reflexes were checked to determine the proper depth of anaesthesia. Each rat was euthanised with a carbon dioxide overdose at the end of the experiment.
Substance P immunohistochemistry
For tissue preparation, the right and left nodose ganglia and dorsal root ganglia (C1-C2 and T1-T4) were excised, fixed in picric acid-formaldehyde for 3 h and rinsed three times with 0.1 M phosphate-buffered saline containing 0.3% Triton X-100 (PBS-Tx) (pH 7.8). The nodose ganglia were then frozen in isopentane, cooled with liquid nitrogen and stored in airtight bags at −80°C. The immunocytochemical procedures for the localisation of substance P immunoreactive neurons were similar to those described previously (Kan et al. 2012). Briefly, cryostat sections (12 μm thickness) of nodose ganglia were mounted on gelatin-coated cover-slips, dried briefly at room temperature and then incubated with rabbit anti-substance P antiserum (Peninsula, Belmont, CA, USA) at a dilution of 1:100 for 60 min in a humidified chamber at 37°C. The coverslips were rinsed with PBS-Tx containing 1% bovine serum albumin (PBS-Tx + BSA) three times, for 5 min per rinse. The sections were then incubated with fluorescein isothiocyanate (FITC)-labelled goat anti-rabbit IgG (ICN Immunobiologicals, Inc., Costa Mesa, CA, USA) at a dilution of 1:100 for 60 min at 37°C. Then, the coverslips were mounted with fluoromount, and the sections were observed under a fluorescence microscope equipped with a fluorescein (excitation wavelengths from 455 to 500 nm and emission wavelengths >510 nm) filter. The controls consisted of testing the specificity of primary antiserum by absorption with 1 μg/ml of the specific antigen. Non-specific background labelling was determined by omission of primary antiserum. The quantitative measurement of fluorescence intensity in nodose ganglia was performed similarly to the authors’ previously described method (Kan et al. 2012).
Western blots
The phosphorylation status of cTnI was examined at 24 h following pulmonary exposure to UFTiO2. Cardiac tissue samples were removed from rats, rapidly frozen and crushed with a mortar and pestle at the temperature of liquid nitrogen for protein extraction. Cardiac tissue was prepared in lysis buffer containing 20 mmol/l Tris-HCl, 20 mmol/l NaCl, 0.1 mmol/l ethylenediaminetetraacetic acid (EDTA), 0.1% Triton X-100 and protease inhibitors and centrifuged at 10,000 g for 20 min at 4°C as described previously (Kan et al. 2012). Equal amounts of protein were loaded onto an 8% SDS PAGE (sodium dodecyl sulfate polyacrylamide gel electrophoresis) and then transferred to a polyvinylidene difluoride (PVDF) membrane (Invitrogen, Carlsbad, CA, USA). Phosphorylation of cTnI was detected by phospho-cardiac troponin-I (Ser 23/24) antibody (Cell Signaling Technology, Danvers, MA, USA). Total cTnI was detected with a rabbit mAb (Cell Signaling Technology).
Statistical analysis
Data were compared using analysis of variance, followed by pairwise comparisons between the control and treated groups using a Student’s t-test. All data were analysed using JMP software (version 9.0) and differences were considered statistically significant at p < 0.05. The values in the figures are expressed as the mean ± SD (standard deviation).
Results
Effects of UFTiO2 and TRP channel blocker on heart rate
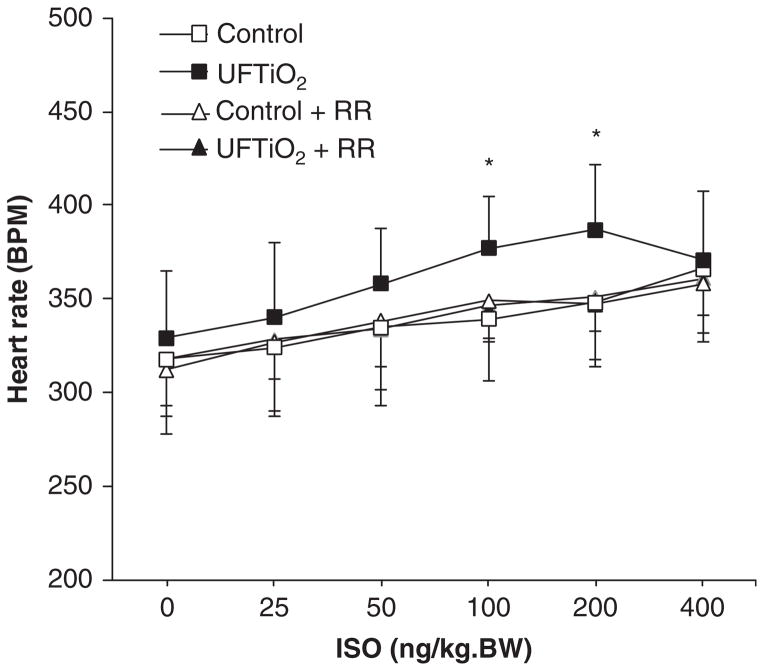
Pulmonary inhalation of UFTiO2 did not change the basal heart rate compared with the control group (exposed to filtered air) at 24 h post-exposure. However, ISO-induced increases in heart rate were greater in rats exposed to UFTiO2 compared with rats exposed to filtered air (Figure 1). Pretreatment of the rats with ruthenium red, a non-selective TRP channel blocker, did not affect the basal heart rate in rats either exposed to filtered air or UFTiO2, but prevented the ISO-induced increase in heart rate in rats exposed to UFTiO2 (Figure 1). In addition, ruthenium red alone had no effect on heart rate in response to ISO (Figure 1).
