Abstract
Left ventricular hypertrophy (LVH) is the most common myocardial structural abnormality associated with heart failure with preserved ejection fraction (HFpEF). LVH is driven by neurohumoral activation, increased mechanical load and cytokines associated with arterial hypertension, chronic kidney disease, diabetes and other co-morbidities. Here we discuss the experimental and clinical evidence that links LVH to diastolic dysfunction and qualifies LVH as one diagnostic marker for HFpEF. Mechanisms leading to diastolic dysfunction in LVH are incompletely understood but may include extracellular matrix changes, vascular dysfunction as well as altered cardiomyocyte mechano-elastical properties. Beating cardiomyocytes from HFpEF patients have not yet been studied, but we and others have shown increased Ca2+ turnover and impaired relaxation in cardiomyocytes from hypertrophied hearts. Structural myocardial remodeling can lead to heterogeneity in regional myocardial contractile function, which contributes to diastolic dysfunction in HFpEF. In the clinical setting of patients with compound co-morbidities, diastolic dysfunction may occur independently of LVH. This may be one explanation why current approaches to reduce LVH have not been effective to improve symptoms and prognosis in HFpEF. Exercise training on the other hand, in clinical trials improved exercise tolerance and diastolic function but did not reduce LVH. Thus, current clinical evidence does not support regression of LVH as a surrogate marker for (short-term) improvement of HFpEF.
Left Ventricular Hypertrophy – Clinical Presentation
Heart failure with preserved ejection fraction (HFpEF) or diastolic heart failure (DHF; as it has been classically referenced) is common, of increasing prevalence, and causes a substantial reduction in prognosis. In the majority of patients with symptomatic HFpEF, a history of hypertensive heart disease including changes in LV geometry such as myocardial hypertrophy can be found. Myocardial hypertrophy is defined as an increase in ventricular myocardial mass. In clinical practice and in animal studies, left ventricular (LV) hypertrophy (LVH) is often assessed by measurement of end-diastolic thickness of septal and LV posterior wall and may be associated with normal or dilated LV cavity. Based on the assessment of the ratio of LV wall thickness and LV internal diameter (relative wall thickness), altered LV geometry in LVH has been classified into three groups: concentric remodeling (enlarged heart with normal relative wall thickness), concentric hypertrophy (increased relative wall thickness, normal internal diameter) and eccentric hypertrophy (increased relative wall thickness, increased internal diameter) (65). In clinical trials, LV mass (LVM) is the most common parameter of LVH and is estimated by algorithms substracting the volume of the LV cavity from the volume enclosed by the epicardium. LVM as assessed by echocardiography is related to body surface area (LVM index, LVMI) and gender (65) and has been shown to correlate well with LV weight at necropsy in mice (51) and men (15).
Causes and Consequences of Left Ventricular Hypertrophy
LVH has long been regarded as a natural response to stabilize LV function in the presence of triggers that increase mechanical (after-)load, such as arterial hypertension or aortic stenosis (31). Indeed, according to Laplace’s Law (LV wall stress = (LV pressure × LV radius) / (2× LV wall thickness)) an increase in LV wall thickness lowers the tension (pressure) acting on the individual myocardial cell. However, the concept of LVH as a compensatory mechanism has been challenged based on clinical observations as well as experimental models. Clinically, the degree of LVH has been associated with worse outcome (34; 56; 67). LVH (by electrocardiography, ECG) has been found to be a predictor of sudden cardiac death and the risk increases with LVH independent of other risk factors (including coronary artery disease and heart failure) (34). In the Framingham Heart Study and other clinical trials, LVH based on ECG or echocardiographic criteria has been suggested as an independent cardiovascular risk factor (34; 67). Furthermore, diuretics, non-nitrate vasodilators (e.g. diltiazem or prazosin) and inotropes that improve symptoms and hemodynamics of hypertensive heart disease but not LVH, are generally not associated with improved prognosis in heart failure (11). Most importantly, in animal models of heart failure, pharmacological and genetic interference with hypertrophic signaling cascades did not promote decompensation but rather were beneficial for LV function and survival (22; 42).
LVH is also observed in athletes as a consequences of repetitive vigorous exercise (or in case of the python snake also by consumption of an extended meal), and during pregnancy (3). However, in the athlete’s heart hypertrophy is not associated with increased fibrosis or apoptosis, results in normal or increased cardiac function and normal survival (12). Experimental data indicates that it is the type of trigger not the duration that initiates signaling for either physiological or maladaptive LVH (92). For instance, chronic exercise training as a physiological stimulus results in an increased level of growth hormone and subsequently IGF-1 which mediates cardiomyocyte growth and survival via the phosphoinositide 3-kinase (PI3K) pathway (59; 97). This type of physiological LVH is not associated with diastolic dysfunction or worse prognosis (8; 69; 119) and not focus of this review.
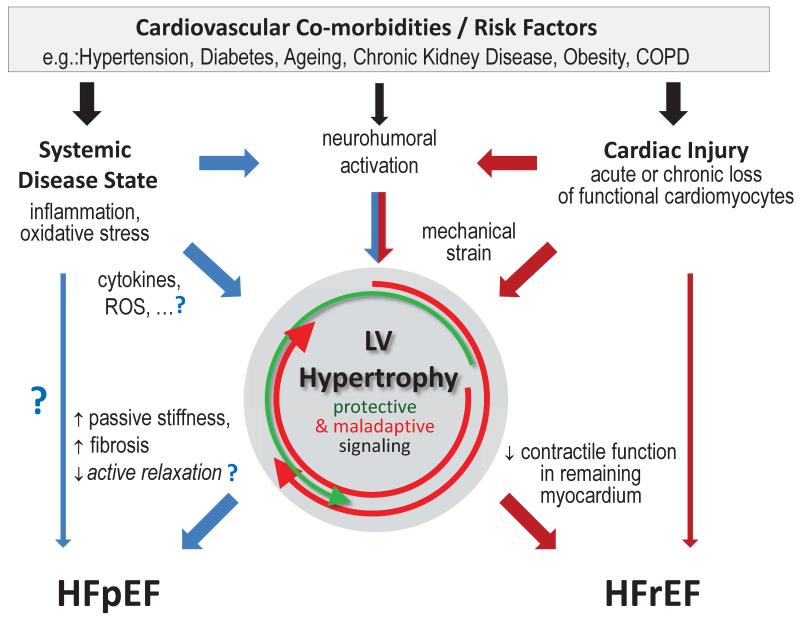
Co-morbidities, such as arterial hypertension, diabetes or chronic kidney disease, which promote LVH (107; 112) are common in heart failure patients with preserved as well as with reduced (HFrEF) ejection fraction. LVH is also often observed in HFpEF (mostly concentric LVH) and HFrEF (often eccentric). However, there is strong and growing cumulative evidence that HFpEF and HFrEF represent different disease entities as reviewed recently (55). Paulus and Tschöpe have recently proposed a new paradigm which suggests fundamental differences in the mechanisms that drive LV remodeling and contractile dysfunction in HFpEF and HFrEF (89). Accordingly, a chronic systemic inflammatory disease state and associated cardiac mesenchymal alterations promote contractile dysfunction in HFpEF, whereas HFrEF is driven by dysfunction intrinsic to the cardiomyocytes. Figure 1 combines these observations and shows the pivotal role of LV hypertrophic remodeling in both disease entities. LVH following loss of cardiomyocytes (e.g. acutely with myocardial infarction or chronically with idiopathic cardiomyopathy) often results in HFrEF (red arrows), which is in line with distinct signaling pathways. Vice versa, concentric LVH as a result of multiple cardiovascular risk factors is a common cause for HFpEF (blue arrows) and in clinical settings (as opposed to many experimental models) only infrequently transitions to HFrEF (14; 70). However, eccentric hypertrophy in HFpEF is also observed and potentially indicates a distinct subgroup of patients that may develop HFrEF (50). Alterations at the cardiomyocyte level during LVH contribute to the heart failure phenotype. Loss of contractile function within the remaining cardiomyocytes during LV remodeling promotes the transition from LVH to HFrEF. On the other hand, in HFpEF, cardiomyocyte and extracellular matrix passive stiffness are increased (Fig. 1, and see section “Cellular Mechanisms”). Triggers of LVH often also activate cardio-protective signaling in cardiomyocytes (e.g. as triggered by natriuretic peptides), however, the maladaptive pathways prevail during the natural course of the disease (see (5) for a more detailed review).
Figure 1.
Role of left ventricular hypertrophy in heart failure. Based on (89), heart failure with preserved (HFpEF) and reduced (HFrEF) ejection fraction are driven by different pathomechanisms (blue and red arrows). While both share some degree of neurohumoral activation (middle), the proposed paradigm suggests systemic low-grade inflammation and oxidative stress are more prominent mediators of HFpEF whereas cardiomyocyte injury is pivotal in HFrEF. Downstream signaling activates some protective (green circular arrow) but overwhelmingly maladaptive (red circular arrows) pathways (5). Left ventricular hypertrophic remodeling is common but not inevitable (thin arrows), however, the cellular phenotype differs in HFpEF vs HFrEF. See text for more details.
Myocardial Dysfunction Associated with Pathological Left Ventricular Hypertrophy
The link between maladaptive LVH and diastolic dysfunction has been established more than 30 years ago (see (72) for review). ECG signs of LVH are a strong predictor of diastolic dysfunction (61). In fact, in HFpEF, LVH is the most frequent structural cardiac abnormality. Arterial hypertension is common as a trigger of LVH and present in the majority of HFpEF patients (Table 1). In HFpEF patients LVH is correlated with hospitalization for heart failure, cardiovascular death or aborted cardiac arrest (37; 106), underscoring the role of LVH as a prognostic marker. However, underlying pathomechanisms that may link LVH to diastolic dysfunction and HFpEF are still not completely understood. Functional effects of LVH have been extensively studied in hypertrophic cardiomyopathy (HCM). In these conditions, global LV systolic function (ejection fraction, LV emptying) is initially augmented indicating a hypercontractile state (13). HFpEF patients reportedly have more pronounced concentric hypertrophy than patients with hypertensive heart disease without HFpEF (80). HFpEF is an exercise-related syndrome, and LVH correlates with an attenuated increase or even decrease in LV ejection fraction during exercise and reduced exercise capacity (81), depending on LVH etiology (104). Patients with a concentric type of LVH performed worst during exercise, attributed to reduced contractile reserve but also to chronotropic incompetence (63). Lam et al. found a significant albeit weak inverse correlation between LVH and exercise capacity (63). Notably, when further adjusting for known confounders such as age, gender, clinical variables, co-morbidities and medication, the association of exercise capacity and LVH was markedly attenuated or no longer detectable (19).
