Abstract
Dimethyl carbonate (DMC) is an industrial chemical, used as a paint and adhesive solvent, with the potential for significant increases in production. Using select immune function assays, the purpose of these studies was to evaluate the immunotoxicity of DMC following dermal exposure using a murine model. Following a 28-day exposure, DMC produced a significant decrease in thymus weight at concentrations of 75% and greater. No effects on body weight, hematological parameters (erythrocytes, leukocytes, and their differentials), or immune cell phenotyping (B-cells, T-cells, and T-cell sub-sets) were identified. The IgM antibody response to sheep red blood cell (SRBC) was significantly reduced in the spleen but not the serum. DMC was not identified to be an irritant and evaluation of the sensitization potential, conducted using the local lymph node assay (LLNA) at concentrations ranging from 50–100%, did not identify increases in lymphocyte proliferation. These results demonstrate that dermal exposure to DMC induces immune suppression in a murine model and raise concern about potential human exposure and the need for occupational exposure regulations.
Keywords: Immunotoxicity, immune suppression, DMC, hypersensitivity
Introduction
Dimethyl carbonate (DMC), also referred to as carbonic acid, dimethyl ester, and methyl carbonate, is an industrial chemical with promising new and useful applications across a broad range of markets. DMC is an organic compound, classified as a carbonate ester with the formula OC(OCH3)2. It is readily hydrolyzed to carbon dioxide and methanol in the environment and presumably in the body via esterases, where methanol is subsequently metabolized to formaldehyde, which is further oxidized to formic acid (OEHHA, 2010). It is a high volume chemical, with production exceeding 1 million pounds annually in the US; Kowa Corporation has proposed use to potentially increase from 2–5 million pounds per year (OEHHA, 2010). It is a colorless, fast evaporating solvent with a substantial polar nature and moderate hydrogen-bonding strength which could be effective in replacing esters, glycol ethers, and ketones in formulations. In addition to being a solvent, it has potential uses as a methylating agent and as a chemical intermediate for use in the production of diphenyl carbonate, isocyanate, allyl diethylene, glycol carbonate, and carbamate pesticides. There is also interest in using this compound as a fuel oxygenate additive and as a solvent of lithium battery electrolytes in the battery industry (Li et al., 2009; Inamoto et al., 2012).
More recently, the use of DMC as a solvent has increased because of its exemption from classification as a volatile organic compound (VOC) under the Clean Air Act in 2009 by the US EPA (2009). This ruling was based on DMC not being classified as a hazardous air pollutant and its low ozone-forming potential. With the growing awareness of environmental protection, low-pollution products with low toxicity have become the trend. The manufacture of solvent-based paints requires a large number of organic solvents, most of which have some degree of toxicity, including: aliphatic hydrocarbons, aromatic hydrocarbons, esters, ketones, and alcohols. DMC has been proposed as a low-toxicity solvent for paints, inks, and adhesives which could replace the currently used and toxic toluene, ethyl acetate, and butyl acetate (American, 2009).
DMC is also readily biodegradable, with a low potential to bio-accumulate or be persistent in the environment (Memoli et al., 2001; Miao et al., 2008); this allows it to be considered as a green reagent. Because of this classification DMC has grown in popularity for use in select applications and as a replacement for other solvents such as methyl ethyl ketone, tert-butyl acetate, and parachlorobenzotrifluoride (American, 2009), and other methylating reagents including phosgene, methyl chloroformate, iodomethane, and dimethyl sulfate (Fabbri et al., 2005).
In spite of the non-toxic claims for DMC, studies describing its toxicity are limited. No long-term, chronic exposure studies have been conducted. DMC was identified to have low acute oral toxicity (LD50 rat = 12,900 mg/kg, LD50 mouse = 6000 mg/kg) and found to be negative in mutagenicity tests (i.e., in vitro Ames and Comet assays) conducted in L-929 mouse fibroblasts (Song, 2002). In a 10-day developmental toxicity study, mated female mice were exposed by inhalation to concentrations of DMC up to 3000 ppm during gestation days 6–15 for 6 h/day (Exxon Corporation, 1992; Bevan, 1995). Maternal and fetal body weights were reduced and the number of growth-stunted and malformed fetuses was increased at 3000 ppm. The No Observed Adverse Effect Level (NOAEL) for maternal and developmental toxicity was determined to be 1000 ppm, virtually identical to the main metabolite of DMC, methanol (NOAEL = 1000 ppm) (Rogers et al., 1993). From the very limited human data, DMC is suspected to be mildly toxic by ingestion (Lewis, 1996; HSDB, 2009). No other data were available in the peer-reviewed literature for chronic exposure of humans to DMC. There is, however, toxicity data available for the metabolites of DMC (methanol, formaldehyde, and formic acid) (NTP, 1992; ATSDR, 1999; NTP-CERHR, 2003).
Due to the high production volume of this chemical, there is the potential for exposure of workers and the general public located near facilities where DMC is used. While DMC is described as a non-toxic alternative, aside from acute toxicity studies, no other organ-specific toxicities have been examined and the MSDS sheet states that the toxicological properties of this chemical have not been tested. The recent increase in occupational use, along with the potential for dermal exposure, raise concerns about related adverse health effects. The purpose of these studies was to determine the immunotoxicity following dermal exposure to DMC using a murine model.