Figure 1.
The dose–response curve of heart rate in response to ISO at 24 h after exposure to UFTiO2 and the effect of ruthenium red (RR) on UFTiO2-induced heart rate increase in response to ISO. Each value represents the mean ± SD of six rats; p < 0.05 compared with the control group (*).
Effects of UFTiO2 and TRP channel blocker on left ventricular function
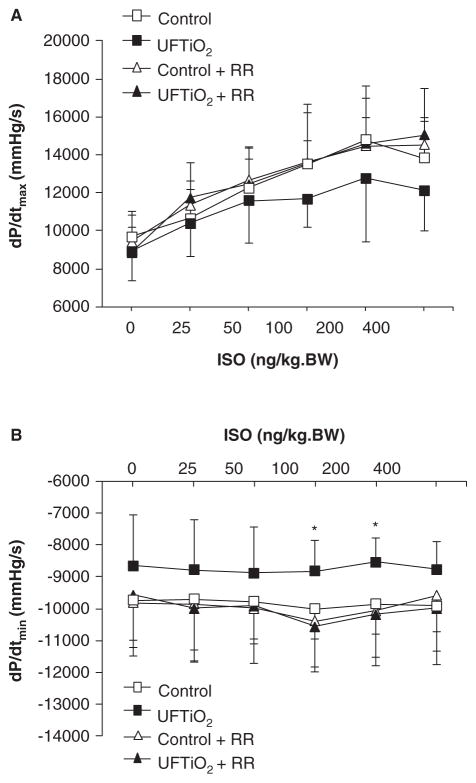
Baseline measures of dP/dtmax, which reflects the systolic function of the left ventricle, were similar in all experimental groups at 24 h post-exposure. Pulmonary inhalation of UFTiO2 slightly reduced dP/dtmax in response to higher dosages of ISO, but the difference did not reach statistical significance when compared with the control group (p > 0.05) (Figure 2A). The dP/dtmin, which indicates the diastolic function of the left ventricle, was decreased in response to ISO and reached statistical significance at higher dosages in rats exposed to UFTiO2 compared with the control group (Figure 2B). Reduced dP/dtmin in response to ISO was prevented by pretreatment of rats with ruthenium red (Figure 2B).
Figure 2.
Effects of UFTiO2 and ruthenium red (RR) on cardiac function. (A) The contractile ability of the heart at 24 h after exposure to UFTiO2 was assessed by measuring the maximum rate of increase in left ventricular pressure, dP/dtmax. (B) The diastolic function of the heart at 24 h after exposure to UFTiO2 was assessed by measuring the minimum rate of decrease in left ventricular pressure, dP/dtmin. Each value represents the mean ± SD of six rats; p < 0.05 compared with the control group (*).
Effects of UFTiO2 and TRP channel blocker on systemic blood pressure
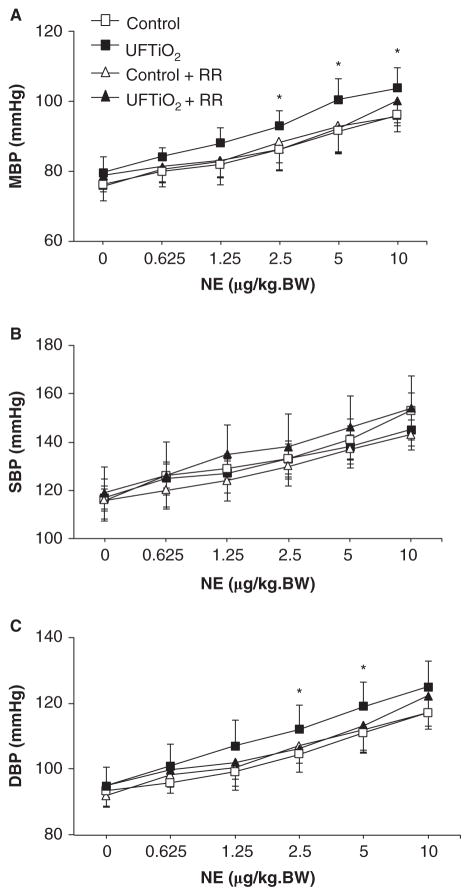
Pulmonary inhalation of UFTiO2 or pretreatment with ruthenium red did not alter basal levels of mean blood pressure compared with the control group (p > 0.05) (Figure 3A). However, UFTiO2 significantly increased mean blood pressure in response to NE, an α-adrenergic receptor agonist. Ruthenium red prevented the increase of mean blood pressure in response to NE in rats exposed to UFTiO2 (Figure 3A). Systolic blood pressure was not affected by pulmonary inhalation of UTFiO2 either at basal levels or in response to NE (Figure 3B). By contrast, diastolic blood pressure in response to NE was significantly increased at higher NE concentrations in rats exposed to UFTiO2 at 24 h post-exposure compared with the control group (Figure 3C). Pretreatment of rats with ruthenium red prevented increased diastolic blood pressure in response to NE in rats exposed to UFTiO2 (Figure 3C).