Table 1.
Prevalence of arterial hypertension and left ventricular hypertophy (LVH) in randomized controlled trials on heart failure with preserved ejection fraction.
| Study Acronym | N | Art. Hypertens. | LVH | Reference |
|---|---|---|---|---|
| RELAX | 216 | 85% | 48% | (98) |
| TOPCAT substudy | 935 | 91% | 47% | (106) |
| CHARM-ES | 312 | 64% | 52% | (94) |
| PARAMOUNT | 279 | 94% | n.a.* | (111) |
| I-PRESERVE substudy | 745 | 92% | 29% | (129) |
| Aldo-DHF | 422 | 92% | n.a.* | (20) |
N.a. = not available.
mean left ventricular mass index was 83±25 g/m2 (males; normal reference range 49-115 g/m2 (64)) and 77±20 g/m2 (females; normal reference range 43-95 g/m2) in I-preserve (30) and 109±28 g/m2 in Aldo-DHF (20).
RELAX: PhosphodiesteRasE-5 Inhibition to Improve CLinical Status And. EXercise Capacity in Diastolic Heart Failure; TOPCAT: Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist; CHARM-ES: Candesartan in Heart failure: Assessment of Reduction in Mortality and morbidity-Preserved Echocardiographic Substudy; PARAMOUNT: Prospective comparison of ARNI with ARB on Management Of heart failUre with preserved ejectioN fraction; I-PRESERVE: Irbesartan in Heart Failure with Preserved Ejection Fraction Study; Aldo-DHF: Aldosterone Receptor Blockade in Diastolic Heart Failure
Altered Regional Contractility in Left Ventricular Hypertrophy and HFpEF
Previous studies have suggested that patients with LV hypertrophy and preserved ejection fraction may have subtle systolic dysfunction not reflected by the ejection fraction (7; 95). In recent years, LV deformation during systole has been quantified in multiple planes using speckle tracking echocardiography or MRI tissue tagging. Planes of deformation have been defined related to myocardial fibre orientation, including longitudinal, radial, and circumferential shortening (strain) (108). As reported earlier in this journal (99) and confirmed in other conditions of hypertrophic cardiac remodeling, an increase in radial strain may conceal a loss of contractile function along the longitudinal heart axis (longitudinal strain) and maintaining a preserved global ejection fraction (58; 60). Deterioration of regional strain correlates with regional LVH (127) and parallels increased LVH in rodents (57), in pigs (115), as well as in patients (120)}. Regional contractile dysfunction is potentially related to increased regional fibrosis (120). In the PARAMOUNT trial impaired longitudinal strain in HFpEF patients was not correlated to other markers of diastolic dysfunction, but associated with NTproBNP, which was interpreted as a sign of systolic dysfunction despite preserved EF (60). However, others found a reduction in global longitudinal strain in hypertensive patients to be strongly associated with diastolic dysfunction but not with LVH (28). Notably, a decrease in longitudinal and increase in radial regional strain in response to myocardial stress is a common pattern and has been observed early (i.e. before the onset of global diastolic dysfunction) as recently confirmed in a large animal model (41) and in patients (86). Impaired regional strain may even occur before LVH manifestation (25). The increase in radial strain may dissipate during the progression of LVH (58). In HFpEF LVH is associated with a higher degree of spatial heterogeneity in longitudinal strain at rest (100) and even more pronounced dyssynchrony in regional contraction during exercise (113). In HCM such regional functional heterogeneity was associated with the distribution of myocardial fibrosis (9). Increased fibrosis has recently been related to the deterioration of regional strain also in a large animal model of hypertensive heart disease (115). It has to be kept in mind that LV relaxation is also a function of LV afterload (ventricular-arterial coupling, see (49) for review). For instance, LV relaxation was more prolonged in hypertensive HFpEF patients than non-hypertensive HFpEF patients related to altered LV-arterial coupling (27). In analogy, changes in regional strain in response to exercise may also respond to exercise-induced alterations in afterload which differ depending on LVH etiology (104).
In summary, LVH is associated with global diastolic dysfunction and HFpEF in experimental and clinical studies, which is the basis for the inclusion of LVH as a diagnostic marker in the clinical algorithm to detect HFpEF (90). Structural remodeling induces regions of reduced regional strain during systole that are compensated by areas of increased contractile function and this heterogeneity may contribute to diastolic dysfunction and exercise limitation with and without LVH.
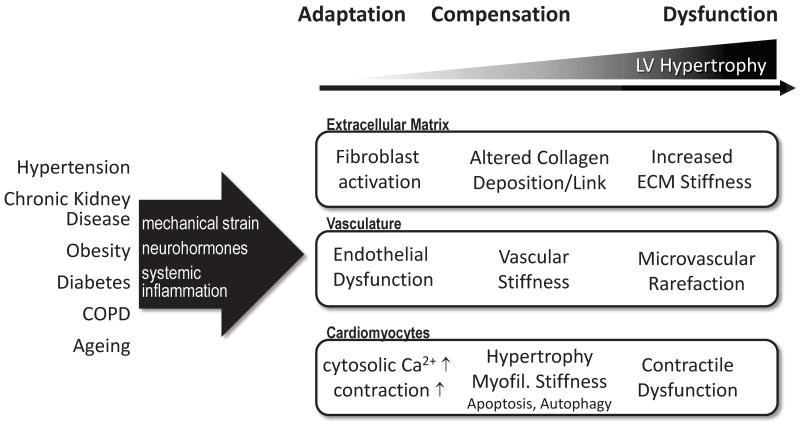
Cellular Mechanisms of Contractile Dysfunction in Left Ventricular Hypertrophy and HFpEF
Altered contractility in LVH at the organ level is related to structural and functional abnormalities involving the extracellular matrix and fibrous tissue, the vasculature as well as the cardiomyocytes themselves.
Figure 2.
Cellular pathomechanisms linking left ventricular hypertrophy to diastolic dysfunction. See text for details.
LVH is often associated with increased fibrosis, mostly reactive interstitial fibrosis, even though replacement fibrosis following cardiomyocyte apoptosis has also been described (29). An increase in total collagen expression and cross-linking was associated with diastolic dysfunction and HFpEF (48). In chronic kidney disease and in diabetic cardiomyopathy LV fibrosis and diastolic dysfunction are not necessarily linked to the presence of LVH (24; 75), but fibrosis may promote the progression of LVH to heart failure (23).
LVH as well as other risk factors such as age, diabetes, obesity and hypertension which are associated with HFpEF have been linked to coronary microvascular rarefaction in animal models and patients (43; 116). Vice versa, reversal of myocardial hypertrophy (121) or vascular endothelial growth factor gene therapy (105) in murine models of HFpEF increased microvascular density along with improvement of diastolic function. In mouse models microvascular rarefaction preceded LVH, suggesting that microvascular dysfunction may also be a cause of diastolic dysfunction independent of LVH (93). On the other hand, in a pig model of HFpEF (aortic banding) capillary density was unchanged in hypertrophied hearts (21). A recent study in human autopsies supported a link between microvascular rarefaction and HFpEF in a cohort with high prevalence (65%) of coronary artery disease (82). In summary, while microvascular dysfunction and vascular remodeling during LVH may promote HFpEF, the role of microvascular rarefaction in human HFpEF with different leading co-morbidities remains to be determined. It has also been postulated that vascular dysfunction, altered extracellular matrix composition and cytokines modulate cardiomyocyte function in HFpEF (89).
Cardiomyocyte contractile function is controlled by Ca2+-dependent myofilament activation and relaxation as well as by passive visco-elastical properties largely determined by the myofilaments (e.g. titin-related stiffness). As in the whole organ, mechanical energy stored in the sarcomeric protein titin during contraction contributes to recoil during relaxation. Vice versa, resting cardiomyocyte tension in diastole is a determinant of contractile force during systole. Thus, the relationship between “systolic” and “diastolic” function at the cellular level is expected to be highly interdependent.
Following seminal studies of Paulus’ group who reported significantly increased resting tension in cardiomyocytes from patients with HFpEF correlating with end-diastolic pressure in vivo (6; 122), increased cardiomyocyte passive stiffness has been confirmed in several animal models with LVH and diastolic dysfunction (36; 45). The large sarcomeric protein titin acts as a molecular spring and is a main determinant of passive stiffness (68). Interestingly, titin-dependent stiffness is increased in patients with arterial hypertension and HFpEF but not in patients with hypertension alone (128), supporting its mechanistic role. As titin-associated proteins also may be involved in mechanosensing and hypertrophic signaling, it is currently unclear whether altered titin function is cause or effect of LV hypertrophic remodeling (62). In addition, hypo-phosphorylation of myofilaments leading to increased Ca2+ sensitivity may also contribute to impaired cardiomyocyte relaxation in HFpEF (35).