Materials and methods
Test articles and chemicals
Dimethyl Carbonate (DMC; Figure 1) [CAS #616-386], α-hexylcinnamaldehyde (HCA) [CAS# 101-86-0], 2,4-dinitrofluorobenzene (DNFB) [CAS# 70-34-8], and cyclophosphamide [CP; CAS# 50-18-0] were purchased from Aldrich Chemical Company, Inc. (Milwaukee, WI).
Figure 1.
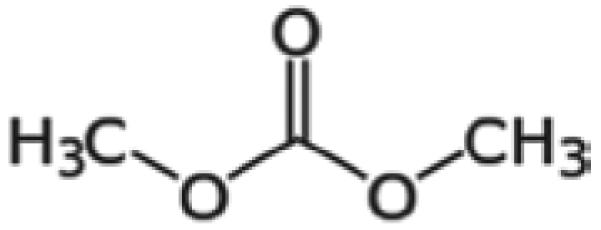
Chemical structure of DMC.
Species selection
Female BALB/c and B6C3F1 mice were used in these studies. BALB/c mice have a T-helper (TH)-2 bias and are commonly used to evaluate potential IgE-mediated sensitization and were therefore used in the hypersensitivity studies (Woolhiser et al., 2000; Klink and Meade, 2003). B6C3F1 mice are the strain of choice for immunotoxicity studies and were used to evaluate the IgM response to sheep red blood cells (SRBC) (Luster et al., 1992a, b).
All mice were purchased from Taconic (Germantown, NY) at 6–8 weeks-of-age. Upon arrival, the animals were allowed to acclimate for a minimum of 5 days. Each shipment of animals was randomly assigned to a treatment group, weighed, and individually identified via tail marking using a permanent marker. A preliminary analysis of variance on body weights was performed to insure an homogeneous distribution of animals across treatment groups. The animals were housed at a maximum of five mice/cage in ventilated plastic shoebox cages with hardwood chip bedding. NIH-31 modified 6% irradiated rodent diet (Harlan Teklad) and tap water was provided from water bottles, ad libitum. The temperature in the animal facility was maintained between 68–72°F and the relative humidity between 36–57%; a light/dark cycle was maintained at 12-h intervals. All animal experiments were performed in an Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC) accredited National Institute for Occupational Safety and Health (NIOSH) animal facility in accordance with an animal protocol approved by the Institutional Animal Care and Use Committee.
Concentration range-finding studies
Concentration range-finding studies were performed to select the concentrations of DMC to be used for dermal exposures. BALB/c mice were exposed topically to acetone vehicle or increasing concentrations of DMC in acetone on the dorsal surface of each ear (25 μl per ear) for three consecutive days. Animals were allowed to rest for 2 days following the last exposure and then weighed and examined for signs of toxicity, such as loss of body weight, fatigue/lack of activity, and ungroomed fur. DMC was tested at concentrations up to 100%. The maximum concentration selected for the subsequent studies was based on limits of toxicity.
DMC exposures
For the hypersensitivity study, BALB/c mice (five mice/group) were topically treated with acetone vehicle, increasing concentrations of DMC or positive control [30% HCA (v/v; sensitization positive control) and 0.3% DNFB (v/v; irritancy positive control)] on the dorsal surface of each ear (25 μl per ear) once a day for three consecutive days. For the immune phenotyping and hematology studies, B6C3F1 mice (n = 5) were topically exposed to acetone or increasing concentrations of DMC (up to 100%) topically on the shaved backs (50 μl) once a day for 28 consecutive days. For analysis of the IgM response to SRBC, B6C3F1 mice (n = 6) were topically exposed to acetone or increasing concentrations of DMC (up to 100%) topically on the shaved backs (50 μl) once a day for 28 consecutive days. Cyclophosphamide (20 mg/kg in isotonic sterile saline) was included as the positive control for the analysis of the IgM response to SRBC and was injected intraperitoneally 4 days prior to sacrifice.
Combined local lymph node and irritancy assay
To determine the irritancy and sensitization potential of DMC, a combined local lymph node assay (LLNA) was conducted. DMC dosing concentrations (50–100%) and vehicle (acetone) were selected based solubility and preliminary concentration range finding studies. A combined LLNA was performed according to the methods previously described in Anderson et al. (2007).
Phenotypic analysis of splenocytes
Spleen phenotypes were analyzed using flow cytometry, as described by Manetz and Meade (1999). Animals were euthanized by CO2 inhalation 24 h after the final exposure, weighed, and examined for gross pathology. Blood was collected in EDTA-coated vacutainer tubes following transection of the abdominal aorta and hematological analysis was conducted (see below). The liver, spleen, kidneys, and thymus were removed, cleaned of connective tissue and weighed. For phenotypic analysis, the spleen was collected in 3 ml phosphate-buffered saline (PBS, pH 7.4) and dissociated using the frosted ends of two microscope slides. Cell counts were performed using a Coulter Counter (Z2 model, Beckman Coulter, Brea, CA), and 1 × 106 cells per sample were added to the wells of a 96-well plate. Cells were washed using staining buffer (1% bovine serum albumin/0.1% sodium azide in PBS) and then incubated with Fc block (clone 2.4G2; BD Pharmingen, San Diego, CA). For analysis of T-cell sub-sets, cells were then treated with 100 µl of anti-mouse CD3e (APC, clone 145-2C11), anti-mouse CD4 (FITC, clone RM4-5), and anti-mouse CD8a (PE, clone 53-6.7) antibody solutions; for analysis of B-cells, cells received anti-mouse CD45R/B220 antibody (FITC, clone RA3-6B2). Parallel sets of cells received appropriate isotype controls diluted in staining buffer. All antibodies and isotype controls were purchased from BD Pharmingen. The cells were then incubated on ice in the dark for 30 min. The cells were then washed and incubated with propidium iodide (PI). After a final wash, cells were re-suspended in staining buffer and analyzed with a Becton Dickinson LSR II flow cytometer using a PI viability gate; all assays were performed using the FACS DIVA software accompanying the flow system. For each sample, a minimum of 10,000 events was acquired.