Figure 3.
Effects of UFTiO2 and ruthenium red (RR) on blood pressure. (A) The dose–response curve of mean blood pressure (MBP) in response to NE at 24 h after exposure to UFTiO2. (B) The dose–response curve of systolic blood pressure (SBP) in response to NE at 24 h after exposure to UFTiO2. (C) The dose–response curve of diastolic blood pressure (DBP) in response to NE at 24 h after exposure to UFTiO2. Each value represents the mean ± SD of six rats; p < 0.05 compared with the control group (*).
Effect of TRP channel blocker on SP synthesis in nodose and dorsal root ganglia and cTnI phosphorylation
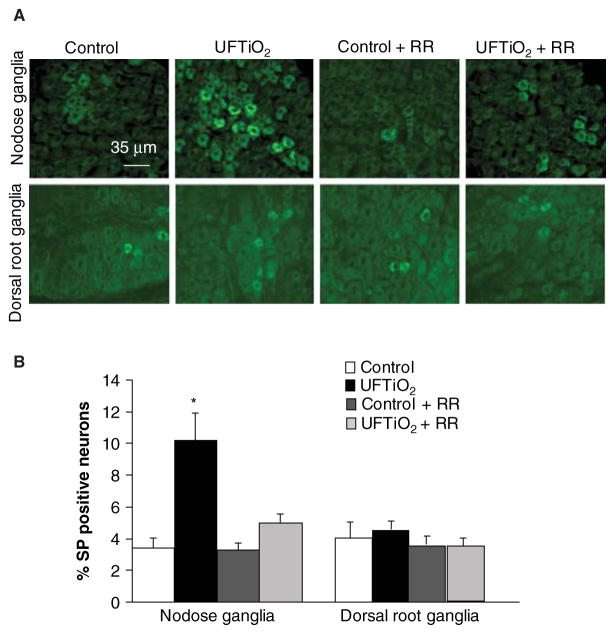
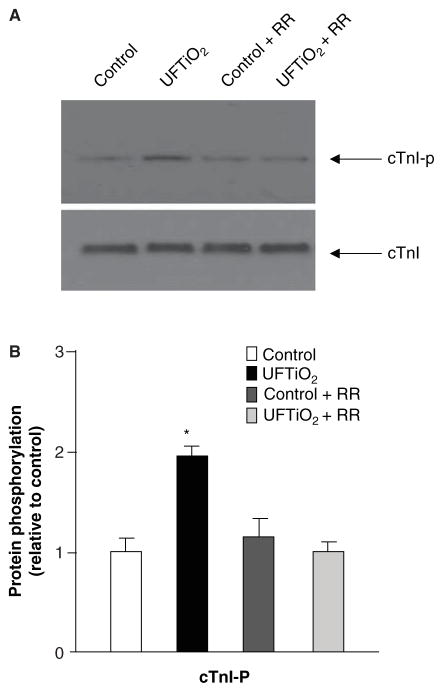
Pulmonary inhalation of UFTiO2 significantly increased neuronal substance P immunoreactivity in the nodose ganglia, but not in the dorsal root ganglia at 24 h post-exposure (Figure 4A). Immunohistochemistry revealed that only 3.42 ± 0.65% of neurons displayed substance P immunoreactivity in control rats, whereas 10.23 ± 1.67% (p ≤ 0.05 compared with the controls) of neurons were substance P positive in rats exposed to UFTiO2. Substance P immunoreactivity in nodose ganglia by UFTiO2 was reduced to 4.98 ± 0.59% in rats pretreated with ruthenium red (Figure 4B). Western blots confirmed that pulmonary inhalation of UFTiO2 increased the phosphorylation level of cTnI in the heart at 24 h post-exposure (Figure 5A and B). However, pretreatment with ruthenium red prevented UFTiO2-elevated phosphorylation levels of cTnI (Figure 5A and B).
Figure 4.
Fluorescence photomicrographs of substance P (SP) immunoreactivity in nodose and dorsal root ganglia. (A) SP immunoreactivity was detected in different groups at 24 h post-exposure. (B) The SP immunoreactivity in nodose and dorsal root ganglia was quantified (B). Each value represents the mean ± SD of 5 rats; p < 0.05 compared with the control group (*).
Figure 5.
The Western blot represents cardiac TnI phosphorylation and expression in different groups at 24 h post-exposure. (A) Each lane of the Western blot shows cardiac TnI phosphorylation and expression in a myocyte preparation from an individual rat. (B) Densitometry values of specific bands were compared between each treatment and the control group. Each value represents the mean ± SD of three different experiments; p < 0.05 compared with the control group (*).
Effect of UFTiO2 on reactive oxygen species in the heart and aortic artery
Pulmonary exposure to nanoparticles or polluted air can increase reactive oxygen species (ROS) generation in the lung and the secondary organs, which causes tissue damage (Lodovici & Bigagli 2011). As reported previously, inhalation exposure to UFTiO2 at levels and duration used in the present study did not alter bronchoalveolar lavage markers of inflammation and lung damage. However, histological evaluation demonstrated alveolar macrophages associated with deposition sites of particles in scattered alveoli (Nurkiewicz et al. 2008). In the present study, the authors also examined the formation of ROS in the heart and aortic artery after UFTiO2 exposure. Hydroxyl radicals were indirectly measured in heart and aorta tissue slices according to methods previously described (Ide et al. 2000). The results indicated that UFTiO2 exposure did not increase the amount of ROS production in the heart and aortic artery (data not shown).