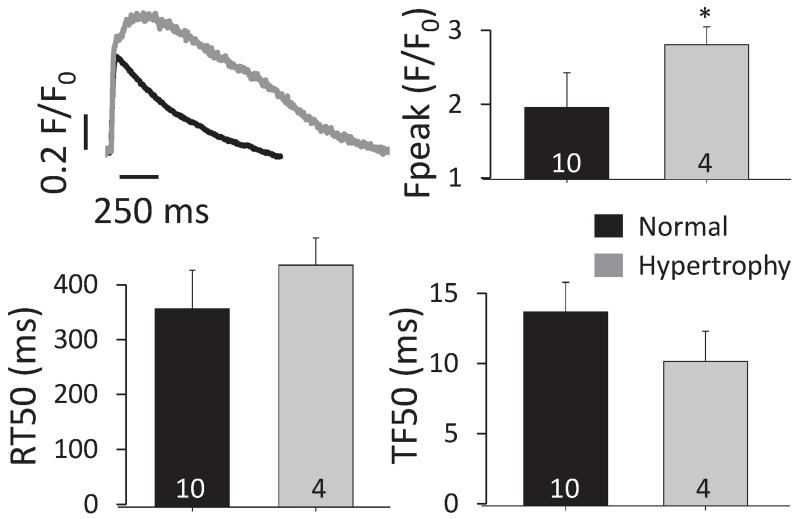
Due to the limited availability of myocardial samples that would allow isolation of functional cardiomyocytes, active cardiomyocyte contraction has not been studied in human HFpEF. In LVH in the absence of ischemia an increase in cardiomyocyte size is achieved by the addition of sarcomeres in parallel in concentric hypertrophy or sequentially (longitudinally) in eccentric hypertrophy (102). Experimental evidence suggests that also at the cellular level hypertrophy is associated with altered contractile function. Studies in several animal models with LVH (e.g. aortic stenosis, hypertension, diabetes or kidney dysfunction) have demonstrated impaired active cardiomyocyte relaxation (26; 73; 78). Cardiomyocytes from animals models with hypertrophied, non-failing hearts show cytosolic Ca2+ transients with normal or increased amplitude, often with slowed Ca2+ decay during diastole and increased diastolic [Ca2+]I indicating increased cytosolic Ca2+ load which may contribute to slowed cardiomyocyte relaxation and promote remodeling (77; 84). However, most small animal models of LVH with signs of diastolic dysfunction and congestion as observed in clinical HFpEF rapidly progress into severe HFrEF. Recently larger animal models mimicking common clinical conditions (e.g. advanced age, hypertension) with preserved EF have been developed and may allow a better understanding of the heterogeneity of regional myocardial contractility and cellular function (35; 41). Yet to date active cellular contractile function in stable HFpEF has not been well studied and a clear correlation between in vitro cardiomyocyte relaxation and diastolic function in vivo is lacking. The few studies on human myocardial biopsies from HFpEF patients did not report on function in intact cardiomyocytes. We have recently compared LV cardiomyocytes from non-failing healthy and from remodeled hypertrophied donor hearts (ejection fraction > 45%, Figure 3, (44; 71)) and found a preserved Ca2+ transient amplitude, with a prolonged cytosolic Ca2+ decay (71), suggesting an early increase in cytosolic Ca2+ load in human LV remodeling. Based on current evidence we propose that in LVH with preserved ejection fraction availability of cytosolic [Ca2+] is not limiting but rather promotes contraction, whereas elevated [Ca2+] may contribute to slowed myofilament relaxation during diastole. Further studies are needed to address the role of cardiomyocyte cytosolic Ca2+ decay in HFpEF.
Figure 3.
Upper left: Example [Ca2+]i transients from healthy (N=3) and remodeled hearts (N=2, ejection fraction ⍰ 45%; 1 with concentric remodeling and 1 with eccentric hypertrophy). Ca2+ transient amplitude (upper right) was significantly increased, changes in time to half maximal release (TF50, lower left) and relaxation (RT50, lower right) did not reach significance (number in bars indicate number of cells, error bars=S.E.M.).
It is fair to say that diastolic dysfunction is also observed in the absence of LVH, as only about half of the patients in clinical HFpEF trials have LVH (Table 1 and Figure 1, see also (91)). In some animal models diastolic dysfunction precedes the development of LVH (17). Risk factors such as insulin resistance (88) and diabetes mellitus (76) may contribute to diastolic dysfunction in the absence of LVH.
Clinical Treatment of Left Ventricular Hypertrophy and its Effects on HFpEF
In a variety of heart failure models (e.g. rodents, rabbit, dogs), interference with LV hypertrophic signaling pathways reliably reduces LVH and improves diastolic function often independent of alterations in blood pressure (22; 38; 110). While these observations support a role for LVH in mediating diastolic dysfunction and as a therapeutic target, many of these models later develop HFrEF which impedes translation of these results to the multifactorial setting of clinical HFpEF.
It has to be kept in mind that diastolic function is a function of afterload thus treatment effects may also at least in part reflect reduced arterial resistance and not improved LV compliance per se (1). A larger number of prospective randomized trials have confirmed regression of LVH with standard antihypertensive therapies, such as angiotensin receptor blockers (ARB), angiotensin converting enzyme inhibitors (ACEI), Ca2+ antagonists or spironolactone (4; 16; 54), but also novel approaches such as renal sympathetic denervation (103). A causal relationship between a reduction in LVH and improved diastolic function, however, is less well established in clinical studies. Early reports suggested that the betablockers teratorol or sotalol improve diastolic function independently from their effects on LVH (46; 118). In the last two decades, smaller uncontrolled studies reported improved diastolic function following LVH reduction induced by current antihypertensive therapy, aortic valve replacement in aortic stenosis or renal sympathetic denervation (47; 101; 103; 117; 124), while others did not (32; 109; 123). Larger randomized controlled trials such as PRESERVE (Prospective Randomized Enalapril Study Evaluating Regression of Ventricular Enlargement; enalapril, (16)) or ASCOT (Anglo-Scandinavian Cardiac Outcomes Trial; amlodipine±perindopril, (2)), however, did not show any association between a reduction in LVH and improvement in diastolic filling, whereas the LIFE study did (Losartan Intervention For Endpoint reduction in Hypertension study; losartan or atenolol, (126)). Mineralocorticoid-receptor antagonists (MRA) such as epleronone and spironolactone, reliably reduce LVH. MRAs were more consistently (e.g. (83; 96)) but also not always (33) associated with improved diastolic function. However, as shown in the Aldo-DHF trial, the decrease of LVMI with aldosterone was not accompanied by an improvement of exercise capacity in patients with mild to moderate HFpEF (20) which is further questioning the “obvious” link between exercise capacity and LVMI. Taken together, inhibition of neurohumoral activation promotes regression of some but not all maladaptive changes leading to contractile dysfunction in LVH.
The multifactorial origin of diastolic dysfunction in clinical settings may also explain the weaker association between LVH regression and improvement of diastolic function. In the RELAX trial the presence of LVH did not affect treatment efficacy with sildenafil (98), indicating that a better understanding of the cellular mechanisms linking LV hypertrophy to HFpEF is warranted to refine therapeutic approaches.
Hypertrophic remodeling is in part counterbalanced by anti-hypertrophic pathways, including cyclic guanosine monophosphate (cGMP) -dependent signaling triggered by nitric oxide or natriuretic peptides (5). HFpEF has been linked to reduced cGMP-mediated signaling, and in experimental conditions, increasing cGMP by inhibition of phosphodiesterase 5 (PDE5, by sildenafil) attenuated LVH and diastolic dysfunction (85). Surprisingly, sildenafil failed to improve diastolic dysfunction or LVH in the RELAX trial (98), questioning PDE5 as a therapeutic target at least in an unselected cohort of HFpEF patients. PDE9 inhibition may be superior to PDE5 by more selectively increasing cGMP related to natriuretic peptide signaling (66). The angiotensin-receptor-neprilysin-inhibitor LCZ696 also reduced LVH and improved diastolic function in experimental conditions (125), a benefit in outcome in HFpEF patients is being evaluated in the ongoing larger PARAGON-HF trial.
Exercise Effects on Left Ventricular Hypertrophy and HFpEF
Disturbed diastolic function and increased vascular stiffness are major contributors to exercise limitation in patients with HFpEF (10; 39; 53). The subsequent rise in LV filling pressure at rest and/or during exercise has been suggested to be directly related to the severity of HF symptoms in HFpEF patients.
Several single center trials addressed the role of exercise training on exercise capacity and cardiac function in patients with HFpEF. Although they demonstrated a significant improvement of exercise capacity and quality of life, they failed to demonstrate an improvement of cardiac systolic or diastolic function or of LVH (40; 52; 114). Similar findings were made under more controlled conditions in a translational large animal model of HFpEF (74). In a prospective clinical approach, the multicenter Exercise Training in Diastolic Heart Failure Pilot study (Ex-DHF-P) randomized patients with New York Heart Association (NYHA) class II-III, left ventricular ejection fraction (LVEF)≥50%,, echocardiographic evidence of diastolic dysfunction, sinus rhythm, and ≥1 additional cardiovascular risk factor to 32 sessions of supervised, combined endurance/resistance exercise training (n=44) or to usual care (n=20) (18). Peak oxygen consumption (VO2) after 3 months (primary endpoint) significantly improved with training, resulting in a between group difference of 3.3mL/kg/min (P<0.001). Also the resting left ventricular filling index (E/e’), the left atrial volume index and different QoL dimensions were improved after follow-up (87). Again, as also reported in previous trials, LVH did not change after training.
Several reasons might contribute to the actual lack of evidence regarding the link between improved exercise capacity, improved cardiac diastolic function and the regression of LVH. In all available studies, the intervention period was limited (12, 16, or 24 weeks). Furthermore, patients were not classified using a comparable diagnostic algorithm as now recommended for the diagnosis of HFpEF (79; 90). Last, the induction of physiological adaption of the myocardium induced by exercise training might cover the beneficial effects of exercise training on detrimental cardiac remodeling processes in this HF population with preserved LVEF. Future studies are therefore urgently needed to further elaborate the effects of exercise training on cardiac structure and function. The ongoing Exercise Training in Diastolic Heart Failure (Ex-DHF) study will randomize n=320 patients (1:1 ratio) to exercise training or usual care and will have an individual 12 months follow-up (ISRCTN 86879094, www.controlled-trials.com). Since LVH is part of the specific inclusion criteria used in Ex-DHF, this study might help to better understand the effects of exercise training on LVH in this condition.
Summary and Conclusion
Experimental and clinical studies indicate that maladaptive LVH, i.e. in the presence of pathological stimuli, can per se induce diastolic dysfunction and thus contribute to the HFpEF phenotype. Mechanisms are diverse and probably etiology-specific and include vascular dysfunction and potentially vascular rarefaction, changes in the extracellular matrix composition including increased fibrosis, and alterations of the intrinsic active and passive contractile properties of the cardiac myocyte. In the multifactorial clinical setting of HFpEF, diastolic dysfunction and HFpEF are also observed in the absence and independently of LVH in a considerable number of patients. A reduction of LVH is not necessarily associated with an improvement of diastolic function. Thus, current clinical evidence does not support regression of LVH as a surrogate marker for short-term improvement of HFpEF.