Hematology
Selected hematological parameters were evaluated using a Hemavet 950 automatic hematology analyzer (Drew Scientific, Waterbury, CT). End-points analyzed included peripheral erythrocyte and leukocyte counts, leukocyte differentials (lymphocytes, neutrophils, monocytes, basophils, and eosinophils), platelet counts, hematocrit, hemoglobin levels, mean corpuscular hemoglobin (MCH) and hemoglobin concentration (MCHC), mean corpuscular volume (MCP), mean platelet volume (MCV), and platelet distribution width (PDW).
Spleen in vivo response to the T-cell-dependent antigen SRBC
The primary IgM response to sheep red blood cells (SRBC) was enumerated using a modified hemolytic plaque assay of Jerne and Nordin (1963). Four days prior to euthanasia (i.e., Day 29), the mice were immunized with 7.5 × 107 SRBC (in 200 µl volume) by intravenous injection. All SRBC for these studies were drawn from a single donor animal (Lampire Laboratories, Pipersville, PA). On the day of sacrifice, mice were euthanized by CO2 asphyxiation, body and organ weights were recorded, and spleens were collected in 3 ml of Hank’s balanced salt solution (HBSS). Blood was also retrieved in serum collection tubes following transection of the abdominal aorta and stored at −20°C for subsequent analysis of serum anti-SRBC IgM levels (see below).
Single cell suspensions of the spleens from individual animals were prepared in HBSS by disrupting the spleen between the frosted ends of microscopic slides. To identify the total number of spleen cells, 20 μl of cells were added to 10 ml of Isoton II diluent (1:500; Beckman Coulter) and two drops of Zap-o-globin (Beckman Coulter) were added to lyse red blood cells. Cells were then counted in the Coulter counter. Dilutions (1:30 and 1:120) of spleen cells were then prepared, and 100 μl of each dilution were added to test tubes containing a 0.5 ml warm agar/dextran mixture (0.5% Bacto-Agar, DIFCO; and 0.05% DEAE dextran; Sigma, St. Louis, MO), 25 μl of 1:1 ratio of SRBC suspension, and 25 μl of 1:4 dilution (1 ml lyophilized) guinea pig complement (Cedarlane Labs, Burlington, Canada). Each sample was vortexed, poured into a petri dish, covered with a microscope coverslip, and incubated for 3 h at 37°C. The plaques (representing antibody-forming B-cells) were then counted. Results were expressed in terms of both specific activity (IgM PFC per 106 spleen cells) and total activity (IgM PFC per spleen).
Serum IgM response to SRBC
Serum samples were analyzed for anti-SRBC IgM using a commercially available ELISA kit (Life Diagnostics, West Chester, PA), according to manufacturer recommendations with modifications. Test serum was diluted (1:200, 1:400, 1:800, and 1:1,600) and incubated in the micro-titer wells for 45 min at 25°C. The wells were subsequently washed, 100 µl horse-radish peroxidase-conjugated secondary antibody was added, and the plates incubated for a further 45 min at 25°C. Thereafter, the wells were washed to remove unbound antibodies, and 100 µl tetramethylbenzidine peroxidase (TMB) reagent was added to each well. The plates were then incubated for 20 min at room temperature before color development was stopped by the addition of 50 µl of kit-provided Stop Solution. Optical density in each well was then measured spectrophotometrically at 450 nm using a Spectra Max M2 plate reader (Molecular Devices, Sunnyvale, CA). The concentration of the anti-SRBC IgM in the test samples was determined by comparison to a standard curve generated in parallel using SoftMax Pro software, and reported as units of anti-SRBC IgM (U/ml) plotted vs absorbance values at 450 nm.
Statistical analyses
For analysis of the data generated from the described animal studies, the data were first tested for homogeneity using the Bartlett’s Chi Square test. If homogeneous, a one-way analysis of variance (ANOVA) was conducted. If the ANOVA showed significance at p < 0.05 or less, the Dunnett’s Multiple Range t-test was used to compare treatment groups with the control group. Linear trend analysis was performed to determine if DMC had exposure concentration-related effects for the specified end-points. Statistical analysis was performed using Graph Pad Prism version 5.0 (San Diego, CA). Statistical significance against value for 0% DMC samples is designated by * p ≤ 0.05 and ** p ≤0.01.
Results
In vivo studies did not identify DMA to be an allergic sensitizer or dermal irritant
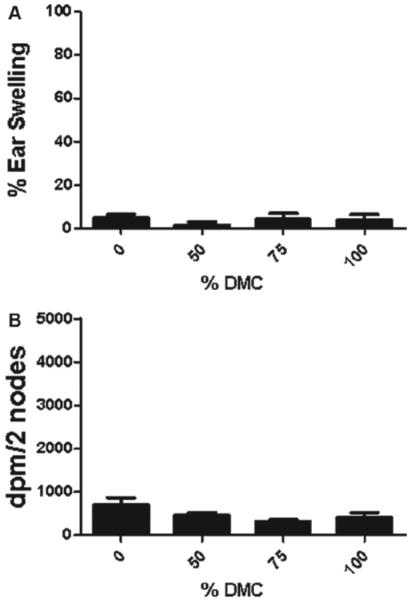
Dermal exposure to DMC was not found to be toxic at any concentration tested (data not shown). For this reason, concentrations of DMC up to 100% were tested in the subsequent studies. No ear swelling was observed in mice after dermal exposure to DMC (Figure 2a). DNFB (0.3%) was used as a positive control for irritancy studies and resulted in an average significant increase of 145% in ear swelling post-application. No increase in auricular draining lymph node proliferation was identified after treatment with DMC (Figure 2b). HCA (30%) was used as a positive control for these experiments and resulted in an average SI value of 9.8.