Discussion and conclusion
The present study reports that pulmonary exposure of rats to UFTiO2 did not increase ROS production in the heart and aortic artery, but significantly increased heart rate, depressed diastolic function of the heart and elevated mean and diastolic blood pressure in response to adrenergic stimuli. Pretreatment of the rats with ruthenium red, a non-selective TRP channel blocker, not only inhibited substance P synthesis in nodose ganglia but also inhibited the phosphorylation level of cTnI and prevented the changes in cardiovascular function associated with substance P synthesis in nodose ganglia induced by UFTiO2.
An increase in heart rate (Figure 1) associated with altered diastolic function in response to ISO at 24 h after exposure to UFTiO2 suggests an acute effect of UFTiO2 on cardiac function (Figures 1 and 2). A change in heart rate usually results from an alteration in autonomic nervous system activity. Alterations in autonomic nervous system activity have been observed in epidemiological studies and are positively correlated with the level of small-sized particles in ambient air (Pope et al. 1999). Increased heart rate resulting from exposure to engineered nanoparticles was first reported in a study using an isolated rodent heart. The increase in heart rate in this experimental model was primarily due to an increase of catecholamine released from adrenergic nerve endings within the heart (Stampfl et al. 2011). In the present study, it is likely that pulmonary inhalation of engineered nanoparticles is able to influence the activity of the autonomic nervous system, which in turn modulates neurotransmitter synthesis or release from neuronal terminals that innervate the heart. This proposed mechanism is based on the finding that pulmonary inhalation of UFTiO2 increased substance P synthesis in nodose ganglia, which may alter the activity of nodose ganglia neurons that project to the medulla and different parts of the heart. Such a mechanism may also partially explain why increases in heart rate are observed during air pollution episodes (Peters et al. 1999).
This study also demonstrated that pulmonary inhalation of UFTiO2 had a significant effect on cardiac function, with diastolic function of the heart affected more than systolic function. This observation, together with the previous finding that inhalation of UFTiO2 resulted in an increase in phosphorylated cTnI, suggests that UFTiO2-induced cTnI phosphorylation may serve as one of the factors underlying the cardiac dysfunction observed in the present study and epidemiological studies of air pollution. In vivo and in vitro studies have confirmed that a sustained increase in cTnI phosphorylation regulated by protein kinase A (PKA) changes myofilament calcium binding affinity and reduces relaxation rate, thereby impairing the diastolic function of the heart (Sakthivel et al. 2005). Other studies have shown that increases of sympathetic nerve activity or catecholamine concentrations in the blood can result in an elevation of cTnI phosphorylation, which indicates a neuron-regulated effect on the phosphorylation status of cTnI (van Dijk et al. 2008). Altered diastolic function of the heart is considered an early pathophysiological indicator of many forms of human heart failure (Krajnak et al. 2011; Satpathy et al. 2006). However, the impact of altered diastolic function on the heart usually produces no symptoms in its early stage, unless it has progressed to the point of diminishing heart function. The alterations in the diastolic function of the heart resulting from pulmonary inhalation of UFTiO2 in this study suggest that pulmonary exposure to nanoparticles may increase the risk of a cardiac event in workers exposed daily to nanoparticles.
Pulmonary inhalation of UFTiO2 also altered vasculature function, as evidenced by an increase in mean blood pressure in response to NE (Figure 3A). This increased mean blood pressure was primarily due to elevated diastolic blood pressure (Figure 3C) because while the increases in systolic blood pressure in response to NE were greater in rats exposed to UFTiO2 (Figure 3B), they did not reach significant levels. The authors and other investigators have reported that inhalation of pulmonary irritants, including UFTiO2, can result in changes in the vascular function of small vessels primarily by increasing vascular tone through a sympathetic nerve-regulated mechanism and/or impairing acetylcholine-induced vasodilation through an endothelium-regulated pathway (Krajnak et al. 2011; LeBlanc et al. 2009; Nurkiewicz et al. 2008). The impact of endothelial dysfunction on blood pressure could be different, as it affects diastolic more than systolic blood pressure in rats (de Belchior et al. 2012). This could be the mechanism of isolated diastolic hypertension, one subtype of hypertension diagnosed in the clinic (Arima et al. 2012).