Acknowledgements
FRH is supported by a research grant of the Fondazione Internazionale Menarini. SS is supported by the Austrian Science Fund (FWF, P27637-B28). Dr. Felix Hohendanner is participant in the Charité Clinical Scientist Program funded by the Charité – Universitätsmedizin Berlin and the Berlin Institute of Health.
References
- 1.Balogun MO, Dunn FG. Systolic and diastolic function following regression of left ventricular hypertrophy in hypertension. J Hypertens Suppl. 1991;9:S51–S55. doi: 10.1097/00004872-199112002-00007. [DOI] [PubMed] [Google Scholar]
- 2.Barron AJ, Hughes AD, Sharp A, Baksi AJ, Surendran P, Jabbour RJ, Stanton A, Poulter N, Fitzgerald D, Sever P, O’Brien E, Thom S, Mayet J. Long-term antihypertensive treatment fails to improve E/e’ despite regression of left ventricular mass: an Anglo-Scandinavian cardiac outcomes trial substudy. Hypertension. 2014;63:252–258. doi: 10.1161/HYPERTENSIONAHA.113.01360. [DOI] [PubMed] [Google Scholar]
- 3.Bernardo BC, Weeks KL, Pretorius L, McMullen JR. Molecular distinction between physiological and pathological cardiac hypertrophy: experimental findings and therapeutic strategies. Pharmacol Ther. 2010;128:191–227. doi: 10.1016/j.pharmthera.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 4.Betocchi S, Chiariello M. Effects of antihypertensive therapy on diastolic dysfunction in left ventricular hypertrophy. J Cardiovasc Pharmacol. 1992;19(Suppl 5):S116–S121. [PubMed] [Google Scholar]
- 5.Bisping E, Wakula P, Poteser M, Heinzel FR. Targeting cardiac hypertrophy: toward a causal heart failure therapy. J Cardiovasc Pharmacol. 2014;64:293–305. doi: 10.1097/FJC.0000000000000126. [DOI] [PubMed] [Google Scholar]
- 6.Borbely A, van d V, Papp Z, Bronzwaer JG, Edes I, Stienen GJ, Paulus WJ. Cardiomyocyte stiffness in diastolic heart failure. Circulation. 2005;111:774–781. doi: 10.1161/01.CIR.0000155257.33485.6D. [DOI] [PubMed] [Google Scholar]
- 7.Borlaug BA, Lam CS, Roger VL, Rodeheffer RJ, Redfield MM. Contractility and ventricular systolic stiffening in hypertensive heart disease insights into the pathogenesis of heart failure with preserved ejection fraction. J Am Coll Cardiol. 2009;54:410–418. doi: 10.1016/j.jacc.2009.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caselli S, Di Paolo FM, Pisicchio C, Pandian NG, Pelliccia A. Patterns of left ventricular diastolic function in Olympic athletes. J Am Soc Echocardiogr. 2015;28:236–244. doi: 10.1016/j.echo.2014.09.013. [DOI] [PubMed] [Google Scholar]
- 9.Chang SA, Lee SC, Choe YH, Hahn HJ, Jang SY, Park SJ, Choi JO, Park SW, Oh JK. Effects of hypertrophy and fibrosis on regional and global functional heterogeneity in hypertrophic cardiomyopathy. Int J Cardiovasc Imaging. 2012;28(Suppl 2):133–140. doi: 10.1007/s10554-012-0141-2. [DOI] [PubMed] [Google Scholar]
- 10.Clark AL, Poole-Wilson PA, Coats AJ. Exercise limitation in chronic heart failure: central role of the periphery. J Am Coll Cardiol. 1996;28:1092–1102. doi: 10.1016/S0735-1097(96)00323-3. [DOI] [PubMed] [Google Scholar]
- 11.Cohn JN, Ferrari R, Sharpe N, Behalf of an International Forum on Cardiac Remodeling Cardiac remodeling--concepts and clinical implications: a consensus paper from an international forum on cardiac remodeling. J Am Coll Cardiol. 2000;35:569–582. doi: 10.1016/s0735-1097(99)00630-0. [DOI] [PubMed] [Google Scholar]
- 12.Colan SD, Sanders SP, MacPherson D, Borow KM. Left ventricular diastolic function in elite athletes with physiologic cardiac hypertrophy. J Am Coll Cardiol. 1985;6:545–549. doi: 10.1016/s0735-1097(85)80111-x. [DOI] [PubMed] [Google Scholar]
- 13.Come PC, Bulkley BH, Goodman ZD, Hutchins GM, Pitt B, Fortuin NJ. Hypercontractile cardiac states simulating hypertrophic cardiomyopathy. Circulation. 1977;55:901–908. doi: 10.1161/01.cir.55.6.901. [DOI] [PubMed] [Google Scholar]
- 14.Desai RV, Ahmed MI, Mujib M, Aban IB, Zile MR, Ahmed A. Natural history of concentric left ventricular geometry in community-dwelling older adults without heart failure during seven years of follow-up. Am J Cardiol. 2011;107:321–324. doi: 10.1016/j.amjcard.2010.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Devereux RB, Alonso DR, Lutas EM, Gottlieb GJ, Campo E, Sachs I, Reichek N. Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol. 1986;57:450–458. doi: 10.1016/0002-9149(86)90771-x. [DOI] [PubMed] [Google Scholar]
- 16.Devereux RB, Palmieri V, Sharpe N, De Q V, Bella JN, de SG, Walker JF, Hahn RT, Dahlof B. Effects of once-daily angiotensin-converting enzyme inhibition and calcium channel blockade-based antihypertensive treatment regimens on left ventricular hypertrophy and diastolic filling in hypertension: the prospective randomized enalapril study evaluating regression of ventricular enlargement (preserve) trial. Circulation. 2001;104:1248–1254. doi: 10.1161/hc3601.095927. [DOI] [PubMed] [Google Scholar]
- 17.Dupont S, Maizel J, Mentaverri R, Chillon JM, Six I, Giummelly P, Brazier M, Choukroun G, Tribouilloy C, Massy ZA, Slama M. The onset of left ventricular diastolic dysfunction in SHR rats is not related to hypertrophy or hypertension. Am J Physiol Heart Circ Physiol. 2012;302:H1524–H1532. doi: 10.1152/ajpheart.00955.2010. [DOI] [PubMed] [Google Scholar]
- 18.Edelmann F, Gelbrich G, Dungen HD, Frohling S, Wachter R, Stahrenberg R, Binder L, Topper A, Lashki DJ, Schwarz S, Herrmann-Lingen C, Loffler M, Hasenfuss G, Halle M, Pieske B. Exercise training improves exercise capacity and diastolic function in patients with heart failure with preserved ejection fraction: results of the Ex-DHF (Exercise training in Diastolic Heart Failure) pilot study. J Am Coll Cardiol. 2011;58:1780–1791. doi: 10.1016/j.jacc.2011.06.054. [DOI] [PubMed] [Google Scholar]
- 19.Edelmann F, Gelbrich G, Duvinage A, Stahrenberg R, Behrens A, Prettin C, Kraigher-Krainer E, Schmidt AG, Dungen HD, Kamke W, Tschope C, Herrmann-Lingen C, Halle M, Hasenfuss G, Wachter R, Pieske B. Differential interaction of clinical characteristics with key functional parameters in heart failure with preserved ejection fraction--results of the Aldo-DHF trial. Int J Cardiol. 2013;169:408–417. doi: 10.1016/j.ijcard.2013.10.018. [DOI] [PubMed] [Google Scholar]
- 20.Edelmann F, Wachter R, Schmidt AG, Kraigher-Krainer E, Colantonio C, Kamke W, Duvinage A, Stahrenberg R, Durstewitz K, Loffler M, Dungen HD, Tschope C, Herrmann-Lingen C, Halle M, Hasenfuss G, Gelbrich G, Pieske B. Effect of spironolactone on diastolic function and exercise capacity in patients with heart failure with preserved ejection fraction: the Aldo-DHF randomized controlled trial. JAMA. 2013;309:781–791. doi: 10.1001/jama.2013.905. [DOI] [PubMed] [Google Scholar]
- 21.Emter CA, Tharp DL, Ivey JR, Ganjam VK, Bowles DK. Low-intensity interval exercise training attenuates coronary vascular dysfunction and preserves Ca(2)(+)-sensitive K(+) current in miniature swine with LV hypertrophy. Am J Physiol Heart Circ Physiol. 2011;301:H1687–H1694. doi: 10.1152/ajpheart.00610.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Esposito G, Rapacciuolo A, Naga Prasad SV, Takaoka H, Thomas SA, Koch WJ, Rockman HA. Genetic alterations that inhibit in vivo pressure-overload hypertrophy prevent cardiac dysfunction despite increased wall stress. Circulation. 2002;105:85–92. doi: 10.1161/hc0102.101365. [DOI] [PubMed] [Google Scholar]
- 23.Falcao-Pires I, Hamdani N, Borbely A, Gavina C, Schalkwijk CG, van d V, van HL, Stienen GJ, Niessen HW, Leite-Moreira AF, Paulus WJ. Diabetes mellitus worsens diastolic left ventricular dysfunction in aortic stenosis through altered myocardial structure and cardiomyocyte stiffness. Circulation. 2011;124:1151–1159. doi: 10.1161/CIRCULATIONAHA.111.025270. [DOI] [PubMed] [Google Scholar]
- 24.Falcao-Pires I, Leite-Moreira AF. Diabetic cardiomyopathy: understanding the molecular and cellular basis to progress in diagnosis and treatment. Heart Fail Rev. 2012;17:325–344. doi: 10.1007/s10741-011-9257-z. [DOI] [PubMed] [Google Scholar]
- 25.Fang ZY, Leano R, Marwick TH. Relationship between longitudinal and radial contractility in subclinical diabetic heart disease. Clin Sci (Lond) 2004;106:53–60. doi: 10.1042/CS20030153. [DOI] [PubMed] [Google Scholar]
- 26.Flagg TP, Cazorla O, Remedi MS, Haim TE, Tones MA, Bahinski A, Numann RE, Kovacs A, Schaffer JE, Nichols CG, Nerbonne JM. Ca2+-independent alterations in diastolic sarcomere length and relaxation kinetics in a mouse model of lipotoxic diabetic cardiomyopathy. Circ Res. 2009;104:95–103. doi: 10.1161/CIRCRESAHA.108.186809. [DOI] [PubMed] [Google Scholar]