Figure 2.
Irritancy and allergic sensitization potential after dermal exposure to DMC. Analysis of the (a) irritancy and (b) allergic sensitization potential of DMC using the combined irritancy/LLNA. DPM represent [3H]-thymidine incorporation into draining lymph node cells of BALB/c mice following exposure to vehicle or concentration of DMC. Bars represent mean (± SE) of five mice (10 ears; irritancy) per group. Levels of statistical significance are denoted (** p < 0.01) as compared to acetone vehicle.
Dermal exposure to DMC for 28 days results in reduced thymus weights
In contrast to body weight data, a statistically significant decrease in thymus weights was observed following exposure to 100% DMC (Table 1). No other significant changes in body or organ weight were observed following exposure to any concentration of DMC (Table 1). Although not statistically significant, there was a slight decrease in spleen weight. Dermal exposure to DMC did not alter any of the analyzed hematological parameters (Table 2), splenocyte numbers, or subpopulations (Table 3).
Table 1.
Body/organ weights of female B6C3F1 mice dermally exposed to dimethyl carbonate for 28 days.
| Dimethyl carbonate |
||||
|---|---|---|---|---|
| Parameter | 0% | 50% | 75% | 100% |
| Body weight (g) | 21.61 ± 0.40 | 20.79 ± 1.10 | 22.33 ± 1.17 | 22.07 ± 1.02 |
| Kidney weight (mg) | 259 ± 5 | 264 ± 18 | 284 ± 15 | 272 ± 9 |
| % bw | 1.19 ± 0.01 | 1.27 ± 0.03 | 1.28 ± 0.08 | 1.23 ± 0.00 |
| Spleen weight (mg) | 88 ± 17 | 75 ± 5 | 74 ± 4 | 67 ± 4 |
| % bw | 0.41 ± 0.09 | 0.36 ± 0.01 | 0.33 ± 0.02 | 0.30 ± 0.02 |
| Thymus weight (mg) | 55 ± 2 | 50 ± 2 | 44 ± 3* | 40 ± 3** |
| % bw | 0.26 ± 0.01 | 0.24 ± 0.02 | 0.20 ± 0.02 | 0.18 ± 0.02* |
| Liver weight (mg) | 1123 ± 74 | 1013 ± 65 | 1090 ± 62 | 1034 ± 60 |
| % bw | 5.20 ± 0.38 | 4.90 ± 0.07 | 4.90 ± 0.31 | 4.70 ± 0.15 |
bw, body weight.
Values are expressed as means (± SE) for each group.
Significantly different from acetone controls at
p < 0.05
p < 0.01.
Table 2.
Hematology parameters of female B6C3F1 mice dermally exposed to dimethyl carbonate for 28 days.
| Dimethyl carbonate |
||||
|---|---|---|---|---|
| Parameter | 0% | 50% | 75% | 100% |
| Hemoglobin (g/dl) | 13.84 ± 0.26 | 12.82 ± 0.79 | 13.50 ± 0.63 | 13.84 ± 0.76 |
| Erythrocytes (M/μl) | 8.96 ± 0.22 | 8.35 ± 0.50 | 8.59 ± 0.43 | 8.85 ± 0.50 |
| Platelets (K/μl) | 291 ± 131 | 471 ± 92 | 297 ± 101 | 384 ± 63 |
| Hematocrit (%) | 47.36 ± 1.05 | 43.74 ± 2.79 | 44.68 ± 2.26 | 46.40 ± 2.78 |
| MCV (fl) | 52.84 ± 0.21 | 52.32 ± 0.30 | 52.02 ± 0.12 | 52.40 ± 0.27 |
| MPV (fl) | 5.66 ± 0.32 | 5.52 ± 0.65 | 5.28 ± 0.44 | 4.80 ± 0.28 |
| MCH (pg) | 15.46 ± 0.12 | 15.36 ± 0.12 | 15.70 ± 0.17 | 15.68 ± 0.11 |
| MCHC (g/dl) | 29.22 ± 0.21 | 29.32 ± 0.19 | 30.26 ± 0.35 | 29.88 ± 0.24 |
| PDW (%) | 23.02 ± 1.06 | 20.24 ± 1.09 | 22.80 ± 1.81 | 22.86 ± 1.13 |
| Leukocytes (K/μl) | 7.00 ± 1.34 | 6.64 ± 1.51 | 7.75 ± 1.42 | 7.26 ± 1.37 |
| % Lymphocytes | 74.62 ± 2.50 | 73.90 ± 3.08 | 81.94 ± 1.15 | 73.87 ± 3.48 |
| % Neutrophils | 20.39 ± 1.95 | 20.39 ± 2.29 | 14.93 ± 1.15 | 21.44 ± 2.13 |
| % Monocytes | 3.76 ± 0.31 | 3.81 ± 0.37 | 2.57 ± 0.33 | 2.80 ± 0.42 |
| % Eosinophils | 1.09 ± 0.37 | 1.57 ± 0.55 | 0.69 ± 0.20 | 1.37 ± 0.85 |
| % Basophils | 0.15 ± 0.05 | 0.34 ± 0.13 | 0.07 ± 0.03 | 0.52 ± 0.31 |
MCH, mean corpuscular hemoglobin; MCV, mean corpuscular volume; MPV, mean platelet volume; PDW, platelet distribution width; MCHC, mean corpuscular hemoglobin concentration.