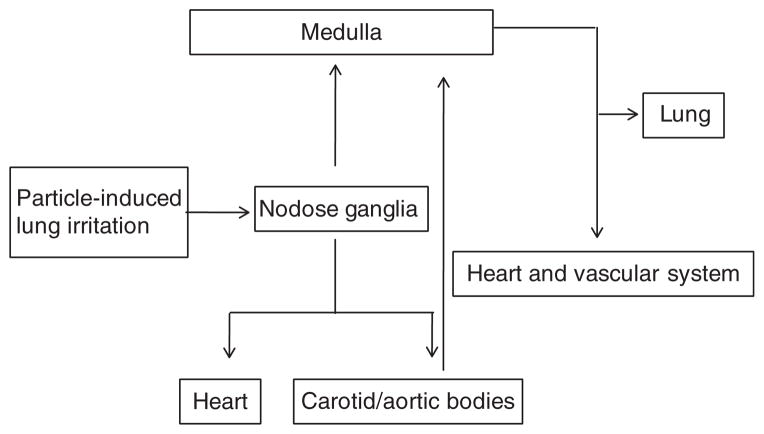
The alterations in cardiovascular reactions in response to adrenergic stimuli in this study indicate that pulmonary inhalation of UFTiO2 may have an influence on the activity of the autonomic nerves that regulate cardiovascular function. Recently, other investigators have reported that local treatment with an α-adrenergic receptor antagonist partially blocked vascular dilatory dysfunction induced by pulmonary exposure to nanoparticles (Brook et al. 2009; Nurkiewicz et al. 2008), which also suggested an involvement of the autonomic nervous system in response to nanoparticle exposure. The present study showed that UFTiO2 exposure-increased neurotransmitter substance P synthesis occurred predominantly in the nodose ganglia, but not in the dorsal root ganglia (Figure 4A), indicating that the sensory neurons in the nodose ganglia are likely associated with cardiovascular functional and biological changes after exposure to UFTiO2. This was supported by the interesting finding that pretreatment of rats with a non-selective TRP channel blocker not only inhibited substance P synthesis in the nodose ganglia but also prevented the cardiovascular functional changes and increases in phosphorylated cTnI (Figures 4 and 5). Both nodose and dorsal root ganglia contain cell bodies of pulmonary sensory neurons, however, the neuronal pathways that functionally transfer signals from the lung to the cardiovascular regulatory centre in the medulla, the heart and the peripheral vasculatures are different. It has been reported that the cardiovascular responses to the inhalation of certain irritants can be blocked by bilateral vagotomy, suggesting that cardiovascular responses induced by certain irritants in the lung can be mediated mainly through nodose ganglia (Wang et al. 1996). In addition, nodose ganglia also contain the cell bodies of the aortic baroreceptor neurons involved in the baroreflex, one of the body’s most important homeostatic mechanisms for maintaining normal blood pressure (Li et al. 2008). Therefore, considering the unique neuronal connections of the nodose ganglia with the cardiovascular system, the observations from the current study indicate that the nodose ganglia play a significant role in the regulation of UTFiO2 exposure-induced changes in the cardiovascular system by acting as an intermediary centre for integrating signalling from the lung and then further transmitting such signals to either the central nervous system or peripheral nerves, which regulate and control cardiovascular function (Figure 6). Nevertheless, these findings are sufficiently compelling enough to warrant more definitive research.
Figure 6.
Schematic diagram describes a nodose ganglia-involved neuronal pathway in the regulation of cardiovascular function after pulmonary exposure to nanoparticles. Activation of TRP channels in the lung by nanoparticles results in transmission of stimulatory signals to nodose ganglia, where signals are integrated and processed. These signals are then transmitted to the cardiovascular centre in the medulla or the peripheral organs through the afferent neurons of nodose ganglia, resulting in modification of cardiovascular function.
Recently, Hazari et al. (2011) demonstrated that TRP channels in the lung play a crucial role in the regulation of cardiac rhythm after exposure to diesel particles, and blockage of TRP channels by ruthenium red abolished diesel exposure-induced cardiac arrhythmias. TRP channels are a group of ion channels (Ca2+ and Na+) that function as cellular sensors. They are expressed in a variety of tissues, including the endings of nodose C-fibres in the lung and cardiomyocytes in the heart (Vennekens 2011), and respond to diverse signals, including intracellular and extracellular messengers, exogenous chemicals, temperature and mechanical stress (Glazebrook et al. 2005). In this study, pretreatment of rats with a non-selective TRP channel blocker prevented UFTiO2 exposure-induced biological and functional changes in the cardiovascular system, which indicates an activation of TRP channels by pulmonary inhalation of UFTiO2. The mechanism by which UTFiO2 exposure activates TRP channels in the lung is unclear and requires further investigation. However, it has been shown that nanoparticles, including engineered nanoparticles like UFTiO2, have high surface activity and can interact with macrophages, epithelial cells or leukocytes that can evoke the production of ROS and pro-inflammatory factors in the lung (Rahman et al. 2002; Scherbart et al. 2011). Therefore, the possible mechanisms responsible for the activation of TRP channels following UTFiO2 exposure include the direct stimulation of TRP channels by UFTiO2 or locally generated ROS and/or inflammatory factors in the alveoli.
It has been reported that TRP channels are also expressed in the heart and vasculature and involved in several fundamental cell functions, such as contraction, proliferation and cell death (Watanabe et al. 2008). Ruthenium red is a non-selective TRP channel blocker, it can block ryanodine receptors in the heart and reduce cardiac muscle contractility in vitro (Aschar-Sobbi et al. 2012). However, the effects of ruthenium red on the cardiovascular function in vivo are uncertain. In the present study, pretreatment of rats with ruthenium red did not affect cardiovascular function either at basal levels or in response to adrenergic stimuli, which suggests that the effects of ruthenium red on preventing UFTiO2 exposure-induced biological and functional changes observed in this study are not likely via the blocking of TRP channels in the heart and vasculature.