- 27.Fujimoto N, Onishi K, Dohi K, Tanabe M, Kurita T, Takamura T, Yamada N, Nobori T, Ito M. Hemodynamic characteristics of patients with diastolic heart failure and hypertension. Hypertens Res. 2008;31:1727–1735. doi: 10.1291/hypres.31.1727. [DOI] [PubMed] [Google Scholar]
- 28.Galderisi M, Lomoriello VS, Santoro A, Esposito R, Olibet M, Raia R, Di Minno MN, Guerra G, Mele D, Lombardi G. Differences of myocardial systolic deformation and correlates of diastolic function in competitive rowers and young hypertensives: a speckle-tracking echocardiography study. J Am Soc Echocardiogr. 2010;23:1190–1198. doi: 10.1016/j.echo.2010.07.010. [DOI] [PubMed] [Google Scholar]
- 29.Galiuto L, Lotrionte M, Crea F, Anselmi A, Biondi-Zoccai GG, De GF, Baldi A, Baldi F, Possati G, Gaudino M, Vetrovec GW, Abbate A. Impaired coronary and myocardial flow in severe aortic stenosis is associated with increased apoptosis: a transthoracic Doppler and myocardial contrast echocardiography study. Heart. 2006;92:208–212. doi: 10.1136/hrt.2005.062422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gori M, Lam CS, Gupta DK, Santos AB, Cheng S, Shah AM, Claggett B, Zile MR, Kraigher-Krainer E, Pieske B, Voors AA, Packer M, Bransford T, Lefkowitz M, McMurray JJ, Solomon SD. Sex-specific cardiovascular structure and function in heart failure with preserved ejection fraction. Eur J Heart Fail. 2014;16:535–542. doi: 10.1002/ejhf.67. [DOI] [PubMed] [Google Scholar]
- 31.Grossman W, Jones D, McLaurin LP. Wall stress and patterns of hypertrophy in the human left ventricle. J Clin Invest. 1975;56:56–64. doi: 10.1172/JCI108079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gupta A, Schiros CG, Gaddam KK, Aban I, Denney TS, Lloyd SG, Oparil S, Dell’Italia LJ, Calhoun DA, Gupta H. Effect of spironolactone on diastolic function in hypertensive left ventricular hypertrophy. J Hum Hypertens. 2015;29:241–246. doi: 10.1038/jhh.2014.83. [DOI] [PubMed] [Google Scholar]
- 33.Gupta A, Schiros CG, Gaddam KK, Aban I, Denney TS, Lloyd SG, Oparil S, Dell’Italia LJ, Calhoun DA, Gupta H. Effect of spironolactone on diastolic function in hypertensive left ventricular hypertrophy. J Hum Hypertens. 2015;29:241–246. doi: 10.1038/jhh.2014.83. [DOI] [PubMed] [Google Scholar]
- 34.Haider AW, Larson MG, Benjamin EJ, Levy D. Increased left ventricular mass and hypertrophy are associated with increased risk for sudden death. J Am Coll Cardiol. 1998;32:1454–1459. doi: 10.1016/s0735-1097(98)00407-0. [DOI] [PubMed] [Google Scholar]
- 35.Hamdani N, Bishu KG, von Frieling-Salewsky M, Redfield MM, Linke WA. Deranged myofilament phosphorylation and function in experimental heart failure with preserved ejection fraction. Cardiovasc Res. 2013;97:464–471. doi: 10.1093/cvr/cvs353. [DOI] [PubMed] [Google Scholar]
- 36.Hamdani N, Franssen C, Lourenco A, Falcao-Pires I, Fontoura D, Leite S, Plettig L, Lopez B, Ottenheijm CA, Becher PM, Gonzalez A, Tschope C, Diez J, Linke WA, Leite-Moreira AF, Paulus WJ. Myocardial titin hypophosphorylation importantly contributes to heart failure with preserved ejection fraction in a rat metabolic risk model. Circ Heart Fail. 2013;6:1239–1249. doi: 10.1161/CIRCHEARTFAILURE.113.000539. [DOI] [PubMed] [Google Scholar]
- 37.Hawkins NM, Wang D, McMurray JJ, Pfeffer MA, Swedberg K, Granger CB, Yusuf S, Pocock SJ, Ostergren J, Michelson EL, Dunn FG. Prevalence and prognostic implications of electrocardiographic left ventricular hypertrophy in heart failure: evidence from the CHARM programme. Heart. 2007;93:59–64. doi: 10.1136/hrt.2005.083949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hayashida W, Donckier J, Van MH, Charlier AA, Pouleur H. Diastolic properties in canine hypertensive left ventricular hypertrophy: effects of angiotensin converting enzyme inhibition and angiotensin II type-1 receptor blockade. Cardiovasc Res. 1997;33:54–62. doi: 10.1016/s0008-6363(96)00194-0. [DOI] [PubMed] [Google Scholar]
- 39.Haykowsky MJ, Brubaker PH, John JM, Stewart KP, Morgan TM, Kitzman DW. Determinants of exercise intolerance in elderly heart failure patients with preserved ejection fraction. J Am Coll Cardiol. 2011;58:265–274. doi: 10.1016/j.jacc.2011.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Haykowsky MJ, Brubaker PH, Stewart KP, Morgan TM, Eggebeen J, Kitzman DW. Effect of endurance training on the determinants of peak exercise oxygen consumption in elderly patients with stable compensated heart failure and preserved ejection fraction. J Am Coll Cardiol. 2012;60:120–128. doi: 10.1016/j.jacc.2012.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hiemstra JA, Liu S, Ahlman MA, Schuleri KH, Lardo AC, Baines CP, Dellsperger KC, Bluemke DA, Emter CA. A new twist on an old idea: a two-dimensional speckle tracking assessment of cyclosporine as a therapeutic alternative for heart failure with preserved ejection fraction. Physiol Rep. 2013;1:e00174. doi: 10.1002/phy2.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hill JA, Karimi M, Kutschke W, Davisson RL, Zimmerman K, Wang Z, Kerber RE, Weiss RM. Cardiac hypertrophy is not a required compensatory response to short-term pressure overload. Circulation. 2000;101:2863–2869. doi: 10.1161/01.cir.101.24.2863. [DOI] [PubMed] [Google Scholar]
- 43.Hoenig MR, Bianchi C, Rosenzweig A, Sellke FW. The cardiac microvasculature in hypertension, cardiac hypertrophy and diastolic heart failure. Curr Vasc Pharmacol. 2008;6:292–300. doi: 10.2174/157016108785909779. [DOI] [PubMed] [Google Scholar]
- 44.Hohendanner F, Ljubojevic S, Macquaide N, Sacherer M, Sedej S, Biesmans L, Wakula P, Platzer D, Sokolow S, Herchuelz A, Antoons G, Sipido K, Pieske B, Heinzel FR. Intracellular dyssynchrony of diastolic cytosolic [Ca(2)(+)] decay in ventricular cardiomyocytes in cardiac remodeling and human heart failure. Circ Res. 2013;113:527–538. doi: 10.1161/CIRCRESAHA.113.300895. [DOI] [PubMed] [Google Scholar]
- 45.Hudson B, Hidalgo C, Saripalli C, Granzier H. Hyperphosphorylation of mouse cardiac titin contributes to transverse aortic constriction-induced diastolic dysfunction. Circ Res. 2011;109:858–866. doi: 10.1161/CIRCRESAHA.111.246819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ibrahim MM, Zaghloul SS, Helmi SM. Effect of regression of left ventricular hypertrophy following sotalol therapy on diastolic function in hypertensive patients. J Hypertens Suppl. 1987;5:S411–S414. [PubMed] [Google Scholar]
- 47.Ikonomidis I, Tsoukas A, Parthenakis F, Gournizakis A, Kassimatis A, Rallidis L, Nihoyannopoulos P. Four year follow up of aortic valve replacement for isolated aortic stenosis: a link between reduction in pressure overload, regression of left ventricular hypertrophy, and diastolic function. Heart. 2001;86:309–316. doi: 10.1136/heart.86.3.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kasner M, Westermann D, Lopez B, Gaub R, Escher F, Kuhl U, Schultheiss HP, Tschope C. Diastolic tissue Doppler indexes correlate with the degree of collagen expression and cross-linking in heart failure and normal ejection fraction. J Am Coll Cardiol. 2011;57:977–985. doi: 10.1016/j.jacc.2010.10.024. [DOI] [PubMed] [Google Scholar]
- 49.Kass DA. Ventricular arterial stiffening: integrating the pathophysiology. Hypertension. 2005;46:185–193. doi: 10.1161/01.HYP.0000168053.34306.d4. [DOI] [PubMed] [Google Scholar]
- 50.Katz DH, Beussink L, Sauer AJ, Freed BH, Burke MA, Shah SJ. Prevalence, clinical characteristics, and outcomes associated with eccentric versus concentric left ventricular hypertrophy in heart failure with preserved ejection fraction. Am J Cardiol. 2013;112:1158–1164. doi: 10.1016/j.amjcard.2013.05.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kiatchoosakun S, Restivo J, Kirkpatrick D, Hoit BD. Assessment of left ventricular mass in mice: comparison between two-dimensional and m-mode echocardiography. Echocardiography. 2002;19:199–205. doi: 10.1046/j.1540-8175.2002.00199.x. [DOI] [PubMed] [Google Scholar]
- 52.Kitzman DW, Brubaker PH, Herrington DM, Morgan TM, Stewart KP, Hundley WG, Abdelhamed A, Haykowsky MJ. Effect of endurance exercise training on endothelial function and arterial stiffness in older patients with heart failure and preserved ejection fraction: a randomized, controlled, single-blind trial. J Am Coll Cardiol. 2013;62:584–592. doi: 10.1016/j.jacc.2013.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kitzman DW, Little WC, Brubaker PH, Anderson RT, Hundley WG, Marburger CT, Brosnihan B, Morgan TM, Stewart KP. Pathophysiological characterization of isolated diastolic heart failure in comparison to systolic heart failure. JAMA. 2002;288:2144–2150. doi: 10.1001/jama.288.17.2144. [DOI] [PubMed] [Google Scholar]