Values are expressed as the means (± SE) for each group.
Table 3.
Effects of dermal exposure to dimethyl carbonate for 28 days on total spleen cell number and of lymphocyte sub-populations in female B6C3F1 mice.
| Dimethyl carbonate |
||||
|---|---|---|---|---|
| Parameter | 0% | 50% | 75% | 100% |
| Spleen nucleated cell number (×106) |
143.6 ± 17.10 | 116.2 ± 4.6 | 123.4 ± 4.8 | 113.6 ± 7.3 |
| B-cell number (×106) | 66.37 ± 7.7 | 55.92 ± 3.5 | 63.53 ± 2.3 | 54.75 ± 4.7 |
| % of splenocytes | 46.30 ± 0.89 | 47.97 ± 1.22 | 51.57 ± 1.28 | 47.96 ± 1.10 |
| T-cell number (×106) | 40.18 ± 1.4 | 37.48 ± 1.4 | 38.06 ± 1.2 | 36.80 ± 1.9 |
| % of splenocytes | 27.94 ± 0.31 | 27.88 ± 0.81 | 26.18 ± 0.73 | 27.47 ± 1.13 |
| CD4+T-cell number (×106) | 30.18 ± 1.43 | 27.48 ± 1.40 | 28.06 ± 1.23 | 26.80 ± 1.86 |
| % of splenocytes | 21.61 ± 1.32 | 23.77 ± 1.27 | 22.71 ± 0.47 | 23.66 ± 0.97 |
| CD8+T-cells (×106) | 15.42 ± 0.81 | 13.80 ± 0.60 | 13.00 ± 0.29 | 13.44 ± 1.01 |
| % of splenocytes | 11.03 ± 0.66 | 11.93 ± 0.38 | 10.58 ± 0.36 | 11.86 ± 0.53 |
Mice were dermally exposed to vehicle (acetone) or different concentrations of DMC for 28 days (times) over 2 weeks. The mice were euthanized 24 h after the final exposure, spleens were removed, and total nucleated splenocytes counted. Numbers of B- and T-cells, and subsets of T-cells (CD4+ and CD8+) were enumerated.
Values represent the means (± SE) for each group.
Dermal exposure to DMC suppressed the splenic—but not serum—IgM response to SRBC
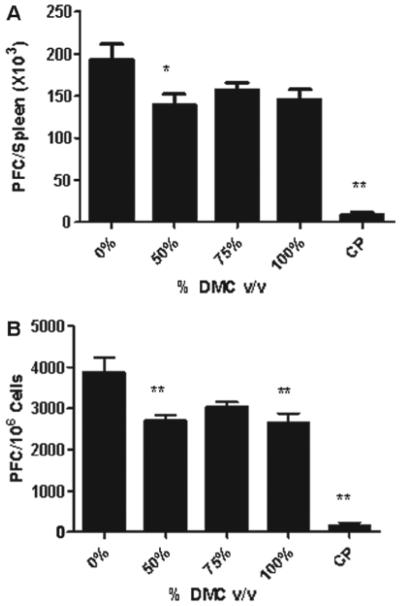
To evaluate if exposure to DMC was immunosuppressive, the murine IgM response to SRBC was examined following a 28-day exposure to DMC. Statistically significant reductions in the PFC/spleen and specific (PFC/106 cells) IgM antibody activity against SRBC were observed after exposure to DMC (Figures 3a and b). Exposure of mice to 100% DMC resulted in a suppression of the values for PFC/spleen and PFC/106 cells (33 and 46%, respectively, vs values for vehicle-treated mice); 50% DMC resulted in suppressions of PFC/spleen (38%) and PFC/106 cells (43%) (Figure 3). While mice exposed to 75% DMC did not have a statistically significant reduction in antibody production, the levels appeared to be reduced compared to those associated with the vehicle controls. Mice exposed to cyclophosphamide had a significantly reduced specific spleen IgM response (78%) and total IgM response (76%) compared to levels noted in vehicle-treated controls. There was no change in serum anti-SRBC IgM antibody levels following exposure to DMC; however, cyclophosphamide (positive control) suppressed the antibody response by 66% relative to control mice values (Figure 4).
Figure 3.
DMC suppresses the spleen IgM response to SRBC. Analysis of antibody producing spleen cells after a 28-day dermal exposure to DMC suppressed the (a) total and (b) specific activity IgM response to SRBC. Bars represent mean fold-change (± SE) of six mice per group. Cyclophosphamide (CP) was included as the positive control.
Figure 4.