In conclusion, the in vivo observations together with the previous findings that pulmonary inhalation of UFTiO2 did not cause significant systemic inflammation and that direct exposure of cardiomyocytes to UFTiO2 did not induce biological changes provide compelling evidence to support a role for nodose ganglia in the neuronal-mediated pathway that regulates cardiovascular function following pulmonary inhalation of UFTiO2 (Kan et al. 2012). Activation of TRP channels appears to be an initial step to trigger this entire event.
The significance of the findings is that this proposed mechanism may not only be critical for engineered nano-particle exposure-regulated alterations in cardiovascular function but may also be an important mechanism underlying the increased incidence of cardiovascular diseases associated with air pollution. Unchanged basal cardiovascular function, but altered cardiovascular reactions in response to adrenergic stimuli after pulmonary inhalation of UTFiO2 indicate that pulmonary inhalation of UFTiO2 or ambient nanoparticles may not pose an immediate health risk to healthy people; however, in people predisposed to cardiovascular diseases, such as coronary artery disease, congestive heart failure or essential hypertension associated with high circulating plasma catecholamine, pulmonary inhalation of nanoparticles may worsen the disease state and trigger a cardiovascular event.
Acknowledgments
The authors would like to thank Rebecca Salmen for her excellent technical support.
Footnotes
Declaration of interest
The opinions expressed in this manuscript are those of the authors and do not necessarily represent the views of the National Institute for Occupational Safety and Health, Center for Disease Control and Prevention of the USA.
References
- Ai J, Wurster RD, Harden SW, Cheng ZJ. Vagal afferent innervation and remodeling in the aortic arch of young-adult Fischer 344 rats following chronic intermittent hypoxia. Neuroscience. 2009;164(2):658–666. doi: 10.1016/j.neuroscience.2009.06.066. [DOI] [PubMed] [Google Scholar]
- Arima H, Murakami Y, Lam TH, Kim HC, Ueshima H, Woo J Asia Pacific Cohort Studies C. Effects of prehypertension and hypertension subtype on cardiovascular disease in the Asia-Pacific Region. Hypertension. 2012;59(6):1118–1123. doi: 10.1161/HYPERTENSIONAHA.111.187252. [DOI] [PubMed] [Google Scholar]
- Aschar-Sobbi R, Emmett TL, Kargacin GJ, Kargacin ME. Phospholamban phosphorylation increases the passive calcium leak from cardiac sarcoplasmic reticulum. Pflugers Arch. 2012;464(3):295–305. doi: 10.1007/s00424-012-1124-9. [DOI] [PubMed] [Google Scholar]
- Bartoli CR, Wellenius GA, Diaz EA, Lawrence J, Coull BA, Akiyama I, et al. Mechanisms of inhaled fine particulate air pollution-induced arterial blood pressure changes. Environ Health Perspect. 2009;117(3):361–366. doi: 10.1289/ehp.11573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brook RD, Urch B, Dvonch JT, Bard RL, Speck M, Keeler G, et al. Insights into the mechanisms and mediators of the effects of air pollution exposure on blood pressure and vascular function in healthy humans. Hypertension. 2009;54(3):659–667. doi: 10.1161/HYPERTENSIONAHA.109.130237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cascio WE, Cozzi E, Hazarika S, Devlin RB, Henriksen RA, Lust RM, et al. Cardiac and vascular changes in mice after exposure to ultrafine particulate matter. Inhal Toxicol. 2007;19(Suppl 1):67–73. doi: 10.1080/08958370701493456. [DOI] [PubMed] [Google Scholar]
- Chen Z, Meng H, Xing G, Yuan H, Zhao F, Liu R, et al. Age-related differences in pulmonary and cardiovascular responses to SiO2 nanoparticle inhalation: nanotoxicity has susceptible population. Environ Sci Technol. 2008;42(23):8985–8992. doi: 10.1021/es800975u. [DOI] [PubMed] [Google Scholar]
- Curran DR, Walsh MT, Costello RW. Interactions between inflammatory cells and nerves. Curr Opin Pharmacol. 2002;2(3):243–248. doi: 10.1016/s1471-4892(02)00155-8. [DOI] [PubMed] [Google Scholar]
- de Belchior ACs, Angeli JK, de O Faria T, Siman FDM, Silveira EA, Meira EF, et al. Post-weaning protein malnutrition increases blood pressure and induces endothelial dysfunctions in rats. PLoS One. 2012;7(4):e34876. doi: 10.1371/journal.pone.0034876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinh QT, Groneberg DA, Peiser C, Mingomataj E, Joachim RA, Witt C, et al. Substance P expression in TRPV1 and trkA-positive dorsal root ganglion neurons innervating the mouse lung. Respir Physiol Neurobiol. 2004;144(1):15–24. doi: 10.1016/j.resp.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Dockery DW, Schwartz J, Spengler JD. Air pollution and daily mortality: associations with particulates and acid aerosols. Environ Res. 1992;59(2):362–373. doi: 10.1016/s0013-9351(05)80042-8. [DOI] [PubMed] [Google Scholar]