- 54.Klingbeil AU, Schneider M, Martus P, Messerli FH, Schmieder RE. A meta-analysis of the effects of treatment on left ventricular mass in essential hypertension. Am J Med. 2003;115:41–46. doi: 10.1016/s0002-9343(03)00158-x. [DOI] [PubMed] [Google Scholar]
- 55.Komajda M, Lam CS. Heart failure with preserved ejection fraction: a clinical dilemma. Eur Heart J. 2014;35:1022–1032. doi: 10.1093/eurheartj/ehu067. [DOI] [PubMed] [Google Scholar]
- 56.Koren MJ, Devereux RB, Casale PN, Savage DD, Laragh JH. Relation of left ventricular mass and geometry to morbidity and mortality in uncomplicated essential hypertension. Ann Intern Med. 1991;114:345–352. doi: 10.7326/0003-4819-114-5-345. [DOI] [PubMed] [Google Scholar]
- 57.Koshizuka R, Ishizu T, Kameda Y, Kawamura R, Seo Y, Aonuma K. Longitudinal strain impairment as a marker of the progression of heart failure with preserved ejection fraction in a rat model. J Am Soc Echocardiogr. 2013;26:316–323. doi: 10.1016/j.echo.2012.11.015. [DOI] [PubMed] [Google Scholar]
- 58.Kouzu H, Yuda S, Muranaka A, Doi T, Yamamoto H, Shimoshige S, Hase M, Hashimoto A, Saitoh S, Tsuchihashi K, Miura T, Watanabe N, Shimamoto K. Left ventricular hypertrophy causes different changes in longitudinal, radial, and circumferential mechanics in patients with hypertension: a two-dimensional speckle tracking study. J Am Soc Echocardiogr. 2011;24:192–199. doi: 10.1016/j.echo.2010.10.020. [DOI] [PubMed] [Google Scholar]
- 59.Koziris LP, Hickson RC, Chatterton RT, Jr., Groseth RT, Christie JM, Goldflies DG, Unterman TG. Serum levels of total and free IGF-I and IGFBP-3 are increased and maintained in long-term training. J Appl Physiol (1985) 1999;86:1436–1442. doi: 10.1152/jappl.1999.86.4.1436. [DOI] [PubMed] [Google Scholar]
- 60.Kraigher-Krainer E, Shah AM, Gupta DK, Santos A, Claggett B, Pieske B, Zile MR, Voors AA, Lefkowitz MP, Packer M, McMurray JJ, Solomon SD. Impaired systolic function by strain imaging in heart failure with preserved ejection fraction. J Am Coll Cardiol. 2014;63:447–456. doi: 10.1016/j.jacc.2013.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Krepp JM, Lin F, Min JK, Devereux RB, Okin PM. Relationship of electrocardiographic left ventricular hypertrophy to the presence of diastolic dysfunction. Ann Noninvasive Electrocardiol. 2014;19:552–560. doi: 10.1111/anec.12166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kruger M, Linke WA. The giant protein titin: a regulatory node that integrates myocyte signaling pathways. J Biol Chem. 2011;286:9905–9912. doi: 10.1074/jbc.R110.173260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lam CS, Grewal J, Borlaug BA, Ommen SR, Kane GC, McCully RB, Pellikka PA. Size, shape, and stamina: the impact of left ventricular geometry on exercise capacity. Hypertension. 2010;55:1143–1149. doi: 10.1161/HYPERTENSIONAHA.109.146845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise J, Solomon S, Spencer KT, St John SM, Stewart W. Recommendations for chamber quantification. Eur J Echocardiogr. 2006;7:79–108. doi: 10.1016/j.euje.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 65.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, Sutton MS, Stewart WJ. Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 66.Lee DI, Zhu G, Sasaki T, Cho GS, Hamdani N, Holewinski R, Jo SH, Danner T, Zhang M, Rainer PP, Bedja D, Kirk JA, Ranek MJ, Dostmann WR, Kwon C, Margulies KB, van Eyk JE, Paulus WJ, Takimoto E, Kass DA. Phosphodiesterase 9A controls nitric-oxide-independent cGMP and hypertrophic heart disease. Nature. 2015;519:472–476. doi: 10.1038/nature14332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Levy D. Clinical significance of left ventricular hypertrophy: insights from the Framingham Study. J Cardiovasc Pharmacol. 1991;17(Suppl 2):S1–S6. doi: 10.1097/00005344-199117002-00002. [DOI] [PubMed] [Google Scholar]
- 68.LeWinter MM, Granzier H. Cardiac titin: a multifunctional giant. Circulation. 2010;121:2137–2145. doi: 10.1161/CIRCULATIONAHA.109.860171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lewis JF, Spirito P, Pelliccia A, Maron BJ. Usefulness of Doppler echocardiographic assessment of diastolic filling in distinguishing “athlete’s heart” from hypertrophic cardiomyopathy. Br Heart J. 1992;68:296–300. doi: 10.1136/hrt.68.9.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lieb W, Gona P, Larson MG, Aragam J, Zile MR, Cheng S, Benjamin EJ, Vasan RS. The natural history of left ventricular geometry in the community: clinical correlates and prognostic significance of change in LV geometric pattern. JACC Cardiovasc Imaging. 2014;7:870–878. doi: 10.1016/j.jcmg.2014.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ljubojevic S, Radulovic S, Leitinger G, Sedej S, Sacherer M, Holzer M, Winkler C, Pritz E, Mittler T, Schmidt A, Sereinigg M, Wakula P, Zissimopoulos S, Bisping E, Post H, Marsche G, Bossuyt J, Bers DM, Kockskamper J, Pieske B. Early remodeling of perinuclear Ca2+ stores and nucleoplasmic Ca2+ signaling during the development of hypertrophy and heart failure. Circulation. 2014;130:244–255. doi: 10.1161/CIRCULATIONAHA.114.008927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lorell BH, Apstein CS, Weinberg EO, Cunningham MJ. Diastolic function in left ventricular hypertrophy: clinical and experimental relationships. Eur Heart J. 1990;11(Suppl G):54–64. doi: 10.1093/eurheartj/11.suppl_g.54. [DOI] [PubMed] [Google Scholar]
- 73.Lovelock JD, Monasky MM, Jeong EM, Lardin HA, Liu H, Patel BG, Taglieri DM, Gu L, Kumar P, Pokhrel N, Zeng D, Belardinelli L, Sorescu D, Solaro RJ, Dudley SC., Jr. Ranolazine improves cardiac diastolic dysfunction through modulation of myofilament calcium sensitivity. Circ Res. 2012;110:841–850. doi: 10.1161/CIRCRESAHA.111.258251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Marshall KD, Muller BN, Krenz M, Hanft LM, McDonald KS, Dellsperger KC, Emter CA. Heart failure with preserved ejection fraction: chronic low-intensity interval exercise training preserves myocardial O2 balance and diastolic function. J Appl Physiol (1985) 2013;114:131–147. doi: 10.1152/japplphysiol.01059.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Martin FL, McKie PM, Cataliotti A, Sangaralingham SJ, Korinek J, Huntley BK, Oehler EA, Harders GE, Ichiki T, Mangiafico S, Nath KA, Redfield MM, Chen HH, Burnett JC., Jr. Experimental mild renal insufficiency mediates early cardiac apoptosis, fibrosis, and diastolic dysfunction: a kidney-heart connection. Am J Physiol Regul Integr Comp Physiol. 2012;302:R292–R299. doi: 10.1152/ajpregu.00194.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Masugata H, Senda S, Goda F, Yoshihara Y, Yoshikawa K, Fujita N, Daikuhara H, Okuyama H, Taoka T, Kohno M. Left ventricular diastolic dysfunction in normotensive diabetic patients in various age strata. Diabetes Res Clin Pract. 2008;79:91–96. doi: 10.1016/j.diabres.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 77.McCrossan ZA, Billeter R, White E. Transmural changes in size, contractile and electrical properties of SHR left ventricular myocytes during compensated hypertrophy. Cardiovasc Res. 2004;63:283–292. doi: 10.1016/j.cardiores.2004.04.013. [DOI] [PubMed] [Google Scholar]
- 78.McMahon AC, Naqvi RU, Hurst MJ, Raine AE, MacLeod KT. Diastolic dysfunction and abnormality of the Na+/Ca2+ exchanger in single uremic cardiac myocytes. Kidney Int. 2006;69:846–851. doi: 10.1038/sj.ki.5000193. [DOI] [PubMed] [Google Scholar]
- 79.McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Bohm M, Dickstein K, Falk V, Filippatos G, Fonseca C, Gomez-Sanchez MA, Jaarsma T, Kober L, Lip GY, Maggioni AP, Parkhomenko A, Pieske BM, Popescu BA, Ronnevik PK, Rutten FH, Schwitter J, Seferovic P, Stepinska J, Trindade PT, Voors AA, Zannad F, Zeiher A, Bax JJ, Baumgartner H, Ceconi C, Dean V, Deaton C, Fagard R, Funck-Brentano C, Hasdai D, Hoes A, Kirchhof P, Knuuti J, Kolh P, McDonagh T, Moulin C, Popescu BA, Reiner Z, Sechtem U, Sirnes PA, Tendera M, Torbicki A, Vahanian A, Windecker S, McDonagh T, Sechtem U, Bonet LA, Avraamides P, Ben Lamin HA, Brignole M, Coca A, Cowburn P, Dargie H, Elliott P, Flachskampf FA, Guida GF, Hardman S, Iung B, Merkely B, Mueller C, Nanas JN, Nielsen OW, Orn S, Parissis JT, Ponikowski P. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2012;14:803–869. doi: 10.1093/eurjhf/hfs105. [DOI] [PubMed] [Google Scholar]