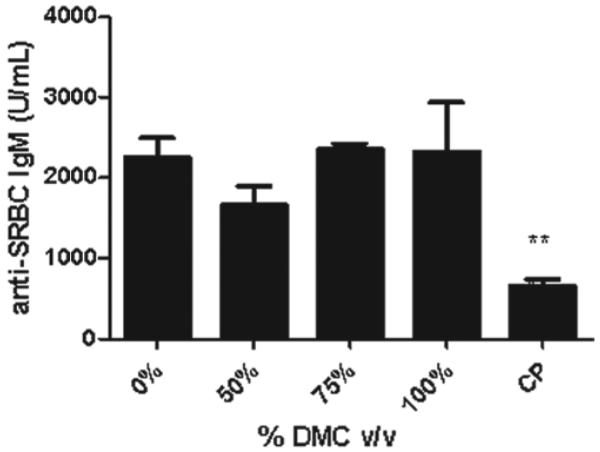
DMC exposure does not alter the serum IgM response to SRBC. Analysis of serum after a 28-day dermal exposure to DMC did not produce alternations in IgM response to SRBC. Bars represent mean fold-change (± SE) of six mice per group. Cyclophosphamide (CP) was included as the positive control.
Discussion
More than 13 million workers in the US are potentially exposed to chemicals that can be absorbed through the skin. Exposures to chemical substances may induce immune abnormalities including immune suppression and autoimmunity, potentially compromising the ability of the immune system to recognize or neutralize infectious agents or neoplastic cells. There is an increasing need for the immunological evaluation of exposure to these chemicals and/or substances due to the potential risk for the development of clinical disease.
In an attempt to fill some of the data gaps associated with DMC exposure-related health effects, the immunotoxicity of DMC was evaluated using a murine model in the studies described here. The results from these studies suggest that DMC is a non-irritating and non-sensitizing chemical, as evidenced by the lack of increase in ear swelling and lymphocyte proliferation. However, suppression of the IgM response to SRBC and reduced thymus weights were observed following a 28-day exposure to this chemical. Statistically significant suppression was observed at 50% and 100% concentrations for the specific activity, while only at 50% for the total activity. Although the antibody response was reduced at the 75% concentration, it was not statistically significant. Based on the lack of concentration dose response and the linearity between exposure group responses, the threshold for suppression may be at a maximum following exposure to concentrations of DMC of 50% and greater.
The T-cell dependent antibody response is one of the most sensitive indicators of immune integrity because it relies on an organized immune response that is dependent on the functional capacity and co-operation of numerous cell types including B-cells, T-cells, and macrophages (Anderson et al., 2006). Immunosuppression determined by the spleen IgM response to SRBC (plaque assay) was observed, but the serum IgM response to SRBC was not decreased. Although the mechanism underlying the contradictory results between the splenic PFC response and serum IgM titers to SRBC is unknown, it has been observed following exposure to other chemicals (Hsieh et al., 1989; Temple et al., 1993; Johnson et al., 2000). The differences in sensitivities may be attributed to the specific end-points that each assay measures. The plaque assay enumerates antibody-producing cells in the spleen, while the ELISA method measures IgM levels in the serum that may be produced in the spleen as well as the lymph nodes and bone marrow. It has also been determined that the murine spleen PFC response to SRBC peaks on day 4 after immunization, while the serum IgM response peaks between days 5 and 6 (Loveless et al., 2007). It should also be noted that, in our studies, the spleen and serum were both collected on the same day (i.e., Day 4) after immunization.
Although exposure to DMC can occur through inhalation and dermal contact during its production and use, there are currently no Occupational Safety and Health Administration (OSHA)-, National Institute for Occupational Safety and Health (NIOSH)-, or American Conference of Governmental Industrial Hygienists (ACGIH)-recommended or required exposure limits. This lack of regulation is in spite of expanded use of DMC that has led to a greater potential for worker exposure. It is important to note that although the concentrations of DMC evaluated in the studies described here were high (50–100%), neat exposure is often required for solvents. In an attempt at regulation, the manufacturer Kowa American has set forth a recommended 8-h personal exposure limit (PEL) of 100 ppm based on OSHA and ACGIH exposure limits for methanol—the expected primary metabolite of DMC.
It has been proposed that DMC would replace some of the more toxic paint solvents, including toluene, ethyl acetate, and butyl acetate. Hsieh et al. (1989, 1990) evaluated the immunotoxic effect of toluene exposure via drinking water in mice. Similar to what was noted with DMC, decreases in thymus weight and suppression of the spleen but not serum, IgM response to SRBC were observed. There were no changes in body weights or hematological parameters following exposure to toluene. Based on a scheme proposed by van Loveren and colleagues that classifies immunotoxic chemicals based on the affected immune responses (i.e., antibody response, organ weights), toluene and DMC would both be categorized as weak immunotoxic agents (Veraldi et al., 2006). Comparable to the DMC developmental toxicity studies, whole body inhalation exposure of rats (gestation day 6–20, at 6 h/day) to concentrations of butyl acetate (up to 3000 ppm) resulted in decreased maternal weight gain and food consumption (Saillenfait et al., 2007). However, while DMC resulted in developmental effects, for butyl acetate, fetal effects were limited to a decreased fetal weight at the 3000 ppm exposure level. A 13-week butyl acetate rat inhalation study also demonstrated decreased body weight, feed consumption, liver, kidney, and spleen weights at concentrations of ≥ 1500 ppm for male rats (David et al., 2001). Acute toxicity and cancer risk assessment for butyl acetate suggest it is a potential carcinogen (Budroe et al., 2004). Limited toxicity data (LD50 rats = 11.3 g/kg) was found for ethyl acetate. Given the similarities between DMC and other commonly used industrial solvents, it is questionable if it really is a non- or less toxic alternative.