- Donaldson K, Stone V, Seaton A, MacNee W. Ambient particle inhalation and the cardiovascular system: potential mechanisms. Environ Health Perspect. 2001;109(Suppl 4):523–527. doi: 10.1289/ehp.01109s4523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glazebrook PA, Schilling WP, Kunze DL. TRPC channels as signal transducers. Pflugers Arch. 2005;451(1):125–130. doi: 10.1007/s00424-005-1468-5. [DOI] [PubMed] [Google Scholar]
- Guic MM, Kosta V, Aljinovic J, Sapunar D, Grkovic I. Characterization of spinal afferent neurons projecting to different chambers of the rat heart. Neurosci Lett. 2010;469(3):314–318. doi: 10.1016/j.neulet.2009.12.016. [DOI] [PubMed] [Google Scholar]
- Hazari MS, Haykal-Coates N, Winsett DW, Krantz QT, King C, Costa DL, et al. TRPA1 and sympathetic activation contribute to increased risk of triggered cardiac arrhythmias in hypertensive rats exposed to diesel exhaust. Environ Health Perspect. 2011;119(7):951–957. doi: 10.1289/ehp.1003200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YC, Ghio AJ. Controlled human exposures to ambient pollutant particles in susceptible populations. Environ health. 2009;8:33. doi: 10.1186/1476-069X-8-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ide T, Tsutsui H, Kinugawa S, Suematsu N, Hayashidani S, Ichikawa K, et al. Direct evidence for increased hydroxyl radicals originating from superoxide in the failing myocardium. Circ Res. 2000;86(2):152–157. doi: 10.1161/01.res.86.2.152. [DOI] [PubMed] [Google Scholar]
- Kan H, Wu Z, Young SH, Chen TH, Cumpston JL, Chen F, et al. Pulmonary exposure of rats to ultrafine titanium dioxide enhances cardiac protein phosphorylation and substance P synthesis in nodose ganglia. Nanotoxicology. 2012;6(7):736–45. doi: 10.3109/17435390.2011.611915. [DOI] [PubMed] [Google Scholar]
- Kang GS, Gillespie PA, Gunnison A, Moreira AL, Tchou-Wong KM, Chen LC. Long-term inhalation exposure to nickel nanoparticles exacerbated atherosclerosis in a susceptible mouse model. Environ Health Perspect. 2011;119(2):176–181. doi: 10.1289/ehp.1002508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanna D, Kan H, Fang Q, Xie Z, Underwood BL, Jain AC, et al. Inducible nitric oxide synthase attenuates adrenergic signaling in alcohol fed rats. J Cardiovasc Pharmacol. 2007;50(6):692–696. doi: 10.1097/FJC.0b013e318158de08. [DOI] [PubMed] [Google Scholar]
- Kosta V, Guic MM, Aljinovic J, Sapunar D, Grkovic I. Immunohistochemical characteristics of neurons in nodose ganglia projecting to the different chambers of the rat heart. Autono Neurosci. 2010;155(1–2):33–38. doi: 10.1016/j.autneu.2010.01.001. [DOI] [PubMed] [Google Scholar]
- Krajnak K, Kan H, Waugh S, Miller GR, Johnson C, Roberts JR, et al. Acute effects of COREXIT EC9500A on cardiovascular functions in rats. J Toxicol Environ Health A. 2011;74(21):1397–1404. doi: 10.1080/15287394.2011.606795. [DOI] [PubMed] [Google Scholar]
- LeBlanc AJ, Cumpston JL, Chen BT, Frazer D, Castranova V, Nurkiewicz TR. Nanoparticle inhalation impairs endothelium-dependent vasodilation in subepicardial arterioles. J Toxicol Environ Health A. 2009;72(24):1576–1584. doi: 10.1080/15287390903232467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legramante JM, Valentini F, Magrini A, Palleschi G, Sacco S, Iavicoli I, et al. Cardiac autonomic regulation after lung exposure to carbon nanotubes. Hum Exp Toxicol. 2009;28(6–7):369–375. doi: 10.1177/0960327109105150. [DOI] [PubMed] [Google Scholar]
- Li YL, Tran TP, Muelleman R, Schultz HD. Blunted excitability of aortic baroreceptor neurons in diabetic rats: involvement of hyper-polarization-activated channel. Cardiovasc Res. 2008;79(4):715–721. doi: 10.1093/cvr/cvn141. [DOI] [PubMed] [Google Scholar]
- Li Z, Hulderman T, Salmen R, Chapman R, Leonard SS, Young SH, et al. Cardiovascular effects of pulmonary exposure to single-wall carbon nanotubes. Environ Health Perspect. 2007;115(3):377–382. doi: 10.1289/ehp.9688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodovici M, Bigagli E. Oxidative stress and air pollution exposure. J Toxicol. 2011;2011:487074. doi: 10.1155/2011/487074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niwa Y, Hiura Y, Sawamura H, Iwai N. Inhalation exposure to carbon black induces inflammatory response in rats. Circ J. 2008;72(1):144–149. doi: 10.1253/circj.72.144. [DOI] [PubMed] [Google Scholar]