- 80.Melenovsky V, Borlaug BA, Rosen B, Hay I, Ferruci L, Morell CH, Lakatta EG, Najjar SS, Kass DA. Cardiovascular features of heart failure with preserved ejection fraction versus nonfailing hypertensive left ventricular hypertrophy in the urban Baltimore community: the role of atrial remodeling/dysfunction. J Am Coll Cardiol. 2007;49:198–207. doi: 10.1016/j.jacc.2006.08.050. [DOI] [PubMed] [Google Scholar]
- 81.Meyer M, McEntee RK, Nyotowidjojo I, Chu G, LeWinter MM. Relationship of exercise capacity and left ventricular dimensions in patients with a normal ejection fraction. An exploratory study. PLoS One. 2015;10:e0119432. doi: 10.1371/journal.pone.0119432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mohammed SF, Hussain S, Mirzoyev SA, Edwards WD, Maleszewski JJ, Redfield MM. Coronary microvascular rarefaction and myocardial fibrosis in heart failure with preserved ejection fraction. Circulation. 2015;131:550–559. doi: 10.1161/CIRCULATIONAHA.114.009625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mottram PM, Haluska B, Leano R, Cowley D, Stowasser M, Marwick TH. Effect of aldosterone antagonism on myocardial dysfunction in hypertensive patients with diastolic heart failure. Circulation. 2004;110:558–565. doi: 10.1161/01.CIR.0000138680.89536.A9. [DOI] [PubMed] [Google Scholar]
- 84.Mukherjee R, Spinale FG. L-type calcium channel abundance and function with cardiac hypertrophy and failure: a review. J Mol Cell Cardiol. 1998;30:1899–1916. doi: 10.1006/jmcc.1998.0755. [DOI] [PubMed] [Google Scholar]
- 85.Nagayama T, Hsu S, Zhang M, Koitabashi N, Bedja D, Gabrielson KL, Takimoto E, Kass DA. Sildenafil stops progressive chamber, cellular, and molecular remodeling and improves calcium handling and function in hearts with pre-existing advanced hypertrophy caused by pressure overload. J Am Coll Cardiol. 2009;53:207–215. doi: 10.1016/j.jacc.2008.08.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Narayanan A, Aurigemma GP, Chinali M, Hill JC, Meyer TE, Tighe DA. Cardiac mechanics in mild hypertensive heart disease: a speckle-strain imaging study. Circ Cardiovasc Imaging. 2009;2:382–390. doi: 10.1161/CIRCIMAGING.108.811620. [DOI] [PubMed] [Google Scholar]
- 87.Nolte K, Herrmann-Lingen C, Wachter R, Gelbrich G, Dungen HD, Duvinage A, Hoischen N, von OK, Schwarz S, Hasenfuss G, Halle M, Pieske B, Edelmann F. Effects of exercise training on different quality of life dimensions in heart failure with preserved ejection fraction: the Ex-DHF-P trial. Eur J Prev Cardiol. 2015;22:582–593. doi: 10.1177/2047487314526071. [DOI] [PubMed] [Google Scholar]
- 88.Novo G, Pugliesi M, Visconti C, Spatafora P, Fiore M, Di MR, Guarneri FP, Vitale G, Novo S. Early subclinical ventricular dysfunction in patients with insulin resistance. J Cardiovasc Med (Hagerstown) 2014;15:110–114. doi: 10.2459/JCM.0b013e3283638164. [DOI] [PubMed] [Google Scholar]
- 89.Paulus WJ, Tschope C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol. 2013;62:263–271. doi: 10.1016/j.jacc.2013.02.092. [DOI] [PubMed] [Google Scholar]
- 90.Paulus WJ, Tschope C, Sanderson JE, Rusconi C, Flachskampf FA, Rademakers FE, Marino P, Smiseth OA, De KG, Leite-Moreira AF, Borbely A, Edes I, Handoko ML, Heymans S, Pezzali N, Pieske B, Dickstein K, Fraser AG, Brutsaert DL. How to diagnose diastolic heart failure: a consensus statement on the diagnosis of heart failure with normal left ventricular ejection fraction by the Heart Failure and Echocardiography Associations of the European Society of Cardiology. Eur Heart J. 2007;28:2539–2550. doi: 10.1093/eurheartj/ehm037. [DOI] [PubMed] [Google Scholar]
- 91.Pavlopoulos H, Grapsa J, Stefanadi E, Kamperidis V, Philippou E, Dawson D, Nihoyannopoulos P. The evolution of diastolic dysfunction in the hypertensive disease. Eur J Echocardiogr. 2008;9:772–778. doi: 10.1093/ejechocard/jen145. [DOI] [PubMed] [Google Scholar]
- 92.Perrino C, Naga Prasad SV, Mao L, Noma T, Yan Z, Kim HS, Smithies O, Rockman HA. Intermittent pressure overload triggers hypertrophy-independent cardiac dysfunction and vascular rarefaction. J Clin Invest. 2006;116:1547–1560. doi: 10.1172/JCI25397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Perrino C, Naga Prasad SV, Mao L, Noma T, Yan Z, Kim HS, Smithies O, Rockman HA. Intermittent pressure overload triggers hypertrophy-independent cardiac dysfunction and vascular rarefaction. J Clin Invest. 2006;116:1547–1560. doi: 10.1172/JCI25397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Persson H, Lonn E, Edner M, Baruch L, Lang CC, Morton JJ, Ostergren J, McKelvie RS. Diastolic dysfunction in heart failure with preserved systolic function: need for objective evidence:results from the CHARM Echocardiographic Substudy-CHARMES. J Am Coll Cardiol. 2007;49:687–694. doi: 10.1016/j.jacc.2006.08.062. [DOI] [PubMed] [Google Scholar]
- 95.Petrie MC, Caruana L, Berry C, McMurray JJ. “Diastolic heart failure” or heart failure caused by subtle left ventricular systolic dysfunction? Heart. 2002;87:29–31. doi: 10.1136/heart.87.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pitt B, Reichek N, Willenbrock R, Zannad F, Phillips RA, Roniker B, Kleiman J, Krause S, Burns D, Williams GH. Effects of eplerenone, enalapril, and eplerenone/enalapril in patients with essential hypertension and left ventricular hypertrophy: the 4E-left ventricular hypertrophy study. Circulation. 2003;108:1831–1838. doi: 10.1161/01.CIR.0000091405.00772.6E. [DOI] [PubMed] [Google Scholar]
- 97.Pretorius L, Owen KL, Jennings GL, McMullen JR. Promoting physiological hypertrophy in the failing heart. Clin Exp Pharmacol Physiol. 2008;35:438–441. doi: 10.1111/j.1440-1681.2008.04893.x. [DOI] [PubMed] [Google Scholar]
- 98.Redfield MM, Chen HH, Borlaug BA, Semigran MJ, Lee KL, Lewis G, LeWinter MM, Rouleau JL, Bull DA, Mann DL, Deswal A, Stevenson LW, Givertz MM, Ofili EO, O’Connor CM, Felker GM, Goldsmith SR, Bart BA, McNulty SE, Ibarra JC, Lin G, Oh JK, Patel MR, Kim RJ, Tracy RP, Velazquez EJ, Anstrom KJ, Hernandez AF, Mascette AM, Braunwald E. Effect of phosphodiesterase-5 inhibition on exercise capacity and clinical status in heart failure with preserved ejection fraction: a randomized clinical trial. JAMA. 2013;309:1268–1277. doi: 10.1001/jama.2013.2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Riordan MM, Kovacs SJ. Stiffness- and relaxation-based quantitation of radial left ventricular oscillations: elucidation of regional diastolic function mechanisms. J Appl Physiol (1985) 2007;102:1862–1870. doi: 10.1152/japplphysiol.01219.2006. [DOI] [PubMed] [Google Scholar]
- 100.Santos AB, Kraigher-Krainer E, Bello N, Claggett B, Zile MR, Pieske B, Voors AA, McMurray JJ, Packer M, Bransford T, Lefkowitz M, Shah AM, Solomon SD. Left ventricular dyssynchrony in patients with heart failure and preserved ejection fraction. Eur Heart J. 2014;35:42–47. doi: 10.1093/eurheartj/eht427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sato A, Hayashi M, Saruta T. Relative long-term effects of spironolactone in conjunction with an angiotensin-converting enzyme inhibitor on left ventricular mass and diastolic function in patients with essential hypertension. Hypertens Res. 2002;25:837–842. doi: 10.1291/hypres.25.837. [DOI] [PubMed] [Google Scholar]
- 102.Sawada K, Kawamura K. Architecture of myocardial cells in human cardiac ventricles with concentric and eccentric hypertrophy as demonstrated by quantitative scanning electron microscopy. Heart Vessels. 1991;6:129–142. doi: 10.1007/BF02058278. [DOI] [PubMed] [Google Scholar]
- 103.Schirmer SH, Sayed MM, Reil JC, Ukena C, Linz D, Kindermann M, Laufs U, Mahfoud F, Bohm M. Improvements in left ventricular hypertrophy and diastolic function following renal denervation: effects beyond blood pressure and heart rate reduction. J Am Coll Cardiol. 2014;63:1916–1923. doi: 10.1016/j.jacc.2013.10.073. [DOI] [PubMed] [Google Scholar]
- 104.Schnell F, Donal E, Bernard-Brunet A, Reynaud A, Wilson MG, Thebault C, Ridard C, Mabo P, Carre F. Strain analysis during exercise in patients with left ventricular hypertrophy: impact of etiology. J Am Soc Echocardiogr. 2013;26:1163–1169. doi: 10.1016/j.echo.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 105.Serpi R, Tolonen AM, Huusko J, Rysa J, Tenhunen O, Yla-Herttuala S, Ruskoaho H. Vascular endothelial growth factor-B gene transfer prevents angiotensin II-induced diastolic dysfunction via proliferation and capillary dilatation in rats. Cardiovasc Res. 2011;89:204–213. doi: 10.1093/cvr/cvq267. [DOI] [PubMed] [Google Scholar]