These are the first studies to evaluate the allergic potential and immunotoxicity induced by dermal exposure to DMC using a murine model. Although significant data gaps still exist for the complete toxicological evaluation of this chemical, these results suggest that DMC, when evaluated in a murine model, is an immunotoxic chemical. The results presented here, along with the increasing production demands for this chemical, raise concern for the need for additional, long-term exposure studies. In an effort to reduce and prevent occupational exposure and potential disease, regulations for the use of this chemical may also need to be considered.
Acknowledgments
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the National Institute for Occupational Safety and Health, Centers for Disease Control and Prevention.
Footnotes
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- American K. Dimethyl Carbonate (DMC) The Newest VOC Exempt Solvent. 2009 www.chemical.kowa.com/download/DIMETHYL%20CARBONATE%20powerpoint.ppt. Available online at: Accessed February 8th, 2012.
- Anderson SE, Munson AE, Meade BJ. Analysis of immunotoxicity by enumeration of antibody-producing B-cells. In: Bus JS, Costa LG, Hodgson E, Lawrence DA, Reed D, Hoboken, editors. Current Protocols in Toxicology. Wiley and Sons; NJ: 2006. pp. 18.11.1–18.11.19.. [DOI] [PubMed] [Google Scholar]
- Anderson SE, Wells JR, Fedorowicz A, Butterworth LF, Meade BJ, Munson AE. Evaluation of the contact and respiratory sensitization potential of volatile organic compounds generated by simulated indoor air chemistry. Toxicol. Sci. 2007;97:355–363. doi: 10.1093/toxsci/kfm043. [DOI] [PubMed] [Google Scholar]
- ATSDR . Toxicological Profile for Formaldehyde. ATSDR; Atlanta, GA: 1999. Agency for Toxic Substances and Disease Registry. PB/99/166654. [PubMed] [Google Scholar]
- Bevan CB. Developmental toxicity evaluation of dimethylcarbonate by inhalation in CD-1 mice. Int. Toxicol. 1995;7:P72. [Google Scholar]
- Budroe JD, Brown JP, Salmon AG, Marty MA. Acute toxicity and cancer risk assessment values for tert-butyl acetate. Regul. Toxicol. Pharmacol. 2004;40:168–176. doi: 10.1016/j.yrtph.2004.07.001. [DOI] [PubMed] [Google Scholar]
- David RM, Tyler TR, Ouellette R, Faber WD, Banton MI. Evaluation of subchronic toxicity of n-butyl acetate vapor. Food Chem. Toxicol. 2001;39:877–886. doi: 10.1016/s0278-6915(01)00021-7. [DOI] [PubMed] [Google Scholar]
- Exxon Corporation . Inhalation Development Toxicity Study in Mice with Dimethylcarbonate. Final Report. Exxon Corporation; East Millstone, NJ: 1992. Project number 107334. [Google Scholar]
- Fabbri D, Baravelli V, Chiavari G, Prati S. Dimethyl carbonate as a novel methylating reagent for fatty acids in analytical pyrolysis. J. Chromatog. A. 2005;1065:257–264. doi: 10.1016/j.chroma.2004.12.077. [DOI] [PubMed] [Google Scholar]
- HSDB (Hazardous Substances Data Bank) Dimethyl Carbonate. 2009 http://toxnet.nlm.nih.gov/cgi-bin/sis/htmlgen?HSDB. Available online at. Accessed February 8, 2012. [Google Scholar]
- Hsieh GC, Parker RD, Sharma RP, Hughes BJ. Subclinical effects of groundwater contaminants. III. Effects of repeated oral exposure to combinations of benzene and toluene on immunologic responses in mice. Arch. Toxicol. 1990;64:320–328. doi: 10.1007/BF01972993. [DOI] [PubMed] [Google Scholar]
- Hsieh GC, Sharma RP, Parker RD. Immunotoxicological evaluation of toluene exposure via drinking water in mice. Environ. Res. 1989;49:93–103. doi: 10.1016/s0013-9351(89)80024-6. [DOI] [PubMed] [Google Scholar]
- Inamoto K, Hasegawa C, Hiroya K, Kondo Y, Osako T, Uozumi Y, Doi T. Use of dimethyl carbonate as a solvent greatly enhances the biaryl coupling of aryl iodides and organoboron reagents without adding any transition metal catalysts. Chem. Commun. 2012;48:2912–2914. doi: 10.1039/c2cc17401d. [DOI] [PubMed] [Google Scholar]
- Jerne NK, Nordin AA. Plaque formation in agar by single antibody-producing cells. Science. 1963;140:405. [PubMed] [Google Scholar]
- Johnson CW, Williams WC, Copeland CB, De Vito MJ, Smialowicz RJ. Sensitivity of the SRBC PFC assay versus ELISA for detection of immunosuppression by TCDD and TCDD-like congeners. Toxicology. 2000;156:1–11. doi: 10.1016/s0300-483x(00)00330-9. [DOI] [PubMed] [Google Scholar]
- Klink KJ, Meade BJ. Dermal exposure to 3-amino-5-mercapto-1,2,4-triazole (AMT) induces sensitization and airway hyper-reactivity in BALB/c mice. Toxicol. Sci. 2003;75:89–98. doi: 10.1093/toxsci/kfg171. [DOI] [PubMed] [Google Scholar]