- Nurkiewicz TR, Porter DW, Hubbs AF, Cumpston JL, Chen BT, Frazer DG, et al. Nanoparticle inhalation augments particle-dependent systemic microvascular dysfunction. Part Fibre Toxicol. 2008;5:1. doi: 10.1186/1743-8977-5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters A, Perz S, Doring A, Stieber J, Koenig W, Wichmann HE. Increases in heart rate during an air pollution episode. Am J Epidemiol. 1999;150(10):1094–1098. doi: 10.1093/oxfordjournals.aje.a009934. [DOI] [PubMed] [Google Scholar]
- Pope CA, 3rd, Verrier RL, Lovett EG, Larson AC, Raizenne ME, Kanner RE, et al. Heart rate variability associated with particulate air pollution. Am Heart J. 1999;138(5 Pt 1):890–899. doi: 10.1016/s0002-8703(99)70014-1. [DOI] [PubMed] [Google Scholar]
- Rahman Q, Lohani M, Dopp E, Pemsel H, Jonas L, Weiss DG, et al. Evidence that ultrafine titanium dioxide induces micronuclei and apoptosis in Syrian hamster embryo fibroblasts. Environ Health Perspect. 2002;110(8):797–800. doi: 10.1289/ehp.02110797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakthivel S, Finley NL, Rosevear PR, Lorenz JN, Gulick J, Kim S, et al. In vivo and in vitro analysis of cardiac troponin I phosphorylation. J Biol Chem. 2005;280(1):703–714. doi: 10.1074/jbc.M409513200. [DOI] [PubMed] [Google Scholar]
- Satpathy C, Mishra TK, Satpathy R, Satpathy HK, Barone E. Diagnosis and management of diastolic dysfunction and heart failure. Am Fam Phys. 2006;73(5):841–846. [PubMed] [Google Scholar]
- Scherbart AM, Langer J, Bushmelev A, van Berlo D, Haberzettl P, van Schooten FJ, et al. Contrasting macrophage activation by fine and ultrafine titanium dioxide particles is associated with different uptake mechanisms. Part Fibre Toxicol. 2011;8:31. doi: 10.1186/1743-8977-8-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simeonova PP, Erdely A. Engineered nanoparticle respiratory exposure and potential risks for cardiovascular toxicity: predictive tests and biomarkers. Inhal Toxicol. 2009;21(Suppl 1):68–73. doi: 10.1080/08958370902942566. [DOI] [PubMed] [Google Scholar]
- Simkhovich BZ, Kleinman MT, Kloner RA. Air pollution and cardiovascular injury epidemiology, toxicology, and mechanisms. J Am Coll Cardiol. 2008;52(9):719–726. doi: 10.1016/j.jacc.2008.05.029. [DOI] [PubMed] [Google Scholar]
- Spyer KM. Central nervous integration of cardiovascular control. J Exp Biol. 1982;100:109–128. doi: 10.1242/jeb.100.1.109. [DOI] [PubMed] [Google Scholar]
- Stampfl A, Maier M, Radykewicz R, Reitmeir P, Gottlicher M, Niessner R. Langendorff heart: a model system to study cardiovascular effects of engineered nanoparticles. ACS Nano. 2011;5(7):5345–5353. doi: 10.1021/nn200801c. [DOI] [PubMed] [Google Scholar]
- Stansfeld CE, Wallis DI. Properties of visceral primary afferent neurons in the nodose ganglion of the rabbit. J Neurophysiol. 1985;54(2):245–260. doi: 10.1152/jn.1985.54.2.245. [DOI] [PubMed] [Google Scholar]
- Stone KC, Mercer RR, Gehr P, Stockstill B, Crapo JD. Allometric relationships of cell numbers and size in the mammalian lung. Am J Respir Cell Mol Biol. 1992;6(2):235–243. doi: 10.1165/ajrcmb/6.2.235. [DOI] [PubMed] [Google Scholar]
- Timonen KL, Vanninen E, de Hartog J, Ibald-Mulli A, Brunekreef B, Gold DR, et al. Effects of ultrafine and fine particulate and gaseous air pollution on cardiac autonomic control in subjects with coronary artery disease: the ULTRA study. J Expo Sci Environ Epidemiol. 2006;16(4):332–341. doi: 10.1038/sj.jea.7500460. [DOI] [PubMed] [Google Scholar]
- van Dijk SJ, Hamdani N, Stienen GJ, van der Velden J. Myocardial adaptations in the failing heart: cause or consequence? J Muscle Res Cell Motil. 2008;29(6–8):159–162. doi: 10.1007/s10974-009-9169-x. [DOI] [PubMed] [Google Scholar]
- Vennekens R. Emerging concepts for the role of TRP channels in the cardiovascular system. J Physiol. 2011;589(Pt 7):1527–1534. doi: 10.1113/jphysiol.2010.202077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang AL, Blackford TL, Lee LY. Vagal bronchopulmonary C-fibers and acute ventilatory response to inhaled irritants. Respir Physiol. 1996;104(2–3):231–239. doi: 10.1016/0034-5687(96)00014-x. [DOI] [PubMed] [Google Scholar]
- Watanabe H, Murakami M, Ohba T, Takahashi Y, Ito H. TRP channel and cardiovascular disease. Pharmacol Ther. 2008;118(3):337–351. doi: 10.1016/j.pharmthera.2008.03.008. [DOI] [PubMed] [Google Scholar]