- 106.Shah AM, Claggett B, Sweitzer NK, Shah SJ, Anand IS, O’Meara E, Desai AS, Heitner JF, Li G, Fang J, Rouleau J, Zile MR, Markov V, Ryabov V, Reis G, Assmann SF, McKinlay SM, Pitt B, Pfeffer MA, Solomon SD. Cardiac structure and function and prognosis in heart failure with preserved ejection fraction: findings from the echocardiographic study of the Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist (TOPCAT) Trial. Circ Heart Fail. 2014;7:740–751. doi: 10.1161/CIRCHEARTFAILURE.114.001583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Shah AM, Shin SH, Takeuchi M, Skali H, Desai AS, Kober L, Maggioni AP, Rouleau JL, Kelly RY, Hester A, Keefe D, McMurray JJ, Pfeffer MA, Solomon SD. Left ventricular systolic and diastolic function, remodelling, and clinical outcomes among patients with diabetes following myocardial infarction and the influence of direct renin inhibition with aliskiren. Eur J Heart Fail. 2012;14:185–192. doi: 10.1093/eurjhf/hfr125. [DOI] [PubMed] [Google Scholar]
- 108.Shah AM, Solomon SD. Myocardial deformation imaging: current status and future directions. Circulation. 2012;125:e244–e248. doi: 10.1161/CIRCULATIONAHA.111.086348. [DOI] [PubMed] [Google Scholar]
- 109.Shahi M, Thom S, Poulter N, Sever PS, Foale RA. Regression of hypertensive left ventricular hypertrophy and left ventricular diastolic function. Lancet. 1990;336:458–461. doi: 10.1016/0140-6736(90)92010-f. [DOI] [PubMed] [Google Scholar]
- 110.Signolet IL, Bousquet PP, Monassier LJ. Improvement of cardiac diastolic function by long-term centrally mediated sympathetic inhibition in one-kidney, one-clip hypertensive rabbits. Am J Hypertens. 2008;21:54–60. doi: 10.1038/ajh.2007.9. [DOI] [PubMed] [Google Scholar]
- 111.Solomon SD, Zile M, Pieske B, Voors A, Shah A, Kraigher-Krainer E, Shi V, Bransford T, Takeuchi M, Gong J, Lefkowitz M, Packer M, McMurray JJ. The angiotensin receptor neprilysin inhibitor LCZ696 in heart failure with preserved ejection fraction: a phase 2 double-blind randomised controlled trial. Lancet. 2012;380:1387–1395. doi: 10.1016/S0140-6736(12)61227-6. [DOI] [PubMed] [Google Scholar]
- 112.Taddei S, Nami R, Bruno RM, Quatrini I, Nuti R. Hypertension, left ventricular hypertrophy and chronic kidney disease. Heart Fail Rev. 2011;16:615–620. doi: 10.1007/s10741-010-9197-z. [DOI] [PubMed] [Google Scholar]
- 113.Tan YT, Wenzelburger F, Lee E, Heatlie G, Leyva F, Patel K, Frenneaux M, Sanderson JE. The pathophysiology of heart failure with normal ejection fraction: exercise echocardiography reveals complex abnormalities of both systolic and diastolic ventricular function involving torsion, untwist, and longitudinal motion. J Am Coll Cardiol. 2009;54:36–46. doi: 10.1016/j.jacc.2009.03.037. [DOI] [PubMed] [Google Scholar]
- 114.Taylor RS, Davies EJ, Dalal HM, Davis R, Doherty P, Cooper C, Holland DJ, Jolly K, Smart NA. Effects of exercise training for heart failure with preserved ejection fraction: a systematic review and meta-analysis of comparative studies. Int J Cardiol. 2012;162:6–13. doi: 10.1016/j.ijcard.2012.05.070. [DOI] [PubMed] [Google Scholar]
- 115.Tee MW, Won S, Raman FS, Yi C, Vigneault DM, Davies-Venn C, Liu S, Lardo AC, Lima JA, Noble JA, Emter CA, Bluemke DA. Regional Strain Analysis with Multidetector CT in a Swine Cardiomyopathy Model: Relationship to Cardiac MR Tagging and Myocardial Fibrosis. Radiology. 2015:142339. doi: 10.1148/radiol.2015142339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Tomanek RJ, Palmer PJ, Peiffer GL, Schreiber KL, Eastham CL, Marcus ML. Morphometry of canine coronary arteries, arterioles, and capillaries during hypertension and left ventricular hypertrophy. Circ Res. 1986;58:38–46. doi: 10.1161/01.res.58.1.38. [DOI] [PubMed] [Google Scholar]
- 117.Trimarco B, De LN, Rosiello G, Ricciardelli B, Betocchi S, Filardi PP, Raponi M, Condorelli M. Improvement of diastolic function after reversal of left ventricular hypertrophy induced by long-term antihypertensive treatment with tertatolol. Am J Cardiol. 1989;64:745–751. doi: 10.1016/0002-9149(89)90758-3. [DOI] [PubMed] [Google Scholar]
- 118.Trimarco B, DeLuca N, Rosiello G, Ricciardelli B, Marchegiano R, Condorelli G, Raponi M, Condorelli M. Effects of long-term antihypertensive treatment with tertatolol on diastolic function in hypertensive patients with and without left ventricular hypertrophy. Am J Hypertens. 1989;2:278S–283S. doi: 10.1093/ajh/2.11.278s. [DOI] [PubMed] [Google Scholar]
- 119.Tumuklu MM, Ildizli M, Ceyhan K, Cinar CS. Alterations in left ventricular structure and diastolic function in professional football players: assessment by tissue Doppler imaging and left ventricular flow propagation velocity. Echocardiography. 2007;24:140–148. doi: 10.1111/j.1540-8175.2007.00367.x. [DOI] [PubMed] [Google Scholar]
- 120.Urbano-Moral JA, Rowin EJ, Maron MS, Crean A, Pandian NG. Investigation of global and regional myocardial mechanics with 3-dimensional speckle tracking echocardiography and relations to hypertrophy and fibrosis in hypertrophic cardiomyopathy. Circ Cardiovasc Imaging. 2014;7:11–19. doi: 10.1161/CIRCIMAGING.113.000842. [DOI] [PubMed] [Google Scholar]
- 121.Urbieta-Caceres VH, Zhu XY, Gibson ME, Favreau FD, Jordan K, Lerman A, Lerman LO. Reversal of experimental renovascular hypertension restores coronary microvascular function and architecture. Am J Hypertens. 2011;24:458–465. doi: 10.1038/ajh.2010.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.van Heerebeek L, Hamdani N, Handoko ML, Falcao-Pires I, Musters RJ, Kupreishvili K, Ijsselmuiden AJ, Schalkwijk CG, Bronzwaer JG, Diamant M, Borbely A, van d V, Stienen GJ, Laarman GJ, Niessen HW, Paulus WJ. Diastolic stiffness of the failing diabetic heart: importance of fibrosis, advanced glycation end products, and myocyte resting tension. Circulation. 2008;117:43–51. doi: 10.1161/CIRCULATIONAHA.107.728550. [DOI] [PubMed] [Google Scholar]
- 123.Villari B, Sossalla S, Ciampi Q, Petruzziello B, Turina J, Schneider J, Turina M, Hess OM. Persistent diastolic dysfunction late after valve replacement in severe aortic regurgitation. Circulation. 2009;120:2386–2392. doi: 10.1161/CIRCULATIONAHA.108.812685. [DOI] [PubMed] [Google Scholar]
- 124.Vizzardi E, D’Aloia A, Fiorina C, Bugatti S, Parrinello G, De CM, Giannini C, Di B V, Petronio AS, Curello S, Ettori F, Dei CL. Early regression of left ventricular mass associated with diastolic improvement after transcatheter aortic valve implantation. J Am Soc Echocardiogr. 2012;25:1091–1098. doi: 10.1016/j.echo.2012.06.010. [DOI] [PubMed] [Google Scholar]
- 125.von Lueder TG, Wang BH, Kompa AR, Huang L, Webb R, Jordaan P, Atar D, Krum H. Angiotensin receptor neprilysin inhibitor LCZ696 attenuates cardiac remodeling and dysfunction after myocardial infarction by reducing cardiac fibrosis and hypertrophy. Circ Heart Fail. 2015;8:71–78. doi: 10.1161/CIRCHEARTFAILURE.114.001785. [DOI] [PubMed] [Google Scholar]
- 126.Wachtell K, Bella JN, Rokkedal J, Palmieri V, Papademetriou V, Dahlof B, Aalto T, Gerdts E, Devereux RB. Change in diastolic left ventricular filling after one year of antihypertensive treatment: The Losartan Intervention For Endpoint Reduction in Hypertension (LIFE) Study. Circulation. 2002;105:1071–1076. doi: 10.1161/hc0902.104599. [DOI] [PubMed] [Google Scholar]
- 127.Yang H, Carasso S, Woo A, Jamorski M, Nikonova A, Wigle ED, Rakowski H. Hypertrophy pattern and regional myocardial mechanics are related in septal and apical hypertrophic cardiomyopathy. J Am Soc Echocardiogr. 2010;23:1081–1089. doi: 10.1016/j.echo.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 128.Zile MR, Baicu CF, Ikonomidis S, Stroud RE, Nietert PJ, Bradshaw AD, Slater R, Palmer BM, Van BP, Meyer M, Redfield M, Bull A, Granzier L, LeWinter MM. Myocardial stiffness in patients with heart failure and a preserved ejection fraction: contributions of collagen and titin. Circulation. 2015;131:1247–1259. doi: 10.1161/CIRCULATIONAHA.114.013215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zile MR, Gottdiener JS, Hetzel SJ, McMurray JJ, Komajda M, McKelvie R, Baicu CF, Massie BM, Carson PE. Prevalence and significance of alterations in cardiac structure and function in patients with heart failure and a preserved ejection fraction. Circulation. 2011;124:2491–2501. doi: 10.1161/CIRCULATIONAHA.110.011031. [DOI] [PubMed] [Google Scholar]