- Lewis RJ, editor. Sax’s Dangerous Properties of Industrial Materials. 9th. 1-3. Van Nostrand Reinhold; New York: 1996. pp. 2205. [Google Scholar]
- Li D, Fang W, Xing Y, Guo Y, Lin R. Effects of dimethyl or diethyl carbonate as an additive on volatility and flash point of an aviation fuel. J. Haz. Mater. 2009;161:1193–1201. doi: 10.1016/j.jhazmat.2008.04.070. [DOI] [PubMed] [Google Scholar]
- Loveless SE, Ladics GS, Smith C, Holsapple MP, Woolhiser MR, White KL, Jr, Musgrove DL, Smialowicz RJ, Williams W. Interlaboratory study of the primary antibody response to sheep red blood cells in outbred rodents following exposure to cyclophosphamide or dexamethasone. J. Immunotoxicol. 2007;4:233–238. doi: 10.1080/15476910701385687. [DOI] [PubMed] [Google Scholar]
- Luster MI, Pait DG, Portier C, Rosenthal GJ, Germolec DR, Comment CE, Munson AE, White K, Pollock P. Qualitative and quantitative experimental models to aid in risk assessment for immunotoxicology. Toxicol. Lett. 1992a;64(65):71–78. doi: 10.1016/0378-4274(92)90174-i. [DOI] [PubMed] [Google Scholar]
- Luster MI, Portier C, Pait DG, White KL, Jr, Gennings C, Munson AE, Rosenthal GJ. Risk assessment in immunotoxicology. I. Sensitivity and predictability of immune tests. Fundam. Appl. Toxicol. 1992b;18:200–210. doi: 10.1016/0272-0590(92)90047-l. [DOI] [PubMed] [Google Scholar]
- Manetz TS, Meade BJ. Development of a flow cytometry assay for the identification and differentiation of chemicals with the potential to elicit irritation, IgE-mediated, or T cell-mediated hypersensitivity responses. Toxicol. Sci. 1999;48:206–217. doi: 10.1093/toxsci/48.2.206. [DOI] [PubMed] [Google Scholar]
- Memoli S, Selva M, Tundo P. Dimethylcarbonate for eco-friendly methylation reactions. Chemosphere. 2001;43:115–121. doi: 10.1016/s0045-6535(00)00331-3. [DOI] [PubMed] [Google Scholar]
- Miao X, Fischmeister C, Bruneau C, Dixneuf PH. Dimethyl carbonate: An eco-friendly solvent in ruthenium-catalyzed olefin metathesis transformations. ChemSusChem. 2008;1:813–816. doi: 10.1002/cssc.200800074. [DOI] [PubMed] [Google Scholar]
- NTP (National Toxicology Program) Toxicity Studies of Formic Acid (CAS No. 64-18-6) Administered by Inhalation to F344/N Rats and B6C3F1 Mice. Tox-19. NIH, Publication; Research Triangle Park, NC: 1992. pp. 92–3324. [Google Scholar]
- NTP-CERHR (National Toxicology Program . Monograph on the Potential Human Reproductive and Developmental Effects of Methanol. NIH, Publication; Research Triangle Park, NC: 2003. Center for the Evaluation of Risks to Human Reproduction) No. 03-4478. [Google Scholar]
- OEHHA (Office of Environmental Health Hazard Assessment) Final Revised Health Assessment for Dimethyl Carbonate. 2010 http://www.valleyair.org/Workshops/postings/2010/12–29-10/04%20AppB%20OEHHA%20assessment. pdf. Available online at. Accessed February 8, 2012.
- Rogers JM, Mole ML, Chernoff N, Barbee BD, Turner CI, Logsdon TR, Kavlock RJ. The developmental toxicity of inhaled methanol in the CD-1 mouse, with quantitative dose-response modeling for estimation of benchmark doses. Teratology. 1993;47:175–188. doi: 10.1002/tera.1420470302. [DOI] [PubMed] [Google Scholar]
- Saillenfait AM, Gallissot F, Sabate JP, Bourges-Abella N, Muller S. Developmental toxic effects of ethylbenzene or toluene alone and in combination with butyl acetate in rats after inhalation exposure. J. Appl. Toxicol. 2007;27:32–42. doi: 10.1002/jat.1181. [DOI] [PubMed] [Google Scholar]
- Song C, Zhang Z, Chen X, Zhang Y, Wang C, Liu K. [Study on three kinds of gasoline oxygenates-induced DNA damage in mice fibroblasts] Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi. 2002;20:362–364. [PubMed] [Google Scholar]
- Temple L, Kawabata TT, Munson AE, White KL., Jr Comparison of ELISA and plaque-forming cell assays for measuring the humoral immune response to SRBC in rats and mice treated with benzo[a]pyrene or cyclophosphamide. Fundam. Appl. Toxicol. 1993;21:412–419. doi: 10.1006/faat.1993.1116. [DOI] [PubMed] [Google Scholar]
- US EPA Air quality: revision to definition of volatile organic compounds – exclusion of propylene carbonate and dimethyl carbonate. Fed. Reg. 2009;74:3437–3441. [Google Scholar]
- Veraldi A, Costantini AS, Bolejack V, Miligi L, Vineis P, van Loveren H. Immunotoxic effects of chemicals: A matrix for occupational and environmental epidemiological studies. Am. J. Ind. Med. 2006;49:1046–1055. doi: 10.1002/ajim.20364. [DOI] [PubMed] [Google Scholar]
- Woolhiser MR, Munson AE, Meade BJ. Comparison of mouse strains using the local lymph node assay. Toxicology. 2000;146:221–227. doi: 10.1016/s0300-483x(00)00152-9. [DOI] [PubMed] [Google Scholar]